Abstract
Background
Antibiotic overuse and misuse in primary care are common, highlighting the importance of antimicrobial stewardship (AMS) efforts in this setting. Audit and feedback (A&F) interventions can improve professional practice and performance in some settings.
Objectives and methods
To leverage the expertise from international members of the Joint Programming Initiative on Antimicrobial Resistance – Primary care Antibiotic Audit and feedback Network (JPIAMR-PAAN). Network members all have experience of designing and delivering A&F interventions to reduce inappropriate antibiotic prescribing in primary care settings. We aim to introduce the network and explore ongoing A&F activities in member regions. An online survey was administered to all network members to collect regional information.
Results
Fifteen respondents from 11 countries provided information on A&F activities in their country, and national/regional antibiotic stewardship programmes or policies. Most countries use electronic medical records as the primary data source, antibiotic appropriateness as the main outcome of feedback, and target GPs as the prescribers of interest. Funding sources varied across countries, which could influence the frequency and quality of A&F interventions. Nine out of 11 countries reported having a national antibiotic stewardship programme or policy, which aim to provide systematic support to ongoing AMS efforts and aid sustainability.
Conclusions
The survey identified gaps and opportunities for AMS efforts that include A&F across member countries in Europe, Canada and Australia. JPIAMR-PAAN will continue to leverage its members to produce best practice resources and toolkits for antibiotic A&F interventions in primary care settings and identify research priorities.
Introduction
Approximately 90% of antibiotics are used in the community and outpatient settings.1,2 Around two-thirds of antibiotics are prescribed by physicians in primary care, making this setting highly relevant for antimicrobial stewardship efforts.3,4 Overuse and misuse of antibiotics contributes to rising rates of antimicrobial resistance (AMR) with an estimated 4.95 million and 541 000 deaths in 2019 associated with bacterial AMR globally and in Europe, respectively.5 Antibiotic overuse can also have severe consequences for public health outcomes (e.g. sustained side effects, increased risk of mortality, prolonged hospital stays, loss of protection against infections) and socioeconomic development (e.g. drain on global economy, higher costs of treatment for patients).6
Effective antimicrobial stewardship (AMS) programmes are multifaceted and include data surveillance, education and quality improvement interventions, such as audit and feedback (A&F), to facilitate behaviour change and reduce inappropriate antibiotic prescribing. A&F is a strategy often used to improve professional practice and performance7 and has been defined as ‘any summary of clinical performance of healthcare over a specified period of time’ given verbally, electronically or in written format.8 In general, A&F is effective; a 2012 Cochrane review observed a 4% median improvement in performance, with greater effects observed when baseline performance was poor, when feedback was delivered by a superior/colleague and given more than once (both verbally and written), and when feedback included targets and an action plan.7 In European countries, A&F has been widely used, both as mandatory government-run initiatives and voluntary local initiatives.9 Historically, the UK and the Netherlands were the first countries to initiate A&F in the 1970s and 1980s as voluntary initiatives, and in the Netherlands, A&F was initiated by GPs in primary care settings.9 Existing research illustrates large variations across countries regarding what is audited, the delivery of feedback, and programme ownership.9 A&F is commonly used in Australia as well, including for antibiotics.10 There are a variety of examples of A&F interventions that are operationalized in standard practice.11 Some studies have assessed the effects of A&F in North America, Australia and Western Europe, but there is limited research comparing the use of A&F across different healthcare systems.9,12,13
The Joint Programming Initiative on Antimicrobial Resistance—Primary care Antibiotic Audit and feedback Network (JPIAMR-PAAN) is an international network of experts initiated in response to a call from JPIAMR with the objective to recommend best practices for the delivery of antibiotic prescribing feedback to community clinicians, using behavioural science. The JPIAMR-PAAN was assembled in May 2021, as a collaboration led by researchers in Canada and Norway representing three interrelated disciplines (AMS, primary care and implementation science). The network has more than 30 members representing 11 different countries (Document S1, available as Supplementary data at JAC-AMR Online). The network members were invited to participate in the network because of their interest and expertise in one or more of the three interrelated disciplines.
The main objectives of the JPIAMR-PAAN include: (i) to develop and implement best practice tools and resources for designing and implementing antibiotic prescribing A&F in primary care; and (ii) to identify and prioritize a research agenda to advance the field and optimize A&F to support appropriate antibiotic prescribing in primary care. Further details are available on our website.14
There is uncertainty about the best way of designing and delivering antibiotic A&F interventions in primary care, making the main objectives of JPIAMR- PAAN highly relevant.
In this paper, we provide an overview of existing and planned A&F initiatives as part of AMS programmes in primary care within JPIAMR-PAAN.
Materials and methods
An online survey was administered to all JPIAMR-PAAN network members with the goal of at least one respondent per country. The survey included 11 questions regarding A&F interventions on antibiotic prescribing, separated into three domains: current A&F activities, planned A&F activities and national antibiotic stewardship programme and policy (Document S2). The survey only collected which country the members represented. No other personalized data were collected.
The survey was sent by e-mail with a survey link, along with an invitation to participate, to all JPIAMR-PAAN-members (33), representing 11 countries. The survey was available from 21 October 2021 to 15 January 2022. During this period, respondents received one invitation to participate and three e-mail reminders to complete the survey. We also asked members to provide additional details, information and edits on two occasions (in July and October 2022). The results from the survey were analysed descriptively and additional details were added from simple web searches of key programmes mentioned in the results.
Results
In total we received responses from all 11 countries (Australia, Canada, Denmark, France, Italy, the Netherlands, Norway, Spain, Sweden, Switzerland and the UK), represented by JPIAMR-PAAN members from 15 different individuals. We received two responses from Italy, Sweden, Switzerland and the UK, and we amalgamated all regional responses from the same country.
Current and planned A&F activities
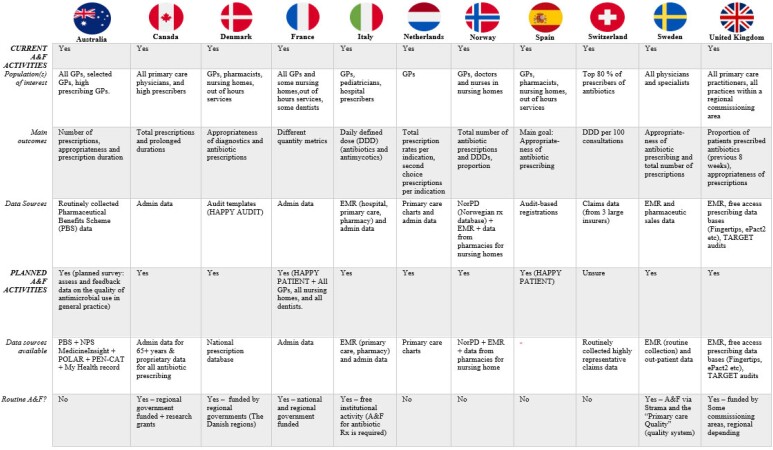
All 11 countries had members who reported current and ongoing A&F work on antibiotic prescribing to primary care providers. Furthermore, 10 out of 11 countries had members who reported planned future work in A&F. In general, the population of interest for A&F was GPs, other primary care physicians, and prescribers in nursing homes (Figure 1).
Figure 1.
Summary of current and planned A&F activities.
Members from Denmark and Spain made reference to the ‘HAPPY PATIENT’ project: a 3 year project with focus on decreasing inappropriate antimicrobial use for common community-acquired infections. The HAPPY PATIENT project was built upon a patient-centred approach, initiated in January 2021, and will be implemented in five target countries (Spain, France, Lithuania, Poland and Greece) with diverse health systems, populations and levels of antibiotic consumption.15,16 The project flyer15 is available in Document S3.
Data sources and outcomes used in A&F activities
Across different countries, significant variability exists in the data sources and outcomes used in A&F activities. The most commonly used data sources were EMRs and claims data and routinely collected administrative data (Figure 1).
Representatives from Denmark reported their use of audit templates as data sources from the ‘HAPPY AUDIT’ project. The HAPPY AUDIT registration sheet is available in Document S4.17 This was a multinational A&F intervention project in the EU, with 15 partners from nine countries.17 The project was initiated in 2008 through quality improvement of GPs in the diagnosis and treatment of respiratory tract infections by developing intervention programmes.17
England reported using ‘Treat Antibiotics Responsibly, Guidance, Education and Tools’ (TARGET) audits for prescriber self-assessment. The TARGET website hosts a range of AMS tools including, for example, patient information leaflets, self-assessment checklists (Document S5), toolkits and resources to help practitioners implement AMS activities.18 Respondents from England also mentioned various free-access monthly practice-level prescribing databases such as ‘Fingertips’ and ‘ePact2’, which allow prescribers in England to compare their practice against local, regional or national settings/average.19,20 Lowering AntiMicrobial Prescribing (LAMP) is a regional UK project, which utilizes EMR data.21 Similarly, the Australian Commission on Safety and Quality in Health Care and the Australian National Centre for Antimicrobial Stewardship also provide a variety of AMS resources for practitioners aiming to implement AMS activities.22,23
The survey showed differences in the outcome metrics used for A&F interventions across countries, but the most common outcomes used were total number of antibiotic prescriptions and antibiotic prescribing appropriateness (Figure 1).
National antibiotic stewardship policy/programme(s)
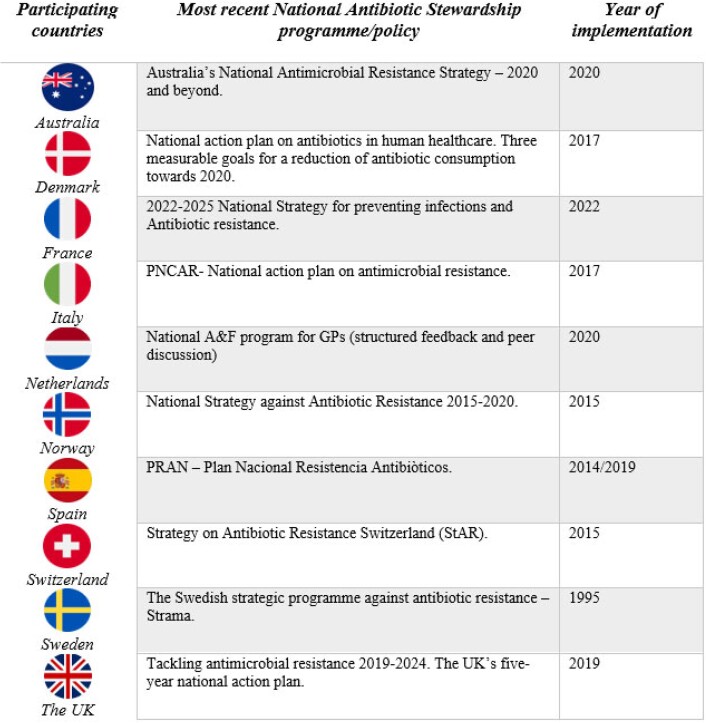
Members from 9 out of 11 countries reported having a national AMS programme or policy. Figure 2 provides an overview of the most recent national antibiotic stewardship programme/policy. We discovered that these national action plans did not have primary care as main focus. One exception was Denmark with two goals aimed at primary care. Several countries had national programmes developed using A&F as a strategy in primary care.
Figure 2.
National AMS programmes/policies.
Australia’s representative reported having a national antimicrobial strategy, which is a 20 year vision to minimize the development and spread of AMR, and that builds upon the 2015 strategy. In addition, one of the pillar objectives is appropriate usage and stewardship practices, with continued work with, for example, GPs to develop AMS initiatives in community settings.24
From France, the presence of a national AMR action plan that uses the One Health approach was indicated, with five objectives focused on awareness, education, research and innovation, monitoring and surveillance, and interministerial and international actions governance,25 and is complemented by an operational plan (2022–25) in human health, which must be supported, developed and deployed regionally and locally, that includes primary care, to achieve a long-term impact.26
Members from Italy also reported having a national AMR control plan (PNCAR) along with regional AMR plans. The Italian PNCAR (2017–20) outlines the strategies for surveillance, prevention and control of healthcare-associated infections, and specific AMR prevention activities.27
The Netherlands has a national voluntary programme for GPs to provide data from their charts and get structured feedback and discussion in small groups.
Norway described a 5 year strategy (2015–20) with goals of reducing total antibiotic use through reductions in inappropriate antibiotic prescribing.28 One of the focus areas of this action plan is to improve prescribing practices in all sectors, with promises to investigate the creation of a feedback system to GPs, among others, on their antibiotic prescribing practice, with peer assessment.28 Following the strategy, a 5 year governmental action plan was in place in 2016,29 leading to the establishment of national interventions based on A&F for GPs and nursing homes (RAK/RASK, more appropriate antibiotic use in municipalities and nursing homes).30,31 See Document S6 for the RAK course.32 A recent study demonstrated a significant reduction in total antibiotic use by the end of 2020; however, the COVID-19 pandemic likely contributed to the observed reduction with reduced use of health services.33
Spain reported having limited stewardship programmes in their country. However, we found the presence of a National Plan against Resistance to Antibiotics (PRAN) (2019–21), with the aim of raising awareness of AMR.34
A prominent stewardship programme in Europe is Strama in Sweden. Strama was founded in 1995 and is now an advisory body to the Public Health Agency of Sweden, and the Strama work consists of networking and interaction and is ‘characterized as a cyclical process with continuous communication with prescribers’.35 A 10 year follow-up analysis of Strama demonstrated its contributions to the reduction in antibiotic use nationally, but called for sustained stewardship efforts.36 In Sweden, Strama has been successfully implemented on a national level, where regional groups provide feedback on antibiotic consumption to prescribers using local data, and carry out local stewardship interventions.37
The UK also published a 5 year national action plan in 2019 to support their 20 year vision on AMR, in which resistance is effectively contained and controlled with three key messages: reducing the need for antimicrobials; optimizing the use of antimicrobials; and investing in innovation, supply and access.38
Denmark also have a national action plan with a main goal of 30% reduction in the following three areas: redeemed antibiotic prescription; broad-spectrum prescriptions in primary care; and consumption of critically important antibiotics in hospitals, whereas the first two goals are aimed at antibiotic consumption in the primary healthcare section.39
Canada and Switzerland reported to have no national antibiotic stewardship programme/policy at the time of the survey, but through online search we found Switzerland’s Strategy on Antibiotic Resistance (StAR) with eight strategic objectives to ensure the effectiveness of antibiotics long term.40
Routine A&F activities and funding sources
Members from 6 out of 11 countries reported routine antibiotic A&F activities in primary care in their country, with notable differences in funding sources. Australia has routine A&F in hospitals, but this does not yet exist in primary care. In Ontario, Canada some activities are government funded, while others are conducted with support from academic research grants. Denmark reported having routine A&F that could be supported by the Danish regions. The five Danish regions were formed during the 2007 reform, and have the primary responsibility for overseeing hospitals, GPs and specialists in private practice.41 In France, activities are primarily coordinated and funded by the national health insurance and the Ministry of Health.42,43 In Italy, A&F for antibiotic prescribing is a required institutional activity in each Local Health Authority. In Sweden, A&F is carried out via Strama (Swedish strategic programme against antibiotic resistance)35 in all 21 Swedish regions and ‘Primary Care Quality’, a quality system for the primary care setting for quality improvement.44 In several Swedish regions the primary healthcare centres deliver an annual report on the outcome of their work to improve rational antibiotic prescribing and in some regions this also includes collegial reflective meetings and discussion of case studies, which was highlighted in a study as important by GPs to achieve rational antibiotic use.35,45 The regional Strama groups are funded by the Swedish regions. The UK also has routine A&F for antibiotic prescribing for primary care, some of which is paid for by Intermediate Care Boards (formerly clinical commissioning groups), but this is region dependent.
In Sweden, A&F occurs as both mandatory and voluntary activities, and similarly, A&F is a required institutional activity in Italy and France. Other programmes mentioned by survey respondents were voluntary A&F activities.
Discussion
Our survey has identified that some European countries, Australia and Canada have implemented heterogeneous AMS programmes in primary care, which commonly feature A&F as a component.
Survey results showed that all participating countries are actively engaged in primary care antibiotic A&F, but with various populations of interest, outcomes and data sources. There was significant variability in the reported data sources and outcomes for A&F activities. Multiple countries utilized EMRs as the main data source to identify outcomes of total number of antibiotic prescriptions and appropriateness of antibiotic prescribing. Such variability may indicate uncertainty on best practices related to the development and implementation of A&F interventions, and antibiotic stewardship programmes at large. Historical context and resource availability may also contribute to the observed variability.
There are a variety of metrics that can be used to measure community antibiotic use, which have different benefits, interpretations and limitations. Antibiotic appropriateness is generally the preferred metric; however, it can be challenging and costly to accurately obtain from administrative or EMR data.46,47 Canada reported the use of prolonged duration as an outcome for A&F interventions and demonstrated increased effectiveness of reducing overall antibiotic use with the inclusion of prolonged duration prescribing in the feedback and action plans.48,49 France is exploring the concept of ‘proxy indicators’, estimating the appropriateness of antibiotic prescribing at prescriber or facility level, based on routine administrative data.26
The WHO has called for all nations to develop AMS action plans.6 In May 2015, the WHO highlighted the crises of systematic overuse and misuse of antimicrobial drugs in both human medicine and food production, leading to the threat of AMR for all countries, ultimately calling for immediate global action to prevent a post-antibiotic era.6
Our survey results showed that Australia and most European countries that participated in this survey responded to the WHO’s call for a national action plan for AMR. Canada reported no national plan at the time of the survey, which may be partly attributed to the separation of health ministries and mandates on a provincial level and delays from the COVID-19 pandemic. Nonetheless, the success and implementation of these action plans vary by regions, and the sustainability of these interventions are unclear. Continuous international collaborations can be beneficial in sharing the results of each national action plan and leveraging expertise to identify the most successful component of these antimicrobial stewardship efforts. The European Commission has recognized AMR as a high priority and allocated a budget of EUR 50 million of direct grants with the one of the expected results being updated national action plans across the EU.50 The JPIAMR-PAAN members who participated in this survey are from countries with publicly funded healthcare systems and (at least partial) publicly funded medication programmes. This may facilitate data collection and operationalizing A&F initiatives on a population level.
The feedback presentation and composition of antibiotic A&F reports varied between the participating countries. For example, Canada, the UK and Australia have published their studies using mailed letters to high-prescribing primary care physicians with good results (see Documents S7–S9 for example letters).10,48,51 These reports had behavioural change messaging with a peer-comparison component. In contrast, studies from Switzerland conducted among primary care physicians demonstrated that A&F intervention with peer comparison had no significant effect on reducing antibiotic use.52,53 Conflicting results on the impact of A&F interventions are likely related to the details of the intervention design, the metrics and comparators used, and how the intervention links the data and messaging to the desired behaviour change.54 Further research is needed that compares various methods of peer-comparison A&F in primary care settings.3 A recent study from Norway among GPs found motivation for quality improvement work to be being better doctors, and discussions in safe peer groups seemed to be a safe arena, and may be an important setting for quality improvement work.55
Value of the paper
This survey may contribute to the literature by comparing and contrasting different approaches to AMS and A&F in primary care from JPIAMR-PAAN member countries, providing opportunities to foster collaboration and for programmes to build off one another. A&F is an important component of AMS in primary care and this paper provides insights into the variability in which A&F is currently being implemented or planned.
Limitations
There are notable limitations in this paper on A&F interventions and AMS efforts.
Survey results for each country were obtained from only one or two representatives, and limited to the 11 countries presented by JPIAMR-PAAN members. Furthermore, web-based search was conducted to confirm or supply survey results. We only received information on our members’ current and planned A&F activities, and not all existing AMS and A&F activities in their country.
Further, these data are not generalizable to low- or middle-income countries as there are unique challenges related to both the burden of drug-resistant infections and response to AMR.56
Conclusions
We hope to encourage further discourse in the field of antibiotic A&F and develop guidance for the development and implementation of A&F interventions. The goals of JPIAMR-PAAN are to provide further insights and recommendations on best practices for antibiotic A&F to primary care clinicians. Further work from this network will outline best practices for development and conducting A&F in primary care, as well as define research priorities to advance the field and improve antibiotic prescribing to slow the emergence of AMR. We will continue to leverage the network’s expertise to explore the design of A&F across different healthcare systems to advance and provide guidance to the field.
Supplementary Material
Acknowledgements
We would like to thank all the members of the JPIAMR-PAAN for participating and providing valuable input from their country. A full list of JPIAMR-PAAN members is in Document S1.
Contributor Information
Benedikte Olsen Michalsen, Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Alice X T Xu, Health Protection, Public Health Ontario, Toronto, Canada; Public Health Sciences, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada.
Sarah L Alderson, Leeds Institute of Health Sciences, University of Leeds, Leeds, UK; Oaklands Health Centre, Holmfirth, UK.
Lars Bjerrum, Section of General Practice and Research Unit for General Practice, Department of Public Health, University of Copenhagen, Copenhagen, Denmark.
Jamie Brehaut, Centre for Practice-Changing Research (CPCR), Ottawa Hospital Research Institute, Ottawa, Canada.
Heiner C Bucher, University of Basel, c/o Division of Clinical Epidemiology, Basel, Switzerland.
Janet Clarkson, University of Dundee, NHS Education for Scotland, Dundee, UK.
Eilidh Duncan, Health Services Research Unit, University of Aberdeen, Aberdeen, UK.
Jeremy Grimshaw, Department of Medicine, University of Ottawa Canada Research Chair in Health Knowledge Transfer and Uptake, Ottawa, Canada.
Ronny Gunnarsson, General Practice/Family medicine, Department of Public Health and Community Medicine, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Goteborg, Sweden; Centre for Antibiotic Resistance Research (CARe) at University of Gothenburg, Gothenburg, Sweden.
Sigurd Høye, Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Noah Ivers, Department of Family Medicine, Women’s College Hospital, Toronto, Canada.
Donna M Lecky, Primary Care & Interventions Unit, UK Health Security Agency, Gloucester, UK.
Morten Lindbæk, Antibiotic Centre for Primary Care, Department of General Practice, Institute of Health and Society, University of Oslo, Oslo, Norway.
Carl Llor, Department is University Institute in Primary Care Research, University Institute in Primary Care Research Jordi Gol, Via Roma Health Centre, Barcelona, Spain; CIBER de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain; Research Unit for General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark.
Pia Touboul Lundgren, Département de Santé Publique, Hôpital de l'Archet, Nice, France.
Denise O’connor, School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, 3004, Australia.
Celiné Pulcini, Public Health Research Unit, Université de Lorraine, APEMAC, Nancy, France; CHRU-Nancy, Université de Lorraine, Service de Maladies Infectieuses et Tropicales, Nancy, France.
Craig Ramsay, Health Services Research Unit, University of Aberdeen, Aberdeen, UK.
Pär-Daniel Sundvall, General Practice/Family medicine, Department of Public Health and Community Medicine, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Research, Education, Development & Innovation, Primary Health Care, Region Västra Götaland, Goteborg, Sweden; Centre for Antibiotic Resistance Research (CARe) at University of Gothenburg, Gothenburg, Sweden.
Theo Verheij, Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrect, The Netherlands.
Kevin L Schwartz, Health Protection, Public Health Ontario, Toronto, Canada; Public Health Sciences, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada; Division of Infectious Diseases, St. Joseph's Health Centre - Unity Health Toronto, Toronto, Ontario, Canada.
JPIAMR-PAAN:
Anna Acampora, Sarah L Alderson, Pablo Alonso Coello, Attila Altiner, Lars Bjerrum, Jamie Brehaut, Benjamin Brown, Heiner C Bucher, Chris Butler, Laura Cavazzuti, Janet Clarkson, Marina Davoli, An De Sutter, Mirko Di Martino, Eilidh Duncan, Nick Francis, Roberto Grilli, Jeremy Grimshaw, Ronny Gunnarsson, Michael Hallsworth, Lars Hemkens, Sigurd Hoye, Noah Ivers, Tasneem Khan, Donna M Lecky, Morten Lindbaek, Jeff Linder, Paul Little, Carl Llor, Fabiano Lorencatto, Denise O’connor, Celine Pulcini, Craig Ramsay, Rosella Saulle, Kevin L Schwartz, Maia Simon, Pär-Daniel Sundvall, Monica Taljaard, Pia Touboul Lundgren, Akke Vellinga, Jan Verbakel, and Theo Verheij
Funding
This work was supported by funding from the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) (Canadian Institute of Health Research grant number 448378) as part of JPIAMR Network Plus 2020.
Transparency declarations
The JPIAMR-PAAN have received funding from the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR). Carl Llor declares having received research grants from Abbott Diagnostics. Jeremy Grimshaw holds a Canada research Chair in Health Transfer and Uptake. All other authors: nothing to declare.
Supplementary data
Documents S1-S9 are available as Supplementary data at JAC-AMR Online.
References
- 1. Bell BG, Schellevis F, Stobberingh Eet al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14: 13. 10.1186/1471-2334-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanchez GV, Fleming-Dutra KE, Roberts RMet al. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65: 1–12. 10.15585/mmwr.rr6506a1 [DOI] [PubMed] [Google Scholar]
- 3. Shuldiner J, Schwartz KL, Langford BJet al. Optimizing responsiveness to feedback about antibiotic prescribing in primary care: protocol for two interrelated randomized implementation trials with embedded process evaluations. Implement Sci 2022; 17: 17. 10.1186/s13012-022-01194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. UK Health Security Agency . English surveillance programme for antimicrobial utilisation and resistance (ESPAUR). Report 2021 to 2022. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1069632/espaur-report-2020-to-2021-16-Nov-FINAL-v2.pdf.
- 5. Mestrovic T, Aguilar G, Swetschinski LRet al. The burden of bacterial antimicrobial resistance in the WHO European region in 2019: a cross-country systematic analysis. Lancet Public Health 2022; 7: e897–913. 10.1016/S2468-2667(22)00225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . Global action plan on antimicrobial resistance. 2016. https://www.who.int/publications/i/item/9789241509763.
- 7. Ivers N, Jamtvedt G, Flottorp Set al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev 2012; 6: CD000259. 10.1002/14651858.CD000259.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jamtvedt G, Young JM, Kristoffersen DTet al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2006; 3: CD000259. 10.1002/14651858.CD000259 [DOI] [PubMed] [Google Scholar]
- 9. Jamtvedt G, Flottorp S, Ivers N. Audit and feedback as a quality strategy. In: Busse R, Klazinga N, Panteli Det al., eds. Improving Healthcare Quality in Europe: Characteristics, Effectiveness and Implementation of Different Strategies. European Observatory on Health Systems and Policies, 2019; Chapter 10. https://www.ncbi.nlm.nih.gov/books/NBK549284/. [PubMed] [Google Scholar]
- 10. Australian Government . Nudge vs Superbugs. A behavioural economics trial to reduce the overprescribing of antibiotics. 2018. https://behaviouraleconomics.pmc.gov.au/sites/default/files/projects/report-nudge-vs-superbugs.pdf.
- 11. Health Quality Ontario . MyPractice Reports. https://www.hqontario.ca/quality-improvement/practice-reports.
- 12. The Ottawa Hospital Research Institute . The Audit & Feedback Metalab. Webinars and Presentations. https://www.ohri.ca/auditfeedback/resources-webinars/.
- 13. Khan T, Alderson S, Francis JJet al. Repeated analyses of national clinical audit reports demonstrate improvements in feedback methods. Implement Sci Commun 2020; 1: 106. 10.1186/s43058-020-00089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. JPIAMR-PAAN . Joint Programming Initiative on Antimicrobial Resistance. Primary Care Antibiotic Audit and Feedback Network. https://www.jpiamr-paan.org/.
- 15. HAPPY PATIENT . The Health Alliance for Prudent Prescription and Yield of Antibiotics from a Patient-Centred Perspective. https://happypatient.eu/. [DOI] [PMC free article] [PubMed]
- 16. Bjerrum A, García-Sangenís A, Modena Det al. Health alliance for prudent prescribing and yield of antibiotics in a patient-centred perspective (HAPPY PATIENT): a before-and-after intervention and implementation study protocol. BMC Prim Care 2022; 23: 102. 10.1186/s12875-022-01710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bjerrum L, Munck A, Gahrn-Hansen Bet al. Health alliance for prudent prescribing, yield and use of antimicrobial drugs in the treatment of respiratory tract infections (HAPPY AUDIT). BMC Fam Pract 2010; 11: 29. 10.1186/1471-2296-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RCGP Learning . TARGET antibiotics toolkit hub. https://elearning.rcgp.org.uk/course/view.php? id=553.
- 19. Office for Health Improvement & Disparities . Fingertips. Public health data. https://fingertips.phe.org.uk/.
- 20. NHS Business Services Authority . ePACT2. https://www.nhsbsa.nhs.uk/access-our-data-products/epact2.
- 21. NHS, West Yorkshire Research and Development . LAMP, Lowering AntiMicrobial prescribing. https://www.westyorksrd.nhs.uk/lamp.
- 22. NCAS . National Centre for Antimicrobial Stewardship. https://www.ncas-australia.org/.
- 23. Australian Commission on Safety and Quality in Health Care. https://www.safetyandquality.gov.au/.
- 24. Australian Government 2020 . Australia’s National Antimicrobial Resistance Strategy. 2020 & beyond. https://www.amr.gov.au/resources/australias-national-antimicrobial-resistance-strategy-2020-and-beyond.
- 25. Ministère des Solidaritès et de la Santè (Ministry of Solidarity and Health) . French national action plan on antimicrobial resistance: innovative measures. 2021.https://solidarites-sante.gouv.fr/IMG/pdf/8_pages_antibioresistance-final-en.pdf.
- 26. Ministère Des Solidarités et de la Santé (Ministry of Solidarity and Health) 2022. 2022-2025 National Strategy for preventing Infections and Antibiotic Resistance. https://solidarites-sante.gouv.fr/IMG/pdf/national_strategy_for_preventing_infections_and_antibiotic_resistance_2022-2025_.pdf.
- 27. Ministero della Salute (Ministry of Health) . PNCAR—National action plan on antimicrobial resistance. 2017.https://www.salute.gov.it/imgs/C_17_opuscoliPoster_362_ulterioriallegati_ulterioreallegato_0_alleg.pdf.
- 28. Norwegian Ministries . National Strategy against Antibiotic Resistance 2015–2020. 2015.https://www.regjeringen.no/contentassets/5eaf66ac392143b3b2054aed90b85210/antibiotic-resistance-engelsk-lavopploslig-versjon-for-nett-10-09-15.pdf.
- 29. Norwegian Ministry of Health and Care Services . Action plan against antibiotic resistance in health care services with the aim of reducing the use of antibiotics in the population by 30 precent by the end of 2020 (Handlingsplan mot anitbiotikaressistens i helsetjenesten med det mål å redusere antibiotikabruken i befolkningen med 30 prosent innen utløpet av 2020). 2016.https://www.regjeringen.no/contentassets/915655269bc04a47928fce917e4b25f5/handlingsplan-antibiotikaresistens.pdf.
- 30. Antibiotic Centre for Primary Care (ASP) . More correct antibiotic use in the municipalities (RAK) [Riktigere Antibiotikabruk i Kommunene (RAK)]. https://www.antibiotika.no/rak-riktigere-antibiotikabruk-i-kommunene/.
- 31. Antibiotic Centre for Primary Care (ASP) . RASK - More correct use of antibiotics for nursing homes in municipalities (RASK—Riktigere antibiotikabruk for sykehjem i kommunene). https://www.antibiotika.no/2017/04/21/rask-2/.
- 32. Høye S. More appropriate antibiotic use in municipalities. (‘Riktigere antibiotikabruk i kommunene’. Utposten: blad for allmenn- og samfunnsmedisin 2017; 46: 16–9. [Google Scholar]
- 33. Blix HS, Høye S. Use of antibiotics during the COVID-19 pandemic. (‘Bruk av antibiotika under covid-19-pandemien’). Tidsskr Nor Laegeforen 2021; 141. [DOI] [PubMed] [Google Scholar]
- 34. The National Plan against Antibiotic resistance (PRAN) . PRAN - Plan Nacional Resistencia Antibiòticos. 2019. https://www.resistenciaantibioticos.es/es/quienes-somos.
- 35. Public Health Agency of Sweden . Swedish work on containment of antibiotic resistance. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/communicable-disease-control/antibiotics-and-antimicrobial-resistance/swedish-work-on-containment-of-antibiotic-resistance/.
- 36. Mölstad S, Erntell M, Hanberger Het al. Sustained reduction of antibiotic use and low bacterial resistance: 10-year follow-up of the Swedish Strama programme. Lancet Infect Dis 2008; 8: 125–32. 10.1016/S1473-3099(08)70017-3 [DOI] [PubMed] [Google Scholar]
- 37. STRAMA . The Swedish strategic programmes against antibiotic resistance (Samverknad mot antibiotikaresistens). https://strama.se/? lang.
- 38. HM Government . Tackling antimicrobial resistance 2019–2024. The UK’s five-year national action plan. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/784894/UK_AMR_5_year_national_action_plan.pdf. [DOI] [PubMed]
- 39. The Danish Ministry of Health . National action plan on antibiotics in human healthcare. Three measurable goals for a reduction of antibiotic consumption towards 2020 (National handlingsplan til mennesker). 2017.https://sum.dk/Media/6/2/National-handlingsplan-for-antibiotika-til-mennesker-UK%20version.pdf.
- 40. The Federal Council . Strategy on Antibiotic Resistance Switzerland (StAR) (trategiebericht StAR). 2015. https://www.star.admin.ch/star/en/home/star/strategie-star.html.
- 41. Schmidt M, Schmidt SAJ, Adelborg Ket al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019; 11: 563–91. 10.2147/CLEP.S179083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ministére De la Transformation et de la Fonction Publiques. Réduire la Prescription D'antibiotiques en médecine ambulatorie (Reducing The Prescribing of Antibiotics in Ambulatory Medicine). 2022. https://www.modernisation.gouv.fr/files/2022-04/CNAM_Antibiotiques_rapport%20final_030322_MC%20%28004%29.pdf.
- 43. Santé publique France, Répias . Surveillance de la consommation d'antibiotiques en Ehpad. Mission SPARES. Données 2018-2019. (Monitoring of antibiotic consumption in nursing homes. SPARES mission. 2018-2019 data). 2021. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/resistance-aux-antibiotiques/documents/rapport-synthese/surveillance-de-la-consommation-d-antibiotiques-en-ehpad.-mission-spares.-donnees-2018-2019#:∼:text=Au%20total%2C%20455%20structures%20EHPAD,de%20rattachement%20de%20l%27EHPAD.
- 44. Sweden’s Municipalities and Regions . Primary Care Quality. 2021.https://skr.se/skr/englishpages/activities/primarycarequality.10073.html.
- 45. Sundvall PD, Skoglund I, Hess-Wargbaner Met al. Rational antibiotic prescribing in primary care: qualitative study of opportunities and obstacles. BJGP Open 2020; 4: bjgpopen20X101079. 10.3399/bjgpopen20X101079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leung V, Langford BJ, Ha Ret al. Metrics for evaluating antibiotic use and prescribing in outpatient settings. JAC-Antimicrobial Resistance. 2021; 3: dlab098. 10.1093/jacamr/dlab098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Versporten A, Gyssens IC, Pulcini Cet al. Metrics to assess the quantity of antibiotic use in the outpatient setting: a systematic review followed by an international multidisciplinary consensus procedure. J Antimicrob Chemother 2018; 73Suppl 6: vi65. 10.1093/jac/dky119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwartz KL, Ivers N, Langford BJet al. Effect of antibiotic-prescribing feedback to high-volume primary care physicians on number of antibiotic prescriptions: a randomized clinical trial. JAMA Intern Med 2021; 181: 1165–73. 10.1001/jamainternmed.2021.2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daneman N, Lee SM, Bai Het al. Population-wide peer comparison audit and feedback to reduce antibiotic initiation and duration in long-term care facilities with embedded randomized controlled trial. Clin Infect Dis 2021; 73: e1296–304. 10.1093/cid/ciab256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. European Commission . Annex II to the Commission Implementing decision. C(2022) 5436 final. 2022.https://health.ec.europa.eu/system/files/2022-07/com_2022-5436_annex2_en.pdf.
- 51. Hallsworth M, Chadborn T, Sallis Aet al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016; 387: 1743–52. 10.1016/S0140-6736(16)00215-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hemkens LG, Saccilotto R, Reyes SLet al. Personalized prescription feedback using routinely collected data to reduce antibiotic use in primary care: a randomized clinical trial. JAMA Intern Med 2017; 177: 176–83. 10.1001/jamainternmed.2016.8040 [DOI] [PubMed] [Google Scholar]
- 53. Aghlmandi S, Halbeisen FS, Saccilotto Ret al. Effect of antibiotic prescription audit and feedback on antibiotic prescribing in primary care: a randomized clinical trial. JAMA Intern Med 2023; 83: 213–20. 10.1001/jamainternmed.2022.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fox CR, Doctor JN, Goldstein NJet al. Details matter: predicting when nudging clinicians will succeed or fail. BMJ 2020; 370: m3256. 10.1136/bmj.m3256 [DOI] [PubMed] [Google Scholar]
- 55. Eide TB, Øyane N, Høye S. Promoters and inhibitors for quality improvement work in general practice: a qualitative analysis of 2715 free-text replies. BMJ Open Qual 2022; 11: e001880. 10.1136/bmjoq-2022-001880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: 629–55. 10.1016/S0140-6736(21)02724-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.