Abstract
Opioids induce respiratory depression resulting in coma or even death during overdose. Naloxone, an opioid antagonist, is the gold standard reversal agent for opioid intoxication, but this treatment is often less successful for fentanyl. While low dosing is thought to be a factor limiting naloxone’s efficacy, the timing between fentanyl exposure and initiation of naloxone treatment may be another important factor. Here, we used oxygen sensors coupled with amperometry to examine the pattern of oxygen responses in the brain and periphery induced by intravenous fentanyl in freely moving rats. At both doses (20 and 60 μg/kg), fentanyl induced a biphasic brain oxygen response—a rapid, strong, and relatively transient decrease (8–12 min) followed by a weaker and prolonged increase. In contrast, fentanyl induced stronger and more prolonged monophasic oxygen decreases in the periphery. When administered before fentanyl, intravenous naloxone (0.2 mg/kg) fully blocked the hypoxic effects of moderate-dose fentanyl in both the brain and periphery. However, when injected 10 min after fentanyl, when most of hypoxia has already ceased, naloxone had minimal effect on central and peripheral oxygen levels, but at a higher dose, it strongly attenuated hypoxic effects in the periphery with only a transient brain oxygen increase associated with behavioral awakening. Therefore, due to the rapid, strong but transient nature of fentanyl-induced brain hypoxia, the time window when naloxone can attenuate this effect is relatively short. This timing limitation is critical, making naloxone most effective when used quickly and less effective when used during the post-hypoxic comatose state after brain hypoxia has already ceased and harm for neural cells already done.
Keywords: synthetic opioids, brain oxygen, high-speed amperometry, respiratory depression, cerebral vasodilation, hypoxia, hyperoxia
1. Introduction
Fentanyl is a highly potent synthetic opioid agonist, which is widely used in clinical practice as an analgesic drug (Peng and Sandler, 1999). However, this drug is known to produce respiratory depression and subsequent hypoxia at clinically relevant and recreational doses (Dahan et al., 2005; Jaffe et al., 1997; Pattinson, 2008; Yeadon and Kitchen, 1989). Fentanyl has been more recently associated as a cutting agent found in heroin and other less potent opioid drugs often leading to unexpectedly strong effects, including coma and death (Compton et al., 2016; McLaughlin, 2017). While the use of naloxone, a potent opioid antagonist, is the primary tool to alleviate respiratory depression induced by all opioid drugs and revive the intoxicated individual (McKenzie et al., 2016; Skolnick, 2018, 2021), this therapeutic strategy is often less effective with respect to fentanyl (Lynn and Galinkin, 2017; Moss and Carlo, 2019). Insufficient doses of naloxone can be a factor limiting its therapeutic effectiveness, but the timing between drug exposure and the initiation of naloxone treatment appears to be another critical variable. Clinically, naloxone is always administered after a certain variable time after the appearance of overdose symptoms. Therefore, the knowledge on the time-course of brain hypoxic effects of fentanyl and its relation to the timing of naloxone intervention appears of critical importance.
Whole-body plethysmography is a commonly used technology to quantify drug-induced changes in breathing activity in animal studies, but rat data with fentanyl are limited (Brown and Pleuvry, 1981; Dahan et al., 2005 Seckler et al., 2022) and data obtained in mice drastically differ from those observed in rats and humans (Hill et al., 2020). While useful, it is unclear how the changes in are breathing activity translate into changes of oxygen levels in brain tissue—a functionally significant parameter. To directly assess drug-induced fluctuations in oxygen levels in the brain’s extracellular space, we used oxygen sensors coupled with amperometry in freely moving rats (Kiyatkin, 2019). This approach provides second-scale temporal resolution and can be used in freely moving rats for multiple sessions. Using this approach, we found that intravenous (iv) fentanyl is roughly 20- to 30-fold stronger than heroin in inducing brain hypoxia that is rapid, strong, but relatively transient (Solis et al., 2017, 2018). Since naloxone is always administered some definite time after appearance of overdose symptoms, this pattern of fentanyl action may limit therapeutic efficiency of naloxone in reversing fentanyl-induced brain hypoxia.
In contrast to our previous studies, which employed acute recording from a single brain site, in this study we used double-sensor chronic recording where one oxygen sensor was implanted in the nucleus accumbens (NAc), a deep brain structure involved in sensorimotor integration and functioning of the motivation-reinforcement circuit (Badiani et al., 2011; Mogenson et al., 1980; Wise and Bozarth, 1989), and another one into the subcutaneous (SC) space. Chronic sensor implantation provides integrity of blood vessels in the recording area, greatly increasing the recording quality, and the use of the double-sensor approach allows simultaneous assessments of drug-induced oxygen changes in the brain and periphery. First, we examined how fentanyl affects oxygen levels in central and peripheral locations and determined the relationship between their changes. Second, we examined how pre-treatment with naloxone affects fentanyl-induced oxygen responses. Third, we examined how naloxone affects fentanyl-induced changes in oxygen levels in the NAc and SC space when administered at a clinically relevant time after iv fentanyl injection. While drug doses used in rats cannot be directly extrapolated to human conditions due to differences in metabolic activity and drug pharmacokinetics, fentanyl was used at two doses (20 and 60 μg/kg) that correspond to the upper limits of human consumption. Naloxone was used at a relatively low clinically relevant dose (0.2 mg/kg or 14 mg/70 kg) that provides robust blockade of μ-opioid receptors (Kang et al., 2022) and fully blocks brain hypoxia induced by iv heroin at modest doses (Perekopskiy et al., 2020).
2. Materials and methods
2.1. Subjects
Fourteen adult male Long-Evans rats (Charles River Laboratories) weighing 440±40 g at the time of surgery were used in this study. Rats were individually housed in a climate-controlled animal colony maintained on a 12–12 light-dark cycle (7AM light on) with food and water available ad libitum. All procedures were approved by the NIDA-IRP Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals (NIH, Publication 865–23) and ARRIVE guidelines. Maximal care was taken to minimize the number of experimental animals and any possible discomfort or suffering at all stages of the study.
2.2. Surgical preparations
Surgical procedures for electrochemical assessment of oxygen have been described in detail elsewhere (Solis et al., 2017a,b). Briefly put, under general anesthesia (ketamine HCl and xylazine HCl 80 and 8 mg/kg, respectively, with additional boosting at 30% of initial doses as necessary), rats were chronically implanted with Pt-Ir oxygen sensors (Model 7002–02; Pinnacle Technology, Inc., Lawrence, KS, USA) in two locations. The first sensor was implanted in the medial segment of the NAc [AP +1.2 mm, ML ±0.8 mm, and DV +7.2–7.6 mm from the skull surface, according to coordinates from the rat brain atlas (Paxinos and Watson, 1998) and the second sensor was implanted in the SC space in the frontal area of the rat’s head. This area is densely vascularized, and the sensor implanted in this area does not move during behavioral activation, so it provides artifact-free electrochemical recording. Both sensors were secured with dental acrylic to three stainless steel screws threaded into the skull. During the same surgery, rats were implanted with a chronic jugular catheter, which ran subcutaneously to the head mount. Rats were allowed a minimum of 5 days of post-operative recovery and at least 3 daily habituation sessions (~6 h each) to the recording environment. Jugular catheters were flushed daily with 0.2 ml heparinized saline to maintain patency.
2.3. Electrochemical detection of oxygen
For in vivo oxygen detection we used Pinnacle oxygen sensors coupled with high-speed amperometry. The principles of electrochemical oxygen detection, construction of oxygen sensors, and their calibration were described in detail elsewhere (Kiyatkin, 2019; Solis et al., 2018).
2.4. Experimental procedures
This manuscript describes the results of two experiments conducted in two groups of rats, which underwent the same surgeries but received different drug treatments. The timelines of these experiments are shown in Supplementary materials (Fig. S1 and Fig. S2). At the onset of each recording session, rats were briefly anesthetized (<2 min) with isoflurane and electrochemical sensors were connected to the potentiostat via an electrically shielded flexible cable and a multi-channel electrical swivel. A catheter extension mounted on the cable was used to allow for stress- and cue-free drug delivery from outside the cage. Since two drugs were used in most recording sessions, they were delivered via two separate catheter extensions, thus limiting possible contamination of one drug by another drug. Testing began 90–120 min after the electrochemical sensors were connected to the recording instrument, allowing for baseline currents to stabilize. Then, for subsequent 4–6 hours rats received drug treatment, which were different in each experiment. After treatments were completed, rats were lightly anesthetized by isoflurane and disconnected from the potentiostat. Rats were allowed to recover from anesthesia and their jugular catheters were flushed with heparinized saline before being returned to the animal colony.
In both experiments, we used two drugs: fentanyl (fentanyl citrate Injection 50 μg/mL; Hospira Inc., Lake Forest, IL) and naloxone (naloxone HCl; Sigma-Aldrich), which were delivered via slow, ~20-s iv injections. In the first experiment, rats received two types of treatment (Naloxone-Fentanyl and Fentanyl-Naloxone) for at least 4 daily sessions. In “Naloxone-Fentanyl” sessions, rats received three fentanyl injections at a 20 μg/kg (60 nM) dose and one injection of naloxone (0.2 mg/kg) delivered 10 min before the second fentanyl injection. In the “Fentanyl-Naloxone” sessions, rats received 4 fentanyl injections at the same 20 μg/kg dose and one naloxone injection at a 0.2 mg/kg dose delivered 10 min after the second fentanyl injection. Two additional fentanyl injections were made at 90- and 150-min post-naloxone to observe the pattern and duration of naloxone’s effects on fentanyl-induced oxygen responses. The order of these treatments was counterbalanced, and each rat received two different drug sessions twice. However, in some sessions we were unable to maintain this protocol due to technical problems with recording quality from one of two sensors or catheter patency.
Based on our previous measurements (Solis et al., 2017c, 2018), the 20 μg/kg dose of fentanyl used in this experiment can be viewed as a modest one resulting in about 50% decrease in brain oxygen levels. This dose is close to generally accepted human lethal dose (2 mg or 29 μg/kg; Dahan et al., 2005), but early data on LD50 for iv administration in rats are much higher (2.91 mg/kg, Van Bever et al., 1974; 1 mg/kg, von Gunten et al., 2010). However, this dose is much larger than that used clinically as an analgesic drug (50–100 μg/70 kg) and it is also larger than doses optimal for maintaining self-administration behavior in rats (3 μg/kg; Wade et a., 2010). The real LD50 for iv fentanyl administration in humans is unknown and its presumed values can greatly vary, being dependent on multiple individual factors, previous use of other neuroactive drugs, and possible contaminations of fentanyl by other less or more potent opioids and other neuroactive drugs. As shown previously, iv fentanyl used in drug-naive conditions dose-dependently decreases NAc oxygen levels, with minimal but significant effects at a 3 μg/kg dose and very strong effects (drop up to 30% of baseline) but no lethality at 40 μg/kg (Solis et al., 2018). The 0.2 mg/kg dose of naloxone chosen in this experiment in higher than used in clinical practice (1–8 mg/70 kg; Lynn and Galinkin, 2018; Moss and Carlo, 2019; Kang et al., 2022) but can be viewed as a moderate one in rats. Naloxone at this dose fully blocks brain hypoxia, brain and body hyperthermia and peripheral vasoconstriction induced by heroin at a self-administering dose (0.1 mg/kg; Perekopskiy et al., 2020). While in clinical settings naloxone is administered by different routes (iv, subcutaneous, intramuscular, intranasal), iv administration used in this study provides the most rapid onset of action and allows stress-free drug delivery to quietly resting rats that is impossible with other routes of drug administration. While 10 minutes is a sufficient time interval for full manifestation of the effects of iv naloxone in the first treatment protocol (Naloxone-Fentanyl), a 10-min interval for the post-fentanyl naloxone injection in the second treatment protocol (Fentanyl-Naloxone) was chosen as the shortest timing of therapeutic intervention in humans after the appearance of clinical symptoms. In real life, this pre-treatment interval may be much longer.
The second experiment was conducted to clarify the results obtained in the first experiment and examine how the effects of naloxone on fentanyl-induced oxygen changes differ for the larger drug dose (60 μg/kg) within the range of possible human overdose (1–3 mg/70 kg). Six rats assigned to this experiment received two drug treatments. In the first treatment protocol, which served as a control for the first experiment, rats received 4 fentanyl injections at a 20 μg/kg dose and one saline injection 10 minutes after the second fentanyl injection. This experiment was identical to “Fentanyl-Naloxone“ test in the first experiment, but instead of naloxone rats received saline injection. This experiment also provided data on possible effects of repeated fentanyl injections used in the first experiment. Since the effects of fentanyl are dose-dependent (Solis et al., 2018), we also tested how naloxone affects brain and peripheral oxygen responses induced by fentanyl at the dose within the range of possible human overdose. In this case, during the same session rats received two fentanyl injections at a 60 μg/kg dose, which were followed by the injections of either naloxone (0.2 mg/kg) or saline (see experimental timeline in Supplementary materials (Fig. S2).
2.5. Histological verification of electrode placements
When experiments were complete, rats were deeply anesthetized with isoflurane, decapitated, and their brains were extracted and stored in 10% formalin solution. Later, the brains were cut on a cryostat and analyzed for verification of the locations of cerebral implants and possible tissue damage at the area of electrochemical recording.
2.6. Data analysis
Electrochemical data was analyzed in a slow (1-min) and rapid (10-s) time resolution. Because each individual sensor differed in substrate sensitivity, currents were first converted into concentrations (μM) based on sensitivity calibrations provided by the manufacturer and then into percent (%) changes in oxygen concentration. Values from one-minute prior to stimulus presentation and drug injections were averaged and set as the 100% baseline for slow and rapid time-course analyses. One-way repeated measure ANOVA (followed by Fisher LSD post-hoc tests) was used to evaluate statistical significance of drug-induced oxygen responses. To assess the relationships between oxygen changes in the NAc and SC space we used time-dependent correlation analyses, which demonstrate how the change in oxygen in one location (brain) is related to changes in oxygen in other location (periphery). For text clarity, quantitative results of most statistical evaluations are shown in the Table in the Supplemental Materials (Supplementary Table S1).
3. Results
Data presented in this study were obtained in 14 rats (44 daily sessions) equipped with two oxygen sensors chronically implanted in the medial segment of the NAc (later verified histologically) and SC space along with an iv catheter. In four of these sessions, the recording quality was not satisfactory due to technical malfunctions, so the data of these sessions were excluded from further analyses.
Behaviorally, the effects of fentanyl at both doses (20 and 60 μg/kg) were characterized by hypoactivity and muscle rigidity (visualized as stiff flexion of the limb and tail erection). The intensity and duration of hypoactivity, rigidity and visually observed decreases in spontaneous respiration were stronger with higher fentanyl doses. Rats injected with the 60 μg/kg dose also presented occasionally with tonic-clonic-like convulsive movements and high-pitched vocalizations that were observed within the first minute following injection onset. Naloxone given 10 minutes after fentanyl at both doses was followed by an immediate recovery from paralysis, lowered respirations, and sedation; these changes were also more pronounced after fentanyl at a higher dose. Rats who had experienced tonic-clonic-like movements following fentanyl administration did not respond as strongly to naloxone in the reversal of hypoactivity and decreased spontaneous respiration.
3.1. Effects of fentanyl
The effects of fentanyl on oxygen levels in the NAc and SC space were examined in 7 rats during 23 daily sessions. This data sample included only the first fentanyl injections of each session, which were analyzed with slow, 1-min and rapid, 10-s time resolutions.
As can be seen in Fig. 1A, fentanyl at a 20 μg/kg dose induced a biphasic change in NAc oxygen levels (F21,1470=14.67, p<0.001), with a rapid and strong decrease (~54% of baseline) followed by a weaker, slower and more prolonged increase above baseline (~130%). Oxygen levels in the SC space also decreased (F16,1120=22.05, p<0.001), but this decrease was monophasic and stronger than in the brain (nadir at ~34% of baseline). While oxygen levels in the NAc reached nadir at the second minute after injection start, it took 3–4 min to reach nadir in the SC space.
Figure 1.
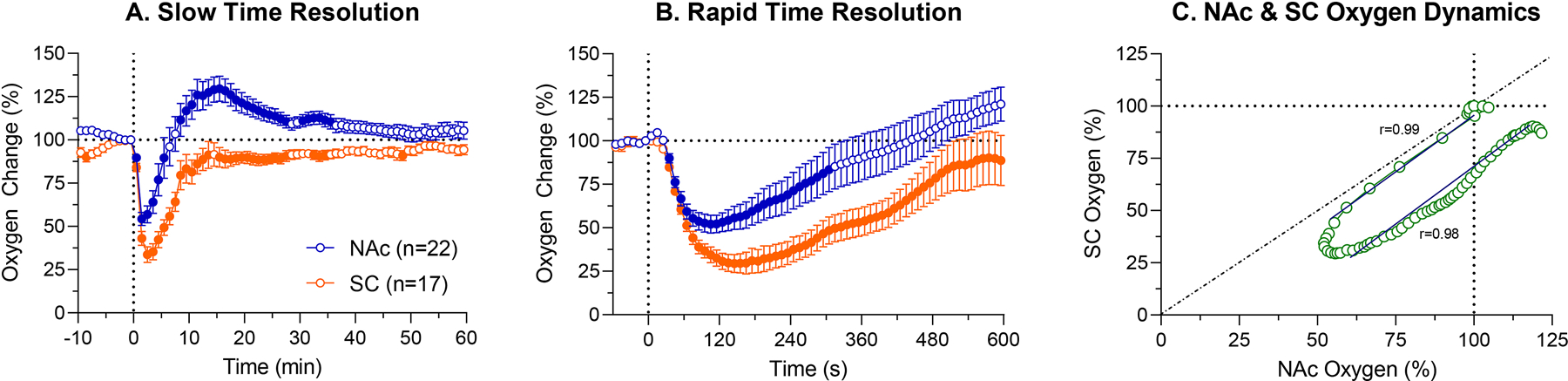
Mean (±SEM) changes in NAc and SC oxygen levels induced by iv injection of fentanyl (20 μg/kg) in awake, freely moving rats. A. = changes averaged with 1-min quantification bin for 60 min post-injection; B. = changes averaged with 10-s quantification bin for 10 min post-injection; C. = Correlative relationships between oxygen changes in the NAc and SC space. Filled symbols show values significantly different (p<0.05) from the last pre-injection value. r = coefficient of correlation.
Since the largest oxygen changes in both locations occurred close to the injection, next we analyzed data with a rapid, 10-s time resolution (Fig. 1B). In this case, oxygen levels in both locations rapidly decreased (NAc: F21,1386=21.84; SC space: F16,1056=34.10; both p<0.001) with a latency ~35 s from the start of a 20-s injection. While both concentration curves were similar during the first minute, they later diverged, with a stronger drop in the SC space and appearance of increase in the NAc. The mean duration of brain hypoxia in this case was ~8 minutes.
To examine the relationships between oxygen changes in the brain and periphery, we used time-dependent correlation analyses, which shows how the change in one parameter is related to changes in another parameter (Fig. 1C). After a ~30 s onset latency for both parameters, both concentration curves decreased with a high negative correlation (r=−0.99, p<0.001). At approximately two minutes, inversion occurred, and both curves correlatively increased from their nadirs toward the pre-injection baseline (r=0.98, p<0.001). In the SC space oxygen levels did not reach baseline suggesting sustained hypoxia in the periphery, but in the brain, oxygen levels increased above baseline, suggesting weak post-hypoxic hyperoxia.
3.2. Naloxone blocks fentanyl-induced hypoxia: differences between the brain and periphery
The first treatment protocol (“Naloxone-Fentanyl”) was used to examine how naloxone injected before fentanyl affects fentanyl-induced oxygen responses. Figure 2A shows mean changes in oxygen levels in the NAc and SC space, when the rat was exposed to fentanyl, naloxone and then injected twice with fentanyl at different time points post-naloxone. These changes are shown in terms of μM oxygen concentrations, which were relatively similar in both the NAc and SC space during the entire recording sessions. Figures 2B–E show relative oxygen responses (percent vs. pre-injection baseline=100%) after each drug injection.
Figure 2.
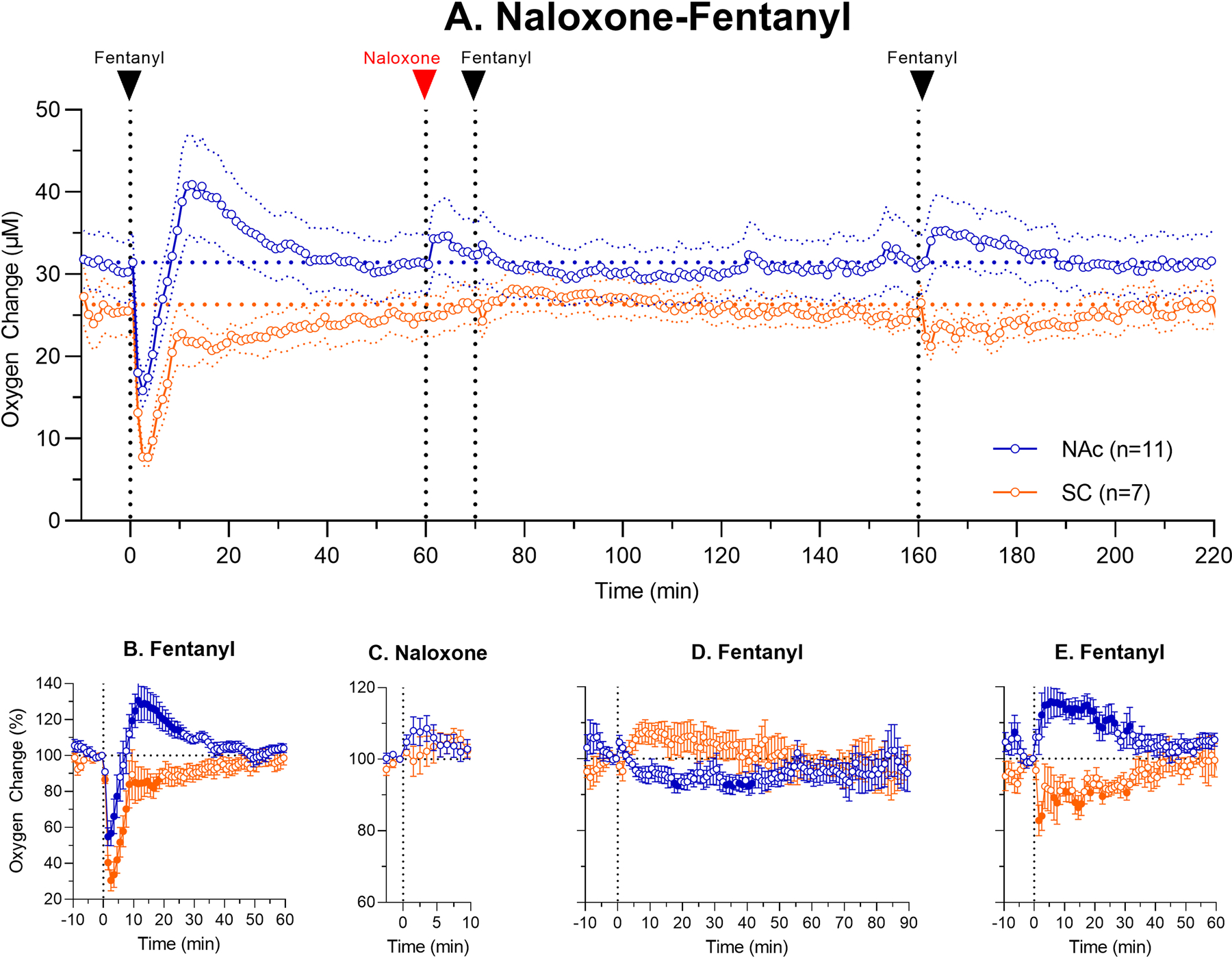
Mean (±SEM) changes in oxygen levels in the NAc and SC space induced by iv fentanyl (20 μg/kg) before and after iv administration of naloxone (0.2 mg/kg). Top graph (A.) shows changes in oxygen levels during the entire session (μM) and lower graphs show relative changes (%) after each subsequent drug injection (B. fentanyl; C. naloxone; D. fentanyl and E. fentanyl). n = numbers of averaged tests. Filled symbols show values significantly different from the pre-injection baseline.
As can be seen, naloxone has no immediate effects on oxygen levels in both the NAc and SC space (Fig. 2C), but brain oxygen levels slightly increased above baseline for ~5 min after the injection. In contrast to the first injection of fentanyl, which induced strong oxygen responses in both NAc and SC space (Fig. 2B), the second fentanyl injection made at 10 min after naloxone, induced minimal changes in both locations (NAc: F10,1000=1.72; SC space: F6,600=1.59; p<0.05 for both). In the NAc, oxygen levels slightly tonically decreased, and in the SC space they tonically increased (Fig. 2D). No signs of hypoxia were also seen after the third injection of fentanyl made 100 min after naloxone injection (Fig. 2E). In this case, fentanyl slightly increased NAc oxygen levels (F10,700=3.42, p<0.001) and decreased these levels in the SC space (F6,420=1.75, p<0.05). Thus, in contrast to brain hypoxia induced by the first injection of fentanyl, this drug injected after naloxone induced weak hyperoxia, while weaker hypoxia was seen in the periphery.
3.3. The effects of naloxone after fentanyl exposure
Figure 3 shows the results of the second treatment protocol, when naloxone was administered 10 min after fentanyl injection. Similar to Fig. 2, top graph (A) shows mean absolute changes in oxygen in each recording location, and lower graphs (B-F) show relative changes after each drug injection.
Figure 3.
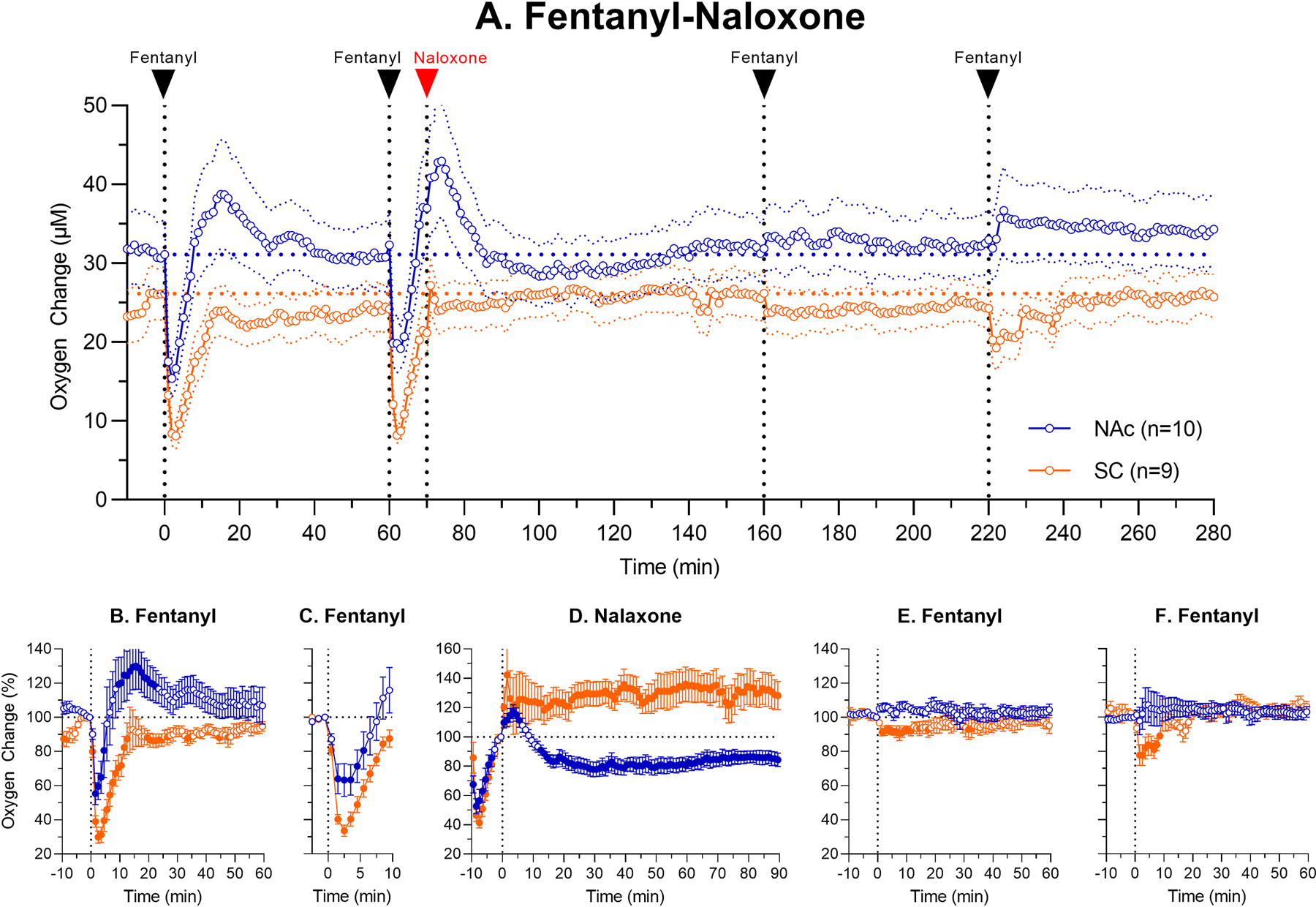
Mean (±SEM) changes in oxygen levels in the NAc and SC space induced by iv fentanyl (20 μg/kg) before and after iv administration of naloxone (0.2 mg/kg). Top graph (A) shows changes in oxygen levels during the entire session ((μM) and lower graphs show relative changes (%) after each subsequent drug injection (B, fentanyl; C, fentanyl; D, naloxone; E, fentanyl and F, fentanyl). Filled symbols show values significantly different from pre-injection baseline (p<0.05).
As can be seen in Fig. 3A–B, the first fentanyl injection induced a typical oxygen response, which was biphasic in the brain and monophasic in the SC space. The second injection of fentanyl induced a similar initial change until naloxone was injected at 10th min post-fentanyl. At this time point, NAc oxygen levels already increased above baseline after their initial decrease, and oxygen levels in the SC space were close to baseline after its initial strong decrease (Fig. 3A, C–D). Although with respect to the baseline at the time of naloxone injection NAc levels tonically decreased and SC levels tonically increased (Fig. 3D), the effect on brain hypoxia was absent because hypoxia already ceased at this time (Fig. 3A). The effect of naloxone on SC oxygen levels was also minimal because oxygen levels in this location were close to the pre-fentanyl baseline at the time of naloxone administration.
Naloxone fully blocked changes in oxygen induced by the third injection administered at 90 min post-naloxone (Fig. 3E). Minimal effects of fentanyl (tendency to increase in the NAc and weak decrease in the SC space) were also found following the last fentanyl injection made at 150 min post-naloxone (Fig. 3F). Therefore, naloxone at this dose has prolonged effects blocking both central and peripheral oxygen responses induced by fentanyl.
3.4. Oxygen responses induced by repeated fentanyl injections: saline control
Data obtained in the first experiment revealed that naloxone injected before fentanyl fully blocks fentanyl-induced oxygen responses for at least two hours. However, the effects of naloxone were minimal when it was injected 10 min post-fentanyl when brain hypoxia had already ceased. Therefore, changes in oxygen observed after naloxone (Fig. 3A, D) could be in fact induced by fentanyl but not naloxone itself. To explore this possibility, we conducted an additional experiment (5 rats in 8 sessions), which was identical to the previous experiment, but instead of naloxone rats received iv saline after the second fentanyl injection.
As can be seen in Figure 4A and B, the first fentanyl injection of a session induced biphasic brain oxygen responses (F7,490=7.82, p<0.001) and more prolonged monophasic decreases in SC oxygen (F7,490=22.41, p<0.001). Similar responses were also seen after each subsequent fentanyl injection (see Table in Supplemental materials), and saline injected 10 min after the second fentanyl injection had no evident effects on oxygen levels in both locations. Like in previous experiment, fentanyl-induced oxygen decreases were stronger and more prolonged in the SC space than in the NAc, where the initial oxygen drop becomes weaker with repeated injections. Like in previous naloxone experiments, brain hypoxia had already ceased and SC hypoxia strongly weakened at 10th min post-fentanyl when saline was injected. When analyzed with respect to a new pre-injection baseline (Fig. 4A, D), we also observed modest decreases in NAc oxygen and tonic increases in SC oxygen levels—the pattern identical to that seen after naloxone in the previous experiment (Fig. 3D).
Figure 4.
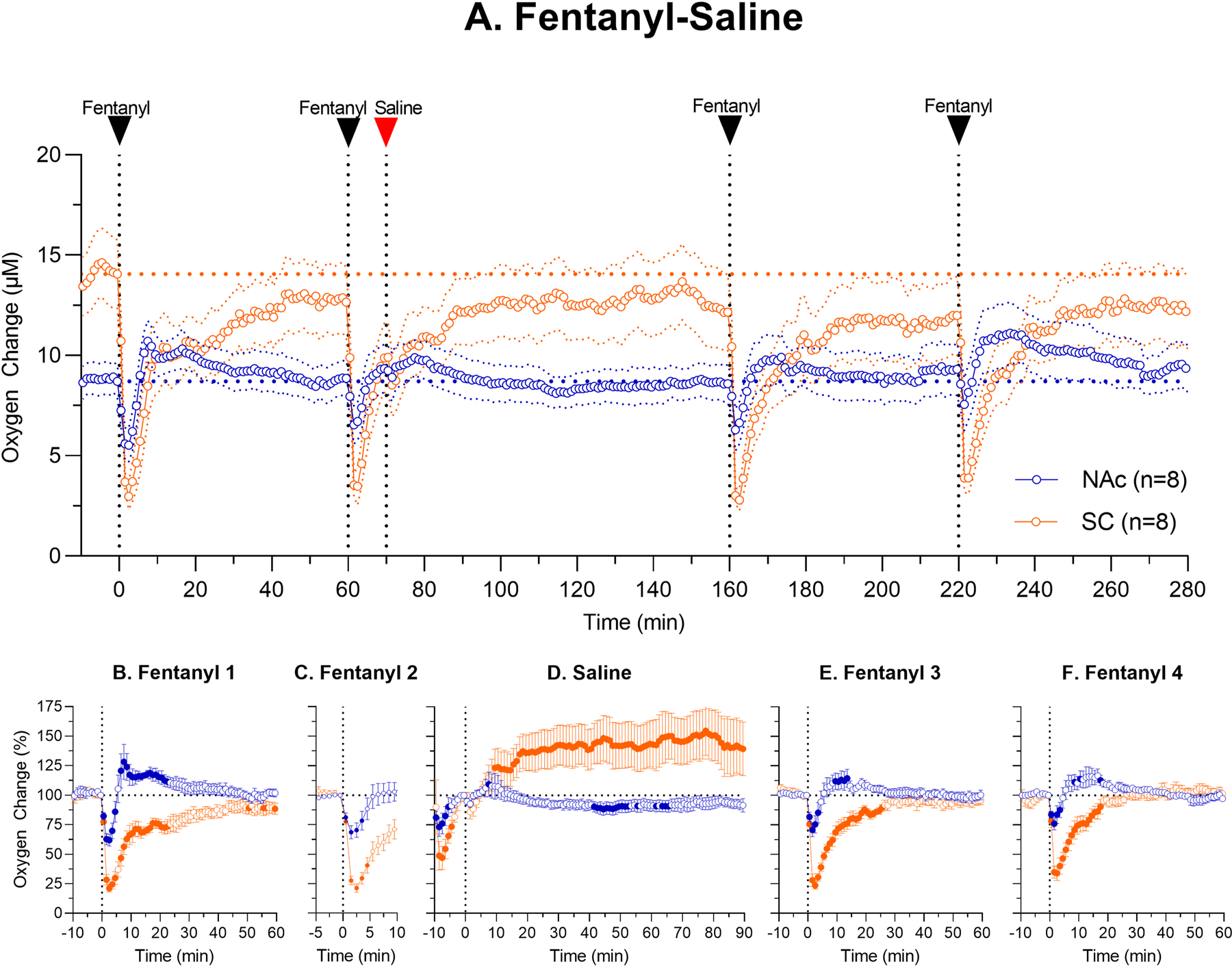
Mean (±SEM) changes in oxygen levels in the NAc and SC space induced by iv fentanyl (20 μg/kg) before and after iv administration of saline. Top graph (A) shows absolute changes in oxygen levels during the entire session (μM) and lower graphs show relative changes after each injection (B, fentanyl; C, fentanyl; D, saline; E, fentanyl and F, fentanyl). n = numbers of averaged tests. Filled symbols show values significantly different from pre-injection baseline.
The similarity in fentanyl-induced oxygen responses after administration of both naloxone and saline may suggest that naloxone administered 10 min after the modest dose of fentanyl has no effects on brain oxygen dynamics. This was confirmed by the direct comparison of relative oxygen responses after injections of naloxone and saline (Fig. 5). In this case, oxygen dynamics in both recording locations was similar and concentration curves were superimposed within the entire 90 min after injections of both naloxone and saline. Minimal differences between two curves were seen within several minutes after the injection, when NAc oxygen levels transiently increased after naloxone when the rat was awakened from fentanyl-induced hypoactivity. However, these between-drug differences were small, and they could be related to differences in effects of fentanyl, which were weaker in the second vs. the first experiment. Quantitative analyses of differences in concentration curves revealed weak, but significant interaction (Time x Injection: NAc F8,120=2.89; SC space F8,120=2.99; both p<0.05), and naloxone-saline difference was significant in the NAc for 5 min after the injection. Minor between-group differences in oxygen dynamics were also seen in the SC space. Oxygen levels slowly returned for ~14 min in the saline group, in the naloxone group they returned to pre-fentanyl baseline within the first minute after its injection.
Figure 5.
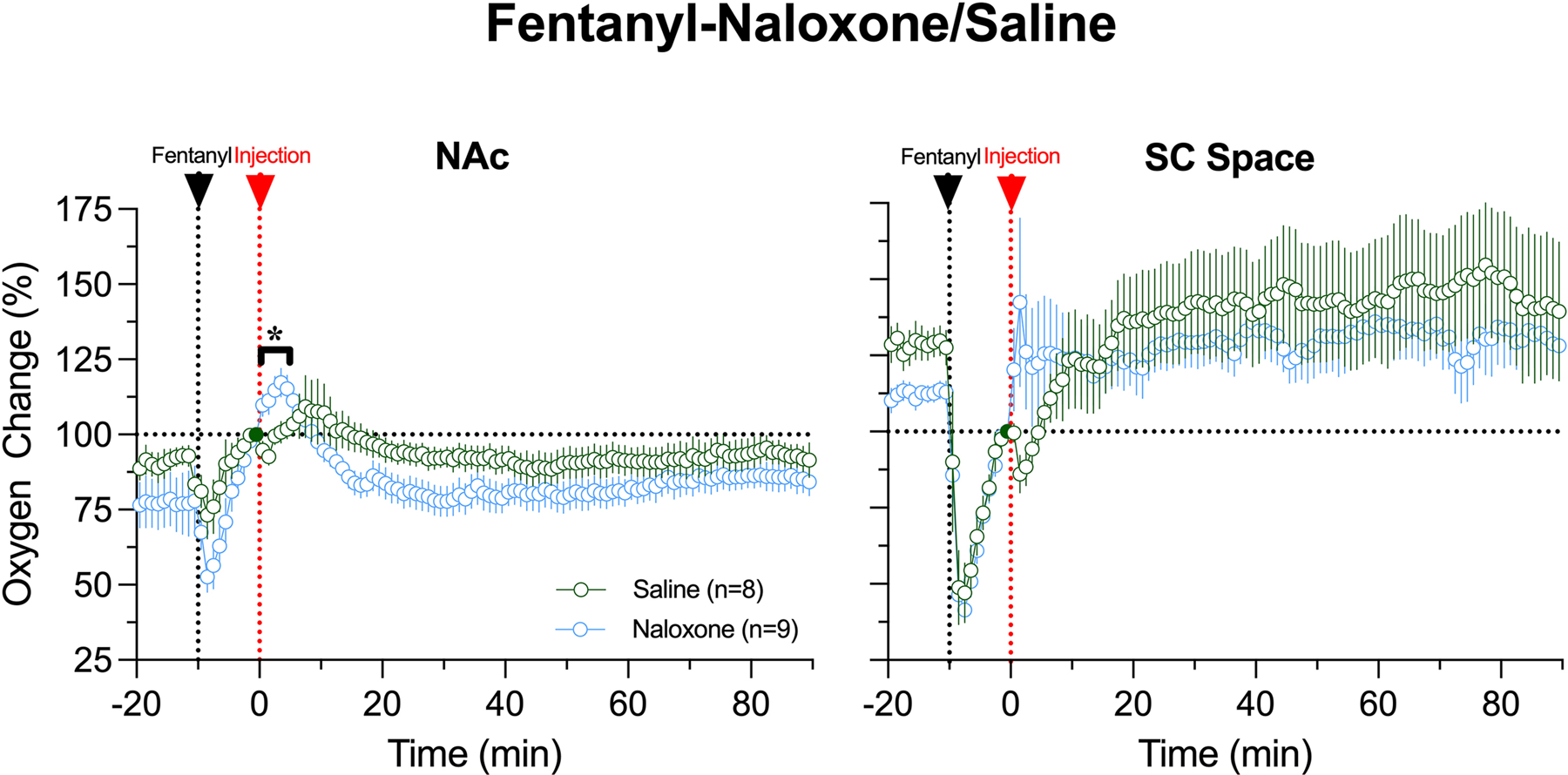
Relative changes in oxygen levels in the NAc (A) and SC space (B) after iv administrations of naloxone and saline analyzed with respect to the levels at 10th minute of fentanyl administration. The first dashed veridical line shows the time of fentanyl injection (−10 min) and the second line shows the time of naloxone or saline injections (0 min). The last pre-injection value (=100%) is shown by filled circle. Black line with asterisk in A shows time interval (~5 min) where two curves were significantly different.
3.5. The effects of naloxone on central and peripheral oxygen responses during fentanyl at a larger dose
Data obtained in the previous experiment revealed that naloxone injected 10 minutes after fentanyl at a modest dose (20 μg/kg), when brain hypoxia had already ceased, has minimal effects on changes in oxygen levels in both the NAc and SC space. However, the effects of fentanyl on oxygen levels are dose-dependent (Solis et al., 2018), and the effects of naloxone could differ after the rats receive fentanyl at a higher dose that induces larger hypoxic responses. To test for this possibility, we examined how naloxone (0.2 mg/kg) will affect oxygen responses induced by fentanyl at a higher dose (60 μg/kg), within the range of possible human overdose. These tests were conducted in 6 rats over 11 sessions. During these sessions, rats received two fentanyl injections with a 120-min inter-injection interval; one injection was followed by naloxone, and another followed by saline. Initially, to exclude the order factor, we planned to counter-balance naloxone and saline tests, but we found that the effects of fentanyl after the use of naloxone are blocked or significantly attenuated for prolonged time interval, making it impossible to conduct the second saline test. Therefore, first in the session we tested the effects of saline and then the effects of naloxone.
Oxygen responses induced by fentanyl at a 60 μg/kg dose followed a similar pattern of changes, with a biphasic, down-up response in the NAc (F10,1010=12.65, p<0.001) and prolonged monophasic decrease in the SC space (F9,909=14.50, p<0.001; Fig. 6 A–B). At a larger fentanyl dose, mean oxygen levels in the SC space dropped to ~20% of baseline, and the return to baseline took ~ 90 minutes; these effects were stronger and more prolonged than those induced by fentanyl at a lower dose (decrease to ~60% below baseline and duration ~35 min). In the NAc, the effects of fentanyl at a 60 μg/kg dose on oxygen levels were also stronger than those with a 20 μg/kg dose and the decrease (~55% of baseline at nadir) was evident only for ~10 minutes vs. ~4 min with a lower dose. The behavioral effects of fentanyl at a 60 μg/kg dose were also stronger and more prolonged that those at a 20 μg/kg dose. Both the amplitude and duration of brain hypoxia seen in individual rats and sessions varied in each dose sample and they were larger for a 60 μg/kg (lowest point: mean=54.2±2.7 %, SD=8.7 % ; range: 43–73 %; duration: 616.0±69.0 s; range 420–960 s) vs. a 20 μg/kg dose (lowest point: mean=69.5±5.6 %; SD=25.5 %; range:31–98 s; duration: mean 340.0±42.0 s; SD=178.2 s; range: 60–540 s).
Figure 6.
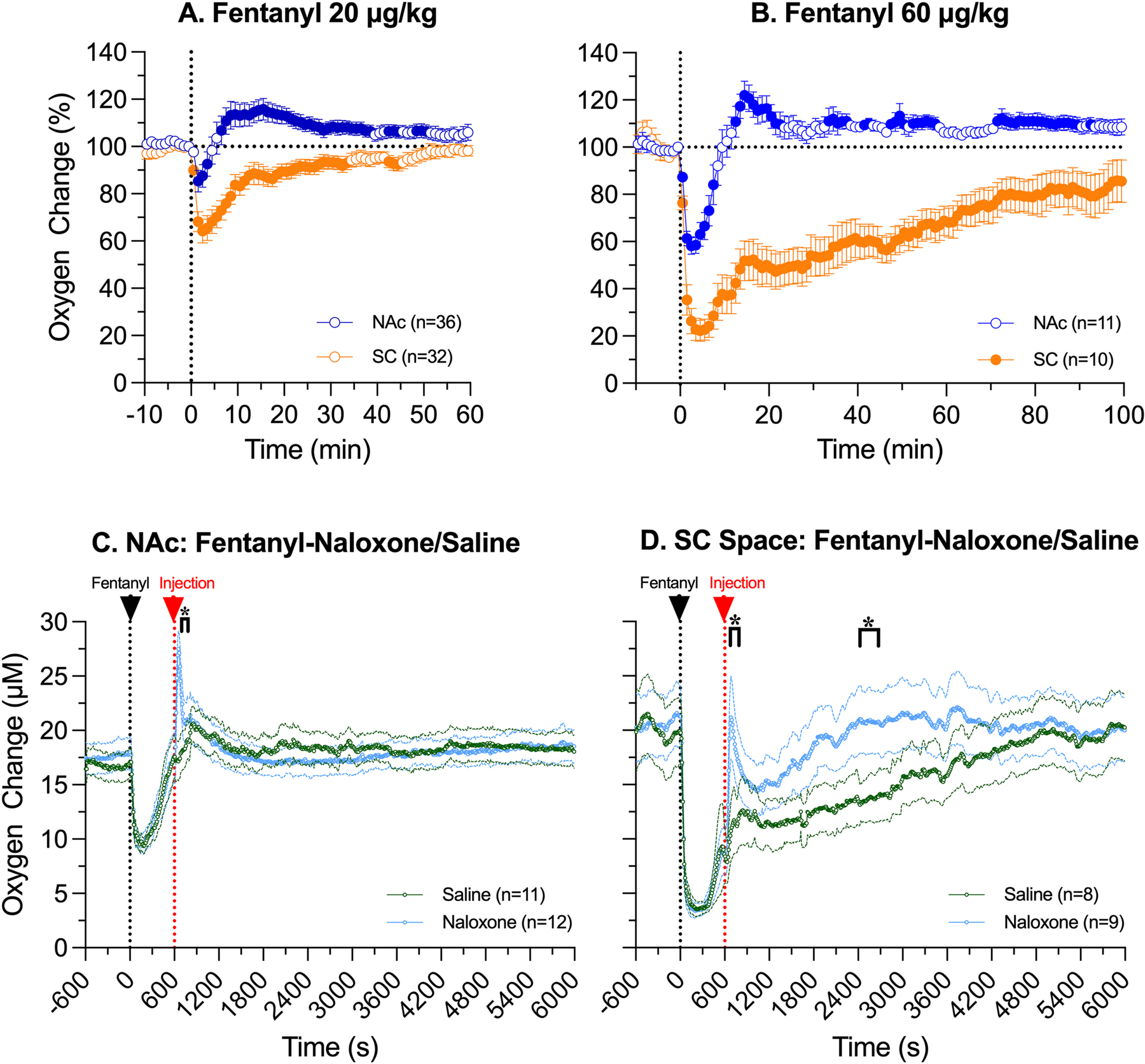
A-B. Relative changes in oxygen levels in the NAc and SC space induced by fentanyl at 20 (A) and 60 μg/kg doses (B). Filled symbols mark values significantly different (p<0.05) from pre-injection baseline. C-D. Changes in oxygen levels (C-NAc; D-SC space) following iv injections of naloxone or saline (time=600 s) 10 minutes after iv injection of fentanyl at 60 μg/kg (time= 0 s). Asterisks show intervals where between-group differences were significant (p<0.05).
The effects of naloxone on oxygen responses induced by fentanyl at a higher, 60 μg/kg dose differed from the effects found at its modest dose, and they were also different in the NAc and SC space (Fig. 6C–D). In the SC space, oxygen levels strongly and similarly decreased after fentanyl injection in both groups, but concentration curves diverged from each other after the injection of naloxone or saline injection, showing significant between-group differences (Two-way ANOVA Time x Treatment interaction: F601,9015=2.53, p<0.001). After saline injection, oxygen levels in the SC space slowly returned to baseline, but they rapidly increased immediately after naloxone injection, when the rat was awakened from deep sedation induced by fentanyl (Fig. 6D). This rapid increase was relatively transient, and it followed by a relative decrease before returning to the pre-fentanyl baseline. However, the total duration of hypoxia (~20 min) in this case was much shorter than that after saline injection (~90 min). In contrast, changes in NAc oxygen after naloxone injection were much weaker, and a significant between-group difference was seen only several minutes after the injection (two-way ANOVA Time x Treatment interaction F601,12621=2.41 p<0.001; Fig. 6C). In this case, NAc oxygen levels rapidly, strongly, but transiently increased above pre-injection baseline within several minutes following naloxone injection when the rat was awakened from fentanyl-induced sedation, while no such increase was seen after saline injection. In both groups, naloxone and saline were injected at 10th min after fentanyl, when NAc oxygen levels already returned to the pre-injection values in both samples.
4. Discussion
While it is known that fentanyl induces respiratory depression, which leads to brain hypoxia, this study provides qualitative and quantitative analyses of fentanyl-induced changes in oxygen levels in both the brain and periphery assessed in rats under physiologically relevant conditions. A similar analysis was conducted for the effects of naloxone, a prototypical drug used to reverse fentanyl-induced hypoxia. Since fentanyl readily crosses the blood-brain barrier, producing exceptionally rapid neural effects, a two-sensor technology and high-resolution analysis of the effects of fentanyl, naloxone, and their interaction allowed us to reveal important differences in drug-induced oxygen dynamics in the brain and periphery. While most technologies used to examine hypoxic effects of opioid drugs assess the mechanical aspects of breathing, use indirect measurements, or examine blood oxygen changes, brain oxygen is a functionally important parameter and drug-induced hypoxia is the primary cause for damage to brain cells and overdose-induced organism’s death. Combination of all these factors allowed us to obtain several novel findings, to substantiate existing findings, and consider essential timing limitations for the use of naloxone--still the best therapeutic tool for attenuating or blocking heath-hazardous effects of fentanyl and other highly potent synthetic opioids. Although our findings were obtained in rats, which have important differences from humans in metabolic activity, basic physiological parameters and drug pharmacokinetics, we believe that these findings are relevant to human conditions.
4.1. Fentanyl: Novel Findings
This study revealed several important features of the effects of fentanyl in the brain (NAc) and periphery (SC space). First, in both recording locations fentanyl rapidly decreased oxygen levels with a ~30–40 s onset latency and maximal change at 2nd and 3rd minutes after the start of a 20-s iv injection. Second, the effects of fentanyl in the brain were essentially biphasic, with the initial rapid, strong, but relatively short-term decrease followed by a weaker, rebound-like increase above the pre-injection baseline. In contrast, fentanyl induced monophasic effects in the SC space, where oxygen levels rapidly and strongly decreased and slowly returned to baseline. The pattern of oxygen responses was similar at modest, behaviorally active doses (20 μg/kg) and at a higher dose (60 μg/kg), but the changes were larger and more prolonged in a latter case. While brain oxygen levels at their nadir dropped to ~50% of baseline, oxygen decrease in the SC space was even stronger, down to 15–20% of baseline.
Plethysmographic studies assessing fentanyl-induced breathing activity produced highly varying estimates for the magnitude and duration of respiratory depression, which depends on the routes of administration, dose and duration of drug delivery, animal species, and their age and weight (Brown and Pleuvry, 1981; Dahan et al., 2005; Hill et al., 2020; Kuo et al., 2015; Laferriere et al., 2005; Varshneya et al., 2022; Seckler et al., 2022). Direct high resolution oxygen measurement in the current study revealed that brain hypoxia assessed in the NAc following iv fentanyl administration in adult rats is strong, but relatively short for a moderate dose of iv fentanyl (~8 min) and becomes only slightly more prolonged for a 60 μg/kg dose (10–12 min, see Fig. 6B). In contrast, fentanyl-induced hypoxia in the periphery is stronger and much more prolonged than in the brain for both doses, with oxygen levels returning to baseline at ~40 and ~80 min post-injection, respectively. While consistent with previous studies (Solis et al., 2017c, 2018), relatively short duration of brain hypoxia and its minor increase with a large dose increase may be surprising. However, such rapid dynamics agrees with the pharmacokinetics of fentanyl examined in humans (Metz et al, 2000). When injected as a 1-s iv bolus at a relatively high analgesic dose (100 μg/70 kg), fentanyl levels in arterial blood peaked at 15 s and rapidly dropped to 10% of a peak at ~150 s. Fentanyl levels in brain tissue increased slightly slower, with~ 35 s to reach 50% of peak brain concentration and 360 s to reach a peak. Similarly rapid pharmacokinetics of iv fentanyl has been shown in rats (Hug and Murphy, 1981). The uptake of fentanyl in this study was extremely rapid with maximum drug levels occurring at or before the first sampling at 1.5 min. Brain hypoxia elicited by iv fentanyl could be longer with other routes of drug administration and following co-use of other neuroactive drugs frequent in recreational settings. As shown previously, a 10% lacing of heroin with fentanyl dramatically enhances duration of brain hypoxia, making it two-fold longer than that with pure heroin (Solis et al., 2017c). Strong potentiation of brain hypoxic effects of heroin also occurred when its injection was preceded by intra-gastric administration of alcohol (Thomas et al., 2021) and midazolam, a benzodiazepine drug (Afzal and Kiyatkin, 2019). The duration of hypoxia could be more prolonged at higher fentanyl doses, but this drug exposure will induce a larger drop in brain oxygen and progressively larger toxic impact of hypoxia on brain cells, decompensation of physiological functions, and organism’s death. Rats can tolerate even strong decreases in brain oxygen if they are transient, but damage of brain cells is greatly progressing if hypoxia becomes prolonged (Bailey, 2019; Busi and Greer, 2010).
While respiratory depression with subsequent drop in blood oxygen is an evident mechanism for fentanyl-induced decreases in oxygen levels in both brain and periphery, two other factors, post-hypoxic brain accumulation of CO2, a powerful vasoconstrictor (Attwell et al., 2010; Battisti-Charbonney et al., 2011; Schmidt and Kety, 1947) and drug-induced peripheral vasoconstriction that results in redistribution of arterial blood from the periphery to the brain and heart (Kiyatkin, 2021) appear to be responsible for post-hypoxic brain hyperoxia induced by fentanyl. These are likely the main factors for cerebral vasodilation/increased cerebral blood flow that enhances oxygen entry into brain tissue despite its lowering in arterial blood. The involvement of these mechanisms explains robust differences in fentanyl-induced oxygen dynamics in the brain and periphery—the appearance of post-hypoxic hyperoxia and much shorter duration of hypoxia in the brain and stronger, much more prolonged hypoxia in the periphery.
A biphasic pattern of brain oxygen response coupled with stronger and more prolonged hypoxia in peripheral tissues is not unique to fentanyl, likely reflecting similarity in the basic mechanisms underlying the effects of highly potent short-acting opioid drugs. Iv heroin within the wild dose ranges (50–600 μg/kg) also rapidly decreased NAc oxygen levels and was also followed by a rebound-like oxygen increase (Afzal and Kiyatkin, 2019; Thomas et al., 2021). However, heroin-induced brain hypoxia was weaker but more prolonged than that for fentanyl at equipotent doses and rats tolerated much higher doses of heroin.
4.2. Naloxone as an efficient blocker of fentanyl’s hypoxic effects
Consistent with our previous studies with heroin (Perekopskiy et al., 2020), naloxone administered at a relatively low dose (0.2 mg/kg or 600 nM) did not affect basal oxygen levels in both brain and periphery but was highly efficient in blocking fentanyl-induced oxygen responses in both locations. In contrast to the pre-naloxone fentanyl injection that induced prominent behavioral effects and strong hypoxia, fentanyl injected 10 min after naloxone did not induce observable behavioral effects and had weak tonic responses with no sign of hypoxia (see Fig. 2D). Brain hypoxia was also absent when fentanyl was injected 100 min after naloxone and brain oxygen levels slightly but significantly increased. At this time point, oxygen levels in the SC space decreased (see Fig. 2E), suggesting re-appearance of weak peripheral hypoxia. Similar changes were found in the second experiment and the effects of fentanyl delivered at 90 and 150 min after naloxone were virtually absent. Only a weak decrease in SC oxygen was evident following the last fentanyl injection in this treatment protocol. Therefore, naloxone has strong and relatively prolonged (>~2-hours) antagonistic effects on fentanyl-induced brain hypoxia but cannot fully prevent peripheral hypoxia at the given dose.
4.3. Naloxone after and too late: A critical timing limitation
As a highly potent blocker of opioid receptors, iv naloxone strongly attenuates or fully blocks multiple behavioral and physiological effects of opioid drugs, including fentanyl. However, the situation becomes more complex in real life when naloxone is administered at a certain variable time after drug exposure and appearance of life-threatening clinical symptoms. In this case, the timing of naloxone delivery (or therapeutic window) becomes the most critical parameter. Developing our treatment protocol, we selected 10 min from iv fentanyl injection as a minimal realistic time when naloxone intervention may begin. While it is generally proclaimed that naloxone treatment should begin as quick as possible, in real-life conditions this post-exposure timing interval greatly varies and could be much longer than 10 min.
As shown in this study, fentanyl in a moderate dose (20 μg/kg) induces strong brain hypoxia, which despite its strength, disappears at about 8 min post-injection and is substituted by a modest oxygen increase when naloxone is injected. In this case, naloxone, which was highly effective to block drug-induced hypoxia when injected before fentanyl, became virtually ineffective because there was nothing to block. Minimal effect of naloxone on brain oxygen levels at this time point was confirmed by control saline injection; changes in brain oxygen levels were identical for both injections (Fig. 5). However, naloxone had weak reversal of fentanyl-induced oxygen decrease in the SC space inducing a transient rise in oxygen and shortening the duration of peripheral hypoxia (see Fig. 3D).
Although naloxone delivered at 10th min after fentanyl had minimal effects on oxygen levels centrally and peripherally, hypoxic effects of fentanyl were strongly attenuated and almost blocked at 90 and 150 min after naloxone (see Fig. 3A), mimicking the effects seen in the trial when fentanyl was delivered after naloxone (see Fig. 2A). Therefore, these findings suggest that naloxone at a modest, human-relevant dose induces strong and prolonged blockade of opioid receptors, thus preventing fentanyl to bind to these receptors and express its behavioral and physiological effects.
Despite more pronounced hypoxia induced by fentanyl at a higher dose (60 μg/kg), its duration in the brain was also relatively short (range 7–16 min), and naloxone was injected when brain oxygen levels were approaching the pre-injection baseline. While ineffective in changing brain hypoxia at this time point, naloxone induced rapid, strong, but transient oxygen increase (hyperoxia), which was related to dramatic changes in animal behavior, which appeared within 15–30 s after the start of naloxone injection. In contrast to the brain, naloxone strongly attenuated peripheral hypoxia. Like in the brain, SC oxygen levels rapidly and strongly increased immediately after naloxone injection. Therefore, in case of iv fentanyl exposure in rats within a wide range of doses there is a limited time window when naloxone “treatment” could be effective. The efficacy of this treatment will progressively decline if naloxone is delivered at times exceeding 8–10 min post-injection. Of course, these quantitative estimates are valid for rats, iv drug delivery, and the range of fentanyl doses used in this study. The therapeutic window of naloxone should be longer in humans, especially when fentanyl base is contaminated with other neuroactive drugs (i.e., alcohol, benzodiazepines, etc.) frequent in real-time recreational settings.
4.4. Limitations of this study
Electrochemical oxygen sensors coupled with high-speed amperometry, were used to examine changes in drug-induced brain oxygenation and clarify their underlying mechanisms. However, the hypoxic effects of opioid drugs are also assessed by plethysmography (Ren et al., 2009), which detects drug-induced decreases in respiratory activity, the primary cause of brain hypoxia. Pulse oximetry is another technique to examine hypoxic effects of opioids (Dahan et al., 2005;), which detect decreases in blood oxygen saturation, an index of diminished oxygen inflow due to respiratory depression. Each of these techniques investigates different parameters and each has own merits and limitations. Therefore, results obtained with these clinically important techniques are uniquely different from electrochemically assessed changes in brain oxygen, which result not only from respiratory depression but from the post-hypoxic changes in the tone of cerebral vessels that oppose decreases in brain oxygenation. The absence of this central contribution may explain the basic similarity between drug-induced monophasic decreases in respiratory activity and decreases in peripheral oxygen levels revealed by electrochemistry. Despite these differences, a recent plethysmographic study of fentanyl (Seckler et al., 2022) revealed basic similarity with our data obtained by using subcutaneous sensor.
Despite the advantages of electrochemical assessments of brain oxygenation in freely moving rats, this technology has also several important limitations. First, in contrast to non-invasive plethysmography and pulse oximetry, which can be used in intact rats, electrochemical studies are relatively complex, requiring a three-part surgical preparation and multi-session recordings in each individual rat. Furthermore, each oxygen sensor has a unique sensitivity, producing highly variable absolute baseline currents and requiring data presentation as a relative change. This complexity of the electrochemical technique affected our experimental protocol and the sample size. While we used iv drug delivery and fentanyl at relatively modest doses, it is may be interesting to examine how our conclusions will hold with other routes of drug administration and higher drug doses within the range of possible overdose. Similarly, we picked only one time interval for post-fentanyl naloxone delivery. While this 10-min time interval was chosen as the minimal time to begin therapeutic intervention, it would be of interest to assess how the fentanyl-induced oxygen response will be affected if naloxone will be administered at shorter or longer time intervals.
4.5. Functional implications
Recent clinical observations suggest that naloxone, to be effective to antagonize fentanyl-induced respiratory depression and revive intoxicated individuals, should be delivered at doses greater than those that are effective to block the effects of other less potent and less rapidly acting opioid drugs such as heroin, oxycodone, and morphine. Current study underscores another critical limitation in using naloxone to treat fentanyl overdose. While fentanyl induced rapid and strong brain hypoxia, this effect is relatively short, greatly succeeding fentanyl-induced behavioral effects and oxygen decreases in peripheral body locations. Due to this transitory nature of fentanyl-induced brain hypoxia, which followed by a rebound-like hyperoxia, the time window, when naloxone is effective to attenuate or block this effect, is relatively short. While the duration of respiratory depression and subsequent hypoxia in humans could be longer than in rats, with other routes of drug administration, and it can be potentiated by concomitant use of other neuroactive drugs (i.e., benzodiazepines. alcohol, ketamine, xylazine, etc.), this timing limitation is critical because naloxone could be very effective when administered quickly, using the most rapid delivery route, and less effective when administered during post-hypoxic comatose state after brain hypoxia is already ceased and harm for neural cells is already done.
Supplementary Material
Preferred variant.
Highlights:
Electrochemistry was used to examine oxygen changes induced by iv fentanyl
Fentanyl induced strong but relatively short (<8–12 min) brain hypoxia and more prolonged hypoxia in the periphery
Iv naloxone (0.2 mg/kg) injected before fentanyl blocked drug-induced oxygen changes in both locations
Naloxone used 10 min post-fentanyl (20 μg/kg) had minimal effects on oxygen levels in both locations
Used at this timing, naloxone strongly attenuated hypoxic effects of fentanyl (60μg/kg) in the periphery with only transient positive effect in the brain
Shorter variant.
Highlights:
Electrochemistry was used to examine fentanyl-induced changes in brain oxygen
Iv fentanyl induced rapid, strong but relatively short (<8–12 min) brain hypoxia
Iv naloxone injected before fentanyl fully blocked brain oxygen responses
Naloxone used 10 min post-fentanyl had minimal effects on brain oxygen changes
In periphery, fentanyl induced prolonged hypoxia, which was attenuated by naloxone
Acknowledgements:
The authors want to thank Dr. Yavin Shaham for valuable suggestions on matters discussed in this manuscript and Shinbe Choi for editorial assistance.
Funding:
The study was supported by the Intramural Research Program of the NIH, NIDA (NIH Grant 1ZIADA000566–12 for Dr. Eugene A. Kiyatkin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
The Authors reports no conflict of interest.
Data availability
Raw data and the results of their primary analyses are available on request from Dr. Eugene A. Kiyatkin (NIDA-IRP, NIH; ekiyatki@intra.nida.nih.gov).
References
- Afzal A, Kiyatkin EA, 2019. Interactions of benzodiazepines with heroin: respiratory depression, temperature effects, and behavior. Neuropharmacology 158, 107677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA, 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y, 2011. Opiate versus psychostimulant addiction: the differences do matter. Nat. Rev. Neurosci 12, 685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, 2019. Oxygen and brain death; back from the brink. Exp. Physiol 104, 1769–79. [DOI] [PubMed] [Google Scholar]
- Battisti-Charbonney A, Fisher JA, Duffin J, 2011. Respiratory, cerebrovascular and cardiovascular responses to isocapnic hypoxia. Respir. Physiol. Neurobiol 179, 259–68. [DOI] [PubMed] [Google Scholar]
- Brown JH, Pleuvry BJ, 1981. Antagonism of the respiratory effects of alfentanil and fentanyl by naloxone in the conscious rabbit. Br. J. Anaesth 10, 1033–7. [DOI] [PubMed] [Google Scholar]
- Busi KM, Greer DM, 2010. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 26, 5–13. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT, 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374, 154–63. [DOI] [PubMed] [Google Scholar]
- Curay CM, Irwin MR,Kiyatkin EA, 2022. Rapid fuctuations in brain oxigenation during glucose-drinking behaviot in trained rats. J. Neurophysiol, 127, 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L et al. 2005. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth 94, 825–34. [DOI] [PubMed] [Google Scholar]
- Hill R, Santhakumar R, Dewey W, Kelly E, Henderson G, 2020. Fentanyl depression of respiration: Comparison with heroin and morphine. Brit. J. Pharmacol 177, 254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug CC, Murphy MR, 1981. Tissue redistribution of fentanyl and termination of its effects in rats. Anesthesiology 55, 369–75. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Knapp CM, Ciraulo DA, 1997. Opiates: Clinical Aspects. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance Abuse, Third edn. Baltimore: Williams & Wilkins,. p. 158–66. [Google Scholar]
- Kang Y, O’Conor KA, Kelleher AC, Ramsey J, Bakhoda A, 2022. Naloxone’s dose‑dependent displacement of [11C]carfentanil and duration of receptor occupancy in the rat brain. Sci. Rep 12, 6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin EA, 2019. Physiological and drug-induced fluctuations in brain oxygen and glucose assessed by substrate-sensitive sensors coupled with high-speed amperometry. In: Wilson GS and Michael AC, editors. Compendium of in vivo monitoring in real-time molecular neuroscience. Volume 3. Probing brain functions, disease and Injury with enhanced optical and electrochemical sensors World Scientific, p. 219–50. [Google Scholar]
- Kiyatkin EA, 2021. Functional role of peripheral vasoconstriction: not only thermoregulation but much more. J. Integr. Neurosci 20, 755–64. [DOI] [PubMed] [Google Scholar]
- Kyo A, Wyse BD, Meutermans W, Smith MT, 2015. In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: no two oopioid have the same profile. Br. J. Pharmacol 172, 532–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferriere A, Colin-Durand J, Moss IR, 2005. Ontogeny of respiratory sensitivity and tolerance to the mu-opioid agonist fentanyl in rat. Brain Res. Dev. Brain. Res 156, 210–7. [DOI] [PubMed] [Google Scholar]
- Lynn RR, Galinkin JL, 2018. Naloxone dosage for opioid reversal: current evidence and clinical implications. Ther. Adv. Drug. Saf 9, 63–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie M, Zed PJ, Ensom MHH, 2016. Opioid pharmacokinetics-pharmacodynamics: clinical implications in acute pain management in trauma. Ann. Pharmacother, 50, 209–18. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY, 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol 14, 69–97. [DOI] [PubMed] [Google Scholar]
- Moody OA, Zhang ER,Arora V, Kato R, Cotton JF,Solt K, 2020. D-amphtamine accelerates recovery of consciousness and respiratory drive after high-dose fentanyl in rats. Front. Phamacol 11–2020, 10.3389/fphar.2020.585356. [DOI] [PMC free article] [PubMed]
- Moss RB, Carlo DJ, 2019. Higher doses of naloxone are needed in the synthetic opioid era. Subst. Abuse Treat. Prev. Policy 14:6. Doi: 10.1186/s13011-019-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K, 2017. Deadly chemistry. Science 355, 1364–6. [DOI] [PubMed] [Google Scholar]
- Pattinson KT, 2008. Opioids and the control of respiration. Br. J. Anaesth 100, 747–58. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1998. The rat brain in stereotaxic coordinates, Academic Press: San Diego. [DOI] [PubMed] [Google Scholar]
- Perekopskiy D, Afzal A, Jackson SN, Muller L, Woods AS, Kiyatkin EA, 2020. The role of peripheral opioid receptors in triggering heroin-induced brain hypoxia. Sci. Rep 10, 833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Ding X, Funk GD, Greer JJ, 2009. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology 110, 1364–1370. [DOI] [PubMed] [Google Scholar]
- Schmidt CF, Kety SS, 1947. Recent studies of cerebral blood flow and cerebral metabolism in man. Trans. Am. Physicians 60, 52–8. [PubMed] [Google Scholar]
- Seckler JM, Grossfield A, May WJ, Getsy PM, Lewis SJ, 2022. Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats. Biomed. Pharmacother 146:112571, doi: 10.1016/j.biopha.2021.112571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, 2018. On the front lines of the opioid epidemic: Rescue by naloxone. Eur. J. Pharmacol 835, 147–53. [DOI] [PubMed] [Google Scholar]
- Skolnick P, 2021.Treatment of overdose in the synthetic opioid era. Pharmacol. Ther Oct 9:108019. doi: 10.1016/j.pharmthera.2021.108019. [DOI] [PubMed] [Google Scholar]
- Peng PW, Sandler AN, 1999. A review of the use of fentanyl analgesia in the management of acute pain in adults. Anesthesiology 90, 576–99. [DOI] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Kiyatkin EA , 2017a. Rapid physiological fluctuations in nucleus accumbens oxygen levels induced by arousing stimuli: relationships with changes in brain glucose and metabolic neural activation. Front. Integr. Neurosci 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Shaham Y, Kiyatkin EA , 2017b. Intravenous heroin induces rapid brain hypoxia and hyperglycemia that precede brain metabolic response. eNeuro 4(3) e0151–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr., Cameron-Burr KT, Kiyatkin EA, 2017c. Heroin contaminated with fentanyl dramatically enhances brain hypoxia and induces brain hypothermia. eNeuro 4(5) e0323–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis E Jr. Cameron-Burr KT, Shaham Y, Kiyatkin EA, 2018. Fentanyl-induced brain hypoxia triggers brain hyperglycemia and biphasic changes in brain temperature. Neuropsychopharmacology 43,810–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Curay CM, Kiyatkin EA, 2021. Relationships between oxygen changes in the brain and periphery following physiological activation and the action of heroin and cocaine. Sci Rep 11:6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SA, Curay CM, Kiyatkin EA, 2021. Effects of alcohol on brain oxygenation and brain hypoxia induced by intravenous heroin. Neuropharmacology 197, 108713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bever WF, Niemegeers CJ, Janssen PA, 1974. Synthetic analgesics. Synthesis and pharmacology of the diastereoisomers of N-(3-methyl-1-(2-phenylethyl)-4-piperidyl)-N-phenylpropanamide and N-(3-methyl-1-(1-methyl-2-phenylethyl)-4-piperidyl)-N-phenylpropanamide. J. Med. Chem 17, 1047–51. [DOI] [PubMed] [Google Scholar]
- Varshneya NB, Hassanien SH, Holt MC, Stevens DL, Layle NK et al. , 2022. Respiratory depressant effects of fentanyl analogs are opioid receptor-mediated. Biochem. Pharmacol 195, 114805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten CF, Bruera E, Pirrello RD, Portenoy RK, 2010. New opioids: expensive distractions or important additions to practice? J. Palliat. Med 13, 505–11. [DOI] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF, 2015. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA, 1987. A psychomotor stimulant theory of addiction. Psychol. Rev 94, 469–92. [PubMed] [Google Scholar]
- Yeadon M, Kitchen I, 1989. Opioids and respiration. Prog. Neurobiol 33, 1–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data and the results of their primary analyses are available on request from Dr. Eugene A. Kiyatkin (NIDA-IRP, NIH; ekiyatki@intra.nida.nih.gov).


