Abstract
Introduction
radiofrequency catheter ablation (RFA) is the first-line therapy for symptomatic Wolff Parkinson White (WPW) patients according to the American Heart Association. We conducted this study to assess the success rate, recurrence rate, and rate of complications associated with the utilization of radiofrequency catheter ablation for managing patients with WPW.
Method
We searched PubMed, Cochrane library, Web of Science and Scopus databases using all identified keywords and index terms through 4 January 2022. We included all studies conducted on WPW patients who were treated with ablation. We conducted the analysis using Open Meta Analyst and MedCalc version 19.1.
Results
Among 2268 unique articles identified, only 11 articles met our inclusion criteria. The pooled effect estimates showed high success rate (94.1%[95%CI:92.3–95.9], p < 0.001)), low recurrence rate (6.2% [95%CI:4.5–7.8, p < 0.001]) and low rate of complications (1%[95%CI:0.4–1.5, p < 0.001]).
Conclusion
RFA showed a high success rate, low recurrence rate and low rate of complications in WPW patients.
Keywords: WPW-Syndrome, Recurrence, Cryoablation, Radiofrequency ablation, Complications
1. Introduction
Wolff Parkinson white (WPW) is a congenital syndrome that affects about 0.1% of the population.1 In this syndrome, an abnormal conducting pathway carries the impulses from the atria to the ventricles outside the range of the AV node. Patients can be diagnosed only from their characteristic ECG patterns. However, high rates of aberrant impulse discharge can occur at this pathway resulting in episodic attacks of tachycardia and a sense of palpitation.2 If the abnormal impulse does not vanish at the end of the electrical pathway, it can re-ascend to the atria causing a re-entry circuit with pre-excitation that can become manifested as ventricular tachycardia, fibrillation, and eventually sudden death.3,4
Management options for WPW syndrome include anti-arrhythmic medications, surgery, and radiofrequency catheter ablation.5 Unlike the other options, radiofrequency ablation is more practical as it is non-invasive and does not require long-term compliance and adherence by the patients. In 2015, the American Heart Association has recommended it as the first-line therapy for symptomatic WPW patients.6 However, it still harbours some risk of recurrence of arrhythmia and other complications such as sudden death. Another issue to be considered is the radiation involved in the procedure which may induce cancer in a small number of cases.7, 8, 9
In our systematic review and meta-analysis, we aim to assess the success rate, recurrence rate, and rate of complications associated with the utilization of radiofrequency catheter ablation for managing patients with WPW.
2. Methods
We followed the PRISMA statement guidelines.10 And performed all steps based on the Cochrane handbook of systematic reviews of intervention.11
2.1. Search strategy
We conducted our search on PubMed, Cochrane library, Web of Science and Scopus databases and analysed the text words found in titles, abstracts, and index terms used to describe each article, using all identified keywords and index terms from inception to 4 January 2022. Our search strategy was [(’‘Wolff-Parkinson-White Syndrome’‘[Mesh] OR ‘‘Wolff Parkinson White Syndrome’’ OR ‘‘WPW Syndrome’‘) AND (’‘Catheter Ablation’‘[Mesh] OR ‘‘catheter ablation’’ OR ‘‘Radiofrequency Ablation’‘[Mesh] OR ‘‘radiofrequency ablation’’ OR ‘‘Cryosurgery’‘[Mesh] OR ‘‘cryosurgery’’ OR ‘‘cryoablation’’ OR ‘‘Pulmonary Vein Isolation’‘[MESH] OR ‘‘pulmonary vein isolation’’ OR ‘‘pulmonary vein ablation’‘)].
2.2. Study selection criteria
Studies were included according to the PICOS strategy. There was no restriction on the year of publications. We included studies with 1) Population: patients with WPW, 2) Intervention: ablation, 3) No comparator 4) Outcomes: articles reporting success rate, recurrence rate, complications, procedure time, fluoroscopy time and radiation dose and 5) Study design: Any interventional or observational study. We excluded: 1) Animal trials, case reports, cross-sectional and case series, 3) Conferences abstract, 4) Studies without relevant population, intervention, or outcomes and 5) non-English studies.
2.3. Screening and quality assessment
Two authors independently screened titles and abstracts of the records retrieved according to the search strategy, to identify studies that potentially meet the inclusion criteria outlined above. The same authors independently assessed the full text of the selected studies for eligibility. Any disagreement between them over the eligibility of studies was resolved through discussion or with a senior author. We used the NIH tool to perform a quality assessment for the included observational studies and non-randomized clinical trials. We assessed the presence of publication bias in Egger's test for funnel plot asymmetry.12
2.4. Data extraction
Two authors independently extracted the data using a formatted excel sheet for data extraction. Any disagreement was resolved through discussion or with a senior author. The extracted data included the following: Summary, baseline characteristics data and outcomes of interest: success rate, recurrence rate, complications, procedure time (min), fluoroscopy time (min) and radiation dose (μGY/cm2).
2.5. Quality assessment
Two authors assessed the quality of included studies using the NIH tool for observational studies. We judged each domain with yes, no, cannot determine (CD), not applicable (NA) or not reported (NR). All domains are reported in supplementary material table 1. The quality of the studies was determined according to quality rating score: good (14–13 points), fair (9–12 points) or poor (8–0 points).
2.6. Data synthesis
We pooled the proportion of dichotomous data and mean and SD for continuous data with a 95% confidence interval using the random effect model and the Der-Simonian Liard method. We used Open Meta Analyst and MedCalc version 19.1.
3. Results
3.1. Literature search results
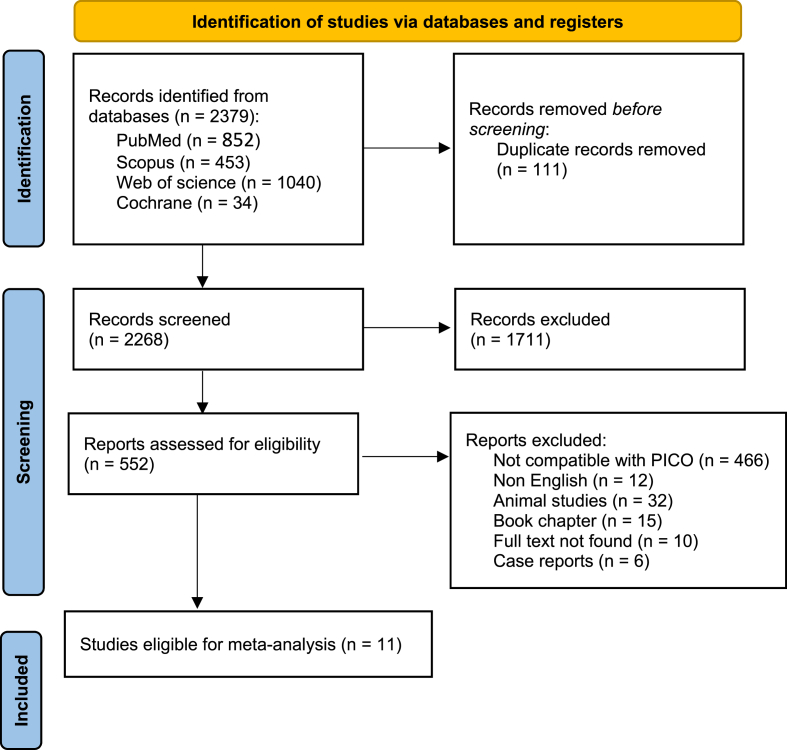
Our search retrieved 2268 unique articles. After the title and abstract screening, 552 articles were retrieved and assesses for eligibility (Fig. 1). Finally, 11 cohort studies13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 were included for quantitative analysis with a total number of patients of 5537. Summary and baseline characteristics of included studies were reported in Table 1 and Table 2; respectively.
Fig. 1.
PRISMA flow diagram.
Table 1.
Summary of included studies.
| ID | Title | Design | Inclusion criteria | Type of ablation | Device used | length of follow up (month) |
|---|---|---|---|---|---|---|
| GULLETTA 2013 | Safety and Efficacy of Open Irrigated-Tip Catheter Ablation of Wolff-Parkinson-White Syndrome in Children and Adolescents | Cohort | Patients less than 18 years old who were referred to San Raffaele Hospital for catheter ablation of WPW between January 2010 and July 2011. a history of paroxysmal tachycardia or syncope. inducible supraventricular tachycardia at the electrophysiologic study. a preexcited RR interval <250 ms during induced atrial fibrillation. an effective refractory period of the AP < 240 ms. |
Radiofrequency ablation, | open irrigatedtip catheter (Celsius Thermocool 3.5 mm, 7 F, Btype, Biosense Webster, Diamond Bar, CA, USA) | 12 |
| P. DiLorenzo 2012 | Ablating the anteroseptal accessory pathway—ablation via the right internal jugular vein may improve safety and efficacy | Cohort | patients less than 21 years of age, who had an accessory pathway in the anteroseptal region of the tricuspid annulus (either concealed AP or WPW), and underwent radiofrequency (RF) or cryoablation (cryo) with the ablation catheter approaching the anteroseptal region via the right internal jugular vein (RIJV). | Radiofrequency, | a 4-mm tip 7Fr EZ Steer Ablation Catheter (Biosense Webster, Diamond Bar, CA, USA) in all cases except in patients weighing less than 20 kg, where a 4-mm tip 5Fr Medtronic Attacker Ablation Catheter (Medtronic, Minneapolis, MN, USA) Cryoablation, 6-mm tip Cryocatheter (Medtronic) | 15 |
| Ceresnak 2015 | Success Rates in Pediatric WPW Ablation Are Improved with 3-Dimensional Mapping Systems Compared with Fluoroscopy Alone: A Multicenter Study | Cohort | All patients 21 years of age with WPW who underwent invasive EP testing and ablation between 2008 and 2012 | Radiofrequency or Cryoablation, | Biosense Webster (NaviStar, Diamond Bar, CA, USA) or Boston Scientific (Blazer, Marlborough, MA, USA) 7 French 4 mm tip catheters. | 17 |
| Brado 2021 | Outcomes of ablation in Wolff-Parkinson-White-syndrome: Data from the German Ablation Registry | Cohort | admissions for catheter ablation of supraventricular or ventricular arrhythmias, all consented patients between January 2007 and January 2010 | Radiofrequency or Cryoablation, | Biosense Webster (NaviStar, Diamond Bar, CA, USA) or Boston Scientific (Blazer, Marlborough, MA, USA) 7 French 4 mm tip catheters. | 12 |
| Pietrzak 2020 | Success rate and safety of catheter ablation in preexcitation syndrome: A comparison between adult and pediatric patients | Cohort | Patients with WPW were referred to electrophysiological study (EPS) and CA between 2016 and 2017 | Radiofrequency: | irrigated tip ablation — power control mode (temperature limit 48 °C, power limit of 30 W; Navistar ThermoCool, Biosense Webster, Diamond Bar, CA, USA) | – |
| Lin 2019 | Trend and risk factors of recurrence and complications after arrhythmias radiofrequency catheter ablation: a nation-wide observational study in Taiwan | Cohort | all targeted patients with arrhythmia who received first RFCA from 2001 to 2010. The targeted arrhythmias were PSVT (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) Code 4270), WPW (426.7), AFL (427.32), AF (427.31) and VT (427.1), | Radiofrequency | – | 51.6 |
| HOCINI 2015 | Focal Arrhythmia Ablation Determined by High-Resolution Noninvasive Maps: Multicenter Feasibility Study | Cohort | patients with symptomatic premature ventricular complexes (PVCs), atrial tachycardia (AT), and manifest accessory pathway (WPW syndrome) were prospectively included at 3 centers (France, England, and Germany). | Radiofrequency, | 4-mmtip (Biosense-Webster, Diamond Bar, CA, USA) ablation catheter | 24.7 |
| T. Brown 2021 | Ablation of manifest septal accessory pathways: a single-center experience | Cohort | Patients undergoing an electrophysiologic study (EPS) with a manifest SAP were identified from a chart review between January of 2008 and August of 2019 | Radiofrequency and cryoablation | , large curve 4-mm tip ablation catheter (Blazer™ Boston Scientific, Marlborough, MA). But for 2 AP used 8-mm RF ablation catheter | 21.5 |
| Mudrić 2019 | Six-month success of radiofrequency ablation in cardiac arrhythmias treatment – experience of our centre | Cohort | consecutive patients with different cardiac arrhythmias in which RFA was performed during 2014 at the Institute for Cardiovascular Diseases “Dedinje”, Belgrade, Serbia,. | Radiofrequency | – | 6 |
| Fujino 2020 | Clinical characteristics of challenging catheter ablation procedures in patients with WPW syndrome: A 10 year single-center experience | Cohort | symptomatic consecutive WPW patients who underwent a catheter ablation procedure to eliminate an AP at between August 2005 and December 2015 | Radiofrequency and cryoablation, | 4-mm non-irrigated tip ablation catheter (BlazerTMII; Boston Scientific, San Jose, CA, USA for right sided ablation or Celsius; Biosense Webster, Diamond Bar, CA, USA for left sided ablation), 7-Fr 6-mm electrode tip cryoablation catheter (Freezor1 Xtra, CryoCath; Medtronic Inc., Minneapolis, MN, USA), Cryoablation, 7-Fr 6-mm electrode tip cryoablation catheter (Freezor1 Xtra, CryoCath; Medtronic Inc., Minneapolis, MN, USA) | 99.6 |
| Uhm 2018 | Accessory pathway-related left ventricular wall motion abnormality and the effects of radiofrequency catheter ablation in patients with Wolff-Parkinson-White syndrome | Cohort | consecutive patients with WPW syndrome who underwent pre-RFCA echocardiography and RFCA for AP at a university hospital between January 2011 and May 2017 |
Radiofrequency, | – | 6 |
Table 2.
baseline characteristics.
| Study ID | N | Age, mean (SD), year | Sex, n(%) |
Structural heart disease n(%) | Multiple accessory pathways n(%) | Location of single accessory pathways n(%) |
Hypertension n(%) | Diabetes n(%) | Smoking n(%) | Renal failure n(%) | Heart failure n(%) | Congenital heart disease n(%) | Ischaemic heart disease n(%) | Valvular heart disease n(%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | Left free wall | Right free wall |
Posteroseptal | Anteroseptal | |||||||||||||
| Brado 2021 | 789 | 42.8 ± 16.3 | 474(60.1) | 315(39.9) | – | – | 70(8.87) | – | – | – | – | 26(3.3) | – | – | – | – | 42(5.4) | 8(1) |
| Fujino 2020 | 475 | 38.2 ± 16.2 | 291(61) | 184(39) | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| GULLETTA 2013 | 41 | 13 ± 2.3047 | 25(61) | 16(39) | – | – | 18(43.9) | 4(9.75) | 12(29.26) | 4(9.75) | – | – | – | – | – | – | – | – |
| HOCINI 2015 | 7 | 45 ± 14.6 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Lin 2019 | 3051 | 36.267 ± 19.950 |
1988 (65.2) | 1063(34.8) | – | – | – | – | – | – | 275 (9.0) | 113 (3.7) | – | 12 (0.4) | 21 (0.7) | 34 (1.11) | 87 (2.9) | – |
| P. DiLorenzo 2012 | 12 | 14.625 ± 3.913 | – | – | – | – | – | – | – | 12(100) | – | – | – | – | – | 1(8.3) | – | – |
| Pietrzak 2020 | 86 | 27.5 ± 10.752907 |
55(63.9) | 31(36.1) | – | – | 53(66.25) | – | – | – | – | – | – | – | – | – | – | – |
| T. Brown 2021 | 33 | 34.9 ± 12.1 | 17 (51.5) | 16(49.5) | 6 (18.2) | – | – | – | 15(45.45) | 13(39.39) | 6 (18.2) | 2 (6.1) | – | – | – | – | 1 (3.0) | – |
| Uhm 2018 | 348 | 37.6 ± 17.3 | 203(58.33) | 145(41.66) | – | 13(3.735) | 176(50.5) | 78(22.41) | – | – | – | – | – | – | – | – | – | – |
| Ceresnak 2015 | 651 | 13 ± 4.0 | 378 (58) | 273(42) | – | 45 (7) | 256 (39) | 395 (61) | – | – | – | – | – | – | – | 59 (9) | – | 2(0.3) |
| Mudrić 2019 | 44 | 32 ± 15 | 29(65.90) | 15(34.09) | 1 (2) | – | 19 (43) | 5 (13) | – | – | 6 (14) | – | – | – | – | – | – | – |
3.2. Risk of bias assessment
The overall quality of six studies13,15,16,18,21,23 was fair and the others14,17,19,20,22 were of poor quality. The detailed quality assessment was reported in supplementary materials Table 1.
3.3. Outcomes
3.3.1. Success rate
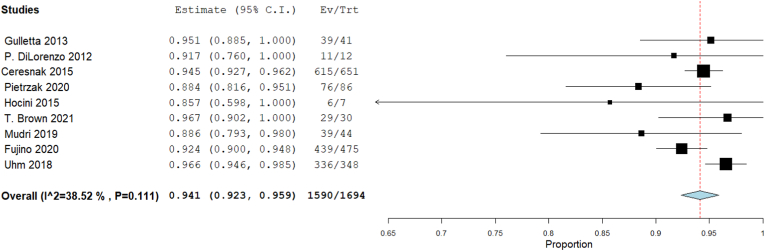
Our single-arm meta-analysis showed that the overall success rate was 94.1% [95% CI: 92.3, 95.9], p < 0.001). The heterogeneity among studies were insignificant (I2 = 38.52%, Chi-Square p = 0.111) (Fig. 2).
Fig. 2.
Forest plot displays success rate in WPW patients.
3.3.2. Recurrence rate
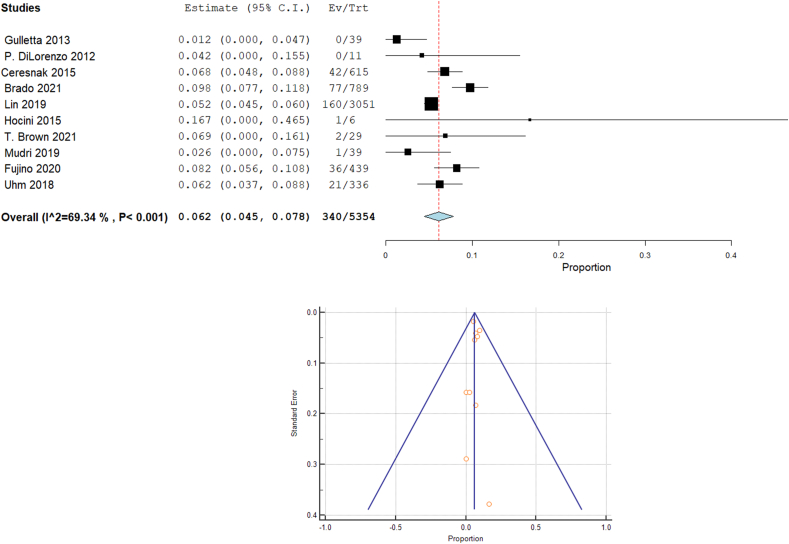
Our analysis revealed that the overall recurrence rate was 6.2% [95% CI: 4.5, 7.8, p < 0.001]. The heterogeneity among studies were significant (I2 = 69.34%, Chi-Square p < 0.001) (Fig. 3A). Fig. 3B showed the funnel plot for publication bias.
Fig. 3.
Forest plot displays recurrence rate in WPW patients, and funnel plot for publication bias.
3.3.3. Complications rate
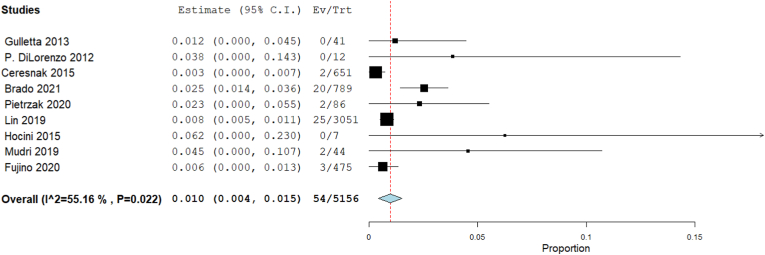
Our analysis revealed that the complications rate was 1% [95% CI: 0.4, 1.5, p < 0.001]. The heterogeneity among studies were significant (I2 = 55.16%, Chi-Square p = 0.022) (Fig. 4).
Fig. 4.
Forest plot displays Complications rate in WPW patients.
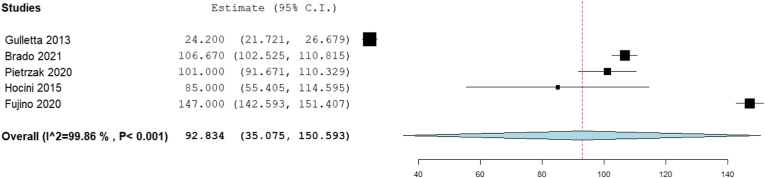
3.3.4. Procedure time (min)
Our analysis showed that the overall procedure time (min) was 92.83 [95% CI: 35.075, 150.593, p = 0.002]. The pooled studies were heterogenous (I2 = 99.86%, Chi-Square p < 0.001) (Fig. 5).
Fig. 5.
Forest plot displays Procedural time (min).
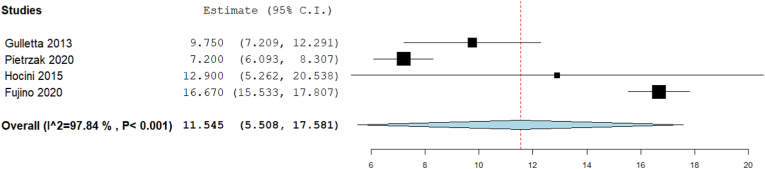
3.3.5. Fluoroscopy time (min)
Our analysis showed that the overall fluoroscopy time (min) was 11.545 [95% CI: 5.5, 17.58, p < 0.001]. The pooled studies were heterogenous (I2 = 97.84%, Chi-Square p < 0.001) (Fig. 6).
Fig. 6.
Forest plot displays Fluoroscopy time (min).
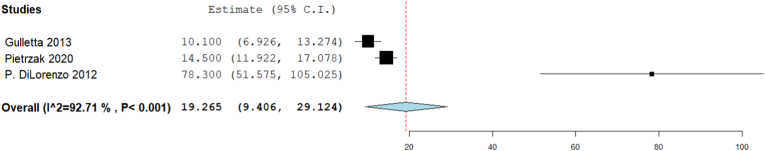
3.3.6. Radiation dose (μGY cm2)
Only three studies reported radiation dose. Analysis showed that the radiation dose (μGY cm2) was 19.265 [95% CI: 9.406, 29.124, p < 0.001]. The pooled studies were heterogenous (I2 = 92.708%, Chi-Square p < 0.001) (Fig. 7).
Fig. 7.
Forest plot displays Radiation dose (μGY cm2).
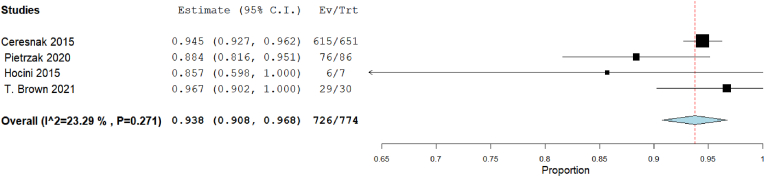
3.3.7. Success rate in 3D electro anatomical approach versus traditional mapping approach
Our analysis showed that the overall success rate in 3D mapping was 93.8% [95% CI: 90.8, 96.8], p < 0.001). The heterogeneity among studies were insignificant (I2 = 23.29%, Chi-Square p = 0.271) (Fig. 8). But in traditional mapping approach was 94.1% [95% CI: 91.2, 97], p < 0.001). The heterogeneity among studies were significant (I2 = 54.88%, Chi-Square p = 0.065) (Fig. 9).
Fig. 8.
Forest plot displays success rate in 3D mapping approach.
Fig. 9.
Forest plot displays success rate in traditional mapping approach.
3.3.8. Recurrence rate in 3D electro anatomical approach versus traditional mapping approach
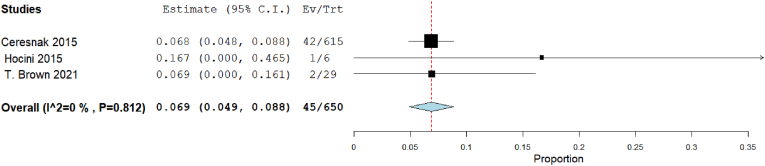
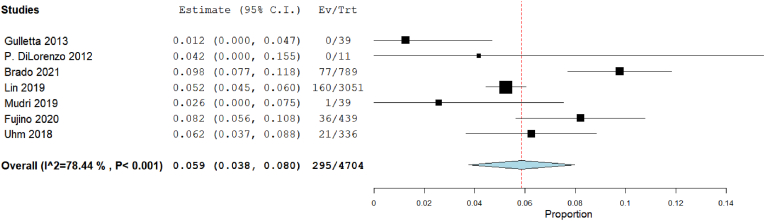
Our analysis showed that the recurrence rate in 3D mapping was 6.9% [95% CI: 4.9, 8.8], p < 0.001). The heterogeneity among studies were insignificant (I2 = 0%, Chi-Square p = 0.812) (Fig. 10). The recurrence rate in traditional mapping approach was 5.9% [95% CI: 3.8, 8], p < 0.001). The heterogeneity among studies were significant (I2 = 54.88%, Chi-Square p = 0.065) (Fig. 11).
Fig. 10.
Forest plot displays recurrence rate in 3D mapping approach.
Fig. 11.
Forest plot displays recurrence rate in traditional mapping approach.
3.3.9. Complication rate in 3D electro anatomical approach versus traditional mapping approach
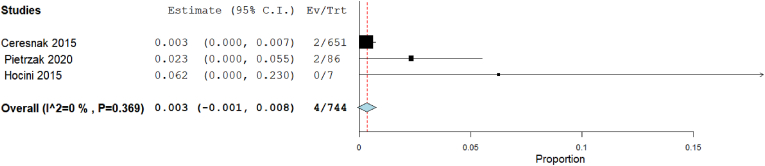
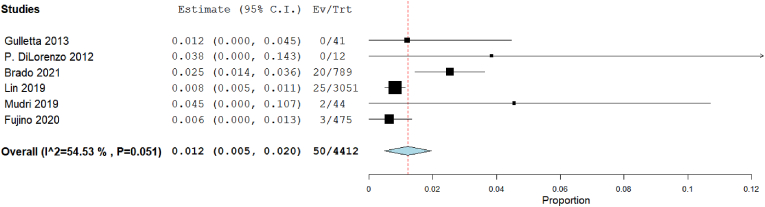
Our analysis showed that the complication rate in 3D mapping was insignificant 0.3% [95% CI: −0.1, 0.8], p = 0.107). The heterogeneity among studies were insignificant (I2 = 0%, Chi-Square p = 0.369) (Fig. 12). But in traditional mapping approach was 1.2% [95% CI: 0.5, 2], p < 0.001). The heterogeneity among studies were significant (I2 = 54.53%, Chi-Square p = 0.051) (Fig. 13).
Fig. 12.
Forest plot displays complication rate in 3D mapping approach.
Fig. 13.
Forest plot displays complication rate in traditional mapping approach,
4. Discussion
Catheter ablation, either through radiofrequency (RFA) or cryoablation, has shown efficacy for WPW syndrome patients. Several centres consider catheter ablation as a first-line treatment, however, there was not sufficient evidence for this approach.24 Recently, the European Heart Rhythm Association (EHRA) has recommended catheter ablation for asymptomatic WPW patients whose accessory pathway has high-risk features, including young patients, multiple accessory pathways, inducible atrioventricular re-entrant tachycardia, and pathway's effective refractory period of <240 ms.25
The results of our analysis revealed that catheter ablation of accessory pathways led to a statistically significant overall success rate of 94.1%. Ablation was also associated with significantly reduced recurrence and complication rates of 6.2% and 1% respectively. These outcomes agree with the results reported in the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) guidelines for the management of asymptomatic young patients with WPW that reported acute success rates of 92%–100% and recurrence rates of 0%–13% for ablation performed in individual pediatric centers.26
As for success rates, the highest rate was observed in Brown et al14 Interestingly, this study targeted septal pathways for ablation that is considered more complex than ablation of other pathways due to the challenging anatomy and close relation to AV node and His bundle that results in a greater risk of heart block and recurrence.27,28 This success may be attributed to the precise choice of the type of ablation for each individual and the use of non-invasive 3-D mapping before the procedure. According to Ceresnak et al, non-invasive 3-D mapping has significantly improved the success rates of ablation in WPW patients.15 Hoccini et al is another included study that used a 3-D mapping, but the success rate wasn't consistent due to the small sample size (only 7 patients). On the other hand, this study revealed zero complications.19
We conducted a further analysis respecting the use of 3-D mapping which was associated with a success rate nearly equal to the traditional ablation procedure. On the other hand, 3-D mapping showed a nonsignificant reduction of the recurrence and complication rates. We believe these results were affected by the small-sized studies that used 3-D mapping and this approach needs to be investigated in larger trials to detect whether it can improve the outcome of the ablation or not.
Regarding the recurrence rates, significant heterogeneity for this outcome is explained by the presence of the small size in Hoccini et al19 that induced publication bias. Also, the variable duration of follow-up among the studies must have induced diverse reporting of patients' recurrence. The approaches of follow-up patients determine the accuracy of the reported recurrence. Brado et al revealed that the telephone-based follow-up in their study led to the underestimation of asymptomatic recurrence.13 Another factor that may have contributed to this heterogeneity is the variable anatomy of the ablated accessory pathways within the same study and among all studies, which is considered to affect patients’ tachycardia and the outcomes of the ablation.29,30
The complication rates showed moderate heterogeneity. Gulletta et al and DiLorenzo et al, which reported zero complications and zero recurrences, performed catheter ablation in patients younger than 21 years.16,18 This supports Blaufox et al who have stated that complications aren't significantly associated with a patient's age, weight, or structural heart disease.31 Minimizing the complications in DiLorenzo et al can be attributed to their novel approach to ablation via the right internal jugular vein. This approach, as explained by the study authors, has maintained catheter stability and signal quality during operating anteroseptal pathways of pediatric patients.16 On the other hand, Gulletta et al depended on a power-controlled conservative protocol according to the ablated pathway to minimize the complications.18 This is consistent with Cohen et al who linked the complication rates to ablation energy, and the duration of the procedure.26 Ceresnak et al is a third pediatric study that produced minimal complications after using a 3-D mapping system that, in turn, reduced the procedure time.15
Regarding the ablation type, four of the included studies13, 14, 15,17 applied both RFA and Cryoablation and all other studies performed RFA only. Both procedures have different rates of complications and recurrence and this factor may be an underlying cause of heterogeneity in these two outcomes.26 Cryoablation was associated with higher recurrence rates than RFA in Atrioventricular nodal re-entrant tachycardia (AVNRT) patients as reported by a meta-analysis that compared both ablation types.32
The analyses of the ablation parameters (procedure time, fluoroscopy time, and radiation dose) were significantly heterogeneous among the studies. Gulletta et al indicated that their short procedure and fluoroscopy time is due to using open irrigated-tip catheters and the small number of patients with right lateral free wall pathways.18 Additionally, the shortest fluoroscopy time achieved by Pietrzak et al is correlated to the use of 3-D non-invasive mapping.22 On the contrary, Fujino et al showed long durations of both procedure and fluoroscopy time, which was significantly associated with increased failure rates.17 To reduce the shortcomings of the traditional ablation procedure, novel approaches were introduced to localize the accessory pathways prior to the intervention. One simple algorithm, which depended on a 12-lead ECG and considered patients' heart axis, showed accuracy in determining the accessory pathway location in both children and adults.33 Such precise localization of the ablated pathways is expected to improve the procedure parameters and patients’ outcomes.
One of our major limitations in conducting this systematic review and meta-analysis is the heterogeneity of the studies in the included population regarding patients' age, location of the accessory pathways, presence of structural abnormalities, and comorbid illnesses. Most of the studies have not reported enough details about patients’ characteristics to support conducting meta-regression analysis based on these variables. Another limitation is the lack of guidelines for performing catheter ablation for all centres to follow, which resulted in the diversity of technical parameters among the studies. These limitations are related to the observational design of the included studies that restricted providing a cause–effect relationship between the intervention and the outcomes. The strengths of our study include searching multiple databases to include all studies applying to our criteria. Moreover, we followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, and we reported this manuscript according to the PRISMA statement.
Eventually, we recommend establishing consolidated guidelines for catheter ablation and providing specific plans for each case. Future interventions following similar guidelines will contribute to yielding stronger evidence.
4.1. Conclusion
Based on the findings the use of RFA showed a high success rate, low recurrence rate and associated with low rate of complications in WPW patients.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ihj.2023.02.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Kobza R., et al. Prevalence of preexcitation in a young population of male Swiss conscripts. Pacing Clin Electrophysiol. 2011;34:949–953. doi: 10.1111/j.1540-8159.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- 2.Fitzsimmons P.J., McWhirter P.D., Peterson D.W., Kruyer W.B. The natural history of Wolff-Parkinson-White syndrome in 228 military aviators: a long-term follow-up of 22 years. Am Heart J. 2001;142:530–536. doi: 10.1067/mhj.2001.117779. [DOI] [PubMed] [Google Scholar]
- 3.Dreifus L.S., Haiat R., Watanabe Y., Arriaga J., Reitman N. Ventricular fibrillation. Circulation. 1971;43:520–527. doi: 10.1161/01.CIR.43.4.520. [DOI] [PubMed] [Google Scholar]
- 4.Timmermans C., et al. Aborted sudden death in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1995;76:492–494. doi: 10.1016/S0002-9149(99)80136-2. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher J.J., Svenson R.H., Sealy W.C., Wallace A.G. The Wolff-Parkinson-white syndrome and the preexcitation dysrhythmias: medical and surgical management. Med Clin. 1976;60:101–123. doi: 10.1016/S0025-7125(16)31922-8. [DOI] [PubMed] [Google Scholar]
- 6.Blomström-Lundqvist C., et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias∗∗This document does not cover atrial fibrillation; atrial fibrillation is covered in the ACC/AHA/ESC guidelines on the management of patients with atrial fibrillation found on the ACC, AHA, and ESC Web sites.—executive summary. J Am Coll Cardiol. 2003;42:1493–1531. doi: 10.1016/j.jacc.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H., et al. Radiation exposure during radiofrequency catheter ablation of accessory atrioventricular connections. Circulation. 1991;84:2376–2382. doi: 10.1161/01.CIR.84.6.2376. [DOI] [PubMed] [Google Scholar]
- 8.Jackman W.M., et al. Catheter ablation of accessory atrioventricular pathways (Wolff–Parkinson–white syndrome) by radiofrequency current. N Engl J Med. 1991;324:1605–1611. doi: 10.1056/NEJM199106063242301. [DOI] [PubMed] [Google Scholar]
- 9.Kugler J.D., Danford D.A., Houston K.A., Felix G. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: comparison of early and recent eras. J Cardiovasc Electrophysiol. 2002;13:336–341. doi: 10.1046/j.1540-8167.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. n71-n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane Handbook for Systematic Reviews of Interventions. Wiley; 2019. [Google Scholar]
- 12.Stuck A.E., et al. Bias in meta-analysis detected by a simple, graphical. BMJ. 1998;316 doi: 10.1136/bmj.316.7129.469. 469-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brado J., et al. Outcomes of ablation in Wolff-Parkinson-White-syndrome: data from the German ablation registry. Int J Cardiol. 2021;323:106–112. doi: 10.1016/j.ijcard.2020.08.102. [DOI] [PubMed] [Google Scholar]
- 14.Brown M.T., et al. Ablation of manifest septal accessory pathways: a single-center experience. J Intervent Card Electrophysiol. 2021;61:349–355. doi: 10.1007/s10840-020-00823-w. [DOI] [PubMed] [Google Scholar]
- 15.Ceresnak S.R., et al. Success rates in pediatric WPW ablation are improved with 3-dimensional mapping systems compared with fluoroscopy alone: a multicenter study. J Cardiovasc Electrophysiol. 2015;26:412–416. doi: 10.1111/jce.12623. [DOI] [PubMed] [Google Scholar]
- 16.DiLorenzo M.P., Pass R.H., Nappo L., Ceresnak S.R. Ablating the anteroseptal accessory pathway—ablation via the right internal jugular vein may improve safety and efficacy. J Intervent Card Electrophysiol. 2012;35:293–299. doi: 10.1007/s10840-012-9699-9. [DOI] [PubMed] [Google Scholar]
- 17.Fujino T., et al. Clinical characteristics of challenging catheter ablation procedures in patients with WPW syndrome: a 10 year single-center experience. J Cardiol. 2020;76:420–426. doi: 10.1016/j.jjcc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Gulletta S., et al. Safety and efficacy of open irrigated-tip catheter ablation of Wolff-Parkinson-white syndrome in children and adolescents. Pacing Clin Electrophysiol. 2013;36:486–490. doi: 10.1111/pace.12086. [DOI] [PubMed] [Google Scholar]
- 19.Hocini M., et al. Focal arrhythmia ablation determined by high-resolution noninvasive maps: multicenter feasibility study. J Cardiovasc Electrophysiol. 2015;26:754–760. doi: 10.1111/jce.12700. [DOI] [PubMed] [Google Scholar]
- 20.Jurcevic-Mudric R., Angelkov L., Tomovic M., Kojic D., Milojevic P. Six-months success of radiofrequency ablation in cardiac arrhythmias treatment: experience of our centre. Vojnosanit Pregl. 2019;76:398–403. doi: 10.2298/VSP170125109J. [DOI] [Google Scholar]
- 21.Lin Y., Wu H.-K., Wang T.-H., Chen T.-H., Lin Y.-S. Trend and risk factors of recurrence and complications after arrhythmias radiofrequency catheter ablation: a nation-wide observational study in Taiwan. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-023487. e023487-e023487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrzak R., et al. Success rate and safety of catheter ablation in preexcitation syndrome: a comparison between adult and pediatric patients. Cardiol J. 2022;29:88–92. doi: 10.5603/CJ.a2020.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uhm J.-S., et al. Accessory pathway-related left ventricular wall motion abnormality and the effects of radiofrequency catheter ablation in patients with Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 2019;30:102–108. doi: 10.1111/jce.13753. [DOI] [PubMed] [Google Scholar]
- 24.Svendsen J.H., et al. Current strategy for treatment of patients with Wolff-Parkinson-White syndrome and asymptomatic preexcitation in Europe: European Heart Rhythm Association survey. Europace. 2013;15:750–753. doi: 10.1093/europace/eut094. [DOI] [PubMed] [Google Scholar]
- 25.Arnar D.O., et al. Management of asymptomatic arrhythmias: a European heart Rhythm association (EHRA) consensus document, endorsed by the heart failure association (HFA), heart Rhythm society (HRS), Asia Pacific heart Rhythm society (APHRS), cardiac arrhythmia society of Southern Africa (CASSA), and Latin America heart Rhythm society (LAHRS) EP Europace. 2019;21:844–845. doi: 10.1093/europace/euz046. [DOI] [PubMed] [Google Scholar]
- 26.Cohen M.I., et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-white (WPW, ventricular preexcitation) electrocardiographic pattern: developed in partnership between the pediatric and congenital electrophysi. Heart Rhythm. 2012;9:1006–1024. doi: 10.1016/j.hrthm.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Tai C.-T., Chen S.-A., Chiang C.-E., Lee S.-H., Chang M.-S. Electrocardiographic and electrophysiologic characteristics of anteroseptal, midseptal, and Para-Hisian accessory pathways. Chest. 1996;109:730–740. doi: 10.1378/chest.109.3.730. [DOI] [PubMed] [Google Scholar]
- 28.Xie B., Heald S.C., Bashir Y., Camm A.J., Ward D.E. Radiofrequency catheter ablation of septal accessory atrioventricular pathways. Heart. 1994;72:281–284. doi: 10.1136/hrt.72.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hikspoors J.P.J.M., et al. Miniseries 1—Part I: the Development of the atrioventricular conduction axis. EP Europace. 2022;24:432–442. doi: 10.1093/europace/euab287. [DOI] [PubMed] [Google Scholar]
- 30.Macías Y., et al. Miniseries 1—Part II: the comparative anatomy of the atrioventricular conduction axis. EP Europace. 2022;24:443–454. doi: 10.1093/europace/euab291. [DOI] [PubMed] [Google Scholar]
- 31.Blaufox A.D., Felix G.L., Saul J.P. Radiofrequency catheter ablation in infants ≤18 Months old. Circulation. 2001;104:2803–2808. doi: 10.1161/hc4801.100028. [DOI] [PubMed] [Google Scholar]
- 32.Hanninen M., et al. Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. J Cardiovasc Electrophysiol. 2013;24:1354–1360. doi: 10.1111/jce.12247. [DOI] [PubMed] [Google Scholar]
- 33.El Hamriti M., et al. EASY-WPW: a novel ECG-algorithm for easy and reliable localization of manifest accessory pathways in children and adults. EP Europace. 2022 doi: 10.1093/europace/euac216. euac216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.