Abstract
BACKGROUND & AIMS:
Cow’s milk protein (CMP) is the most common trigger of inflammation in children and adults with eosinophilic esophagitis (EoE). We sought to assess the clinical, endoscopic, and histologic efficacy of dietary elimination of all CMP-containing foods in EoE.
METHODS:
We performed a prospective observational study in children with EoE treated with the 1-food elimination diet (1FED), excluding all CMP. Children and their caretakers were educated by a registered dietitian regarding dietary elimination of all CMP-containing foods, with substitutions to meet nutritional needs for optimal growth and development, and daily meal planning. Upper endoscopy with biopsies was performed after 8 to 12 weeks of treatment. The primary end point was histologic remission, defined as fewer than 15 eosinophils per high-power field. Secondary end points were symptomatic, endoscopic, and quality-of-life (QOL) improvements.
RESULTS:
Forty-one children (76% male; ages, 9 ± 4 years; 88% white) underwent 1FED education and post-treatment endoscopy with biopsies. Histologic remission occurred in 21 (51%) children, with a decrease in peak eosinophils per high-power field from a median of 50 (interquartile range, 35–70) to a median of 1 (interquartile range, 0–6; P < .0001). Endoscopic abnormalities improved in 24 (59%) patients, while symptoms improved in 25 (61%). Improved symptoms included chest pain, dysphagia, and pocketing/spitting out food. Parents perceived worse QOL, while children perceived improved QOL with the 1FED.
CONCLUSIONS:
One-food elimination of CMP-containing foods from the diet induced histologic remission in more than 50% of children with EoE and led to significant improvement in symptoms and endoscopic abnormalities. The ease of implementation and adherence supports the 1FED as first-line dietary treatment.
Keywords: EoE, Treatment, Response, Dairy, Children, Dietary, Antigen
Eosinophilic esophagitis (EoE) is a chronic antigen-mediated allergic inflammatory disorder with significant treatment-associated impact on quality of life (QOL).1 In a seminal publication, Kelly et al2 established the role of food antigen(s) in triggering eosinophilia in EoE. Subsequent elemental diet as treatment studies showed remission in 88% to 95% of subjects.3–5 An exclusive elemental diet is challenging to maintain, and requires numerous subsequent endoscopies to identify safe foods6; thus, less-restrictive diets are necessary. Kagalwalla et al7 showed that a 6-food elimination diet (SFED) achieved remission in 75% of children with EoE, and similar response rates were shown in adults.8,9 The superiority of a SFED over an allergy-test–directed elimination diet was shown by a meta-analysis.10
Despite high efficacy, adherence to a SFED can be challenging,11 and there remains intense interest in less-restrictive dietary elimination strategies. Our group, in a prospective multicenter study, established the efficacy of a 4-food elimination diet (4FED) with histologic remission in 65% of children with EoE.12 Even less restrictive 2-food group elimination diets are effective, but also can be difficult to implement and maintain.13 We and others have identified cow’s milk protein (CMP) as the most commonly identified trigger of eosinophilia.7,14–21 However, prior studies were limited by retrospective designs, small sample sizes, or age restrictions, and may not have estimated their efficacy accurately because of selection bias. Furthermore, CMP elimination studies have not used a histologic scoring system to assess inflammatory and structural changes, or addressed predictive factors of treatment response. Thus, studies to validate a 1-food elimination diet (1FED) of all CMP for EoE are necessary.
In this prospective study, we assessed the histologic, symptom, endoscopic, and quality-of-life outcomes in response to dietary elimination of all CMP in children with EoE. We also assessed predictive factors of treatment response.
Methods
Patient Recruitment
Patients ages 2 to 18 years with a previously confirmed diagnosis pf proton pump inhibitor (PPI)-refractory EoE per 2011 consensus guidelines22 were recruited prospectively from gastroenterology clinics at the Ann and Robert H. Lurie Children’s Hospital of Chicago from March 2012 to May 2017. Patients previously treated with swallowed steroids underwent a baseline endoscopy 3 months after discontinuing the medication to confirm recurrent esophageal eosinophilia (≥15 eosinophils per high-power field [eos/hpf]). Patients on PPI at study enrollment continued the medication once daily. Intranasal steroids for allergic rhinitis and inhaled steroids for asthma were continued. Patients on oral prednisone and those with non-EoE eosinophilic gastrointestinal disease, celiac disease, or inflammatory bowel disease were excluded from this study. The Institutional Review Board at the Ann and Robert H. Lurie Children’s Hospital approved this study. Informed consent/assent was provided by the participants/their parents at the time of study enrollment.
Study Design
A 3-day pretreatment diet log was reviewed at enrollment to provide guidance about food substitution to ensure optimal growth. A single registered dietitian created standardized educational materials used for the duration of the study. Patients and parents were instructed on complete avoidance of all foods and supplements containing CMP. Meal planning used appropriate substitutions to meet the individual participant’s nutritional needs. Food label reading was advised to avoid cross-contact. Printed instructions detailing specific guidance to avoid food contamination was provided to each family (Supplementary Tables 1–4). Avoidance of other animal milks such as goat or sheep was recommended, however, consumption of these milks (as cheese) was not noted on the 3-day pretreatment diet logs. Diet logs also were collected midway through the diet elimination to ensure compliance with food elimination and establish avoidance of contamination and, after review, the patient was cleared for follow-up endoscopy. Contamination or cross-contact with CMP resulted in delaying endoscopy for 4 weeks after removal of the contamination. Endoscopy therefore was performed 8 to 12 weeks after enrollment. The recruitment goal was 54 patients to power the study at 80%, with an a value of .05 for 51% of patients to have fewer than 15 eos/hpf.
Outcomes
The primary outcome was histologic response. Four samples each from the proximal, mid-, and distal esophagus were collected. H&E assessment of standard-of-care esophageal biopsy specimens was performed to determine the peak eosinophil count (PEC), defined as the maximal count of intra-epithelial eosinophils per high-powered field (0.23 mm2). Patients with post-treatment biopsy specimens showing fewer than 15 or 15 or more eos/hpf were classified as 1FED responders or nonresponders, respectively.23
The secondary outcomes were improvements in symptoms, endoscopic findings, and QOL. Symptoms of esophageal dysfunction were collected at enrollment and post-treatment endoscopy. We used a 15-point scale that previously identified a response to 4FED.12 This scale measures the presence or absence of the following: abdominal pain, chest pain, dysphagia, early satiety, feeding aversion, food impaction, gag, heartburn, odynophagia, pocket/spit out food, poor appetite, reflux/regurgitation, slow eating, and vomiting. Endoscopic features such as edema, rings, exudate, furrows, and stricture were scored as absent or present. PedsQL (Mapi Research Trust, Lyon, France) Generic and EoE-specific questionnaires were added before enrollment of the 24th patient. The questionnaires were collected for the following age groups: 5 to 7 years, 8 to 12 years, and 13 to 18 years. Further description of the PedsQL questionnaires is detailed in the Supplementary Methods section.
Statistics
Baseline dichotomous characteristics including demographics, symptoms, endoscopic abnormalities, atopy/comorbidities, concurrent medications, and allergy tests were summarized by frequency, and compared between histologic responders and nonresponders by the Fisher exact test (dichotomous variables) or the Student t test (continuous variables). Baseline and post-treatment PEC were summarized as the median (interquartile range [IQR]) and compared between histologic responders and nonresponders by the Wilcoxon rank-sum test. Changes before and after 1FED in PEC, EoE–histologic scoring system (HSS), symptom sum, endoscopic composites, and QOL scores were compared by the Wilcoxon signed-rank test. A binary logistic regression model was generated with treatment responder status as dependent and age at enrollment and carries EpiPen (Viatris, Canonburg, PA) as independent variables. Statistical analysis was performed using SAS/STAT version 9.4 (SAS Institute, Cary, NC) and R 3.5.3 (R Core team, Vienna, Austria).
Additional methods are described in the Supplemental Methods section of the Supplementary Materials.
Results
Patient Characteristics
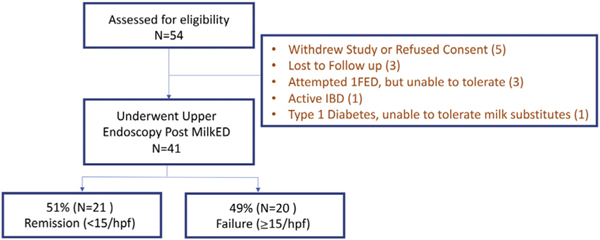
Fifty-four patients were enrolled. Of these, 41 patients completed endoscopy 8 to 12 weeks after a 1FED (Figure 1). Seventy-five percent of these patients were male, with a mean age of 9 years (IQR, 6–13 y) (Table 1). Eighty-eight percent of patients were Caucasian with frequent personal or family histories of atopic diseases (Table 1). IgE-mediated food allergy was present in 32% of patients; allergic rhinitis was the most common atopic comorbidity identified in 66% of patients and in 63% of family members. Sensitization to foods by skin prick tests was present in 65%, this was food-specific IgE in 78%. CM-specific IgE was present in 43%, skin prick test to CM was positive in only 11%. The median baseline eosinophil count was 50 eos/hpf (IQR, 35–80 eos/hpf) (Table 2). The baseline EoE-HSS grade/stage score was 9 (IQR, 8–12)/9 (IQR, 6–10) in the distal esophagus, 9 (IQR, 6–13)/9 (IQR, 5–11) in the midesophagus, and 7 (IQR, 3–11)/6 (IQR, 4–10) in the proximal esophagus (Table 2). A score of 9 defines mild-moderate severity because the maximum score is 21, whereas a score of 3 or less has been proposed as an EoE-HSS Remission Score.24 Dysphagia (44%), early satiety (43%), slow eating (50%), and vomiting (34%) were the most frequent symptoms (Table 3). Endoscopic abnormalities were present, including edema and furrowing in 78%, exudates in 59%, and rings in 20% (Table 3), although no strictures were identified.
Figure 1.
Consort diagram showing study population. 1FED, 1-food elimination diet; hpf, high-power field; IBD, inflammatory bowel disease; MilkED, milk elimination diet.
Table 1.
Baseline Demographic, Clinical, Endoscopic, and Histologic Characteristics
| Overall (N = 41) | |
|---|---|
|
| |
| Demographics, n (%) | |
| Age, y, mean [IQR] | 9 [6–13] |
| Male | 31 (76) |
| White | 36 (88) |
| Hispanic | 2 (5) |
| Atopy/comorbidities, n (%) | |
| Allergic rhinitis | 27 (66) |
| Asthma | 14 (34) |
| Conjunctivitis | 2 (5) |
| Drug allergy | 6 (15) |
| Eczema | 10 (24) |
| Gastroesophageal reflux disease | 6 (15) |
| IgE-mediated food allergy | 13 (32) |
| Family history, n (%) | |
| Allergic rhinitis | 26 (63) |
| Asthma | 22 (54) |
| Celiac disease | 6 (15) |
| Colitis | 2 (5) |
| Crohn’s disease | 2 (5) |
| Eczema | 13 (32) |
| EoE | 6 (15) |
| Esophageal stricture | 5 (12) |
| IgE-mediated food allergy | 15 (37) |
| Gastroesophageal reflux disease | 21 (51) |
| Hiatal hernia | 7 (17) |
| Medications at enrollment, n (%) | |
| Proton pump inhibitor | 37 (90) |
| Antihistamine | 11 (27) |
| Bronchodilator | 8 (20) |
| EpiPen (carries) | 15 (37) |
| Inhaled corticosteroid | 5 (12) |
| Intranasal corticosteroid | 7 (17) |
| Montelukast | 4 (10) |
| Topical steroid | 0 (0) |
| Skin prick test, n (%) | |
| Any abnormal (n = 23) | 15 (65) |
| Milk abnormal (n = 18) | 2 (11) |
| Food sensitization on RAST, n (%) | |
| Any abnormal (n = 23) | 18 (78) |
| Milk abnormal (n = 21) | 9 (43) |
| Prior treatment, n (%) | |
| Swallowed steroida | 8 (20) |
EoE, eosinophilic esophagitis; IQR, interquartile range; RAST, radioallergosorbent test.
Swallowed steroids included fluticasone inhaler or viscous budesonide.
Table 2.
Change in PEC and EoE-HSS Score After 1FED in Pediatric EoE
| n | Pretreatment | n | Post-treatment | P valuea | |
|---|---|---|---|---|---|
|
| |||||
| Histology, median [IQR] | |||||
| Overall | 41 | 50 [35–80] | 41 | 12 [1–40] | <.001 |
| Distal esophagus | 41 | 35 [20–45] | 41 | 10 [1–35] | <.001 |
| Midesophagus | 41 | 30 [18–60] | 41 | 4 [0–17] | <.001 |
| Proximal esophagus | 30 | 35 [11–50] | 40 | 1 [0–7] | <.001 |
| EoE HSS score | |||||
| Grade | |||||
| Distal esophagus | 39 | 9 [8–12] | 39 | 5 [2–6] | <.001 |
| Midesophagus | 38 | 9 [6–13] | 38 | 4 [1–5] | <.001 |
| Proximal esophagus | 27 | 7 [3–11] | 27 | 2 [1–5] | <.001 |
| Stage | |||||
| Distal esophagus | 39 | 9 [6–10] | 39 | 3 [1–5] | <.001 |
| Midesophagus | 38 | 9 [5–11] | 38 | 2 [0–5] | <.001 |
| Proximal esophagus | 27 | 6 [4–10] | 27 | 1 [0–4] | <.001 |
NOTE. Significant values are bolded P < .05.
EoE-HSS, eosinophilic esophagitis histologic scoring system; IQR, interquartile range; 1FED, 1-food elimination diet; PEC, peak eosinophil count.
Comparison by Wilcoxon signed-rank test.
Table 3.
Change in Endoscopic Abnormalities and Symptoms With 1FED in Pediatric EoE
| n | Pretreatment | n | Post-treatment | P valuea | |
|---|---|---|---|---|---|
|
| |||||
| Endoscopic findings, n (%) | |||||
| Edema | 41 | 32 (78) | 41 | 22 (54) | .01 |
| Exudate | 41 | 24 (59) | 41 | 10 (24) | .001 |
| Furrows | 41 | 32 (78) | 41 | 22 (54) | .02 |
| Rings | 41 | 8 (20) | 41 | 16 (39) | .04 |
| Stricture | 41 | 0 (0) | 41 | 1 (2) | NDb |
| Inflammatory sum,c median [IQR] | 41 | 2 [2–3] | 41 | 1 [0–2] | <.001 |
| Sum,b median [IQR] | 41 | 3 [2–3] | 41 | 2 [1–3] | .009 |
| Symptoms, n (%) | |||||
| Abdominal pain | 41 | 13 (32) | 38 | 16 (42) | .45 |
| Chest pain | 39 | 10 (26) | 37 | 3 (8) | .039 |
| Dysphagia | 39 | 17 (44) | 38 | 6 (16) | .002 |
| Early satiety | 35 | 15 (43) | 37 | 16 (43) | 1.00 |
| Feeding difficulties | 39 | 10 (26) | 37 | 9 (24) | 1.00 |
| Food impaction | 38 | 10 (26) | 38 | 5 (13) | .15 |
| Gagging/choking | 37 | 7 (19) | 37 | 2 (5) | .22 |
| Heartburn | 37 | 9 (24) | 37 | 8 (22) | 1.00 |
| Nausea | 38 | 10 (26) | 38 | 10 (26) | .77 |
| Odynophagia | 37 | 5 (14) | 37 | 1 (3) | .22 |
| Pockets or spits out food | 36 | 10 (28) | 37 | 3 (8) | .039 |
| Poor appetite | 37 | 10 (27) | 37 | 5 (14) | .13 |
| Reflux or regurgitation | 36 | 11 (31) | 36 | 8 (22) | .69 |
| Slow eating | 38 | 19 (50) | 37 | 11 (30) | .11 |
| Vomiting | 41 | 14 (34) | 38 | 7 (18) | .15 |
| Night wakening | 35 | 7 (19) | 35 | 3 (9) | .51 |
| Symptom sum,d median [IQR] | 41 | 4 [3–6] | 38 | 3 [1–5] | .003 |
NOTE. The Wilcoxon signed-ranked test was used for paired endoscopy and symptom scores. Significant values are bolded, P < .05.
IQR, interquartile range; 1FED, 1-food elimination diet; ND, not determined.
Comparison by McNemar test was performed for dichotomous paired data.
Sum of edema, exudate, rings, furrows.
Sum of edema, exudate, and furrows.
Sum of symptoms that are present.
Change in Peak Eosinophil Count and Histology Scores
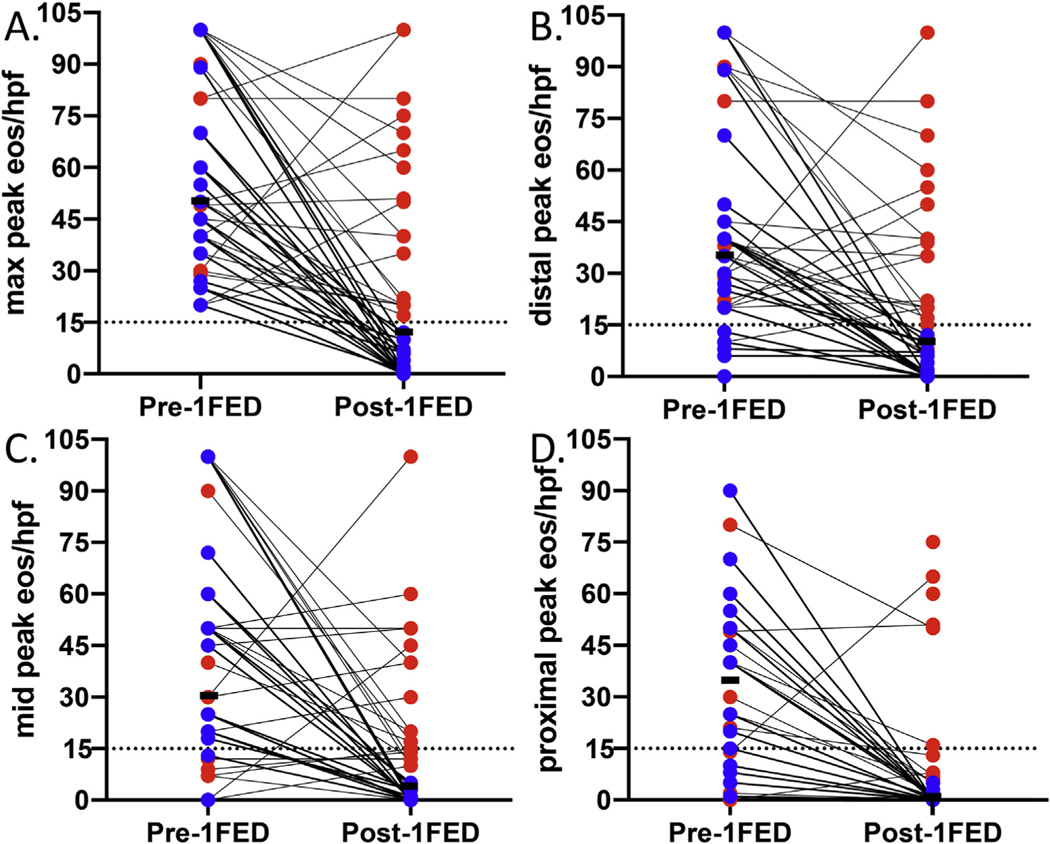
Of the 41 patients who underwent endoscopy after a 1FED, 21 (51%) achieved the primary histologic remission outcome of fewer than 15 eos/hpf (Figure 2A). Sixteen (39%) patients had 6 or fewer eos/hpf and 11 (27%) patients had 1 or fewer. We performed an intention-to-treat analysis by including all patients assessed for eligibility except those who withdrew or refused consent. By intention-to-treat analysis, 21 of 49 (43%) achieved histologic remission. The median PEC was reduced to 12 (IQR, 1–40; P < .001) (Table 2). The distal esophagus had the highest PEC and the proximal esophagus had the lowest after a 1FED (Table 2). A few histologic nonresponders had a decrease in eosinophilia in the proximal esophagus only (Figure 2B–D).
Figure 2.
(A) Peak eosinophil counts before and after 1FED. Reduced eosinophilia with milk elimination in the (B) distal, (C) mid-, and (D) proximal esophagus. The median is shown in black. Histologic responders are shown in blue, and nonresponders are shown in red. 1FED, 1-food elimination diet; eos/hpf, eosinophils per high-power field. Dotted line at histologic threshold for active EoE (15 eos/hpf).
We found a significant reduction in the EoE-HSS grade and stage scores in the distal, mid-, and proximal esophagus after a 1FED (Table 2). There was a significant reduction in both the eosinophil and epithelial composite grade and stage scores in the proximal, mid-, and distal esophagus (Supplementary Table 5). Although lamina propria fibrosis was not a common finding among our cohort, if present, it improved significantly in the proximal esophagus, and had a nonsignificant trend toward improvement in the midesophagus and distal esophagus (Supplementary Table 5).
Endoscopic and Symptomatic Response
Exudates improved after treatment in 58% of patients (P = .001), furrows in 31% (P = .02), and edema in 31% (P = .01) (Table 3). The number of endoscopic abnormalities was reduced from a median of 3 (IQR, 2–3) to a median of 2 (IQR, 1–3) (P < .01) (Table 3). Inflammatory findings were reduced from a median of 2 (IQR, 2–3) to a median of 1 (IQR, 0–2) (P < .001) (Table 3). Rings were more frequent at the post-treatment endoscopy (8 vs 16; P = .04). There was a 25% reduction in the median number of symptoms: 4 (IQR, 3–6) vs 3 (IQR, 1–5) (P = .003) (Table 3). Several symptoms improved: chest pain (before: 10 [26%] vs after: 3 [8%]; P = .04), dysphagia (before: 17 [44%] vs after: 6 [16%]; P < .005), and pocketing/spitting out food (before: 10 [28%] vs after: 3 [8%]; P = .04).
Resolution of all endoscopic abnormalities occurred in 7 of 21 (33%) histologic responders while this occurred in only 3 of 30 (15%) nonresponders (Table 4). Resolution of at least 1 symptom occurred in 25 (61%) patients after a 1FED, while 12 (29%) patients had resolution of all symptoms (Table 4). We did not observe a significant difference in resolution of at least 1 symptom between histologic responders (15 of 21; 71%) and nonresponders (10 of 20; 50%; P = .21).
Table 4.
Outcomes of 1FED on Histologic, Endoscopic, and Clinical Metrics in Pediatric EoE
| Overall | Respondera | Nonrespondera | |
|---|---|---|---|
|
| |||
| EGD to assess histologic response to CM elimination, n | 41 | 21 | 20 |
| Resolution of ≥1 endoscopy finding(s), n (%)b | 24 (59) | 13 (62) | 11 (55) |
| Resolution of all endoscopic findings, n (%)b | 10 (24) | 7 (33) | 3 (15) |
| Resolution of ≥1 symptoms, n (%)b | 25 (61) | 15 (71) | 10 (50) |
| Resolution of all symptoms, n (%)b | 12 (29) | 4 (19) | 8 (40) |
CM, cow’s milk; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; 1FED, 1-food elimination diet.
A responder was defined by a peak eosinophil count fewer than 15 eosinophils per high-power field on esophageal biopsy specimens after a 1FED.
The percent denominator is the column total.
Quality-of-Life Changes With Elimination of Cow’s Milk
Paired data points before and after treatment were collected for 14 children (self-report) and 15 caregivers (Supplementary Table 6). Although not significant, we found a trend toward improved QOL and a moderate-large effect size for self-reported Generic Core Scales: overall (78 vs 82; P = .38; effect size, 0.48), psychosocial (72 vs 78; P = .40; effect size, 0.42), and social (80 vs 98; P = .1; effect size, 0.88), which met the threshold for a minimal clinically important difference. There was a trend for lower parent-reported scores after a 1FED with a moderate-to-large effect size for domains: social (95 vs 80; P = .18; effect size, 0.53), psychosocial (90 vs 78; P = .29; effect size, 0.37), and emotional (90 vs 70; P = .31; effect size, 0.38), which met the threshold for a minimal clinically important difference. Thus, we found a dissociation of overall QOL between children and their parents/caregivers. For the self-reported PedsQL EoE module, there was a trend toward higher scores with a moderate-to-large effect size for the Symptoms II (66 vs 78; P = .17; effect size, 0.47) and Worry (69 vs 77; P = .06; effect size, 0.76) subscales. There were no significant differences before and after the 1FED for the EoE-QOL module completed by parents/caregivers.
Predictors of Treatment Response
Lastly, we explored potential predictors of treatment response. No differences were seen in sex, race, or ethnicity. Histologic responders had a nonsignificant trend toward being older: 12 years (IQR: 6–15 y) vs 7 years (IQR: 5–10 y) (P = .06) (Supplementary Table 7). No differences were noted in atopic comorbidities, although a nonsignificant trend toward decreased IgE-mediated food allergy was seen with histologic responders (19% vs 45%; P = .10). Among the family histories, there was a nonsignificant increase in celiac disease for histologic nonresponders (5% vs 25%; P = .09). Regarding medication use at the time of enrollment, histologic nonresponders more commonly carried an EpiPen (14% vs 60%; P = .004) and took inhaled corticosteroids (0% vs 25%; P < .05). Skin prick testing and food-specific IgE testing did not have predictive capacity for the response to a 1FED (Supplementary Table 7). A binary logistic regression model identified age and carrying an EpiPen as independent predictors of treatment response (Supplementary Table 8). Thus, the 1FED was more successful in older patients without anaphylactic food allergy.
Discussion
Dietary therapy for EoE is recommended as first-line treatment for EoE,25 but highly restrictive diets can be difficult to adhere to long term.11,26 Current standard-of-care elimination dietary treatment protocols in EoE exclude 4 to 8 foods from the diet.7,12,27 However, excluding multiple foods from the diet, even temporarily, is challenging to maintain because it imposes a significant burden for adequate nutrient and micronutrient intake. Therefore, identifying less-restrictive diets is of interest. In this study, we showed that exclusion of all CMP induces histologic remission in more than 50% of children with EoE. This was a large single-center prospective study assessing the efficacy of a 1FED (milk) for the treatment of EoE.
Prior work has identified variable histologic remission rates of 44% to 65% to milk elimination.14,17–21 Kruszewski et al17 found improvement in 65% of children (13 of 20), however, in their cohort, PPI-refractory EoE was not established with an endoscopy and their patients were treated concurrently with both PPI and milk elimination. In a retrospective cohort, Wong et al18 reported 58 of 102 children who had histologic remission after 8 to 12 weeks of a 1FED, consistent with our findings. A recent prospective randomized study assessed the efficacy of 1FED vs 4FED and found similar improvements in validated symptom, histology, and quality-of-life scores. This study was restricted to children ages 6 and up, and did not extend the treatment duration for cross-contamination.19 Our study validates CMP as a critical food trigger of eosinophilic inflammation, and removal of CMP alone reduces eosinophilia in more than half of children with EoE. We observed endoscopic improvement in 59%, with complete resolution of all endoscopic abnormalities in 24%. This is similar to Wong et al,18 who reported resolution of inflammatory endoscopic abnormalities in 24% of patients treated with 1FED. However, there was a surprising increase in the frequency of rings after a 1FED. Rings, an early sign of fibrostenosis, were found in 20% of patients at baseline, and mild rings may have been under-recognized in the presence of inflammatory changes. Finally, we observed symptomatic improvement in 61% of patients and resolution of all symptoms in 29% of patients. Kliewer et al19 reported significantly improved symptom scores with a 1FED, although there was greater improvement with a 4FED. Interestingly, we saw greater symptom improvement as well in our prospective 4FED study.28 Thus, this study validates that the 1FED (milk) is sufficient to achieve all aspects of remission, clinical, endoscopic, and histologic, in a meaningful group of children with EoE.
Elimination diets are difficult, particularly when elimination of multiple types of food is needed. Diet elimination therapy increases the risk of malnutrition and nutritional deficiencies because patients often face a significant loss of calories and micronutrients, such as calcium and vitamin D.27 Although a number of patients dropped out of the study before the second endoscopy, only 3 patients were unable to tolerate the diet. A key reason for this was the nutritional guidance by a dietitian to ensure calorie and micronutrient needs and assess for cross-contamination and this was also a key strength of our study. Notably, a subset of patients reported improved QOL for social and worry domains, suggesting the psychosocial effects of a 1FED may be limited, as compared with broader elimination.11 Further studies are needed to understand long-term QOL in children treated with a 1FED.
We previously identified female sex and asthma as predictors of a successful response to a 4FED, while family history of food allergy and food sensitization by IgE were predictors of poor response.12 In this study we found younger patients and patients with IgE-mediated food allergy were more likely to fail a 1FED. Finally, we found allergy testing had a poor correlation with identified EoE food triggers, similar to prior results from our group and others.9,12,29
The major strengths of this study were the prospective design and the number of subjects recruited, which improve generalizability. In addition, we used the validated EoE-HSS to show structural improvements in basal zone hyperplasia and dilated intercellular spaces in addition to eosinophilia. The EoE-HSS was evaluated at 3 levels of the esophagus (proximal, mid-, and distal), and identified a variable response, with the proximal esophagus most responsive to treatment, and the distal esophagus the least responsive to treatment. Incorporation of validated QOL measures identified novel perspectives of the 1FED between patients and their parents/caregivers. We identified discordant QOL scores for various overall and EoE-specific domains. Although small sample size may play a role, this finding raises interesting questions that future studies can address.
Our study had limitations as well. The study design intended to enroll 54 patients over 5 years, yet only 41 underwent endoscopy. This may have limited the significant differences we identified; however, this was a large single-center report of children with EoE following a 1FED prospectively. We did not randomize patients to placebo or another treatment arm. A placebo effect has been seen in medication trials,30 but there are no placebo (or sham-diet) controlled studies of dietary elimination in EoE. The lack of a validated symptom questionnaire may have limited our ability to detect symptom improvement, although dysphagia, a core EoE symptom, improved in a significant number of patients. We saw a reduction in the number of symptoms in our subjects. In addition, our study may have experienced selection bias because recruitment of this study overlapped with our 4FED study.12 Both studies identified CMP as the predominant trigger of EoE. Finally, our study did not determine the impact of PPIs with a 1FED because most patients continued PPI during the study. A recent abstract identified improved histologic response to a PPI with the addition of a 4FED.31 However, it remains unknown if stopping a PPI before starting a 1FED would have altered the treatment response. Studies that randomize to diet vs diet/PPI could address the possible additive impact of PPI to response.
In summary, we prospectively identified histologic remission in 1 of 2 children with EoE with a significantly less-restrictive diet than SFED or 4FED. Significant improvement in symptoms, and endoscopic and histologic abnormalities, were achieved with the 1FED in a majority of patients. Notably, a 1FED does not require food re-introduction, thus transition to maintenance occurs more quickly without additional endoscopy. This has significant implications on the standard of care, and further studies could assess the optimal “step-up” approach, which has been shown previously to be feasible in adults.13 Our results provide evidence that CMP elimination can be offered as an alternative to other elimination diets as first-line treatment for EoE.
Supplementary Material
What You Need to Know.
Background
Eosinophilic esophagitis (EoE) is a chronic allergic–inflammatory disorder of the esophagus mostly triggered by food antigen(s); elimination diets excluding 2, 4, and 6 foods are effective but difficult to implement and maintain. Cow’s milk protein is the most common identified trigger of inflammation.
Findings
Exclusive elimination of cow’s milk protein induces histologic remission and improves endoscopic abnormalities and symptoms in more than 50% of children with EoE.
Implications for patient care
This single-milk-protein elimination diet represents an easy-to-accomplish, first-line approach to dietary elimination therapy for EoE.
Funding
This work was supported primarily by the Buckeye Foundation, United States (A.F.K.). Additional support was provided by K08DK097721 (J.B.W.), institutional funds from the Ann and Robert H. Lurie Children’s Hospital of Chicago, and the Consortium of Eosinophilic Gastrointestinal Researchers. Joshua B. Wechsler is a Consortium of Eosinophilic Gastrointestinal Researchers scholar trainee. The Consortium of Eosinophilic Gastrointestinal Researchers (U54 AI117804) (J.B.W.) is part of the Rare Disease Clinical Research Network, an initiative of the Office of Rare Diseases Research, National Center for Advancing Translational Research, and is funded through collaboration between the National Institute of Allergy and Infectious Diseases, National Institute of Diabetes and Digestive and Kidney Diseases, and National Center for Advancing Translational Research. The Consortium of Eosinophilic Gastrointestinal Researchers also is supported by patient advocacy groups including the American Partnership for Eosinophilic Diseases, Campaign Urging Research for Eosinophilic Diseases, and the Eosinophil Family Coalition.
Abbreviations used in this paper:
- 1FED
1-food elimination diet
- 4FED
4-food elimination diet
- CMP
cow’s milk protein
- EoE
eosinophilic esophagitis
- eos/hpf
eosinophil per high-power field
- HSS
histologic scoring system
- IQR
interquartile range
- PEC
peak eosinophil count
- PPI
proton pump inhibitor
- QOL
quality of life
- SFED
6-food elimination diet
Footnotes
CRediT Authorship Contributions
Amir F Kagalwalla (Conceptualization: Lead; Data curation: Lead; Formal analysis: Lead; Funding acquisition: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Lead; Software: Supporting; Supervision: Lead; Validation: Lead; Visualization: Equal; Writing – original draft: Equal; Writing – review & editing: Lead)
Joshua B. Wechsler (Data curation: Equal; Formal analysis: Equal; Funding acquisition: Supporting; Investigation: Equal; Software: Lead; Supervision: Equal; Validation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Sally Schwartz (Data curation: Supporting; Formal analysis: Supporting; Writing – original draft: Supporting)
Nicoleta C. Arva (Data curation: Equal; Formal analysis: Equal; Writing – original draft: Supporting)
Kwang-Youn A. Kim PhD (Data curation: Equal; Formal analysis: Equal; Methodology: Supporting; Validation: Supporting)
Liqi Chen (Data curation: Supporting; Formal analysis: Supporting; Writing – original draft: Supporting)
Melanie Makhija (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting)
Katie Amsden (Data curation: Equal)
Kaitlin Keeley (Data curation: Supporting)
Saeed Mohammed (Formal analysis: Supporting; Writing – original draft: Supporting)
Evan S Dellon (Formal analysis: Supporting; Writing – review & editing: Equal)
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.03.049.
Conflicts of interest
This author discloses the following: Joshua B. Wechsler serves on the medical advisory board of the Campaign Urging Research for Eosinophilic Diseases. The remaining authors disclose no conflicts.
References
- 1.Hiremath G, Kodroff E, Strobel MJ, et al. Individuals affected by eosinophilic gastrointestinal disorders have complex unmet needs and frequently experience unique barriers to care. Clin Res Hepatol Gastroenterol 2018;42:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995;109:1503–1512. [DOI] [PubMed] [Google Scholar]
- 3.Markowitz JE, Spergel JM, Ruchelli E, et al. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol 2003;98:777–782. [DOI] [PubMed] [Google Scholar]
- 4.Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013;108:759–766. [DOI] [PubMed] [Google Scholar]
- 5.Warners MJ, Vlieg-Boerstra BJ, Verheij J, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther 2017;45:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson CJ, Abonia JP, King EC, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2012;129:1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006; 4:1097–1102. [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves N, Yang GY, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012; 142:1451–1459 e1; quiz e14–e15. [DOI] [PubMed] [Google Scholar]
- 9.Lucendo AJ, Arias A, Gonzalez-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013; 131:797–804. [DOI] [PubMed] [Google Scholar]
- 10.Arias A, Gonzalez-Cervera J, Tenias JM, et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146:1639–1648. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Hirano I, Doerfler B, et al. Assessing adherence and barriers to long-term elimination diet therapy in adults with eosinophilic esophagitis. Dig Dis Sci 2018;63:1756–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagalwalla AF, Wechsler JB, Amsden K, et al. Efficacy of a 4-food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2017;15:1698–1707 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina-Infante J, Arias A, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2–4-6 study. J Allergy Clin Immunol 2018;141:1365–1372. [DOI] [PubMed] [Google Scholar]
- 14.Kagalwalla AF, Amsden K, Shah A, et al. Cow’s milk elimination: a novel dietary approach to treat eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2012;55:711–716. [DOI] [PubMed] [Google Scholar]
- 15.Kagalwalla AF, Shah A, Li BU, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Pediatr Gastroenterol Nutr 2011;53:145–149. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012;130:461–467 e5. [DOI] [PubMed] [Google Scholar]
- 17.Kruszewski PG, Russo JM, Franciosi JP, et al. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis. Dis Esophagus 2016;29:377–384. [DOI] [PubMed] [Google Scholar]
- 18.Wong J, Goodine S, Samela K, et al. Efficacy of dairy free diet and 6-food elimination diet as initial therapy for pediatric eosinophilic esophagitis: a retrospective single-center study. Pediatr Gastroenterol Hepatol Nutr 2020;23:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliewer K, Aceves SS, Atkins D, et al. Efficacy of 1-food and 4-food elimination diets for pediatric eosinophilic esophagitis in a randomized multi-site study. Gastroenterology 2019; 156:S172–S173. [Google Scholar]
- 20.Teoh T, Mill C, Chan E, et al. Liberalized versus strict cow’s milk elimination for the treatment of children with eosinophilic esophagitis. J Can Assoc Gastroenterol 2019;2:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoofien A, Dias JA, Malamisura M, et al. Pediatric eosinophilic esophagitis: results of the European Retrospective Pediatric Eosinophilic Esophagitis Registry (RetroPEER). J Pediatr Gastroenterol Nutr 2019;68:552–558. [DOI] [PubMed] [Google Scholar]
- 22.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20 e6; quiz 21–22. [DOI] [PubMed] [Google Scholar]
- 23.Dellon ES, Gupta SK. A conceptual approach to understanding treatment response in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:2149–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins MH, Martin LJ, Wen T, et al. Eosinophilic esophagitis histology remission score: significant relations to measures of disease activity and symptoms. J Pediatr Gastroenterol Nutr 2020;70:598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020;158:1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed CC, Fan C, Koutlas NT, et al. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;46:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bashaw H, Schwartz S, Kagalwalla AF, et al. Tutorial: nutrition therapy in eosinophilic esophagitis-outcomes and deficiencies. JPEN J Parenter Enteral Nutr 2020;44:600–609. [DOI] [PubMed] [Google Scholar]
- 28.Kaur S, Rosen JM, Kriegermeier AA, et al. Utility of gastric and duodenal biopsies during follow-up endoscopy in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2017;65:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philpott H, Nandurkar S, Royce SG, et al. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment Pharmacol Ther 2016;44:223–233. [DOI] [PubMed] [Google Scholar]
- 30.Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 2019;156:592–603.e10. [DOI] [PubMed] [Google Scholar]
- 31.Heine R, Peters R, Cameron D, et al. Effect of a 4-food elimination diet and omeprazole in children with eosinophilic esophagitis – a randomized, controlled trial. J Allergy Clin Immunol 2019;143:AB309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.