Abstract
Heart disease is the primary cause of death worldwide. Even though enormous research has been done, and many pharmacological and surgical treatments have been introduced to treat heart disease, the mortality rate still remains high. Gene therapy is widely used to understand molecular mechanisms of myocadiac infarction and to treat cardiomyocyte loss. It was reported that adult cardiomyocytes proliferate at a very low rate, thus targeting their proliferation has become a new regenerative therapeutic target. Currently, re-activating cardiomyocyte proliferation seems one of the most promising methods to promote adult cardiomyocyte renewal. In this review, we highlight gene therapeutic targets of cell proliferation presently being pursued to re-activate the cell cycle of cardiomyocytes, including cell cycle regulators, transcription factors, micro RNAs, signal transduction, and other contributing factors. We also summarize gene delivery vectors that have been used in cardiac research and ongoing major challenges to be overcome in the translation to the clinical approach and future directions.
1. Introduction
Heart disease is the primary cause of death in developed countries, accounting for approximately 20% of total deaths in the United States in 2020 [1]. Heart disease results in the loss of cardiomyocytes, causing ventricular dysfunction and heart failure. Tremendous research has been conducted to treat heart disease for decades; however, it still remains the leading cause of mortality in the world. A major reason is that following myocardial infarction, the lost cardiomyocytes are replaced with fibrotic scar tissue because the heart is one of the least regenerative organs in the body [2, 3]. Unlike neonatal hearts where cardiomyocytes divide at the first a few days, adult cardiomyocytes exit cell cycle and lack the ability to recover impaired cardiac function [4–7]. In the past decades, it seemed impractical to replace the lost cardiomyocytes or restore the function of cardiomyocytes, despite the existence of conventional treatments. Therefore, heart transplantation had been considered the only treatment to cure heart disease at the end stage of heart failure [8]. Even though the outcome of heart transplantation results in an improvement in patient survival, there are serious limitations facing the field such as the number of available donor hearts, rejection of the donor heart, and primary graft failure [9]. For these reasons, there have been increasing efforts in the development of alternative strategies to treat the damaged myocardium including achieving cardiomyocyte renewal [10–12].
Gene therapy is being explored to treat heart disease by transferring potential therapeutic genetic information to cardiomyocytes and modifying the expression and levels of proteins. Over the past 30 years, the field of cardiac gene therapy has developed significantly, and it is one of the extensively investigated methods to improve cardiomyocyte renewal [13]. Many previous studies have investigated gene therapy for cardiac regeneration in vitro and in vivo, however, it has been challenging when it comes to clinical settings due to the low efficiency of gene transference and the lack of significant clinical results [14, 15]. In this review, we discuss gene therapeutic strategies presently being pursued to promote cardiomyocyte proliferation. We also discuss ongoing major challenges to be overcome in the translation to the clinical approach and future directions. The unique feature of the current review is to present current cardiac gene therapy strategies specifically to promote cell proliferation, targeting cell cycle re-entry. A literature search was performed using PubMed using the keywords “cardiomyocyte proliferation”, “gene therapy”, and “cell cycle”.
2. Activators of cardiomyocyte proliferation are potential therapeutic targets for heart regeneration
2.1. Cell cycle regulators determine the proliferative capacity
In mammals, the heart grows via the cell division of cardiomyocytes during embryonic and fetal development [16]. Shortly after birth, cardiomyocytes exit the cell cycle, resulting in the enlargement of myocardial volume via cardiac hypertrophy instead of hyperplasia [16]. In detail, mice have cardiac regeneration capacity within the first seven days after birth, and pigs can regenerate from preexisting cardiomyocytes within the first two days postnatally [6, 7, 17]. In humans, it was generally believed that cardiomyocytes exit the cell cycle after birth and stay in a quiescent state, completing mitosis. However, the integration of 14C demonstrated that the cardiomyocyte turnover rate is approximately 1% at the age of 20, constantly decreasing annually to 0.3% at the age of 75 [18]. Even though it is minimal, cell cycle re-activation in postnatal cardiomyocytes has become a potential therapeutic intervention for promoting cardiomyocyte proliferation after cardiac injury. Therefore, cell cycle regulators were targeted to promote cell cycle re-entry and endogenous cardiomyocyte proliferation (Figure 1).
Figure 1.
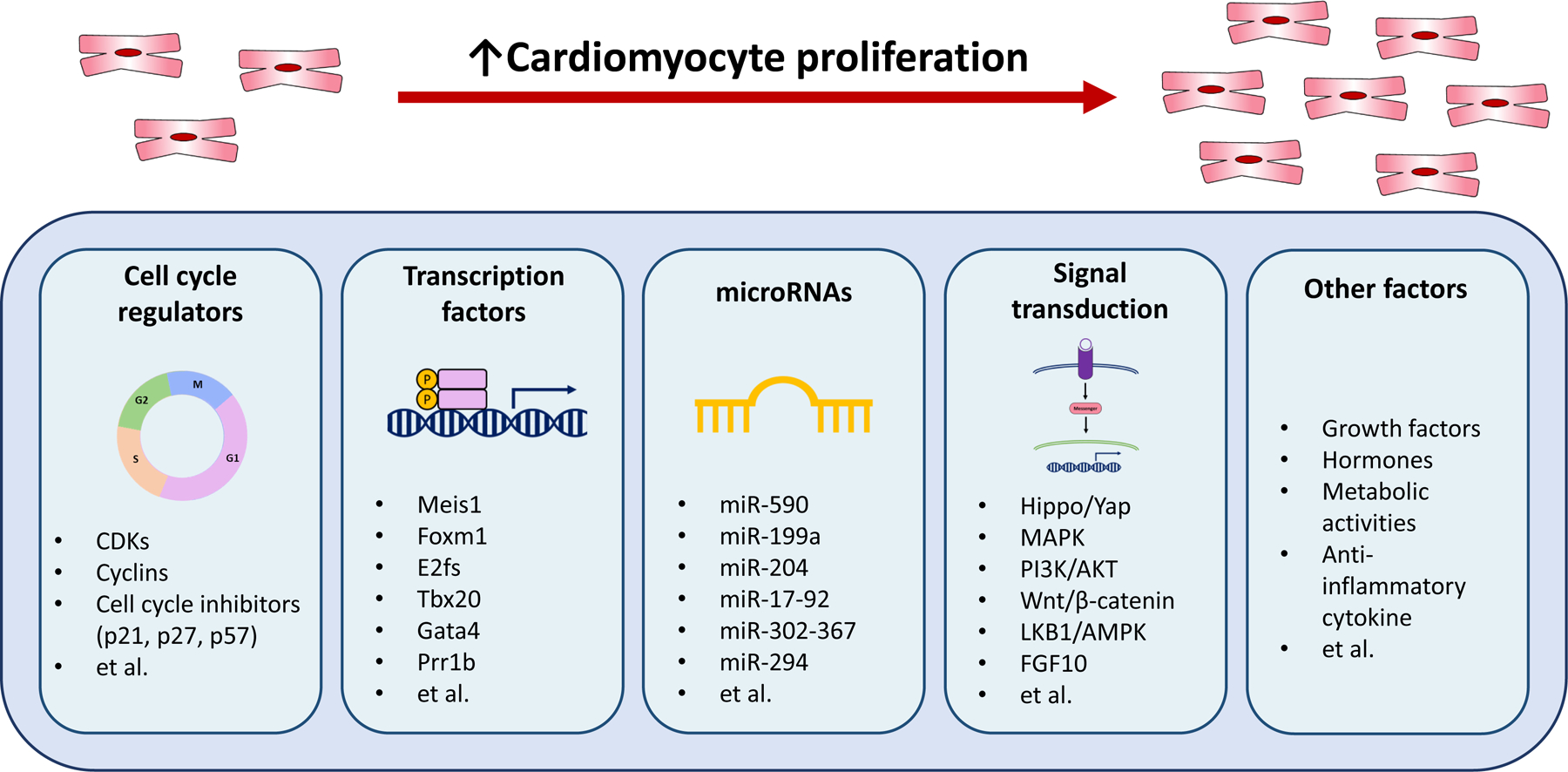
Molecular targets that promote cardiomyocyte cell cycle and proliferation. The figure summarizes the molecules that have been shown to regulate cardiomyocyte cell cycle and proliferation.
The cell cycle is tightly regulated by multiple factors such as cyclins and the cyclin-dependent kinases (CDKs) at different phases, thus it is expected that manipulating the expression of the cell cycle regulators would induce cell cycle re-entry. For instance, cyclin A2-induced cell cycle activation increases border zone myofilament density and myocardial function in the damaged rat hearts [19] as well as mediating mitosis and hyperplasia in mice [20]. Cyclin D1 overexpression increased cardiomyocyte DNA synthesis and multinucleation in adult mice [21]. In addition, cyclin D2 overexpressing adult transgenic mice maintained cell cycle activity and reduced scar size after myocardial infarction [22]. Furthermore, a combination of cell cycle activators including CDK1, CDK4, cyclin B1, and cyclin D1 efficiently improved cell division and cardiac function in mouse, rat, and human cardiomyocytes after myocardial infarction [23]. The researchers reported that a combination of the cell cycle regulators might efficiently activate the proliferative potential in adult cardiomyocytes. In addition to cell cycle activators, cell cycle inhibitors including p21, p27, and p57 have been used to manipulate the cell cycle in adult cardiomyocytes, and silencing the expression of the inhibitors induces re-entry of the cell cycle in cardiomyocytes [24, 25].
2.2. Transcription factors control the expression of cell cycle regulators
There are several transcription factors that regulate the cell cycle (Figure 1), and various research have been done on these transcription factors as potential targets to activate cardiomyocyte renewal. First, myeloid ecotropic viral integration site 1 (Meis1) regulates cell cycle arrest and is necessary to induce transcription of the CDK inhibitors including p15, p16, and p21 [26]. Meis1 knockout in mice showed improvement in postnatal cardiomyocyte proliferation capacity. Forkhead box M1 (Foxm1), a major proliferation-associated transcription factor, regulates S-phase and M-phase entry, and it is essential for mitosis and progression in cardiomyocytes [27]. A recent study reported that Foxm1 deletion in adult zebrafish resulted in a decrease in cardiomyocyte proliferation and significantly increased scar area after ventricular amputation [28]. In addition, the E2f family is known to regulate many cell cycle genes, with E2f1, E2f2, and E2f4 being the most investigated [29–31]. Intramyocardial injection of E2f1 via adenoviral delivery in adult mice re-activated the cell cycle and completed DNA synthesis in postmitotic cardiac muscle [29]. Similarly, E2f2 overexpression in the heart re-activated proliferation by inducing cyclin A and cyclin E in adult mouse cardiomyocytes [30]. Another study found that adenovirus-mediated delivery of E2f2 and E2f4 but not E2f1 and E2f3 induced S-phase entry, activating DNA synthesis without apoptosis in neonatal cardiomyocytes from both rats and mice [31]. Additionally, overexpression of the T-box transcription factor (Tbx20) in adult mouse cardiomyocytes promoted cardiomyocyte proliferation, cardiac function, and survival rate after myocardial infarction by activating the expression of proliferation positive regulators [32]. Gata binding protein 4 (Gata4) plays a significant role in neonatal cardiac development, promoting cardiac hypertrophy and angiogenesis and the maintenance of postnatal cardiac function [33, 34]. Cardiomyocyte Gata4 knockout mice showed reduced angiogenesis and cardiomyocyte proliferation after cryoinjury whereas Gata4 overexpression induced cardiomyocyte proliferation and cardiac regeneration in mice 7 days after cryoinjury [35]. The paired related homeobox 1 (Prrx1b) is a transcription factor that participates in wound healing and limb regeneration [36]. Prrx1b knockout resulted in excessive fibrosis and impaired proliferation in zebrafish cardiomyocytes, suggesting that Prrx1b is necessary for balancing fibrosis and cardiomyocyte renewal.
2.3. microRNAs regulate cell cycle in cardiomyocytes
Various microRNAs (miRNAs) play a pivotal role in regulating the cell cycle in cardiomyocytes (Figure 1). Eulalio et al. screened various functional miRNAs regulating neonatal cardiomyocyte proliferation, and they found that forty miRNAs significantly enhanced DNA synthesis as well as cytokinesis in both mouse and rat cardiomyocytes [37]. Among them, has-miR-590 and has-miR-199a significantly promoted postnatal cardiomyocyte renewal by increasing proliferation and reducing infarct size in adult mice after myocardial infarction. In addition, overexpression of miR-204, miR-17–92, miR-302–367, and miR-294 promoted cardiomyocyte proliferation in adult mice [38–41]. On the other hand, inhibition of miR-195 and miR-128 improved cardiomyocyte proliferation of preexisting cardiomyocytes and left ventricular systolic function in adult mice after myocardial infarction [42–44].
2.4. The signaling pathways activate postnatal cardiomyocyte proliferation
Various intracellular developmental signaling pathways play a pivotal role in regulating cardiomyocyte proliferation (Figure 1). Hippo/Yes-associated protein (YAP) signaling is essential in embryonic heart growth as well as postnatal cardiac function [45]. Cardiac specific YAP knockout in mice resulted in impairment of neonatal cardiac regeneration post-myocardial infarction [46]. In addition, YAP overexpression in transgenic mice enhanced cardiomyocyte proliferation in the postnatal heart after cardiac injury [46]. The mitogen-activated protein kinases (MAPK) signaling is a core pathway that controls cellular proliferation, differentiation, development, and apoptosis in mammalian cells [47]. In 2015, Zhao and Peng found that MAPK1 promotes the proliferation of H9C2 rat cardiomyocytes by activating phosphoinositide-3-kinase (PI3K)/AKT signaling pathway [48]. PI3K/AKT signaling pathway is linked to the Hippo/YAP signaling through PI3K catalytic subunit beta (Pi3kcb), a critical enzyme that regulates cell growth and metabolic activity to promote adult cardiomyocyte proliferation and survival [49]. The Wnt/β-catenin signaling pathway is known to participate in embryonic development and adult tissue homeostasis [50]. Cardiomyocyte-specific deletion of low-density lipoprotein receptor-related protein 6 (LRP6), a Wnt co-receptor, increased cardiomyocyte proliferation in mice after myocardial infarction [51]. Liver kinase B1 (LKB1), a major upstream kinase for AMP-activated protein kinase, was also identified as a potential therapeutic target, showing that LKB1 knockdown improves postnatal cardiomyocyte renewal [52]. Furthermore, thyroid hormone signaling may regulate cardiac regeneration [53]. Cardiomyocyte-specific inhibition of thyroid hormone signaling postponed cell cycle exit, restoring cardiac regenerative potential [53, 54]. Additionally, fibroblast growth factor (FGF) 10 is known to promote cardiomyocyte renewal [55, 56]. A previous study found that conditional overexpression of FGF10 in adult mice hearts promoted cardiomyocyte cell cycle re-entry [55]. The study suggested that FGF10 signaling induces Forkhead Box O3 (FOXO3) phosphorylation, resulting in the inhibition of CDK inhibitor such as P27 and thus the activation of proliferation.
2.5. Other contributing factors
Besides the direct cell cycle regulators, there are indirect factors that regulate the cell cycle (Figure 1). Myeloid-derived growth factor (Mydgf) improved cardiomyocyte proliferation as well as heart regeneration in adult mice after myocardial infarction, reducing fibrotic area and increasing the survival rate by activating c-Myc/FoxM1 signaling pathway compared to the control group [57]. Neuregulin 1 (NRG1), FGF1, and periostin significantly increased DNA synthesis in primary adult rat cardiomyocytes, inducing cell-cycle reentry [58, 59]. The enhancer of zeste homolog 2 (Ezh2) is required to activate the platelet-derived growth factor receptor- β (PDGFR-β) to induce cardiomyocyte proliferation, and the activation of PDGFR-β resulted in the improvement of adult heart regeneration after myocardial infarction by decreasing scar size as well as increasing left ventricular systolic function [60]. Interestingly, metabolic activity also affects the cardiomyocyte cell cycle after myocardial infarction. Neonatal cardiomyocytes switch metabolism from glycolysis to oxidative phosphorylation, resulting in reactive oxygen species production and DNA damage and eventually causing cardiomyocyte cell cycle exit [61]. Bae et al. reported that malonate to inhibit succinate dehydrogenase can activate adult cardiomyocyte proliferation, revascularization, and heart regeneration by reprogramming the metabolism in the adult cardiomyocytes [62]. Lastly, an anti-inflammatory cytokine, IL-13 stimulated the cell cycle and heart regeneration in neonatal mice cardiomyocytes by activating ERK1/2 and AKT downstream signaling [63]. Furthermore, after myocardial infarction, it appears that promoting IL-13 binding to type II IL4Rα/IL13Rα1 receptor increased cardiomyocyte DNA synthesis and recovery from myocardial infarction at the age when heart regeneration is not active [64].
3. Gene therapy vectors
Gene therapy is to treat and/or improve a patient’s disease condition by manipulating the expression of a gene in the patient’s target cells. Since naked DNA molecules have a relatively large size and hydrophilic characteristics, they are not able to enter the cells efficiently [65]. Therefore, in gene therapy, a gene can be delivered by a carrier, called a vector. Generally, gene delivery to target cells can be achieved using either viral vectors or non-viral vectors (Figure 2).
Figure 2.
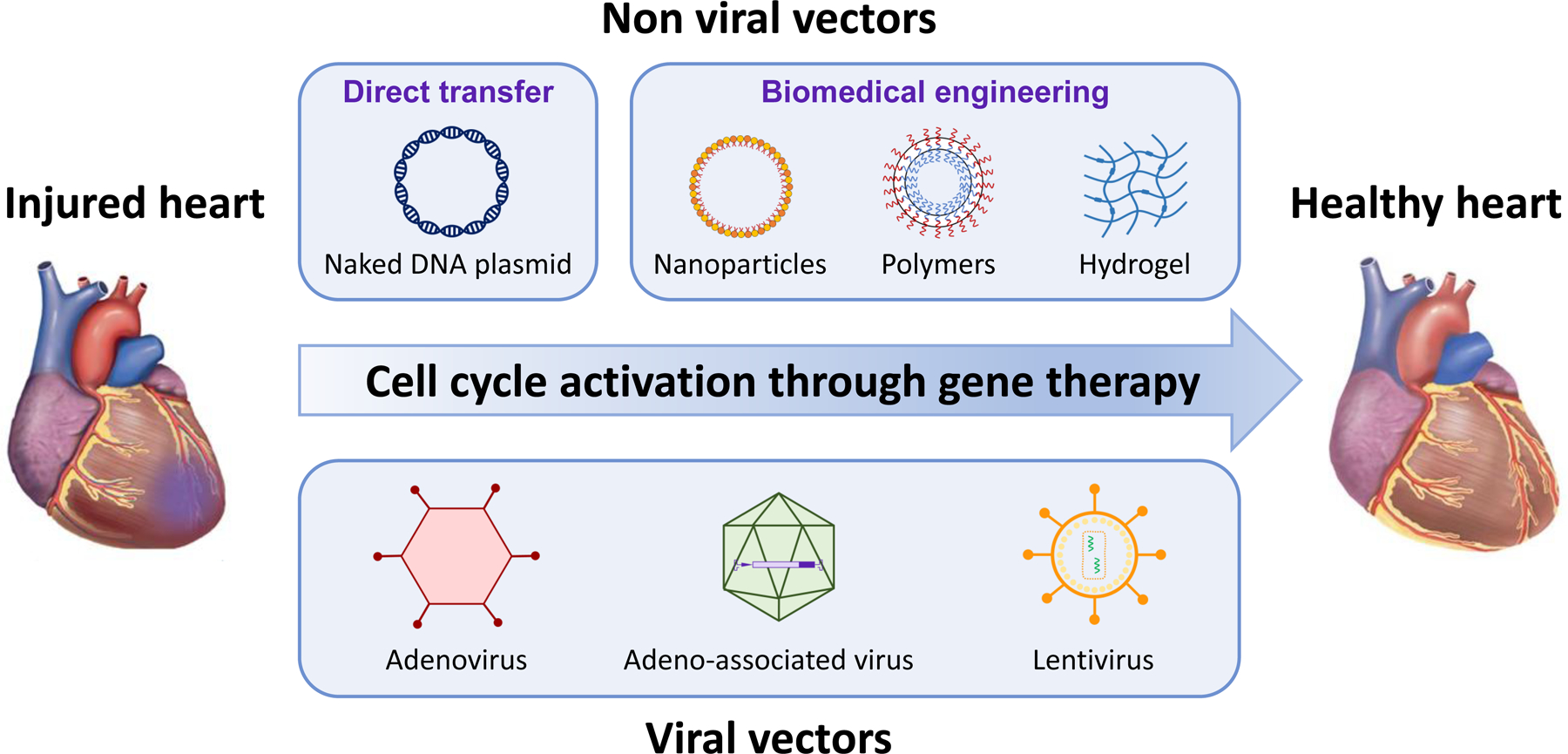
Approaches for cardiac gene therapy. The figure outlines the viral- and nonviral-based approaches to deliver genes and cardioprotective agents for promoting cell cycle activation.
3.1. Non-viral vectors
The non-viral gene delivery employs synthetic or natural compounds to deliver a gene into cells instead of viruses. The non-viral vectors carry plasmid DNA, mRNA, and nucleic acid complexes [66]. Non-viral gene delivery has critical limitations such as low gene transfection efficiencies due to the limited cellular uptake, low transient expression, and short-term expression period [65]. Nevertheless, there are many beneficial features of non-viral vectors including less toxicity, low immunogenicity, no packaging limitations, and low cost of production [67]. In heart disease research, various non-viral vectors have been used (Table 1). The easiest non-viral transfection method is the direct transfer with DNA plasmids. Direct intramyocardial injection of a plasmid encoding vascular endothelial growth factors (VEGF) A-165 was successful to improve heart conditions in injured hearts in pigs, sheep, and humans [68–70].
Table 1.
Non-viral vectors to deliver genes into injured hearts.
| Delivery method | Target gene | Species | Reference |
|---|---|---|---|
| DNA plasmid | VEGF A-165 | Human, Pig, Sheep | [66–68] |
| PEGylated-NP | Schisandrin B | Rat | [77] |
| PEGylated-NP | Baicalin | Rat | [78] |
| PEGylated-NP | Puerarin | Rat | [79] |
| PLGA-NP | Cyclosporine A | Mouse | [80] |
| PLGA-NP | Irbesartan | Mouse | [81] |
| PLGA-NP | Pitavastatin | Mouse, Rat, Pig | [82–84] |
| PLGA-NP | VEGF | Mouse | [85] |
| PLGA-NP | IGF1 | Mouse | [86] |
| Conjugate chitosan-graft-PEI-eprosartan | VEGF | Rat | [87] |
| PDMA | EGFP | Mouse | [88] |
| Hydrogel | miR-302 | Mouse | [91] |
| Hydrogel | miR-199a-3p | Rat | [92] |
| Hydrogel | miR-21–5p | Pig | [93] |
| UTM | VEGF | Mouse | [94] |
PEGylated-NP, nanoparticles with polyethylene glycol; PLGA-NP, a copolymer (lactic-co-glycolic acid); PEI, polyethylenimine; PDMA, a cationic poly(beta-amino ester); UTM, the ultrasound-targeted microbubble; VEGF, vascular endothelial growth factor; IGF, insulin-like growth factor; EGFP, enhanced green fluorescent protein.
To increase the stability of plasmid DNA, the biomedical engineering approach is introduced to carry the naked plasmid DNA to the heart. Previously, nanoparticles have shown safe and effective gene delivery in cardiovascular disease models [71]. In particular, ionizable lipid nanoparticles have shown high transfection efficacy in cardiomyocytes with less toxicity in vitro as well as in vivo [71], and many studies have used lipid nanoparticles to deliver their target genes to repair infarcted myocardial tissue [72–74]. Liposomes or lipoplex nanoparticles surround negatively charged DNA with positively charged lipids and detergents to increase cellular uptake through endocytosis [75]. However, the liposomal DNA complexes are rapidly removed from the systemic circulation and do not prevent intracellular degradation in endosomes [76]. In addition, the liposome delivery method presents critical limitations such as high toxicity because of the interaction with the cell membrane and the release of the DNA from the endosomes to the cytoplasm [77, 78]. To reduce the aggregation of lipid nanoparticles and nonspecific endocytosis, polyethylene glycol-modified (PEGylated) lipid nanoparticle conjugates were synthesized and showed a promising delivery capacity in the myocardial ischemia model [79–81]. Another nanoparticle that has been used in a heart disease model is poly-lactide/glycolide acid (PLGA). Ikeda et al. successfully delivered their target gene, cyclosporine A, to cardiomyocyte mitochondria through intravenous injection of PLGA nanoparticles in a murine myocardial ischemia-reperfusion model [82]. Furthermore, PLGA-mediated intravenous delivery of irbesartan and pitavastatin ameliorated left ventricular remodeling after myocardial ischemia-reperfusion injury in various animal models [83–86]. Besides intravenous injection, PLGA-mediated intramyocardial delivery of VEGF and insulin-like growth factor 1 (IGF1) to the mice hearts promoted recovery from myocardial infarction by improving vascular density and left ventricular contractile function and reducing infarct size [87, 88].
Another plasmid DNA delivery strategy is to use polymers. Various polymers have been introduced such as poly-L-lysine (PLL) and polyethylenimine (PEI) to treat heart disease because of their low immunogenicity; however, the poor circulatory half-lives and transfection efficiency are the major drawbacks in the gene therapy field [77]. Thus, many researchers have tried to overcome the obstacles. Previously, a copolymer conjugate chitosan-graft-PEI-eprosartan to VEGF plasmid showed strong therapeutic impacts in cardiomyocytes in myocardial ischemia rats [89]. To increase the transfection efficiency and reduce the toxicity of polymers, Xu et al. synthesized a cationic poly (β-amino ester) with a degradable backbone (PDMA), and the PDMA carrying plasmid EGFP showed a higher transfection rate and cell viability than PEI alone in neonatal mouse cardiomyocytes [90]. Additionally, dexamethasone-conjugated PEI was suggested as a potential therapeutic gene carrier in cardiomyocytes since it showed strong transfection efficiency and anti-apoptotic effect in rat cardiomyocytes [91].
To increase gene delivery sustainability and localization with minimal disturbance in the cardiac function, injectable hydrogel encapsulation was introduced. Hydrogels are crosslinked hydrophilic polymers and have great benefits for the heart because they show similar features to the heart including viscoelasticity and structures, resulting in less immune response [92]. Intramyocardial delivery of miR-302 via a hydrogel promoted cardiomyocyte proliferation and regeneration after myocardial infarction in mice [93]. Polymeric nanoparticles with a shear-thinning hydrogel encapsulating miR-199a-3p showed lower toxicity than lipid nanoparticles, and the injection of hydrogel with miR-199a-3p significantly improved cardiac functions after myocardial infarction in rats [94]. miR-21–5p carrying mesoporous silica nanoparticles encapsulated into hydrogel matrix improved vascularization and reduced the scar size after myocardial infarction in pig hearts [95].
As a physical delivery method, the ultrasound-targeted microbubble (UTM) was introduced, and the method showed increased cell membrane permeability, less toxicity, and low immunogenicity. UTM delivery of VEGF, which is an angiogenic gene, into the heart increased capillary and arteriolar density, myocardial perfusion, and cardiac function after myocardial infarction in mice [96]. However, there are still challenges such as low transfection efficiency and toxicity that must be overcome for using non-viral vectors in large animal models and eventually clinical trials [97, 98]. As we discussed in this section, there are only a few studies that investigated the effects of non-viral vector delivery on cardiomyocyte renewal [69, 70, 93]. More research needs to be undertaken into the use of non-viral vectors as a therapeutic method in large animals and then clinical settings.
3.2. Viral vectors
In gene therapy, most research has focused on viral gene delivery rather than non-viral vectors over the past years. As the efficiency of gene transfer is the most challenging obstacle in gene therapy, their high gene transfer efficiency took the spotlight. Specifically many studies in heart disease have used viral vectors to explore cardiomyocyte function owing to longer transgene expression and higher gene transfer capacity than non-viral vectors [99]. We summarize the advantages and disadvantages of major viral vectors that are used in cardiomyocyte research such as adenovirus, adeno-associated viruses (AAVs), and lentivirus (Table 2).
Table 2.
Viral vectors to deliver genes into injured hearts.
| Virus | Target gene | Species | Reference |
|---|---|---|---|
| Adenovirus | VEGF-DΔNΔC | Human | [100] |
| Adenylyl cyclase 6 | Human | [101] | |
| VEGF, Angiopoietin-1 | Pig | [102] | |
| Proline/arginine-rich peptide 39 | Pig | [103] | |
| FGF4 | Pig, Human | [104, 105] | |
| VEGF121 | Human | [106] | |
| Cyclin A2 | Pig | [107] | |
| Cyclin D1, CDK4 | Rat | [108] | |
| CDK1, CDK4, Cyclin B1, Cyclin D1 | Mouse | [21] | |
| AAV | SERCA2a | Human | [115] |
| BNP116 | Human | ClnicalTrials.gov Identifier: NCT04179643 | |
| Glycoprotein 130 | Mouse | [116] | |
| miRNA-LRP6 | Mouse | [117] | |
| Lysophosphatidic acid 3 | Mouse | [118] | |
| miR-199a | Pig | [119] | |
| Lentivirus | miR302–367 | Mouse | [127] |
| CDK1, CDK4, CyclinB1, CyclinD1 | Rat, Pig | [128] | |
| Integrin β1 | Rat | [129] |
AAV, adeno-associated virus; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; CDK, cyclin-dependent kinases; SERCA2a, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; LRP6, low-density lipoprotein receptor-related protein 6.
Adenovirus
The adenoviral vector consists of non-enveloped, double-stranded DNA (dsDNA), and the dsDNA is translocated into the nucleus followed by gene transduction. Adenovirus has a high DNA packaging capacity up to 36 kb [76]. The adenovirus delivery method was widely used in clinical trials at the beginning of gene therapy. The expression of transgene through adenoviral vectors is very vigorous for the first few days, however, it decreases within 2 weeks [100]. A major disadvantage of the adenovirus vector is that it promotes high antibody and inflammatory responses [76]. Adenovirus-mediated immunogenicity was further increased with myocardial ischemia-reperfusion [101]. Nevertheless, several animal and clinical trials have been done with an adenoviral vector carrying angiogenic factors. A few showed positive therapeutic effects by inducing angiogenesis and vasculogenesis, but most of the trials showed no significant difference compared to the placebo group [102–108]. Cardiomyocyte renewal has recently gained attention as a therapeutic target for heart failure, thus there are only a handful number of studies that used adenovirus to improve cardiomyocyte renewal. In 2014, Shapiro et al. found that intramyocardial injection of replication-deficient adenovirus vector with cyclin A2 induced cardiac regeneration after myocardial infarction by increasing cytokinesis of cardiomyocytes in adult pigs [109]. In addition, adenoviral delivery of cyclin D1 with cyclin-dependent kinase 4 (CDK4) into the myocardium induced cell cycle re-entry, resulting in cell division of cardiomyocytes in adult rats [110]. Similarly, adenoviral transfection of a combination of CDK1, CDK4, cyclin B1, and cyclin D1 improved cardiac function by increasing cardiomyocyte proliferation after myocardial infarction in mice [23].
Adeno-associated viruses
Adeno-associated viruses (AAVs) are nonenveloped, single-stranded DNA viruses and require a helper virus such as adenovirus to replicate [111]. AAVs enter the cytoplasm of the target cells through endocytosis by binding to receptors. Then, the virus translocates into the nucleus followed by transcription after synthesizing the double-strand genome. Since AAVs use a helper virus, it produces rapid and persistent AAV capsids, resulting in the degradation of the infected cells [112]. Therefore, AAVs are considered relatively reliable when it comes to immunogenicity and transfection compared to other viral vectors [113, 114]. However, AAVs have limited packaging capacity (~4.7 kb), which limits the size of the transgene [76]. There are more than 100 serotypes of AAVs have been identified, and AAV6 and AAV9 have been shown to be the most efficient AAV serotype in the heart [115, 116]. Despite positive results in vitro and in vivo studies that targeted angiogenic cardiac gene therapy using AAVs to treat ischemic heart disease, the translation into clinical trials has been disappointing. In the first AAV clinical trial, epicardial coronary artery infusion of AAV1 with sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2a) showed no significant clinical outcomes compared to the placebo group [117]. Ever since then, no further clinical trials with cardiac AAV gene therapy have been reported, except for the one currently ongoing (ClinicalTrials.gov Identifier: NCT04179643). The study is a phase 1, sequential dose escalation study to test the safety and effectiveness of intracoronary infusion of a new chimeric AAV vector, BNP116, in patients with heart failure.
In preclinical research, a previous study reported that the AAV9 infection of glycoprotein 130, which is necessary for neonatal cardiomyocyte proliferation, enhanced cardiomyocyte proliferation and regeneration after myocardial infarction by activating the Yap pathway in adult mice [118]. Moreover, overexpression of AAV9-mediated miRNA-low-density lipoprotein receptor-related protein 6 (LRP6) and lysophosphatidic acid 3 reduced myocardial infarction size and ameliorated left ventricular systolic function by increasing cardiomyocyte proliferation in mice [119, 120]. In addition, Gabisonia et al. found that injection of AAV6-mediated human miRNA-199a increased cardiomyocyte proliferation after myocardial infarction in pigs [121]. Robust research has investigated gene therapy through AAVs for the therapeutic purpose of heart repair and regeneration in vitro and in small animals. As the results seem promising in cardiomyocyte renewal, investigation in large animal models should be undertaken to allow the move forward into clinical trials.
Lentivirus
Lentiviruses are enveloped, single-stranded RNA retroviruses and have up to 10 kb packaging capacity [76]. A unique characteristic of lentivirus is that it can transduce non-dividing cells without an immune response against the transduced cells and present long-term gene expression with high transduction efficiency [122]. Because cardiomyocytes are non-replicating cells after birth, lentiviral gene delivery has the potential as an effective transfection method in cardiomyocytes. A few clinical studies have used lentiviral vectors in patients with HIV, Wiskott-Aldrich syndrome, β-hemoglobinopathies, and leukodystrophies [123–127]. Even though there is no clinical study that has used lentiviral vectors in cardiac tissue as yet, many researchers have used lentiviral gene transfection to investigate cardiomyocyte proliferation in vitro as well as in vivo models [128]. Lentiviral delivery of miR302–367 induced cardiomyocyte renewal in adult mice after myocardial infarction by promoting cardiomyocyte proliferation and reducing scar formation [129]. Intramyocardial injection of lentivirus encoding four cell cycle factors including CDK1, CDK4, CCNB1, and CCND1 after myocardial infarction in rats and pigs improved left ventricular ejection fraction and scar size [130]. In addition, lentiviral vector-mediated overexpression of integrin β1 increased cardiomyocyte survival after myocardial infarction in rats [131]. The biggest concern of using lentivirus vectors is their safety. Lentivirus has a potential risk to cause insertional oncogenesis because it integrates into the target cell randomly with a preference to the coding regions of the gene [132]. Therefore, lentiviral vectors need further improvements in safety matter before they can be used in cardiomyocytes in a clinical setting.
4. Conclusive summary, limitations, and future directions
Understanding the underlying mechanism behind cardiomyocyte proliferation has become significantly important to treat myocardial infarction and heart failure. After heart injury, the heart significantly loses cardiomyocytes and thus functions. Recent research proved that using gene therapy, cardiomyocytes can proliferate by re-entering the cell cycle. Therefore, genetically editing genes involved in the cell cycle may improve adult cardiomyocyte proliferation and renewal. Thus, gene therapy targeting the cell cycle to restore the function of cardiomyocytes can now be considered a potential therapeutic method for heart failure. However, there is limited evidence that shows the cell cycle activation results in the generation of new cardiomyocytes restoring the lost function [13, 133]. Cell proliferation is necessary for the renewal of cardiomyocytes, yet it remains to be confirmed if those proliferated cells differentiate into and regenerate fully functioning cardiomyocytes.
The current review intended to provide comprehensive gene therapeutic strategies that have been used to activate the cell cycle. We mainly focused on the genetic targets specifically to induce cardiomyocyte proliferation along with gene delivery vectors. The limitation is that we have not discussed other contributing factors that are known to affect cardiomyocyte proliferation such as angiogenesis, gender, and age. However, the current review is unique since it provides a comprehensive discussion on gene therapeutic targets of the cell cycle to activate cardiomyocyte proliferation as there is growing evidence that targeting the cell cycle enables regeneration in injured hearts.
The past and ongoing clinical trials that used gene therapy to treat heart failure are mostly focusing on the regulation of intracellular calcium and angiogenesis [134]. Even though preclinical studies showed promising results in the improvement of cardiac function by delivering genes that are involved in angiogenesis and calcium regulation, similar results were not shown in humans. As stated in this review, recently, multiple studies have shown promising results in activating cell proliferation in small animal models using gene therapy. A clinical trial has been done with a cell cycle activator, recombinant human neuregulin1, via intravenous infusion in chronic heart failure patients [135]. The patients who received recombinant human neuregulin1 showed increased left ventricular function and structure compared to the placebo group even though it was not statistically significant. Although promoting the cell cycle provides hope to treat heart disease, it is yet premature to administrate gene therapy that promotes the cell cycle into clinical practice due to the lack of sufficient preclinical studies to assess efficacy and safety. Even though small animal models play significant roles in translational medicine, there are still physiological and anatomical differences between the small animals and humans, therefore meticulous consideration has to be given during data analysis. Taken together, more intensive research with large animal models and proper preclinical study design are required to establish a more practical translational method to reduce the risk of the failure of clinical translation.
Key Points.
It is important to understand the underlying mechanism behind cardiomyocyte cell cycle exits in adult mammalian hearts.
Gene therapy targeting the cell cycle genes increases adult cardiomyocyte proliferation.
Promoting the cell cycle appears to be a viable therapeutic strategy for cardiomyocyte renewal.
Acknowledgments
We thank the National Institutes of Health and the American Heart Association for funding support. In addition, Figure 1 and Figure 2 were created with Biorender.com
Funding:
NIH R01 HL142627, HL156855, AHA 20TPA35490001.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
Data availability:
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Ahmad FB, Anderson RN. The Leading Causes of Death in the US for 2020. JAMA. 2021;325(18):1829–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzahor E, Poss KD. Cardiac regeneration strategies: staying young at heart. Science. 2017;356(6342):1035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161(7):1566–75. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y, Zharkinbekov Z, Sarsenova M, Yeltay G, Saparov A. Recent Advances in Gene Therapy for Cardiac Tissue Regeneration. Int J Mol Sci. 2021;22(17):9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011. Feb 25;331(6020):1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, et al. Regenerative Potential of Neonatal Porcine Hearts. Circulation. 2018. Dec 11;138(24):2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesse M, Welz A, Fleischmann BK. Heart regeneration and the cardiomyocyte cell cycle. Pflug Arch EUR J Phy. 2018;470(2):241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonsho M, Michel S, Ahmed Z, Alessandrini A, Madsen JC. Heart transplantation: challenges facing the field. Cold Spring Harb Perspect Med. 2014;4(5):a015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awada HK, Hwang MP, Wang Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: Aspects to consider and proteins to deliver. Biomaterials. 2016. Mar;82:94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12(6):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leach JP, Martin JF. Cardiomyocyte proliferation for therapeutic regeneration. Curr Cardiol Rep. 2018;20(8):1–8. [DOI] [PubMed] [Google Scholar]
- 13.López AE, del Rosario Bauza M, Cuniberti L, Crottogini AJ, Olea FD, Locatelli P. Gene therapy: targeting cardiomyocyte proliferation to repopulate the ischemic heart. J Cardiovasc Pharmacol. 2021;78(3):346–60. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto H, Olson EN, Bassel-Duby R. Therapeutic approaches for cardiac regeneration and repair. Nat Rev Cardiol. 2018 2018/October/01;15(10):585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z, Pu WT. Strategies for Cardiac Regeneration and Repair. Sci Transl Med. 2014 2014/June/04;6(239):239rv1–rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M-T, Ye S, Su J, Garg V. Cardiomyocyte proliferation and maturation: two sides of the same coin for heart regeneration. Front Cell Dev Biol. 2020;8:594226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo YJ, Panlilio CM, Cheng RK, Liao GP, Atluri P, Hsu VM, et al. Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation. 2006. Jul 4;114(1 Suppl):I206–13. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004. Aug 20;279(34):35858–66. [DOI] [PubMed] [Google Scholar]
- 21.Soonpaa MH, Koh GY, Pajak L, Jing S, Wang H, Franklin MT, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997. Jun 1;99(11):2644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005. Jan 7;96(1):110–8. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, et al. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018. Mar 22;173(1):104–16.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Stefano V, Giacca M, Capogrossi MC, Crescenzi M, Martelli F. Knockdown of cyclin-dependent kinase inhibitors induces cardiomyocyte re-entry in the cell cycle. J Biol Chem. 2011. Mar 11;286(10):8644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tane S, Ikenishi A, Okayama H, Iwamoto N, Nakayama KI, Takeuchi T. CDK inhibitors, p21(Cip1) and p27(Kip1), participate in cell cycle exit of mammalian cardiomyocytes. Biochem Biophys Res Commun. 2014. Jan 17;443(3):1105–9. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013. May 9;497(7448):249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007. Dec;388(12):1257–74. [DOI] [PubMed] [Google Scholar]
- 28.Zuppo DA, Missinato MA, Santana-Santos L, Li G, Benos PV, Tsang M. Foxm1 drives cardiomyocyte proliferation in adult zebrafish after cardiac injury. bioRxiv. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agah R, Kirshenbaum LA, Abdellatif M, Truong LD, Chakraborty S, Michael LH, et al. Adenoviral delivery of E2F-1 directs cell cycle reentry and p53-independent apoptosis in postmitotic adult myocardium in vivo. J Clin Invest. 1997;100(11):2722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebelt H, Zhang Y, Kampke A, Xu J, Schlitt A, Buerke M, et al. E2F2 expression induces proliferation of terminally differentiated cardiomyocytes in vivo. Cardiovasc Res. 2008;80(2):219–26. [DOI] [PubMed] [Google Scholar]
- 31.Ebelt H, Hufnagel N, Neuhaus P, Neuhaus H, Gajawada P, Simm A, et al. Divergent siblings: E2F2 and E2F4 but not E2F1 and E2F3 induce DNA synthesis in cardiomyocytes without activation of apoptosis. Circ Res. 2005. Mar 18;96(5):509–17. [DOI] [PubMed] [Google Scholar]
- 32.Xiang FL, Guo M, Yutzey KE. Overexpression of Tbx20 in Adult Cardiomyocytes Promotes Proliferation and Improves Cardiac Function After Myocardial Infarction. Circulation. 2016. Mar 15;133(11):1081–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisping E, Ikeda S, Kong Sek W, Tarnavski O, Bodyak N, McMullen Julie R, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci. 2006 2006/September/26;103(39):14471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, et al. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006. Mar 31;98(6):837–45. [DOI] [PubMed] [Google Scholar]
- 35.Malek Mohammadi M, Kattih B, Grund A, Froese N, Korf-Klingebiel M, Gigina A, et al. The transcription factor GATA4 promotes myocardial regeneration in neonatal mice. EMBO Mol Med. 2017. Feb;9(2):265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Bakker DEM, Bouwman M, Dronkers E, Simões FC, Riley PR, Goumans MJ, et al. Prrx1b restricts fibrosis and promotes Nrg1-dependent cardiomyocyte proliferation during zebrafish heart regeneration. Development. 2021. Oct 1;148(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012. Dec 20;492(7429):376–81. [DOI] [PubMed] [Google Scholar]
- 38.Liang D, Li J, Wu Y, Zhen L, Li C, Qi M, et al. miRNA-204 drives cardiomyocyte proliferation via targeting Jarid2. Int J Cardiol. 2015. Dec 15;201:38–48. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Huang ZP, Seok HY, Ding J, Kataoka M, Zhang Z, et al. mir-17–92 cluster is required for and sufficient to induce cardiomyocyte proliferation in postnatal and adult hearts. Circ Res. 2013. Jun 7;112(12):1557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barroso-delJesus A, Romero-López C, Lucena-Aguilar G, Melen GJ, Sanchez L, Ligero G, et al. Embryonic stem cell-specific miR302–367 cluster: human gene structure and functional characterization of its core promoter. Mol Cell Biol. 2008. Nov;28(21):6609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borden A, Kurian J, Nickoloff E, Yang Y, Troupes CD, Ibetti J, et al. Transient Introduction of miR-294 in the Heart Promotes Cardiomyocyte Cell Cycle Reentry After Injury. Circ Res. 2019. Jun 21;125(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011. Sep 2;109(6):670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013. Jan 2;110(1):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W, Feng Y, Liang J, Yu H, Wang C, Wang B, et al. Loss of microRNA-128 promotes cardiomyocyte proliferation and heart regeneration. Nat Commun. 2018. Feb 16;9(1):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Y, Leach J, Wang J, Martin JF. Hippo/Yap signaling in cardiac development and regeneration. Curr Treat Options Cardiovasc Med. 2016;18(6):1–9. [DOI] [PubMed] [Google Scholar]
- 46.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci USA. 2013;110(34):13839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002 2002/March/01;12(1):9–18. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Li L, Peng L. MAPK1 up-regulates the expression of MALAT1 to promote the proliferation of cardiomyocytes through PI3K/AKT signaling pathway. Int J Clin Exp Pathol. 2015;8(12):15947–53. [PMC free article] [PubMed] [Google Scholar]
- 49.Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022 2022/January/03;7(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Zhou L, Liu H, Duan R, Zhou H, Zhang F, et al. LRP6 downregulation promotes cardiomyocyte proliferation and heart regeneration. Cell Res. 2021 2021/April/01;31(4):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qu S, Liao Q, Yu C, Chen Y, Luo H, Xia X, et al. LKB1 suppression promotes cardiomyocyte regeneration via LKB1-AMPK-YAP axis. Bosn J Basic Med Sci. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirose K, Payumo AY, Cutie S, Hoang A, Zhang H, Guyot R, et al. Evidence for hormonal control of heart regenerative capacity during endothermy acquisition. Science. 2019. Apr 12;364(6436):184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Payumo AY, Chen X, Hirose K, Chen X, Hoang A, Khyeam S, et al. Adrenergic-thyroid hormone interactions drive postnatal thermogenesis and loss of mammalian heart regenerative capacity. Circulation. 2021;144(12):1000–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hubert F, Payan SM, Pelce E, Bouchard L, Sturny R, Lenfant N, et al. FGF10 promotes cardiac repair through a dual cellular mechanism increasing cardiomyocyte renewal and inhibiting fibrosis. Cardiovasc Res. 2022;118(12):2625–37. [DOI] [PubMed] [Google Scholar]
- 56.Hubert F, Payan SM, Rochais F. FGF10 signaling in heart development, homeostasis, disease and repair. Front Genet. 2018;9:599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Li Y, Feng J, Liu W, Li Y, Liu J, et al. Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration. Theranostics. 2020;10(20):9100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–70. [DOI] [PubMed] [Google Scholar]
- 59.Kühn B, Del Monte F, Hajjar RJ, Chang Y-S, Lebeche D, Arab S, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13(8):962–9. [DOI] [PubMed] [Google Scholar]
- 60.Yue Z, Chen J, Lian H, Pei J, Li Y, Chen X, et al. PDGFR-β signaling regulates cardiomyocyte proliferation and myocardial regeneration. Cell Rep. 2019;28(4):966–78. [DOI] [PubMed] [Google Scholar]
- 61.Lindgren IM, Drake RR, Chattergoon NN, Thornburg KL. Down-regulation of MEIS1 promotes the maturation of oxidative phosphorylation in perinatal cardiomyocytes. Faseb j. 2019. Jun;33(6):7417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bae J, Salamon RJ, Brandt EB, Paltzer WG, Zhang Z, Britt EC, et al. Malonate promotes adult cardiomyocyte proliferation and heart regeneration. Circulation. 2021;143(20):1973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wodsedalek DJ, Paddock SJ, Wan TC, Auchampach JA, Kenarsary A, Tsaih SW, et al. IL-13 promotes in vivo neonatal cardiomyocyte cell cycle activity and heart regeneration. Am J Physiol Heart Circ Physiol. 2019. Jan 1;316(1):H24–h34. [DOI] [PubMed] [Google Scholar]
- 64.Paddock SJ, Swift SK, Alencar-Almeida V, Kenarsary A, Alvarez-Argote S, Flinn MA, et al. IL4Rα signaling promotes neonatal cardiac regeneration and cardiomyocyte cell cycle activity. J Mol Cell Cardiol. 2021. Dec;161:62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Al-Dosari MS, Gao X. Nonviral gene delivery: principle, limitations, and recent progress. Aaps J. 2009. Dec;11(4):671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Godbey WT, Mikos AG. Recent progress in gene delivery using non-viral transfer complexes. J Control Release. 2001. May 14;72(1–3):115–25. [DOI] [PubMed] [Google Scholar]
- 67.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy- an overview. J Clin Diagn Res. 2015. Jan;9(1):Ge01–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gyöngyösi M, Khorsand A, Zamini S, Sperker W, Strehblow C, Kastrup J, et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005. Aug 30;112(9 Suppl):I157–65. [DOI] [PubMed] [Google Scholar]
- 69.Laguens R, Cabeza Meckert P, Vera Janavel G, Del Valle H, Lascano E, Negroni J, et al. Entrance in mitosis of adult cardiomyocytes in ischemic pig hearts after plasmid-mediated rhVEGF165 gene transfer. Gene Ther. 2002. Dec;9(24):1676–81. [DOI] [PubMed] [Google Scholar]
- 70.Vera Janavel G, Crottogini A, Cabeza Meckert P, Cuniberti L, Mele A, Papouchado M, et al. Plasmid-mediated VEGF gene transfer induces cardiomyogenesis and reduces myocardial infarct size in sheep. Gene Ther. 2006. Aug;13(15):1133–42. [DOI] [PubMed] [Google Scholar]
- 71.Scalzo S, Santos AK, Ferreira HAS, Costa PA, Prazeres P, da Silva NJA, et al. Ionizable Lipid Nanoparticle-Mediated Delivery of Plasmid DNA in Cardiomyocytes. Int J Nanomedicine. 2022;17:2865–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou LS, Zhao GL, Liu Q, Jiang SC, Wang Y, Zhang DM. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J Inflamm (Lond). 2015;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yin Q, Pei Z, Wang H, Zhao Y. Cyclosporine A-nanoparticles enhance the therapeutic benefit of adipose tissue-derived stem cell transplantation in a swine myocardial infarction model. Int J Nanomedicine. 2014;9:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ben-Mordechai T, Kain D, Holbova R, Landa N, Levin LP, Elron-Gross I, et al. Targeting and modulating infarct macrophages with hemin formulated in designed lipid-based particles improves cardiac remodeling and function. J Control Release. 2017. Jul 10;257:21–31. [DOI] [PubMed] [Google Scholar]
- 75.Su C-H, Wu Y-J, Wang H-H, Yeh H-I. Nonviral gene therapy targeting cardiovascular system. Am J Physiol Heart Circ Physiol. 2012;303(6):H629–H38. [DOI] [PubMed] [Google Scholar]
- 76.Rincon MY, VandenDriessche T, Chuah MK. Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc Res. 2015;108(1):4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scimia MC, Cannavo A, Koch WJ. Gene therapy for heart disease: molecular targets, vectors and modes of delivery to myocardium. Expert Rev Cardiovasc Ther. 2013. Aug;11(8):999–1013. [DOI] [PubMed] [Google Scholar]
- 78.Saffari M, Moghimi HR, Dass CR. Barriers to Liposomal Gene Delivery: from Application Site to the Target. Iran J Pharm Res. 2016. Winter;15(Suppl):3–17. [PMC free article] [PubMed] [Google Scholar]
- 79.Shao M, Yang W, Han G. Protective effects on myocardial infarction model: delivery of schisandrin B using matrix metalloproteinase-sensitive peptide-modified, PEGylated lipid nanoparticles. Int J Nanomedicine. 2017;12:7121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Wang J, Pan J. Baicalin-loaded PEGylated lipid nanoparticles: characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016. Nov;23(9):3696–703. [DOI] [PubMed] [Google Scholar]
- 81.Dong Z, Guo J, Xing X, Zhang X, Du Y, Lu Q. RGD modified and PEGylated lipid nanoparticles loaded with puerarin: Formulation, characterization and protective effects on acute myocardial ischemia model. Biomed Pharmacother. 2017. May;89:297–304. [DOI] [PubMed] [Google Scholar]
- 82.Ikeda G, Matoba T, Nakano Y, Nagaoka K, Ishikita A, Nakano K, et al. Nanoparticle-Mediated Targeting of Cyclosporine A Enhances Cardioprotection Against Ischemia-Reperfusion Injury Through Inhibition of Mitochondrial Permeability Transition Pore Opening. Sci Rep. 2016 2016/February/10;6(1):20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakano Y, Matoba T, Tokutome M, Funamoto D, Katsuki S, Ikeda G, et al. Nanoparticle-Mediated Delivery of Irbesartan Induces Cardioprotection from Myocardial Ischemia-Reperfusion Injury by Antagonizing Monocyte-Mediated Inflammation. Sci Rep. 2016. Jul 11;6:29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Y, Koga JI, Tokutome M, Matoba T, Ikeda G, Nakano K, et al. Nanoparticle-Mediated Delivery of Pitavastatin to Monocytes/Macrophages Inhibits Left Ventricular Remodeling After Acute Myocardial Infarction by Inhibiting Monocyte-Mediated Inflammation. Int Heart J. 2017. Aug 3;58(4):615–23. [DOI] [PubMed] [Google Scholar]
- 85.Nagaoka K, Matoba T, Mao Y, Nakano Y, Ikeda G, Egusa S, et al. A New Therapeutic Modality for Acute Myocardial Infarction: Nanoparticle-Mediated Delivery of Pitavastatin Induces Cardioprotection from Ischemia-Reperfusion Injury via Activation of PI3K/Akt Pathway and Anti-Inflammation in a Rat Model. PLoS One. 2015;10(7):e0132451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ichimura K, Matoba T, Nakano K, Tokutome M, Honda K, Koga J, et al. A Translational Study of a New Therapeutic Approach for Acute Myocardial Infarction: Nanoparticle-Mediated Delivery of Pitavastatin into Reperfused Myocardium Reduces Ischemia-Reperfusion Injury in a Preclinical Porcine Model. PLoS One. 2016;11(9):e0162425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, et al. VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol Heart Circ Physiol. 2018. Feb 1;314(2):H278–h84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang MY, Yang YJ, Chang CH, Tang AC, Liao WY, Cheng FY, et al. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release. 2013. Sep 10;170(2):287–94. [DOI] [PubMed] [Google Scholar]
- 89.Yu K, Wu S, Li H. A chitosan-graft-PEI-eprosartan conjugate for cardiomyocyte-targeted VEGF plasmid delivery in myocardial ischemia gene therapy. J Exp Nanosci. 2016;11(2):81–96. [Google Scholar]
- 90.Xu P, Li SY, Li Q, Ren J, Van Kirk EA, Murdoch WJ, et al. Biodegradable cationic polyester as an efficient carrier for gene delivery to neonatal cardiomyocytes. Biotechnol Bioeng. 2006. Dec 5;95(5):893–903. [DOI] [PubMed] [Google Scholar]
- 91.Kim H, Kim HA, Bae YM, Choi JS, Lee M. Dexamethasone-conjugated polyethylenimine as an efficient gene carrier with an anti-apoptotic effect to cardiomyocytes. J Gene Med. 2009. Jun;11(6):515–22. [DOI] [PubMed] [Google Scholar]
- 92.Camci-Unal G, Annabi N, Dokmeci MR, Liao R, Khademhosseini A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014 2014/May/01;6(5):e99–e. [Google Scholar]
- 93.Wang LL, Liu Y, Chung JJ, Wang T, Gaffey AC, Lu M, et al. Local and sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischemic injury. Nat Biomed Eng. 2017;1:983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang H, Qin X, Wang H, Zhao X, Liu Y, Wo HT, et al. An in Vivo miRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano. 2019. Sep 24;13(9):9880–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, Chen X, Jin R, Chen L, Dang M, Cao H, et al. Injectable hydrogel with MSNs/microRNA-21–5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci Adv. 2021. Feb;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujii H, Sun Z, Li SH, Wu J, Fazel S, Weisel RD, et al. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging. 2009. Jul;2(7):869–79. [DOI] [PubMed] [Google Scholar]
- 97.Zhou W, Ma T, Ding S. Non-viral approaches for somatic cell reprogramming into cardiomyocytes. Semin Cell Dev Biol. 2022. Feb;122:28–36. [DOI] [PubMed] [Google Scholar]
- 98.Djurovic S, Iversen N, Jeansson S, Hoover F, Christensen G. Comparison of nonviral transfection and adeno-associated viral transduction on cardiomyocytes. Mol Biotechnol. 2004 2004/September/01;28(1):21–31. [DOI] [PubMed] [Google Scholar]
- 99.Yerevanian A, Yerevanian A, Hajjar RJ. Progress in gene therapy for heart failure. J Cardiovasc Pharmacol. 2014. Feb;63(2):95–106. [DOI] [PubMed] [Google Scholar]
- 100.Hajjar RJ. Potential of gene therapy as a treatment for heart failure. J Clin Invest. 2013. Jan;123(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmid C, Heemann U, Tilney NL. Factors contributing to the development of chronic rejection in heterotopic rat heart transplantation. Transplantation. 1997;64(2):222–8. [DOI] [PubMed] [Google Scholar]
- 102.Hartikainen J, Hassinen I, Hedman A, Kivelä A, Saraste A, Knuuti J, et al. Adenoviral intramyocardial VEGF-DΔNΔC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur Heart J. 2017;38(33):2547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hammond HK, Penny WF, Traverse JH, Henry TD, Watkins MW, Yancy CW, et al. Intracoronary Gene Transfer of Adenylyl Cyclase 6 in Patients With Heart Failure: A Randomized Clinical Trial. JAMA Cardiol. 2016. May 1;1(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tao Z, Chen B, Tan X, Zhao Y, Wang L, Zhu T, et al. Coexpression of VEGF and angiopoietin-1 promotes angiogenesis and cardiomyocyte proliferation reduces apoptosis in porcine myocardial infarction (MI) heart. Proc Natl Acad Sci U S A. 2011. Feb 1;108(5):2064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Post MJ, Sato K, Murakami M, Bao J, Tirziu D, Pearlman JD, et al. Adenoviral PR39 improves blood flow and myocardial function in a pig model of chronic myocardial ischemia by enhancing collateral formation. Am J Physiol Regul Integr Comp Physiol. 2006. Mar;290(3):R494–500. [DOI] [PubMed] [Google Scholar]
- 106.Gao MH, Lai NC, McKirnan MD, Roth DA, Rubanyi GM, Dalton N, et al. Increased regional function and perfusion after intracoronary delivery of adenovirus encoding fibroblast growth factor 4: report of preclinical data. Hum Gene Ther. 2004. Jun;15(6):574–87. [DOI] [PubMed] [Google Scholar]
- 107.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105(11):1291–7. [DOI] [PubMed] [Google Scholar]
- 108.Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006. Nov;13(21):1503–11. [DOI] [PubMed] [Google Scholar]
- 109.Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med. 2014. Feb 19;6(224):224ra27. [DOI] [PubMed] [Google Scholar]
- 110.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, Adachi S, Hiroe M, Shimizu M, et al. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res. 2003. Jan 10;92(1):e12–9. [DOI] [PubMed] [Google Scholar]
- 111.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004. Jun;78(12):6381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002. Sep 3;99(18):11854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno‐associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3(3):81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zacchigna S, Zentilin L, Giacca M. Adeno-associated virus vectors as therapeutic and investigational tools in the cardiovascular system. Circ Res. 2014;114(11):1827–46. [DOI] [PubMed] [Google Scholar]
- 115.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010. Jun;3(3):81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006. Jul;14(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016. Mar 19;387(10024):1178–86. [DOI] [PubMed] [Google Scholar]
- 118.Li Y, Feng J, Song S, Li H, Yang H, Zhou B, et al. gp130 Controls Cardiomyocyte Proliferation and Heart Regeneration. Circulation. 2020. Sep 8;142(10):967–82. [DOI] [PubMed] [Google Scholar]
- 119.Wu Y, Zhou L, Liu H, Duan R, Zhou H, Zhang F, et al. LRP6 downregulation promotes cardiomyocyte proliferation and heart regeneration. Cell Res. 2021. Apr;31(4):450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang F, Liu S, Pei J, Cai L, Liu N, Liang T, et al. LPA(3)-mediated lysophosphatidic acid signaling promotes postnatal heart regeneration in mice. Theranostics. 2020;10(24):10892–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature. 2019. May;569(7756):418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kieserman JM, Myers VD, Dubey P, Cheung JY, Feldman AM. Current Landscape of Heart Failure Gene Therapy. J Am Heart Assoc. 2019. May 21;8(10):e012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McGarrity GJ, Hoyah G, Winemiller A, Andre K, Stein D, Blick G, et al. Patient monitoring and follow-up in lentiviral clinical trials. J Gene Med. 2013. Feb;15(2):78–82. [DOI] [PubMed] [Google Scholar]
- 124.Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010. Sep 16;467(7313):318–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009. Nov 6;326(5954):818–23. [DOI] [PubMed] [Google Scholar]
- 126.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013. Aug 23;341(6148):1233158. [DOI] [PubMed] [Google Scholar]
- 127.Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013. Aug 23;341(6148):1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu W, Zhao M, Mattapally S, Chen S, Zhang J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ Res. 2018. Jan 5;122(1):88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015. Mar 18;7(279):279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Abouleisa RRE, Salama ABM, Ou Q, Tang XL, Solanki M, Guo Y, et al. Transient Cell Cycle Induction in Cardiomyocytes to Treat Subacute Ischemic Heart Failure. Circulation. 2022. Apr 26;145(17):1339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li L, Guan Q, Dai S, Wei W, Zhang Y. Integrin β1 Increases Stem Cell Survival and Cardiac Function after Myocardial Infarction. Front Pharmacol. 2017;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schlimgen R, Howard J, Wooley D, Thompson M, Baden LR, Yang OO, et al. Risks Associated With Lentiviral Vector Exposures and Prevention Strategies. J Occup Environ Med. 2016. Dec;58(12):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Auchampach J, Han L, Huang GN, Kühn B, Lough JW, O’Meara CC, et al. Measuring cardiomyocyte cell-cycle activity and proliferation in the age of heart regeneration. Am J Physiol Heart Circ Physiol. 2022;322(4):H579–H96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ylä-Herttuala S, Baker AH. Cardiovascular Gene Therapy: Past, Present, and Future. Mol Ther. 2017. May 3;25(5):1095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, et al. A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55(18):1907–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


