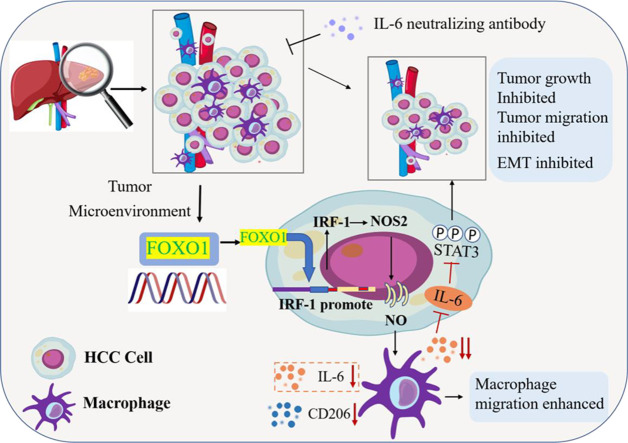
Highlights
-
•
FOXO1 impairs HCC progression indirectly through macrophages.
-
•
FOXO1 transcriptional target IRF-1 increased tumor cells NO expression.
-
•
NO recruits macrophages and suppressing IL-6 releasing from it.
-
•
The shaped macrophages inhibited HCC progression by inhibiting IL-6/STAT3 activation.
Keywords: Hepatocellular carcinoma, Macrophages, FOXO1, Inflammation
Abstract
Tumor heterogeneity dominates tumor biological behavior and shapes the tumor microenvironment. However, the mechanisms of tumor genetic features modulate immunity response were not clearly clarified. Tumor associated macrophages (TAMs) exert distinct immune functions in the progression of hepatocellular carcinoma (HCC) based on the inducible phenotype. FOXO family members sense changes in the extracellular or intracellular environment by activating a series of signaling pathways. FOXO1, a transcription factor that a common suppressor in hepatocellular carcinoma, correlated with a better tumor biological behavior in HCC through shaping macrophages anti-tumour response. Here, we found that human HCC tissue microarray (TMA) slides were employed to showed tumor derived FOXO1 negatively related with distribution of protumour macrophages. This phenomenon was confirmed in mouse xenograft model and in vitro. HCC-derived FOXO1 inhibits tumorigenesis not only by targeting tumor cells but also by synchronizing with re-educated macrophages. These effects may be partially dependent on FOXO1 transcriptionally modulates IRF-1/nitrio oxide (NO) axis in exerting effects in macrophages and decreasing IL-6 releasing from macrophages in tumor microenvironment indirectly. This feedback suppressed the progression of HCC by inactivation of IL-6/STAT3 in HCC. It implicates the potential role of FOXO1 in the therapeutic effects for modulating immune response by targeting macrophages.
Graphical abstract
Introduction
Hepatocellular carcinoma (HCC) is an inflammation-related malignancy and one of the major leading causes of cancer death worldwide. More than ninety percent of HCC is progressively initiated and unfolded in the context of hepatocyte injury, inflammation reaction and regeneration [1]. The major risk factor for HCC is chronic inflammation originating from viral hepatitis and nonviral liver diseases. Although the etiology of HCC is unclear, the huge molecular heterogeneity resulting from the accumulation of genetic mutations in the passenger process makes it difficult to clarify the molecular mechanisms in the progression of HCC.
The molecular heterogeneity of HCC not only regulates tumor cell biological behavior but also shapes immunogenicity in the tumor microenvironment (TME) [2]. FOXO1 is a transcription factor that belongs to the forkhead box family (FOXO), which is broadly expressed in the liver, immune system, and muscle and has diverse functions, including cell cycle, cell growth, cell metabolism, tumor suppression, and immunity, in a tissue-specific manner [3,4]. FOXO1 regulates the immune response by affecting celluar senescence, immune cell migration and cytokine expression in macrophages and T cells [5], [6], [7]. FOXO family members sense changes in the extracellular or intracellular environment by activating a series of signaling pathways, such as the PI3K/AKT, IGF and p53 pathways. This tight regulation coordinates FOXO1 expression with surrounding microenvironment changes [8].
Recruitment of immune cells such as macrophages in tumors has been correlated with the different prognoses of patients [9,10]. We found that there was a difference in the allocation of macrophages in HCC tissues with various FOXO1 expression levels. FOXO1 regulates macrophage M1 differentiation by positively activating TLR2 and TLR4 signaling and upregulating MHC II expression, which suppresses HCC tumor progression [6,11]. Whether FOXO1 expression derived from tumor cells could affect tumor immunity in HCC has not been clarified. Here, we investigated whether FOXO1 shows a crosstalk role in tumor cells and macrophages in the progression of HCC.
Materials and Methods
Cell culture and reagents
Hepa1-6, Huh7, THP-1, and 293T cells were purchased from ATCC company and cultured in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (Sigma Aldrich, St. Louis, USA) and cultured under 5% carbon dioxide according to the protocols. THP-1 cells were stimulated with 200 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma‒Aldrich, St. Louis, USA) for 24 hours to induce macrophage differentiation as described [12]. SYBR green real-time PCR assay kit (Thermo Fisher, Waltham, MA, USA), and transwell cell culture plate (Sigma Aldrich).
Mice
Wild-type C57BL/6J mice and C57BL/6 SCID mice were purchased from Jackson Laboratory (Jackson Laboratory, Bar Harbor, Maine, USA). All the mice were male and between 8 and 10 weeks old.
Mouse peritoneal macrophage (PM) collection
Peritoneal macrophages were collected by injecting 10 ml of sterilized PBS into the peritoneum of wild-type C57BL/6 mice after sacrifice. Peritoneal exudate cells were isolated with a mouse monocyte isolation kit (Stem Cell, Vancouver, Canada) as described [13].
Xenograft in a mouse model
One million different groups of Hepa1-6 cells were injected subcutaneously into the right upper flank of C57BL/6 SCID mice with 200 μl of PBS. In the coinjection groups, peritoneal macrophages were mixed with Hepa1-6 cells (1:1) [14,15]. Tumor size was measured every three days. Volume was calculated as length × width × width/2. Two weeks later, the mice were sacrificed to obtain tumor samples.
Human HCC tissues
Thirty-two paired fresh frozen HCC samples, diagnosed by pathology, were obtained from HCC patients who underwent hepatectomy between 2010 and 2017 at the liver cancer center, Department of Surgery, University of Pittsburgh Medical Center (UPMC). This study was approved by the ethics committee of UPMC. The pathological characteristics are presented in Table 1. Every tissue microarray (TMA) of 51 paired tumor and nontumor tissue paraffined samples from patients who underwent hepatectomy at the Second Hospital of Anhui Medical University was prepared for immunohistochemistry staining (IHC). This study protocol was approved by the Hospital Institutional Review Committee.
Table 1.
Correlation between FOXO1 expression and clinicopathologic characteristics of HCC patients from UPMC.
| Clincalpathologcial characteristics | FOXO1 expression |
P value | ||
|---|---|---|---|---|
| High (17) | Low (15) | |||
| Etiology | 0.096 | |||
| Non-alcoholic steatohepatitis | 1 | 8 | ||
| Viral hepatitis | 10 | 2 | ||
| No | 6 | 5 | ||
| Tumor size (cm) | 6.65±5.25 | 7.49±4.67 | 0.492 | |
| Differentiation | 0.911 | |||
| High | 1 | 10 | ||
| Moderate | 5 | 15 | ||
| Poor | 1 | 0 | ||
| Vascular invasion | 0.036* | |||
| Yes | 1 | 16 | ||
| No | 6 | 9 | ||
| TMN Stage | 0.966 | |||
| T1 | 3 | 2 | ||
| T2 | 9 | 9 | ||
| T3/T4 | 5 | 4 | ||
P < 0.05.
Immunohistochemistry
The sections of TMAs were 4-μm thick, and immunohistochemistry staining was performed according to a previous protocol [16]. The polink-2 plus polymer HRP detection system (PV-9001, ZSBIO Company, Beijing, China) was used. Primary antibodies against FOXO1 (Abcam), CD68 (Abcam), CD206 (Abcam), IL-6 (Abcam), Vimentin (Sigma), and phospho-STAT3 (Tyr705) (CST) were incubated at 4°C overnight. The secondary antibody (SPN9001, ZSBIO Company, Beijing, China) was incubated at room temperature for 1 hour. Two independent pathologists determined and recorded FOXO1 expression levels of all tissue slides.
Immunofluorescence analysis
Frozen tissues from human and mouse HCC samples were embedded in optimum cutting temperature (OCT) compound (Sakura, USA), and 8-μm-thick sections were obtained. Two percent paraformaldehyde was used to fix samples. Then, 0.1% Triton X-100 and 10% FBS in PBS were employed as the permeabilization solution. Then, primary antibodies were incubated overnight. F4/80 (Abcam), CD206 (Abcam), and IL-6 (Abcam). After washing, specific secondary antibodies or F-actin labeled with Alexa Fluor 488 (Thermo Fisher) and DAPI were used for staining. Photos were observed and acquired by a Nikon A1 confocal microscope (Nikon, Melville, NY, USA).
Western blotting
Whole cell lysates and cell nuclei protein were prepared with cell lysis buffer and a cell fractionation kit (CST). BCA protein assays and Western blotting were performed as described [17]. Anti-FOXO1 (Abcam, Cambridge, MA, USA), IRF-1 (CST, Beverly, MA, USA), NOS2 (Santa Cruz, Dallas, TX, USA), Vimentin (Sigma), N-cadherin (Sigma), β-actin (Sigma), Lamin A/C (CST), phospho-STAT3 (Tyr705) (CST), and STAT3 (CST).
Cell transfection
The human FOXO1 lentiviral vector plasmid, negative control vector plasmid, and lentivirus packaging assay kit were purchased from ABM (ABM, Richmond, BC, Canada). Mouse FOXO1 lentiviral-expressing particles and negative control lentivirus particles were purchased from Amsbio (Milton Park, Abingdon, UK). Human FOXO1 lentiviral-expressing particles were established according to the manufacturer's instructions in 293T cells. Blasticidin 35 μg/ml (InvivoGen, San Diego, CA, USA) and puromycin 0.6 μg/ml (InvivoGen) were used to select transfected Hepa1-6 and Huh7 cells for 3 weeks, respectively. The adenoviral IRF-1 expression construct was constructed as previously described [18]. HCC cells were transfected with an adenoviral IRF-1 expression construct for 48 hours.
Cell proliferation assay
A cell proliferation assay was employed by a cell counting kit-8 assay (MedChemExpress, Monmouth Junction, NY, USA). Hepa1-6 and Huh7 cells were plated in 96-well plates at 2 × 103, and CCK8 was added and incubated according to the manufacturer's instructions. Absorbance was set at 450 nM and measured using a microplate reader (BioTek, Winooski, VT, USA).
Chromatin immunoprecipitation (ChIP) and dual-luciferase reporter assay
A chromatin immunoprecipitation assay kit (Active motif, Carlsbad, CA, USA) was used to monitor the interaction between FOXO1 and the IRF-1 promoter as described [19]. IRF-1 dual-reporter promoter vectors and a nonpromoter sequence negative control vector, pEZX-LvPG04 (Genecopoeia, Rockville, MD, USA) were transfected into Lv-control-Hepa1-6 and Lv-FOXO1-Hepa1-6 cells with Lipofectamine 2000 (Invitrogen, San Diego, CA, USA) according to the manufacturer's instructions. A Gaussia luciferase assay kit (Genecopoeia) was used to detect the signal intensity by microplates (Biotek, Winooski, VT, USA).
Cell migration assay
The FOXO1 inhibitor AS1842856 (MediChemExpress) was added to Lv-FOXO1-Hepa1-6 and Lv-control-Huh7 for 18 hours [20]. Different groups of HCC cells were seeded at a 2 × 103 density in the upper chamber of a 0.8 μm 12-well transwell plate (Thermo Fisher) and then cultured for 12 hours. Then, 200 μl of 10% FBS DMEM complete culture medium was added to the lower chambers. Conditioned culture medium (CM) was collected from different groups of HCC cells at a 1 × 106 density after culturing for 48 hours and then purified by a 0.22 µm filter. Macrophage migration assays were performed as follows: mouse and human macrophages were plated at 2 × 103 and 4 × 103/well in the upper chamber. One hundred microliters of HCC CM supplemented with 100 μl of 10% FBS fresh complete culture medium was added to the lower chamber and then cultured for 12 hours. HCC cells (5 × 105) were plated and cultured for 48 hours. Then, 1 ml of HCC CM was added to 1 ml of 10% FBS complete fresh culture medium and collected to treat 5 × 105 macrophages for 24 hours. After renewal with 2 ml of the new complete DMEM culture medium and culture for 24 hours, CM was collected after filtering with a 0.22 µm filter. Two hundred microliters of macrophage were added to the lower chamber with or without IL-6 neutralizing antibody at 19 µg/ml according to the manufacturer's instructions. HCC cells were plated in the upper chamber and cocultured for 12 hours [21]. The migrated cells were then stained with 0.1% crystal violet. The cells were counted under a microscope (200 ×) [22].
Quantitative real-time PCR
Total RNA was extracted from tissue samples or cells, and reverse transcription was performed as previously described [23]. The mRNA levels were detected by SYBR Green Master Mix (Thermo Fisher) using a real-time PCR system (ABI). GAPDH or β-actin was used as an internal control for human and mouse. The nitric oxide donor DETA-NONOate (Cayman, Ann Arbor, Michigan, USA) was added at 100 nm and 200 nm in culture medium. For primers, see the Supplemental Material.
Enzyme-linked immunosorbent assay (ELISA)
CM from mouse macrophages was collected as mentioned above, and a mouse IL-6 ELISA (R&D, Minneapolis, MN, USA) and nitric oxide assay kit (Thermo Fisher) were performed according to the protocols. Measurement was performed by setting the wavelength at 540 nm or 450 nm with correction at 540 nm.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software program (Version 7.04, GraphPad Software, San Diego, CA, USA). Continuous variables were expressed by median and range. Experiments were performed at least in triplicate. The unpaired two-tailed Student's test and the one-way Anova with Tukey's multiple comparison test were performed for the difference of continuous variables in two or multiple groups. Chi-square test was used for comparison of categorical variables. Comparisons between groups of non-normal distributed were using Mann-Whitney test. The survival curve was performed with the Kaplan–Meier method and differences were assessed using the log-rank test. P value < 0.05 was considered as statistical significance.
Results
FOXO1 was negatively correlated with HCC and decreased CD206-positive cells in HCC tissues
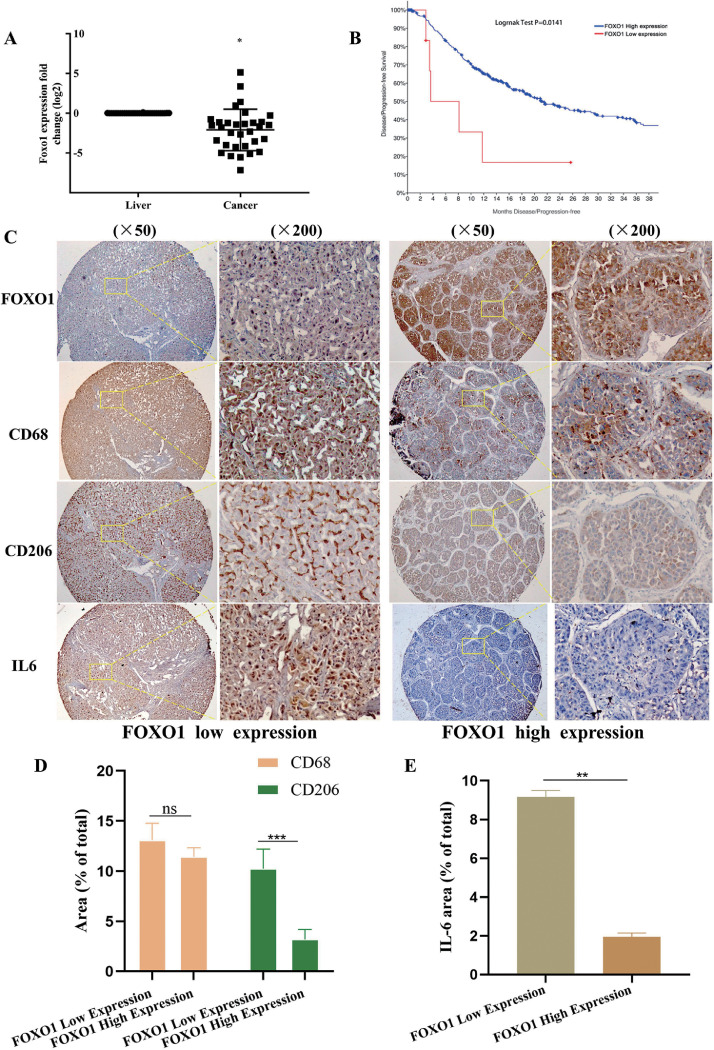
We investigated the expression of FOXO1 in HCC from 32 patients who had undergone liver resection between 2011 and 2017 at the University of Pittsburgh Medical Center. FOXO1 mRNA expression was higher than that in corresponding noncancerous liver tissues (Fig. 1A). Significantly low expression of FOXO1 was observed in 47% of HCC patients. By analyzing the relationship between FOXO1 expression levels and clinicopathological characteristics in these patients and the patients from the The Second Hospital of Anhui Medical University, it was found that FOXO1 expression was negatively associated with vascular invasion (Table 1, 2). We further analyzed the relationship between FOXO1 expression and clinical prognosis in 440 HCC patients from the TCGA dataset (TCGA-2 V-A95S-01). FOXO1 expression positively correlated with the disease-free survival rate (Fig. 1B)24. In human HCC samples, fewer CD 206-positive cells were present in the high FOXO1 expression group than in the low FOXO1 expression group (Fig. 1C). The IL-6 expression level was also investigated because it is a protumorigenic inflammatory cytokine that promotes HCC progression by synchronizing the interaction between HCC cells and macrophages(Fig. 1D) [24]. Less IL-6 expression was detected in the high FOXO1 expression group (Fig. 1 E, Fig. S1A, B).
Fig. 1.
High FOXO1 expression negatively correlated with HCC progression. A, FOXO1 mRNA expression in non-HCC liver tissues and HCC tissues from HCC patients in UPMC (n = 32 patients). B, Correlation between FOXO1 expression and disease-free survival rate in HCC patients from the TCGA database (n=400 patients). Patients with low FOXO1 expression in HCC tissues have a poor disease progression free rate. C, Representative IHC images showed FOXO1, CD68, CD206 and IL-6 expression in HCC patients (n= 51 paitents). FOXO1 expression showed negatively related with CD206 positive cells, and IL6 expression in HCC tissues (Original magnification of IHC images, × 50, × 200). D, The relationship between FOXO1 expression and CD68 positive and CD 206 positive cells. E, The relationship between FOXO1 expression and the IL-6 expression. (Mean±SEM, *P<0.05, *** P<0.001, ns, nonsignificant).
Table 2.
Correlation between FOXO1 expression and clinicopathologic characteristics of HCC patients from The second hospital of Anhui Medical University.
| Clinicopathologic characteristics | FOXO1 expression |
P value | |
|---|---|---|---|
| High (n=34) | Low (n=17) | ||
| Age(years) | 0.463 | ||
| ≤65 | 26 | 15 | |
| >65 | 8 | 2 | |
| Sex | 0.593 | ||
| male | 32 | 15 | |
| female | 2 | 2 | |
| Cirrhosis | 0.728 | ||
| No | 7 | 5 | |
| yes | 27 | 12 | |
| AFP | 0.404 | ||
| Low | 15 | 8 | |
| High | 19 | 9 | |
| Vascular invasion | 0.028* | ||
| No | 26 | 7 | |
| Yes | 8 | 10 | |
| Tumor size(cm) | |||
| ≤3/>3 | 11/23 | 2/15 | 0.175 |
| ≤5/>5 | 20/14 | 8/9 | 0.553 |
P < 0.05.
High FOXO1 expression in HCC inhibits HCC cell growth and migration but enhances migration and suppresses CD206 and IL-6 expression in macrophages
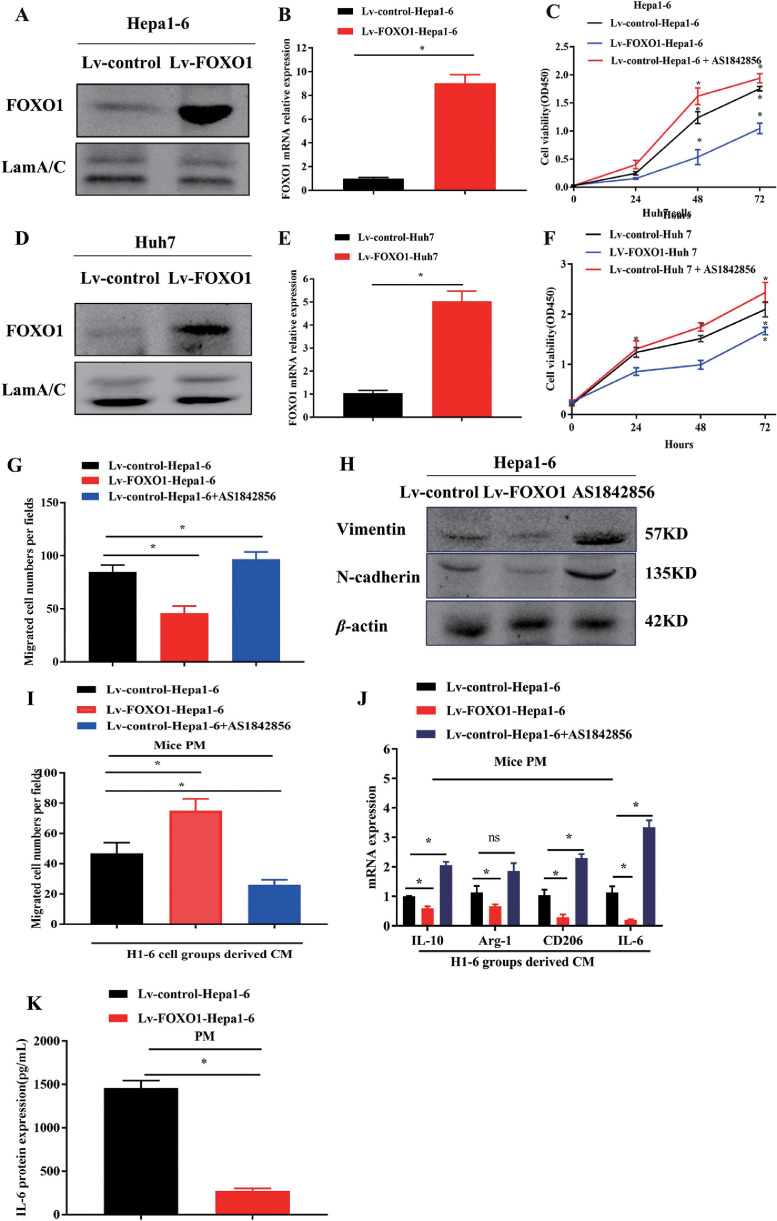
To confirm the effect of tumor-derived FOXO1 on tumor and macrophage biological behaviors, we performed growth and migration or invasion assays. We generated cell lines that stably upregulated FOXO1 by transfecting Huh7 cells (Lv-FOXO1-Huh7) and Hepa1-6 (Lv-FOXO1-Hepa1-6) cells using lentivirus carrying the FOXO1 gene (Fig. 2A, B, D, E). Increasing FOXO1 expression inhibited HCC cell proliferation, while inhibiting FOXO1 with the FOXO1 inhibitor AS1842856 promoted Hepa1-6 and Huh7 cell proliferation (Fig. 2C, F). Increasing FOXO1 expression obviously suppressed HCC cell passage through the membrane and inhibited Vimentin and N-cadherin expression, while decreasing FOXO1 expression with a FOXO1 inhibitor increased HCC migration and Vimentin and N-cadherin expression (Fig. 2G, H, Fig. S2A-D). THP-1 cells were induced to differentiate into macrophages with PMA as previously reported. Culture medium from highly FOXO1-expressing Huh7 and Hepa1-6 HCC cells enhanced mouse PM- and THP-1-induced macrophage migration through the membrane in a transwell coculture system. CM from HCC cells with low FOXO1 expression inhibited macrophage migration (Fig. 2I, Fig. S2E-G). CM from HCC cells with high FOXO1 expression reduced IL-6, CD206 and IL-10 expression in macrophages. (Fig. 2J, K, Fig. S2H, I)
Fig. 2.
FOXO1 expression inhibited HCC progression and enhanced macrophage migration. A, B, Expression of endogenous FOXO1 in Lv-FOXO1-Hepa1-6 and Lv-control-Hepa1-6 cells were analyzed by western blot and qPCR. C, Proliferation assay performed in Lv-control-Hepa1-6, Lv-FOXO1-Hepa1-6 cells and Lv-control-Hepa1-6 treated with FOXO1 inhibitor. D, E, Expression of endogenous FOXO1 in Lv-control-Huh7, Lv-FOXO1-Huh7 cells groups were evaluated by western blot and qPCR. F, Proliferation assay performed in Lv-control-Huh7, Lv-FOXO1-Huh7 and Lv-control-Huh7 treated with FOXO1 inhibitor. G, Quantification of migrated Lv-control-Hepa1-6, Lv-FOXO1-Hepa1-6, and Lv-control-Hepa1-6 treated with FOXO1 inhibitor cells by cell migration assay. H, Vimentin and N-cadherin expression was detected in Hepa1-6 cells with different FOXO1 expression levels by western blot. I, Qualification of migrated PM cells treated with CM from Hepa1-6 with different FOXO1 expression levels. J, Quantification of mRNA expression of IL-6, CD206, IL-10, Arg-1 in PM cocultured with CM from Hepa1-6 cells with different FOXO1 expression levels was detected by qPCR. K, IL-6 protein expression in CM from PMs cocultured with Hepa1-6 with different FOXO1 expression levels by ELISA. (Mean±SEM, *P<0.05).
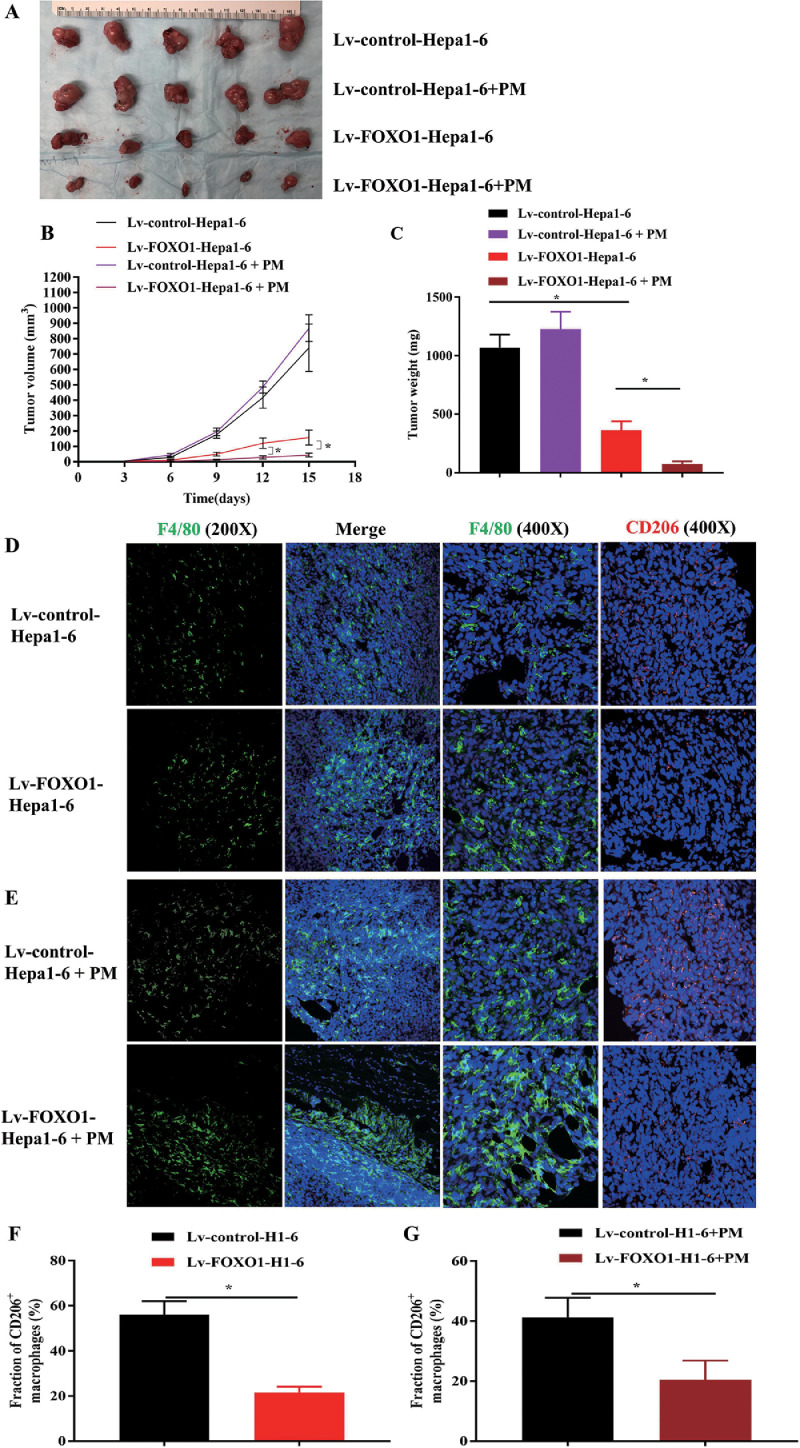
Increasing FOXO1 expression in HCC cells inhibits tumor growth and CD206-positive tumor-associated macrophage infiltration in vivo
The effect of FOXO1 on the progression of HCC was also explored in a xenograft model. Here, we found that tumor growth was inhibited in mice transplanted with Lv-FOXO1-Hepa1-6 cells compared to mice implanted with Lv-control-Hepa1-6 cells (Fig. 3A-C). It was also found that the number of macrophages (F4/80 positive) increased in FOXO1 high expression tumors, while the percentage of CD206 positive cells decreased (Fig. 3D, F). To further confirm this, we transplanted Lv-control-Hepa1-6 and Lv-FOXO1-Hepa1-6 cells combined with mouse peritoneal macrophages into mice. Tumor growth was significantly inhibited (Fig. 3A-C). The percentage of CD206-positive cells in tumors was significantly reduced in the Lv-FOXO1-Hepa1-6 combined with PM-injected group (Fig. 3E, G).
Fig. 3.
FOXO1 suppressed xenograft tumor growth and enhanced macrophage recruitment. A, Images of Lv-control-Hepa1-6 and Lv-FOXO1-Hepa1-6 cells co-injected with or without PM in the right flank of mice are shown (n=5). B, C The tumor volume and weight were measured in different groups. D, E, F4/80 and CD206 immunofluorescence staining was performed in these tumors. F, G, Ratio of CD206-positive cells in F4/80-positive cells. Original magnification of IHC images, × 200, × 400. (Mean±SEM, *P<0.05).
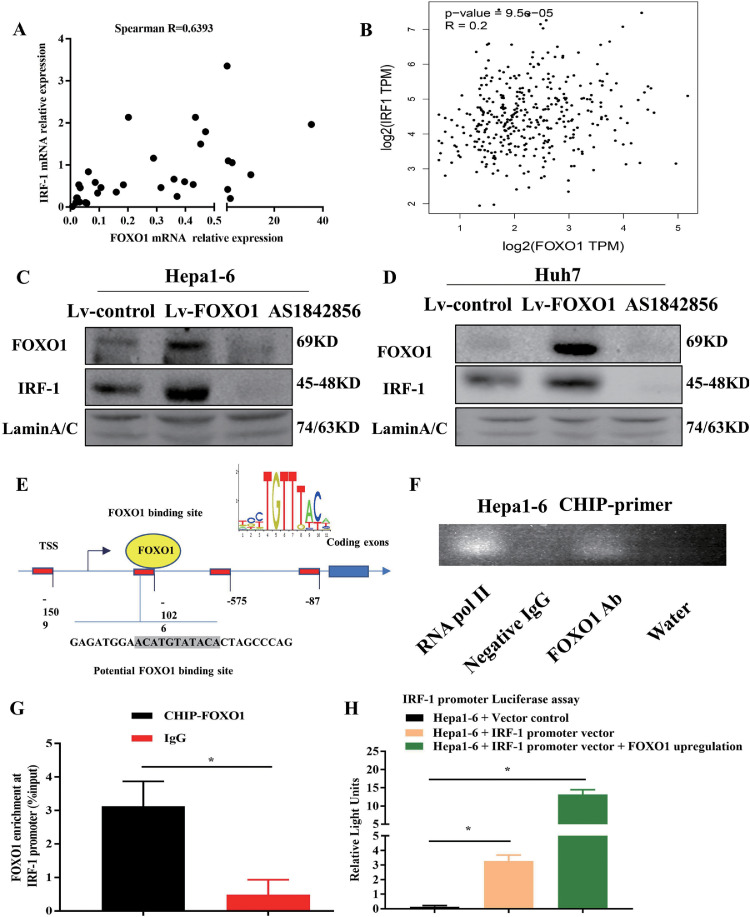
FOXO1 positively regulates IRF-1 expression by directly binding to its promoter
In previous research, we revealed that IRF-1 derived from tumor cells inhibited tumor progression by priming the immune response and inducing antitumor immune cell infiltration18. Nitric oxide, which is closely associated with the polarization of macrophages, is directly regulated by IRF1 [25,26]. Here, we explored the relationship between IRF-1 and FOXO1 expression. In human HCC tumor samples, FOXO1 expression positively correlated with IRF-1 expression (Fig. 4A-B) [27]. Regulation of FOXO1 expression positively regulates IRF-1 expression in HCC cells (Fig. 4C, D). To solidify the direct regulatory relationship between IRF-1 and FOXO1, the JASPAR database was employed to predict the potential binding sites for FOXO1 in the promoter of IRF-1 (Fig. 4E). Chromatin immunoprecipitation was then performed to confirm this finding (Fig. 4F, G). The promoter of the IRF-1 gene was cloned and linked to the pEZX-LvPG04 vector. The results of the luciferase assay showed that the relative luciferase activity of the IRF-1 promoter was significantly induced by overexpression of FOXO1 (Fig. 4H).
Fig. 4.
Increasing FOXO1 expression in HCC upregulated IRF-1 by binding to the promoter. A, B, The correlation between FOXO1 and IRF-1 mRNA expression in HCC samples. C, D, Expression of IRF-1 was detected in Hepa1-6 and Huh7 cells with different FOXO1 expression levels. E, The mechanic graph shows the predicted binding sites of FOXO1 located in the promoter of IRF-1; F, PCR products of ChIP performed on Hepa1-6 cells ran on a 1% agarose gel. RNA pol II group was the positive control, Negative IgG group and water group were the negative control, FOXO1 antibody enriched in the DNA promoters. G, IRF-1 was confirmed to be the promoter binding by FOXO1 by qPCR through specific primers, IgG was the negative control group. H, Luciferase reporter assays were performed to show the activity of IRF-1 promoter transfection (mean±SEM, *P<0.05).
FOXO1 derived from HCC regulates macrophage function through IRF-1/NO expression
Ad-virus-IRF1 vectors were used to reverse the inhibition of migration by downregulating FOXO1 expression in macrophages. CM from HCC cells with high IRF-1 expression enhanced macrophage migration (Fig. 5A, B, Fig. S3A-D) and reduced CD206 and IL-6 expression in macrophages (Fig. 5C, D, Fig. S3E, F). NO is an important inflammatory product in the microenvironment that mediates the interaction between tumor cells and macrophages [28]. In a previous study, we found that IRF-1 is dependent on the production of NO in inflammatory reactions [29]. To confirm the possible role of NO in macrophage polarization in this research, we tested the production of NOS2 and NO in HCC cells. FOXO1 and IRF-1 positively regulated the production of NO in Hepa1-6 and Huh7 cells, respectively (Fig. 5E-H, Fig. S3G-J). We used DETA NONOate as an NO donor to mimic the effects on macrophages. DETA NONOate inhibited IL-6 and CD206 expression in PM- and THP-1-induced macrophages and promoted their migration capacity (Fig. 5I-K, Fig. S3K-M).
Fig. 5.
FOXO1 promoted nitric oxide expression in HCC cells through IRF-1/NOS2, which inhibited CD206 and IL-6 expression and enhanced migration in PM cells. A, IRF-1 expression were detected in Hepa1-6 cells in different groups by western blot, Ad-IRF1(adenovirus-IRF-1), Ad-shIRF-1(adenovirus-shRNA-IRF1); B, Quantification of migrated PM cocultured with CM from HCC cells with different levels of IRF-1 expression; C, D, Quantification of CD206 and IL-6 mRNA in PM cocultured with CM from HCC cells transfected with Ad-IRF-1 and Ad-control; E-H, NOS2 and nitric oxide expression were detected in Hepa1-6 cells with different FOXO1 and IRF-1 expression levels by western blot and nitric oxide assay kit; I, J Quantification of IL-6 and CD206 mRNA expression was conducted in PM treated with the nitric oxide donor DETA NONOate. K, Quantification of migrated PM treated with nitric oxide donor DETA NONOate. (Mean±SEM, *P<0.05).
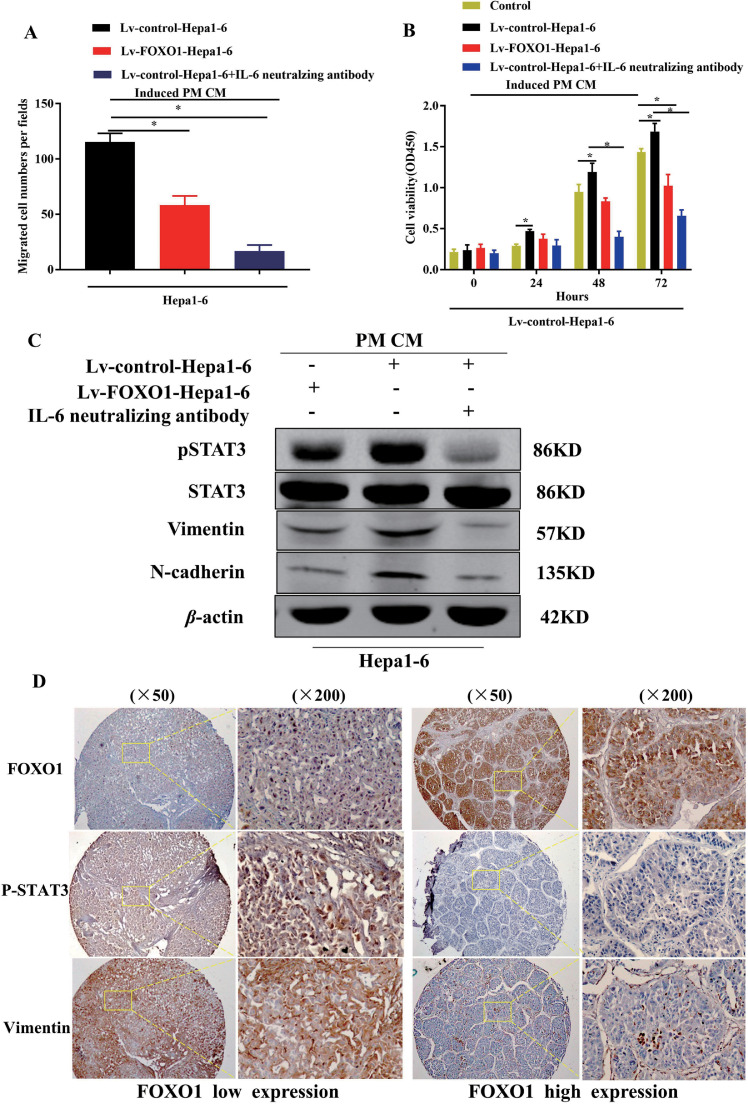
Macrophages treated with high FOXO1 expression culture medium inhibited HCC cell proliferation and migration
We collected CM from macrophages cocultured with Lv-control-Hepa1-6 and Lv-FOXO1-Hepa1-6 and then treated Hepa1-6 and Huh7 cells with this CM for 24 hours. The CM from macrophages pretreated with Lv-control-Hepa1-6 or Lv-control-Huh7 promoted HCC cell migration and proliferation (Fig. 6A, B, Fig. S4A-D) in contrast to CM from macrophages pretreated with the high FOXO1 expression HCC cells by increasing phosphorylated STAT3, vimentin, and N-cadherin expression, which were blocked by using neutralizing IL-6 antibody (Fig. 6C, Fig. S4E).
Fig. 6.
IL-6/STAT3 was activated in the interaction between different FOXO1 HCC cell groups and induced macrophages. A, Quantification of migrated Hepa1-6 cells toward CM from mice PM by transwell assay; B, Proliferation assays were performed in Hepa1-6 cells treated with CM from PM induced by Lv-control-Hepa1-6, Lv-Foxo1-Hepa1-6, combined with IL-6 neutralizing antibody or not; C, p-STAT3, STAT3, Vimentin, N-cadherin expression were detected in Hepa1-6 cells treated with CM from Lv-control-Hepa1-6, Lv-FOXO1-Hepa1-6 induced macrophages combined with IL-6 neutralizing antibody or not by western blot. D, IHC staining of FOXO1-, P-STAT3-, and Vimentin was performed in different FOXO1 expression groups in human HCC samples. (Mean±SEM, *P<0.05).
FOXO1 derived from HCC cells inhibited IL-6 expression in macrophages and suppressed IL-6/STAT3 activation in HCC cells
The protumorigenic effects and clinical significance of TAMs producing IL-6 in HCC have been well documented in previous studies [22,30]. Here, we found that HCC cells cocultured with macrophages promoted IL-6 expression in macrophages and activation of STAT3 in HCC cells. Culture medium from HCC cells with high FOXO1 expression inhibited IL-6 expression in macrophages. In contrast to HCC cells cocultured with macrophages, phosphorylation of STAT3 was suppressed in HCC cells cocultured with macrophages with high FOXO1 expression. A neutralizing antibody against IL-6 was used to block the effects of IL-6 from macrophages, and it was found that the phosphorylation of STAT3 in HCC cells was inhibited and that N-cadherin and vimentin expression was reduced (Fig. 6C, Fig. S4E). The same results were also obtained in HCC tissues. HCC tissues with high FOXO1 expression contained fewer p-STAT3-positive cells and lower Vimentin expression, while tissues with low FOXO1 expression contained more p-STAT3-positive cells and higher Vimentin expression (Fig. 6D).
Discussion
Over 90% of liver cancers develop under chronic inflammatory conditions [31]. Oncogenic factors that promote carcinogenesis include liver matrix changes, unbalanced interactions between liver cells and nonparenchymal cells, and immune response dysfunction. In this study, we explored the additional function of the tumor suppressor FOXO1 in HCC to induce antitumor immunity by regulating macrophage differentiation to inhibit tumor progression. In previous research, FOXO1 has been considered to exert antitumorigenic effects in different types of cancer by inhibiting cancer cell proliferation, apoptosis, and metabolism. Notably, FOXO1 has also been proven to function in antitumor immunity reactions by activating innate immune cells directly or inducing TME alteration [32]. Here, we found that increased FOXO1 expression predicts a good prognosis for HCC patients and is negatively correlated with vascular invasion. In addition, the number of CD206-positive macrophages (M2-polarized) decreased in high FOXO1 expression tumors compared to low FOXO1 expression tumors. This phenomenon has also been demonstrated in mouse xenograft assays. High FOXO1 expression in tumor cells recruits macrophages and reduces the ratio of M2-polarized macrophages. Based on this, we hypothesized that FOXO1 derived in HCC may inhibit tumor progression by regulating TAM polarization.
As a major player in the TME, TAMs are well known to exert oncogenic functions and promote the EMT process in tumor cells directly or indirectly [33,34] Furthermore, TAMs can also be reeducated by the tumor microenvironment, providing antitumor function by shaping their differentiation [35]. IL-6 is involved in inflammation and is closely linked to the prognosis of cancer patients. Chronically elevated levels of the proinflammatory cytokine IL-6, which promotes tumor cell survival, are a poor prognostic factor in patients with many types of cancers, including melanoma [10,36,37]. We cocultured HCC cells with macrophages in vitro. High FOXO1 expression in tumor cells suppressed CD206, IL-10, Arg-1 and IL-6 mRNA expression in macrophages in contrast to the control. Culture medium from HCC cells with high FOXO1 expression also enhanced the migration capacity of macrophages, whereas FOXO1 inhibited migration in HCC cells. We injected HCC cells in combination with macrophages into nude mice. Tumor size was further reduced in comparison to the group in which only cells with high FOXO1 expression were injected. In the control group, injected macrophages combined with tumor cells showed no significant change in tumor size. These results might demonstrate that FOXO1 inhibits tumor progression not only by targeting tumor cells but also by inducing antitumor differentiation in macrophages. As a key transcription factor, FOXO1 control a wide spectrum of genes regulate cell cycle, cell metabolism, angiogenesis, senescence through PI3K/AKT pathway. Intrinsic factors in tumors either enhance or inhibit related oncogenic pathways, such as PI3K/AKT, p38/MAPK, and CDK related pathway [38], [39], [40]. Through these mechanisms, it regulates the releasing of cytokines, chemokines or senescence-associated secretory phenotypes such as nitric oxide, CCL2, TGF-β, IL-6 and et, al. These changes remodel immune state in TME and regulate immune cell infiltration [41].
In addition to its regulatory effects on cancer cell intrinsic factors, FOXO1 also positively regulates antitumor immunity through immune cells and other cells in the TME by inhibiting c-Myc, VEGF, and PDL-1 directly or indirectly [32,42]. These intrinsic tumor factors are dependent on shaping macrophage differentiation. In addition, another regulatory pathway, FOXO1, was found to function through IRF-1. As a key regulator in immunity, IRF-1 transcriptionally targets iNOS, class I and II MHC genes, enhancing the antitumor effects in primary and adaptive immune responses [43], [44], [45]. By regulating FOXO1 expression in HCC cells, FOXO1 regulates NOS2 and NO expression by directly binding to the IRF-1 promoter, which may partially account for the antitumor differentiation of macrophages. To confirm this, an NO donor was employed to test the induced effects. NO enhanced macrophage infiltration, shaped antitumor polarization and inhibited IL-6 expression, which is in line with previous research [40,46]. In the TME, IL-6/STAT3 is commonly constitutively activated in HCC during the interaction between tumor cells and immune cells. Exogenous or TAM-derived IL-6 promotes HCC proliferation, EMT and metastasis [30,47]. We treated HCC cells with CM from induced macrophages. Macrophages induced by HCC cells promoted proliferation and migration in HCC cells. The protumorigenic effect induced by TAMs was suppressed by blocking the effects of IL-6. In contrast, the CM from macrophages treated with HCC cells with high FOXO1 expression exerted a similar antitumor effect by suppressing the phosphorylation of STAT3 [37]. IL-6 is expressed in both autocrine and exocrine manners in the HCC TME. Although it is difficult to identify the activation of STAT3 in an autocrine manner, IL-6 secreted by TMA was thought to be more important for the activation of STAT3 than other cells in HCC [47,48].
Conclusion
In summary, our study reveals that FOXO1 derived from HCC exerts tumor suppressor effects through enhanced macrophage infiltration and antitumorigenic polarization through positive upregulation of the IRF-1/NO pathway. Polarized macrophages inhibit HCC proliferation and migration by inhibiting IL6/STAT3 activation. This further highlights the potential role of tumor cell intrinsic factors in shaping immune responses. This study provides an improved understanding of tumor-derived FOXO1 that restricts carcinogenesis in HCC and a promising strategy for treating HCC.
CRediT authorship contribution statement
Xiao Cui: Conceptualization, Methodology, Writing – original draft, Funding acquisition. Huiyong Zhao: Writing – original draft, Visualization. Sheng Wei: Methodology, Investigation. Qiang Du: Resources, Conceptualization. Kun Dong: Resources. Yihe Yan: Resources. David. A Geller: Conceptualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We Thank Dr. Zhengzhong Feng and Dr. Xian Wang for their help in pathological diagnosis and experiments. This work was supported by the National Institutes of Health (Grant Number: HHSN276201200017C), Nature Science Foundation of Anhui Medical University (Grant Number: 2022XKJ182), Anhui Provincial Natural Science Foundation (Grant Number: 1808085MH270).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2023.100900.
Contributor Information
Xiao Cui, Email: cx_2077@bjmu.edu.cn.
Huiyong Zhao, Email: drzhy2020@163.com.
Sheng Wei, Email: ws940803@163.com.
Qiang Du, Email: qiangdu@pitt.edu.
Kun Dong, Email: dongkun007@163.com.
Yihe Yan, Email: yanyihe12@sr.gxmu.edu.cn.
David. A Geller, Email: gellerda@upmc.edu.
Appendix. Supplementary materials
References
- 1.Vogel A, Meyer T, Sapisochin G, et al. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–1362. doi: 10.1016/S0140-6736(22)01200-4. [DOI] [PubMed] [Google Scholar]
- 2.Sia D, Villanueva A, Friedman SL, et al. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152(4):745–761. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 3.Yadav RK, Chauhan AS, Zhuang L, et al. FOXO transcription factors in cancer metabolism. Seminars Cancer Biol. 2018;50:65–76. doi: 10.1016/j.semcancer.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo CT, Liao W, Dadi S, et al. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529(7587):532–536. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik S, Sadhu S, Elesela S, et al. Transcription factor Foxo1 is essential for IL-9 induction in T helper cells. Nat. Commun. 2017;8(1):815. doi: 10.1038/s41467-017-00674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JB, Zhao ZB, Liu QZ, et al. FOXO1 is a regulator of MHC-II expression and anti-tumor effect of tumor-associated macrophages. Oncogene. 2018;37(9):1192–1204. doi: 10.1038/s41388-017-0048-4. [DOI] [PubMed] [Google Scholar]
- 7.Delpoux A, Marcel N, Hess Michelini R, et al. FOXO1 constrains activation and regulates senescence in CD8 T cells. Cell Rep. 2021;34(4) doi: 10.1016/j.celrep.2020.108674. [DOI] [PubMed] [Google Scholar]
- 8.Webb AE, Kundaje A, Brunet A. Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell. 2016;15(4):673–685. doi: 10.1111/acel.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Ye YC, Chen Y, et al. Crosstalk between hepatic tumor cells and macrophages via Wnt/beta-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death. Dis. 2018;9(8):793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa da Silva M, Breckwoldt MO, Vinchi F, et al. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages. Front. Immunol. 2017;8:1479. doi: 10.3389/fimmu.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YC, Ma HD, Yin XY, et al. Forkhead Box O1 Regulates Macrophage Polarization Following Staphylococcus aureus Infection: Experimental Murine Data and Review of the Literature. Clin. Rev. Allergy Immunol. 2016;51(3):353–369. doi: 10.1007/s12016-016-8531-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YL, Li Q, Yang XM, et al. SPON2 promotes M1-like macrophage recruitment and inhibits hepatocellular carcinoma metastasis by distinct integrin-rho GTPase-hippo pathways. Cancer Res. 2018;78(9):2305–2317. doi: 10.1158/0008-5472.Can-17-2867. [DOI] [PubMed] [Google Scholar]
- 13.Linehan E, Dombrowski Y, Snoddy R, et al. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13(4):699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Poppoe F, Chen J, et al. Macrophages Polarized by Expression of ToxoGRA15II Inhibit Growth of Hepatic Carcinoma. Front. Immunol. 2017;8:137. doi: 10.3389/fimmu.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu DK, Tse AP, Xu IM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat. Commun. 2017;8(1):517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou H, Li WX, Cui X, et al. CARMA3/NF-κB signaling contributes to tumorigenesis of hepatocellular carcinoma and is inhibited by sodium aescinate. World J. Gastroenterol. 2019;25(36):5483–5493. doi: 10.3748/wjg.v25.i36.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du Q, Zhang X, Cardinal J, et al. Wnt/beta-catenin signaling regulates cytokine-induced human inducible nitric oxide synthase expression by inhibiting nuclear factor-kappaB activation in cancer cells. Cancer Res. 2009;69(9):3764–3771. doi: 10.1158/0008-5472.CAN-09-0014. [DOI] [PubMed] [Google Scholar]
- 18.Yang MQ, Du Q, Varley PR, et al. Interferon regulatory factor 1 priming of tumour-derived exosomes enhances the antitumour immune response. Br. J. Cancer. 2018;118(1):62–71. doi: 10.1038/bjc.2017.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerur N, Fukuda S, Banerjee D, et al. cGAS drives noncanonical-inflammasome activation in age-related macular degeneration. Nat. Med. 2018;24(1):50–61. doi: 10.1038/nm.4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Sun C, Xiao G, et al. S-nitrosylation of the Peroxiredoxin-2 promotes S-nitrosoglutathione-mediated lung cancer cells apoptosis via AMPK-SIRT1 pathway. Cell Death. Dis. 2019;10(5):329. doi: 10.1038/s41419-019-1561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Yu G, Chu H, et al. Macrophage-Associated PGK1 Phosphorylation Promotes Aerobic Glycolysis and Tumorigenesis. Mol. Cell. 2018;71(2):201–215. doi: 10.1016/j.molcel.2018.06.023. e7. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Wang GZ, Wang Y, et al. Hypoxia-induced HMGB1 expression of HCC promotes tumor invasiveness and metastasis via regulating macrophage-derived IL-6. Exp. Cell. Res. 2018;367(1):81–88. doi: 10.1016/j.yexcr.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Du Q, Zhang X, Liu Q, et al. Nitric oxide production upregulates Wnt/β-catenin signaling by inhibiting Dickkopf-1. Cancer Res. 2013;73(21):6526–6537. doi: 10.1158/0008-5472.Can-13-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kamijo R, Harada H, Matsuyama T, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263(5153):1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 26.Chistiakov DA, Myasoedova VA, Revin VV, et al. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223(1):101–111. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic. Acids. Res. 2017;45(W1):W98–w102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Tsung A, Stang MT, Ikeda A, et al. The transcription factor interferon regulatory factor-1 mediates liver damage during ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(6):G1261–G1268. doi: 10.1152/ajpgi.00460.2005. [DOI] [PubMed] [Google Scholar]
- 30.Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llovet JM, Villanueva A, Lachenmayer A, et al. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat. Rev. Clin. Oncol. 2015;12(7):408–424. doi: 10.1038/nrclinonc.2015.103. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Wang F, Hughes T, et al. FOXOs in cancer immunity: Knowns and unknowns. Semin. Cancer Biol. 2018;50:53–64. doi: 10.1016/j.semcancer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang C, Dou R, Wei C, et al. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol. Ther. 2021;29(6):2088–2107. doi: 10.1016/j.ymthe.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Q, Fang Y, Lai Q, et al. CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling. J. Exp. Clin. Cancer Res. 2020;39(1):132. doi: 10.1186/s13046-020-01637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genard G, Lucas S, Michiels C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front. Immunol. 2017;8:828. doi: 10.3389/fimmu.2017.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashwah H, Bertram K, Stirm K, et al. The IL-6 signaling complex is a critical driver, negative prognostic factor, and therapeutic target in diffuse large B-cell lymphoma. EMBO Mol. Med. 2019;11(10):e10576. doi: 10.15252/emmm.201910576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heichler C, Scheibe K, Schmied A, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69(7):1269–1282. doi: 10.1136/gutjnl-2019-319200. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Chen P, Liu K, et al. CDK1/2/5 inhibition overcomes IFNG-mediated adaptive immune resistance in pancreatic cancer. Gut. 2021;70(5):890–899. doi: 10.1136/gutjnl-2019-320441. [DOI] [PubMed] [Google Scholar]
- 39.Haas L, Elewaut A, Gerard CL, et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer. 2021;2(7):693–708. doi: 10.1038/s43018-021-00221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chand V, Liao X, Guzman G, et al. Hepatocellular carcinoma evades RB1-induced senescence by activating the FOXM1-FOXO1 axis. Oncogene. 2022;41(30):3778–3790. doi: 10.1038/s41388-022-02394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48(3):399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Kim SY, Ko YS, Park J, et al. Forkhead Transcription Factor FOXO1 Inhibits Angiogenesis in Gastric Cancer in Relation to SIRT1. Cancer Res. Treat. 2016;48(1):345–354. doi: 10.4143/crt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann. Oncol. 2018;29(11):2232–2239. doi: 10.1093/annonc/mdy399. [DOI] [PubMed] [Google Scholar]
- 44.Hou J, Chen SN, Gan Z, et al. In Primitive Zebrafish, MHC Class II Expression Is Regulated by IFN-γ, IRF1, and Two Forms of CIITA. J. Immunol. 2020;204(9):2401–2415. doi: 10.4049/jimmunol.1801480. [DOI] [PubMed] [Google Scholar]
- 45.Demel UM, Böger M, Yousefian S, et al. Activated SUMOylation restricts MHC class I antigen presentation to confer immune evasion in cancer. J. Clin. Invest. 2022;132(9) doi: 10.1172/jci152383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philipp D, Suhr L, Wahlers T, et al. Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res. Ther. 2018;9(1):286. doi: 10.1186/s13287-018-1039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng YS, Tseng HY, Chen YA, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer. 2019;18(1):42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg N, Van Haele M, Lanton T, et al. Combined hepatocellular-cholangiocarcinoma derives from liver progenitor cells and depends on senescence and IL-6 trans-signaling. J. Hepatol. 2022;77(6):1631–1641. doi: 10.1016/j.jhep.2022.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.