We and others have previously reported suboptimal antibody responses in lung transplant recipients (LTRs) after 2 doses of SARS-CoV-2 mRNA vaccine.1,2 Immunosuppressive regimens that include antimetabolite therapy (AMT, mycophenolate or azathioprine)3 and higher doses of AMT4 have been associated with poorer antibody responses. Safety and efficacy of holding AMT around a third dose of vaccine (D3) is being studied in a randomized trial in kidney and liver transplant recipients (NCT05077254), but the effects in LTRs are unknown. At our center, LTRs who were >1 y posttransplant with no acute cellular or antibody-mediated rejection within 12 mo, were offered the option to hold AMT for 1 wk before and 2 wks after D3 as part of clinical care and not a clinical trial. We performed a retrospective study of LTR who received D3 through October 2021 and who did or did not hold AMT. Demographics, clinical data, donor-specific antibody (HLA-DSA) levels, pulmonary function tests, lung biopsies, and SARS-CoV-2 antibody levels (on EUROIMMUN EIA, positive ≥1.1 arbitrary units, or qualitative DiaSorin assay), obtained as part of clinical care, were extracted from the electronic medical record. This study was approved by the Johns Hopkins Institutional Review Board.
Of 35 LTRs, AMT was held by 29 (82.9%) and continued by 6 (17.1%) peri-D3. Of these, 23 (65.7%) received mRNA-1273 vaccine, 11 (31.4%) received BNT162b2 vaccine, and 1 received Ad26.COV2.S vaccine for D3. All but 3 received D3 after the FDA’s Emergency Use Authorization on August 12, 2021, whereas 3 received D3 of their own volition before that (neither given nor encouraged by our center). Median (interquartile range [IQR]) time between D2 and D3 was 164 (146–188) d (Table 1).
TABLE 1.
Clinical characteristics of lung transplant recipients who held and did not hold perivaccination antimetabolite therapy
| Overall (n = 35) | Non-AMT hold (n = 6) | AMT hold (n = 29) | |
|---|---|---|---|
| Age, median (IQR) | 56 (40–65) | 66 (45–71) | 49 (40–65) |
| Female sex, no. (%) | 20 (57) | 4 (67) | 16 (55) |
| Non-White, no. (%) | 5 (14) | 1 (17) | 4 (14) |
| Hispanic/Latino, no. (%) | 1 (3) | 1 (17) | 0 (0) |
| Diagnosis, no. (%) | |||
| CF/bronchiectasis | 12 (34) | 1 (17) | 11 (38) |
| ILD/IPF | 10 (29) | 2 (33) | 8 (28) |
| COPD/A1ATD | 4 (11) | 2 (33) | 2 (7) |
| PH/sarcoidosis/HP/BOd | 6 (17) | 1 (17) | 5 (17) |
| Other | 3 (9) | 0 (0) | 3 (10) |
| Medication included in immunosuppression regimen, no. (%) | |||
| Azathioprine | 6 (17) | 2 (33) | 4 (14) |
| Cyclosporine | 4 (11) | 1 (17) | 3 (10) |
| Everolimus | 2 (6) | 2 (33) | 0 (0) |
| Glucocorticoida | 35 (100) | 6 (100) | 29 (100) |
| Mycophenolate mofetil | 29 (83) | 4 (67) | 25 (86) |
| Tacrolimus | 30 (86) | 5 (83) | 26 (90) |
| Triple Immunosuppression, no. (%) | 33 (94) | 6 (100) | 27 (93) |
| Mycophenolate total daily dose (mg/d), median (IQR) | 1000 (360–1500) | 720 (0–1000) | 1000 (720–1500) |
| Glucocorticoid total daily dose >10 mg/d, no. (%) | 0 (0) | 0 (0) | 0 (0) |
| Years since transplant, median (IQR) | 4.4 (2.9–8.8) | 3.3 (2.5–8.3) | 4.9 (3.2, 8.8) |
| Days between D2 and D3, median (IQR) | 164 (146–188) | 148 (99–177) | 166 (150–188) |
| D3 vaccine type, no. (%) | |||
| BNT162b2 | 11 (31) | 2 (33) | 9 (31) |
| mRNA-1273 | 23 (66) | 3 (50) | 20 (69) |
| Ad26.COV2.S | 1 (3) | 1 (17) | 0 (0) |
| Preexisting positive increase DSA post-D3, no. (%)b,c | 1 (7) | 0 (0) | 1 (8) |
Glucocorticoid includes prednisone and prednisone equivalents.
The denominator differs from the total N as only 15 participants had pre- and post-D3 DSA.
Preexisting positive increase DSA post-D3 denotes that the patient had a positive HLA prior pre-D3 that was increased when measured post-D3.
A1ATD, alpha-1 antitrypsin deficiency; AMT, antimetabolite therapy; BO, bronchiolitis obliterans; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; DSA, donor-specific antibody; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; PH, pulmonary hypertension.
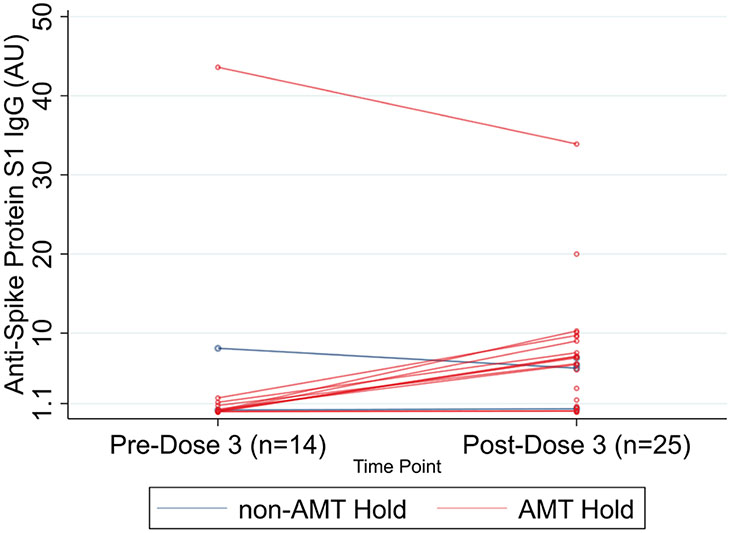
For the AMT-hold group, the median (IQR) anti-S1 post-D3 (6.09 AU [0.58–9.29; n = 24]) was significantly higher than the median (IQR) anti-S1 pre-D3 (0.14 AU [0.08–0.87] [n = 15]) (P = 0.003; 1-sided Wilcoxon matched-pairs signed-rank test) (Figure 1). HLA-DSA testing before and after D3 was available for 15 LTRs. HLA-DSA increased after D3 in only 1 of 12 (8.3%) who held AMT versus 0 of 3 who did not hold AMT (P > 0.9). Five had lung biopsies post-D3; only 1 (non-AMT–hold group) showed acute rejection.
FIGURE 1.
Pre- and post-D3 antibody titers among LTRs stratified by AMT hold. AMT, antimetabolite therapy; LTR, lung transplant recipient.
Pulmonary function tests results within 90 d pre-D3 and 90 ± 45 d post-D3 were available for 24 LTRs (19 who held AMT). Median (IQR) change in FEV1 was −0.01 L (−0.08 to 0.08) for the AMT–hold group and 0.13 L (0.05–0.15) in the non-AMT–hold group.
Limitations include small sample size, especially the non-AMT–hold group, retrospective design, lack of neutralization assays and T-cell and memory B-cell data; however, these preliminary results, including stable DSA and FEV1, lack of acute rejection, and increase in post-D3 SARS-CoV-2 antibody levels in the AMT-hold group, suggest that a brief peri-D3 hold of AMT may be promising for future study. Moreover, increased SARS-CoV-2 antibody levels after D3 may portend a greater increase after D4.5 We hope this report will support the design of future clinical trials for rigorous evaluation of the AMT-hold strategy to improve antibody responses to SARS-CoV-2 vaccines in LTRs.
Acknowledgments
This work was made possible by the generous support of the Ben Dov family. This work was supported by grant number T32DK007713 (Alejo) from the National Institute of Diabetes and Digestive and Kidney Diseases, K24AI144954 (Segev), U01AI138897 and K23AI157893 (Werbel) from National Institute of Allergy and Infectious Diseases, and F32AG067642-01A1 (Ruck) from National Institute on Aging. The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
D.L.S. has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallincrodt, Thermo Fisher Scientific, AstraZeneca, Regeneron. R.K.A. has the following financial disclosures: grant and research support from Aicuris, Astellas, Chimerix, Merck, Oxford Immunotec, Qiagen, Regeneron, Takeda/Shire. The other authors declare no conflicts of interest.
REFERENCES
- 1.Hallett AM, Greenberg RS, Boyarsky BJ, et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J Heart Lung Transplant. 2021;40:1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman TW, Meek B, Rijkers GT, et al. Poor serologic response to 2 doses of an mRNA-based SARS-CoV-2 vaccine in lung transplant recipients. Transplantation. 2022;106:e103–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell J, Chiang TP, Alejo JL, et al. Effect of mycophenolate mofetil dosing on antibody response to SARS-CoV-2 vaccination in heart and lung transplant recipients. Transplantation. 2022;106:e269–e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell J, Alejo J, Chiang TP-Y et al. Antibody response to a fourth dose of SARS-CoV-2 vaccine in organ transplant recipients: an update. Transplantation. 2021;105:e280–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]