Abstract
Background
The 2022 Global Initiative for Asthma guidelines emphasise the inhaled long-acting β2-agonist formoterol as part of the first treatment step, and therefore formoterol use among athletes will probably increase. However, prolonged supratherapeutic use of inhaled β2-agonists impairs training outcomes in moderately trained men. We investigated whether inhaled formoterol, at therapeutic doses, imposes detrimental effects in endurance-trained individuals of both sexes.
Methods
51 endurance-trained participants (31 male, 20 female; mean±sd maximal oxygen consumption (V̇O2max) 62±6 mL·min−1·kg bw−1 and 52±5 mL·min−1·kg bw−1, respectively) inhaled formoterol (24 µg; n=26) or placebo (n=25) twice daily for 6 weeks. At baseline and follow-up, we assessed V̇O2max and incremental exercise performance during a bike-ergometer ramp-test; body composition by dual-energy X-ray absorptiometry; muscle oxidative capacity by high-resolution mitochondrial respirometry, enzymatic activity assays and immunoblotting; intravascular volumes by carbon monoxide rebreathing; and cardiac left ventricle mass and function by echocardiography.
Results
Compared to placebo, formoterol increased lean body mass by 0.7 kg (95% CI 0.2–1.2 kg; treatment×trial p=0.022), but decreased V̇O2max by 5% (treatment×trial p=0.013) and incremental exercise performance by 3% (treatment×trial p<0.001). In addition, formoterol lowered muscle citrate synthase activity by 15% (treatment×trial p=0.063), mitochondrial complex II and III content (treatment×trial p=0.028 and p=0.007, respectively), and maximal mitochondrial respiration through complexes I and I+II by 14% and 16% (treatment×trial p=0.044 and p=0.017, respectively). No apparent changes were observed in cardiac parameters and intravascular blood volumes. All effects were sex-independent.
Conclusion
Our findings demonstrate that inhaled therapeutic doses of formoterol impair aerobic exercise capacity in endurance-trained individuals, which is in part related to impaired muscle mitochondrial oxidative capacity. Thus, if low-dose formoterol fails to control respiratory symptoms in asthmatic athletes, physicians may consider alternative treatment options.
Short abstract
Prolonged treatment with inhaled formoterol impairs aerobic exercise capacity in endurance-trained individuals. Physicians may consider alternative treatment options if low-dose formoterol fails to control airway symptoms in asthmatic athletes. https://bit.ly/3vLsjHM
Introduction
The 2022 Global Initiative for Asthma (GINA) [1] guidelines emphasise the inhaled long-acting β2-agonist, formoterol, combined with inhaled corticosteroids (ICS) as the preferred first step in asthma treatment. Therefore, we can expect a surge in the use of formoterol among athletes [2, 3]. This is further emphasised by the fact that current antidoping regulations (i.e. the 2022 prohibited list) permit athletes to inhale formoterol at doses of up to 54 µg daily, which is the maximum recommended dose of as-needed formoterol in combination with ICS in 1 day as per 2022 GINA guidelines [1], irrespective of objective indication of asthma and asthma-related conditions [4].
Inhaled β2-agonists are commonly used by endurance athletes for whom performance is mostly dominated by aerobic rather than anaerobic capacity [5]. Paradoxically, recent studies indicate that prolonged treatment with β2-agonists may in fact impair aerobic exercise capacity. For example, we recently observed that daily inhalation of terbutaline at high doses (4 mg·day−1) abolished enhancement of maximal oxygen consumption (V̇O2max) and exercise capacity with 4 weeks of endurance training in healthy moderately trained men [6]. V̇O2max is important across a range of endurance sports as it is directly related to the maximal rate of energy production sustainable by aerobic processes, thus postponing reliance on anaerobic energy production [7]. And while V̇O2max only partly predicts endurance exercise performance [8], professional cyclists may sustain workloads at ∼90% V̇O2max for up to an hour [9], and thus it can reasonably be assumed that a decrease in V̇O2max is detrimental to endurance exercise performance. These early findings suggest that overuse or unwarranted use of β2-agonists can be cause for concern in the treatment of athletes.
However, some important aspects must be addressed to guide future practices in asthma treatment in athletic populations. These aspects relate primarily to real-life practice applicability, as prior studies on aerobic exercise performance employed high doses of short-acting β2-agonists (terbutaline and salbutamol) [6, 10, 11]. And while the acute effects of formoterol on aerobic exercise performance have been investigated [12–14], no data are available on the effects of prolonged treatment with formoterol on aerobic exercise outcomes in doses resembling those used clinically. In addition, participants in prior studies were all moderately trained males [6, 10, 11], which does not accurately reflect athletic populations comprising highly trained males and females. Lastly, there are no available data from human studies on the physiological mechanisms that underlie the potential detrimental effects of β2-agonists.
A significant fraction of the inhaled β2-agonist dose reaches the systemic circulation [15–17] and could therefore affect any physiological system of importance for aerobic exercise performance. This includes both oxygen transport (i.e. cardiac function, blood oxygen-carrying capacity and muscle capillarisation) and peripheral oxygen utilisation (i.e. muscle mitochondrial content and function). In terms of oxygen transport, only data from rodents are available, but these consistently show that systemic administration of β2-agonists induces cardiac hypertrophy [18–20] and impairs cardiac function [19]. For example, formoterol induces cardiac hypertrophy in rats with doses as low as 0.003 mg·kg−1·day−1 [21], potentially limiting cardiac output during strenuous exercise. As inhaled β2-agonists induce muscle hypertrophy in humans [22], this could conceivably affect cardiac muscle too. In terms of peripheral oxygen utilisation, β2-agonists can impair the oxidative capacity of skeletal muscle during periods with [6] or without [11] exercise training. However, these early studies utilised surrogate immunoblotting markers of mitochondrial function and not the gold-standard methodology of measuring ex vivo respiratory capacity using high-resolution respirometry [23, 24].
Therefore, our main objective was to investigate the effect of prolonged treatment with inhaled formoterol for 6 weeks on V̇O2max and exercise performance in healthy female and male endurance-trained participants. Other objectives were to examine factors of importance for aerobic exercise capacity including body composition, cardiac structure, blood oxygen-carrying capacity and muscle oxidative capacity and function. We hypothesised that formoterol would increase muscle mass, but impair V̇O2max, exercise performance during an incremental test to exhaustion, and muscle oxidative capacity.
Methods
Study design and ethics approval
The study was a randomised, double-blinded, placebo-controlled, parallel-group study. Participants completed one experimental trial day at baseline and after 6 weeks of daily formoterol or placebo treatment. The study was registered in clinicaltrials.gov (identifier NCT03565302). The study was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the regional ethics committee of Copenhagen (H-18007889). All participants were informed about possible risks involved and gave their oral and written consent before inclusion.
Participants
All trials were conducted at the Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark. Inclusion criteria were age 18–45 years, body mass index <26 kg·m−2 and V̇O2max >50 and >55 mL·min−1·kg bw-1 for females and males, respectively. Exclusion criteria were asthma or exercise-induced bronchoconstriction, smoking, use of β2-agonists in the past 5 years, chronic use of prescription medication (excluding contraceptives) or chronic illness.
Assessment of eligibility criteria
We assessed eligibility criteria during a medical examination with electrocardiography (ECG-2150; Nihon Kohden, Rosbach, Germany), blood pressure measurements (M7 Intellisense; OMRON, Kyoto, Japan) and lung function (EasyOne Air; NDD Medical Technologies, Andover, MA, USA). Participants completed a ramp test on a bicycle ergometer (Monark LC6; Monark, Vansbro, Sweden) for determination of V̇O2max by indirect calorimetry (Oxycon Pro; CareFusion, San Diego, CA, USA). The ramp test was preceded by three 4-min bouts at 75, 100 and 125 W and 100, 150 and 200 W for females and males, respectively. Participants performed lung function testing of forced expiratory volume in 1 s (FEV1) 5, 10, 15 and 20 min after conclusion of the ramp test to exclude exercise-induced bronchoconstriction (defined as a >10% decrease in FEV1). After the last FEV1 measurement, participants inhaled 800 µg salbutamol (onset of action ≈5 min [25]) and FEV1 was assessed once more after 10 min after inhalation to test for reversibility, defined as a 12% and 200 mL increase in FEV1 according to European Respiratory Society guidelines [26].
Randomisation and blinding
Upon inclusion, participants were allocated into two groups receiving either formoterol or placebo. Randomisation was performed by minimisation for sex, V̇O2max and lean body mass to ensure group homogeneity at baseline for the main outcome measures [27, 28]. Allocation was performed by personnel not involved in enrolment or conducting experimental trials. Formoterol and placebo were administered in identical-looking inhalers and the intervention was identical for both treatments, and thus allocation was concealed to assessors and participants throughout the whole intervention.
In subgroups of participants, we measured muscle mitochondrial respiration (n=20), muscle protein abundance of mitochondrial proteins (n=41) and cardiac structure and resting function (n=20). These were selected based on predefined allocations, where muscle biopsies were collected in the first 41 participants and mitochondrial respiration was assessed in the first 10 participants allocated to each treatment, whereas cardiac structure and resting function were assessed in the final 10 participants allocated to each treatment.
Outcome measures and sample size
The main response outcome measure was change in V̇O2max during a ramp test to exhaustion on a bike ergometer. Other outcome measures were changes in incremental exercise performance; lean mass and fat mass measured by dual-energy X-ray absorptiometry; left ventricle mass and function measured by echocardiography; oxygen-carrying capacity in blood measured by carbon monoxide rebreathing; and muscle oxidative capacity measured by high-resolution mitochondrial respirometry. All measurements were conducted at both the pre- and post-intervention experimental trial days.
Sample size was determined for the main outcome measure in GPower 3.1.9.3 with an α-level of 0.05 and β-level of 0.8 for a linear mixed model repeated-measures design, which resulted in a required sample size of 24 participants per group. Effect size and standard deviation were estimated for change in lean mass from a previous study [22], as no data were available for change in V̇O2max.
Intervention and study drugs
The intervention period consisted of 6 weeks of twice daily inhalation of formoterol (Foradil; Medical Valley Invest, Höllviken, Sweden) or placebo. Formoterol and placebo inhalers were delivered by the regional pharmacy of Copenhagen, Denmark. During the intervention, participants were instructed to maintain their physical activity levels and not to make major changes in their normal training or nutrition regimens. In addition, participants were asked not to donate blood during the intervention. Participants were asked to report their physical activity levels and any potential illness during the intervention at the post-intervention trial. On trial days, participants kept a detailed nutritional log and replicated nutritional intake to minimise inter-day variation in study outcome measurements.
For the first week of the intervention the administered dose of formoterol was 12 µg twice daily (total daily dose 24 µg), after which the administered dose was 24 µg twice daily (total daily dose 48 µg) for the remaining weeks. The lower dosage in the first week was chosen to maintain participant treatment blinding and to minimise discomfort from potential side-effects of formoterol, which include slight muscle tremor, headaches and restlessness.
Participants abstained from study drugs for 2 days before both experimental trials (mean±sd 2.3±0.9 days) to avoid acute effects of treatment on test procedures (duration of action of formoterol ≈12 h [29–31]). Both experimental trials were completed at the same time of day (mean±sd deviation between trial time of day 0.5±0.5 h).
During the intervention, participants were instructed to inhale the study drug twice daily between 06:00 and 11:00, and between 16:00 and 20:00. All inhalations were supervised via video monitoring (e.g. Skype/FaceTime).
Pre- and post-intervention trials
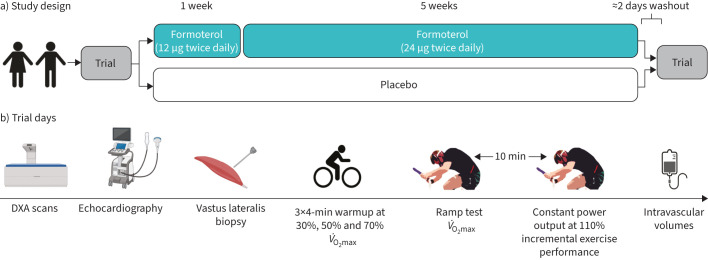
Before and at follow-up, we measured body composition using dual-energy X-ray absorptiometry followed by assessment of cardiac structure by echocardiography. Next, we collected a biopsy from the left vastus lateralis muscle under local anaesthesia, and then participants completed a standardised warmup of three sets of 4 min at 30%, 50% and 70% of V̇O2max, respectively, as calculated from the screening ramp test. Following this, participants completed a bike ergometer ramp test to task failure for determination of V̇O2max and incremental exercise performance, starting from 125 and 150 W for females and males, respectively, with workload continuously increasing every second for an average increase of 25 and 30 W·min−1 for females and males, respectively, until task failure, which was defined as a drop below 70 rpm despite strong verbal encouragement for >3 s. After the ramp test, participants rested for 10 min before completing a constant power output test at 110% incremental exercise performance from the prior ramp test to exhaustion (as suggested by Poole and Jones [32] for more accurate V̇O2max assessment).
V̇O2max was determined as the highest mean value recorded in any consecutive 30-s period during either the ramp test or constant power output test at 110% incremental exercise performance. Incremental exercise performance was determined as the highest power output attained during the ramp test. Power output eliciting V̇O2max was determined as the power output at the time of V̇O2max.
After ≈20 min rest, we assessed intravascular volumes (blood volume and plasma volume) and total haemoglobin mass. After the first experimental trial, participants were instructed in correct inhaler technique and kept the inhaler for home administration of subsequent doses. A flowchart depicting the experimental trials and study intervention is shown in figure 1.
FIGURE 1.
Overview of a) study design and b) trial days. DXA: dual-energy X-ray absorptiometry; V̇O2max: maximal oxygen consumption.
Experimental procedures
Detailed descriptions of experimental procedures are available in the supplementary material.
Statistics
We used SPSS (version 28; IBM Software, Chicago, IL, USA) for statistical analyses. Normally distributed data are presented as mean±sd and were verified with the Shapiro–Wilk test and Q-Q plots. We estimated the effect of formoterol versus placebo with a linear mixed model analysis of the variance where treatment (formoterol/placebo) and trial (pre-/post-) were modelled as fixed effects and participants as a random effect for a full factorial design. We estimated sex differences in the response to formoterol with the same analysis of the variance model with sex added as a third factor. Effect size of outcome measures is presented as mean change with 95% confidence interval (i.e. change in the formoterol group versus change in the placebo group) and exact p-values (except when <0.001) to represent probability.
Results
Participants
The experimental trials were conducted from June 2018 to January 2022. In total, 51 healthy endurance-trained participants (20 females and 31 males) completed the study distributed to either a formoterol or placebo group (table 1). Participants were engaged in running (n=35) or cycling (n=25), as well as light resistance training (n=25). None of the participants presented with post-exercise reductions in FEV1 or a positive bronchodilator reversibility test after exercise (table 1). A participant flow diagram is presented in the supplementary results.
TABLE 1.
Baseline characteristics of study participants
| Formoterol (n=26) | Placebo (n=25) | |||
| Male | Female | Male | Female | |
| Participants | 16 | 10 | 15 | 10 |
| Age (years) | 26±6 | 25±4 | 28±8 | 25±3 |
| Height (cm) | 183±7 | 168±6 | 182±6 | 168±7 |
| Weight (kg) | 77.2±10.1 | 62.8±8.8 | 74.8±7.5 | 62.7±8.2 |
| Lean mass (kg) | 64.2±8.5 | 45.4±5.7 | 61.7±6.1 | 45.1±4.9 |
| Fat percentage (%) | 13.6±3.8 | 24.9±4.4 | 14.4±3.9 | 25.1±3.6 |
| V̇O2max (mL·min−1) | 4830±715 | 3282±623 | 4604±397 | 3163±441 |
| V̇O2max (mL·min−1·kg bw−1) | 62.8±5.2 | 52.0±4.5 | 61.8±6.5 | 50.9±6.1 |
| Incremental exercise performance# (W) | 408±54 | 285±43 | 391±29 | 289±33 |
| Power output eliciting V̇O2max (W) | 391±53 | 270±44 | 377±36 | 277±31 |
| FVC (L) | 6.1±1.5 | 4.1±0.5 | 5.4±0.7 | 4.2±0.5 |
| FEV1 (L) | 4.8±1.1 | 3.6±0.4 | 4.5±0.5 | 3.5±0.3 |
| FEV1/FVC | 0.79±0.07 | 0.87±0.04 | 0.84±0.06 | 0.84±0.06 |
| Post-exercise change in FEV1 (%) | −0.6±3.0 | −2.9±4.0 | −1.6±2.1 | −2.7±4.4 |
| Change in FEV1 after β2-agonist (%) | 1.1±5.6 | 3.5±3.5 | 4.1±3.2 | 1.2±2.6 |
Data are presented as n or mean±sd. V̇O2max: maximal oxygen consumption; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s. #: the highest power output achieved during the ramp test to exhaustion.
Compliance, blinding and side-effects
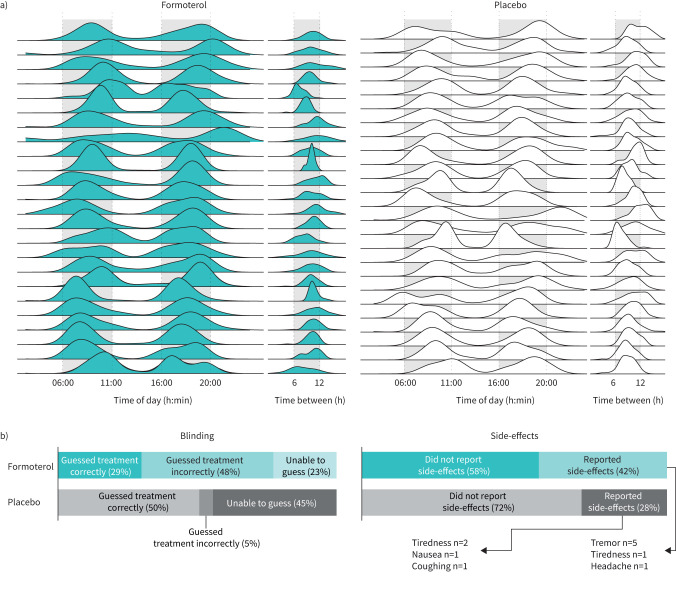
Mean compliance was 99.5±1.3% and 99.4±1.3% in the formoterol and placebo group, respectively, with the corresponding lowest compliances being 93.3% and 95.2%. Administration time points distributed successfully in the pre-determined time frames with daily administrations being separated by 10±2 h on average (figure 2a).
FIGURE 2.
Compliance, blinding and side-effects. a) Ridge plot of daily inhalations in the formoterol and placebo groups. Participants were instructed to administer inhalations between the daily hours of 06:00 and 11:00 as well as 16:00 and 20:00. All participants’ daily inhalations were supervised by online video calls. Specific inhalation time points for two participants in the formoterol group are missing. b) Blinding and side-effects.
Participants performed 7±5 and 8±3 h of structured endurance training per week in the formoterol and placebo group, respectively, which did not change during the intervention (p<0.8 for both treatment groups).
Body composition
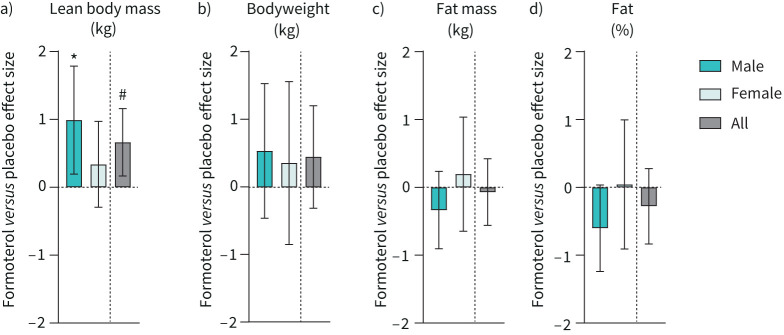
Formoterol increased lean body mass (treatment×trial, p=0.022; figure 3a) independent of sex (sex×treatment×trial, p=0.223), but had no apparent effect on bodyweight (treatment×trial, p=0.238; figure 3b), fat mass (treatment×trial, p=0.714; figure 3c) or fat percentage (treatment×trial, p=0.272; figure 3d) compared with placebo.
FIGURE 3.
Change in body composition after 6 weeks of twice-daily inhalation of formoterol (48 µg·day−1) in endurance-trained males (n=16) and females (n=10) versus placebo (males n=15, females n=10). Pooled data from males and females are shown (all). a) Lean body mass; b) bodyweight; c) body fat mass; d) body fat percentage. Values are effect sizes compared with placebo with 95% confidence intervals. *: p<0.05 (different from placebo within-sex); #: p<0.05 (treatment×trial interaction effect).
Aerobic exercise capacity
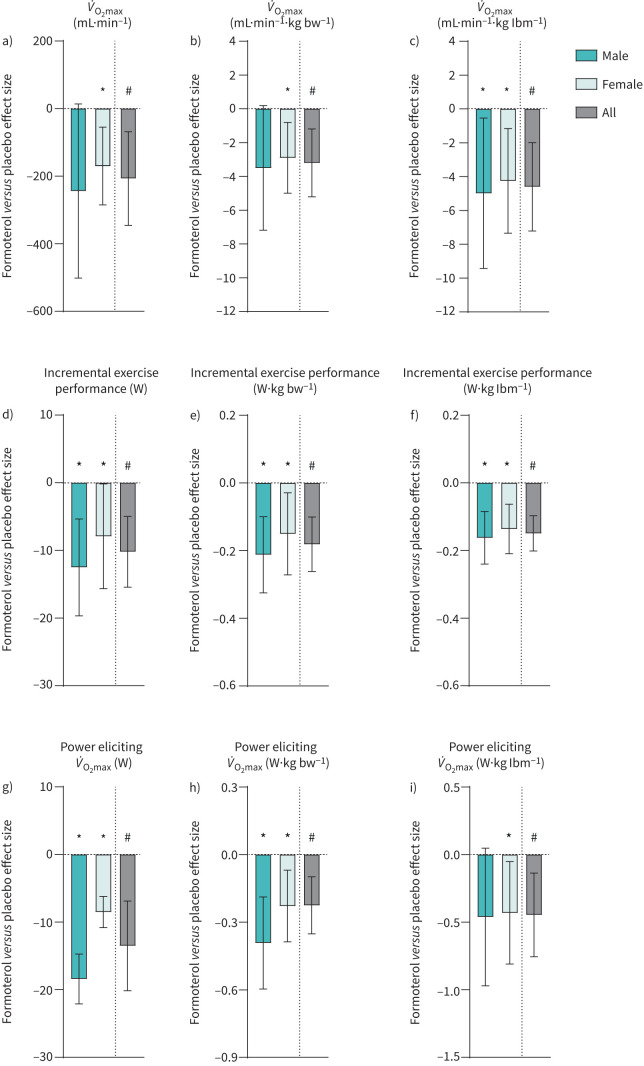
Formoterol treatment decreased V̇O2max by 5% (treatment×trial, p=0.013; figure 4a) independent of sex (sex×treatment×trial, p=0.650). When expressed relative to bodyweight and lean body mass, formoterol treatment decreased V̇O2max by 5% (treatment×trial, p=0.009; figure 4b) and 6% (treatment×trial, p=0.002; figure 4c), respectively. Incremental exercise performance declined by 3% with formoterol treatment (treatment×trial, p<0.001; figure 4d), independent of sex (sex×treatment×trial, p=0.395) and also when expressed relative to bodyweight and lean body mass (both p<0.001; figure 4e,f). V̇O2max was elicited at a lower power output with formoterol treatment, decreasing by 3% (treatment×trial, p=0.002; figure 4g), which was independent of sex (sex×treatment×trial, p=0.114). When expressed relative to bodyweight and lean body mass, formoterol treatment decreased the power output eliciting V̇O2max by 7% (treatment×trial, p<0.001; figure 4h) and 7% (treatment×trial, p<0.001; figure 4i), respectively.
FIGURE 4.
Change in aerobic exercise capacity during a ramp test to exhaustion after 6 weeks of twice-daily inhalation of formoterol (48 µg·day−1) in endurance-trained males (n=16) and females (n=10) versus placebo (males n=15, females n=10). Pooled data from males and females are shown (all). a) Maximal oxygen consumption (V̇O2max); b) V̇O2max relative to bodyweight (bw); c) V̇O2max relative to lean body mass (lbm); d) incremental exercise performance; e) incremental exercise performance relative to bw; f) incremental exercise performance relative to lbm, g) power output eliciting V̇O2max; h) power output eliciting V̇O2max relative to bw; i) power output eliciting V̇O2max relative to lbm. Values are effect sizes compared with placebo with 95% confidence intervals. *: p<0.05 (different from placebo within-sex); #: p<0.05 (treatment×trial interaction effect).
Content and function of muscle oxidative enzymes
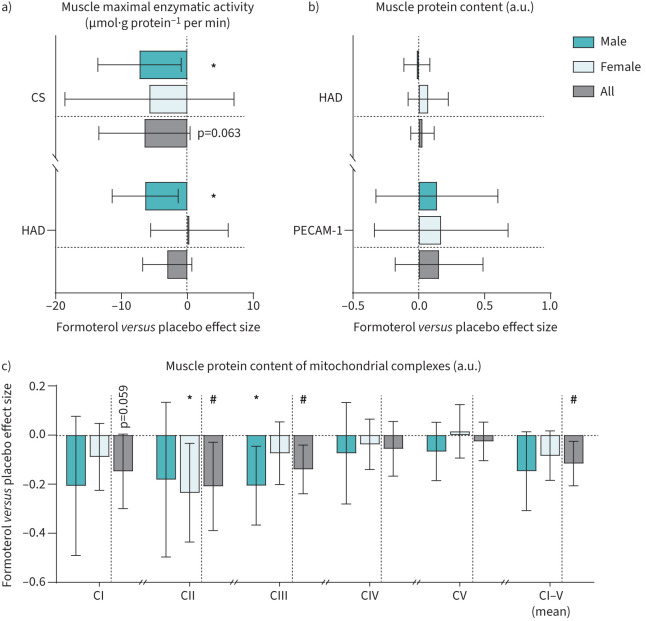
In a subgroup of 41 participants (formoterol 11 males and 10 females; placebo 10 males and 10 females), we assessed maximal muscle citrate synthase activity, which declined by 15% with formoterol treatment (treatment×trial, p=0.063; figure 5a) independently of sex (sex×treatment×trial, p=0.820).
FIGURE 5.
Change in maximal enzymatic activity and protein content in muscle samples after 6 weeks of twice-daily inhalation of formoterol (48 µg·day−1) in endurance-trained males (n=11) and females (n=10) versus placebo (males n=10, females n=10). Pooled data from males and females are shown (all). a) Maximal activity of mitochondrial enzymes citrate synthase (CS) and hydroxyacyl-CoA dehydrogenase (HAD); b) muscle protein content of HAD and platelet endothelial cell adhesion molecule-1 (PECAM-1); c) muscle protein content of mitochondrial complexes I–V (CI–V). Values are effect sizes compared with placebo with 95% confidence intervals. a.u.: arbitrary units. *: p<0.05 (different from placebo within-sex); #: p<0.05 (treatment×trial interaction effect).
Maximal activity of hydroxyacyl-CoA dehydrogenase (HAD) activity declined by 7% with formoterol treatment (treatment×trial, p=0.105; figure 5a). Protein content of mitochondrial complexes decreased with formoterol treatment for complex I (p=0.059), complex II (treatment×trial, p=0.028) and complex III (p=0.007), whereas there was no apparent effect on complexes IV (p=0.329) and V (p=0.507; figure 5). Mean complex I–V expression was decreased with formoterol treatment (treatment×trial, p=0.019).
Protein content of HAD (treatment×trial, p=0.513) and platelet endothelial cell adhesion molecule-1 (PECAM-1) (p=0.366) did not change with formoterol treatment (figure 5b). None of the changes were sex-dependent.
Muscle mitochondrial respiration
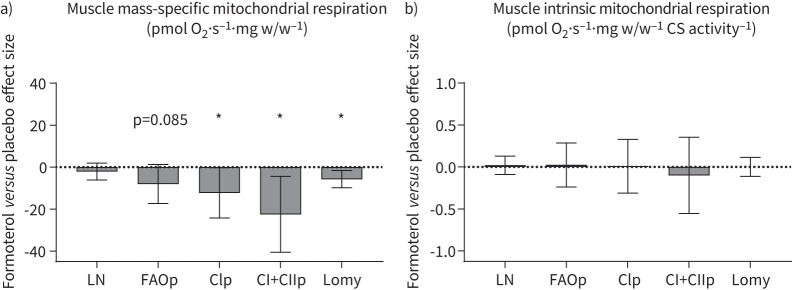
In a subgroup of 20 participants (formoterol: five males, five females; placebo: five males, five females), we further assessed mitochondrial respiration. In this subgroup, formoterol treatment attenuated complex I and complex I+II coupled respiration by 14% (treatment×trial, p=0.044) and 16% (treatment×trial, p=0.017), respectively. In addition, formoterol treatment decreased fatty acid oxidation (treatment×trial, p=0.085) and oligomycin-induced leak respiration (treatment×trial, p=0.009). Formoterol treatment had no apparent effect on mass-specific leak respiration (treatment×trial, p=0.293).
When mitochondrial respiration values were adjusted for citrate synthase activity (intrinsic mitochondrial respiration), formoterol had no effect compared with placebo (all treatment×trial p>0.6; figure 6b). Absolute data for muscle mitochondrial respiration are available in the supplementary results.
FIGURE 6.
Change in muscle mitochondrial respiration after 6 weeks of twice-daily inhalation of formoterol (48 µg·day−1) in endurance-trained males (n=5) and females (n=5) versus placebo (males n=5, females n=5). Data are pooled for sexes. a) Mass-specific respiration; b) intrinsic respiration (adjusted for citrate synthase (CS) activity). O2: oxygen; LN: leak respiration; FAOp: fatty acid oxidation; CIp: complex I coupled respiration; CI+CIIp: complex I+II coupled respiration; Lomy: oligomycin-induced LN. Values are effect sizes compared with placebo with 95% confidence intervals. *: p<0.05 (treatment×trial interaction effect).
Cardiac structure, resting function and intravascular volumes
In a subgroup of 20 participants (formoterol: nine males and one female; placebo: 10 males), we conducted cardiac echocardiography and measured total haemoglobin mass and intravascular volumes at baseline and after 6 weeks of treatment. Formoterol had no apparent effect on cardiac structure. In addition, total haemoglobin and intravascular volumes were unchanged with formoterol (table 2). Details on cardiac function and further haematological measurements are available in supplementary results.
TABLE 2.
Cardiac structure and function and intravascular volumes before (pre-) and after (post-) 6 weeks of twice-daily administration of formoterol (48 µg·day−1) or placebo in endurance-trained participants (19 males, one female)
| Formoterol | Placebo | Formoterol versus placebo effect size mean change (95% CI) | Interaction p-value | |||
| Pre | Post | Pre | Post | |||
| Participants | 10 | 10 | ||||
| Cardiac parameters | ||||||
| LV mass (g) | 226±52 | 233±45 | 225±32 | 224±27 | 8 (−3 to 20) | 0.158 |
| LV mass index (g·m−2) | 115±25 | 118±22 | 115±15 | 115±11 | 4 (−2 to 10) | 0.154 |
| Total haemoglobin mass and intravascular volumes | ||||||
| Blood volume (L) | 6.2±0.6 | 6.3±0.8 | 6.3±0.6 | 6.3±0.4 | 0.1 (−0.4 to 0.4) | 0.796 |
| Plasma volume (L) | 3.7±0.4 | 3.6±0.5 | 3.6±0.5 | 3.6±0.3 | 0.1 (−0.4 to 0.3) | 0.792 |
| Total haemoglobin mass (g) | 897±128 | 904±135 | 897±76 | 897±76 | 7 (−24 to 39) | 0.631 |
Data are presented as n or mean±sd, unless otherwise stated. LV: left ventricle.
Discussion
The first key finding of the present study is that 6 weeks of treatment with the inhaled long-acting β2-agonist formoterol impairs aerobic exercise capacity, as reflected by reductions in V̇O2max, incremental exercise performance and power output eliciting V̇O2max in young, endurance-trained males and females. The second key finding is that these changes are accompanied by decreased muscle mitochondrial density and respiratory capacity, but not by altered cardiac structure, total haemoglobin mass and intravascular volumes.
The decline in aerobic exercise outcomes in the formoterol group was homogeneous, as 21 out of 26 participants had a reduction in V̇O2max, incremental exercise performance and power output eliciting V̇O2max. Adjusting for both lean mass and bodyweight did not affect the outcomes, highlighting the likely detrimental effects of prolonged formoterol treatment on real-life aerobic exercise performance in weight-bearing sports such as cycling or running where reductions in bodyweight are desired to minimise the cost of movement [33]. The detrimental effects are noteworthy as we employed a dose of only 24 µg formoterol twice daily, which lies within normal therapeutic range for persons with uncontrolled asthma (2022 GINA guidelines maximum recommended dose of as-needed formoterol with ICS: 54 µg per day in mild asthma [1]). The dose is also within the permitted range by the World Anti-Doping Agency (2022 prohibited list: 54 µg inhaled formoterol per day [4]). Thus, the present findings uncover a point of caution when employing prolonged treatment with formoterol in athletes competing in aerobic-dominated sports.
A few other studies have investigated effects of prolonged treatment with inhaled β2-agonists on aerobic-dominated exercise performances. However, these were performed in moderately trained populations with exclusively male participants and short-acting β2-agonists. We have found terbutaline, at high inhaled doses (4 mg·day−1; normal therapeutic range 0.5–2 mg·day−1) not only to reduce V̇O2max [11], but also to blunt the effect of a 4-week endurance training programme on V̇O2max and incremental exercise performance in moderately trained men [6]. In contrast, Dickinson et al. [10] reported no effect of inhaled salbutamol (unsupervised doses of 400 μg four times daily) on V̇O2max or 3-km time-trial performance during a 6-week combined endurance and strength training intervention in 16 moderately trained men. Discrepancies between the studies may relate to the combined strength/endurance training protocol employed in Dickinson et al., and by drug compliance. In particular, compliance decreases progressively with split doses to as little as 50% with four daily doses [34]. For this reason, we closely monitored compliance of every participant using mobile video tools and achieved almost 100% compliance for both drug administration and time of administration. With the present study, we not only extend the findings in moderately trained males to a larger sample size of endurance-trained males and females, but demonstrate that even inhaled therapeutic doses of β2-agonists can impair aerobic performance outcomes when used daily for a prolonged period.
While studies in rodents have indicated that the detrimental effects of β2-agonists lie in their ability to induce concentric cardiac hypertrophy [18, 19, 35, 36], we observed no apparent effect of formoterol on left ventricle mass, wall thicknesses, or function. Rather, our findings suggest that formoterol impairs the capacity of skeletal muscle to utilise oxygen. Using gold-standard high-resolution mitochondrial respirometry, we observed that formoterol attenuated respiration through complex I and complex I+II, which are considered markers of maximal mitochondrial respiratory capacity [37]. This impairment was largely related to a decline in mitochondrial content as intrinsic respiration (i.e. respiration normalised to citrate synthase activity) negated the decrease with formoterol. Indeed, decreases in citrate synthase activity and mitochondrial complex abundances suggested that mitochondrial density was compromised by formoterol. The latter observations coincide with previous findings in males treated with terbutaline while undertaking a period of endurance [6] or resistance training [11] and suggests that the detrimental effect is a class-specific effect of the drug and not necessarily an interaction with training. Because we observed no apparent changes in blood oxygen-carrying capacity and total haemoglobin mass, as well as no change in PECAM-1 expression (a marker of muscle capillarisation), our data collectively indicate that the impairment of muscle oxidative capacity is a key mechanism driving the impairment of aerobic exercise outcomes with formoterol.
Compared to prior studies on the effect of prolonged treatment with β2-agonists on aerobic exercise outcomes, the strengths of the present study were the larger sample size of endurance-trained individuals representing both sexes, the close monitoring of drug adherence, and the treatment regimen being applicable to treatment of asthma in athletes. In addition, our study utilised an array of physiological measures, providing coherence of findings. However, an important consideration is that we included healthy, rather than asthmatic participants, and that we did not co-administer an inhaled corticosteroid as per first-line treatment (e.g. budesonide). The reason for this was to investigate the isolated effect of formoterol in the absence of confounding factors such as tolerance development after potentially years of β2-agonist use or side-effects associated with prolonged corticosteroid use. In addition, participants could not use β2-agonists in the days prior to follow-up and this limited the inclusion of asthmatic participants. Nevertheless, as pharmacokinetics and pharmacodynamics of β2-agonists appear to be similar for asthmatic and nonasthmatic participants [38, 39], there is little reason to believe that our results are not applicable to asthmatic athletes.
In conclusion, the present study demonstrates that inhaled therapeutic doses of the long-acting β2-agonist formoterol can impose detrimental effects on aerobic exercise outcomes in endurance-trained individuals, irrespective of sex, and that these effects are associated with impaired muscle oxidative capacity and function. These findings should guide future antidoping policy-making to protect athlete health, and should be worth considering for physicians treating asthmatic athletes. While low-dose treatment with formoterol is essential in the treatment of asthmatic athletes, in cases where low-dose formoterol fails to control respiratory symptoms, physicians may consider other treatment options before stepping up to high-dose treatment (such as 54 µg·day−1: the maximum recommended dose by the 2022 GINA guidelines and the maximal permitted dose by current antidoping regulations), as this may compromise aerobic exercise outcomes. Instead, treatment options such as anticholinergics and leukotriene receptor antagonists may be considered.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods 00643-2022.supplementary_methods (94.8KB, pdf)
Supplementary results 00643-2022.supplementary_results (234.7KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
This study is registered at www.clinicaltrials.gov with identifier number NCT03565302. Individual deidentified participant data are available on reasonable request to the corresponding author.
Conflict of interest: All authors have nothing to disclose.
Support statement: This work was supported by grants from the World Anti-Doping Agency and Anti Doping Denmark. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Global Initiative for Asthma (GINA) . 2022 GINA Report, Global Strategy for Asthma Management and Prevention. https://ginasthma.org/gina-reports/
- 2.Boulet LP, O'Byrne PM. Asthma and exercise-induced bronchoconstriction in athletes. N Engl J Med 2015; 372: 641–648. doi: 10.1056/NEJMra1407552 [DOI] [PubMed] [Google Scholar]
- 3.Weiler JM, Bonini S, Coifman R, et al. American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol 2007; 119: 1349–1358. doi: 10.1016/j.jaci.2007.02.041 [DOI] [PubMed] [Google Scholar]
- 4.World Anti-Doping Agency . Prohibited List 2022. www.wada-ama.org/sites/default/files/2022-01/2022list_final_en_0.pdf Date last accessed: 3 May 2022.
- 5.Dickinson JW, Whyte GP, McConnell AK, et al. Impact of changes in the IOC-MC asthma criteria: a British perspective. Thorax 2005; 60: 629–632. doi: 10.1136/thx.2004.037499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hostrup M, Onslev J, Jacobson GA, et al. Chronic β2-adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J Physiol 2018; 596: 231–252. doi: 10.1113/JP274970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc 2000; 32: 70–84. doi: 10.1097/00005768-200001000-00012 [DOI] [PubMed] [Google Scholar]
- 8.Grant S, Craig I, Wilson J, et al. The relationship between 3 km running performance and selected physiological variables. J Sports Sci 1997; 15: 403–410. doi: 10.1080/026404197367191 [DOI] [PubMed] [Google Scholar]
- 9.Jeukendrup AE, Craig NP, Hawley JA. The bioenergetics of World Class Cycling. J Sci Med Sport 2000; 3: 414–433. doi: 10.1016/S1440-2440(00)80008-0 [DOI] [PubMed] [Google Scholar]
- 10.Dickinson J, Molphy J, Chester N, et al. The ergogenic effect of long-term use of high dose salbutamol. Clin J Sport Med 2014; 24: 474–481. doi: 10.1097/JSM.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 11.Lemminger AK, Jessen S, Habib S, et al. Effect of beta2-adrenergic agonist and resistance training on maximal oxygen uptake and muscle oxidative enzymes in men. Scand J Med Sci Sports 2019; 29: 1881–1891. doi: 10.1111/sms.13544 [DOI] [PubMed] [Google Scholar]
- 12.Carlsen KH, Hem E, Stensrud T, et al. Can asthma treatment in sports be doping? The effect of the rapid onset, long-acting inhaled β2-agonist formoterol upon endurance performance in healthy well-trained athletes. Respir Med 2001; 95: 571–576. doi: 10.1053/rmed.2001.1105 [DOI] [PubMed] [Google Scholar]
- 13.Tjørhom A, Riiser A, Carlsen KH. Effects of formoterol on endurance performance in athletes at an ambient temperature of −20°C. Scand J Med Sci Sports 2007; 17: 628–635. doi: 10.1111/j.1600-0838.2006.00628.x [DOI] [PubMed] [Google Scholar]
- 14.Riiser A, Tjørhom A, Carlsen KH. The effect of formoterol inhalation on endurance performance in hypobaric conditions. Med Sci Sports Exerc 2006; 38: 2132–2137. doi: 10.1249/01.mss.0000233801.46906.9b [DOI] [PubMed] [Google Scholar]
- 15.Dyreborg A, Krogh N, Backer V, et al. Pharmacokinetics of oral and inhaled terbutaline after exercise in trained men. Front Pharmacol 2016; 7: 150. doi: 10.3389/fphar.2016.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jessen S, Becker V, Rzeppa S, et al. Pharmacokinetics of salmeterol and its main metabolite α-hydroxysalmeterol after acute and chronic dry powder inhalation in exercising endurance-trained men: implications for doping control. Drug Test Anal 2021; 13: 747–761. doi: 10.1002/dta.2978 [DOI] [PubMed] [Google Scholar]
- 17.Haase CB, Backer V, Kalsen A, et al. The influence of exercise and dehydration on the urine concentrations of salbutamol after inhaled administration of 1600 μg salbutamol as a single dose in relation to doping analysis. Drug Test Anal 2016; 8: 613–620. doi: 10.1002/dta.1828 [DOI] [PubMed] [Google Scholar]
- 18.Duncan ND, Williams DA, Lynch GS. Deleterious effects of chronic clenbuterol treatment on endurance and sprint exercise performance in rats. Clin Sci 2000: 98: 339–347. doi: 10.1042/CS19990069 [DOI] [PubMed] [Google Scholar]
- 19.Gregorevic P, Ryall JG, Plant DR, et al. Chronic β-agonist administration affects cardiac function of adult but not old rats, independent of β-adrenoceptor density. Am J Physiol Heart Circ Physiol 2005; 289: H344–H349. doi: 10.1152/ajpheart.01254.2004 [DOI] [PubMed] [Google Scholar]
- 20.Ryall JG, Sillence MN, Lynch GS. Systemic administration of β2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br J Pharmacol 2006; 147: 587–595. doi: 10.1038/sj.bjp.0706669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koziczak-Holbro M, Rigel DF, Dumotier B, et al. Pharmacological characterization of a novel 5-hydroxybenzothiazolone-derived β2-adrenoceptor agonist with functional selectivity for anabolic effects on skeletal muscle resulting in a wider cardiovascular safety window in preclinical studies. J Pharmacol Exp Ther 2019; 369: 188–199. doi: 10.1124/jpet.118.255307 [DOI] [PubMed] [Google Scholar]
- 22.Jessen S, Onslev J, Lemminger A, et al. Hypertrophic effect of inhaled beta2-agonist with and without concurrent exercise training: a randomized controlled trial. Scand J Med Sci Sports 2018; 28: 2114–2122. doi: 10.1111/sms.13221 [DOI] [PubMed] [Google Scholar]
- 23.Lanza IR, Nair KS. Mitochondrial metabolic function assessed in vivo and in vitro. Curr Opin Clin Nutr Metab Care 2010; 13: 511–517. doi: 10.1097/MCO.0b013e32833cc93d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard M, Taivassalo T, Ritchie D, et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 2011; 6: e18317. doi: 10.1371/journal.pone.0018317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Noord JA, Smeets JJ, Maesen FP. A comparison of the onset of action of salbutamol and formoterol in reversing methacholine-induced bronchoconstriction. Respir Med 1998; 92: 1346–1351. doi: 10.1016/S0954-6111(98)90140-8 [DOI] [PubMed] [Google Scholar]
- 26.Louis R, Satia I, Ojanguren I, et al. European Respiratory Society guidelines for the diagnosis of asthma in adults. Eur Respir J 2022; 60: 2110585. doi: 10.1183/13993003.01585-2021 [DOI] [PubMed] [Google Scholar]
- 27.Scott NW, McPherson GC, Ramsay CR, et al. The method of minimization for allocation to clinical trials. A review. Control Clin Trials 2002; 23: 662–674. doi: 10.1016/S0197-2456(02)00242-8 [DOI] [PubMed] [Google Scholar]
- 28.Treasure T, MacRae KD. Minimisation: the platinum standard for trials? Randomisation doesn't guarantee similarity of groups; minimisation does. BMJ 1998; 317: 362–363. doi: 10.1136/bmj.317.7155.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecaillon JB, Kaiser G, Palmisano M, et al. Pharmacokinetics and tolerability of formoterol in healthy volunteers after a single high dose of Foradil dry powder inhalation via Aerolizer. Eur J Clin Pharmacol 1999; 55: 131–138. doi: 10.1007/s002280050607 [DOI] [PubMed] [Google Scholar]
- 30.Ringdal N, Derom E, Wåhlin-Boll E, et al. Onset and duration of action of single doses of formoterol inhaled via Turbuhaler. Respir Med 1998; 92: 1017–1021. doi: 10.1016/S0954-6111(98)90348-1 [DOI] [PubMed] [Google Scholar]
- 31.Bartow RA, Brogden RN. Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma. Drugs 1998; 55: 303–322. doi: 10.2165/00003495-199855020-00016 [DOI] [PubMed] [Google Scholar]
- 32.Poole DC, Jones AM. Measurement of the maximum oxygen uptake V̇O2max: V̇O2peak is no longer acceptable. J Appl Physiol 2017; 122: 997–1002. doi: 10.1152/japplphysiol.01063.2016 [DOI] [PubMed] [Google Scholar]
- 33.Hoon MW, Haakonssen EC, Menaspà P, et al. Racing weight and resistance training: perceptions and practices in trained male cyclists. Phys Sportsmed 2019; 47: 421–426. doi: 10.1080/00913847.2019.1607602 [DOI] [PubMed] [Google Scholar]
- 34.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001; 23: 1296–1310. doi: 10.1016/S0149-2918(01)80109-0 [DOI] [PubMed] [Google Scholar]
- 35.Ingalls CP, Barnes WS, Smith SB. Interaction between clenbuterol and run training: effects on exercise performance and MLC isoform content. J Appl Physiol 1996; 80: 795–801. doi: 10.1152/jappl.1996.80.3.795 [DOI] [PubMed] [Google Scholar]
- 36.Sillence MN, Matthews ML, Spiers WG, et al. Effects of clenbuterol, ICI118551 and sotalol on the growth of cardiac and skeletal muscle and on β2-adrenoceptor density in female rats. Naunyn Schmiedebergs Arch Pharmacol 1991; 344: 449–453. doi: 10.1007/BF00172585 [DOI] [PubMed] [Google Scholar]
- 37.Vincent G, Lamon S, Gant N, et al. Changes in mitochondrial function and mitochondria associated protein expression in response to 2-weeks of high intensity interval training. Front Physiol 2015; 6: 51. doi: 10.3389/fphys.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch S, MacInnis MJ, Sporer BC, et al. Inhaled salbutamol does not affect athletic performance in asthmatic and non-asthmatic cyclists. Br J Sports Med 2015: 49: 51–55. doi: 10.1136/bjsports-2013-092706 [DOI] [PubMed] [Google Scholar]
- 39.Krogh N, Backer V, Rzeppa S, et al. Pharmacokinetics of nebulized and oral procaterol in asthmatic and non-asthmatic subjects in relation to doping analysis. Drug Test Anal 2016: 8: 1056–1064. doi: 10.1002/dta.1935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary methods 00643-2022.supplementary_methods (94.8KB, pdf)
Supplementary results 00643-2022.supplementary_results (234.7KB, pdf)