Abstract
Background:
The purpose of this study was to estimate the lifetime direct medical costs per incident case of genital herpes in the United States.
Methods:
We used medical claims data to construct a cohort of people continuously enrolled in insurance for at least 48 consecutive months between 2010 and 2018. From this cohort, we identified initial genital herpes diagnoses as well as the cost of related clinical visits and medication during the 36 months following an initial diagnosis. Lifetime costs beyond 36 months were estimated based on treatment use patterns observed in the 36 months of follow-up.
Results:
The present value of lifetime direct medical costs of genital herpes was estimated to be $972 per treated case or $165 per infection (2019 dollars), not including costs associated with prevention or treatment of neonatal herpes. The clinical visit at which genital herpes was first diagnosed accounted for 27% of lifetime costs. Subsequent clinical visits and medications related to genital herpes accounted for an additional 13% and 60% of lifetime costs, respectively.
Conclusions:
The results from this study can inform cost-effectiveness analysis of GH control interventions as well as help quantify the cost burden of sexually transmitted infections in the United States.
Keywords: genital herpes, cost, burden of disease, MarketScan, health economics
Short Summary
Based on medical claims data, the average lifetime direct medical cost of genital herpes was estimated to be $972 (per treated case) and $165 (per infection) in 2019 dollars.
Introduction
Genital herpes (GH) is caused by anogenital infection with the herpes simplex virus (HSV). HSV has two distinct serotypes, HSV-1 and HSV-2. In the United States, HSV-2 alone was estimated to have a national seroprevalence of 12.1% in 2016.1 Symptomatic GH is most often characterized by the appearance of painful vesicular-ulcerative lesions. While there are treatments to shorten the duration of symptoms and reduce the frequency of recurrences, GH is a lifelong condition. The Centers for Disease Control and Prevention’s (CDC) 2015 STD Treatment Guidelines recommend GH be treated with suppressive therapy taken continuously or episodic treatment for recurrent flare-ups.2 Despite often being asymptomatic, GH can result in numerous complications, including extragenital lesions, aseptic meningitis, herpes simplex encephalitis, and neonatal herpes through maternal-fetal transmission.3,4 Evidence also suggests that HSV-2 infection may increase the risk of acquiring HIV.5,6
GH carries a cost burden attributable to the direct medical costs of diagnosing and treating infection, as well as potential hospitalization costs for treating sequelae. Because HSV cannot be cured, infection can impose long-term or even lifelong costs such as costs associated with suppressive therapy. Little is known about the lifetime cost of GH. Of the few studies that have examined costs related to GH, most estimated national total costs for a specific calendar year or set of years.7–9 To our knowledge, the most recent study to estimate the lifetime cost per HSV-2 infection was by Fisman and colleagues in 2002.10 More recent papers have cited those 2002 estimates and adjusted them for inflation.11,12 In this study, which was part of a larger project to estimate the incidence, prevalence, and cost of STIs acquired in 2018,13,14 we sought to update and expand upon the existing literature by estimating the direct medial cost per incident case of GH in the United States using expenditure data for GH-related medical care.
Methods
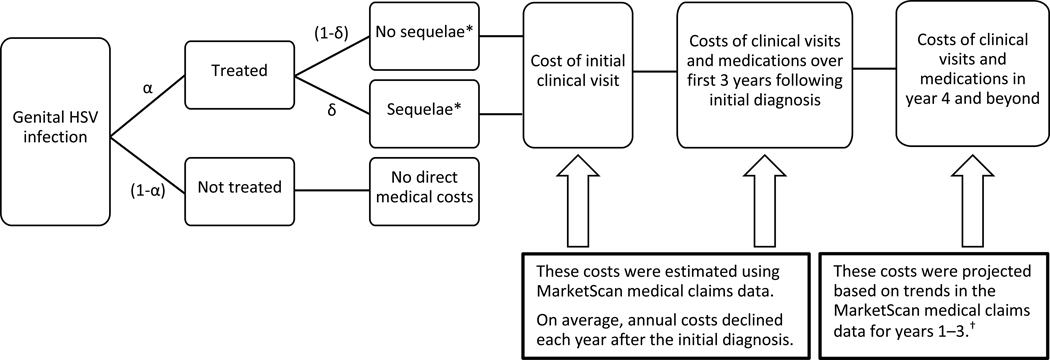
To estimate the lifetime medical cost per GH infection, regardless of whether caused by HSV-1 or HSV-2, we calculated the cost of an initial clinical visit during which GH was first diagnosed, all GH-related costs observed during the three years following that initial clinical visit, and projected costs for years four and beyond (Figure 1). We estimated the cost per treated case of GH separately for cases without sequelae and cases with sequelae. Lifetime cost per infection was calculated based on (1) the probability that an infection would be treated and (2) for treated cases, the probability that sequelae costs would be incurred (Figure 1).
Figure 1:
Cost Calculation Logic Flow
Note: The probability that a given GH infection is treated is denoted by α. The probability that a treated case will involve sequelae is denoted by δ. Both α and δ appear in Equation (2).
* We stratified people by those who did and did not have any of the sequelae listed in Table 1. People with sequelae had higher annual costs on average than those without sequelae.
† We assumed that over the person’s remaining life expectancy, the annual number of GH-related clinical visits and medication costs would continue to decline at the same rate as observed from year two to year three in the MarketScan data.
Data sources and case selection
We obtained data on medical costs related to GH using IBM® Watson Health™ MarketScan Inpatient, Outpatient, Outpatient Pharmaceutical Drug, and Annual Enrollment Commercial Databases for people enrolled in a health plan for at least 48 consecutive months between 2010 and 2018.15 MarketScan databases consisted of over 25 million enrollees with commercial health plans annually. Databases captured person-specific enrollment and medical service information for outpatient visits, outpatient medication prescriptions, inpatient admissions, diagnosis codes, and billing information. MarketScan data consisted of de-identified people with distinct enrollee IDs, allowing people to be anonymously linked across MarketScan datasets. CDC Institutional Review Board exemption status was obtained.
People with GH-related medical expenses between 2011 and 2015 were found using the International Classification of Disease Ninth and Tenth Revisions (ICD-9 and ICD-10). People in the inpatient database could receive a primary diagnosis code for hospital admission and up to 15 additional ICD codes. People in the outpatient database could receive up to four diagnosis codes. To begin, we extracted claims for inpatient and outpatient clinical visits that included any diagnosis code indicating GH or HSV-related sequelae (Table 1). A person could receive a GH-related diagnosis code presumptively, regardless of test positivity, for reimbursement purposes. Given that MarketScan data lacked lab results, we merged people’s clinical visits with their medication claims and assumed any prescriptions a person had for GH treatment medications around the time of a GH-related diagnosis code were indicative of a true case of GH. GH treatment medications were identified by the generic names “acyclovir,” “valacyclovir,” or “famciclovir” as listed in CDC STD treatment guidelines.2,16 As with other recent studies using insurance claims data, we allowed medication claims to fall in a window between seven days before a GH-related diagnosis code up to 30 days after in order to account for delays in processing or data entry errors.17 After we identified people with at least one GH diagnosis code supported by a medication claim, henceforth referred to as “probable GH cases,” we used the date of each person’s earliest medication supported GH diagnosis code as their index date.
Table 1:
Diagnosis Codes Related to GH Infection; International Classification of Disease Ninth and Tenth Revisions (ICD-9 and ICD-10)
| Code | Version | |
|---|---|---|
|
| ||
| Panel A: Codes Used to Identify Initial and Subsequent Visits | ||
| Visits related to treatment of GH | ||
| Genital herpes, unspecified | 054.1 | ICD-9 |
| Genital herpes, genitalia and urogenital tract | A60.0 | ICD-10 |
| Herpes viral infection of perianal skin and rectum | A60.1 | ICD-10 |
| Anogenital herpes viral infection unspecified | A60.9 | ICD-10 |
| Visits related to treatment of sequelae of GH | ||
| Herpetic meningoencephalitis | 054.3 | ICD-9 |
| Herpes simplex with unspecified ophthalmic complications | 054.4 | ICD-9 |
| Herpetic septicemia | 054.5 | ICD-9 |
| Herpes viral meningitis | B00.3 | ICD-10 |
| Herpes viral encephalitis | B00.4 | ICD-10 |
| Herpes viral ocular disease | B00.5 | ICD-10 |
| Disseminated herpes viral disease | B00.7 | ICD-10 |
|
| ||
| Panel B: Additional Codes Used to Identify Subsequent Visits | ||
| Herpes simplex with other specified complications | 054.7 | ICD-9 |
| Herpes simplex with unspecified complication | 054.8 | ICD-9 |
| Herpes simplex without mention of complication | 054.9 | ICD-9 |
| Herpes viral vesicular dermatitis | B00.1 | ICD-10 |
| Other forms of herpes viral infection | B00.8 | ICD-10 |
| Herpes viral infection, unspecified | B00.9 | ICD-10 |
Note: People with diagnosis codes before October 1, 2015 received an ICD-9 diagnosis and those after October 1, 2015 received an ICD-10 diagnosis. Codes listed in this table were inclusive of all sub-codes. For example, 054.4 included 054.40, 054.41 and 054.49. To be retained in our sample, a person needed to have an initial GH diagnosis beginning with one of the codes listed in Panel A. For those people, any future claims were considered subsequent GH-related clinical visits if they were recorded using a diagnosis code beginning with any of the codes listed in Panel A or Panel B. People with obstetrical diagnosis codes were excluded so as not to conflate costs associated with the treatment of GH with costs associated with the prevention of neonatal herpes.
Once all probable GH cases were compiled, we limited our sample to people whose index date likely reflected their first-ever GH diagnosis, not simply the first diagnosis that happened to fall within our sample period. Accurate classification of first-ever GH diagnosis was important for two reasons: 1) because GH is a lifelong condition, the timing of the initial diagnosis determines the length of treatment; and 2) because newly acquired GH tends to decrease in severity over time, the cost of treatment tends to be frontloaded.10,18 To increase the likelihood that people’s index dates reflected new diagnoses, we linked each probable GH case to MarketScan Annual Summary of Enrollment databases and imposed the following criteria: 1) a person needed to be continuously enrolled for 12 months prior to their index date; and 2) they needed to have no medication claims for the aforementioned GH-related antiviral medications during that period. People meeting both criteria were classified as having “confirmed index dates.” To identify patterns in GH-related costs following an initial diagnosis, we retained only people who were also continuously enrolled for 36 months following their confirmed index date. Requiring continuous enrollment ensured that our sample was not missing GH-related medical events due to lapses in coverage. The remaining sample was then linked to all subsequent inpatient, outpatient, and medication claims falling with those 36 months.
Estimating costs associated with GH was complicated by the fact that many HSV-related diagnosis codes a clinician might use to code for GH are ambiguous with respect to site of infection, and could be used to code either genital or oral HSV infections. To increase confidence that our sample consisted of HSV cases which began as anogenital infections, we retained only people with a diagnosis code on their index date that was either unambiguously a code specific to GH or a code for HSV-related sequelae (Table 1, Panel A). Any claims from the inpatient or outpatient claims databases following a person’s index date were considered “subsequent clinical visits” if they contained any of the diagnosis codes listed in Panel A or Panel B of Table 1. Expanding the possible diagnosis codes for subsequent visits allowed us to capture ambiguously-coded GH visits among people determined to have previous anogenital infections. Because costs were not separable by diagnosis code, any clinical visits coded with both GH and non-GH related diagnoses codes were excluded when calculating average costs. People who ever had a clinical visit dropped due to a confounding diagnosis code were excluded from the average number of subsequent clinical visits per year and probability of an initial clinical visit being inpatient. Everyone, regardless of confounding diagnosis codes, was included in estimating the probability that a treated GH infection involved sequelae. Prescriptions from the medication claims database were retained if they were related to the treatment of GH. Finally, any claims with costs ≤ $1 were discarded, as they were likely data entry errors.17,19,20
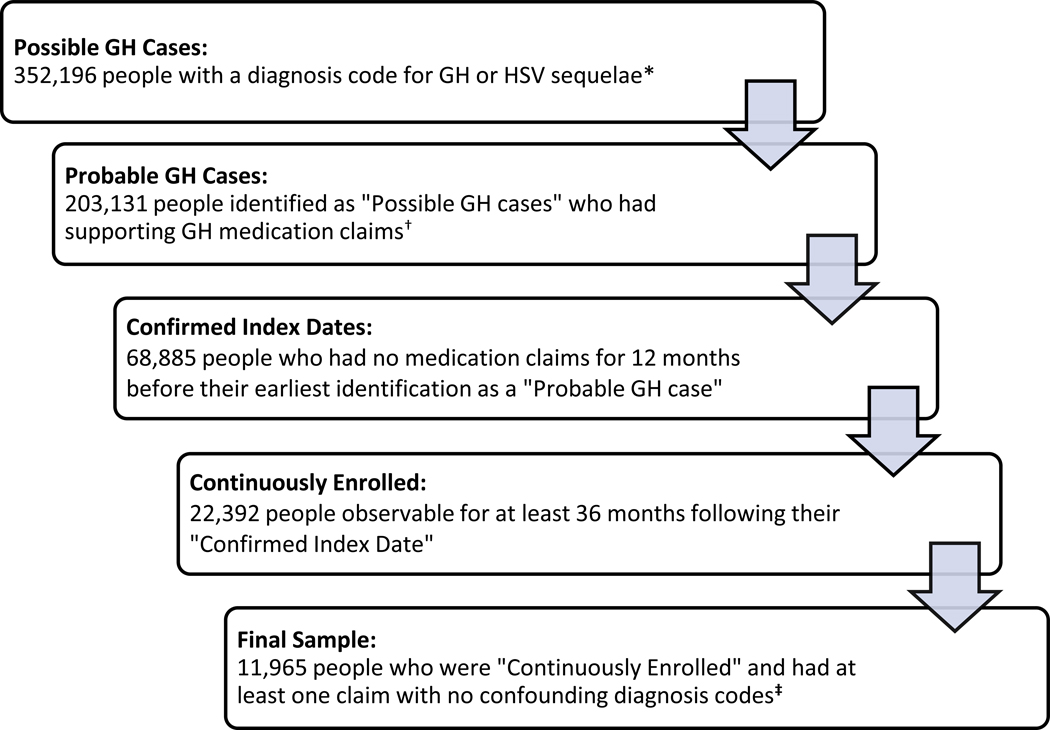
Imposing the restrictions listed above yielded a sample of people with: 1) a probable GH case supported by a GH medication claim; 2) 12 months of continuous observation prior to the first observed probable GH case, during which there were no other GH-related prescriptions or diagnosis codes; 3) 36 months of continuous observation after their first observed probable GH case; and 4) at least one GH-related clinical visit with no confounding diagnosis codes (Figure 2).
Figure 2:
Sample Selection Process; People Ages 15–49 Years; MarketScan Sample 2010–2018
* Diagnosis codes for GH and HSV related sequelae are listed in Table 1.
† A person’s clinical visit was considered to have had a supporting medication claim if that same person had a medication claim listed by the generic name “acyclovir,” “valacyclovir,” or “famciclovir” in the general timeframe of the clinical visit.
‡ People who ever had a visit with codes for both a GH diagnosis and non-GH diagnosis were excluded from the average number of subsequent clinical visits per year and probability of an initial clinical visit being inpatient. Any clinical visits such people had that were free from confounding diagnosis codes were retained in order to inform the average cost per initial or subsequent clinical visit. All people, regardless of the presence of confounding diagnosis codes, were used in estimating the probability that a treated GH infection involved sequelae.
Lifetime cost per treated case of GH
Costs were converted to 2019 dollars using the Personal Consumption Expenditure health component price index, per Dunn et al. 2018.21 Lifetime medical cost per GH infection was estimated by multiplying the probability that a GH infection is treated by the probability-weighted sum of expected lifetime medical cost per treated case of GH with and without sequelae (Figure 1). We computed lifetime costs for each of four separate groups: males who did not experience sequelae, females who did not experience sequelae, males who experienced sequelae, and females who experienced sequelae. For all four groups, lifetime medical cost per treated case was calculated as the sum of 1) the cost of the initial GH-related clinical visit, 2) the costs of subsequent clinical visits and medications related to GH observed in the first three years after the initial visit, and 3) an estimation of the costs of GH-related clinical visits and medications four or more years after the initial visit. The first two components were estimated directly from the MarketScan cohort. Because the cohort was followed for 36 months after the initial visit, we did not have data on costs incurred four or more years after the initial visit. We used the MarketScan data to calculate the annual percent decline in GH-related clinical visits and medication costs between years two and three after an initial diagnosis and assumed this rate of decline would continue in year four through the remaining life expectancy. Specifically, the lifetime medical cost () per treated case of GH was estimated according to the following:
| (1) |
where s denotes male or female sex and x denotes whether a case involved sequelae. is the average cost of an initial GH visit, y denotes year relative to initial diagnosis, and converts future costs to present value by discounting 3% annually. The first three years of follow-up costs were added in the first summation of Equation (1) using sample averages for each year. The average number of subsequent GH-related clinical visits () was multiplied by the average cost of a subsequent clinical visit () then added to the average expenditure on GH-related medications (). While the first summation adds observed costs from MarketScan, the second summation of Equation (1) adds estimated costs from year four after an initial diagnosis up to the average remaining life expectancy () for people of sex s and sequelae status x.22 The second summation in the lifetime medical cost equation is similar to the first summation, except that it replaces the observed year-specific average cost of a subsequent clinical visit with an average across years one, two, and three (). In addition, the second summation also includes two adjustment terms, one to account for decreasing frequency of clinical visits over time () and one to account for decreasing medication expenditures over time ().
Lifetime cost per GH infection
We estimated overall lifetime medical cost per GH infection () by taking the estimated lifetime medical cost per treated case of GH without sequelae multiplied by the probability of having no sequelae plus the estimated lifetime medical cost per treated case of GH with sequelae multiplied by the probability of having sequelae, all multiplied by the probability that a case of GH is treated. The calculation of lifetime costs using the cost per treated case from Equation (1) is mathematically expressed as follows:
| (2) |
where is the probability that someone with a GH infection receives treatment, is the estimated probability that a treated case will involve sequelae, and is the estimated lifetime medical cost per treated case of GH for people of sex s and sequelae status x calculated according to Equation (1). Our base-case value of was 17%, based on a previous estimate of the percentage of GH infections that are symptomatic.10 We applied a base-case probability of sequelae of 15.5% (=15.5%), based on the number of people in our sample with HSV-related sequelae likely attributable to GH divided by the total number of GH cases. In our baseline estimation, a case with HSV-related sequelae was considered likely attributable to GH if a person had any HSV-related diagnosis codes that were not clearly related to oral infection on or prior to the date of their diagnosis code for HSV-related sequelae.
Probabilistic sensitivity analysis
We simulated our sample 10,000 times by redrawing all values for each person from appropriate distributions generated using the true sample characteristics. A person’s initial GH-related clinical visit cost, subsequent clinical visit cost, and medication costs were drawn from log-normal distributions with means and standard deviations equal to the true sample values for the respective variables. The number of subsequent clinical visits was redrawn from a Poisson distribution. Whether a person’s initial clinical visit was inpatient or not was based upon whether a draw from a uniform distribution from 0 to 1 was below or above the sample mean. The probability that a GH infection is treated and the probability that a case involves sequelae were each drawn from beta distributions. The simulated samples were then used to estimate lifetime medical cost per GH infection 10,000 times. The 5th and 95th percentiles of the resulting 10,000 cost estimates were used as the lower and upper bounds of our confidence interval, respectively. These bounds were also used to conduct one-way sensitivity analyses in which each set of parameters within the lifetime medical cost equations were varied one at a time holding all other parameters constant at their base-case values.
Results
From our initial sample of medical claims, we classified 352,196 people ages 15 to 49 years with “possible GH cases” having a diagnosis code for GH or HSV-related sequelae (Figure 2). Of those, 203,131 were further classified as “probable GH cases” due to the presence of a supporting medication claim. 68,885 people were classified as having “confirmed index dates” due to having no related medication claims for 12 months before their earliest identification as a “probable GH case.” 22,392 people classified as having “confirmed index dates” were “continuously enrolled,” meaning they were observable for 36 continuous months following their initial diagnosis. Lastly, clinical visits with potentially confounding diagnosis codes were removed, yielding our final sample of 11,965 people. Our sample had a median age of 33 years at initial diagnosis. At the time of first diagnosis, 59.4% were covered by preferred provider organization (PPO) insurance (Table 2).
Table 2:
Sample Characteristics; People Ages 15–49 Years; MarketScan Sample 2010–2018
| (1) | (2) | (3) | |
|---|---|---|---|
| Females | Males | Total | |
|
| |||
| Median age | 33 | 36 | 33 |
| Share with sequelae | 10.9% | 25.6% | 14.4% |
| Insurance types | |||
| Comprehensive | 1.8% | 2.0% | 1.8% |
| EPO | 1.0% | 0.7% | 0.9% |
| HMO | 13.3% | 12.7% | 13.2% |
| POS | 9.5% | 8.9% | 9.4% |
| PPO | 58.8% | 61.0% | 59.4% |
| CDHD\HDHP | 14.9% | 13.9% | 14.6% |
| N | 9,110 | 2,855 | 11,965 |
Note: Summary statistics reported for age and insurance type reflect the value at the time of initial diagnosis. The unabbreviated insurance types are: comprehensive, exclusive provider organization (EPO), health maintenance organization (HMO), point-of-service (POS), preferred provider organization (PPO), and consumer-driven health plan or high-deductible health plan (CDHD\HDHP). Insurance types do not necessarily sum to 100% due to rounding and missing data.
Costs for people without sequelae
Among people treated for GH who do not develop sequelae, the present value lifetime direct medical cost per treated GH infection was estimated to be $906 for females and $864 for males (Table 3). The average clinical visit associated with an initial GH diagnosis cost $220 for females and $168 for males. Subsequent GH-related clinical visits cost $153 and $146 for females and males, respectively. Females had an average of 0.228 subsequent visits in the first year following initial diagnosis, 0.036 in year two, and 0.029 in year three. Males had an average of 0.249 subsequent visits in the first year, 0.093 in the second, and 0.062 in the third. For females, average expenditure on GH-related medication was $280 in year one, $107 in year two, and $77 in year three. For males, medication costs were $232 in year one, $105 in year two, and $80 in year three.
Table 3:
Estimated Lifetime Medical Costs per Treated Case of GH without Sequelae; People Ages 15–49 Years; MarketScan Sample 2010–2018; 2019 Dollars
| (1) | (2) | (3) | |
|---|---|---|---|
| Females | Males | Total | |
|
| |||
| Initial visit cost (IC) | $220 ($207-$234) | $168 ($155-$171) | $209 ($198-$220) |
| Subsequent visit cost (SC) | $153 ($146-$161) | $146 ($141-$152) | $152 ($146-$158) |
| Number of subsequent visits (SV) | |||
| Year 1 | 0.228 (0.220–0.237) | 0.249 (0.232–0.267) | 0.233 (0.225–0.241) |
| Year 2 | 0.036 (0.035–0.038) | 0.093 (0.086–0.100) | 0.048 (0.046–0.050) |
| Year 3 | 0.029 (0.028–0.030) | 0.062 (0.058–0.067) | 0.036 (0.035–0.037) |
| Antiviral medication cost (DC) | |||
| Year 1 | $280 ($271-$289) | $232 ($216-$250) | $270 ($262-$278) |
| Year 2 | $107 ($104-$110) | $105 ($98-$113) | $107 ($103-$110) |
| Year 3 | $77 ($75-$80) | $80 ($75-$86) | $78 ($76-$80) |
| Observed medical costs for 3 years after initial GH diagnosis | $721 ($701-$741) | $634 ($599-$661) | $703 ($685-$719) |
| Estimated lifetime medical cost per treated case of GH without sequelae () | $906 ($882-$931) | $864 ($814-$907) | $897 ($875-$918) |
| N | 8,114 | 2,125 | 10,239 |
Note: Means (confidence interval). Estimated lifetime medical costs per treated case of GH without sequelae were calculated according to Equation (1). Total lifetime cost cannot be replicated directly from the data in this table because the total lifetime cost includes costs incurred beyond year three.
Costs for people with sequelae
For people treated for GH who develop sequelae, the present value lifetime direct medical cost per treated GH infection was estimated to be $1,488 and $1,225 for females and males, respectively (Table 4). Clinical visits during which GH was first diagnosed cost $638 for females and $490 for males. Subsequent GH-related visits cost $291 and $200 for females and males, respectively. Females had an average of 1.476 subsequent clinical visits in the first year following initial diagnosis, 0.178 in year two, and 0.146 in year three. Males had an average of 1.406 subsequent clinical visits in the first year, 0.230 in the second, and 0.154 in the third. Medication costs for females were $185, $57, and $36 in years one, two, and three, respectively. Medication costs for males were $153, $65, and $37.
Table 4:
Estimated Lifetime Medical Costs per Treated Case of GH with Sequelae; People Ages 15–49 Years; MarketScan Sample 2010–2018; 2019 Dollars
| (1) | (2) | (3) | |
|---|---|---|---|
| Females | Males | Total | |
|
| |||
| Initial visit cost (IC) | $638 ($458-$839) | $490 ($315-$687) | $575 ($446-$712) |
| Subsequent visit cost (SC) | $291 ($223-$389) | $200 ($143-$285) | $253 ($203-$323) |
| Number of subsequent visits (SV) | |||
| Year 1 | 1.476 (1.415–1.539) | 1.406 (1.334–1.477) | 1.446 (1.399–1.494) |
| Year 2 | 0.178 (0.170–0.185) | 0.230 (0.219–0.242) | 0.200 (0.193–0.206) |
| Year 3 | 0.146 (0.140–0.152) | 0.154 (0.146–0.161) | 0.149 (0.144–0.154) |
| Antiviral medication cost (DC) | |||
| Year 1 | $185 ($159-$216) | $153 ($131-$180) | $172 ($154-$193) |
| Year 2 | $57 ($49-$66) | $65 ($55-$76) | $60 ($54-$67) |
| Year 3 | $36 ($31-$42) | $37 ($32-$43) | $36 ($32-$41) |
| Observed medical costs for 3 years after initial GH diagnosis | $1,279 ($1,068-$1,523) | $1,129 ($907-$1,387) | $1,216 ($1,055-$1,393) |
| Estimated lifetime medical cost per treated case of GH with sequelae () | $1,488 ($1,255-$1,764) | $1,225 ($992-$1,500) | $1,377 ($1,202-$1,575) |
| N | 996 | 730 | 1,726 |
Note: Means (confidence interval). Estimated lifetime medical costs per treated case of GH with sequelae were calculated according to Equation (1). Total lifetime cost cannot be replicated directly from the data in this table because the total lifetime cost includes costs incurred beyond year three.
Combined costs
In our sample of “probable GH cases,” 15.5% had sequelae, yielding a probability-weighted average lifetime direct medical cost per treated case of $996 for females, $920 for males, and $972 overall (Table 5). Assuming 17% of GH infections are ever treated, the estimated lifetime direct medical cost per GH infection is $169 for females and $156 for males, or $165 overall.10 On average, the clinical visit at which genital herpes was first diagnosed accounted for 27% of lifetime costs. Subsequent clinical visits and medications related to GH accounted for an additional 13% and 60% of lifetime costs, respectively. In the probabilistic sensitivity analysis, the 5th and 95th percentiles of our 10,000 estimations of the overall lifetime direct medical cost per treated case were $936 and $1,009, respectively. The 5th and 95th percentiles of the cost per infection were $90 and $258, respectively. In one-way sensitivity analyses, our estimated cost per infection was most sensitive to variation in α, the probability that a case of GH is treated. Varying α from 10% to 35% yielded cost per infection estimates ranging from $97 to $340. Individually varying the remaining variables resulted in estimates within 5% of the base case (Table 6).
Table 5:
Estimated Lifetime Medical Costs per GH Infection; People Ages 15–49 Years; MarketScan Sample 2010–2018; 2019 Dollars
| (1) | (2) | (3) | |
|---|---|---|---|
| Females | Males | Total | |
|
| |||
| Estimated lifetime medical cost per treated case of GH without sequelae (Table 3) | $906 ($882-$931) | $864 ($814-$907) | $897 ($875-$918) |
| Estimated lifetime medical cost per treated case of GH with sequelae (Table 4) | $1,488 ($1,255-$1,764) | $1,225 ($992-$1,500) | $1,377 ($1,202-$1,575) |
| Probability that a treated GH case involves sequelae () | 15.5% (11.4%−19.6%) | 15.5% (11.4%−19.6%) | 15.5% (11.4%−19.6%) |
| Overall estimated lifetime medical cost per treated case of GH () | $996 ($950-$1,049) | $920 ($861-$975) | $972 ($936-$1,009) |
| Probability that a GH infection is treated () | 17% (10%−35%) | 17% (10%−35%) | 17% (10%−35%) |
| Estimated lifetime medical cost per GH infection () | $169 ($78-$287) | $156 ($71-$260) | $165 ($90-$258) |
| N | 9,110 | 2,855 | 11,965 |
Note: Means (confidence interval). Estimated lifetime medical costs per treated case of GH both with and without sequelae were calculated according to Equation (1). Estimated lifetime medical costs per GH infection were calculated according to Equation (2).
Table 6:
One-way Sensitivity Analyses: Estimated Lifetime Medical Costs per GH Infection (Total Sample, Females and Males, Ages 15–49 Years) When Varying One Parameter Value At a time, 2019 Dollars
| Parameter varied | (1) | (2) |
|---|---|---|
| Lifetime cost when lower bound value of parameter is applied | Lifetime cost when upper bound value of parameter is applied | |
|
| ||
| None (base-case analysis) | $165 | $165 |
| Probability that a GH infection is treated ()* | $97 | $340 |
| Probability that a treated GH case involves sequelae ()† | $162 | $168 |
| Probability initial clinical visit was inpatient | $159 | $172 |
| Cost of initial inpatient clinical visit | $164 | $166 |
| Cost of initial outpatient clinical visit | $164 | $166 |
| Number of subsequent clinical visits (SV) | $161 | $171 |
| Cost of subsequent clinical visits (SC) | $165 | $165 |
| Cost of medication (DC) | $160 | $170 |
| Rate of decrease in costs years 4 onward (%ΔSV, %ΔDC)‡ | $133 | $455 |
Note: MarketScan Sample 2010–2018. All values listed are estimations of lifetime direct medical cost per GH infection. Unless stated otherwise, all lower and upper bound parameter values applied reflect the 5th and 95th percentiles from 10,000 simulations of the MarketScan sample (as shown in the confidence intervals in Table 3 and Table 4).
The probability that a GH infection is treated () was varied from 10% to 35%, the range of people with HSV-2 estimated to be aware of having a GH infection.29,30 The lifetime cost per infection can be calculated for any value of by multiplying by the lifetime cost per treated case.
The probability that a treated GH case involves sequelae (), was calculated as the number of people in our sample with HSV-related sequelae likely attributable to GH divided by the total number of GH cases. The base case (=15.5%) assumed that initial diagnosis codes ambiguous about the site of infection were likely attributable to GH. The lower bound (=11.4%) assumed that cases with ambiguous coding with respect to site of infection were attributable to GH in the same proportions as unambiguous cases. The upper bound (=19.6%) was assumed to be symmetric to the lower bound with respect to the base-case value.
Lower bound values for percent decreases in subsequent visits and medication costs suppose decreases of 100%. That is, they represent a scenario in which there are no additional costs in years four onward. The upper bound values assume percent decreases of 0%, meaning the number of subsequent visits and the cost of medications remain constant from year three throughout the remainder of a person’s life.
Discussion
Our estimated lifetime direct medical costs per GH infection of $169 and $156 for females and males, respectively, are lower than previous estimates by Fisman and colleagues published in 2002 ($974 and $1,184 after adjusting for inflation). Much of that difference is attributable to Fisman and colleagues’ inclusion of costs associated with lost productivity and neonatal transmission. A more substantive contributor is a discrepancy in the number of subsequent clinical visits per treated GH case. Fisman and colleagues assumed that those on suppressive therapy had a clinical visit every three to six months and those on episodic therapy had a visit every three to five flare-ups (with a median of 4 or 5 flare-ups in year one and an annual decline of 0.7 afterwards). In our insurance claims sample, patients with GH infections had far fewer visits, averaging 0.408 in year one. It may be that people in our sample had more recurrences between visits than Fisman and colleagues expected. For the estimated rates of recurrence, Fisman and colleagues cited studies such as Benedetti (1994) conducted in research clinic settings in Washington, in which follow-up visits were encouraged among study participants and might therefore have been more frequent than is typical in routine practice.23 It might also be that the average number of recurrences is lower at the time of this writing than it was in 2002. More recent work has identified substantial increases in the share of GH infections resulting from HSV-1, which tends to have less frequent recurrences than GH caused by HSV-2.23,24
While we believe these estimates based on medical claims data constitute a meaningful data-driven contribution to the literature on GH-related costs, the use of medical claims data introduces potential limitations. First, medical claims data do not include costs for those who are uninsured or who have public insurance. To the extent that uninsured people diagnosed with GH are less likely to receive ongoing treatment, our estimates would be biased upwards. Related to this point is the exclusion of individuals due to our 36-month continuous enrollment sample criteria. As with uninsured people, those with intermittent coverage may have lower healthcare utilization, and therefore lower lifetime medical costs. One-way sensitivity analyses suggest that bias resulting from overestimation of ongoing medication costs is unlikely to have a substantial impact on the final estimated lifetime cost per GH infection (Table 6). A second limitation of claims data is the lack of information about test results. To address this limitation, we restricted our sample to those people with initial GH diagnosis codes that were accompanied by an appropriate medication claim. Third, because the costs reported in insurance claims were not separable by diagnosis code, clinical visits with non-HSV related diagnosis codes had to be excluded from cost averages. If non-medication related costs of treating GH were higher for visits with confounding diagnosis codes, conditional on sequelae status, then our estimates would be biased downwards. Fourth, pharmaceutical claims data do not indicate what condition a medication was prescribed to treat. We assumed any medications with the generic names “acyclovir,” “valacyclovir,” or “famciclovir” prescribed to people classified with possible GH cases were prescribed for treating GH. This is unlikely to bias our results substantially as these medications are used almost exclusively for treatment relating to herpes viruses. Our estimates of medication costs for cases in which sequelae develop may be underestimated in that they did not include costs of medications that may have been prescribed in addition to the three antiviral medications listed above. However, due to the nature of insurance claims data, our clinical visit cost estimates are inclusive of any medication administered in inpatient settings. Our study could not determine whether medication costs could be attributed to suppressive vs. episodic therapy. Fortunately, this issue does not impact our average lifetime cost estimates, which reflect the average level of use of suppressive and episodic therapy in the population we observed.
It should be noted that while this work focused on direct medical costs associated with the clinical and pharmacological treatment of GH in an infected adolescent or adult, neonatal HSV infection resulting from GH may constitute a substantial cost burden. A recent study using Medicaid data identified 4.5 per 10,000 neonates as being born with HSV infections, resulting in a median Medicaid payout of $93,296 (2019 dollars) within 6 months of birth.4 In addition to not including medical costs associated with prevention or treatment of neonatal herpes, our estimates do not include costs relating to the increased risk of acquiring HIV. Nor do they include costs associated with prevention of GH, such as screening costs, or non-medical costs, such as those arising from GH-induced productivity losses. Perhaps the most substantial cost associated with GH is the psychosocial burden carried by those who are infected. Oftentimes anxieties about the implications of a GH diagnosis for one’s sexual and romantic lives negatively impact mental health, inflicting a nontrivial toll on one’s quality of life.25–28 Future work should build on our estimated direct medical cost by incorporating the various cost domains excluded from this analysis in order to arrive at a more holistic understanding of the burden inflicted by GH. Finally, differences between the estimates presented here and those of previous studies should not be interpreted as changes in the lifetime cost of GH over time, as any discrepancies may be due to methodological differences. We hope that our updated study will prove as useful as these previous studies not only to inform cost-effectiveness analyses of GH control interventions but also to help quantify the cost burden of sexually transmitted infections in the United States.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflicts of Interest and Source of Funding: The authors declared no conflicts of interest.
References
- 1.McQuillan G, Kruszon-Moran D, Flagg EW, Paulose-Ram R. Prevalence of Herpes Simplex Virus Type 1 and Type 2 in Persons Aged 14–49: United States, 2015–2016. NCHS Data Brief 2018; (304): 1–8. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines. MMWR Morb Mortal Wkly Rep 2015. 2015; 64(No. RR-3): 1–137.25590678 [Google Scholar]
- 3.Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes 2004; 11 Suppl 2: 57A–64A. [PubMed] [Google Scholar]
- 4.Mahant S, Hall M, Schondelmeyer AC, Berry JG, Kimberlin DW, Shah SS. Neonatal Herpes Simplex Virus Infection Among Medicaid-Enrolled Children: 2009–2015. Pediatrics 2019; 143(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey L, Wald A. Genital Herpes. In: Holmes K, Sparling F, Stamm W, et al. , eds. Sexually Transmitted Diseases. 4th ed. New York, NY: McGraw-Hill; 2008: 399–437. [Google Scholar]
- 6.Looker KJ, Elmes JAR, Gottlieb SL, et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis 2017; 17(12): 1303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szucs TD, Berger K, Fisman DN, Harbarth S. The estimated economic burden of genital herpes in the United States. An analysis using two costing approaches. BMC Infect Dis 2001; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Xia F, Fuhlbrigge M, Dommasch E, Joyce C, Mostaghimi A. Cost of Routine Herpes Simplex Virus Infection Visits to U.S. Emergency Departments 2006–2013. West J Emerg Med 2018; 19(4): 689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao G, Kassler WJ, Rein DB. Medical care expenditures for genital herpes in the United States. Sex Transm Dis 2000; 27(1): 32–8. [DOI] [PubMed] [Google Scholar]
- 10.Fisman DN, Lipsitch M, Hook EW 3rd, Goldie SJ. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United States. Sex Transm Dis 2002; 29(10): 608–22. [DOI] [PubMed] [Google Scholar]
- 11.Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The Estimated Direct Medical Cost of Sexually Transmitted Diseases Among American Youth, 2000. Perspectives on Sexual and Reproductive Health 2004; 36(1): 11–9. [DOI] [PubMed] [Google Scholar]
- 12.Owusu-Edusei K Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40(3): 197–201. [DOI] [PubMed] [Google Scholar]
- 13.Chesson HW, Spicknall IH, Bingham A, et al. The Estimated Direct Lifetime Medical Costs of Sexually Transmitted Infections Acquired in the United States in 2018. Special Issue of Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Special Issue of Sex Transm Dis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen L The Truven Health MarketScan Databases for life sciences researchers. 2017. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines. MMWR Morb Mortal Wkly Rep 2010. 2010; 59(No. RR-12): 1–110.20075837 [Google Scholar]
- 17.Kumar S, Chesson H, Gift T . Estimating the Direct Medical Outpatient Costs of Diagnosis and Treatment of Trichomoniasis among Commercially Insured Patients in the United States, 2016–2018. Sexually Transmitted Diseases 2020; Volume Publish Ahead of Print - Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benedetti JK, Zeh J, Corey L. Clinical reactivation of genital herpes simplex virus infection decreases in frequency over time. Ann Intern Med 1999; 131(1): 14–20. [DOI] [PubMed] [Google Scholar]
- 19.Owusu-Edusei K Jr, Flagg EW, Gift TL. Hospitalization cost per case of neonatal herpes simplex virus infection from claims data. J Pediatr Nurs 2015; 30(2): 346–52. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Chesson H, Gift T. Estimating the direct medical costs and productivity loss of outpatient chlamydia and gonorrhea treatment. Sexually Transmitted Diseases 2020; Publish Ahead of Print - Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn A, Grosse SD, Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res 2018; 53(1): 175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arias E, Xu J. United States Life Tables, 2017. In: Statistics DoV, editor. National Vital Statistics Reports; 2019. p. 10. [PubMed] [Google Scholar]
- 23.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 1994; 121(11): 847–54. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein DI, Bellamy AR, Hook EW 3rd, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 2013; 56(3): 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luby ED, Klinge V. Genital herpes. A pervasive psychosocial disorder. Arch Dematol 1985; 121(4): 494–7. [PubMed] [Google Scholar]
- 26.Mindel A. Psychological and psychosexual implications of herpes simplex virus infections. Scand J Infect Dis Suppl 1996; 100: 27–32. [PubMed] [Google Scholar]
- 27.Goldmeier D, Johnson A, Byrne M, Barton S. Psychosocial implications of recurrent genital herpes simplex virus infection. Genitourin Med 1988; 64(5): 327–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenthal SL, Zimet GD, Leichliter JS, et al. The psychosocial impact of serological diagnosis of asymptomatic herpes simplex virus type 2 infection. Sex Transm Infect 2006; 82(2): 154–7; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groves MJ. Genital Herpes: A Review. Am Fam Physician 2016; 93(11): 928–34. [PubMed] [Google Scholar]
- 30.Fleming DT, McQuillan GM, Johnson RE, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 1997; 337(16): 1105–11. [DOI] [PubMed] [Google Scholar]