Abstract
Immuno-oncology has traditionally focused on the cellular arm of the adaptive immune response, while attributing tumor-promoting activity to humoral responses in tumor-bearing hosts. This view stems from mouse models that do not necessarily recapitulate the antibody response process consistently observed in most human cancers. In recent years, the field has reconsidered the coordinated action of T and B cell responses in the context of anti-tumor immunity, as in any other immune response. Thus, recent studies in human cancer identify B cell responses with better outcome, typically in association with superior T cell responses. An area of particular interest is tertiary lymphoid structures, where germinal centers produce isotype switched antibodies and B cells and T lymphocytes interact with other immune cell types. The presence of these lymphoid structures is associated with better immunotherapeutic responses and remain poorly understood. Here, we discuss recent discoveries on how coordination between humoral and cellular responses is required for effective immune pressure against malignant progression, providing a perspective on the role of tertiary lymphoid structures and interventions to elicit their formation in unresectable tumors.
Keywords: B cell, Plasma cell, Cancer antibodies, Tertiary lymphoid structure, Tumor immunology
1. Introduction
Immuno-oncology has traditionally focused on understanding and targeting the role of αβ T cells in anti-tumor immunity, while the contribution of humoral responses, the other arm of the adaptive immune system, has been associated with accelerated tumor progression. This T cell-centric vision stems, on the one hand, from the pre-conception that antibodies cannot target intracellular antigens, which are considered inaccessible due to the large size of immunoglobulins. On the other hand, previous publications in mouse models attributed a cancer-promoting, immunosuppressive role to B cells at tumor beds. However, a flurry of recent studies in human tumors is rapidly changing previous views. Thus, independent studies have recently associated B and plasma cell infiltration with better clinical outcome in many human cancers, as opposed to the dominant regulatory activity associated with mouse tumors. This includes patients with ovarian cancer [1], endometrial cancer [2], cutaneous melanoma [3], colorectal cancer [4,5], breast carcinoma [6], hepatocarcinoma [7] and sarcoma [8]. In addition, correlations between mRNA expression of markers of the B cell lineage and increased survival were reported for non-small cell lung [9] and gastric [10] cancers. Furthermore, a T follicular helper (Tfh) cell signature, indicative of germinal center activity, has been also associated with better outcome in head and neck human cancer [11]. Finally, beyond scattered B cell infiltration at tumor beds, the presence of B cell-rich structures that recapitulate the architecture of lymph nodes, termed tertiary lymphoid structures (TLS), has been also associated with better outcomes in more than 10 different types of human cancer [12–19].
On the other hand, we have recently demonstrated that a fraction of antibodies spontaneously produced at human tumor beds recognize secreted molecules or molecules with a transmembrane domain, with measurable anti-tumor activity, as these targets are either neutralized or targeted for antibody-mediated cellular phagocytosis [1]. Spontaneous coating of the tumor cell surface by antibodies was subsequently confirmed by independent studies, also in ovarian cancer [20]. In addition, antigens carried in tumor-promoting exosomes [21] could be also effectively targeted by antibodies, even if they are expressed in the cytoplasm or the nucleus of tumor cells.
In this review, we will discuss emerging discoveries on the contribution of humoral immune responses to the abrogation of malignant progression in human cancer, emphasizing possible differences between the immunobiology of human and mouse tumors and commenting on recent discoveries about the role of tertiary lymphoid structures (TLS) in anti-tumor immunity. Changing perspectives in immuno-oncology are forging a new framework to treat and prevent human cancer [22].
2. Differences in humoral responses between human cancer and quickly progressing mouse models
With the obvious exception of lymphoma, the numerous independent associations between B cell infiltration and accelerated tumor growth [23–27] have been restricted to mouse tumor models. Tumor-promoting activity has been primarily attributed to populations of B cells with immunosuppressive activity, known as regulatory B cells or Breg cells. In the absence of a tumor, Breg cells represent less than 10% of circulating B cells [28,29]. Along with Treg cells, they contribute to sustain peripheral tolerance. The T cell inhibitory activity of Breg cells has been associated with the secretion of different cytokines, including IL-10 [30–32], TGF-β and IL-35 [31,33–40]. Both IL-10- and IL-35-producing Breg cells exist in humans, but they are primarily found in autoimmune disease, and they have been proposed as tools in this setting [41]. Based in part on the pre-conception that overall B cell activity promotes, rather than oppose, malignant progression, clinical trials depleting B cells using the anti-CD20 mAb Rituximab were conducted in patients with renal cell carcinoma and melanoma on IL-2 therapy, without beneficial effects [42].
In contrast, recent studies, including our reports, demonstrate that accumulation of B cells and antibody-producing cells in human cancer is consistently associated with better outcome and superior immunotherapeutic responses [1–8,12–19,43]. This begs the question of how faithfully mouse tumor models recapitulate anti-tumor humoral responses in patients with cancer. A major difference between human cancer and mouse models is that the latter are engineered to progress much faster and generate an inflammatory response that likely favors Breg cell development [44,45]. As a result, the Ig isotypes dominant in transplantable tumor models, and in the majority of transgenic tumor-prone mice - which, in general, are already poorly immunogenic - correspond to IgM variants. In contrast, combinations of isotype-switched IgA and IgG dominate non-isotype-switched IgM antibodies in human malignancies such as ovarian [1], endometrial [46], breast [47,48] and colon cancer [49], as well as melanoma [50]. This is important because different isotypes bind to different Fc receptors, which are in turn expressed in different cell types. In addition, IgM is very effective at activating the complement cascade, which could also have tumor-promoting activity [51].
There are, nevertheless, tumor mouse models that reflect the pathophysiology of human responses in human cancer. For instance, using immunogenic mouse models of triple-negative breast cancer, Hollern and colleagues demonstrated the crucial role of B cell activation and antibody production for the effectiveness of immune checkpoint blockers [52]. Furthermore, we recently demonstrated that the progression of intraperitoneal ovarian carcinosarcomas is also partially dependent on the anti-tumor activity of B cells, because B cell depletion accelerates tumor growth [53]. Supporting these observations, enhanced Tfh differentiation upon genetic engineering of T cells resulted in the assembly of tertiary lymphoid structures in virtually all tumors, which was associated with significant delays in malignant progression [53]. Together, these recent studies suggest the major discrepancy between results derived from mouse models and those from cancer patients, as humoral responses could progress differently from most human tumors.
2.1. How do isotype-switched antibodies exert immune pressure in human cancer?
Further supporting the crucial role of humoral immunity in human cancer, recent analyses of hundreds of human tumors in The Cancer Genome Atlas (TCGA) datasets confirmed extensive B cell clonal expansions, isotype switching and hypersomatic mutation across 32 human cancer types [54]. However, the targets recognized by tumor-derived antibodies remained elusive until recently. Using B cells immortalized from 9 different human ovarian carcinomas and arrays that contain > 80% of the human proteome, we demonstrated that IgA and IgG spontaneously produced at tumor beds recognizes hundreds of self-antigens, which include > 10% of secreted molecules, or molecules with a transmembrane domain, which are therefore accessible to these antibodies in the extracellular space [1]. These targets included members of the tetraspanin family or secreted targets such as BDNF, which are expressed in other healthy tissues. We also identified antibodies reacting against olfactory receptors such as OR5V1, which are overexpressed in carcinoma cells, which prompted us to develop novel CAR T cells targeting other members of this family of transmembrane molecules [55]. Interestingly, we could not find antibodies reacting with these molecules in peripheral blood, suggesting that tumor-derived antibodies primarily target local tumor antigens [1]. It is also possible that some of these autoantibodies could recognize glycosylation patterns, rather than unmodified amino acids, thus providing tumor specificity and enlarging the antigenic repertoire. In any case, IgA redirects otherwise immunosuppressive myeloid cells against tumor cells, resulting in antibody-dependent cellular phagocytosis. Presumably, these effects will be stronger in human tumors, because mice, unlike rats and hamsters, lack CD89, which redirects neutrophils against cognate antigens. Although we did not formally test this hypothesis, tumor-derived IgG targeting the same molecules could have similar effects through NK cell activity. Subsequently, Mazor and colleagues also identified targeting autoantigens on the human ovarian cancer cell surface [20], although the study primarily focused on IgGs. Supporting the original studies of the Nelson group [56], as well as our subsequent studies [1], intra-tumoral antibody secreting cells were found in most ovarian cancers analyzed. These cells were clonally expanded and some of them secreted antibodies that targeted MMP14 on the tumor cell surface. Interestingly, the study identified a class of tumor-binding antibodies that undergoes somatic hypermutations; and a second class of autoreactive germline-encoded antibodies [20]. It is important to note that we have only screened our collections of tumor-derived antibodies against self-antigens, which likely break tolerance through overexpression. However, there could be an array of immune relevant neoepitopes generated by specific mutations, which remain uninvestigated and could represent new targets for therapy. Importantly, we found that the predictive value of intra-epithelial T cells in ovarian cancer [57–59] is only relevant if T cells are associated with B cell infiltration [1]. Together, these findings suggest that immunotherapies that boost humoral immunity could be more effective than interventions exclusively focused on T cells, particularly for malignancies that are resistant to checkpoint inhibitors.
A second mechanism of anti-tumor activity mediated by at least IgA produced at tumor beds is mediated through the binding of dimeric IgA (including a J chain) to polymeric IgA (PIGR) receptors that we found to be quasi-universally expressed on the surface of ovarian cancer cells [1]. Similar to enterocytes, binding of dimeric IgA to PIGR triggers bona fide transcytosis through malignant epithelial cells, which elicits transcriptional changes that antagonize the RAS pathway and sensitize tumor cells to cytolytic killing by T cells [1] (Fig. 1). This previously unrecognized mechanism of anti-tumor activity is not restricted to ovarian cancer, because we subsequently found universal PIGR expression in a cohort of 107 patients with different histological subtypes of endometrial carcinoma [2,60]. We found that PIGR occupancy by IgA elicits the activation of IFN and TNF pathways in tumor cells, in association with apoptotic and endoplasmic reticulum stress (i.e., CHOP-dependent) pathways [2]. In addition, accumulation of B cells and plasma cells, which primarily produce IgA, followed by IgG, predicted better survival in all histological subtypes of endometrial cancer, without any significant predictive value for tumor-infiltrating T cells in high-grade endometrioid type and serous tumors [2]. Together, independently of antigen recognition, PIGR-dependent IgA transcytosis through tumor cells clearly antagonizes malignant progression. Although future studies should clarify whether the role of IgA transcytosis extends beyond gynecologic malignancies, analysis of PIGR expression in TCGA datasets reveals a dichotomy between PIGR+ epithelial tumors and PIGR−/low malignancies such as melanoma, sarcoma, glioblastoma or leukemia. It is therefore likely that most human carcinomas have the capacity to transcytose IgA. It remains to be clarified why tumors cells do not evolve to lose PIGR expression. There are several not mutually exclusive possibilities: First, recent in vitro studies in hepatocellular cancer cells suggest that PIGR expression promotes hepatic cell transformation and proliferation through interactions with the YES Src family kinase [61]. Furthermore, other studies using in vitro organotypic models have associated PIGR expression with cancer cell invasion and increased stromal activity [62]. Finally, the immunosuppressive IL10 gene is in the same genomic locus as PIGR, which could result in a cost for deletions of that genomic region. Whatever the reasons, most carcinomas express PIGR, and at least ovarian and endometrial cancer cells have the capacity to induce IgA transcytosis.
Fig. 1.
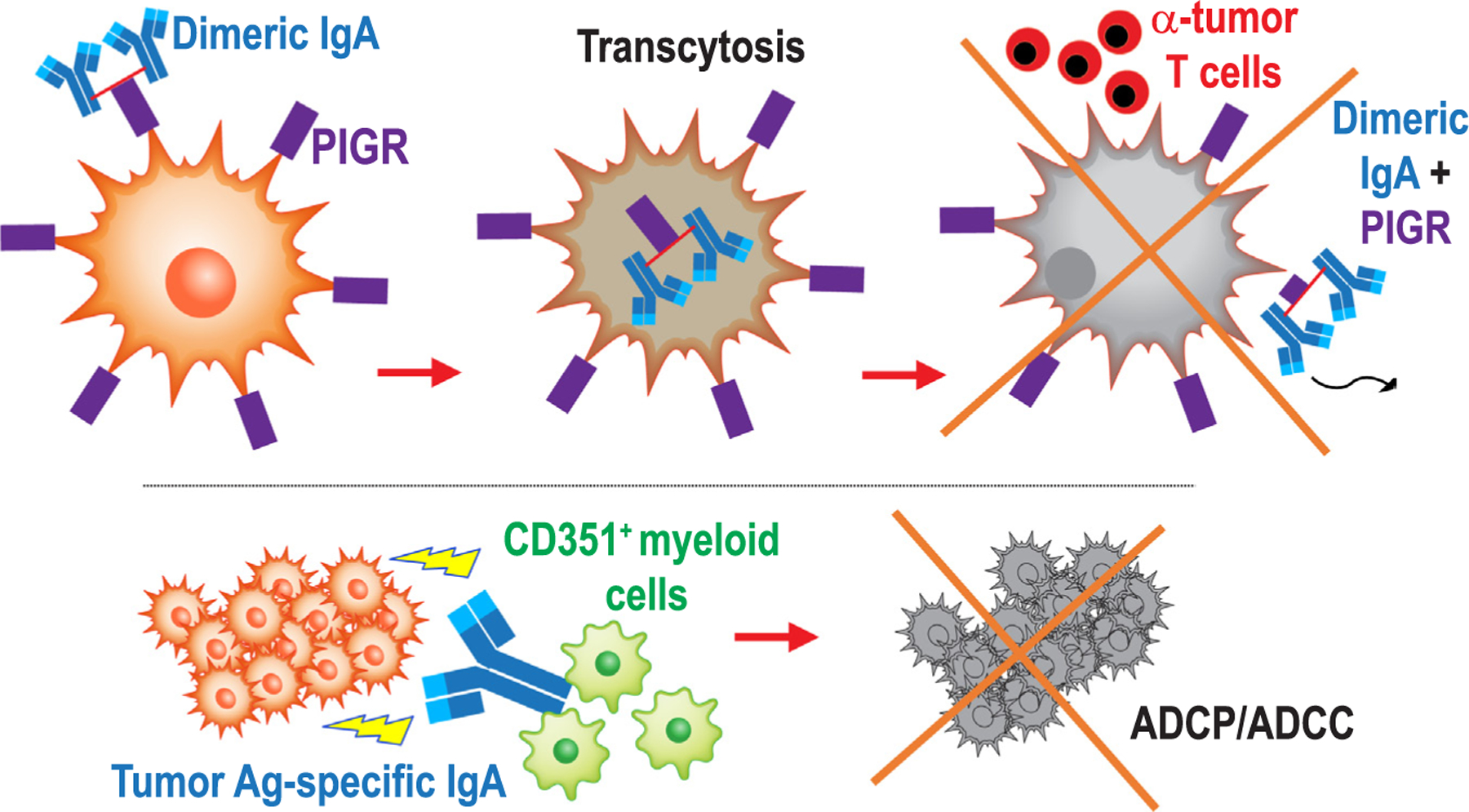
Antigen-dependent and independent mechanisms of anti-tumor activity elicited by IgA at tumor beds. IgA dominates the antibody response in ovarian and endometrial cancer, driving anti-tumor immunity through a dual mechanism: On one hand (top), dimeric IgA transcytoses through tumor cells, which quasi-universally express the IgA/IgM receptor PIGR, which sensitizes tumor cells to T cell-mediated killing. On the other hand, IgA targeting multiple tumor cell transmembrane molecules re-directs myeloid cells against malignant cells, resulting in ADCP-mediated killing.
2.2. TLS predict both immunotherapeutic efficacy and increased survival in multiple human tumors
Lymphoid aggregates consisting of adjacent conglomerates of T cells and B cells, with variable degrees of structural complexity, are assembled inside many human solid cancers. The most mature structures recapitulate the architecture of secondary lymph nodes and are generically termed tertiary lymphoid structures (TLS). Mature TLS therefore include a T cell zone composed by CD4 and CD8 T cells, adjacent to germinal centers with B cells, long-lived plasma cells and interdigitating Tfh cells and follicular dendritic cells, PNAd+ high endothelial venules and some neutrophils and macrophages [13,63] (Fig. 2). Because many less organized lymphoid aggregates of variable size are identified in the tumor microenvironment, categorizing these structures is going to require consensus and guidelines from the field, which currently does not exist. Similar TLS are also found in conditions of inflammation and active immune responses such as autoimmunity [13].
Fig. 2.
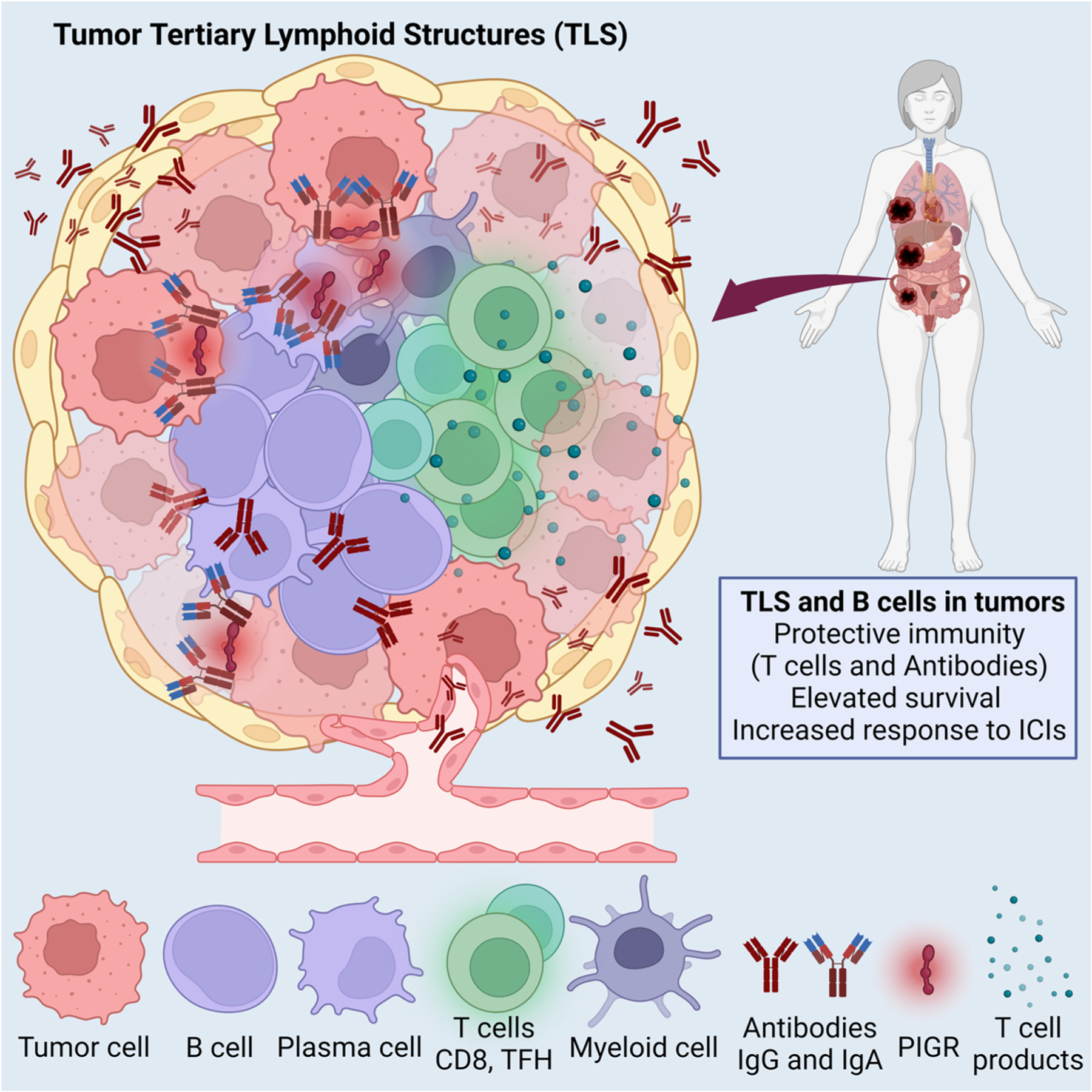
Schematic depiction of TLS elements and proposed mechanisms of enhanced immune protection associated with the presence of TLS.
In virtually all studies of human tumors investigated, which include more than 10 histological types of cancer, the presence of TLS is associated with superior survival and better immunotherapeutic responses [12–19,64], further supporting that superior immune pressure requires cooperation between both arms of the adaptive immune system. For instance, the presence of TLS, which is found in ~23% of ovarian cancers, is associated with denser CD8 T cell infiltration [56]. Similarly, TLS are associated with immune cell infiltration and activation in breast cancer [65–67]. Other studies have identified a positive association between the presence of TLS and outcome in pancreatic [19], colorectal [15], bladder [68], renal [69,70] and non-small cell lung cancer [71]. The presence of TLS at baseline, and B cell infiltration in general, is also predictive of superior responses to different forms of immunotherapy. For instance, in melanoma renal cancer and sarcoma, TLS predict the response to immune checkpoint blockade [8,69,70,72]. Denser B cell infiltration and intra-tumoral TLS also predict durable clinical responses in patients with recurrent ovarian cancer receiving a hypomethylating agent plus immune checkpoint blockade [73]. Furthermore, a B and plasma cell signature, correlating with the presence of TLS, was also identified in patients with lung cancer treated with anti-PD-L1 blockade [74]. In another recent independent study, the presence of mature TLS was associated with increased overall survival, independently of PD-L1 expression and T cell accumulation [75]. B cells, plasma cells and TLS formation therefore emerge as biomarkers to identify superior responders to anti-cancer immunotherapies.
TLS trend to be located in the periphery of the tumor or the stroma [63]. Likely due to the quick progression of mouse tumor models, TLS are rarely found in tumor-bearing mice. However, studies from the Engelhard group and our own observations indicate that intraperitoneal tumor models are more prone than flank tumors to orchestrate TLS [53, 76]. This may have to do with a peritoneal lymphatic network that is more prone to obstruction and favors that T and B cells get stacked at tumor beds, in addition to the vicinity of the spleen, which is a major reservoir of both cell types. In TLS+ tumors, the Fridman group has recently reported 2 different patterns of immune cell infiltration [77]: On the one hand, tumors with an immune structured microenvironment include the production of both (isotype-switched) IgG and IgA by plasma cells in TLS. Predominant production of both isotypes in TLS is supported by other independent studies. For instance, in melanoma metastases [78], while tumor antigen-reactive IgA is associated with TLS formation in breast cancer [79]. On the other hand, in tumors with an immune excluded microenvironment, immune cells and TLS are located outside the tumor beds, although TLS-derived antibodies penetrate the tumor microenvironment and also target tumor cells. Besides antibodies produced in TLS, we found evidence of plasma cells and plasmablasts scattered through the parenchyma of many tumors [1].
While it is becoming increasingly clear that TLS formation and antibody production is associated with the magnitude of anti-tumor immunity and predicts better immunotherapeutic responses, understanding the mechanisms behind these predictors is in its infancy. Clarifying the immunobiology of TLS would open new avenues to induce TLS formation in, for instance, metastatic tumors, which could render them more sensitive to existing and future immunotherapies. Some possible mechanisms of immune protection are discussed below.
2.3. How do intra-tumoral TLS promote spontaneous or immunotherapeutically-driven anti-tumor immunity?
As aforementioned, why the presence of TLS is associated with immune protection, or whether their presence is cause or consequence of a more immunogenic milieu, remains unknown. The most obvious mechanism of anti-tumor activity is the production of isotype-switched antibodies [77–79] that, as commented above, could target the tumor cell surface. Because there are plasma cells and plasmablasts outside TLS in the tumor microenvironment, the anti-tumor activity of specifically TLS-derived antibodies needs to be formally demonstrated. In addition, many other questions regarding antibodies produced in TLS remain open. For instance, what is the clonality of antibodies produced in these structures? Similarly, nature of the antigens recognized by TLS-derived antibodies (i.e., proteins of glycosylation patterns), and whether they are only locally expressed in the adjacent milieu or can target other tumor masses needs to be clarified. Isolation of viable cells from TLS is not technically feasible at the moment, but genomic analyses of laser-capture micro-dissected TLS, or genome-wide transcriptional analyses of these structures, could provide some insight into these questions.
Another mechanism of potential immune protection that has been suggested is to shield tumor-reactive T cells from immunosuppressive signals (i.e., PD-L1 or CD277 [80], or those derived from immunosuppressive myeloid cells) in the adjacent tumor microenvironment, under the filter of high endothelial venules [81]. In addition, basal activating signals generated through the antigen-presenting activity of adjacent B cells, or their expression of co-stimulatory molecules, could maintain these lymphocytes active and prevent paralysis induced by cellular stress or other intrinsic pathways. Of note, both this possible mechanism and obviously antibody production imply a dominant anti-tumor activity for B cell responses in human cancer, as opposed to the tumor-promoting effects that the field has attributed B cells for years.
A possible third mechanism of immune protection could be related to bystander effects in adjacent tumor tissue, resulting in an inflammatory microenvironment that is less permissive for tumor cell growth than other tumor areas. This could be investigated with novel spatial molecular profiling technologies that provide transcriptomic analyses of regions of interest, identified through the staining of multiple markers.
Alternatively, TLS formation could just be the reflection of a more permissive, immunogenic milieu, which allows the recruitment of the right immune cell types and their assembly into lymph node-like structures. The mechanisms behind the crosstalk between different immune cells and their products at TLS need to be urgently clarified, to pave the way for new interventions that leverage these interdependent responses.
2.4. Can TLS assembly be induced as a form of immunotherapy?
If, as suggested by independent strong associations between TLS and superior anti-tumor immunity, TLS provide a hub where immune cells maintain superior anti-tumor activity, understanding the best way to recapitulate the assembly of TLS in unresectable tumors (i.e., metastatic disease or tumors adjacent to the aorta or other vital organs) could lead to novel therapeutic interventions, alone or to boost existing immunotherapies.
While there is consensus about the formation of TLS in a milieu of sustained inflammation [82], different authors have followed different approaches to drive TLS assembly. Seminal studies from the Storkus group, for instance, placed the emphasis on tumor vascular normalization promoted by STING agonists, which leads to TLS formation [81]. Oncolytic virotherapy could have similar effects in promoting a cytokine and chemokine milieu conducive of the recruitment of all players that interact in mature TLS, with the advantage of enhanced antigen spreading [83].
Other studies have demonstrated that the member of the TNF-α family LIGHT, when effectively delivered to tumor vessels using a vascular targeting peptide, also promotes TLS orchestration. TLS assembly in this system occurs after an influx of T cells, and can be boosted by checkpoint inhibition, resulting in increased survival in preclinical models [84].
Not mutually exclusive, our recent work identified that the seminal event leading to the orchestration of all elements that lead to TLS assembly (or at least one of the ways of forming TLS) is the differentiation of activated CD4+ T cells into Tfh cells [53]. This occurs through intrinsic decreased expression of SATB1, a genomic organizer that represses ICOS and is required for effective T Follicular Regulatory cell differentiation, a cell type that antagonizes the activity of Tfh cells [53]. Tfh cells generate a chemokine milieu that promotes spontaneous assembly of TLS in ovarian cancer models, including the secretion of LIGHT and production of IL-21, which activates B cells recruited in response to CXCL13, which is also produced by Tfh cells and was found to be crucial for this process. Accordingly, intra-tumoral administration of autologous Tfh cells was sufficient to elicit TLS formation, while the administration of naïve CD4 T cells did not induce these effects. Tfh cells could be therefore theoretically administered into metastatic cancers or tumors adhered to unresectable locations to drive TLS assembly, generating a permissive environment for T cells rescued through, for instance PD-1 blockade, overall delaying malignant progression.
The crucial role of Tfh cells in the genesis of TLS is further supported by independent studies in preclinical models of colorectal cancer, which also unveiled a role for the microbiota in this process [85]. Through the introduction of Helicobacter hepaticus, Overacre-Delgoffe and colleagues elicited anti-tumor immune responses that were dependent on CD4 T cells and B cells, in association with bacteria-specific Tfh cell differentiation, which promoted TLS assembly [85].
The shared conclusion of these studies is that TLS formation requires the orchestration of a highly inflammatory milieu with the right cytokines and chemokines. There could be therefore different ways of orchestrating these lymphoid structures, but the role of CXCL13 and Tfh cells appears to be particularly important, at least for germinal center formation and maintenance.
2.5. Pro- vs. anti-tumor roles of memory B cells in different malignancies
The role of B cell populations at different stages of differentiation within tumor beds of malignancies of different histological origins is another area that remains incompletely understood. This includes a better characterization of the distinct role of naïve vs. memory B cells in different tumors. Thus, memory B cells are a dynamic population of mature B cells that can reenter germinal centers (and therefore TLS) in response to persistent tumor antigens. Memory B cells can quickly give rise to antibody-secreting cells, which are typically found in multiple tumor areas. For instance, in ovarian cancer, at least 80% of tumors contain > 1% of CD19+CD138−CD38highCD27+ plasmablasts [1]. Memory B cells are also long-lived and can persist for decades. B cell memory responses could be therefore more sustained and effective than responses driven by T cells, which eventually become exhausted, including for the prevention of recurrences after initial clinical responses. In addition, memory B cells could present antigen to local T cells, which may also enable tumor regression. This effect could be particularly important in TLS, where T cells could receive activating signals that maintain a basal tone of effector activity, while high endothelial venules could protect them from immunosuppressive networks outside the TLS.
The role of memory B cells, however, could be opposite in the context of lymphomas. For instance, using an elegant model a mouse model that recapitulates the hallmark BCL2-activating translocation of follicular lymphoma, Sungalee et al. demonstrated that repeated entries into germinal centers were associated with the acquisition of malignant features, and were required for malignant progression of this disease [86]. In addition, recent studies suggest that aberrant memory B cells, rather than plasmablasts, are the cells-of-origin of diffuse B cell lymphoma [87].
3. Concluding remarks
Immuno-oncology has remained narrowly focused on αβ T cell responses for decades. In fact, the role of humoral responses in cancer has been associated with accelerated malignant progression, primarily due to mouse models that do not reflect the isotypes, slower progression, different inflammatory mechanisms and mutational burdens of the human disease that they are supposed to model. Improved preclinical models that better resemble the B cell and plasma cell response of most human tumors are urgently needed, because an array of recent clinical studies indicates that B cell and plasma cell infiltration, along with the isotype-switched antibodies that these cells produced in the tumor microenvironment, are consistently associated with better outcome and superior responses to existing immunotherapies. In fact, T cell infiltration only has predictive value when is associated with concurrent B cell accumulation in some tumors.
Unlike mouse models, antibody production in human tumors appears to be dominated by a combination of IgA and IgG isotypes, which coat the tumor cell surface and are therefore truly tumor-reactive. IgA and IgG are also the isotypes predominantly produced by TLS, which recapitulate the architecture of lymph nodes at tumor beds and are also predictive of better outcome and enhanced immunotherapeutic responses in a variety of tumors. The field urgently needs guidelines to categorize lymphoid aggregates with different degrees of complexity, which could reflect different stages of TLS maturation and have different functions. How TLS contribute to spontaneous or immunotherapeutically driven anti-tumor immunity is also the subject of intense investigation in many laboratories. Understanding the roles of TLS at tumor beds and how they are orchestrated could lead to novel immunotherapies aimed to recapitulate their protective activity in unresectable tumors.
Overall, immuno-oncology is changing the prevailing view of how B cell responses, including the production of antibodies at tumor beds, contribute to anti-tumor immunity. Given that most, if not all vaccines that have worked so far in large populations, including those against viral diseases, depend on the production of on the magnitude of the antibody response, understanding and targeting humoral responses in cancer promises to open new avenues for more effective anti-cancer vaccines and immunotherapies that elicit coordinated immune control of human cancer by the 2 arms of adaptive immunity.
Acknowledgements
This study was supported by R01CA157664, R01CA124515, R01CA178687, R01CA211913 and U01CA232758 to JRCG; and R01CA184185; R01CA233512; R01CA262121; R01CA27303; and P01CA250984 to PCR.
References
- [1].Biswas S, Mandal G, Payne KK, Anadon CM, Gatenbee CD, Chaurio RA, Costich TL, Moran C, Harro CM, Rigolizzo KE, Mine JA, Trillo-Tinoco J, Sasamoto N, Terry KL, Marchion D, Buras A, Wenham RM, Yu X, Townsend MK, Tworoger SS, Rodriguez PC, Anderson AR, Conejo-Garcia JR, IgA transcytosis and antigen recognition govern ovarian cancer immunity, Nature 591 (7850) (2021) 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mandal G, Biswas S, Anadon CM, Yu X, Gatenbee CD, Prabhakaran S, Payne KK, Chaurio RA, Martin A, Innamarato P, Moran C, Powers JJ, Harro CM, Mine JA, Sprenger KB, Rigolizzo KE, Wang X, Curiel TJ, Rodriguez PC, Anderson AR, Saglam O, Conejo-Garcia JR, IgA-dominated humoral immune responses govern patients’ outcome in endometrial cancer, Cancer Res 82 (5) (2022) 859–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ladanyi A, Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma, Pigment Cell Melanoma Res 28 (5) (2015) 490–500. [DOI] [PubMed] [Google Scholar]
- [4].Meshcheryakova A, Tamandl D, Bajna E, Stift J, Mittlboeck M, Svoboda M, Heiden D, Stremitzer S, Jensen-Jarolim E, Grunberger T, Bergmann M, Mechtcheriakova D, B cells and ectopic follicular structures: novel players in antitumor programming with prognostic power for patients with metastatic colorectal cancer, PLoS One 9 (6) (2014), e99008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, McMillan DC, The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer, Br. J. Cancer 106 (12) (2012) 2010–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Z, Zhu Y, Wang Z, Zhang T, Wu P, Huang J, Yin-yang effect of tumor infiltrating B cells in breast cancer: from mechanism to immunotherapy, Cancer Lett 393 (2017) 1–7. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Z, Ma L, Goswami S, Ma J, Zheng B, Duan M, Liu L, Zhang L, Shi J, Dong L, Sun Y, Tian L, Gao Q, Zhang X, Landscape of infiltrating B cells and their clinical significance in human hepatocellular carcinoma, Oncoimmunology 8 (4) (2019), e1571388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH, B cells are associated with survival and immunotherapy response in sarcoma, Nature 577 (7791) (2020) 556–560. [DOI] [PubMed] [Google Scholar]
- [9].Ho KH, Chang CJ, Huang TW, Shih CM, Liu AJ, Chen PH, Cheng KT, Chen KC, Gene landscape and correlation between B-cell infiltration and programmed death ligand 1 expression in lung adenocarcinoma patients from The Cancer Genome Atlas data set, PLoS One 13 (12) (2018), e0208459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hennequin A, Derangere V, Boidot R, Apetoh L, Vincent J, Orry D, Fraisse J, Causeret S, Martin F, Arnould L, Beltjens F, Ghiringhelli F, Ladoire S, Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients, Oncoimmunology 5 (2) (2016), e1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cillo AR, Kurten CHL, Tabib T, Qi Z, Onkar S, Wang T, Liu A, Duvvuri U, Kim S, Soose RJ, Oesterreich S, Chen W, Lafyatis R, Bruno TC, Ferris RL, Vignali DAA, Immune landscape of viral- and carcinogen-driven head and neck cancer, Immunity 52 (1) (2020) 183–199 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sautes-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC, Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention, Front. Immunol 7 (2016) 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dieu-Nosjean MC, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautes-Fridman C, Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers, Immunol. Rev 271 (1) (2016) 260–275. [DOI] [PubMed] [Google Scholar]
- [14].McMullen TP, Lai R, Dabbagh L, Wallace TM, de Gara CJ, Survival in rectal cancer is predicted by T cell infiltration of tumour-associated lymphoid nodules, Clin. Exp. Immunol 161 (1) (2010) 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F, Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers, Clin. Cancer Res 20 (8) (2014) 2147–2158. [DOI] [PubMed] [Google Scholar]
- [16].Ladanyi A, Kiss J, Somlai B, Gilde K, Fejos Z, Mohos A, Gaudi I, Timar J, Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor, Cancer Immunol. Immunother 56 (9) (2007) 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mule JJ, 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci. Rep 2 (2012) 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E, Characterisation and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma, BMC Clin. Pathol 14 (2014) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K, Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer, Br. J. Cancer 112 (11) (2015) 1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mazor RD, Nathan N, Gilboa A, Stoler-Barak L, Moss L, Solomonov I, Hanuna A, Divinsky Y, Shmueli MD, Hezroni H, Zaretsky I, Mor M, Golani O, Sabah G, Jakobson-Setton A, Yanichkin N, Feinmesser M, Tsoref D, Salman L, Yeoshoua E, Peretz E, Erlich I, Cohen NM, Gershoni JM, Freund N, Merbl Y, Yaari G, Eitan R, Sagi I, Shulman Z, Tumor-reactive antibodies evolve from non-binding and autoreactive precursors, Cell 185 (7) (2022), 1208–1222 e21. [DOI] [PubMed] [Google Scholar]
- [21].Biswas S, Mandal G, Roy Chowdhury S, Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X, Conejo-Garcia JR, Bhattacharyya A, Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive M2-polarized macrophages in breast cancer, J. Immunol 203 (12) (2019) 3447–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Engelhard V, Conejo-Garcia JR, Ahmed R, Nelson BH, Willard-Gallo K, Bruno TC, Fridman WH, B cells and cancer, Cancer Cell (2021). [DOI] [PubMed]
- [23].de Visser KE, Korets LV, Coussens LM, De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent, Cancer Cell 7 (5) (2005) 411–423. [DOI] [PubMed] [Google Scholar]
- [24].Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, de Visser KE, De Palma M, Coussens LM, FcRgamma activation regulates inflammation-associated squamous carcinogenesis, Cancer Cell 17 (2) (2010) 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Medler TR, Murugan D, Horton W, Kumar S, Cotechini T, Forsyth AM, Leyshock P, Leitenberger JJ, Kulesz-Martin M, Margolin AA, Werb Z, Coussens LM, Complement C5a fosters squamous carcinogenesis and limits T cell response to chemotherapy, Cancer Cell 34 (4) (2018), 561–578 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M, Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity, Nature 551 (7680) (2017) 340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Z, Lu Z, Lin S, Xia J, Zhong Z, Xie Z, Xing Y, Qie J, Jiao M, Li Y, Wen H, Zhao P, Zhang D, Zhou P, Qian J, Luo F, Wang L, Yu H, Liu J, Gu J, Liu R, Chu Y, Leucine-tRNA-synthase-2-expressing B cells contribute to colorectal cancer immunoevasion, Immunity 55 (6) (2022), 1067–1081 e8. [DOI] [PubMed] [Google Scholar]
- [28].Esteve-Sole A, Luo Y, Vlagea A, Deya-Martinez A, Yague J, Plaza-Martin AM, Juan M, Alsina L, B regulatory cells: players in pregnancy and early life, Int. J. Mol. Sci 19 (7) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C, CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients, Immunity 32 (1) (2010) 129–140. [DOI] [PubMed] [Google Scholar]
- [30].Mauri C, Menon M, The expanding family of regulatory B cells, Int. Immunol 27 (10) (2015) 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, Mauri C, Coussens LM, Balkwill FR, B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis, Proc. Natl. Acad. Sci. USA 108 (26) (2011) 10662–10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Inoue S, Leitner WW, Golding B, Scott D, Inhibitory effects of B cells on antitumor immunity, Cancer Res 66 (15) (2006) 7741–7747. [DOI] [PubMed] [Google Scholar]
- [33].Ganti SN, Albershardt TC, Iritani BM, Ruddell A, Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth, Sci. Rep 5 (2015) 12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lykken JM, Candando KM, Tedder TF, Regulatory B10 cell development and function, Int. Immunol 27 (10) (2015) 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sarvaria A, Basar R, Mehta RS, Shaim H, Muftuoglu M, Khoder A, Sekine T, Gokdemir E, Kondo K, Marin D, Daher M, Alousi AM, Alsuliman A, Liu E, Oran B, Olson A, Jones RB, Popat U, Hosing C, Champlin R, Shpall EJ, Rezvani K, IL-10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation, Blood 128 (10) (2016) 1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Das S, Bar-Sagi D, BTK signaling drives CD1d(hi)CD5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis, Oncogene 38 (17) (2019) 3316–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, Shin SU, Cho HM, Al Bayati A, Pimentel A, Rosenblatt JD, Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses, Int. Immunol 28 (9) (2016) 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC, Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia, Cancer Discov 6 (3) (2016) 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yuen GJ, Demissie E, Pillai S, B lymphocytes and cancer: a love-hate relationship, Trends Cancer 2 (12) (2016) 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, Araki Y, Miyoshi I, Yang L, Trinchieri G, Biragyn A, Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells, Cancer Res 75 (17) (2015) 3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SHE, Anderton SM, Fillatreau S, IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases, Nature 507 (7492) (2014) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Aklilu M, Stadler WM, Markiewicz M, Vogelzang NJ, Mahowald M, Johnson M, Gajewski TF, Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma, Ann. Oncol 15 (7) (2004) 1109–1114. [DOI] [PubMed] [Google Scholar]
- [43].Conejo-Garcia JR, Biswas S, Chaurio R, Humoral immune responses: unsung heroes of the war on cancer, Semin. Immunol 49 (2020), 101419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rosser EC, Mauri C, Regulatory B cells: origin, phenotype, and function, Immunity 42 (4) (2015) 607–612. [DOI] [PubMed] [Google Scholar]
- [45].Mauri C, Bosma A, Immune regulatory function of B cells, Annu. Rev. Immunol 30 (2012) 221–241. [DOI] [PubMed] [Google Scholar]
- [46].Mandal G, Biswas S, Anadon CM, Yu X, Gatenbee CD, Prabhakaran S, Payne KK, Chaurio RA, Martin A, Innamarato P, Moran C, Powers JJ, Harro CM, Mine JA, Sprenger KB, Rigolizzo KE, Wang X, Curiel TJ, Rodriguez PC, Anderson ARA, Saglam O, Conejo-Garcia JR, IgA-dominated humoral immune responses govern patients’ outcome in endometrial cancer, Cancer Res (2021). [DOI] [PMC free article] [PubMed]
- [47].Harris RJ, Cheung A, Ng JCF, Laddach R, Chenoweth AM, Crescioli S, Fittall M, Dominguez-Rodriguez D, Roberts J, Levi D, Liu F, Alberts E, Quist J, Santaolalla A, Pinder SE, Gillett C, Hammar N, Irshad S, Van Hemelrijck M, Dunn-Walters DK, Fraternali F, Spicer JF, Lacy KE, Tsoka S, Grigoriadis A, Tutt ANJ, Karagiannis SN, Tumor-infiltrating B lymphocyte profiling identifies IgG-biased, clonally expanded prognostic phenotypes in triple-negative breast cancer, Cancer Res 81 (16) (2021) 4290–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx JN, Boisson A, Duvillier H, Craciun L, Ameye L, Veys I, Paesmans M, Larsimont D, Piccart-Gebhart M, Willard-Gallo K, Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer, JCI Insight 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mullins CS, Gock M, Krohn M, Linnebacher M, Human colorectal carcinoma infiltrating B lymphocytes are active secretors of the immunoglobulin isotypes A, G, and M, Cancers 11 (6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bosisio FM, Wilmott JS, Volders N, Mercier M, Wouters J, Stas M, Blokx WA, Massi D, Thompson JF, Scolyer RA, van Baren N, van den Oord JJ, Plasma cells in primary melanoma. Prognostic significance and possible role of IgA, Mod. Pathol 29 (4) (2016) 347–358. [DOI] [PubMed] [Google Scholar]
- [51].Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD, Modulation of the antitumor immune response by complement, Nat. Immunol 9 (11) (2008) 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, He X, Garay JP, Carey-Ewend K, Marron D, Ford J, Liu S, Vick SC, Martin M, Parker JS, Vincent BG, Serody JS, Perou CM, B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer, Cell 179 (5) (2019), 1191–1206 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chaurio RA, Anadon CM, Lee Costich T, Payne KK, Biswas S, Harro CM, Moran C, Ortiz AC, Cortina C, Rigolizzo KE, Sprenger KB, Mine JA, Innamarato P, Mandal G, Powers JJ, Martin A, Wang Z, Mehta S, Perez BA, Li R, Robinson J, Kroeger JL, Curiel TJ, Yu X, Rodriguez PC, Conejo-Garcia JR, TGF-beta-mediated silencing of genomic organizer SATB1 promotes Tfh cell differentiation and formation of intra-tumoral tertiary lymphoid structures, Immunity 55 (1) (2022), 115–128 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, Wang B, Gu S, Jiang P, Fan J, Ying X, Zhang J, Carroll MC, Wucherpfennig KW, Hacohen N, Zhang F, Zhang P, Liu JS, Li B, Liu XS, Landscape of B cell immunity and related immune evasion in human cancers, Nat. Genet 51 (3) (2019) 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Martin AL, Anadon CM, Biswas S, Mine JA, Handley KF, Payne KK, Mandal G, Chaurio RA, Powers JJ, Sprenger KB, Rigolizzo KE, Innamarato P, Harro CM, Mehta S, Perez BA, Wenham RM, Conejo-Garcia JR, Olfactory receptor OR2H1 is an effective target for CAR T cells in human epithelial tumors, Mol. Cancer Ther 21 (7) (2022) 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kroeger DR, Milne K, Nelson BH, Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer, Clin. Cancer Res 22 (12) (2016) 3005–3015. [DOI] [PubMed] [Google Scholar]
- [57].Anadon CM, Yu X, Hanggi K, Biswas S, Chaurio RA, Martin A, Payne KK, Mandal G, Innamarato P, Harro CM, Mine JA, Sprenger KB, Cortina C, Powers JJ, Costich TL, Perez BA, Gatenbee CD, Prabhakaran S, Marchion D, Heemskerk MHM, Curiel TJ, Anderson AR, Wenham RM, Rodriguez PC, Conejo-Garcia JR, Ovarian cancer immunogenicity is governed by a narrow subset of progenitor tissue-resident memory T cells, Cancer Cell 40 (5) (2022), 545–557 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G, Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer, N. Engl. J. Med 348 (3) (2003) 203–213. [DOI] [PubMed] [Google Scholar]
- [59].Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K, Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer, Proc. Natl. Acad. Sci. USA 102 (51) (2005) 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Osorio JC, Zamarin D, Beyond T cells: IgA incites immune recognition in endometrial cancer, Cancer Res 82 (5) (2022) 766–768. [DOI] [PubMed] [Google Scholar]
- [61].Yue X, Ai J, Xu Y, Chen Y, Huang M, Yang X, Hu B, Zhang H, He C, Yang X, Tang W, Peng X, Dong L, Wang H, Fan J, Ding J, Geng M, Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma, Hepatology 65 (6) (2017) 1948–1962. [DOI] [PubMed] [Google Scholar]
- [62].Arumugam P, Bhattacharya S, Chin-Aleong J, Capasso M, Kocher HM, Expression of polymeric immunoglobulin receptor and stromal activity in pancreatic ductal adenocarcinoma, Pancreatology 17 (2) (2017) 295–302. [DOI] [PubMed] [Google Scholar]
- [63].Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH, Tertiary lymphoid structures in the era of cancer immunotherapy, Nat. Rev. Cancer (2019). [DOI] [PubMed]
- [64].Schumacher TN, Thommen DS, Tertiary lymphoid structures in cancer, Science 375 (6576) (2022) eabf9419. [DOI] [PubMed] [Google Scholar]
- [65].Lee HJ, Park IA, Song IH, Shin SJ, Kim JY, Yu JH, Gong G, Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer, J. Clin. Pathol 69 (5) (2016) 422–430. [DOI] [PubMed] [Google Scholar]
- [66].Lee M, Heo SH, Song IH, Rajayi H, Park HS, Park IA, Kim YA, Lee H, Gong G, Lee HJ, Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis, Mod. Pathol 32 (1) (2019) 70–80. [DOI] [PubMed] [Google Scholar]
- [67].Solinas C, Garaud S, De Silva P, Boisson A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitoria J, Richard F, Migliori E, Noel G, Duvillier H, Craciun L, Veys I, Awada A, Detours V, Larsimont D, Piccart-Gebhart M, Willard-Gallo K, Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer, Front. Immunol 8 (2017) 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pfannstiel C, Strissel PL, Chiappinelli KB, Sikic D, Wach S, Wirtz RM, Wullweber A, Taubert H, Breyer J, Otto W, Worst T, Burger M, Wullich B, Bolenz C, Fuhrich N, Geppert CI, Weyerer V, Stoehr R, Bertz S, Keck B, Erlmeier F, Erben P, Hartmann A, Strick R, Eckstein M, Bridge Consortium G, Bridge Consortium G, Bridge Consortium G, Bridge Consortium G, The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes, Cancer Immunol. Res 7 (6) (2019) 923–938. [DOI] [PubMed] [Google Scholar]
- [69].Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA, B cells and tertiary lymphoid structures promote immunotherapy response, Nature 577 (7791) (2020) 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Meylan M, Petitprez F, Becht E, Bougouin A, Pupier G, Calvez A, Giglioli I, Verkarre V, Lacroix G, Verneau J, Sun CM, Laurent-Puig P, Vano YA, Elaidi R, Mejean A, Sanchez-Salas R, Barret E, Cathelineau X, Oudard S, Reynaud CA, de Reynies A, Sautes-Fridman C, Fridman WH, Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer, Immunity 55 (3) (2022), 527–541 e5. [DOI] [PubMed] [Google Scholar]
- [71].Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P, Curioni-Fontecedro A, Foukas P, Levesque MP, Moch H, Line A, van den Broek M, Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma, Cancer Res 78 (5) (2018) 1308–1320. [DOI] [PubMed] [Google Scholar]
- [72].Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jonsson G, Tertiary lymphoid structures improve immunotherapy and survival in melanoma, Nature 577 (7791) (2020) 561–565. [DOI] [PubMed] [Google Scholar]
- [73].Chen S, Xie P, Cowan M, Huang H, Cardenas H, Keathley R, Tanner EJ, Fleming GF, Moroney JW, Pant A, Akasha AM, Davuluri RV, Kocherginsky M, Zhang B, Matei D, Epigenetic priming enhances anti-tumor immunity in platinum resistant ovarian cancer, J. Clin. Investig (2022). [DOI] [PMC free article] [PubMed]
- [74].Patil NS, Nabet BY, Muller S, Koeppen H, Zou W, Giltnane J, Au-Yeung A, Srivats S, Cheng JH, Takahashi C, de Almeida PE, Chitre AS, Grogan JL, Rangell L, Jayakar S, Peterson M, Hsia AW, O’Gorman WE, Ballinger M, Banchereau R, Shames DS, Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer, Cancer Cell 40 (3) (2022), 289–300 e4. [DOI] [PubMed] [Google Scholar]
- [75].Vanhersecke L, Brunet M, Guegan JP, Rey C, Bougouin A, Cousin S, Moulec SL, Besse B, Loriot Y, Larroquette M, Soubeyran I, Toulmonde M, Roubaud G, Pernot S, Cabart M, Chomy F, Lefevre C, Bourcier K, Kind M, Giglioli I, Sautes-Fridman C, Velasco V, Courgeon F, Oflazoglu E, Savina A, Marabelle A, Soria JC, Bellera C, Sofeu C, Bessede A, Fridman WH, Loarer FL, Italiano A, Mature tertiary lymphoid structures predict immune checkpoint inhibitor efficacy in solid tumors independently of PD-L1 expression, Nat. Cancer 2 (8) (2021) 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL Jr., Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity, J. Immunol 200 (2) (2018) 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Fridman WH, Meylan M, Petitprez F, Sun CM, Italiano A, Sautes-Fridman C, B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome, Nat. Rev. Clin. Oncol 19 (7) (2022) 441–457. [DOI] [PubMed] [Google Scholar]
- [78].Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N, Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases, Cancer Res 72 (16) (2012) 3997–4007. [DOI] [PubMed] [Google Scholar]
- [79].Garaud S, Zayakin P, Buisseret L, Rulle U, Silina K, de Wind A, Van den Eyden G, Larsimont D, Willard-Gallo K, Line A, Antigen specificity and clinical significance of IgG and IgA autoantibodies produced in situ by tumor-infiltrating B cells in breast cancer, Front. Immunol 9 (2018) 2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Payne KK, Mine JA, Biswas S, Chaurio RA, Perales-Puchalt A, Anadon CM, Costich TL, Harro CM, Walrath J, Ming Q, Tcyganov E, Buras AL, Rigolizzo KE, Mandal G, Lajoie J, Ophir M, Tchou J, Marchion D, Luca VC, Bobrowicz P, McLaughlin B, Eskiocak U, Schmidt M, Cubillos-Ruiz JR, Rodriguez PC, Gabrilovich DI, Conejo-Garcia JR, BTN3A1 governs antitumor responses by coordinating alphabeta and gammadelta T cells, Science 369 (6506) (2020) 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Filderman JN, Appleman M, Chelvanambi M, Taylor JL, Storkus WJ, STINGing the tumor microenvironment to promote therapeutic tertiary lymphoid structure development, Front. Immunol 12 (2021), 690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li R, Berglund A, Zemp L, Dhillon J, Putney R, Kim Y, Jain RK, Grass GD, Conejo-Garcia J, Mule JJ, The 12-CK score: global measurement of tertiary lymphoid structures, Front. Immunol 12 (2021), 694079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Li R, Zhang J, Gilbert SM, Conejo-Garcia J, Mule JJ, Using oncolytic viruses to ignite the tumour immune microenvironment in bladder cancer, Nat. Rev. Urol 18 (9) (2021) 543–555. [DOI] [PubMed] [Google Scholar]
- [84].Johansson-Percival A, He B, Li ZJ, Kjellen A, Russell K, Li J, Larma I, Ganss R, De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors, Nat. Immunol 18 (11) (2017) 1207–1217. [DOI] [PubMed] [Google Scholar]
- [85].Overacre-Delgoffe AE, Bumgarner HJ, Cillo AR, Burr AHP, Tometich JT, Bhattacharjee A, Bruno TC, Vignali DAA, Hand TW, Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer, Immunity 54 (12) (2021), 2812–2824 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sungalee S, Mamessier E, Morgado E, Gregoire E, Brohawn PZ, Morehouse CA, Jouve N, Monvoisin C, Menard C, Debroas G, Faroudi M, Mechin V, Navarro JM, Drevet C, Eberle FC, Chasson L, Baudimont F, Mancini SJ, Tellier J, Picquenot JM, Kelly R, Vineis P, Ruminy P, Chetaille B, Jaffe ES, Schiff C, Hardwigsen J, Tice DA, Higgs BW, Tarte K, Nadel B, Roulland S, Germinal center reentries of BCL2-overexpressing B cells drive follicular lymphoma progression, J. Clin. Investig 124 (12) (2014) 5337–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Venturutti L, Melnick AM, The dangers of deja vu: memory B cells as the cells of origin of ABC-DLBCLs, Blood 136 (20) (2020) 2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]


