Abstract
Depression and cardiovascular disease are common and associated with one another in HIV disease. This study aimed to determine the frequency and everyday functioning implications of the clinical syndrome of vascular depression among people living with HIV (PLWH). Participants in this cross-sectional study included 536 PLWH and 272 seronegative individuals who completed a biomedical and psychiatric research evaluation. Vascular depression was operationalized as the current presence of: 1) two or more vascular conditions; and 2) depression as determined by a normative elevation on the Depression/Dejection subscale of the Profile of Mood States or a diagnosis of Major Depressive Disorder per the International Diagnostic Interview. Everyday functioning was measured by both self- and clinician-rated activities of daily living. A logistic regression model showed that HIV was associated with a three-fold increased risk of vascular depression, independent of potential confounding factors. A second logistic regression model within the PLWH sample showed that PLWH with vascular depression had significantly greater odds of dependence in everyday functioning as compared to PLWH with either vascular disease or depression alone. The elevated frequency of vascular depression in PLWH is consistent with the vascular depression hypothesis from the late-life depression literature. The high rate of functional dependence among PLWH with vascular depression highlights the clinical importance of prospective work on this syndrome in the context of HIV disease.
Keywords: human immunodeficiency virus, mood disorder, depressive symptoms, cardiovascular, functional impairment, IADL
Depression is among the most common neuropsychiatric disorders in people living with HIV (PLWH; Chichetto et al., 2021; Dew et al., 1997; Rabkin, 2008). The prevalence and severity of depressive symptoms is higher among PLWH than in seronegative individuals (Ciesla and Roberts, 2001; Sherr et al., 2011) and PLWH are significantly more likely to meet criteria for Major Depressive Disorder (Bryant et al., 2015; Dew et al., 1997). Depression is a risk factor for HIV infection due to increased HIV risk behavior (Armstrong et al., 2013) and can occur in the early stages of HIV disease (Weber et al., 2013). There are a variety of potential contributors to depression in PLWH, including socioenvironmental factors (e.g., limited access to care, isolation), neurobiology (e.g., impaired neurogenesis, dopamine dysregulation; Matt and Gaskill, 2019), and both positive (e.g., resilience; Rooney et al., 2019) and negative (e.g., stigma, coping, and life stress; Amare et al., 2018) psychological influences (Arseniou et al., 2014). Among PLWH, the presence of depression is associated with poorer health behaviors and worse outcomes, such as suboptimal medication adherence (Gonzalez et al., 2011; Wagner et al., 2011), lower health-related quality of life (Nanni et al., 2015), and greater disease progression and mortality (So-Armah et al., 2019).
Cardiovascular disease (CVD) may be an important element in understanding the etiology of depression among PLWH (Sico et al., 2021). CVD is one of the leading causes of morbidity and mortality in PLWH (Miller et al., 2014). Some of the most common CVDs experienced among PLWH are heart failure, myocardial infarction, and stroke (Alonso et al., 2019; Triant, 2013). Potential risk factors for CVD in PLWH appear to involve an interplay of the high prevalence of modifiable behaviors and health conditions (e.g., smoking, hypertension, type 2 diabetes, dyslipidemia; Hemkens and Bucher, 2014) and non-modifiable CVD-related changes that occur during the progression of the disease (e.g., abnormal fat distribution, chronic inflammation; Friis-Moller et al., 2003). Furthermore, tobacco and heavy alcohol use among PLWH has been associated with coronary artery plaque, myocardial infarction, and general vascular dysfunction (Feinstein et al., 2019). The downstream effects of CVD on the brain in PLWH can promote neurobehavioral changes (e.g., bradyphrenia, executive dysfunction), perhaps by way of immune activation, endothelial cell dysregulation, and chronic inflammation (Boerwinkle et al., 2020; Cysique and Brew, 2019).
As such, it is plausible that CVD plays a role in depression among PLWH. However, to our knowledge, only a few studies have directly examined the complex interplay of depression and CVD in the context of HIV disease (Abou Hassan et al., 2022; Castilho et al., 2020; Chichetto et al., 2021; Horvat Davey et al., 2020; Levy et al., 2020; Park et al., 2021; Parruti et al., 2013; Sico et al., 2021; Stewart et al., 2020; Zuniga et al., 2020). For example, Park et al. (2021) found that higher levels of depressive symptoms were associated with a three-fold increased risk of CVD among a large sample of Korean adults with HIV. Biomarkers of vascular disease, including glucose, hemoglobin A1C, and soluble CD14 are associated with depression among PLWH (Stewart et al., 2020; Zuniga et al., 2020). PLWH with depression tend to show increased carotid plaques (Levy et al., 2020; cf. Parruti et al., 2013) and a higher rate of hypertension (Castilho et al., 2020). Importantly, PLWH with depression are also at higher risk of incident stroke (Sico et al., 2021), suggesting a connection between depression and cerebrovascular injury in HIV disease.
The association of CVD with depression in PLWH mirrors the late-life depression literature. The frequent co-occurrence of CVD and depression in older adults led to the vascular depression (VasDep) hypothesis, which posits that vascular disease may “predispose, precipitate, or perpetuate” late-life depression (Alexopoulos et al., 1997). Because chronic CVD can eventually impact the brain, particularly the accumulation of white matter lesions, it is thought that vascular depression is caused by disruption of mood-related brain networks in people with a history of CVD. Krishnan et al. (1997) proposed an imaging-based definition that uses the presence of deep white matter lesions as evidence of cerebrovascular disease, which are also common in HIV (Mina et al., 2021). Although the hypothesis infers that vascular disease precedes depression, evidence suggests that depression may also predispose individuals to vascular disease or exacerbate pre-existing conditions (Baldwin, 2005; Dotson et al., 2013; Kirton et al., 2014).
According to a national probability sample of 16,423 adults, the population prevalence of VasDep in adults over the age of 50 is 3.4%, compared to 12.2% for major depression without CVD (Gonzalez et al., 2012). The same study found that of those with a lifetime history of major depression, 22.1% met study criteria for VasDep. Although the prevalence of VasDep in older adults is lower than that of non-vascular depression, the consequences of the former appear to be greater than the latter (Aizenstein et al., 2016). VasDep is associated with greater cognitive impairment than depression without comorbid CVD. This is particularly true for executive functions, which in turn contributes to disproportionate functional disability in VasDep. These sequelae are not surprising considering evidence that the clinical presentation of VasDep includes key symptoms of diminished energy, processing speed, and self-initiation; anhedonia; psychomotor retardation; and lack of insight (Aizenstein et al., 2016). VasDep also tends to respond poorly to antidepressant treatment.
VasDep has traditionally been considered a subtype of late-life depression due to the increasing rates of chronic CVD at older ages. Less work has examined VasDep in medical populations that are characterized by high rates of CVD, such as PLWH. The current study addressed this gap in the literature by determining the prevalence of operationally defined VasDep in HIV seropositive and seronegative individuals and examining associations of VasDep with functional impairment in this sample.
Materials and Methods
Participants
Participants included 536 HIV seropositive and 272 seronegative individuals who were enrolled in a study of prospective memory (Woods et al., 2008; Woods et al., 2020) or internet navigation skills (Woods et al., 2017) at an HIV research center in urban Southern California. Participants were recruited from local clinics, community-based organizations, public fliers, and word-of-mouth and were evaluated between July 2005 and June 2018. HIV serostatus was confirmed via MedMira rapid test or Western blot/ELISA. Potential participants were excluded if they endorsed a history of (1) neurological disorders (e.g., seizure disorder, stroke with neurological sequelae), (2) head injury with loss of consciousness for more than 30 mins, (3) central nervous system opportunistic infection, (4) severe psychiatric diagnosis (e.g., schizophrenia), or (5) current substance dependence. Likewise, individuals with a positive breathalyzer or urine toxicology screen for illicit drugs (except marijuana) on the day of testing were excluded. See Table 1 for participant characteristics.
Table 1.
Demographic and clinical characteristics of the study participants.
| HIV Serostatus | HIV+ | HIV− |
|---|---|---|
| N | 536 | 272 |
| Demographics | ||
| Sex (% women) | 13.8 | 36.4 |
| Race/ethnicity (%) | ||
| Black | 22.0 | 19.9 |
| Hispanic/Latin@ | 16.8 | 16.9 |
| White | 59.0 | 58.5 |
| Other | 2.1 | 4.4 |
| Age | 47.8 (11.3) | 46.1 (15.05) |
| Education | 13.6 (2.6) | 14.22 (2.53) |
| Psychiatric (%) | ||
| Substance Use Disorder | 73.3 | 55.5 |
| Generalized Anxiety Disorder | 14.0 | 5.1 |
| Medical | ||
| Non-vascular conditions | 0.4 (0.6) | 0.2 (0.4) |
| Estimated HIV duration (years) | 14.6 (8.9) | |
| Plasma RNA Detectable (%) | 24.1% | |
| Current CD4 count (cells/μL)2 | 601.5 (306.2) | |
| Nadir CD4 count (cells/μL) 2 a | 208.7 (182.6) | 924.34 (302.0) |
| AIDS (%) | 57.8 | |
| Prescribed ART (%) | 86.8 | |
| NNRTI | 30.1 | |
| NRTI | 12.6 | |
| PI | 42.3 | |
| Other | 15.0 |
Note. Values are means (standard deviation) or valid sample % values. HIV = human immunodeficiency virus; RNA = ribonucleic acid; CD4 = cluster of differentiation 4 cell; AIDS = acquired immune deficiency syndrome; ART = antiretroviral therapy; NNRTI = non-nucleoside reverse transcriptase inhibitors; NRTI = nucleoside reverse transcriptase inhibitor; PI = protease inhibitor.
Research in the era of antiretroviral therapies shows that nadir (i.e., the lowest historically reported) CD4 count relates to brain structure and function at broadly small-to-medium effect sizes (e.g., Everall et al., 2009; Jernigan et al., 2011; Walker and Brown, 2018).
Materials and Procedure
All participants provided written, informed consent prior to completing a comprehensive medical, psychiatric, and neuropsychological evaluation.
Operationalizing Vascular Disease and Depression
Vascular Disease.
Presence of current and historical vascular conditions was determined by research nurse interview. Participants were specifically asked about their histories of chronic pulmonary disease, congestive heart failure, transient ischemic attack, type 2 diabetes mellitus, hyperlipidemia, hypertension, myocardial infarction, and peripheral vascular disease. Participants were assigned a score of one for each historical vascular condition endorsed (sample range = 0–2) and a score of two for a current vascular condition (sample range = 0–4). Consistent with prior research in vascular depression (see Bogoian & Dotson (2022) for a review), participants with a total weighted score (range = 0–10) of two or more were assigned to the vascular group (n = 198).
Depression.
Depression was operationalized using two well-validated indicators. Participants completed the Composite International Diagnostic Interview (CIDI version 2.128; World Health Organization, 1998), a structured lay interview for major depressive disorder (MDD). Participants were also administered the 15-item Depression/Dejection scale of the Profile of Mood States (POMS; McNair et al., 1981), on which they indicated how they have been feeling over the past week (e.g., unhappy, sorry for things done) using a five-point Likert-type scale ranging from zero (“not at all”) to four (“extremely”). Possible scores ranged from zero to 60 (Cronbach’s alpha = 0.952, sample range = 0–59). POMS scores were transformed into z-scores (range = −0.9 to 5.6) using age- and sex-based normative data (Nyenhuis et al., 1999), such that higher z-scores reflected worse depressive symptoms. Individuals who obtained a score of 1.5 or higher on the POMS (n = 113) or met criteria for current Major Depressive Disorder (MDD) based on the CIDI (n = 75) were classified as depressed (Patterson et al., 2006). We used a multimodal operationalization of depression for two reasons. First, the frequency of current MDD diagnoses per the CIDI was fairly low, which diminishes both power and reliability. Second, the inclusion of both self-report and interview-based indicators of depression arguably increases the robustness of our measurement of the construct. Consistent with prior studies (Patterson et al., 2006; Woods et al., 2021), individuals with elevated POMS scores were more than six times likely to meet current criteria for depression on the CIDI (odds ratio = 6.9 [4.1, 11.4]). A total of 35.1% of participants with depression were prescribed antidepressant medication.
Vascular Depression.
Participants were stratified based on vascular factors and depression status, which produced a factorial classification that included persons: (1) without vascular disease or depression (V−D−) (n = 330), (2) with vascular disease, but without depression (V+D−) (n = 324), (3) with depression, but without vascular disease (V−D+) (n = 57), and (4) with both vascular disease and depression (V+D+) (n = 97).
Everyday Functioning
Everyday functioning was assessed using a combination of two well-validated measures of self- and clinician rating of activities of daily living (ADLs). All participants completed the Heaton et al. (2004) version of the Lawton Instrumental Activities of Daily Living Scale, which examines basic and instrumental ADLs such as finance management, grocery shopping, cooking, transportation, shopping, medication management, social activity planning, housekeeping,/cleaning, laundry, home repairs, bathing, and dressing. This scale requires participants to rate their current and best levels of functioning on each item with a “1” denoting they have experienced decline from “best” to “now” and a “0” indicating no observable decline. Scores were calculated by summing the number of items with decline out of 16, whereby higher values denote poorer functioning (sample range = 0–6, Cronbach’s alpha = 0.848). Furthermore, ADLs were assessed by a certified nurse using the Karnofsky Scale of Performance Status (KPS; Karnofsky, 1949) which ranges from 100 (i.e., able to carry out normal activity) to zero (i.e., dead; sample range = 50–100). Consistent with prior work in HIV (Casaletto et al., 2017), participants who endorsed two or more ADL domain declines or who were rated as < 90 per the clinician were classified as ADL dependent.
Covariates.
We also measured a variety of potential covariates that are relevant to HIV disease, vascular disease, and depression. A blood draw and research nurse interview provided information about common clinical HIV disease and treatment factors, as well as the frequency of non-vascular medical conditions (e.g., hepatitis C, renal disease, liver disease). Lifetime diagnoses of generalized anxiety disorder (GAD) and substance use disorders (SUD) were obtained from the CIDI. Antidepressant use was not considered as a covariate since use did not differ significantly between V−D+ and V+D+ groups and was not associated with ADLs in PLWH with depression (ps > 0.05). See Table 1 for a comprehensive list of covariates.
Data Analysis
A series of t-tests and chi-square tests were used to determine potential covariates, which were selected using a confound approach (Field-Fote, 2019). Specifically, any variables listed in Tables 1 and 2 that significantly related to both the independent and dependent variables were included in the statistical models. The primary hypotheses were tested by logistic regression. Planned post-hoc analyses consisted of 1) pair-wise odds ratios, which were focused specifically on the V+D+ study cell to reduce Type I error risk, and 2) analyses to determine the impact of antiretroviral therapy (ART) and viral suppression at the time of assessment on the results. A critical alpha was set at 0.05 and all analyses were conducted in JMP 16.0 (SAS, Cary, NC).
Table 2.
Vascular and depression characteristics of the study participants.
| HIV Serostatus | HIV+ | HIV− |
|---|---|---|
| N | 536 | 272 |
| Total Vascular Conditionsa | 2.1 (2.2) | 1.2 (1.8) |
| Hypertension (%) | 37.1 | 22.4 |
| Hyperlipidemia (%) | 35.6 | 17.6 |
| Chronic pulmonary disease (%) | 20.0 | 15.1 |
| Type 2 diabetes mellitus (%) | 10.3 | 7.7 |
| TIA (%) | 2.6 | 1.1 |
| Myocardial infarction (%) | 1.7 | 0.4 |
| Congestive heart failure (%) | 0.8 | 0.7 |
| Peripheral vascular disease (%) | 0.6 | 0 |
| Depression | ||
| Current MDD (%) | 12.1 | 3.7 |
| Recency | ||
| < 2 weeks | 76.9 | 60.0 |
| < 4 weeks | 23.1 | 40.0 |
| Age at first depressive episode | 32.1 (15.2) | 33.7 (20.5) |
| POMS current depressed (%) | 17.4 | 7.4 |
| POMS depression z-scores | 0.4 (1.4) | −0.1 (1.0) |
Note. Values are means (standard deviation) or valid sample % values. HIV = human immunodeficiency virus; y = current or past history; n = no current or past history; TIA = transient ischemic attack; MDD = major depressive disorder; POMS = Profile of Mood States
Vascular conditions include vascular risk factors (e.g., hypertension, hyperlipidemia) in addition to vascular diseases (e.g., congestive heart failure, peripheral artery disease).
Results
Estimated Frequency of Vascular Depression by HIV Serostatus
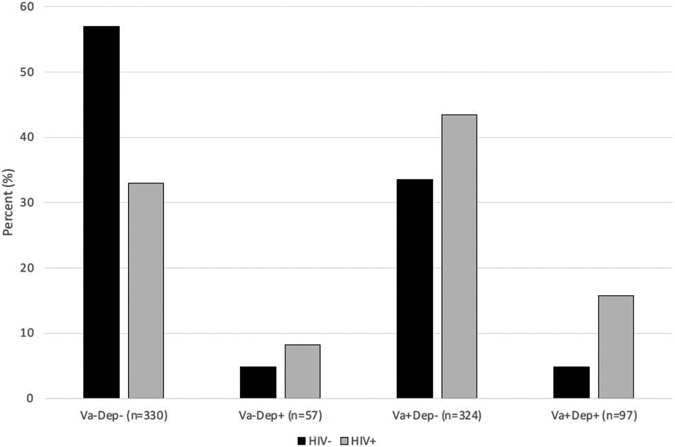
As shown in Table 1, sex, education, non-vascular medical conditions, GAD, and SUD were all significantly associated with HIV serostatus in the expected directions (ps < 0.05). Of these five potential covariates, only non-vascular medical conditions, GAD, and SUD were also significantly associated with VasDep (ps < 0.05) and were therefore included as covariates. The overall logistic regression model with HIV as a predictor of VasDep was significant (df = 12, χ2 = 1171.6, p < 0.0001). All predictor variables within this model were significant contributors, including HIV serostatus (df = 3, χ2 = 37.0, p < 0.0001), GAD (df = 3, χ2 = 25.0, p < 0.0001), SUD (df = 3, χ2 = 9.3, p = 0.025), and non-vascular medical conditions (df = 3, χ2 = 8.4, p = 0.038). Figure 1 shows that 86.6% of the V+D+ cases were among PLWH; in other words, the frequency of VasDep was three times higher among PLWH (15.7%) versus those without HIV (4.8%). Results were similar in pattern and significance when depression was defined only using the CIDI or only using the POMS. Follow-up post-hoc comparisons showed that positive HIV serostatus was associated with higher odds of V+D+ versus V−D− (odds ratio [OR] = 5.7, 95% confidence interval = 3.1, 10.7), V+D− (OR = 2.5 [1.3, 4.8]), and V−D+ (OR = 1.9 [0.8, 4.5]).
Figure 1.
Frequency of vascular depression in persons with and without HIV disease. V−D− without vascular disease or depression, V+D− with vascular disease but without depression, V−D+ with depression but without vascular disease, V+D+ with both vascular disease and depression.
Vascular Depression and Everyday Functioning in PLWH
Table 2 shows that VasDep was associated with older age, GAD, AIDS, longer estimated duration of HIV, and lower nadir CD4 count within the PLWH sample (ps < 0.05). All five of these variables were also associated with ADL dependence (ps < 0.05) and were therefore included as covariates in a logistic regression in which VasDep was a predictor of ADL status in PLWH. The overall model was significant (df = 8, χ2 = 77.7, p < 0.0001) and showed independent contributions of both VasDep (df = 3, χ2 = 44.5, p < 0.0001) and GAD (df = 1, χ2 = 7.9, p = 0.005) in the expected directions. No other covariate made a significant contribution to this model (all ps > 0.10). Follow-up post-hoc comparisons showed that VasDep was associated with higher odds of ADL dependence versus V−D− (OR = 9.6 [5.1, 17.9]), V+D− (OR = 7.1 [4.0, 12.6]) and V−D+ (OR = 5.4 [2.6, 11.5]).
Effect of ART with Viral Suppression
To examine the role of ART and viral suppression in the findings, we replicated the logistic regression models in the subset of 383 PLWH who were on ART and virally suppressed at the time of assessment. HIV serostatus was a strong, independent predictor of VasDep in this subset of participants (df = 3, χ2 = 28.9, p < 0.0001), and VasDep significantly predicted ADL dependence (df = 3, χ2 = 25.7, p < 0.001). We also conducted a one-sample χ2 test and confirmed that the frequencies of V+D−, V−D+, and V+D+ in this subset of PLWH did not differ from the full sample of PLWH (df = 3, χ2 = 1.0, p = 0.789).
Discussion
Depression and vascular disease commonly co-occur among PLWH, but we know little about the specific frequency of VasDep in the context of HIV disease. Findings from this retrospective clinical study are the first to suggest that HIV is associated with an increased risk of VasDep. HIV disease was associated with a three-fold increased rate of VasDep as compared to the seronegative sample, whose rates were broadly comparable to national prevalence estimates (Gonzalez et al., 2012). The association between HIV and VasDep was not confounded by sociodemographics, medical comorbidities, or other psychiatric factors measured in this sample, and findings remained significant in a subsample of PLWH who were on ART and virally suppressed at the time of assessment. These findings extend prior work on the association between vascular disease and depression in HIV (e.g., Horvat Davey et al., 2020) by specifically highlighting their convergence in the clinically relevant neuropsychiatric syndrome of VasDep (Alexopoulos et al., 1997). This preliminary finding generates numerous important questions about the neuropathogenesis, course, clinical features (e.g., neuroimaging and neurocognitive correlates), and mediating/moderating factors (e.g., race, age) of VasDep in PLWH.
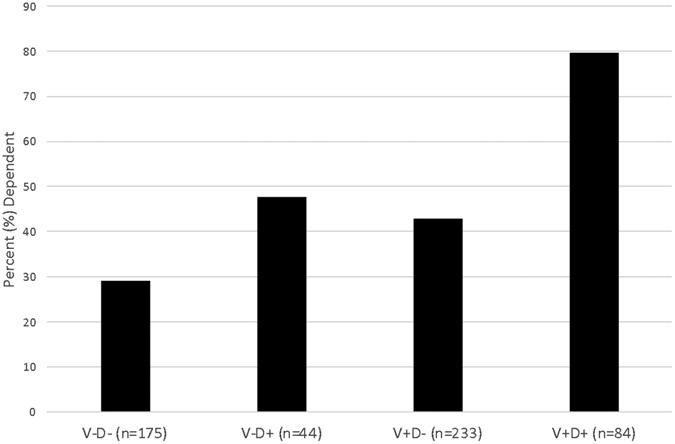
Findings from this study also support the clinical relevance of the increased frequency of VasDep in HIV. Specifically, 80% of the PLWH with VasDep were functionally dependent, nearly double the rates of dependence observed in persons with either depression or vascular disease alone. Moreover, PLWH with VasDep were more than nine times more likely to be functionally dependent as compared to PLWH with neither depression nor vascular disease. Importantly, the higher frequency of functional dependence among PLWH with VasDep was not better explained by sociodemographics, HIV disease severity, or other comorbidities. Thus, a majority of PLWH with VasDep experience greater dependence in their activities of daily living. Functional dependence was operationalized using both clinician- and self-rated declines in ADLs, so future work is needed to examine the effects of VasDep on performance-based measures of functional capacity (e.g., medication management) and objective behavioral indicators (e.g., pharmacy refills, retention in healthcare). It will also be important determine risk (e.g., executive dysfunction) and protective (e.g., environmental supports) factors for functional dependence among PLWH with VasDep.
VasDep is generally considered a subtype of late-life depression, largely due to the strong association between older age and higher risk of cardiovascular and cerebrovascular diseases (Dai et al., 2015). Even though VasDep research has generally been limited to older adults, older age is not a prerequisite for the condition since the presumed mechanism is not age per se, but rather, disruption of mood networks in the brain due to chronic vascular disease. Vascular risk factors (e.g., high blood pressure, obesity) and vascular diseases (e.g., coronary artery disease, chronic heart failure) are associated with white matter lesions in the brain (Wang et al., 2021), seen as white matter hyperintensities (WMH) on magnetic resonance imaging scans. More severe WMH have been reported in individuals with late-life depression and vascular complaints (Tamura and Araki, 2015; van Dijk et al., 2004). In particular, WMH within mood related fasciculi that connect fronto-striatal and limbic areas have been linked to increased depressive symptoms (Aizenstein et al., 2016; Steffens et al., 2011; Taylor et al., 2013). The presence of WMH is a necessary condition for MRI-defined VasDep (Krishnan et al., 1997) and is presumed to be the underlying mechanism in clinically defined VasDep, which bases the diagnosis on the presence of vascular risk factors. As such, individuals with any condition associated with chronic vascular disease could be at increased risk for VasDep. Since WMH are a common finding in PLWH (Mina et al., 2021) and have been shown to be correlated with the presence of vascular conditions (Mina et al., 2021; Wu et al., 2018), our finding of higher VasDep risk in PLWH is consistent with the VasDep hypothesis that has traditionally been tested in late-life depression. In light of evidence that vascular conditions differ in the strength of their relationship with WMH (Alber et al., 2019), our understanding of VasDep in PLWH and other populations could be enhanced by studies that examine the link between individual conditions and depression. The low frequency of several vascular conditions precluded such an examination in the current study.
The functional deficits related to VasDep in the current study parallel consistent findings in the VasDep literature. Both clinically-defined and MRI-defined VasDep predict greater functional disability (Chang et al., 2016). In fact, there is evidence that VasDep is a prodrome of frailty, a condition marked by pronounced difficulty performing instrumental activities of daily living (Paulson and Lichtenberg, 2013). Frailty data are not available for the current sample, but previous work in PLWH found that out of 12 medical and neuropsychiatric comorbidities, elevated depressive symptoms conferred the highest risk for frailty, with the comorbidity of depression with either diabetes, hypertension, or obesity—all vascular risk factors—predicting the highest risk of frailty (Lorenz et al., 2021). Future work should examine the links between clinically or MRI-defined VasDep, functional disability, and frailty in PLWH. Functional deficits in late-life VasDep are associated with executive dysfunction, similar to what has been shown in the larger aging literature (McAlister et al., 2016). WMHs likely underly the interrelationships amongst vascular burden, depression, cognitive deficits, and functional dependence, as WMHs have been associated with each of these variables (Puzo et al., 2019). WMHs predict worse cognitive outcomes in PLWH (Mina et al., 2021; Wu et al., 2018), but thus far, no work has linked WMHs and cognitive outcomes to VasDep in PLWH. This will be an important focus of future research. Future work in PLWH should focus not just on WMH volume, but on the associations between regional WMH volume in mood networks in the brain and depressive symptoms, and whether serostatus modifies this relationship. Also important will be studies that examine demographic (e.g., age, race, socioeconomic status) and clinical (e.g., comorbid conditions, depression severity) factors that may moderate the risk for VasDep in PLWH as well as the cognitive and functional correlates of VasDep.
A few additional limitations to this study deserve consideration in interpreting the findings. First, neuroimaging data were not available, thus we were not able to confirm the presence of WMHs that are the presumed cause of VasDep. Second, the sample was predominantly White, male, and had post-high school education levels; thus, the generalizability of these data to other sociodemographic groups remains to be determined. Similarly, the low frequency of many of the vascular and non-vascular conditions in the sample could impact external validity of the current findings. Third, the study design was observational and cross-sectional. Nonetheless, this study provides initial evidence that PLWH are vulnerable to VasDep, a disorder that has traditionally been studied only in older adults. The increased risk of VasDep in PLWH supports previous assertions that HIV contributes to accelerated aging (e.g., Sheppard et al., 2017). The demonstration of a vulnerability to VasDep and an association between VasDep and functional dependence in the total sample, which includes include persons not on ART and who have detectable viral load, increases the generalizability of the findings to the most vulnerable PLWH. The replication of the findings in the subsample of virally suppressed PLWH on ART suggests the observed findings are not attributable to HIV viral load.
Considering that VasDep in older adults is associated with greater cognitive deficits, functional disability, and treatment resistance than non-vascular depression, these results highlight a critical need for more research on VasDep in PLWH as well as other medical populations that are marked by high vascular burden. Clinically, results suggest depressed PLWH may benefit from adjunctive treatments that target vascular health. For example, lifestyle interventions such as exercise and nutritional changes may be combined with pharmacotherapy or psychotherapy to improve outcomes in VasDep (Jellinger, 2021; Taylor et al., 2018). Lifestyle interventions may also provide effective prevention strategies for PLWH to reduce the risk of developing VasDep.
Figure 2.
Frequency of dependence in activities of daily living in 536 persons living with HIV disease. V−D− without vascular disease or depression, V+D− with vascular disease but without depression, V−D+ with depression but without vascular disease, V+D+ with both vascular disease and depression.
Table 3.
Demographic and clinical characteristics of PLWH with and without vascular disease and depression (N=536).
| Va− Dep− | Va− Dep+ | Va+ Dep− | Va+ Dep+ | |
|---|---|---|---|---|
| N | 175 | 44 | 233 | 84 |
| Demographics | ||||
| Sex (% women) | 10.9 | 13.6 | 15.0 | 16.7 |
| Race/ethnicity (%) | ||||
| Black | 20.6 | 27.3 | 22.8 | 20.2 |
| Hispanic/Latin@ | 23.4 | 22.7 | 13.7 | 8.3 |
| White | 54.2 | 47.7 | 60.5 | 70.2 |
| Other | 1.7 | 2.3 | 3.0 | 1.2 |
| Age | 43.1 (9.8) | 40.7 (12.7) | 51.5 (10.6) | 50.9 (10.1) |
| Education | 13.4 (2.6) | 13.2 (2.2) | 13.9 (2.5) | 13.5 (2.8) |
| Psychiatric (%) | ||||
| Substance use | 70.3 | 75.0 | 72.5 | 81.0 |
| Generalized anxiety | 8.0 | 29.5 | 10.3 | 28.6 |
| Medical | ||||
| Non-vascular conditions | 0.3 (0.6) | 0.2 (0.4) | 0.4 (0.6) | 0.4 (0.6) |
| Est. HIV duration (years) | 12.39 (8.6) | 9.6 (8.3) | 16.3 (8.5) | 17.0 (8.6) |
| Plasma RNA (% detectable) | 23.8% | 31.8% | 21.3% | 28.0% |
| Current CD4 (cells/μL) | 565.1 (303.1) | 598.0 (265.8) | 623.0 (307.8) | 620.9 (325.8) |
| Nadir CD4 (cells/μL) 2 | 226.1 (183.0) | 252.0 (191.0) | 185.5 (168.5) | 214.1 (208.1) |
| AIDS (%) | 49.1 | 47.7 | 62.7 | 67.9 |
| Prescribed ART (%) | 86.7 | 81.8 | 88.7 | 84.3 |
Note. Values are means (standard deviation) or valid sample % values. HIV = human immunodeficiency virus; RNA = ribonucleic acid; CD4 = cluster of differentiation 4 cell; AIDS = acquired immune deficiency syndrome; ART = antiretroviral therapy; PLWH = persons living with hiv disease; Va = vascular; Dep = depression.
Acknowledgements
This study was supported by NIH grants R01-MH073419, R21-MH098607 and P30-MH62512. VMD is supported by the National Institute on Aging (U19AG073172, U19AG065169, R21AG077307), the National Science Foundation (2112455), and the Alzheimer’s Association (AARG-NTF-21-852145). The authors are grateful to the UC San Diego HIV Neurobehavioral Research Program (HNRP) Group (I. Grant, PI) for their infrastructure support; in particular, we thank Donald Franklin, Dr. Erin Morgan, Clint Cushman, and Stephanie Corkran for their assistance with data processing, Marizela Verduzco for her assistance with study management, Drs. Scott Letendre and Ronald J. Ellis for their assistance with the neuromedical aspects of the parent project, and Dr. J. Hampton Atkinson and Jennifer Marquie Beck and their assistance with participant recruitment and retention. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The authors thank the study volunteers for their participation.
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou Hassan FF, Bou Hamdan MA, El Asmar K, Mokhbat JE, Melhem NM, 2022. Trends & predictors of non-AIDS comorbidities among people living with HIV and receiving antiretroviral therapy in Lebanon. Medicine (Baltimore) 101(13), e29162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, Jellinger KA, Kruglov LS, Meshandin IA, Mijajlovic MD, Niklewski G, Pospos S, Raju K, Richter K, Steffens DC, Taylor WD, Tene O, 2016. Vascular depression consensus report - a critical update. BMC Med 14(1), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber J, Alladi S, Bae HJ, Barton DA, Beckett LA, Bell JM, Berman SE, Biessels GJ, Black SE, Bos I, Bowman GL, Brai E, Brickman AM, Callahan BL, Corriveau RA, Fossati S, Gottesman RF, Gustafson DR, Hachinski V, Hayden KM, Helman AM, Hughes TM, Isaacs JD, Jefferson AL, Johnson SC, Kapasi A, Kern S, Kwon JC, Kukolja J, Lee A, Lockhart SN, Murray A, Osborn KE, Power MC, Price BR, Rhodius-Meester HFM, Rondeau JA, Rosen AC, Rosene DL, Schneider JA, Scholtzova H, Shaaban CE, Silva N, Snyder HM, Swardfager W, Troen AM, van Veluw SJ, Vemuri P, Wallin A, Wellington C, Wilcock DM, Xie SX, Hainsworth AH, 2019. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y) 5, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M, 1997. 'Vascular depression' hypothesis. Arch Gen Psychiatry 54(10), 915–922. [DOI] [PubMed] [Google Scholar]
- Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V, 2019. HIV infection and incidence of cardiovascular diseases: An analysis of a large healthcare database. J Am Heart Assoc 8(14), e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amare T, Getinet W, Shumet S, Asrat B, 2018. Prevalence and associated factors of depression among PLHIV in Ethiopia: Systematic review and meta-analysis, 2017. AIDS Res Treat 2018, 5462959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseniou S, Arvaniti A, Samakouri M, 2014. HIV infection and depression. Psychiatry Clin Neurosci 68(2), 96–109. [DOI] [PubMed] [Google Scholar]
- Baldwin RC, 2005. Is vascular depression a distinct sub-type of depressive disorder? A review of causal evidence. Int J Geriatr Psychiatry 20(1), 1–11. [DOI] [PubMed] [Google Scholar]
- Boerwinkle AH, Strain JF, Burdo T, Doyle J, Christensen J, Su Y, Wisch JK, Cooley SA, Vaida F, Smith MD, Jafri H, Paul RH, Benzinger TLS, Ances BM, 2020. Comparison of [11C]-PBR28 binding between persons living with HIV and HIV-uninfected individuals. J Acquir Immune Defic Syndr 85(2), 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoian HR, Dotson VM, 2022. Vascular depression in Black Americans: A systematic review of the construct and its cognitive, functional, and psychosocial correlates. Clin Neuropsychol 36(2), 431–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant VE, Whitehead NE, Burrell LE 2nd, Dotson VM, Cook RL, Malloy P, Devlin K, Cohen RA, 2015. Depression and apathy among people living with HIV: Implications for treatment of HIV associated neurocognitive disorders. AIDS Behav 19(8), 1430–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaletto KB, Weber E, Iudicello JE, Woods SP, 2017. Real-world impact of HIV-associated neurocognitive impairment, Changes in the brain: Impact on daily life. Springer-Verlag Publishing, New York, NY, US, pp. 211–245. [Google Scholar]
- Castilho JL, Rebeiro PF, Shepherd BE, Nash R, Adams RS, Turner M, Furukawa SS, Hulgan T, Koethe JR, Sterling TR, 2020. Mood disorders and increased risk of noncommunicable disease in adults with HIV. J Acquir Immune Defic Syndr 83(4), 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KJ, Hong CH, Kim SH, Lee KS, Roh HW, Kang DR, Choi SH, Kim SY, Na DL, Seo SW, Kim DK, Lee Y, Chung YK, Lim KY, Noh JS, Son SJ, 2016. MRI-defined versus clinically-defined vascular depression; comparison of prediction of functional disability in the elderly. Arch Gerontol Geriatr 66, 7–12. [DOI] [PubMed] [Google Scholar]
- Chichetto NE, Kundu S, Freiberg MS, Koethe JR, Butt AA, Crystal S, So-Armah KA, Cook RL, Braithwaite RS, Justice AC, Fiellin DA, Khan M, Bryant KJ, Gaither JR, Barve SS, Crothers K, Bedimo RJ, Warner A, Tindle HA, Veterans Aging Cohort, S., 2021. Association of syndemic unhealthy alcohol use, smoking, and depressive symptoms on incident cardiovascular disease among veterans with and without HIV-infection. AIDS Behav 25(9), 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE, 2001. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 158(5), 725–730. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ, 2019. Vascular cognitive impairment and HIV-associated neurocognitive disorder: a new paradigm. J Neurovirol 25(5), 710–721. [DOI] [PubMed] [Google Scholar]
- Dai X, Hummel SL, Salazar JB, Taffet GE, Zieman S, Schwartz JB, 2015. Cardiovascular physiology in the older adults. J Geriatr Cardiol 12(3), 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew MA, Becker JT, Sanchez J, Caldararo R, Lopez OL, Wess J, Dorst SK, Banks G, 1997. Prevalence and predictors of depressive, anxiety and substance use disorders in HIV-infected and uninfected men: a longitudinal evaluation. Psychol Med 27(2), 395–409. [DOI] [PubMed] [Google Scholar]
- Dotson VM, Zonderman AB, Kraut MA, Resnick SM, 2013. Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. Int J Geriatr Psychiatry 28(1), 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, Moore D, Ellis R, Cherner M, Gelman B, Morgello S, Singer E, Grant I, Masliah E, National Neuro, A.T.C., 2009. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol 15(5-6), 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS, 2019. Characteristics, prevention, and management of cardiovascular disease in people living with HIV: A scientific statement from the American Heart Association. Circulation 140(2), e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EE, 2019. Mediators and moderators, confounders and covariates: Exploring the variables that illuminate or obscure the "active ingredients" in neurorehabilitation. J Neurol Phys Ther 43(2), 83–84. [DOI] [PubMed] [Google Scholar]
- Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD, Data Collection on Adverse Events of Anti, H.I.V.D.S.G., 2003. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 349(21), 1993–2003. [DOI] [PubMed] [Google Scholar]
- Gonzalez HM, Tarraf W, Whitfield K, Gallo JJ, 2012. Vascular depression prevalence and epidemiology in the United States. J Psychiatr Res 46(4), 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, Safren SA, 2011. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr 58(2), 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group, H., 2004. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10(3), 317–331. [DOI] [PubMed] [Google Scholar]
- Hemkens LG, Bucher HC, 2014. HIV infection and cardiovascular disease. Eur Heart J 35(21), 1373–1381. [DOI] [PubMed] [Google Scholar]
- Horvat Davey C, Perazzo JD, Vest M, Josephson RA, Oliveira VHF, Sattar A, Webel AR, 2020. The relationship of cardiorespiratory function, fatigue and depressive symptoms in PLHIV. AIDS Care 32(7), 877–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, 2021. Pathomechanisms of vascular depression in older adults. Int J Mol Sci 23(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR Jr., Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group, C., 2011. Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 17(3), 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky DA, 1949. The clinical evaluation of chemotherapeutic agents in cancer, in: Maclead CM (Ed.) Evaluation of Chemotherapeutic Agents. Columbia University Press, pp. 191–205. [Google Scholar]
- Kirton JW, Resnick SM, Davatzikos C, Kraut MA, Dotson VM, 2014. Depressive symptoms, symptom dimensions, and white matter lesion volume in older adults: a longitudinal study. Am J Geriatr Psychiatry 22(12), 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG, 1997. MRI-defined vascular depression. Am J Psychiatry 154(4), 497–501. [DOI] [PubMed] [Google Scholar]
- Levy ME, Anastos K, Levine SR, Plankey M, Castel AD, Molock S, Sen S, Asch FM, Milam J, Aouizerat B, Weber KM, Golub ET, Kaplan RC, Kassaye S, 2020. Depression and psychosocial stress are associated with subclinical carotid atherosclerosis among women living with HIV. J Am Heart Assoc 9(13), e016425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz DR, Mukerji SS, Misra V, Uno H, Gelman BB, Moore DJ, Singer EJ, Morgello S, Gabuzda D, 2021. Multimorbidity networks associated with frailty among middle-aged and older people with HIV. AIDS 35(15), 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt SM, Gaskill PJ, 2019. Dopaminergic impact of cART and anti-depressants on HIV neuropathogenesis in older adults. Brain Res 1723, 146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister C, Schmitter-Edgecombe M, Lamb R, 2016. Examination of variables that may affect the relationship between cognition and functional status in individuals with mild cognitive impairment: A meta-analysis. Arch Clin Neuropsychol 31(2), 123–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF, 1981. Manual for the Profile of Mood States (POMS). Educational and Industrial Testing Service, San Diego. [Google Scholar]
- Miller CJ, Baker JV, Bormann AM, Erlandson KM, Huppler Hullsiek K, Justice AC, Neuhaus J, Paredes R, Petoumenos K, Wentworth D, Winston A, Wolfson J, Neaton JD, Group, I.S.S., Group, E.S., 2014. Adjudicated morbidity and mortality outcomes by age among individuals with HIV infection on suppressive antiretroviral therapy. PLoS One 9(4), e95061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina Y, Wu T, Hsieh HC, Hammoud DA, Shah S, Lau CY, Ham L, Snow J, Horne E, Ganesan A, Rapoport SI, Tramont EC, Reich DS, Agan BK, Nath A, Smith BR, Consortium, N.-D.N., 2021. Association of white matter hyperintensities with HIV status and vascular risk factors. Neurology 96(14), e1823–e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L, 2015. Depression in HIV infected patients: a review. Curr Psychiatry Rep 17(1), 530. [DOI] [PubMed] [Google Scholar]
- Nyenhuis DL, Yamamoto C, Luchetta T, Terrien A, Parmentier A, 1999. Adult and geriatric normative data and validation of the profile of mood states. J Clin Psychol 55(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Park KS, Hwang SY, Choi BY, Kim J, Kim SI, Kim WJ, Kang C, 2021. Associations of depression and anxiety with cardiovascular risk among people living with HIV/AIDS in Korea. Epidemiol Health 43, e2021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parruti G, Vadini F, Sozio F, Mazzott E, Ursini T, Polill E, Di Stefano P, Tontodonati M, Verrocchio MC, Fulcheri M, Calella G, Santilli F, Manzoli L, 2013. Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort study. PLoS One 8(1), e54555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Young C, Woods SP, Vigil O, Grant I, Atkinson JH, Group, H.I.V.N.R.C., 2006. Screening for major depression in persons with HIV infection: the concurrent predictive validity of the Profile of Mood States Depression-Dejection Scale. Int J Methods Psychiatr Res 15(2), 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D, Lichtenberg PA, 2013. Vascular depression: an early warning sign of frailty. Aging Ment Health 17(1), 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzo C, Labriola C, Sugarman MA, Tripodis Y, Martin B, Palmisano JN, Steinberg EG, Stein TD, Kowall NW, McKee AC, Mez J, Killiany RJ, Stern RA, Alosco ML, 2019. Independent effects of white matter hyperintensities on cognitive, neuropsychiatric, and functional decline: a longitudinal investigation using the National Alzheimer's Coordinating Center Uniform Data Set. Alzheimers Res Ther 11(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, 2008. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep 5(4), 163–171. [DOI] [PubMed] [Google Scholar]
- Rooney AS, Moore RC, Paolillo EW, Gouaux B, Umlauf A, Letendre SL, Jeste DV, Moore DJ, Program, H.I.V.N.R., 2019. Depression and aging with HIV: Associations with health-related quality of life and positive psychological factors. J Affect Disord 251, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DP, Iudicello JE, Morgan EE, Kamat R, Clark LR, Avci G, Bondi MW, Woods SP, Group, H.I.V.N.R.P., 2017. Accelerated and accentuated neurocognitive aging in HIV infection. J Neurovirol 23(3), 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Clucas C, Harding R, Sibley E, Catalan J, 2011. HIV and depression--a systematic review of interventions. Psychol Health Med 16(5), 493–527. [DOI] [PubMed] [Google Scholar]
- Sico JJ, Kundu S, So-Armah K, Gupta SK, Chang CH, Butt AA, Gibert CL, Marconi VC, Crystal S, Tindle HA, Freiberg MS, Stewart JC, 2021. Depression as a risk factor for incident ischemic stroke among HIV-positive veterans in the Veterans Aging Cohort Study. J Am Heart Assoc 10(13), e017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So-Armah K, Gupta SK, Kundu S, Stewart JC, Goulet JL, Butt AA, Sico JJ, Marconi VC, Crystal S, Rodriguez-Barradas MC, Budoff M, Gibert CL, Chang CC, Bedimo R, Freiberg MS, 2019. Depression and all-cause mortality risk in HIV-infected and HIV-uninfected US veterans: a cohort study. HIV Med 20(5), 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Denny KL, Bergman SR, Wang L, 2011. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One 6(7), e22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JC, Polanka BM, So-Armah KA, White JR, Gupta SK, Kundu S, Chang CH, Freiberg MS, 2020. Associations of total, cognitive/affective, and somatic depressive symptoms and antidepressant use with cardiovascular disease-relevant biomarkers in HIV: Veterans Aging Cohort Study. Psychosom Med 82(5), 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Araki A, 2015. Diabetes mellitus and white matter hyperintensity. Geriatr Gerontol Int 15 Suppl 1, 34–42. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS, 2013. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 18(9), 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Schultz SK, Panaite V, Steffens DC, 2018. Perspectives on the management of vascular depression. Am J Psychiatry 175(12), 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, 2013. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep 10(3), 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Launer LJ, Hofman A, Consortium, C., 2004. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 44(5), 625–630. [DOI] [PubMed] [Google Scholar]
- Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni J, Bangsberg DR, Liu H, Investigators M, 2011. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med 42(3), 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Brown GG, 2018. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. J Clin Exp Neuropsychol 40(4), 357–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen Q, Chen J, Yang N, Zheng K, 2021. Risk factors of cerebral small vessel disease: A systematic review and meta-analysis. Medicine (Baltimore) 100(51), e28229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P, Flepp M, Kowalska J, Ledergerber B, Swiss, H.I.V.C.S., 2013. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 14(4), 195–207. [DOI] [PubMed] [Google Scholar]
- Woods SP, Babicz M, Shahani L, Colpo GD, Morgan EE, Teixeira AL, 2021. Brain-derived neurotrophic factor (BDNF) is associated with depressive symptoms in older adults with HIV disease. J Neurovirol 27(1), 70–79. [DOI] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Morgan EE, Verduzco M, Smith TV, Cushman C, Group, H.I.V.N.R.P., 2017. Household everyday functioning in the internet age: Online shopping and banking skills are affected in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc 23(7), 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Carey CL, Dawson MS, Iudicello JE, Gibson S, Grant I, Atkinson JH, Group, H.I.V.N.R.C., 2008. Prospective memory in HIV infection: is "remembering to remember" a unique predictor of self-reported medication management? Arch Clin Neuropsychol 23(3), 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Loft S, Matchanova A, Verduzco M, Cushman C, 2020. Supporting strategic processes can improve time-based prospective memory in the laboratory among older adults with HIV disease. Neuropsychology 34(3), 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 1998. Composite International Diagnostic Interview (CIDI, version 2.1). World Health Organization, Geneva, Switzerland. [Google Scholar]
- Wu M, Fatukasi O, Yang S, Alger J, Barker PB, Hetherington H, Kim T, Levine A, Martin E, Munro CA, Parrish T, Ragin A, Sacktor N, Seaberg E, Becker JT, Neuropsychology Working Group of the Multicenter, A.C.S., 2018. HIV disease and diabetes interact to affect brain white matter hyperintensities and cognition. AIDS 32(13), 1803–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga JA, Harrison ML, Henneghan A, Garcia AA, Kesler S, 2020. Biomarkers panels can predict fatigue, depression and pain in persons living with HIV: A pilot study. Appl Nurs Res 52, 151224. [DOI] [PubMed] [Google Scholar]