Abstract
Background
As messenger RNA (mRNA)-based vaccines for coronavirus disease 2019 (COVID-19) have been administered to millions of individuals worldwide, cases of de novo and relapsing glomerulonephritis after mRNA COVID-19 vaccination are increasing in the literature. While most previous publications reported glomerulonephritis after the first or second dose of an mRNA vaccine, few reports of glomerulonephritis occurring after the third dose of an mRNA vaccine currently exist.
Case presentation
We report a case of rapidly progressive glomerulonephritis in a patient following the third dose of an mRNA COVID-19 vaccine. A 77-year-old Japanese man with a history of hypertension and atrial fibrillation was referred to our hospital for evaluation of anorexia, pruritus, and lower extremity edema. One year before referral, he received two mRNA vaccines (BNT162b2) for COVID-19. Three months before the visit, he received a third mRNA vaccine (mRNA-1273) for COVID-19. On admission, the patient presented severe renal failure with a serum creatinine level of 16.29 mg/dL, which had increased from 1.67 mg/dL one month earlier, prompting us to initiate hemodialysis. Urinalysis showed nephrotic-range proteinuria and hematuria. Renal biopsy revealed mild mesangial proliferation and expansion, a lobular appearance, and double contours of the glomerular basement membrane. Renal tubules had severe atrophy. Immunofluorescence microscopy showed strong mesangial staining for IgA, IgM, and C3c. Electron microscopy exhibited mesangial and subendothelial electron-dense deposits, leading to a diagnosis of IgA nephropathy with membranoproliferative glomerulonephritis-like changes. The kidney function remained unchanged after steroid therapy.
Conclusions
Although the link between renal lesions and mRNA vaccines remains unclear, a robust immune response induced by mRNA vaccines may play a role in the pathogenesis of glomerulonephritis. Further studies of the immunological effects of mRNA vaccines on the kidney are warranted.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-023-03169-3.
Keywords: IgA nephropathy, Rapidly progressive glomerulonephritis, mRNA COVID-19 vaccine, Hemodialysis, Case report
Background
As an important measure to combat the coronavirus disease 2019 (COVID-19) pandemic, messenger RNA (mRNA)-based vaccines, including BNT162b2 (Pfizer) and mRNA-1273 (Moderna), have been administered to millions of individuals worldwide [1]. Because of the decline in their effectiveness over time, a third dose of the mRNA vaccine has been recommended in many countries [2, 3]. In Japan, vaccination with a third dose started in December 2021, mainly for the elderly population and healthcare workers [4].
Cases of de novo and relapsing glomerulonephritis after mRNA and adenovirus-vectored COVID-19 vaccination have been reported in the literature [5–8]. Although a causal relationship between mRNA vaccines and renal injury has not been proven, the temporal association suggests a potential link [5, 6]. The induction of a robust immune response by mRNA vaccines and potential cross-reactivity between human severe acute respiratory syndrome coronavirus 2 antibodies and human tissue antigens may increase the risk of autoimmunity, potentially leading to the development of glomerulonephritis [5, 9]. Previous case reports and case series have reported glomerulonephritis after the first or second dose of an mRNA vaccine [5–8]. Only a few reports of glomerulonephritis occurring after the third dose of the mRNA vaccine have been published.
Here, we report a case of rapidly progressive glomerulonephritis in an elderly Japanese man a few months after the administration of the third dose of an mRNA vaccine for COVID-19. The patient presented with severe renal failure that required hemodialysis. A renal biopsy revealed immunoglobulin A (IgA) nephropathy with unique membranoproliferative glomerulonephritis-like changes.
Case presentation
A 77-year-old man with a history of hypertension and atrial fibrillation was referred to our hospital for evaluation of anorexia, pruritus, and lower-extremity edema. Six years before the visit, his serum creatinine level was 1.37 mg/dL (estimated glomerular filtration rate (eGFR), 40.4 mL/min/1.73m2), and urinalysis results were negative for proteinuria and hematuria. The cause of renal impairment was not investigated at the time. One year before the visit, he received two doses of an mRNA vaccine (BNT162b2) for COVID-19 without apparent side effects. Three months before the visit, the patient received a third dose of an mRNA vaccine (mRNA-1273) for COVID-19 without immediate side effects. One month before the presentation, he had a scheduled outpatient visit to the Cardiology Department of our hospital, where his serum creatinine level was 1.67 mg/dL (eGFR, 31.8 mL/min/1.73m2). Three weeks before the presentation, he developed edema in the lower extremities and pruritus in his trunk and limbs. His appetite declined two weeks before the visit, and he could not eat for 1 week before the visit. On admission, the patient appeared drowsy. His current medications included bisoprolol (2.5 mg), amlodipine (10 mg), furosemide (20 mg), edoxaban tosylate hydrate (30 mg), and herbal medicine called Shakuyaku-kanzo-to for muscle cramps. The patient did not smoke cigarettes or drink alcohol. He did not report any recent infections. He had never contracted COVID-19 infection previously. On physical examination, he had jugular vein distension, bilateral lower extremity edema, and pruritic rashes on his trunk and limbs. He was afebrile and had a blood pressure of 156/74 mmHg, a pulse rate of 70 beats per minute, and an oxygen saturation of 97% while breathing ambient air. His blood test results showed marked renal impairment (urea nitrogen, 155.3 mg/dL; creatinine, 16.29 mg/dL; eGFR, 2.6 mL/min/1.73m2), hyperkalemia, hyperphosphatemia, hypocalcemia, and metabolic acidosis, which are findings equivalent to end-stage kidney disease (ESKD) (Table 1). Chest X-ray radiography and a chest CT scan demonstrated cardiomegaly and bilateral pleural effusions (shown in Supplementary Fig. 1). Echocardiography did not find evidence of the left ventricular wall asynergy or significant valvular lesions. A computed tomography (CT) scan of the abdomen did not show hydronephrosis or enlargement of the bladder, ruling out a post-renal cause (shown in Supplementary Fig. 1). Apparent tumors were absent on the CT scan. The kidneys were slightly enlarged (right, 113 × 46 mm; left, 119 × 57 mm). Fractional excretions of sodium and urea nitrogen were 2.8% and 27.1%, respectively. The inferior vena cava appeared enlarged on the CT scan. Urinalysis revealed the presence of nephrotic-range proteinuria (8.57 g/g creatinine) and hematuria (Table 1).
Table 1.
Laboratory findings on admission
| Blood counts | Reference range | |||
|---|---|---|---|---|
| White blood cell | 6,400 | /µL | 3,300–8,600 | /µL |
| Red blood cell | 390 | X 104/µL | 435–555 | X 104/µL |
| Hemoglobin | 11.7 | g/dL | 13.7–16.8 | g/dL |
| Platelet | 13.8 | X 104/µL | 15.8–34.8 | X 104/µL |
| Reticulocyte | 11.3 | ‰ | 8.0–22.0 | ‰ |
| Coagulation | Reference range | |||
| PT-INR | 1.12 | 0.90–1.10 | ||
| APTT | 27.5 | seconds | 26.9 ± 25% | seconds |
| Fibrinogen | 433 | mg/dL | 200–400 | mg/dL |
| FDP | 8.3 | µg/mL | < 5.0 | µg/mL |
| Venous blood gas | Reference range | |||
| HCO3− | 12 | mmol/L | 22–26 | mmol/L |
| Blood chemistry | Reference range | |||
| Sodium | 145 | mEq/L | 138–145 | mEq/L |
| Potassium | 6.2 | mEq/L | 3.6–4.8 | mEq/L |
| Chloride | 113 | mEq/L | 101–108 | mEq/L |
| Calcium | 6.8 | mg/dL | 8.8–10.1 | mg/dL |
| Phosphorus | 11.4 | mg/dL | 2.7–4.6 | mg/dL |
| Magnesium | 2.3 | mg/dL | 1.8–2.4 | mg/dL |
| Urea nitrogen | 155.3 | mg/dL | 8.0–20.0 | mg/dL |
| Creatinine | 16.29 | mg/dL | 0.65–1.07 | mg/dL |
| Total protein | 6.8 | g/dL | 6.6–8.1 | g/dL |
| Albumin | 2.9 | g/dL | 4.1–5.1 | g/dL |
| LDH | 373 | U/L | 124–222 | U/L |
| AST | 19 | U/L | 13–30 | U/L |
| ALT | 30 | U/L | 10–42 | U/L |
| ALP | 104 | U/L | 38–113 | U/L |
| γGTP | 93 | U/L | 13–64 | U/L |
| CK | 355 | U/L | 59–248 | U/L |
| CK-MB | 7.9 | ng/mL | ≤ 3.8 | ng/mL |
| Troponin-I | 39 | pg/mL | ≤ 23.4 | pg/mL |
| BNP | 751.9 | pg/mL | ≤ 18.4 | pg/mL |
| C reactive protein | 1.28 | mg/dL | ≤ 0.14 | mg/dL |
| Intact PTH | 250.0 | pg/mL | 10.3–65.9 | pg/mL |
| Total cholesterol | 192 | mg/dL | 142–248 | mg/dL |
| HDL cholesterol | 49 | mg/dL | 38–90 | mg/dL |
| LDL cholesterol | 115 | mg/dL | 65–163 | mg/dL |
| Triglyceride | 111 | mg/dL | 40–234 | mg/dL |
| Blood glucose | 109 | mg/dL | 73–109 | mg/dL |
| HbA1c | 5.4 | % | 4.9–6.0 | % |
| IgG | 1504 | mg/dL | 861–1747 | mg/dL |
| IgA | 347 | mg/dL | 93–393 | mg/dL |
| IgM | 66 | mg/dL | 33–183 | mg/dL |
| Rheumatoid factor | 27.7 | U/mL | ≤ 15.0 | U/mL |
| Anti-nuclear antibody | < 40 | fold | < 40 | fold |
| CH50 | 58 | /mL | 32–58 | U/mL |
| C3 | 92 | mg/dL | 73–138 | mg/dL |
| C4 | 26 | mg/dL | 11–31 | mg/dL |
| Cryoglobulin | Negative | Negative | ||
| ASO | 23 | U/mL | ≤ 239 | U/mL |
| ASK | 80 | fold | ||
| PR3-ANCA | < 1.0 | U/mL | < 3.5 | U/mL |
| MPO-ANCA | < 1.0 | U/mL | < 3.5 | U/mL |
| Anti-GBM antibody | < 2.0 | U/mL | < 3.0 | U/mL |
| Serum electrophoresis | All negative | All negative | ||
| Urine electrophoresis | All negative | All negative | ||
| HBs antibody | Negative | Negative | ||
| HBc antibody | Negative | Negative | ||
| HC antibody | Negative | Negative | ||
| HIV antibody | Negative | Negative | ||
| TP antibody | Negative | Negative | ||
| Urine test | Reference range | |||
| Urine protein | 4 + | Negative | ||
| Urine blood | 3 + | Negative | ||
| Urine white blood cell | Negative | Negative | ||
| Specific gravity | 1.015 | 1.005–1.030 | ||
| Urine pH | 5.5 | 5.0–7.5 | ||
| Urine urobilinogen | ± | ± | ||
| Urine bilirubin | Negative | Negative | ||
| Urine sediment | Reference range | |||
| Red blood cell | > 100 | /hpf | < 4 | /hpf |
| White blood cell | 10–19 | /hpf | < 4 | /hpf |
| Urine chemistry | Reference range | |||
| Urinary protein to creatinine ratio | 8.57 | g/gCr | ||
| NAG | 87.4 | U/L | ≤ 11.5 | U/L |
| Beta-2 microglobulin | 418 | µg/L | ≤ 289 | µg/L |
Based on these findings, rapidly progressive glomerulonephritis was suspected. We started sodium zirconium cyclosilicate and sodium bicarbonate administration on the day of admission; additionally, we initiated hemodialysis the following day. His anorexia and edema gradually resolved thereafter. Subsequent serum and urinary screening tests for glomerulopathy revealed an elevated rheumatoid factor level, and the protein fraction test showed a peak in the gamma region (Table 1). Although multiple myeloma, amyloidosis, and monoclonal gammopathy of renal significance were included in our differential diagnoses, serum and urinary immunofixation electrophoresis did not reveal any M-bows. A bone marrow biopsy did not show any monoclonal proliferation of plasma cells, making it unlikely that hematological disease was the cause of his renal dysfunction. The selectivity index was 0.60, suggesting low selectivity. To identify the cause of his renal dysfunction, a percutaneous renal biopsy was performed.
Renal pathology
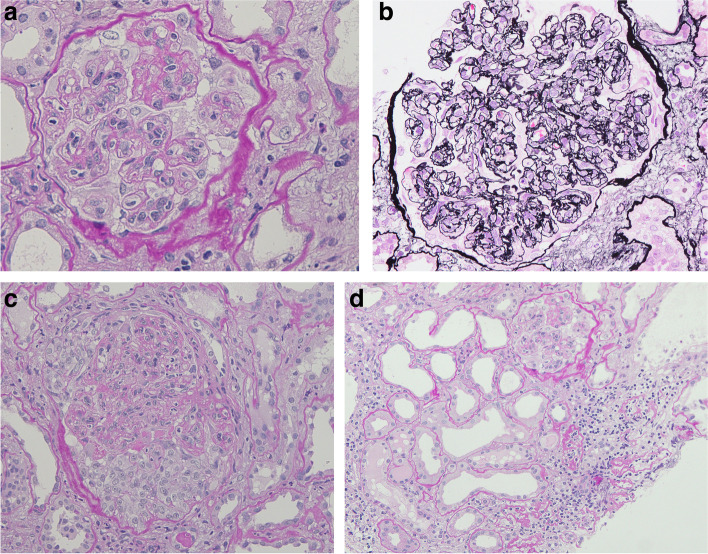
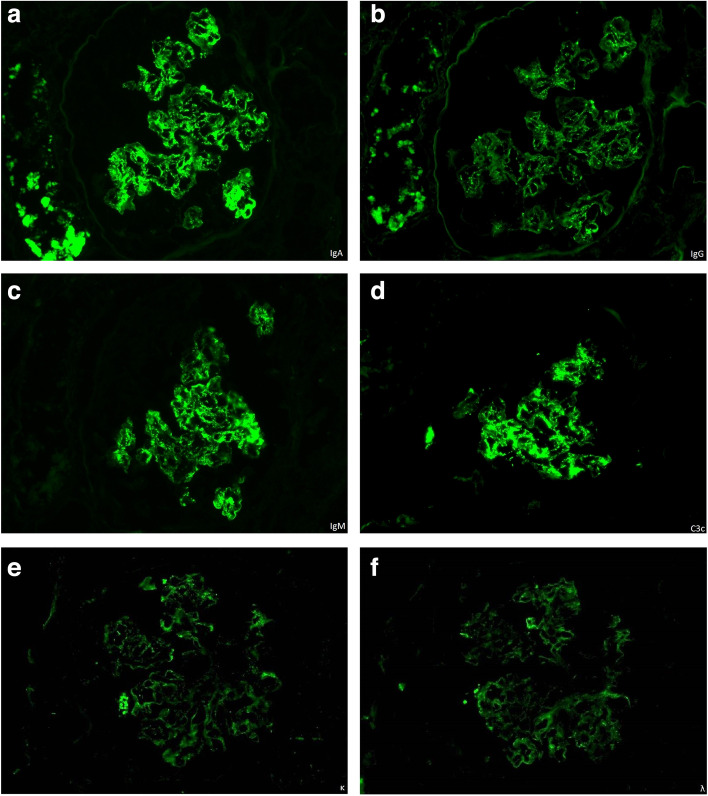
A total of 13 glomeruli were observed on light microscopy. Seven glomeruli showed global sclerosis, and one glomerulus had segmental sclerosis. Most glomeruli exhibited mild mesangial proliferation and expansion with a lobular appearance, with one glomerulus showing a crescent formation (shown in Fig. 1). Double contours of the glomerular basement membrane were present in some of the glomeruli. The renal tubules showed severe atrophy with mild interstitial fibrosis. Congo-red and direct fast scarlet 4BS staining yielded negative results. Immunofluorescence microscopy demonstrated strong mesangial staining of IgA, IgM, and C3c, and weak mesangial staining of IgG (shown in Fig. 2). Positive staining for IgA and C3c was also observed in the basement membrane. Weak staining of kappa and lambda light chains was also present. Electron microscopy revealed the presence of extensive electron-dense deposits in the mesangial regions, with some deposits in the subendothelial regions (shown in Fig. 3). Podocyte effacement was also observed.
Fig. 1.
a Periodic acid Schiff staining of kidney sections from the patient. Mesangial proliferation and expansion with a lobular appearance are observed (original magnification, × 400). b Periodic acid-methenamine-silver staining of kidney sections from the patient. Double contours of the glomerular basement membrane are present (original magnification, × 400). c Periodic acid Schiff staining of kidney sections from the patient. The crescent formation is observed at the bottom of the glomerulus (original magnification, × 200). d Periodic acid Schiff staining of kidney sections from the patient. Renal tubules showed severe atrophy (original magnification, × 200)
Fig. 2.
Immunofluorescence images of kidney sections from the patient. Strong mesangial staining of IgA, IgM, and C3 and weak staining of IgG are observed. Weak staining of kappa and lambda light chains is also observed
Fig. 3.
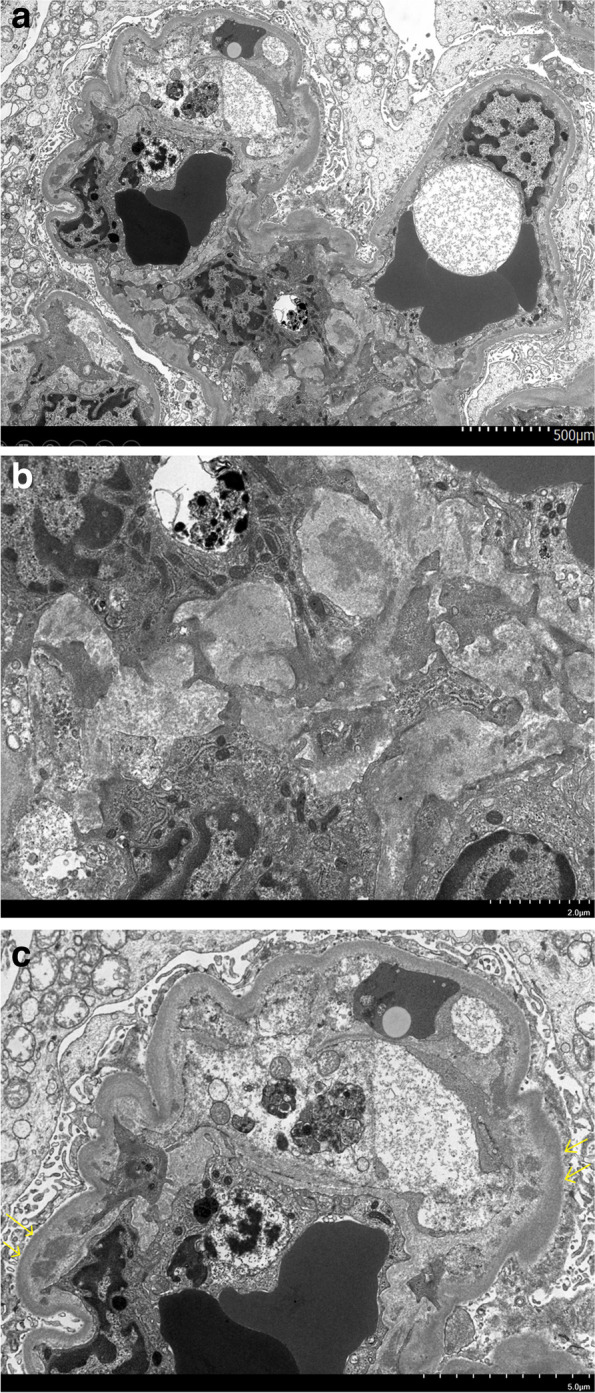
Electron microscopy images of kidney sections from the patient. a Mesangial and subendothelial deposits are visible. b Mesangial deposits are observed (original magnification, × 3000). c Yellow arrows point to subendothelial deposits (original magnification, × 2000)
Differential diagnoses
Considering the presence of proliferative glomerulonephritis with IgA deposits, our differential diagnoses included IgA nephropathy, IgA-dominant postinfectious glomerulonephritis, IgA-dominant membranoproliferative glomerulonephritis (MPGN), and IgA-proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID). Given the absence of monoclonality of immunoglobulins on immunofluorescence, the possibility of PGNMID was unlikely. Postinfectious glomerulonephritis was deemed less likely because of the absence of a recent infection history, normal complement levels, and lack of subepithelial deposits or humps on electron microscopy [10]. Based on the strong staining of IgA, IgM, and C3c in the mesangial regions, the most probable diagnosis was IgA nephropathy. Paraneoplastic IgA nephropathy was less likely as imaging studies and a bone marrow biopsy found no apparent tumor lesions, and his symptoms resolved after starting hemodialysis.
Final diagnosis
IgA nephropathy with MPGN-like changes, grade III(A/C) M1E1S1T1C1.
Treatment and clinical course
Suspecting IgA nephropathy and MPGN based on light microscopy and immunofluorescence findings, we started a 3-day intravenous methylprednisolone pulse followed by 0.8 mg/kg/day (50 mg) of oral prednisolone. Once we found the electron microscopy findings consistent with IgA nephropathy, we administered two additional courses of methylprednisolone pulse therapy and oral steroid therapy. His renal function remained largely unchanged after 1 month of steroid therapy. For hemodialysis vascular access, we created an arteriovenous fistula in the right forearm; however, its maturation was poor. Another attempt to create a fistula was unsuccessful. Eventually, a long-term vascular catheter was inserted in the right jugular vein. The dose of prednisolone was reduced to 15 mg/day, and the patient was discharged without any symptoms on Day 51.
Discussion and conclusions
We report a case of rapidly progressive renal dysfunction in an elderly patient following three doses of mRNA vaccines. Our patient exhibited three unique features. First, the rate of decline in renal function was very rapid. Second, renal pathology revealed mesangial IgA deposition with MPGN-like changes. Third, he received the third dose of an mRNA COVID-19 vaccine 3 months before presentation to our hospital.
The patient’s clinical course and pathological findings supported the diagnosis of rapidly progressive IgA nephropathy, defined as IgA nephropathy with > 50% decline in the eGFR within 3 months after excluding reversible causes and other causes of RPGN [11]. Notably, our patient's renal function decline rate was much faster than that reported in previous studies [12, 13]. For example, a Japanese study of rapidly progressive IgA nephropathy showed a mean initial serum creatinine level of 1.5 mg/dL [12]. One potential reason for the rapid decline in renal function was the concomitant presence of significant tubular damage, as indicated by severe tubular atrophy on renal pathology and marked elevation of renal tubular markers. One contributing factor was decreased renal perfusion, as suggested by the reduced fractional excretion of urea nitrogen. We considered the possibility of dehydration because of anorexia before admission, intravascular volume reduction caused by hypoalbuminemia resulting from nephrotic syndrome, and heart failure. However, he reported that he had drunk fluids before admission. Additionally, it is questionable whether his mild hypoalbuminemia significantly reduced intravascular volume. While his heart failure could cause renal impairment, the patient was hemodynamically stable without apparent left ventricular wall asynergy or significant valvular lesions, suggesting the possibility of the cardio-renal syndrome was less likely. Another factor that may cause tubular damage is the administration of new medications; however, the patient did not recall the use of new medications during recent months. We were unable to identify the cause of significant tubular damage.
Uniquely, our patient’s renal pathology showed mesangial IgA deposition and electron-dense deposits in the mesangial and subendothelial regions with double contours of the basement membrane. Relatively few previous reports have presented similar pathological findings [14, 15]. One case report described a 55-year-old man who presented with rapidly progressive renal failure and proteinuria in the nephrotic range and demonstrated IgA and C3 deposits on immunofluorescence and extensive subendothelial electron-dense deposits with double contours of the basement membrane on electron microscopy [14]. While the possibility of both IgA-dominant MPGN and IgA nephropathy with an MPGN-like pattern existed, the final diagnosis was IgA-dominant MPGN due to the lack of mesangial/paramesangial deposits. Another report of two children showed deposition of IgA and C3 along the capillary wall of glomeruli with type-I MPGN histological findings [15]. One previous study of 244 patients with IgA nephropathy found no cases with MPGN-like changes, whereas subepithelial and/or subendothelial deposits were observed in 23% of cases [16]. Considering these prior studies, IgA nephropathy with an MPGN-like pattern, as observed in our patient, is likely to be very rare.
Although the most probable diagnosis was IgA nephropathy, the possibility of IgA-dominant postinfectious glomerulonephritis, which occurs most frequently in elderly men and can cause acute renal failure with hematuria and proteinuria [10], could not be excluded. Some previous reports showed atypical cases of postinfectious glomerulonephritis, which did not entail hypocomplementemia or a recent infection history [10, 17, 18], similar to our patient’s presentation. While the overlapping clinical and pathological features of IgA nephropathy, IgA-dominant postinfectious glomerulonephritis, and IgA-dominant MPGN posed a diagnostic challenge [14, 17], we considered IgA nephropathy with MPGN-like changes most likely given the presence of mesangial and subendothelial deposits, strong staining for IgA, normal complement levels, and lack of an overt infection [10].
One potential trigger of glomerulonephritis in our patient was the administration of the third dose of an mRNA vaccine. Recent reports have described de novo and relapsing IgA nephropathy following the first or second dose of an mRNA vaccine [5, 6]. Cases of rapidly progressive IgA nephropathy within 3 months of COVID-19 vaccination have also been reported [5, 19]. Two elderly men developed rapidly progressive IgA nephropathy after the first dose of the adenovirus vector vaccine (Astra Zeneca) [5]. Both patients presented with renal failure and nephrotic syndrome, and one patient required dialysis treatment. One adolescent without a pertinent medical history developed rapidly progressive IgA nephropathy within 24 h after the first dose of the mRNA vaccine [19]. She required hemodialysis temporarily; however, her renal function improved after the initiation of steroid therapy. Another potential trigger of our patient’s glomerulonephritis was the administration of different types of mRNA vaccines (BNT162b2 and mRNA-1273). However, the number of published reports on the topic has been very few, precluding us from evaluating its possibility. One retrospective cohort study in Canada demonstrated an increased risk of glomerulonephritis relapse after the second or third dose of the COVID-19 vaccine [20]. While 10% of patients in the study had received one BNT162b2 dose and one mRNA-1273 dose, the study did not evaluate the effect of multiple vaccine types on the risk of relapse. Whether the administration of different types of mRNA vaccines increases glomerulonephritis risk merits further investigation.
Regarding a plausible mechanism that links mRNA vaccines and IgA nephropathy, some researchers hypothesized that Toll-like receptor (TLR) signaling induced by mRNA vaccines might be implicated in the formation of aberrantly glycosylated IgA1, which could be deposited in the glomeruli and cause glomerulonephritis [21, 22]. One previous study showed that the increased expression of TLR-7, which recognizes single-stranded RNAs in the endosome [23, 24], led to greater production of galactose-deficient IgA1 and inflammatory cytokines in the kidneys [25]. Therefore, single-stranded RNA from mRNA vaccines may activate TLR-7 and have a role in the development of IgA nephropathy. Another plausible mechanism relates to a hypothesized postinfectious glomerulonephritis mechanism: single-stranded RNA from vaccines acts as a superantigen, which binds to the major histocompatibility class II molecules on antigen-presenting cells and T cell receptors, leading to T cell activation and significant inflammation [10, 26]. Further studies are warranted to elucidate the mechanistic link between glomerulonephritis and mRNA vaccines.
Previous studies indicated a poor renal prognosis for patients with IgA nephropathy who had a rapidly progressive course, tubular atrophy, or MPGN-like changes [13, 27, 28]. One multicenter cohort study of 113 Chinese patients with crescentic IgA nephropathy showed that approximately 70% of patients developed end-stage renal disease within 5 years, regardless of immunosuppressive therapy [13]. The initial serum creatinine level strongly predicted ESKD, as > 95% of patients with initial serum creatinine > 6.8 mg/dL developed ESKD during follow-up. Another cohort study of 858 Japanese patients with IgA nephropathy demonstrated a poor response to steroid therapy in patients with renal tubular atrophy [27]. One study of 27 patients with IgA deposition on immunofluorescence and MPGN features found a poor renal prognosis [28]. Our patient’s renal prognosis was likely poor because of his high initial serum creatinine level (16.29 mg/dL) and severe renal tubular atrophy found in renal pathology. Although we decided to initiate steroid therapy based on the rapidly progressive course and a mild degree of interstitial fibrosis, his renal function remained largely unchanged after 1 month of steroid treatment, suggesting a poor renal prognosis.
In summary, we present a case of rapidly progressive IgA nephropathy with MPGN-like lesions in an elderly man after the third dose of an mRNA COVID-19 vaccine. While we do not have evidence proving causality, a strong immune response induced by the mRNA vaccines might be involved in the development of significant renal damage. The accumulation of similar case reports may help elucidate the potential link between mRNA vaccines and glomerulonephritis.
Supplementary Information
Additional file 1: Supplementary Fig. 1. (a) Chest computed tomography (CT) scan. Bilateral pulmonary effusion was present. (b) Abdominal CT scan. Hydronephrosis was absent. Apparent tumor lesions were absent.
Acknowledgements
The authors would like to thank Dr. Suzu Yoshida and Dr. Ayaka Kumagai for their support in clinical practice.
Abbreviations
- COVID-19
Coronavirus disease 2019 (COVID-19)
- mRNA
Messenger RNA
- IgA
Immunoglobulin A
- eGFR
Estimated glomerular filtration rate
- ESKD
End-stage kidney disease
- PT-INR
Prothrombin time-international normalized ratio
- APTT
Activated partial thromboplastin time
- FDP
Fibrinogen degradation products
- LDH
Lactate dehydrogenase
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- ALP
Alkaline phosphatase
- γGTP
Gamma-glutamyl transferase
- CK
Creatine kinase
- BNP
Brain natriuretic peptide
- PTH
Parathyroid hormone
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- HbA1c
Hemoglobin A1c
- ASO
Antistreptolysin O
- ASK
Anti-streptokinase
- ANCA
Antineutrophil cytoplasmic antibody
- GBM
Glomerular basement membrane
- HB
Hepatitis B
- HC
Hepatitis C
- HIV
Human immunodeficiency virus
- TP
Treponema pallidum
- hpf
High power field
- NAG
N-acetyl-beta-D-glucosaminidase
- CT
Computed tomography
- MPGN
Membranoproliferative glomerulonephritis
- PGNMID
Proliferative glomerulonephritis with monoclonal immunoglobulin deposits
- TLR
Toll-like receptor
Authors’ contributions
NM, TM, SS, and HW were in charge of the patient care and made the clinical diagnosis. TT and KO performed the histological examination of the kidney. NM, TM, SS, HW, KS, KM, AY, AH, YA, TF, SM, YM, FA, KS, SI, SN, ES, and SU analyzed the patient's clinical and laboratory findings and decided the treatment plan. NM wrote the initial draft of the manuscript. All authors revised the draft critically for important intellectual content, and read and approved the final manuscript.
Funding
This work did not receive any financial support.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal. The research was conducted ethically in accordance with the Declaration of Helsinki.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):200. doi: 10.1186/s12916-022-02397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monge S, Rojas-Benedicto A, Olmedo C, Mazagatos C, José Sierra M, Limia A, et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022; Available from: http://dx.doi.org/10.1016/S1473-3099(22)00292-4 [DOI] [PMC free article] [PubMed]
- 4.COVID-19 Vaccine Booster Shots (3rd Dose). Japanese Ministry of Health, Labour and Welfare. Accessed on July 23, 2022. Available at: https://www.mhlw.go.jp/stf/covid-19/booster.html
- 5.Fenoglio R, Lalloni S, Marchisio M, Oddone V, De Simone E, Del Vecchio G, et al. New onset biopsy-proven nephropathies after COVID vaccination. Am J Nephrol. 2022;53(4):325–330. doi: 10.1159/000523962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 Vaccination and glomerulonephritis. Kidney Int Rep. 2021;6(12):2969–2978. doi: 10.1016/j.ekir.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo WK, Chan KW. Gross haematuria after mRNA COVID-19 vaccination in two patients with histological and clinical diagnosis of IgA nephropathy. Nephrology. 2022;27(1):110–111. doi: 10.1111/nep.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bomback AS, Kudose S, D’Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78(4):477–480. doi: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2020;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr SH, D’Agati VD. IgA-dominant postinfectious glomerulonephritis: a new twist on an old disease. Nephron Clin Pract. 2011;119(1):c18–25. doi: 10.1159/000324180. [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100(4S):S1–S276. [DOI] [PubMed]
- 12.Shimizu A, Takei T, Moriyama T, Itabashi M, Uchida K, Nitta K. Clinical and pathological studies of IgA nephropathy presenting as a rapidly progressive form of glomerulonephritis. Intern Med. 2013;52(22):2489–2494. doi: 10.2169/internalmedicine.52.0420. [DOI] [PubMed] [Google Scholar]
- 13.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol. 2013;24(12):2118–2125. doi: 10.1681/ASN.2012101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal V, Kaul A, Ranade RS, Sharma RK. Immunoglobulin A dominant membranoproliferative glomerulonephritis in an elderly man: A case report and review of the literature. Indian J Nephrol. 2015;25(3):168–170. doi: 10.4103/0971-4065.145425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iitaka K, Yamamoto A, Ogawa N, Sekine T, Tamai S, Motoyama O. IgA-associated glomerulonephritis with membranoproliferative glomerulonephritis-like pattern in two children. Clin Exp Nephrol. 2003;7(4):284–289. doi: 10.1007/s10157-003-0253-z. [DOI] [PubMed] [Google Scholar]
- 16.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29(6):829–842. doi: 10.1016/S0272-6386(97)90456-X. [DOI] [PubMed] [Google Scholar]
- 17.Wallace E, Maillard N, Ueda H, Hall S, Fatima H, Novak J, et al. Immune profile of IgA-dominant diffuse proliferative glomerulonephritis. Clin Kidney J. 2014;7(5):479–483. doi: 10.1093/ckj/sfu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim YJ, Kim SI, Choi YJ, Kang MW. Immunoglobulin A-dominant postinfectious glomerulonephritis in a patient with acute HIV syndrome. AIDS. 2012;26(13):1725–1726. doi: 10.1097/QAD.0b013e32835612f0. [DOI] [PubMed] [Google Scholar]
- 19.Niel O, Florescu C. IgA nephropathy presenting as rapidly progressive glomerulonephritis following first dose of COVID-19 vaccine. Pediatr Nephrol. 2022;37(2):461–462. doi: 10.1007/s00467-021-05351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canney M, Atiquzzaman M, Cunningham AM, Zheng Y, Er L, Hawken S, et al. A Population-Based Analysis of the Risk of Glomerular Disease Relapse after COVID-19 Vaccination. J Am Soc Nephrol. 2022;33(12):2247–2257. doi: 10.1681/ASN.2022030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makita Y, Suzuki H, Kano T, Takahata A, Julian BA, Novak J, et al. TLR9 activation induces aberrant IgA glycosylation via APRIL- and IL-6-mediated pathways in IgA nephropathy. Kidney Int. 2020;97(2):340–349. doi: 10.1016/j.kint.2019.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nihei Y, Kishi M, Suzuki H, Koizumi A, Yoshida M, Hamaguchi S, et al. IgA nephropathy with gross hematuria following COVID-19 mRNA vaccination. Intern Med. 2022;61(7):1033–1037. doi: 10.2169/internalmedicine.8787-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartorius R, Trovato M, Manco R, D’Apice L, De Berardinis P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. NPJ Vaccines. 2021;6(1):127. doi: 10.1038/s41541-021-00391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng N, Xie K, Ye H, Dong Y, Wang B, Luo N, et al. TLR7 in B cells promotes renal inflammation and Gd-IgA1 synthesis in IgA nephropathy. JCI Insight. 2020;5(14). Available from: 10.1172/jci.insight.136965 [DOI] [PMC free article] [PubMed]
- 26.Koyama A, Kobayashi M, Yamaguchi N, Yamagata K, Takano K, Nakajima M, et al. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int. 1995;47(1):207–216. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 27.Itami S, Moriyama T, Miyabe Y, Karasawa K, Nitta K. A novel scoring system based on Oxford classification indicating steroid therapy use for IgA nephropathy. Kidney Int Rep. 2022;7(1):99–107. doi: 10.1016/j.ekir.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andeen NK, Jefferson JA, Akilesh S, Alpers CE, Bissonnette ML, Finn LS, et al. IgA-dominant glomerulonephritis with a membranoproliferative pattern of injury. Hum Pathol. 2018;81:272–280. doi: 10.1016/j.humpath.2018.06.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Fig. 1. (a) Chest computed tomography (CT) scan. Bilateral pulmonary effusion was present. (b) Abdominal CT scan. Hydronephrosis was absent. Apparent tumor lesions were absent.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.