Abstract
Cannabidiol (CBD) is a major cannabinoid present in extracts of the plant Cannabis sativa (marijuana). While the therapeutic effects of CBD on epilepsy have been demonstrated, less is understood regarding its potential adverse effects. Recent studies revealed that CBD induced toxicity in the male reproductive system of animal models. In this study, we used TM4, an immortalized mouse Sertoli cell line, and primary human Sertoli cells to evaluate the toxicities of CBD and its main metabolites, 7-carboxy-CBD and 7-hydroxy-CBD. CBD induced concentration- and time-dependent cytotoxicity in mouse and human Sertoli cells, which mainly resulted from the inhibition of the G1/S-phase cell cycle transition. CBD also inhibited DNA synthesis and downregulated key cell cycle proteins. Moreover, CBD reduced the mRNA and protein levels of a functional marker, Wilms’ tumor 1. Similar to CBD, 7-carboxy-CBD and 7-hydroxy-CBD inhibited cellular proliferation and decreased DNA synthesis. 7-Carboxy-CBD was less cytotoxic than CBD, while 7-hydroxy-CBD showed comparable cytotoxicity to CBD in both mouse and human Sertoli cells. Compared to mouse Sertoli cells, CBD, 7-hydroxy-CBD, and 7-carboxy-CBD were more cytotoxic in human Sertoli cells. Our results indicate that CBD and its main metabolites can inhibit cell proliferation in mouse and human Sertoli cells.
Keywords: Cannabidiol, 7-Carboxy-CBD, 7-Hydroxy-CBD, Male reproductive toxicity, Sertoli cells, Cell cycle arrest, DNA synthesis, Wilms’ tumor 1
Introduction
Cannabidiol (CBD) is one of the major non-psychoactive constituents found in the plant Cannabis sativa L. (marijuana) (Adams et al., 1940; Campos et al., 2012; Huestis, 2007). Beneficial effects of CBD in neuronal physiology have been reported (Grotenhermen, 2003), such as neuroprotective activity via anti-inflammatory and anti-oxidative properties (Costa et al., 2004; Hampson et al., 1998; Silvestro et al., 2020; Ward et al., 2014), sedative effects that decrease anxiety (Crippa et al., 2004; Zuardi et al., 1993), and an anti-epileptic effect that reduces seizure frequency (Elliott et al., 2019; Lattanzi et al., 2018). Recently, CBD has also been implicated to have potentially positive effects for treating ischemic stroke (Hayakawa et al., 2010), psychosis, such as schizophrenia (Batalla et al., 2019; McGuire et al., 2018), and neurodegenerative diseases, such as Alzheimer’s disease (Libro et al., 2017; Watt and Karl, 2017). In 2018, a CBD-based drug (Epidiolex®) was approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older. In 2020, FDA approved Epidiolex oral solution for the treatment of seizures associated with tuberous sclerosis complex in patients one year of age and older.
CBD has been suggested to be generally well tolerated by humans because the reported adverse events, such as diarrhea, nausea, headache, and somnolence, are mild (Taylor et al., 2018). However, concerns have been raised about the adverse effects of CBD on male reproductive physiology. For example, in sexually mature rhesus monkeys, oral administration of 30–300 mg/kg body weight/day CBD for 90 days caused a reduction of testicular size and an inhibition of spermatogenesis (Rosenkrantz et al., 1981). In 21-day-old male Swiss mice, 15 or 30 mg/kg body weight/day CBD administered orally for 34 consecutive days followed by a 35-day recovery period caused a decrease in the number of Sertoli cells, abnormalities in sperm morphology, and decreases in plasma testosterone levels (Carvalho et al., 2020; Carvalho et al., 2018). A number of studies have reported that preincubation of sperm with CBD at 0.1–10 μM inhibited fertilization in sea urchins, a model to study fertilization because of their similarity to human embryos in the early developmental stages (Schuel et al., 1991; Schuel et al., 1987). However, the underlying mechanisms of how CBD negatively influences the male reproductive system in animals have not been elucidated. In addition, reports evaluating CBD-induced male reproductive toxicity in humans remain scarce.
In humans, following oral administration, first pass metabolism of CBD is catalyzed mainly by hepatic cytochrome P450 (CYP) 2C19 and 3A4 (Jiang et al., 2011). Subsequent Phase II biotransformation of CBD occurs via glucuronidation by UDP-glucuronosyltransferase (UGT) 1A9, UGT2B7, and UGT2B17 (Mazur et al., 2009; Ujváry and Hanuš, 2016). One of the main routes of CBD metabolism is oxidation to form 7-hydroxy-CBD, which circulates in human plasma at a concentration of approximately 50% of CBD (Harvey and Mechoulam, 1990; Ujváry and Hanuš, 2016; Wall et al., 1976). 7-Hydroxy-CBD can be further oxidized to 7-carboxy-CBD (Kicman and Toczek, 2020). In humans, 7-carboxy-CBD is the most abundant circulating product in plasma, followed by CBD and 7-hydroxy-CBD. Following a single dose of a 1,500 mg CBD oral solution in healthy volunteers, the Cmax and half-life of CBD, 7-hydroxy-CBD, and 7-carboxy-CBD, were 0.9 μM and 19 h, 0.7 μM and 26 h, and 8.9 μM and 19 h, respectively (Taylor et al., 2018).
The biotransformation of CBD varies considerably between species. For instance, in mice dosed orally with CBD, the production of 7-carboxy-CBD and 7-hydroxy-CBD occurred to similar levels, whereas in rats and humans dosed orally with CBD, the production of 7-carboxy-CBD was significantly higher than 7-hydroxy-CBD (CDER/FDA, 2017). In addition, despite the considerable levels of 7-carboxy-CBD and 7-hydroxy-CBD found in plasma in both animals and humans, an evaluation of the toxicity of these metabolites, particularly, male reproductive toxicity, has not been reported.
Sertoli cells have two main functions in reproductive physiology (Petersen and Söder, 2006). First, they orchestrate testicular development during embryonic and postnatal stages (Brennan and Capel, 2004). Second, Sertoli cells are the “nurse” epithelial cells that provide structural and nutritional support to the germ cells and therefore are essential for the process of spermatogenesis (Hutchison et al., 2008). Damage to Sertoli cells can lead to a disruption of spermatogenesis that can result in a low sperm count and a decrease in sperm quality, which could jeopardize male fertility.
In vitro culturing of Sertoli cells has been demonstrated to be a reliable method to determine cellular targets and molecular pathways that mediate drug-induced toxicity on male reproductive health (Mather, 1980; Reis et al., 2015). In this study, we used immortalized mouse Sertoli cells TM4 and primary human Sertoli cells as models to evaluate the toxic effects and the underlying mechanisms of CBD and its main metabolites. Our experimental approach also provides a cross-comparison of CBD toxicity between mice and humans.
Materials and Methods
Test chemicals
CBD (Batch # NQSS1951, stated purity 100%), 7-carboxy-CBD (Batch # BDG11603, stated purity 97.4%), and 7-hydroxy-CBD (Batch # BDG11596, stated purity 97.6%) were purchased from Purisys (Athens, GA). The identity of each compound was confirmed by in-house 1H-nuclear magnetic resonance and mass spectral analyses. Dimethyl sulfoxide (DMSO) was used as the vehicle and purchased from MilliporeSigma (St. Louis, MO). The chemicals were dissolved in DMSO and stored as 1,000× stock solution at −20 °C.
Cell culture and chemicals treatments
The mouse Sertoli cell line, TM4 (ATCC® CRL-1715™), was purchased from ATCC (Manassas, VA) and cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F-12) media (MilliporeSigma) supplemented with 5% horse serum (Gibco, Gaithersburg, MD), 2.5% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, GA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Gibco). Primary human Sertoli cells were purchased from ScienCell Research Laboratories (Carlsbad, CA) and cultured in Sertoli cell medium (ScienCell Research Laboratories) supplemented with 5% FBS, 1% Sertoli cell growth supplement, and 1% antibiotic solution (ScienCell Research Laboratories) in vessels pre-coated with poly-L-lysine (ScienCell Research Laboratories). The identification of the primary human Sertoli cells was conducted by the manufacturer using immunofluorescence staining for GATA-4 and SOX-9, markers for Sertoli cells. The TM4 and human Sertoli cells were cultured at a seeding density of 5,000 cells/cm2 in 100 mm tissue culture dishes and subcultured by trypsinization (0.05% trypsin-EDTA solution, ScienCell Research Laboratories) at 95% confluency every three to four days for up to 10 passages. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. Prior to the addition of complete media containing the tested chemicals or vehicle (0.1% DMSO), the cells were seeded at a density of 1.7 × 104 cells/cm2 and cultured for 24 h.
Serum protein-binding assay
The serum protein binding of CBD in DMEM/F12 media was determined by equilibrium dialysis using a Rapid Equilibrium Dialysis (RED) Device Single-Use Plate with Inserts (Thermo Fisher Scientific, Waltham, MA). Briefly, complete media containing 100 μM CBD was dialyzed for 4 h and the concentration of CBD inside and outside the dialysis chamber was assessed by reversed phase HPLC with UV detection. The %free CBD was calculated as follows: %Free CBD = (Concentration outside the dialysis chamber/Concentration inside the dialysis chamber) × 100%. %Bound CBD = 100% - %Free CBD. The assay was conducted in triplicate on three separate days.
Measurement of cell viability
MTS (CellTiter 96® AQueous One Solution Cell Proliferation reagents, Promega, Madison, WI) and CellTiter-Blue (CellTiter-Blue® Cell Viability reagents, Promega) assays were used to measure the cell viability of TM4 and human Sertoli cells as described previously (Chen et al., 2018; Li et al., 2021). The percentage of cell viability was calculated from normalization to the control group.
Immunofluorescence staining
TM4 and human Sertoli cells were seeded in pre-coated 18-well chamber slides (ibidi, Martinsried, Planegg, Germany) for 24 h prior to CBD treatment. After CBD exposure, cells were washed with 1 × PBS and fixed with 4% formaldehyde for 10 min at room temperature. Triton™X-100 (0.5%) in 1 × PBS was used to permeabilize the cellular membrane and then cells were blocked with 1 × Blocker™ BSA in PBS (Thermo Fisher Scientific) for 30 min. After blocking, the cells were incubated with DyLight 488-Phalloidin (Thermo Fisher Scientific) in 1 × Blocker™ BSA in PBS for 30 min. Prior to image acquisition, cell nuclei were stained for 10 min with Hoechst 33342 (Thermo Fisher Scientific). For each sample, the images from at least three randomly selected fields were captured using a Cytation 5 Cell Imaging Reader (BioTek, Winooski, VT).
Cell cycle analysis
To analyze cell cycle progression, propidium iodide (MilliporeSigma) was used to stain the nucleic acids for flow cytometry. TM4 and human Sertoli cells were seeded in 6-well plates for 24 h prior to treatment with CBD or the vehicle. After a 24-h exposure, cells were harvested and adjusted to 106 cells/sample and then fixed in cold 50% ethanol for 1 h on ice. RNA in the cells was digested with 200 μg/mL RNase A at 37°C for 1 h. To stain the DNA, 10 μg/mL propidium iodide was added and the cells were incubated at 4°C overnight. A FACSCanto™ flow cytometer with FACSDiva™ software (BD Biosciences, San Jose, CA) was used to detect propidium iodide signals and FlowJo® software (FlowJo, LLC, Ashland, OR) was used to determine the percentage of cells in each cell cycle phase.
EdU proliferation assay
To measure the proliferative ability of the TM4 and human Sertoli cells, DNA synthesis was monitored using 5-ethynyl-2´-deoxyuridine (EdU) staining. EdU, a thymidine analog nucleoside, is incorporated into DNA and is correlated with DNA synthesis activity (Buck et al., 2008). Cells were seeded in 6-well plates for 24 h and then exposed to CBD or its metabolites for 24 h. A Click-iT® EdU Flow Cytometry Assay kit (Thermo Fisher Scientific) was used to supply 10 μM EdU to the cells for 2 h before DNA synthesis detection, which was based on the copper-catalyzed reaction between the azide moiety in Alexa Fluor® 488 dye and the alkyne group in EdU. Before staining, the number of cells was adjusted to 106 cells/sample. Fixation, permeabilization, and Click-iT® reaction steps were performed following the manufacturer’s protocol. The FACSCanto™ flow cytometer with FACSDiva™ software was used to detect Alexa Fluor® 488 signals and FlowJo® software was used to determine the percentage of cells in the S phase.
RNA isolation and quantitative real-time PCR
Quantitative real-time PCR was used to examine the expression level of a functional gene marker in the Sertoli cells. Murine TM4 and human Sertoli cells were seeded in 6-well plates and cultured for 24 h prior to treatment with CBD or the vehicle. Total RNA was isolated using a RNeasy Mini kit (Qiagen, Valencia, CA). The yield of the extracted RNA was determined using a NanoDrop™ 8000 spectrophotometer (Thermo Fisher Scientific). The purity and quality of RNA were measured using an RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA) and an Agilent 2100 Bioanalyzer (Agilent Technologies). Complementary DNA (cDNA) was generated by reverse transcription of 1 μg total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR assays for Wilms’ tumor 1 (WT1) were conducted using a Bio-Rad CFX96TM Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Reactions were prepared using TaqMan® Gene Expression Assays (Thermo Fisher Scientific), including mouse wt1 (Mm01337048_m1), mouse gapdh (Mm99999915_g1), human WT1 (Hs01103751_m1), and human GAPDH (Hs02758991_g1). Assays were run in three technical replicates per experimental condition with FastStart™ Universal Probe Master (Rox) (Roche, Basel, Switzerland), using universal cycling conditions (10 min at 95 °C; 15 s at 95 °C, 1 min 60 °C, 40 cycles). The relative expression level of WT1 was calculated using the 2−ΔΔCt method and normalized to the expression level of the housekeeping gene, GAPDH.
Western blot analysis
Primary antibodies targeting cyclin D1 (#2978), cyclin D3 (#2936), CDK2 (#2546), CDK4 (#12790), CDK6 (#3136), and GAPDH (#5174) were purchased from Cell Signaling Technology (Danvers, MA). Primary antibody for Wilms’ tumor 1 (ab224806) was purchased from Abcam (Cambridge, MA). Secondary horseradish peroxidase (HRP)-conjugated antibodies (sc-2357 and sc-516102) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). TM4 and human Sertoli cells were seeded in 100 mm dishes and cultured for 24 h prior to treatment with CBD or the vehicle. After 24 h of CBD treatment, the cells were harvested, lysed, and the targeted proteins were analyzed by Western blotting as previously described (Li et al., 2020). The protein band intensity was quantified by AlphaView software (San Jose, CA).
Statistical analyses
All data are presented as the mean ± standard deviation (SD) and calculated from three independent experiments. Statistical analyses were conducted by one-way or two-way analysis of variance (ANOVA), as appropriate. Comparisons between groups were conducted by the Holm-Sidak method. If necessary, to maintain an equal variance or normal data distribution, the data were natural log (ln) transformed before conducting the analyses. Concentration-related trends were assessed by linear regression analyses. A p < 0.05 was considered significant.
Results
CBD decreased cell viability in TM4 and human Sertoli cells
It has been reported that the extent of CBD binding with plasma proteins in humans ranges from 86.7 to 92.2% (Tayo et al., 2020). To determine the protein binding of CBD in complete culture media, a Rapid Equilibrium dialysis was conducted. The %Free CBD was < 0.3 ±0.1% and the %Bound CBD was > 99.7%. Thus, to perform our study under a more clinically relevant condition, CBD treatment was performed in the presence of serum in the culture media.
The cytotoxicity of CBD in TM4 and human Sertoli cells was assessed by cell viability measurements using MTS and CellTiter-Blue assays. In TM4 mouse Sertoli cells, treatment with CBD at 0, 10, 12.5, 15, 17.5, and 20 μM for 24 and 48 h decreased cell viability in a concentration- and time-dependent manner as assessed by the MTS assay (Figure 1A). At 48 h, cell viability was significantly decreased by 12.5, 15, 17.5, and 20 μM CBD as compared to both control cultures and cells treated with the same concentrations of CBD for 24 h. Compared to the control group at 48 h, 20 μM CBD reduced cell viability to 57% (Figure 1A). Similarly, when assessed with the CellTiter-Blue assay, treatment with CBD at 0, 10, 12.5, 15, 17.5, and 20 μM for 24 and 48 h decreased cell viability in a concentration- and time-dependent manner (Figure 1B). At 48 h, cell viability in TM4 mouse Sertoli cells treated with 20 μM CBD was decreased to 40% of the DMSO control cells (Figure 1B) indicating a significant decrease in the number of healthy cells; the IC50 of CBD was 15.8 μM (Table 1).
Figure 1. CBD induces cytotoxicity in TM4 and human Sertoli cells.
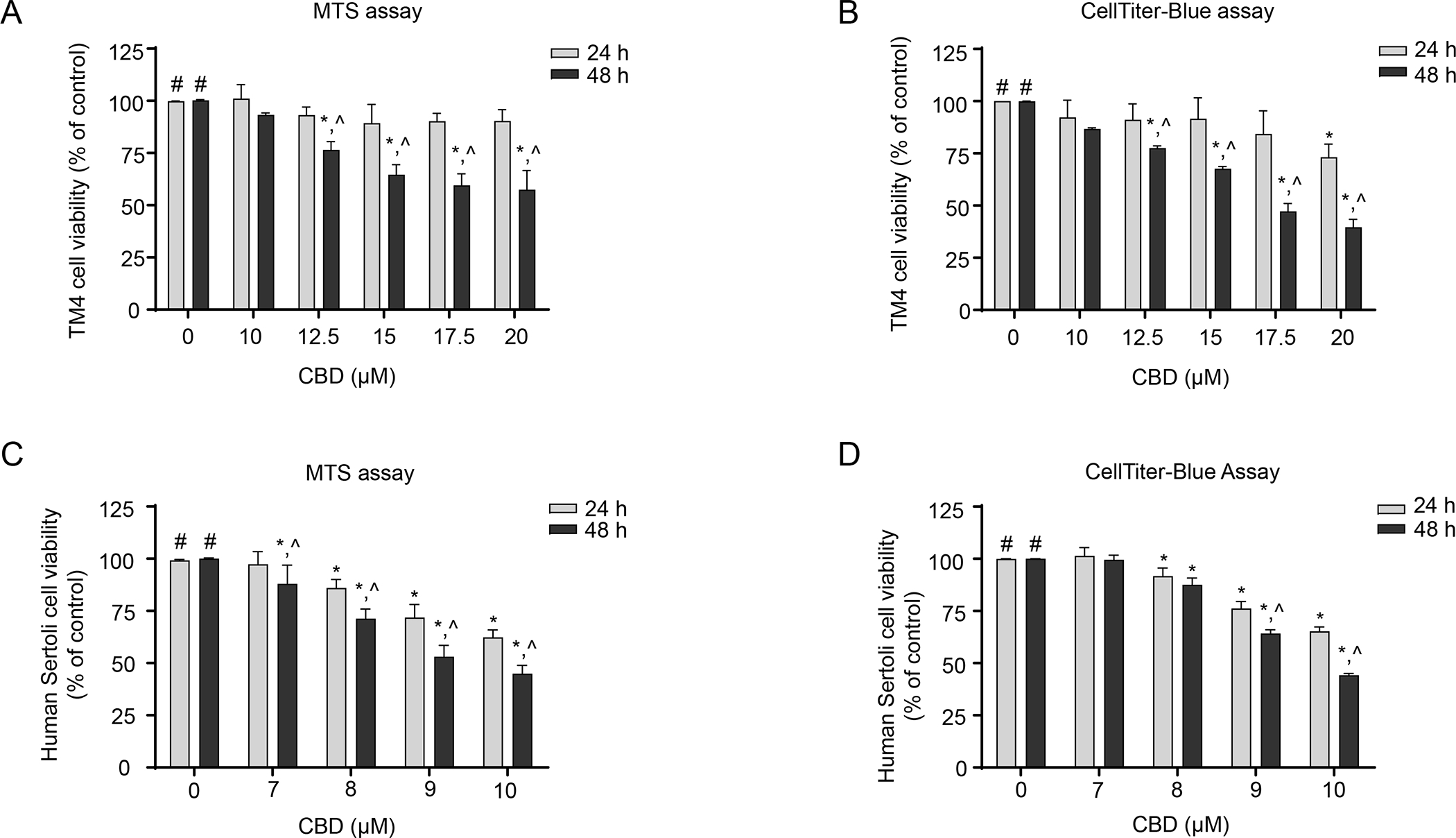
TM4 and human Sertoli cells were treated with DMSO (0 μM) or the indicated CBD concentrations for 24 h or 48 h. Cytotoxicity was determined using MTS assays (A and C) and CellTiter-Blue assays (B and D). Bar graphs are means ± SD (n=3). #, significant concentration-related linear trend. *, significantly different from the DMSO control at the same time point. ^, significantly different from the same concentration at 24 h.
Table1.
The half inhibitory concentration (IC50) of CBD, 7-hydroxy-CBD, and 7-carboxy-CBD on cell viability.a
| TM4 cells | Human Sertoli cells | |||
|---|---|---|---|---|
| IC50 (μM) | 95% confidence intervals (μM) | IC50 (μM) | 95% confidence intervals (μM) | |
|
| ||||
| CBD | 15.8 | 15.1 – 16.7 | 9.8Δ | 8.0 – 11.9 |
| 7-Hydroxy-CBD | 16.3 | 14.4 – 18.5 | 10.7Δ | 10.0 – 12.0 |
| 7-Carboxy-CBD | 70.3*, # | 68.3 – 72.4 | 55.3*, #, Δ | 52.4 – 56.0 |
Significantly different from cells treated with CBD
significantly different from cells treated with 7-hydroxy-CBD
significantly different from TM4 cells treated with the same compound.
TM4 and human Sertoli cells were incubated with various concentrations of CBD, 7-hydroxy-CBD, or 7-carboxy-CBD for 48 h. The IC50 values were obtained from the CellTiter-Blue viability curve, using GraphPad Prism 6.0. Data are presented as the mean IC50 and 95% confidence intervals of three independent experiments.
In human Sertoli cells, CBD at concentrations between 7–10 μM induced concentration- and time-dependent decreases in cell viability (Figures 1C and D). In MTS and CellTiter-Blue assays, 10 μM CBD treatment for 48 h decreased the cell viability to 45% (Figure 1C) and 44% (Figure 1D) of the DMSO control. The IC50 of CBD was 9.8 μM (Table 1). These results indicate that similar to TM4 mouse Sertoli cells, CBD caused significant decreases in cell viability in human Sertoli cells.
CBD induced G1 cell cycle arrest in TM4 and human Sertoli cells
To investigate the possible mechanisms of CBD-induced decreases in cell viability in TM4 and human Sertoli cells, a flow cytometric cell cycle analysis was conducted using propidium iodide staining. In both TM4 and human Sertoli cells, CBD induced a significant increase in the percentage of cells in G1 phase, with a concomitant decrease in the percentage of cells in S phase. The proportion of G2/M phase cells remained approximately the same in controls and CBD-treated samples (Figures 2A–D). In TM4 cells at 24 h, 20 μM CBD increased the percentage of cells in G1 phase to 52% as compared to 39% in the DMSO control, while the percentage of cells in S phase decreased to 11% as compared to 28% in the control (Figure 2B). In human Sertoli cells treated with 10 μM CBD, the percentage of cells in G1 phase increased to 59% as compared to 41% in the control, while the percentage of cells in S phase decreased to 7% as compared to 25% in the control (Figure 2D). These results indicate that CBD induced a G1 cell cycle arrest and inhibited the cell cycle progression in a concentration-dependent manner in TM4 and human Sertoli cells.
Figure 2. CBD arrests TM4 and human Sertoli cells in G1 to S phase transition.
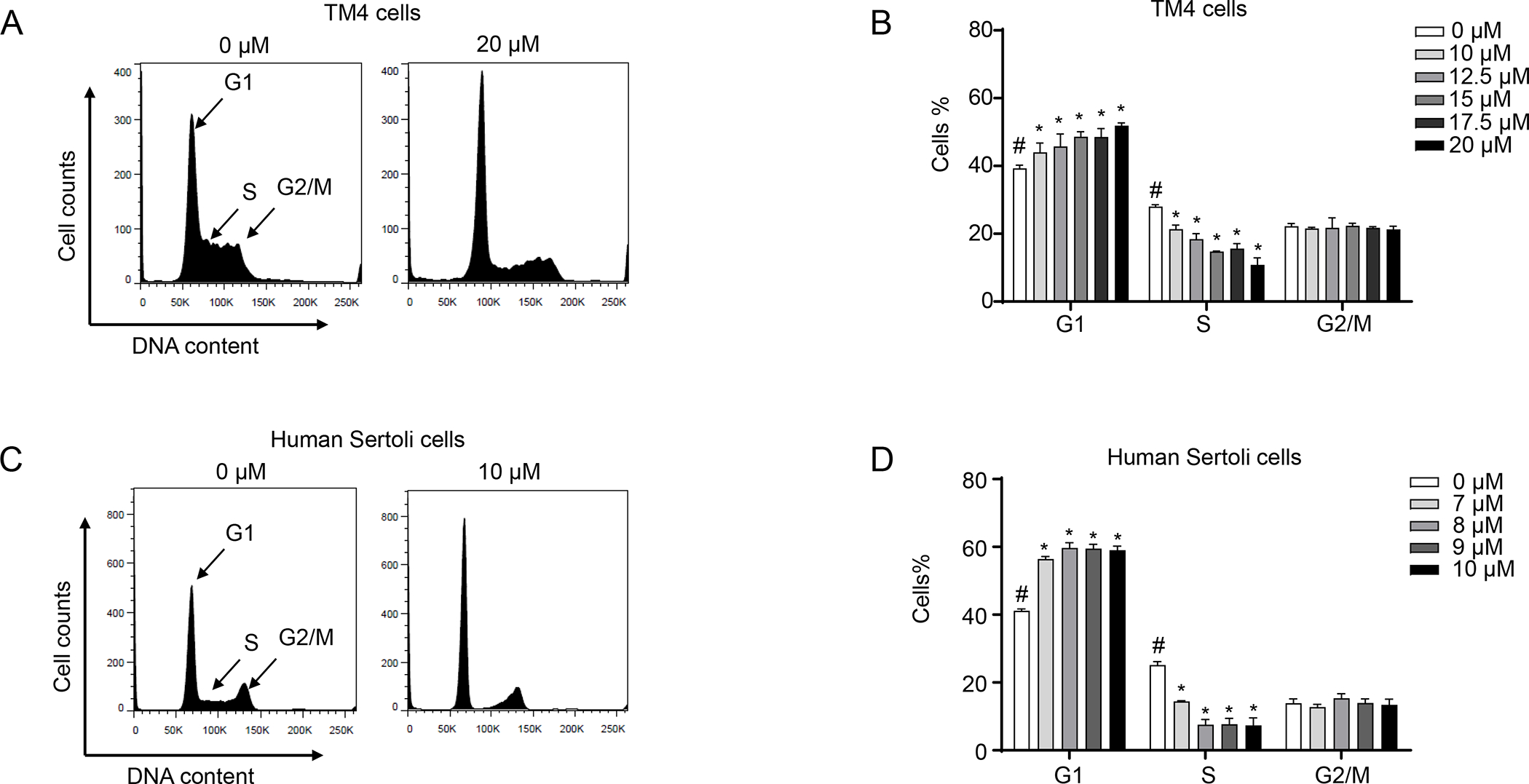
TM4 and human Sertoli cells were treated with DMSO (0 μM) or the indicated concentrations of CBD for 24 h. Cells were stained with propidium iodide and analyzed by flow cytometry. (A and C) Representative histograms show DNA content analyses. (B and D) The bar graph represents the mean percentage of each cell cycle phase ± SD (n=3). #, significant concentration-related linear trend. *, significantly different from the DMSO control.
We next investigated the mechanisms underlying CBD-induced cell cycle disruption in TM4 and human Sertoli cells. To directly measure DNA synthesis during S phase, we applied EdU staining, coupled with flow cytometric analysis. As shown in Figures 3A and B, the percentage of EdU-positive TM4 cells was reduced to 28% as compared to 51% in the control after a 20 μM CBD exposure for 24 h. As shown in Figures 3C and D, 10 μM CBD decreased the percentage of EdU-positive human Sertoli cells to 2% as compared to 32% in the control. These findings suggest that DNA replication was disrupted by the CBD treatment and therefore dividing cells became the minority of the population. These results further confirmed our previous results suggesting CBD induced cell cycle arrest (Figure 2) and therefore support the notion that CBD inhibited proliferation of TM4 and human Sertoli cells via arresting cell cycle progression from G1 to S phase.
Figure 3. CBD inhibits DNA synthesis in TM4 and human Sertoli cells.
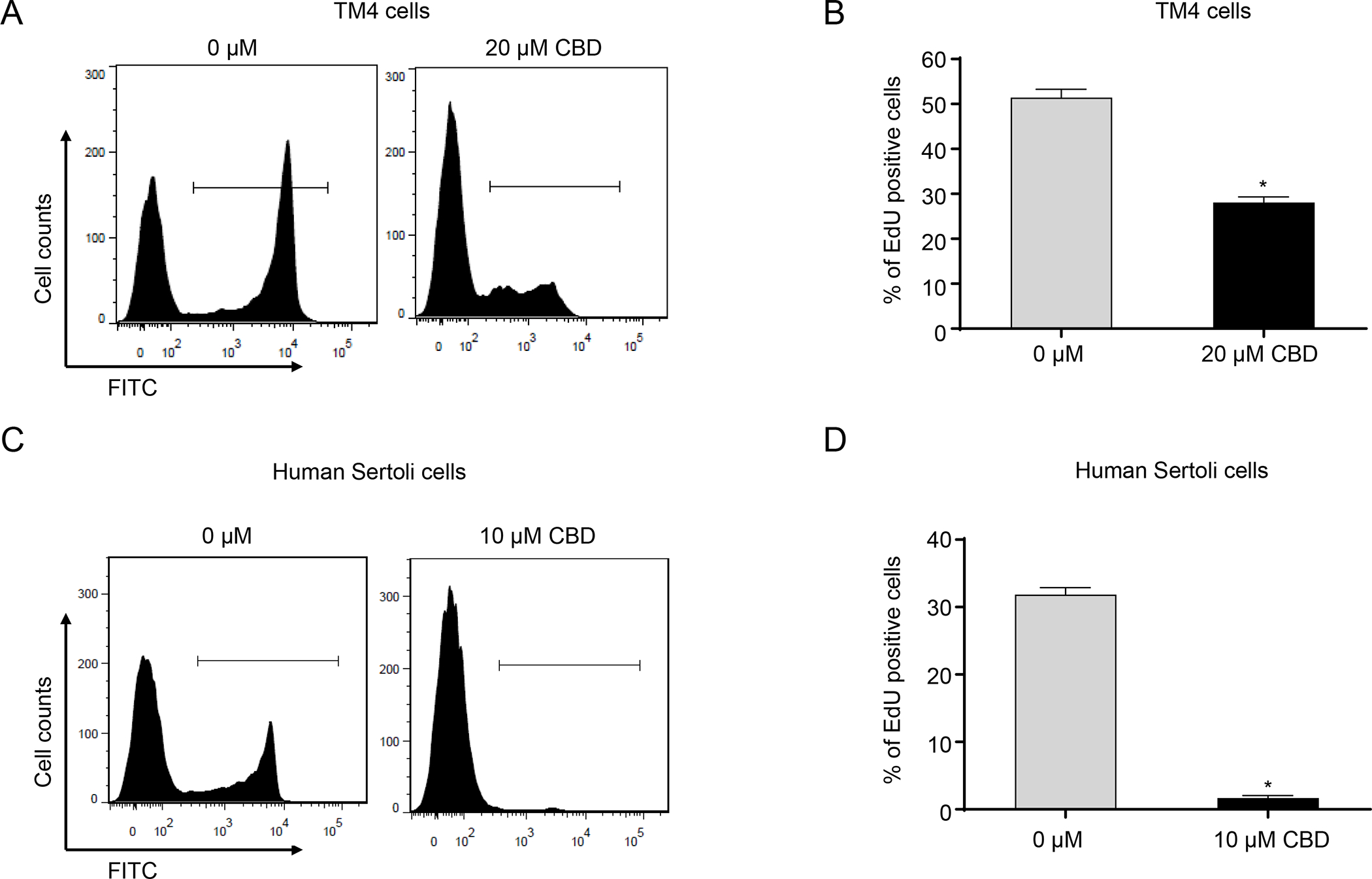
Representative histograms show EdU staining for DNA synthesis in DMSO (0 μM)- and 20 μM CBD-treated TM4 cells (A) and in DMSO (0 μM)- and 10 μM CBD-treated human Sertoli cells (C) for 24 h. (B and D) The bar graph shows the mean percentage of EdU positive cells ± SD (n=3). *, significantly different from the DMSO control.
We then investigated the changes in cell cycle-related proteins, using Western blot analyses. CDK4/6 forms a complex with cyclin D that stimulates expression of genes required for G1 progression and the transition from G1 to S phases (Goel et al., 2018; Sherr, 1993). CDK2 in late G1 phase also plays a role in promoting cells to the S phase (Sherr, 1993). In both TM4 and human Sertoli cells, the protein levels of cyclin D1, cyclin D3, CDK2, CDK4, and CDK6 decreased in a concentration-dependent manner upon CBD treatment for 24 h (Figures 4A–D), suggesting that CBD disturbed cell cycle checkpoints via downregulation of cell cycle-related proteins. Thus, these results indicate CBD treatment disrupted cell cycle checkpoint controls, inhibited cellular DNA synthesis, and arrested TM4 and human Sertoli cells in G1 phase.
Figure 4. CBD decreases cell cycle-related proteins in TM4 and human Sertoli cells.
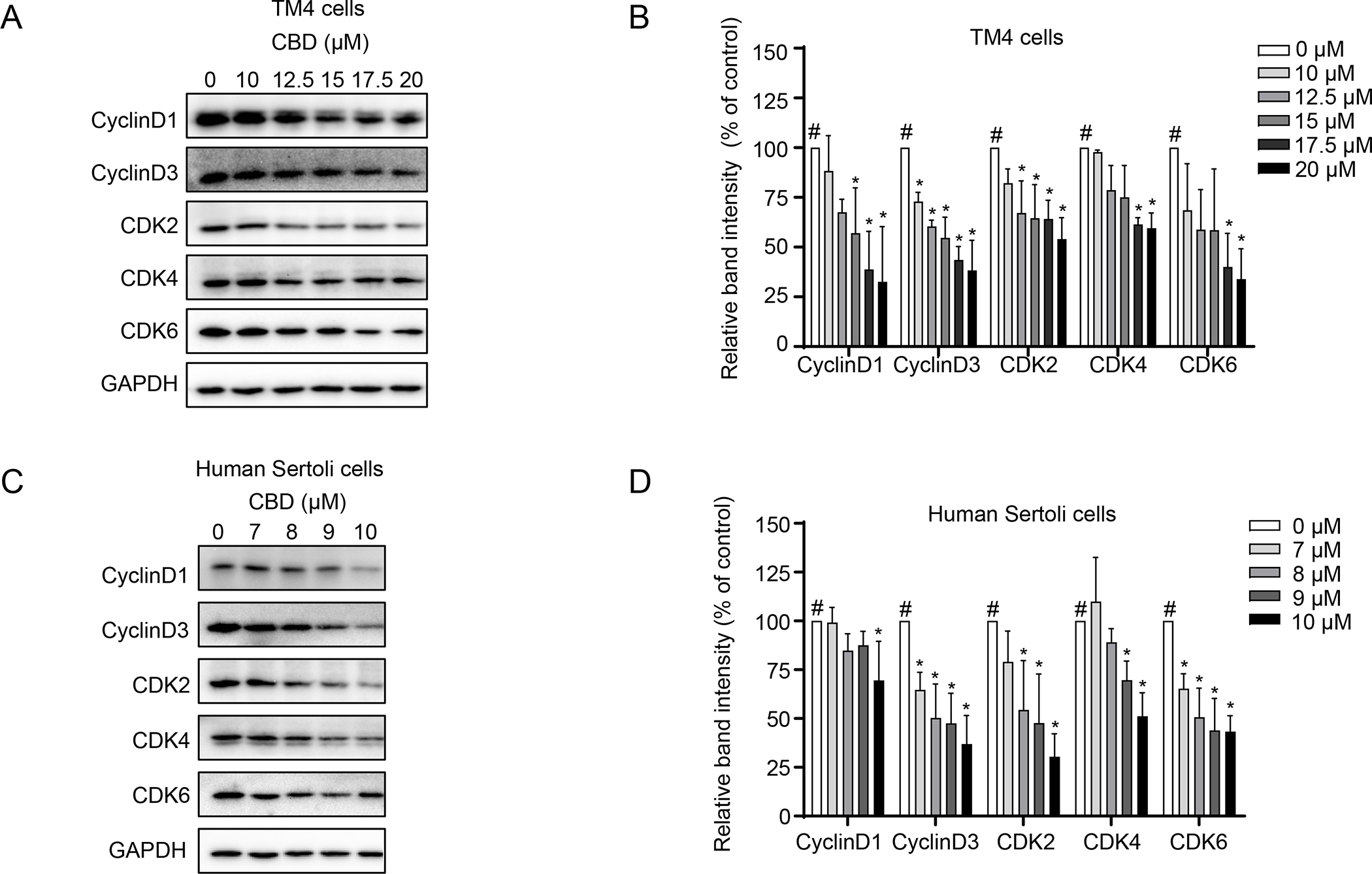
Total cellular proteins were extracted from TM4 and human Sertoli cells after a 24 h CBD treatment. The levels of cyclin D1, cyclin D3, CDK2, CDK4, and CDK6 were determined by Western blotting. GAPDH was used as an internal control. Representative Western blots are shown in A and C, and quantification is presented in B and D. The results represent means ± SD (n=3). The intensity of each protein band was normalized to its individual GAPDH band. #, significant concentration-related linear trend. *, significantly different from the DMSO control.
CBD induced cellular morphological changes in TM4 and human Sertoli cells
To explore whether CBD affects cellular morphology in TM4 and human Sertoli cells, we assessed the distribution of a cytoskeletal protein, filamentous actin (F-actin), using phalloidin fluorescence staining. In DMSO-treated TM4 and human Sertoli cells, F-actin appeared uniformly distributed in the cytoplasm (Figures 5A and B). Treatment with 20 μM CBD in TM4 cells and treatment with 10 μM CBD in human Sertoli cells for 24 h resulted in F-actin cytoskeleton reorganization, as evident by thick F-actin bundles or F-actin aggregation in the cytoplasm (Figures 5A and B). Since F-actin is essential for important cellular functions, such as the motility and contraction of cells during cell division, F-actin reorganization may be an upstream event of CBD-induced inhibition of cellular proliferation.
Figure 5. CBD induces cellular morphological changes in TM4 and human Sertoli cells.
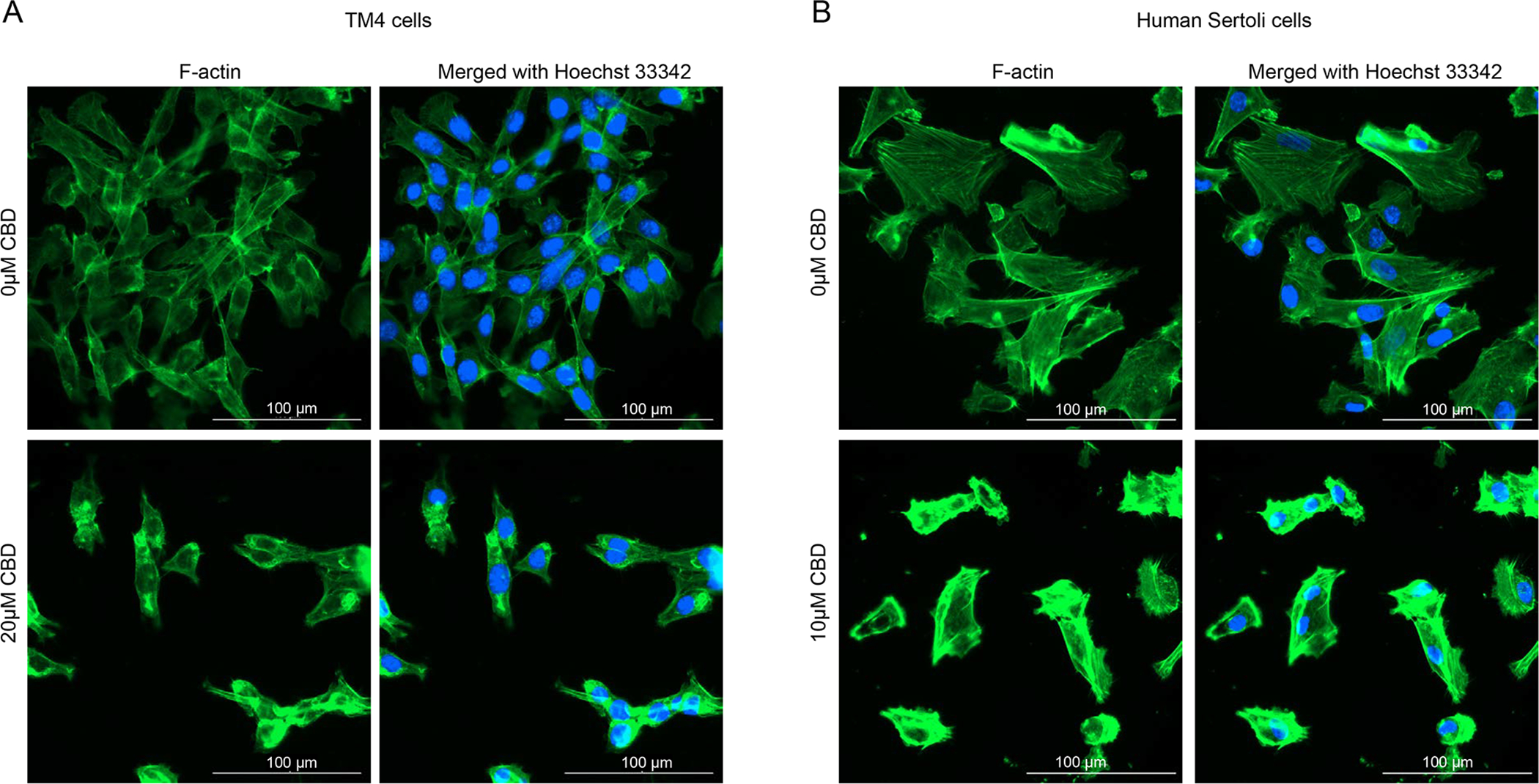
(A) TM4 cells were treated with DMSO (0 μM) or 20 μM CBD for 24 h. (B) Human Sertoli cells were treated with DMSO (0 μM) or 10 μM CBD for 24 h. Cells were stained for F-actin (green, DyLight 488-phalloidin) and nuclear DNA (blue, Hoechst 33342). Immunofluorescent images are representative of three independent experiments. Scale bar is 100 μm.
Analysis of the expression of Wilms’ tumor 1 in CBD treated TM4 and human Sertoli cells
Wilms’ tumor 1 (Wt1) is a marker gene that plays an essential role in male sexual development and the regulation of spermatogenesis (Call et al., 1990; Gao et al., 2006; Wang et al., 2013). To examine whether CBD-induced toxicity interferes with the spermatogenic functions of Sertoli cells, we examined the mRNA and protein expression levels of WT1 following CBD treatment, using quantitative real-time PCR and Western blotting assays. As shown in Figures 6A–D, CBD decreased the expression of Wt1 at both transcriptional and protein levels in a concentration-dependent manner in both TM4 and human Sertoli cells. When TM4 cells were exposed to 20 μM CBD for 24 h, the Wt1 mRNA level decreased to 28% and the WT1 protein level reduced to 48% of the DMSO control (Figures 6A and B). When human Sertoli cells were exposed to 10 μM CBD for 24 h, the WT1 mRNA level decreased to 39% and the WT1 protein level reduced to 49% of the DMSO control (Figures 6C and D). These results suggest that spermatogenic functions in TM4 and human Sertoli cells may be impaired by CBD via downregulation of WT1.
Figure 6. Effects of CBD on the mRNA and protein levels of WT1 in TM4 and human Sertoli cells.
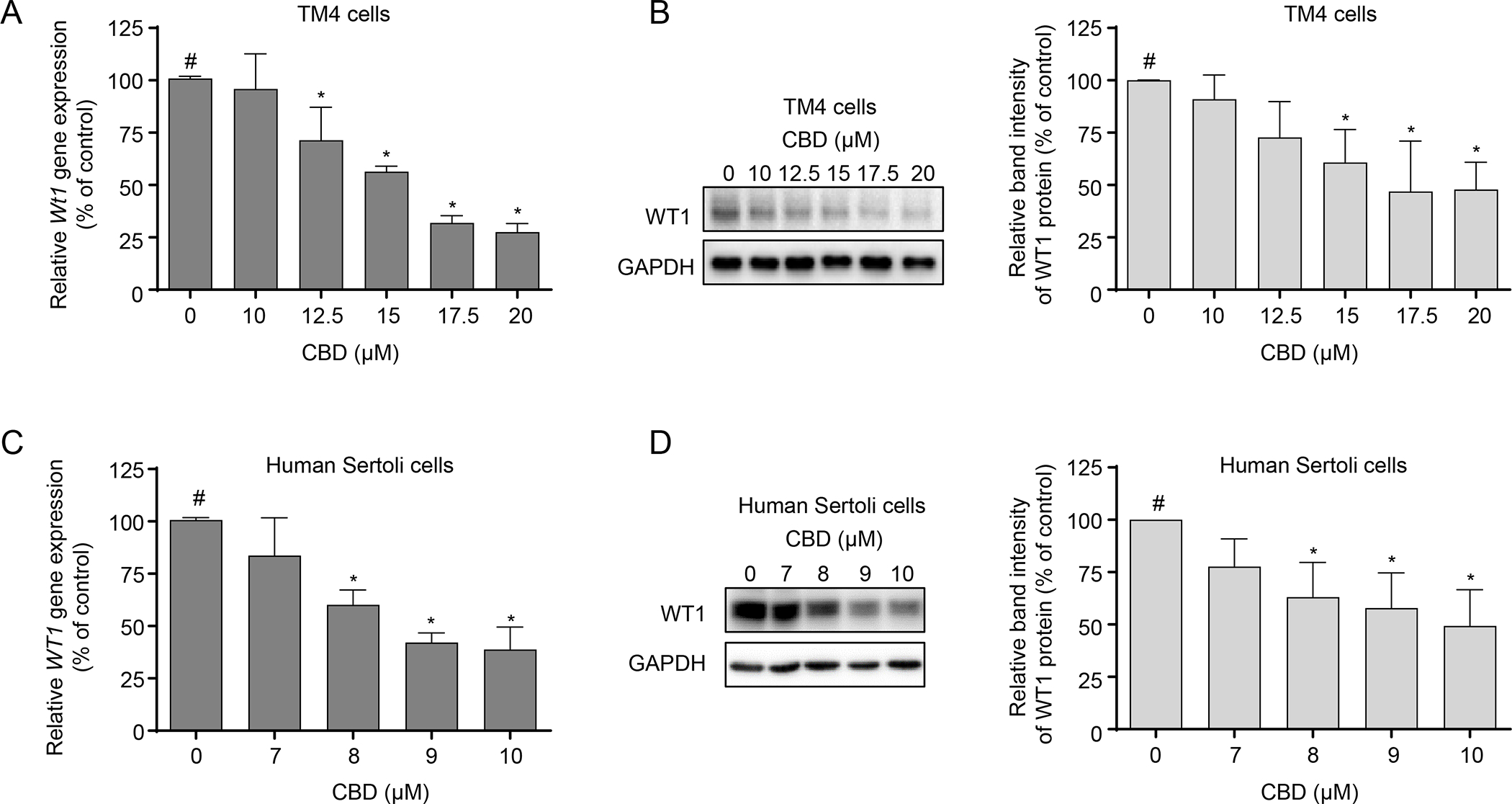
TM4 and human Sertoli cells were treated with DMSO (0 μM) or the indicated CBD concentrations for 24 h. Relative expression of Wt1 was determined using real-time PCR in CBD-treated TM4 (A) and human Sertoli cells (C). The results shown are means ± SD (n=3). #, significant concentration-related linear trend. *, significantly different from the DMSO control. Western blot analysis of WT1 was conducted in CBD-treated TM4 (B) and human Sertoli cells (D). Representative Western blots are shown in the left panel, and quantification is shown in the right panel. The results represent means ± SD (n=3). #, significant concentration-related linear trend. *, significantly different from the DMSO control.
7-Carboxy-CBD and 7-hydroxy-CBD induced cytotoxicity in TM4 and human Sertoli cells
To study the cytotoxicity of the metabolites of CBD, we examined the percentage of viable cells in mouse and human Sertoli cells exposed to 7-carboxy-CBD and 7-hydroxy-CBD, using the CellTiter-Blue assay. As shown in Figures 7A and B, both 7-carboxy-CBD and 7-hydroxy-CBD caused a concentration- and time-dependent decrease in cell viability in TM4 cells. After a 48-h treatment, at the highest tested concentration, 100 μM 7-carboxy-CBD and 20 μM 7-hydroxy-CBD reduced the cell viability in TM4 cells to 8% and 35% of the DMSO control, respectively (Figures 7A and B). The IC50 values of 7-carboxy-CBD and 7-hydroxy-CBD were 70.3 and 16.3 μM, respectively (Table 1). As indicated earlier, the IC50 of CBD in TM4 cells was 15.8 μM (Table 1). Therefore, 7-hydroxy-CBD exhibited comparable toxicity to CBD, while 7-carboxy-CBD was less toxic than CBD and 7-hydroxy-CBD in TM4 mouse Sertoli cells.
Figure 7. 7-Carboxy-CBD and 7-hydroxy-CBD induce cytotoxicity in TM4 and human Sertoli cells.
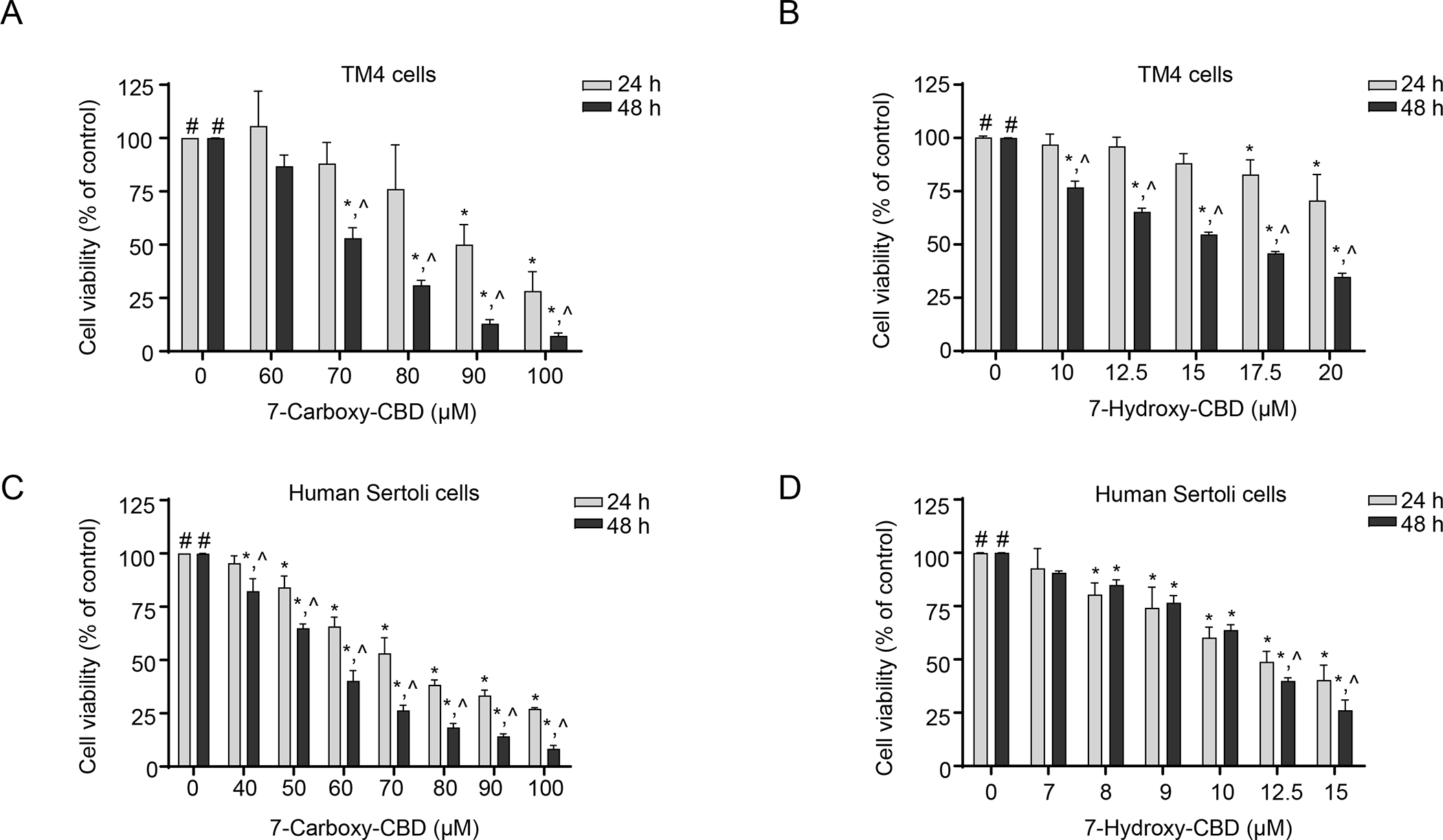
(A and B) TM4 cells were exposed to 60–100 μM 7-carboxy-CBD (A) or 10–20 μM 7-hydroxy-CBD (B) for 24 h or 48 h. (C and D) Human Sertoli cells were exposed to 40–100 μM 7-carboxy-CBD (C) or 7–15 μM 7-hydroxy-CBD (D) for 24 h or 48 h. Cell viability was quantified using a CellTiter-Blue assay. The results represent means ± SD (n=3). #, significant concentration-related linear trend. *, significantly different from the DMSO control at the same time point. ^, significantly different from the same concentration at 24 h.
In human Sertoli cells, after a 48-h treatment, 100 μM 7-carboxy-CBD and 15 μM 7-hydroxy-CBD decreased the cell viability to 8% and 26% of the control, respectively (Figures 7C and D). The IC50 values of 7-carboxy-CBD and 7-hydroxy-CBD were 55.3 and 10.7 μM, respectively (Table 1), as compared to an IC50 of 9.8 μM for CBD (Table 1). Thus, in human Sertoli cells, 7-carboxy-CBD was less toxic than either 7-hydroxy-CBD or CBD.
To analyze further the possible cytotoxic mechanisms of 7-carboxy-CBD and 7-hydroxy-CBD, we used EdU staining to examine the changes in S phase DNA synthesis in both mouse and human Sertoli cells at 24 h. In TM4 cells, 70 μM 7-carboxy-CBD and 20 μM 7-hydroxy-CBD decreased the EdU positive cells to 37% and 19%, respectively, as compared to 54% in the control (Figures 8A and B). These data suggest that the mechanism of cytotoxicity for the two metabolites was similar to CBD, i.e., an inhibition of DNA synthesis during S phase of the cell cycle. In human Sertoli cells, 60 μM 7-carboxy-CBD and 10 μM 7-hydroxy-CBD decreased the EdU positive cells to 26% and 25%, respectively, as compared to 32% in the controls (Figures 8C and D). By comparison, 10 μM CBD decreased the EdU positive cells to 2% (Figure 3D). Thus, in human Sertoli cells, CBD was more potent than its metabolites in disrupting DNA synthesis.
Figure 8. 7-Carboxy-CBD and 7-hydroxy-CBD inhibit DNA synthesis in TM4 and human Sertoli cells.
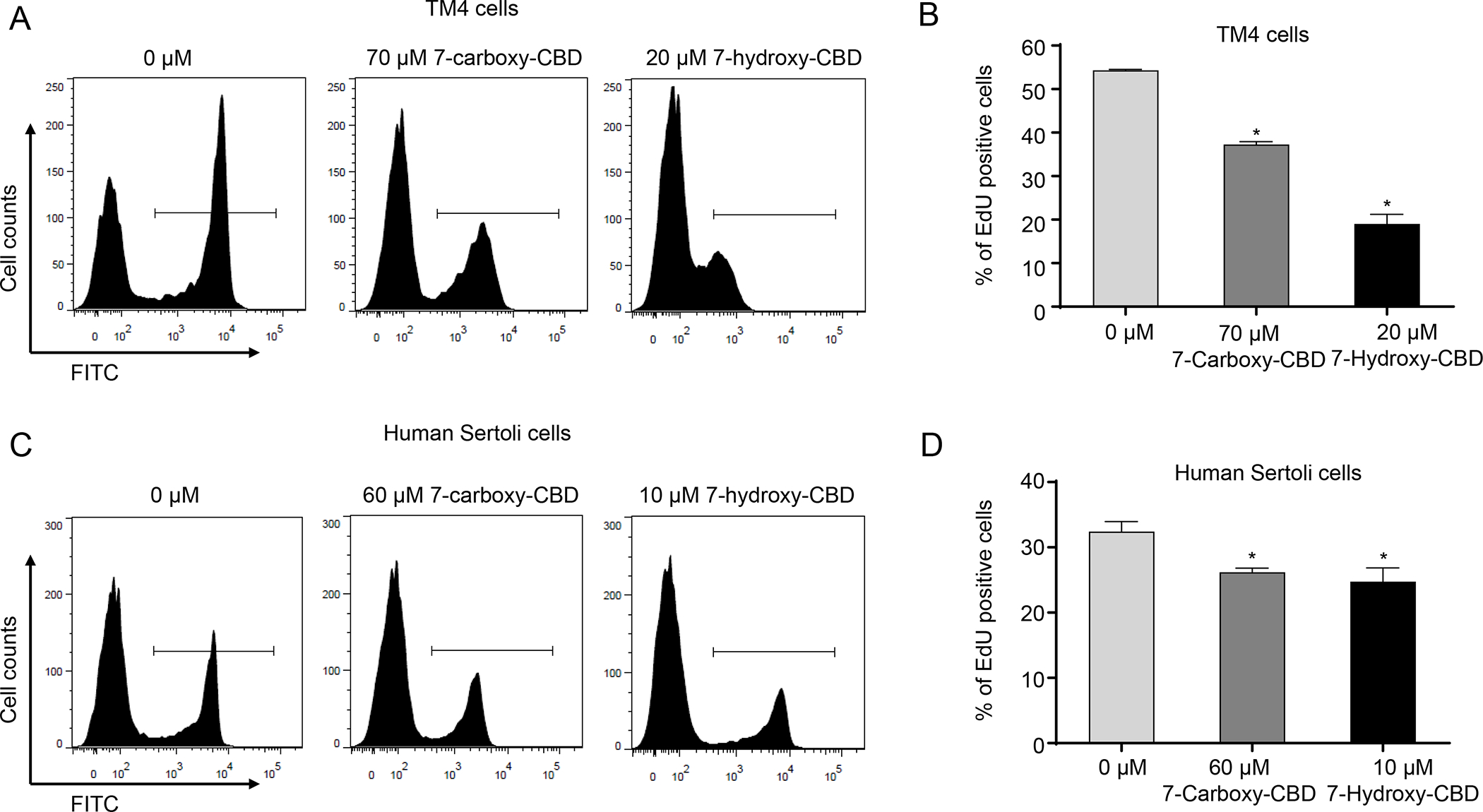
TM4 and human Sertoli cells were treated with DMSO (0 μM), 7-carboxy-CBD, or 7-hydroxy-CBD at the indicated concentrations for 24 h. Representative histograms show EdU staining for DNA synthesis in DMSO, 7-carboxy-CBD, and 7-hydroxy-CBD-treated TM4 (A) and human Sertoli cells (C). (B and D) The bar graphs show the mean percentage of EdU positive cells ± SD (n=3). *, significantly different from the DMSO control.
Discussion
The proliferation of Sertoli cells in rodents past the perinatal phase and into pre-pubertal and adult life maintains the Sertoli cell numbers within a proper range (Figueiredo et al., 2016). Recently, Carvalho et al. (Carvalho et al., 2018) reported that chronic exposure to CBD induced a significant decrease in the number of Sertoli cells in male Swiss mice during adolescence. In good agreement with these findings, our data indicate that CBD inhibits the proliferation of Sertoli cells in vitro. Specifically, we demonstrated that CBD inhibited S phase DNA synthesis and induced G1-phase arrest in mouse and human Sertoli cells, which likely inhibited cellular proliferation. In addition, we observed a decrease in the nuclear transcription factor WT1, a functional marker of Sertoli cells, in CBD-treated mouse and human Sertoli cells following 24 h of exposure. Moreover, the main CBD metabolites found circulating in serum, 7-carboxy-CBD and 7-hydroxy-CBD, were also cytotoxic to mouse and human Sertoli cells, inhibiting DNA synthesis during the S phase.
Necrosis and apoptosis are two common outcomes of cytotoxicity and cellular injury. These are not likely to be the major mechanisms responsible for the decrease in Sertoli cells following CBD, 7-carboxy-CBD, or 7-hydroxy-CBD treatments because we did not observe increases in LDH release or caspase 3/7 enzymatic activity at the 24-h and 48-h timepoints (data not shown). Currently, it is difficult to pinpoint the specific molecular target of CBD in Sertoli cells that results in cytotoxicity. To understand the underlying molecular mechanisms of CBD-induced toxicity in Sertoli cells, experiments, such as RNA sequencing analyses, are ongoing in our laboratory.
CBD and 7-hydroxy-CBD induced cytotoxicity at similar levels in mouse and human Sertoli cells (Table 1); 7-carboxy-CBD was less cytotoxic by a factor of about five in both types of cells. Compared to mouse Sertoli cells, CBD, 7-hydroxy-CBD, and 7-carboxy-CBD were slightly more cytotoxic in human Sertoli cells. The reasons for the differences in CBD toxicity between mice and human Sertoli cells are not known but could be due to differences in cellular uptake of CBD between the two species. Using a liquid chromatography tandem mass spectrometry method (unpublished), we detected higher levels of CBD in human Sertoli cells compared to mouse Sertoli cells under the same treatment conditions (Supplemental Figure 1). To confirm our findings, further bioavailability/permeability studies are necessary.
Another significant finding of our study is that CBD treatment significantly decreased WT1 at both transcriptional and protein levels in mouse and human Sertoli cells. WT1 is highly expressed in Sertoli cells in the seminiferous epithelium of the adult testis and is essential at multiple steps in testicular development. For instance, deletion of Wt1 in Sertoli cells disrupted tubular architectural maintenance in the developing testis (Gao et al., 2006). Deletion of Wt1 in adult mice caused a dramatic reduction in testes size and atrophy of seminiferous tubules, with significant cell loss, and a damaged blood-testis barrier (Wang et al., 2013). WT1 mutations have also been found in human non-obstructive azoospermia patients (Wang et al., 2013). Our findings of decreased WT1 expression following CBD treatment further support the notion that CBD might have negative effects on the male reproductive system.
The recommended dose of CBD for treating patients with epilepsy is 5–20 mg/kg body weight/day (equivalent to 375–1,500 mg/day for a 75 kg adult). In a pharmacokinetics study with healthy volunteers who consumed 1,500 mg/day CBD (750 mg CBD twice daily) for seven consecutive days, the Cmax of CBD in plasma was 0.9 μM on the morning of Day 1, 2.3 μM on the afternoon of Day 1, and 1.1 μM on the morning of Day 7 (Taylor et al., 2018). In the same individuals, the 7-hydroxy-CBD Cmax was 0.6 μM and the 7-carboxy-CBD Cmax was 15.4 μM on the afternoon of Day 1. On the morning of Day 7, the 7-hydroxy-CBD Cmax was 0.5 μM and the 7-carboxy-CBD Cmax reached 28.5 μM. Following a high-fat breakfast, the Cmax of CBD, 7-hydroxy-CBD, and 7-carboxy-CBD increased 5-, 3-, and 2-fold compared to a fasted state (Taylor et al., 2018). In addition, preexisting liver diseases may alter the Cmax. For instance, in hepatic-impaired human subjects, the CBD Cmax was increased in parallel with the severity of hepatic impairment (Taylor et al., 2019). In the present study, the concentrations of CBD (7–10 μM), 7-carboxy-CBD (60–100 μM), and 7-hydroxy-CBD (8–15 μM) used in the incubations with the human Sertoli cells approached human plasma concentrations, and thus are clinically relevant.
In summary, our study demonstrated that CBD inhibited cellular proliferation, disrupted the G1 to S phase transition in cell cycle, altered cytoskeleton organization, and suppressed the expression of WT1. 7-Carboxy-CBD and 7-hydroxy-CBD, the main metabolites of CBD, also induced inhibition of cellular proliferation and decreased DNA synthesis during S phase of the cell cycle in human and mouse Sertoli cells.
Supplementary Material
Acknowledgments:
This work was supported by U.S. Food and Drug Administration’s intramural grant program. XL and YL were supported by appointments to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Compliance with ethical standards
Disclaimer: This article reflects the views of the authors and does not necessarily reflect those of the U.S. Food and Drug Administration. Any mention of commercial products is for clarification only and is not intended as approval, endorsement, or recommendation.
References
- Adams R, Hunt M, Clark JH, 1940. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J Am Chem Soc 62, 196–200 DOI: 10.1021/ja01858a058. [DOI] [Google Scholar]
- Batalla A, Janssen H, Gangadin SS, Bossong MG, 2019. The potential of cannabidiol as a treatment for psychosis and addiction: who benefits most? A systematic review. J Clin Med 8, 1058 DOI: 10.3390/jcm8071058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J, Capel B, 2004. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet 5, 509–521 DOI: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Buck SB, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A, 2008. Detection of S-phase cell cycle progression using 5-ethynyl-2’-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2’-deoxyuridine antibodies. BioTechniques 44, 927–929 DOI: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, Jones C, Housman DE, 1990. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 60, 509–520 DOI: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS, 2012. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367, 3364–3378 DOI: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho RK, Andersen ML, Mazaro-Costa R, 2020. The effects of cannabidiol on male reproductive system: a literature review. J Appl Toxicol 40, 132–150 DOI: 10.1002/jat.3831. [DOI] [PubMed] [Google Scholar]
- Carvalho RK, Santos ML, Souza MR, Rocha TL, Guimarães FS, Anselmo-Franci JA, Mazaro-Costa R, 2018. Chronic exposure to cannabidiol induces reproductive toxicity in male Swiss mice. J Appl Toxicol 38, 1215–1223 DOI: 10.1002/jat.3731. [DOI] [PubMed] [Google Scholar]
- CDER/FDA, 2017. Non-clinical review. Application number:210365Orig1s000. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000PharmR.pdf.
- Chen S, Ren Z, Yu D, Ning B, Guo L, 2018. DNA damage-induced apoptosis and mitogen-activated protein kinase pathway contribute to the toxicity of dronedarone in hepatic cells. Environ Mol Mutagen 59, 278–289 DOI: 10.1002/em.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B, Colleoni M, Conti S, Parolaro D, Franke C, Trovato AE, Giagnoni G, 2004. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol 369, 294–299 DOI: 10.1007/s00210-004-0871-3. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JEC, McGuire PK, Busatto GF, 2004. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology 29, 417–426 DOI: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, Alexander C, Repetski AE, Shukla V, McCoy B, Wells GA, 2019. Cannabis-based products for pediatric epilepsy: a systematic review. Epilepsia 60, 6–19 DOI: 10.1111/epi.14608. [DOI] [PubMed] [Google Scholar]
- Figueiredo AFA, França LR, Hess RA, Costa GMJ, 2016. Sertoli cells are capable of proliferation into adulthood in the transition region between the seminiferous tubules and the rete testis in Wistar rats. Cell Cycle 15, 2486–2496 DOI: 10.1080/15384101.2016.1207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Maiti S, Alam N, Zhang Z, Deng JM, Behringer RR, Lécureuil C, Guillou F, Huff V, 2006. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci U S A 103, 11987–11992 DOI: 10.1073/pnas.0600994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, DeCristo MJ, McAllister SS, Zhao JJ, 2018. CDK4/6 inhibition in cancer: beyond cell cycle arrest. Trends Cell Biol 28, 911–925 DOI: 10.1016/j.tcb.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F, 2003. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42, 327–360 DOI: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Axelrod J, Wink D, 1998. Cannabidiol and (−)Δ9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A 95, 8268–8273 DOI: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DJ, Mechoulam R, 1990. Metabolites of cannabidiol identified in human urine. Xenobiotica 20, 303–320 DOI: 10.3109/00498259009046849. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Fujiwara M, 2010. Therapeutic potential of non-psychotropic cannabidiol in ischemic stroke. Pharmaceuticals (Basel) 3, 2197–2212 DOI: 10.3390/ph3072197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, 2007. Human cannabinoid pharmacokinetics. Chem Biodivers 4, 1770–1804 DOI: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison GR, Scott HM, Walker M, McKinnell C, Ferrara D, Mahood IK, Sharpe RM, 2008. Sertoli cell development and function in an animal model of testicular dysgenesis syndrome. Biol Reprod 78, 352–360 DOI: 10.1095/biolreprod.107.064006. [DOI] [PubMed] [Google Scholar]
- Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K, 2011. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci 89, 165–170 DOI: 10.1016/j.lfs.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Kicman A, Toczek M, 2020. The effects of cannabidiol, a non-intoxicating compound of cannabis, on the cardiovascular system in health and disease. Int J Mol Sci 21, 6740 DOI: 10.3390/ijms21186740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, Silvestrini M, 2018. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs 78, 1791–1804 DOI: 10.1007/s40265-018-0992-5. [DOI] [PubMed] [Google Scholar]
- Li X, He X, Chen S, Guo X, Bryant MS, Guo L, Manjanatha MG, Zhou T, Witt KL, Mei N, 2020. Evaluation of pyrrolizidine alkaloid-induced genotoxicity using metabolically competent TK6 cell lines. Food Chem Toxicol 145, 111662 DOI: 10.1016/j.fct.2020.111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, He X, Chen S, Le Y, Bryant MS, Guo L, Witt KL, Mei N, 2021. The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells. Toxicol Lett 344, 58–68 DOI: 10.1016/j.toxlet.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libro R, Diomede F, Scionti D, Piattelli A, Grassi G, Pollastro F, Bramanti P, Mazzon E, Trubiani O, 2017. Cannabidiol modulates the expression of Alzheimer’s disease-related genes in mesenchymal stem cells. Int J Mol Sci 18, 26 DOI: 10.3390/ijms18010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather JP, 1980. Establishment and characterization of two distinct mouse testicular epithelial cell lines. Biol Reprod 23, 243–252 DOI: 10.1095/biolreprod23.1.243. [DOI] [PubMed] [Google Scholar]
- Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, Finel M, Miller GP, Radominska-Pandya A, Moran JH, 2009. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos 37, 1496–1504 DOI: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S, 2018. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 175, 225–231 DOI: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- Petersen C, Söder O, 2006. The Sertoli cell - a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm Res 66, 153–161 DOI: 10.1159/000094142. [DOI] [PubMed] [Google Scholar]
- Reis MMS, Moreira AC, Sousa M, Mathur PP, Oliveira PF, Alves MG, 2015. Sertoli cell as a model in male reproductive toxicology: advantages and disadvantages. J Appl Toxicol 35, 870–883 DOI: 10.1002/jat.3122. [DOI] [PubMed] [Google Scholar]
- Rosenkrantz H, Fleischman RW, Grant RJ, 1981. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol Appl Pharmacol 58, 118–131 DOI: 10.1016/0041-008x(81)90122-8. [DOI] [PubMed] [Google Scholar]
- Schuel H, Chang MC, Berkery D, Schuel R, Zimmerman AM, Zimmerman S, 1991. Cannabinoids inhibit fertilization in sea urchins by reducing the fertilizing capacity of sperm. Pharmacol Biochem Behav 40, 609–615 DOI: 10.1016/0091-3057(91)90371-8. [DOI] [PubMed] [Google Scholar]
- Schuel H, Schuel R, Zimmerman AM, Zimmerman S, 1987. Cannabinoids reduce fertility of sea urchin sperm. Biochem Cell Biol 65, 130–136 DOI: 10.1139/o87-018. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, 1993. Mammalian G1 cyclins. Cell 73, 1059–1065 DOI: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Silvestro S, Schepici G, Bramanti P, Mazzon E, 2020. Molecular targets of cannabidiol in experimental models of neurological disease. Molecules 25, 5186 DOI: 10.3390/molecules25215186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Crockett J, Tayo B, Morrison G, 2019. A Phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol 59, 1110–1119 DOI: 10.1002/jcph.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Gidal B, Blakey G, Tayo B, Morrison G, 2018. A Phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32, 1053–1067 DOI: 10.1007/s40263-018-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayo B, Taylor L, Sahebkar F, Morrison G, 2020. A Phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet 59, 747–755 DOI: 10.1007/s40262-019-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujváry I, Hanuš L, 2016. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res 1, 90–101 DOI: 10.1089/can.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M, Brine DR, Perez-Reyes M, 1976. The metabolism of cannabinoids in man. Pharmacology of Marijuana, Raven Press, New York, pp. 93–113. [Google Scholar]
- Wang XN, Li ZS, Ren Y, Jiang T, Wang YQ, Chen M, Zhang J, Hao JX, Wang YB, Sha RN, Huang Y, Liu X, Hu JC, Sun GQ, Li HG, Xiong CL, Xie J, Jiang ZM, Cai ZM, Wang J, Wang J, Huff V, Gui YT, Gao F, 2013. The Wilms tumor gene, Wt1, is critical for mouse spermatogenesis via regulation of Sertoli cell polarity and is associated with non-obstructive azoospermia in humans. PLoS Genet 9, e1003645 DOI: 10.1371/journal.pgen.1003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, McAllister SD, Kawamura R, Murase R, Neelakantan H, Walker EA, 2014. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 171, 636–645 DOI: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G, Karl T, 2017. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer’s disease. Front Pharmacol 8, 20 DOI: 10.3389/fphar.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Guimarães FS, Moreira AC, 1993. Effect of cannabidiol on plasma prolactin, growth hormone and cortisol in human volunteers. Braz J Med Biol Res 26, 213–217. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


