Abstract
Introduction:
The sheep was evaluated as a potential model for preclinical evaluation of urethral slings in vivo based on: (1) anatomical measurements of the sheep vagina and (2) histological tissue integration and host response to polypropylene (PP) slings.
Methods:
Eight female, multiparous sheep were utilized. Three of 8 animals underwent surgery mimicking human tension-free vaginal tape protocols for midurethral slings and were euthanized at 6 months. The following measurements were obtained: vaginal length, maximum vaginal width with retraction, symphysis pubis length, and distance from the pubic bone to incision. Explanted sling samples from sheep and human were stained with hematoxylin and eosin for host reaction assessment.
Results:
Geometric measurements were similar between humans and sheep. Sheep vaginal anatomy allowed sling placement similar to procedures in human surgeries, and all sheep recovered without problems. Comparative histology between the sheep and human indicated similar host reaction and collagen deposition around implants, confirming suitability of the sheep model for biomaterial response assessment.
Conclusion:
Sheep vaginal length is comparable to humans. Tissue integration and host response to PP slings showed chronic inflammation with rich collagen deposition around the material in both sheep and human specimens, highlighting the sheep as a potential animal model for preclinical testing of midurethral slings.
Keywords: Stress urinary incontinence, Midurethral sling, Sheep model, Preclinical testing
Introduction
Stress urinary incontinence (SUI) affects ~40% of adult women [1] and impacts social, psychological, and hygienic wellness of patients. Various treatment options are available for SUI management. While there exist nonsurgical treatment options such as lifestyle changes, vaginal cones, pelvic floor rehabilitation, and bladder training [2], surgical treatment remains the most effective option for many SUI patients. Surgical implantation of retropubic and transobturator midurethral slings is a commonly performed procedure and remains as gold-standard treatment with cure rates of 80–90% [3]. Despite success rates, erosion, extrusion, obstruction, voiding dysfunction, and recurrent urinary tract infections are reported [4]. In part, these complications are attributed to synthetic mesh materials. Therefore, we continue to seek improvements in biomaterial selection with the aim of developing the ideal sling material for SUI management [5].
Novel biomaterials could be employed, particularly in revision surgeries. At present, biomaterials such as electrocompacted collagen [6] and poly-l-lactic acid [7] are being assessed for next-generation slings. Preclinical testing of these novel materials in animal models is imperative to understanding biocompatibility, tissue integration, long-term mechanical robustness, and efficacy in restoring continence [8]. Appropriate animal model selection is critical for evidence-based clearance of these materials prior to clinical use, which led to the development of midurethral slings [9, 10]. Various SUI animal models of suburethral sling implantation were reported[11]. The key limitation of rodents is that slings are undersized, limiting assessment of human-sized sling characteristics (porosity continuum and dimensions). While dogs are more appropriately sized than rodents, vaginal morphology of dogs is smaller than humans. In female pigs, urination and birth occur through a single urogenital opening, different from humans where the urethra and vagina have two separate canals [12]. Nonhuman primates are closely related to humans, but ethical concerns related to their use are a concern [13]. Therefore, there is no reported large animal model suitable for testing human-sized slings in vivo. A promising candidate animal model is sheep. Sheep cadavers were used for hands-on training of sling placement because sheep pelvic anatomy is acceptably similar to humans in terms of emulating surgical conditions [14]. However, to the best of our knowledge, the sheep model has yet to be used in testing of biological responses to suburethral slings. The above stated merits warrant a more thorough evaluation of the sheep model as a potential candidate for studying sling biomaterials.
The purpose of this study was to examine whether the sheep model is suitable for preclinical evaluation of midurethral slings in vivo. Specifically, our aims were to: (1) compare anatomical dimensions of sheep vagina to human and (2) assess tissue integration and biological response to polypropylene (PP) slings in sheep versus human. Anatomical and histological assessment of large animal models is crucial for future standardization and preclinical testing of new biomaterials in urogynecological reconstructive surgeries.
Materials and Methods
Human-sized midurethral slings were implanted under general anesthesia, mimicking the tension-free transvaginal technique using a retropubic approach (online suppl. material. 1; see www.karger.com/doi/10.1159/000522138 for all online suppl. material). Standard, commercially available, clinical-grade PP slings and trocars (Boston Scientific Corporation, Marlborough, MA, USA) were used in surgeries. Eight female breeder sheep were used in the study (160–175 pounds, Hunter’s Dorset, Lafayette, IN, USA). Sheep were multiparous, and each had at least 4 lambs. All 8 animals were used for anatomical dimension measurements (shown in Fig. 1a-c). Three of 8 animals used for surgical placement (shown in Fig. 2a-f) of midurethral slings were euthanized at 6 months following surgical implantation to observe long-term host response.
Fig. 1.
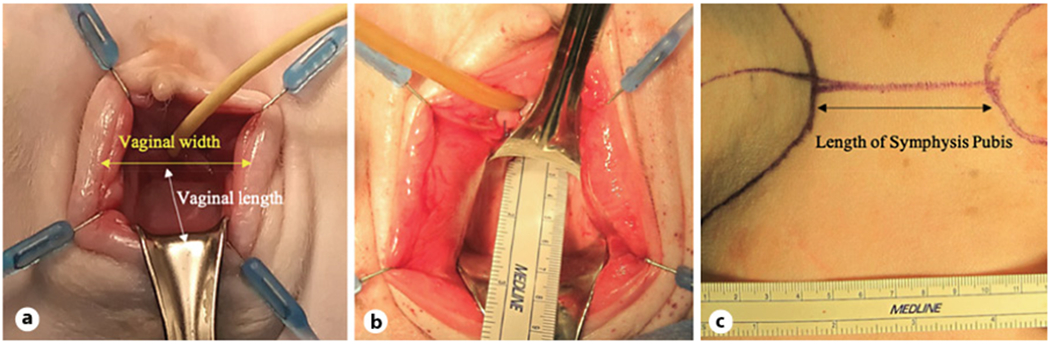
Measurements of pelvic landmarks. a Schematic description of measured dimensions of the sheep vagina. b Depicts measurement of sheep vaginal length. c Measurement of pubic bone length.
Fig. 2.
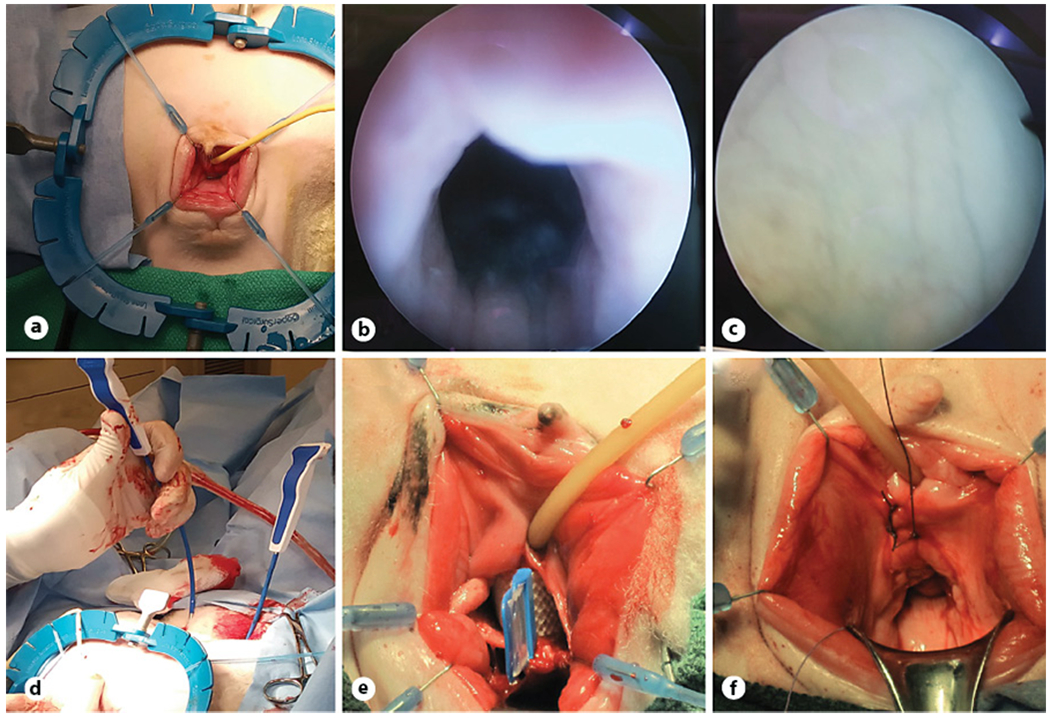
Midurethral sling implantation. a Demonstration of the sheep’s preoperative positioning and the allocation of the human-sized self-retaining retractor and stays. Cystoscopy image of the urethra (b) and bladder (c). d Insertion of trocars. e Sling was inserted under the urethra through a vaginal incision before deployment. f Closure of vaginal incisions.
Euthanasia and Tissue Harvest
Three animals were euthanized at 6 months following surgery using 10–15 mL per 100 lb sodium pentobarbital (FatalPlus, Vorthech Pharmaceuticals, Dearborn MI, USA). A circular perineal incision was made around the urethra and vagina (shown in Fig. 3a). Dissection was performed in the perineal region to separate the posterior vaginal wall from the rectum (shown in Fig. 3b, c). Bilateral perineal dissection was made around the vagina and urethra to reach the pelvis. Pubectomy was performed using a limb and branch lopper (21” to 33,” Corona Tools, Corona, CA, USA) to expose the urethra (shown in Fig. 3d, e). Combined pelvic and perineal dissection was performed to retrieve the bladder, urethra, vagina, uterus, and midurethral sling en bloc (shown in Fig. 3f). Midurethral slings (MUS) were detached from pelvic bones.
Fig. 3.
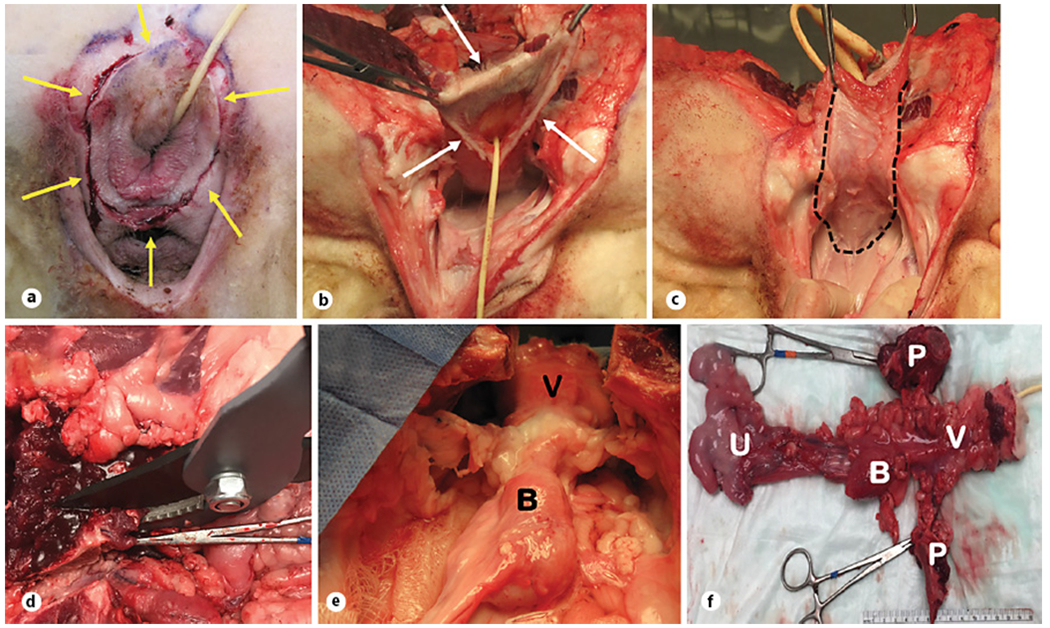
Harvest procedure. a Demonstration of a circular incision made around the urethra and vagina (yellow arrows). b, c Dissection was performed in the perineal region to separate the posterior vaginal wall from the rectum (white arrows). d Pubectomy was performed using a bypass lopper to expose the urethra. e Midurethral sling was detached from the pelvic bones (“B,” bladder and “V,” vagina). f Depicts explanted samples from the sheep. B, bladder; V, vagina; U, uterus; P, pubic bones.
Histology of Tissue Response to Sling Material (Sheep and Human)
Explanted, formalin-fixed, paraffin-embedded mesh specimens were stained with hematoxylin and eosin to evaluate host response. Images of stained slides were collected with a light microscope (Olympus BX51) and scanned digitally (PrimeHisto XE Slide Scanner, Carolina Biological Supply, Burlington, NC, USA). After obtaining an exemption from the IRB committee, human histology slides were retrieved from the pathology department repository in our institute for assessment and review by a senior pathologist (G.M.).
Statistical Methods
Mean ± SD values for sheep measured in this study were compared to those reported in the literature [15, 16] by using t-test with significance set at p < 0.05 (Review Manager, Version 3.5.2, Cochrane).
Results
Morphological measurements from the sheep vagina indicated all geometric measures except for that symphysis pubis length were similar between human and sheep (Table 1). Sheep vaginal anatomy allowed successful placement of slings in a fashion nominally comparable to human surgical procedures, and all sheep recovered smoothly. Mean operative time was 44 ± 12 min. Blood loss was observed to be less than 50 cc for all animals. All animals urinated normally without hematuria or obstruction beginning from the first postoperative day. There were no indications of vaginal infection or sling rejection at any point in the study. Additionally, the proposed dissection method successfully isolated the urethra, vagina, bladder, uterus, and sling en bloc (shown in Fig. 3f). Histology of en bloc tissues confirmed the presence of the MUS between the vagina and urethra (shown in Fig. 4a) with no apparent adverse reactions in the surrounding tissue for all animals. Similar healing patterns and host responses for human and sheep were observed in hematoxylin-and-eosin slides based on general appearance (cell nuclei, fibrous capsule around the PP sling, collagen deposition, etc.) (Fig. 4b). New collagen deposition and cellular filtration were reported in the region around the material in both sheep and human.
Table 1.
Dimensions of the human female pelvis taken from the literature and used for comparisons to measurements from sheep
| Human, n | Human, cm | Sheep, n | Sheep, cm | p value | |
|---|---|---|---|---|---|
| Vaginal length [15] | 50 | 9.6±1.5 | 8 | 8.4375±1.99 | 0.14 |
| Maximum vaginal width with retraction [15] | – | N/R | – | 7±0.80 | – |
| Length of the symphysis pubis [16] | SR | 2.6–4.6 | 8 | 6.775±0.55 | – |
| Distance from the pubic bone to incision [15] | – | N/R | 8 | 6.2875±0.70 | – |
N/R, not reported; SR, systematic review.
Fig. 4.
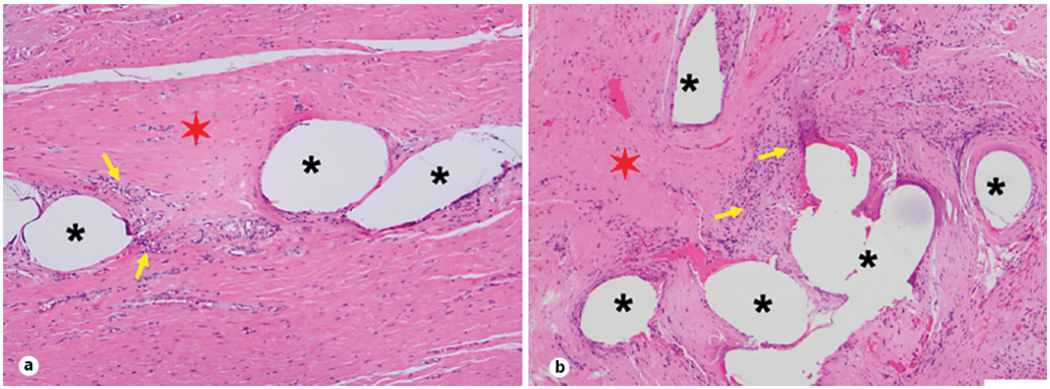
H&E-stained explanted sling specimens. a H&E-stained sheep sling images at 6 months. b H&E-stained human sling images at 6 months. Asterisks denote sling material; red, six-pointed stars demonstrate collagen deposition in and around the sling material; yellow arrows indicate cellular infiltration in and around the sling. The scale bar is 100 μm. H&E, hematoxylin and eosin.
Discussion
Previously, sheep cadavers were reported as suitable for training surgeons on MUS [14] or pelvic organ prolapse (POP) surgeries [17]. Urbankova et al. [18] provided a detailed surgical protocol for implanting rectangular biomaterial patches in the rectovaginal space as a guideline for researchers. This surgical model was used by researchers who implanted experimental electrospun biomaterial patches and PP controls in sheep and examined host response for up to 6 months [19]. However, assessment of MUS or POP implants in sheep, as opposed to biomaterial patches, appears limited in the literature. To the best our knowledge, there are no studies utilizing sheep for evaluation of in vivo response to full length MUS. We were able to demonstrate slings could be inserted in vivo successfully, following which animals recovered without significant complications. Furthermore, resulting healing patterns and host responses in sheep were comparable to humans [20, 21].
Availability and acceptance of the sheep model by researchers make it suitable for preclinical studies, but there are certain limitations associated with this sheep model. First, compared to rodents, sheep are more costly. Furthermore, antibodies for immunohistochemistry or primers for in situ hybridization analyses may not be readily available [22]. As a result, sheep may be suitable for functional implant assessment, whereas utilization of sheep in studies testing biological hypotheses may not be straightforward.
We reported anatomical dimensions of sheep pelvic landmarks and similarities to humans and successfully implanted human-grade slings in sheep with no intraoperative complications. The only anatomical measurement that differed was symphysis pubis length, which was accounted for by cephalad positioning of the abdominal incision approximately 7 cm cranial to the symphysis pubis. This distance is greater than employed in human surgical procedures. In future studies, incisions can be positioned close to the symphysis pubis by using custom trocars with reduced curvature as compared to commercially available trocars in order to match sheep anatomy. Urethral catheterization and medium episiotomy were straightforward. Cystoscopy appeared similar to humans, and urethral ducts were in the same position.
Historically, smaller animal models [11] have been utilized in various SUI research. Some models induce incontinence, whereas others focus on biocompatibility alone. Main limitations with primates or dogs stem from public sensitivity and ethical considerations. This might represent a valid argument for selecting a species such as the pig or sheep in the place of dogs or primates as experimental animals [23]. Recently, pigs were used to explore the retroperitoneum using the transvaginal natural orifice transluminal endoscopic surgery technique. This report promotes the utilization of pigs in retroperitoneal organ research [24]. However, a single urogenital opening in pigs renders them less preferable for urogynecology research experiments. Dogs have been used but were abandoned for poor training purposes due to small vaginal size [25]. These considerations leave the research community with the rodents as one of the few animal models for SUI research. While rodent models remain beneficial and economical for initial biomaterial assessment, from a preclinical translation point of view, implant size limitation is the main drawback of rodent models as elucidated by others [26]. Mechanical testing of small-sized biomaterials might not reflect properties when translated to human-sized slings.
Similar to humans, sheep can develop vaginal prolapse. In this study, we observed spontaneous prolapse (stage 1) at the time of surgery. It has been well documented that sheep acquire spontaneous prolapse [27, 28]. A few epidemiologic studies noted prevalence of POP in sheep to be as high as 15% [29]. Similar to humans, POP incidence in sheep also increases with number of births and aging. These similarities suggest the pathophysiology of POP in sheep and humans may have common mechanisms, indicating the sheep is also a good surrogate model for POP research.
There are limitations in this study. First, the number of animals was limited in the experimental design. However, the focus was on protocol development rather than a hypothesis-driven research study. Another limitation was the lack of incontinence in the current sheep model. Future studies are needed to assess the efficacy of pudendal nerve transection/crush in inducing incontinence in sheep. Furthermore, to be a viable incontinence model, a reliable method to assess incontinence outcome requires development.
In conclusion, the current study indicates (1) feasibility of using human-sized slings in sheep for biomaterial testing and (2) tissue integration and host responses to PP slings in sheep was comparable to that observed in humans. The implantation and dissection protocols developed in this study might be useful to other researchers interested in evaluating novel biomaterials for SUI treatment with MUS or for those who are seeking a suitable surgical practice training model.
Supplementary Material
Funding Sources
We gratefully acknowledge funding from University Hospitals of Cleveland, UH Ventures LLC, and the State of Ohio’s Third Frontier TVSF.
Footnotes
Statement of Ethics
Animal experimental protocol (#18-01-191) was approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University in compliance with the Guide for the Care and Use of Laboratory Animals.
Conflict of Interest Statement
Subbakrishna Shankar is the chief technology officer and holds equity in CollaMedix Inc. Ozan Akkus is the chief scientific officer of and holds equity in CollaMedix Inc. Adonis Hijaz is the chief medical officer and holds equity in CollaMedix Inc. The other authors declare no conflicts of interest.
Data Availability Statement
All data analyzed during this study are included in this article. Any additional raw data can be accessed upon request by contacting the corresponding author.
References
- 1.Isali I, Mahran A, Khalifa AO, Sheyn D, Neudecker M, Qureshi A, et al. Gene expression in stress urinary incontinence: a systematic review. Int Urogynecol J. 2020. Jan;31(1):1–14. [DOI] [PubMed] [Google Scholar]
- 2.Rovner ES, Wein AJ. Treatment options for stress urinary incontinence. Rev Urol. 2004;6 Suppl 3(Suppl 3):S29–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Moldovan CP, Marinone ME, Staack A. Transvaginal retropubic sling systems: efficacy and patient acceptability. Int J Womens Health. 2015;7:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah HN, Badlani GH. Mesh complications in female pelvic floor reconstructive surgery and their management: a systematic review. Indian J Urol. 2012. Apr-Jun;28(2):129–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colaco M, Mettu J, Badlani G. The scientific basis for the use of biomaterials in stress urinary incontinence (SUI) and pelvic organ prolapse (POP). BJU Int. 2015. Jun;115(6):859–66. [DOI] [PubMed] [Google Scholar]
- 6.Chapin K, Khalifa A, Mbimba T, McClellan P, Anderson J, Novitsky Y, et al. In vivo biocompatibility and time-dependent changes in mechanical properties of woven collagen meshes: a comparison to xenograft and synthetic mid-urethral sling materials. J Biom Mater Res B Appl Biomater. 2019. Apr;107(3):479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangir N, Hillary CJ, Chapple CR, MacNeil S. Oestradiol-releasing biodegradable mesh stimulates collagen production and angiogenesis: an approach to improving biomaterial integration in pelvic floor repair. Eur Urol Focus. 2019. Mar;5(2):280–89. [DOI] [PubMed] [Google Scholar]
- 8.Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des Devel Ther. 2018;12:3117–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petros PE, Ulmsten UI, Papadimitriou J. The autogenic ligament procedure: a technique for planned formation of an artificial neo-ligament. Acta Obstet Gynecol Scand Suppl. 1990;153:43–51. [DOI] [PubMed] [Google Scholar]
- 10.Petros P Creating a gold standard surgical device: scientific discoveries leading to TVT and beyond: Ulf Ulmsten memorial lecture 2014. Int Urogynecol J. 2015. Apr;26(4):471–6. [DOI] [PubMed] [Google Scholar]
- 11.Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol. 2011;(202):45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzen E, Follmann F, Jungersen G, Agerholm JS. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet Res. 2015;46:116–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijkstrom M, Bottino R, Cooper DK. Limitations of the pig-to-non-human primate islet transplantation model. Xenotransplantation. 2013. Jan-Feb;20(1):2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerbage Y, Giraudet G, Rubod C, Garabedian C, Rivaux G, Cosson M. Feasibility and benefits of the ewe as a model for vaginal surgery training. Int Urogynecol J. 2017. Oct;28(10):1573–7. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd J, Crouch NS, Minto CL, Liao LM, Creighton SM. Female genital appearance: “normality” unfolds. BJOG. 2005. May;112(5):643–6. [DOI] [PubMed] [Google Scholar]
- 16.Becker I, Woodley SJ, Stringer MD. The adult human pubic symphysis: a systematic review. J Anat. 2010. Nov;217(5):475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansoor A, Curinier S, Campagne-Loiseau S, Platteeuw L, Jacquetin B, Rabischong B. Development of an ovine model for training in vaginal surgery for pelvic organ prolapse. Int Urogynecol J. 2017. Oct;28(10):1595–7. [DOI] [PubMed] [Google Scholar]
- 18.Urbankova I, Callewaert G, Sindhwani N, Turri A, Hympanova L, Feola A, et al. Transvaginal mesh insertion in the ovine model. J Vis Exp. 2017. Jul 27;(125):55706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hympánová L, Rynkevic R, Román S, Mori da Cunha MGMC, Mazza E, Zündel M, et al. Assessment of electrospun and ultra-lightweight plypropylene meshes in the sheep model for vaginal surgery. Eur Urol Focus. 2020. Jan 15;6(1):190–8. [DOI] [PubMed] [Google Scholar]
- 20.Smith TM, Smith SC, Delancey JO, Fenner DE, Schimpf MO, Roh MH, et al. Pathologic evaluation of explanted vaginal mesh: interdisciplinary experience from a referral center. Female Pelvic Med Reconstr Surg. 2013. Jul-Aug;19(4):238–41. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Wang X, Park JY, Chen H, Wang Y, Zheng W. Pathological findings in explanted vaginal mesh. Hum Pathol. 2017. Nov;69:46–54. [DOI] [PubMed] [Google Scholar]
- 22.Turner AS. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg. 2007. Sep-Oct;16(5 Suppl):S158–63. [DOI] [PubMed] [Google Scholar]
- 23.Webster J Ethical and animal welfare considerations in relation to species selection for animal experimentation. Animals. 2014;4(4):729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharopoulou C, Nassif J, Allemann P, Dallemagne B, Perretta S, Marescaux J, et al. Exploration of the retroperitoneum using the transvaginal natural orifice transluminal endoscopic surgery technique. J Minim Invasive Gynecol. 2009. Mar-Apr;16(2):198–203. [DOI] [PubMed] [Google Scholar]
- 25.Herrera-Imbroda B, Lara MF, Izeta A, Sievert KD, Hart ML. Stress urinary incontinence animal models as a tool to study cell-based regenerative therapies targeting the urethral sphincter. Adv Drug Deliv Rev. 2015;82–83:106–16. [DOI] [PubMed] [Google Scholar]
- 26.Vandamme TF. Use of rodents as models of human diseases. J Pharm Bioallied Sci. 2014. Jan-Mar;6(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zacharin RF. Genital prolapse in ruminants. Aust N Z J Obstet Gynaecol. 1969. Nov;9(4):236–9. [DOI] [PubMed] [Google Scholar]
- 28.Couri BM, Lenis AT, Borazjani A, Paraiso MF, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012. May 1;7(3):249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Low JC, Sutherland HK. A census of the prevalence of vaginal prolapse in sheep flocks in the borders region of Scotland. Vet Rec. 1987. Jun 13;120(24):571–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed during this study are included in this article. Any additional raw data can be accessed upon request by contacting the corresponding author.


