Abstract
Introduction and Objective:
Amyloidoses are a heterogeneous group of disorders resulting from deposition of amyloid fibrils into extracellular tissues. While the kidneys are one of the most frequent sites of amyloid deposition, amyloid deposits can also affect a wide range of organ systems, including the heart, liver, gastrointestinal tract, and peripheral nerves. The prognosis of amyloidosis, especially with cardiac involvement, remains poor; however, a collaborative approach applying new tools for diagnosis and management may improve outcomes. In September 2021, the Canadian Onco-Nephrology Interest Group hosted a symposium to discuss diagnostic challenges and recent advances in the management of amyloidosis from the perspectives of the nephrologist, cardiologist, and onco-hematologist.
Methods and Sources of Information:
Through structured presentations, the group discussed a series of cases highlighting the varied clinical presentations of amyloidoses affecting the kidney and heart. Expert opinions, clinical trial findings, and publication summaries were used to illustrate patient-related and treatment-related considerations in the diagnosis and management of amyloidoses.
Key findings:
(1) Overview of the clinical presentation of amyloidoses and the role of specialists in performing timely and accurate diagnostic workup; (2) review of best practices for multidisciplinary management of amyloidosis, including prognostic variables and determinants of treatment response; and (3) update on new and emerging treatments in the management of light chain and amyloid transthyretin amyloidoses.
Limitations:
This conference featured multidisciplinary discussion of cases, and learning points reflect the assessments by the involved experts/authors.
Implications:
Identification and management of amyloidoses can be facilitated with a multidisciplinary approach and higher index of suspicion from cardiologists, nephrologists, and hemato-oncologists. Increased awareness of clinical presentations and diagnostic algorithms for amyloidosis subtyping will lead to more timely interventions and improved clinical outcomes.
Keywords: amyloidosis, amyloid deposits, AL, ATTR
Abrege
Introduction et objectifs:
Les amyloïdoses sont un groupe hétérogène de troubles résultant du dépôt de fibrilles amyloïdes dans les tissus extracellulaires. Les reins sont un des sites les plus fréquents de dépôts amyloïdes, mais ces derniers peuvent également affecter un large éventail de systèmes et d’organes, notamment le cœur, le foie, le tractus gastro-intestinal et les nerfs périphériques. Le pronostic de l’amyloïdose, en particulier en cas d’atteinte cardiaque, est mauvais. Les résultats peuvent cependant être améliorés par une approche collaborative utilisant de nouveaux outils de diagnostic et de prise en charge. En septembre 2021, le Canadian Onco-Nephrology Interest Group (groupe canadien d’intérêt en onco-néphrologie) a organisé un symposium pour discuter des défis liés au diagnostic de l’amyloïdose et des récents progrès dans la gestion de cette maladie du point de vue du néphrologue, du cardiologue et de l’hémato-oncologue.
Méthodologie et sources de l’information:
Au moyen de présentations structurées, le groupe a discuté d’une série de cas mettant en évidence les diverses présentations cliniques d’amyloïdoses affectant les reins et le cœur. Les opinions d’experts, les résultats des essais cliniques et les résumés des publications ont été utilisés pour illustrer les facteurs liés au patient et au traitement à considérer dans le diagnostic et la prise en charge des amyloïdoses.
Principaux résultats:
1) Aperçu de la présentation clinique des amyloïdoses et du rôle des spécialistes dans la réalisation d’un bilan diagnostic précis et en temps opportun (2) Examen des meilleures pratiques de gestion multidisciplinaire de l’amyloïdose, y compris des variables pronostiques et des déterminants de la réponse au traitement (3) Mise à jour sur les traitements nouveaux et émergents dans la prise en charge des amyloïdoses à chaîne légère (AL) et à transthyrétine (ATTR)
Limites:
Ce symposium a donné lieu à une discussion multidisciplinaire de cas; les points d’apprentissage reflètent les évaluations des experts/auteurs concernés.
Conclusion:
L’identification et la prise en charge des amyloïdoses peuvent être facilitées par une approche multidisciplinaire et un indice de suspicion plus élevé de la part des cardiologues, des néphrologues et des hémato-oncologues. Une meilleure connaissance des présentations cliniques et des algorithmes de diagnostic pour le sous-typage de l’amyloïdose permettra d’intervenir plus rapidement et d’améliorer les résultats cliniques.
Introduction and Objective
Amyloidoses are a group of disorders characterized by the extracellular deposition of misfolded proteins in insoluble, organized fibrils (“amyloid”). 1 Deposition of amyloid fibrils can cause progressive organ damage in tissues such as the heart, kidneys, and liver.2-4 Disease pathophysiology involves not only the misfolded proteins but also the interactions of extracellular chaperones, matrix proteins, proteases, and other cofactors that stabilize the amyloid deposits. Prefibrillar aggregates have also been shown to cause direct cytotoxic damage, underscoring the need for early intervention. 5 Thirty-six causative precursor proteins have been identified to date, each leading to different subtypes of amyloidosis with different clinical features and organ involvement. 6
In North America, the most prevalent systemic amyloidosis, accounting for more than 50% of all cases, 7 is immunoglobulin light chain (AL) amyloidosis, a malignant hematologic disorder caused by light chain–producing clonal plasma cells.1,8 More than half of patients with AL amyloidosis have a combination of kidney and cardiac involvement. 9 Other significant amyloidoses include serum amyloid A (AA) amyloidosis (also called reactive amyloidosis) caused by the precursor serum amyloid A (SAA) protein, often in response to underlying chronic inflammation, and amyloid transthyretin (ATTR) amyloidoses caused by misfolding of the precursor protein transthyretin (TTR). 1 The emergence of biologic therapies to control a variety of chronic inflammatory disorders has coincided with a decline in the prevalence of AA amyloidosis, now accounting for 3% of all amyloidosis cases. 7 In addition to AL amyloidosis, ATTR amyloidoses are a significant cause of heart failure. 3
The prognosis for amyloidoses remains poor.3,10 Amyloidoses are under-recognized and diagnosed late in the disease process when there may be fewer interventions that can change the natural history or prognosis of the condition.1-3 Greater organ dysfunction later in the disease may also compromise patient’s vital signs, increasing the challenges of management.10,11 Therefore, timely diagnosis and intervention are essential to improving outcomes in amyloidoses.
The multisystemic nature of these conditions warrants a multidisciplinary approach to both diagnosis and treatment. To address this need, the annual Canadian Onco-Nephrology Interest Group Symposium convened a multispecialty group of speakers including nephrology, cardiology, and hemato-oncology experts to discuss the clinical impact of recent advances in the diagnosis and multidisciplinary management of amyloidoses.
Methods and Sources of Information
The fifth annual Canadian Onco-Nephrology Interest Group Symposium was held virtually on September 29, 2021. The meeting was co-moderated by Dr Abhijat Kitchlu and Dr Charmaine Lok. Through a series of challenging case studies, Dr Kenar D. Jhaveri, a nephrologist, illustrated the challenges of timely diagnosis of different amyloidoses affecting the kidney. Dr Diego Delgado, a cardiologist, highlighted studies in the management of cardiac amyloidosis and reviewed the Canadian Cardiovascular Society (CCS)/Canadian Heart Failure Society (CHFS) joint recommendations for the diagnostic evaluation, management, and monitoring of cardiac amyloidosis. 3 Dr Vishal Kukreti, an onco-hematologist, provided an update on the diagnosis and management of AL amyloidosis through a structured presentation of expert opinions, clinical trial findings, and publication summaries. The 3 presenters then engaged in a moderated multidisciplinary panel discussion addressing attendee questions.
Key Findings
Discussions at the 2021 Canadian Onco-Nephrology Interest Group Symposium focused on the timely diagnosis and molecular subtyping of amyloidoses from the nephrology, cardiology, and onco-hematology perspectives; best practices for multidisciplinary management of amyloidoses; and emerging therapies for amyloidoses.
Kidney Involvement
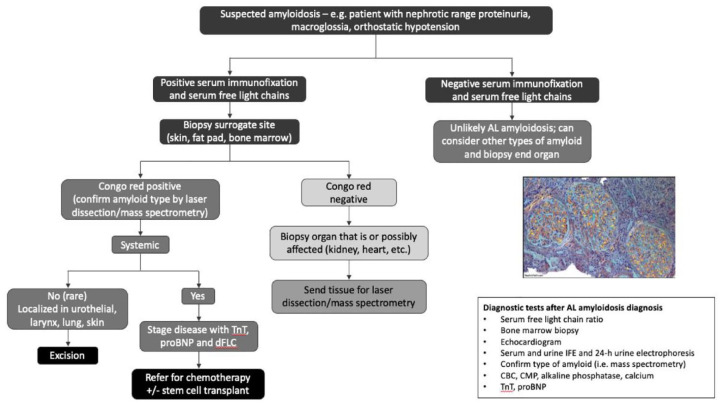
Kidney amyloidosis should be suspected in patients with nephrotic range proteinuria, macroglossia, and orthostatic hypotension. Because AL amyloidosis is the most common presentation affecting the kidneys, workup should include serum immunofixation (SIFE) and quantification of serum free light chains (sFLCs) (Figure 1). The sensitivity for detecting monoclonal protein increases by using a combination of serum protein electrophoresis and immunofixation, sFLC, and urine immunofixation. 10 If immunofixation of serum and urine is negative and the sFLC kappa/lambda ratio is normal, AL amyloidosis is unlikely5,10; however, it is important to consider other subtypes of amyloidoses, including AA amyloidosis and hereditary amyloidoses. 12
Figure 1.
Diagnostic algorithm for the evaluation of suspected kidney amyloidosis.
Source. Reproduced with permission from Deshpande and Jhaveri. 12
Hereditary amyloidoses subtypes are rare on their own, but collectively, they account for approximately 10% of all systemic amyloidoses currently diagnosed. 13 The most common hereditary amyloidosis is caused my pathogenic variants in the TTR gene (hATTR amyloidosis), which typically infiltrates the heart. 1 Fibrinogen A-alpha (AFib) amyloidosis, an amyloidosis caused by a fibrinogen A-alpha pathogenic variant, targets the kidneys, and though being the most common hereditary subtype in Europe, it is uncommon in North America (1%). 1 Other less common hereditary amyloid fibrils that clinically affect the kidneys include apolipoprotein-AI, apolipoprotein-AII, gelsolin, and lysozyme.1,6 Most hereditary amyloidoses associated with kidney or cardiac manifestations are dominantly inherited heterozygous disorders, with amyloid deposits containing both wild-type proteins and pathogenic variants. 14
Diagnosis of kidney amyloidoses requires tissue confirmation of amyloid deposits by Congo red staining showing apple-green birefringence under polarized light or electron micrograph (EM) findings of randomly arranged 8 to 10 nm fibrils. Subsequent identification of the specific amyloid protein type is necessary to determine appropriate treatment.1,5,15 Immunohistochemistry techniques may only usefully identify fibril type in 60% to 70% of cases. 16 Increasingly, clinical trials favor mass spectrometry with laser dissection as the gold standard approach for typing of amyloid fibrils. Centers with available expertise may perform immunogold EM rather than mass spectrometry. 5 Benson et al 17 highlight some of the pros and cons of both immunohistochemistry and laser microdissection/mass spectrometry approaches.
Physicians may consider biopsy of a surrogate site such as a fat pad, rectal biopsy, minor salivary gland, or bone marrow if it is not safe to biopsy the affected organ.12,15 However, because the sensitivity of Congo red staining in more accessible tissues can be substantially lower than that of symptomatic organs, evaluation of surrogate sites should happen at experienced centers. 1 Congo red negativity is not sufficient to rule out a diagnosis of amyloidosis; therefore, a high index of suspicion should lead to subsequent biopsy of the affected organ.5,15 Following biopsy, a systemic workup should look for other areas of amyloidosis, followed by staging (particularly when there is a cardiac component), and finally a referral for appropriate treatment of the underlying condition (based on confirmed subtype). 12
Although the diagnosis is usually based on clinical presentation, laboratory tests, and imaging studies, genetic testing may be considered when there is an atypical presentation or an uncertain diagnosis after a comprehensive diagnostic workup. The results of immunohistochemistry or mass spectrometry analysis should guide the selection of the gene to sequence. Genetic testing is also recommended if there is a strong suspicion of a hereditary form of amyloidosis based on family history and personal medical history. A diagnosis of hereditary amyloidosis should prompt genetic counseling and possible genetic screening of family members, as early detection may allow for better disease management. 18
The diagnosis of kidney amyloidosis can be challenging. Nephrologists should have heightened awareness of the following under-recognized signs and symptoms of amyloidoses:
Orthostatic hypotension. Patients with orthostatic hypotension are frequently started on angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) without suspicion or workup for possible systemic amyloidosis. These drugs are typically poorly tolerated in patients with amyloidosis, 3 and this approach may delay appropriate diagnosis and intervention.
Clinical presentation of nonproteinuric amyloidoses. Amyloid deposits in the kidney are predominantly glomerular, leading most patients with kidney amyloidosis to present with proteinuria, with up to half of patients presenting in the nephrotic range. 5 However, in rare cases, amyloid deposits may be vascular, tubular-interstitial, or lead to intratubular cast nephropathies. Amyloidosis subtypes with more vascular or tubular-interstitial deposition of amyloid (such as leukocyte chemotactic factor 2 and familial AApoA variants) are less likely to result in proteinuria and are frequently undiagnosed.1,5,15
Monoclonal gammopathy of undetermined significance (MGUS) findings in the bone marrow. While AL amyloidosis is the most common kidney amyloidosis, MGUS is not always indicative of AL or heavy chain (HL) subtypes. For example, as many as 39% to 49% of patients with ATTR amyloidosis may also have abnormalities in sFLC ratio or SIFE that indicate MGUS. 19 Subtyping of tissue deposits is essential for accurate typing of amyloidoses and appropriate treatment selection. 1
Multidisciplinary Perspective.
While AL amyloidosis is the most common subtype affecting the kidneys, nephrologists should also consider the possibility of hereditary amyloidoses. Importantly, some of these subtypes will present with nonglomerular findings. Nephrologists should work closely with pathologists to ensure that kidney biopsies are stained with Congo red, even in the absence of glomerular findings or proteinuria.
Cardiac Involvement
Amyloid deposition can affect all structures of the heart, including the myocardium, endocardium, pericardium, valves, and coronary arteries. The most common clinical presentation of cardiac amyloidosis is heart failure, typically with preserved left ventricular ejection fraction. 3 Early diagnosis and intervention in cardiac amyloidoses are essential because of the rapidly progressive nature of the disease and the difficulty of managing patients with advanced organ damage. 20
Two systemic amyloidoses typically present with cardiac involvement: AL amyloidosis, in which more than half of the patients experience cardiac manifestations, and ATTR amyloidosis, caused by misfolding of the tetrameric protein transthyretin, which is produced mainly in the liver.8,21,22 Amyloid transthyretin amyloidoses are further classified as hereditary (hATTR), in which a TTR pathogenic variant is present, or wild-type (wtATTR), observed in predominantly older patients. 22 Hereditary ATTR amyloidoses exhibit a high degree of phenotypic heterogeneity and usually affect the cardiac and/or neurologic systems, while kidney involvement is rare.23,24 Wild-type ATTR is an underdiagnosed cause of heart failure in older patients and is estimated to be the most common type of cardiac amyloidosis. 1 Despite being underdiagnosed, the incidence of wtATTR is reported to have increased from 3% of all amyloidosis cases prior to 2009 to 25% of all cases in more recent years, likely owing to the growing awareness of amyloidosis in cardiac diagnostic workups. 7
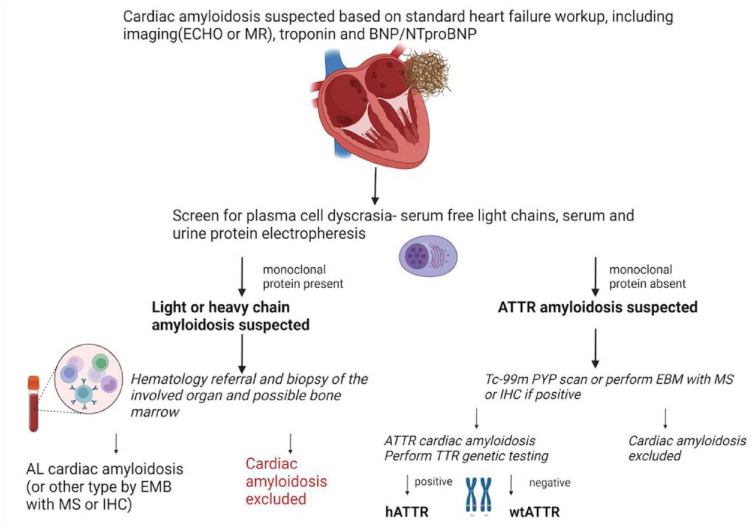
Heart failure symptoms without any clear etiology should trigger clinical suspicion of amyloidosis. 3 The diagnostic algorithm for suspected cardiac amyloidosis includes standard heart failure workup, measurement of cardiac biomarkers troponin and brain natriuretic peptide(BNP)/N-terminal (NT)-pro hormone BNP (NT-proBNP), and cardiac imaging (Figure 2). Either echocardiography with left ventricular (LV) longitudinal strain measurement or cardiac magnetic resonance (CMR) with late gadolinium enhancement and T1 mapping is considered appropriate; the choice of imaging technique should consider local availability, expertise, and any patient-specific contraindications.3,9 Importantly, neither echocardiogram nor CMR can reliably differentiate the subtype of amyloidosis, and in isolation, these tests are not considered confirmatory. A combination of imaging, symptoms, biomarkers, and biopsy findings is required to reach a final diagnosis. 3
Figure 2.
Diagnostic algorithm for the evaluation of suspected cardiac amyloidosis
Source. Adapted from Fine et al. 3
If cardiac amyloidosis is suspected based on a combination of clinical symptoms, cardiac imaging, and biomarker evaluation, patients should be screened for plasma cell dyscrasia using serum and urine protein electrophoresis with immunofixation and sFLC assay. If the findings of cardiac investigations suggest AL amyloidosis, patients should be referred to hematology for a final diagnosis. 3
In patients confirmed negative for AL amyloid, a 99mTc-pyrophosphate nuclear scintigraphy (PYP nuclear scan) can provide high sensitivity, specificity, and positive predictive value for the identification of ATTR amyloidosis. (AL amyloidosis must be ruled out as approximately 8% of patients with AL amyloidosis may also have a positive PYP nuclear scan.) Subsequent genetic testing is recommended to differentiate hATTR from wtATTR. 3 This has implications for prognosis, extracardiac manifestations, the need for familial cascade screening, and eligibility for ATTR-targeted therapies. 3
Cardiac biopsy has traditionally been considered the gold standard for diagnosis of cardiac amyloidosis. In recent years, biopsy has been replaced by the noninvasive evaluation methods described above; however, it still has a role in distinguishing equivocal or discordant results with a clinical suspicion of amyloidosis. 3
Multidisciplinary Perspective.
Cardiac manifestations of amyloidosis are usually part of a systemic disease. For patients with severe cardiac amyloidosis, there may only be a period of weeks to months to successfully intervene. 20 As such, cardiologists, nephrologists, and onco-hematologists should have a low threshold to suspect cardiac amyloidosis and should work together to expedite timely workup and diagnosis.
Illustrative Case Presentations
Cases were used to illustrate the role of the multidisciplinary team in efficient and complete diagnostic workup and to elevate clinical suspicion of amyloidoses for nephrologists and cardiologists.
Case 1: Nephrology Suspicion of AL Amyloidosis
Presentation: A 67-year-old man with a history of hypertension was referred for proteinuria.
Clinical findings: Initial workup revealed a slightly high lambda predominance light chain ratio in the serum and a urine protein-to-creatinine ratio of 340 mg/mmol (3 g/g). Kidney biopsy showed focal segmental glomerulosclerosis with moderate vascular disease, but no signs of Congo red positivity or monoclonal process.
Several months later, the patient reported paresthesia in both legs in a stocking distribution and underwent a fat pad biopsy, which was also Congo red–negative. Sural nerve biopsy and CMR were also negative for amyloidosis. Serum protein electrophoresis showed a serum lambda predominance, and over the next 2 years, urine immunofixation revealed Bence Jones protein.
Two years after the initial workup, a sustained increase in proteinuria (to 7 g/day) without hypertension and with poor tolerance of ACEi/ARBs led physicians to again suspect amyloidosis. A repeat kidney biopsy with Congo red staining revealed spicules in the vasculature and parts of the glomeruli. Immunofluorescence demonstrated a clear predominance of lambda over kappa, and EM revealed amyloid fibrils of 8 to 10 nm.
Diagnostic focus and assessment: Monoclonal gammopathy of renal significance and AL amyloidosis, lambda chain.
Discussion: This case underscores the importance of maintaining a high index of suspicion for amyloidosis. Although a first kidney biopsy did not reveal the presence of amyloid, sustained clinical suspicion and a repeat kidney biopsy almost 2 years later revealed the subtle presence of amyloid. Initial tissue biopsy, particularly when assessed with less sensitive techniques (ie, staining rather than mass spectrometry), may not reveal the presence of amyloid. If amyloidosis is suspected but a kidney biopsy is negative, nephrologists should continue with systemic investigations.
Multidisciplinary Perspective.
AL amyloidosis occurs in the context of underlying plasma cell dyscrasia. As a result, hematologists play a key role in amyloidosis suspicion, diagnostic workup, and management. 25 While paraproteins, abnormal free light chains, and clonally restricted plasma cells with significant nephrotic range proteinuria may arouse suspicion for hematologists, a tissue diagnosis is necessary for definitive diagnosis and subtyping. Hematologists should thus be prepared to make appropriate referrals to cardiology and nephrology colleagues for tissue diagnoses. For patients with MGUS, hematologists may also consider screening for cardiac biomarkers, proteinuria, and alkaline phosphatase (ALP) to improve early detection of AL amyloidosis.
Case 2: AL Amyloidosis With Suspected Cardiac Involvement
Presentation: A 75-year-old man with a medical history of smoking and benign prostatic hyperplasia presented with 1 year of progressive lower extremity edema and shortness of breath on exertion. The patient complained of fatigue, weight loss, and feeling light-headed on standing. He denied any gastrointestinal (GI) symptoms, neuropathy, syncopal events, macroglossia, history of sleep apnea, or any bleeding or bruising. On physical examination, his seated blood pressure was 84/50 mm Hg and heart rate was 70 bpm with no significant postural change. The patient was not on any medication. A general practitioner noted kidney impairment with proteinuria and referred him to a nephrologist.
Clinical findings: The patient’s serum creatinine was 170 µmol/L and serum albumin was 26 g/L. All other lab findings and kidney ultrasound were normal. A 24-hour urine protein electrophoresis showed 5.8 g of albumin per day. Serum protein electrophoresis and immunofixation showed 250 mg/L lambda light chains and 20 mg/L kappa light chains. Urine immunofixation revealed lambda light chains, and bone marrow biopsy showed 10% lambda-restricted plasma cells. Cardiac biomarkers were elevated (troponin I [hs] = 73 ng/L, BNP = 2000 ng/L). He had a jugular venous pressure of 6 cm and bilateral pleural effusions.
Diagnostic focus and assessment: Given the high clinical suspicion of AL amyloidosis, the nephrologist ordered a kidney biopsy. Biopsied tissue was Congo red–positive with apple-green birefringence on polarized light microscopy, confirming amyloidosis. Skeletal survey ruled out myeloma-related bone lesions. Mass spectrometry confirmed the AL amyloidosis subtype.
Clinical findings of lower extremity edema, pleural effusions, and shortness of breath led to suspicion of cardiac involvement, so the patient was sent to a cardiologist for additional workup. Chest radiograph was consistent with congestive heart failure and bilateral pleural effusions. Echocardiogram showed increased LV mass and a normal ejection fraction, diastolic dysfunction, interventricular septum thickness in diastole 16 mm, and pulsed-wave Doppler 16 mm. Holter monitor was normal. Electrocardiogram revealed low voltages classic for advanced cardiac amyloidosis. Cardiac MR with gadolinium was consistent with amyloidosis.
Discussion: Taken together, this patient was determined to have multiorgan AL amyloidosis affecting the kidneys and heart. After a diagnosis of AL amyloidosis was established in the kidney, additional examinations quickly confirmed organ involvement in the heart. These subsequent examinations are important for staging amyloidosis and understanding a patient’s prognosis. Prognostic factors in AL amyloidosis include more sites of organ involvement and elevated cardiac biomarkers and kidney markers that are important for predicting the risk of progressing to dialysis.
The risk of end-stage kidney disease (ESKD) is greatest when both proteinuria and kidney insufficiency are present. Palladini et al 26 developed a 3-stage system that estimates the risk of ESKD in patients with AL amyloidosis, as well early assessment of kidney response to therapy. Patients with estimated glomerular filtration rate <50 mL/min/1.73m2 and 24-hour urine ≥5 g (stage III) are at high risk of ESKD, regardless of the AL amyloidosis intervention.26,27 In this case, the patient’s kidney function (stage II) suggests a 2-year risk for dialysis of 11% to 25%.26,27
The well-defined Mayo staging system considers 3 prognostic factors for overall survival (OS): a difference between involved and uninvolved free light chains (dFLC) ≥0.18 g/L (18 mg/dL), troponin T ≥0.025 µg/L, and NT-proBNP ≥1800 ng/L. The OS of patients is associated with the number of prognostic factors present (0 to all 3 factors = stages 1 to 4). For patients with all 3 factors (stage 4), 1-year OS is as low as ~33%. 28 Similar prognostic outcomes are seen using a scoring system from Boston that assesses risk based on BNP >81 ng/L and troponin I >0.1 µg/L. 29 With both scoring systems, there is a sharp drop in OS within 12 months of diagnosis for patients with the worst prognostic scores, illustrating the need for urgent initiation of therapy and for treatments that work quickly. The patient in this case has a high Mayo score (stage 3). The median OS from time of diagnosis is 14 months and 5-year OS is estimated to be 20%.
Multidisciplinary Perspective.
In AL amyloidosis, patients typically have more than one organ involved. Comprehensive history-taking is critical when seeing patients with amyloidosis, including age, ethnicity, family history, and a full review of systems.
Cardiologists should work closely with hematologists and nephrologists to ensure timely and appropriate ordering and interpretation of screening tests for AL amyloidosis. 3 In this case where kidney biopsy confirmed the diagnosis of amyloidosis, characteristic findings on CMR combined with clinical and serum biomarker assessment were sufficient to conclude the presence of cardiac involvement without the need for further testing. 3
Subsequent examinations following diagnosis of AL amyloidosis allow for prognostic staging. Discussions about prognosis are important to include in patient conversations about optimal management, goals of treatment, and expected outcomes. (In contrast to AL amyloidosis, staging systems for ATTR amyloidosis-related cardiomyopathy are not well validated or used consistently. 3 )
Case 3: hATTR Amyloidosis
Presentation: A 77-year-old man was seen for acute kidney injury and proteinuria in the setting of recently noted anemia. His medical history was significant for 3 previous cardiac arrests and a diagnosis of nonischemic cardiomyopathy. At presentation, he had an implantable cardioverter-defibrillator and was taking cardiac and anti-hypertensive medications. Three years ago, he was diagnosed with congestive heart failure (CHF). Since then, he experienced a slow rise in serum creatinine from 106 µmol/L (1.2 mg/dL) to 141 µmol/L (1.6 mg/dL). There was no family history of cardiac disease reported.
Clinical findings: The patient’s serum creatinine continued to rise to 168 µmol/L (1.9 mg/dL), but he had minimal proteinuria (urine protein-to-creatinine ratio: 0.06 mg/mmol [0.5 mg/g]; repeat 24-hour protein: 600 mg). Bence Jones proteins were noted in the urine, and SIFE showed IgA lambda sFLC ratio of 0.14 (kappa = 3 mg/L, lambda = 22 mg/L). Suspecting amyloidosis, a bone marrow biopsy was performed, which showed 10% plasma cells with IgA lambda predominance. Periosteum was positive for Congo red staining. Echocardiogram showed LV hypertrophy with left atrial enlargement.
Diagnostic focus and assessment: The differential diagnosis considered chronic vascular kidney disease from cardiac disease, vascular amyloidosis (given minimal proteinuria), and early AL amyloidosis. A subsequent kidney biopsy showed moderate nonspecific chronic damage, no active inflammation, and was negative for Congo red and both lambda and kappa immunofluorescence, inconsistent with AL amyloidosis. Kidney injury suggested vascular injury from cardiac disease. However, ongoing concern for systemic amyloidosis led to bone marrow investigation by mass spectrometry, which confirmed ATTR amyloidosis. The patient also had a positive cardiac amyloid scan. A molecular genetic test later confirmed a hereditary form of ATTR, with the Val122Ile pathogenic variant being detected, after which treatment was initiated. As the patient had no living children, cascade familial screening was not done, but otherwise would have been pursued.
Discussion: hATTR amyloidosis is an autosomal-dominant disease, associated with at least 100 different TTR pathogenic variants. In the United States, the most common TTR pathogenic variant is Val122Ile, affecting 3% to 4% of African Americans. 30 Each variant may have a different pattern of organ involvement; for example, while the Val122Ile is predominantly cardiopathic, the Val30Met is predominantly neuropathic. 1 Although peripheral neuropathy and cardiomyopathy are broadly described for hATTR amyloidosis, kidney impairment and proteinuria are rare clinical features. 24 Moreover, clinical differences between different kindreds carrying the same pathogenic variants suggest an important role for environmental and genetic factors, which contribute to the variable penetrance and age of onset observed with hATTR amyloidosis. For instance, the median age of onset of hATTR in the United States is 68 years, but 32 years in Portugal and 52 years in Sweden. 1 An explanation for the variability in the degree of cardiac infiltration and age of onset is the composition of the fibrils (different mixtures of wild-type and pathogenic variants). 31
This case illustrates the importance of avoiding the assumption that all amyloidoses are of the light or heavy chain subtypes. Congo red amyloid in the kidney and MGUS in the bone marrow are not unequivocal for a diagnosis of AL amyloidosis. It is essential that amyloid fibrils be typed and if needed, confirmed by mass spectrometry. In patients with bone marrow findings of smoldering myeloma or MGUS and a kidney biopsy with some amyloid, it is essential to properly type the amyloid deposits. If not appropriately typed, patients with ATTR amyloidosis may be inappropriately treated for myeloma or AL amyloidosis, with poor consequences. 1 For patients with a diagnosis of ATTR cardiac amyloidosis, genetic testing to differentiate hATTR from wtATTR is recommended to guide selection of ATTR-targeted therapy (eg, inotersen or patisiran is only indicated for hATTR). 3 Although data on kidney involvement in ATTR amyloidosis are sparse, 2 recent reports suggest that about one-third of symptomatic patients have kidney involvement, commonly abnormal urine protein excretion.32,33
Multidisciplinary Perspective.
Bone marrow biopsies performed by hematologists can identify the presence of clonally restricted plasma cells and may identify amyloid in about 60% of cases. 34 However, to proceed to subtyping by laser dissection with mass spectrometry, bone marrow biopsy samples may not provide sufficient tissue to reach a diagnosis. Interdisciplinary dialogue is important to ensure that care teams reach a strategy for tissue biopsy that will provide the most efficient time to diagnosis. Hematologists may perform immunologic assays and bone marrow biopsy as a starting point, with other disciplines ready to biopsy other sites if bone marrow is not sufficient for diagnosis and subtyping.
Case 4: AA (Reactive) Amyloidosis
This case also appears as part of a case series published in the Journal of Onco-Nephrology. 35
Presentation: An 81-year-old man presented with rising creatinine and proteinuria. He had a history of localized prostate cancer requiring radiation (in remission) and more recent metastatic melanoma treated with immunotherapy via the PD-1 inhibitor, nivolumab, for almost 1 year. A positron emission tomography scan showed no recurrence of either cancer.
Clinical findings: A year and a half later, the patient’s creatinine was around 440 µmol/L (5 mg/dL), and he had 4.5 g of proteinuria and low serum albumin. A hospital admission led to a finding of left-side unilateral hydronephrosis. A percutaneous nephrostomy tube was placed with no change in serum creatinine. Given progression of uremia, the patient was started on dialysis. Later workup revealed high C-reactive protein, and all serology was normal, including negative SIFE and normal sFLCs.
Diagnostic focus and assessment: Kidney biopsy revealed nodular lesions and Congo red positivity with apple-green birefringence. Immunofluorescence was negative for kappa and lambda light chain deposition but was positive for Protein A. Electron micrograph revealed the presence of amyloid fibrils. The patient was diagnosed with AA amyloidosis with tubular atrophy, diffuse interstitial fibrosis, and severe arterial and arteriolar sclerosis.
Discussion: Many chronic inflammatory conditions (including rheumatoid arthritis, juvenile chronic polyarthritis, ankylosing spondylitis, Crohn’s disease, chronic infections) and malignancies (kidney cell cancer, Hodgkin or other lymphomas, Castleman’s disease) can lead to elevation and tissue deposition of SAA protein, an acute phase reactant. Heritable disorders with periodic fevers can also lead to AA amyloidosis. Amyloidosis caused by the acute phase reactant SAA protein is the most common subtype globally. 1
In this case, the use of an immune checkpoint inhibitor led to a systemic inflammatory response. CTLA-4, PD-1, and PD-L1 inhibitors have been shown to activate the immune system to trigger an inflammatory response, classically an acute interstitial nephritis and rarely glomerular disease.36,37 Four cases of AA amyloidosis associated with immunotherapy have been reported in the literature.35,38 A systemic review of immune checkpoint inhibitor–associated glomerular disease identified amyloidosis as a possible adverse outcome. 39
Multidisciplinary Perspective.
As immune checkpoint inhibitors are used more frequently, nephrologists should consider the possibility of AA amyloidosis and ensure that pathologists investigate Protein A in biopsy samples.
Case 5: Hereditary AFib Amyloidosis
Presentation: A 68-year-old man was investigated for proteinuria. He had type 2 diabetes, anti-nuclear antibody 1:160, and normal complements. Serum immunofixation serum kappa was 5 mg/L and lambda was 26 mg/L. He presented with creatinine 128 µmol/L (1.45 mg/dL) and a urine protein-to-creatinine ratio of 0.45 mg/mmol (4 mg/g).
Clinical findings: The patient was asymptomatic and all other serology was normal.
Diagnostic focus and assessment: Given the high suspicion of amyloidosis, a kidney biopsy was performed. Biopsy revealed amyloidosis, but immunofluorescence staining for subtyping was slightly positive for IgG and negative for IgA and Protein A. Kappa and lambda staining were also negative. Staining for fibrinogen was positive, and mass spectrometry confirmed AFib amyloidosis. Genetic testing later confirmed a fibrinogen Aα-chain (FIBA) pathogenic variant.
Discussion : Fibrinogen A-alpha amyloidosis is a common cause of hereditary amyloidosis affecting the kidneys, and nephrologists should consider hereditary origins of the disease when performing diagnostic workup for amyloidosis. The median age of presentation is 55 years, often accompanied by nephrotic syndrome and hypertension. 1 Mass spectrometry is useful to confirm the AFib subtype. This case highlights the importance of accurate subtyping for appropriate treatment decisions: the fibrinogen amyloid protein is made in the liver; therefore, kidney transplant alone is not sufficient for treatment. Patients must undergo liver-kidney transplant to prevent recurrence. 40 Genetic testing and cascade familial screening would be appropriate consideration in these cases.
Multidisciplinary Approach to Diagnosis
As these case studies illustrate, early diagnosis is critical for patients with amyloidosis but continues to pose a significant clinical challenge. Amyloidoses can affect multiple organs, may closely mimic both heart failure and kidney failure, and often present with nonspecific symptoms such as fatigue.41,42 Therefore, multidisciplinary review of the clinical presentation with a high index of suspicion is necessary for early diagnosis. Collaborative discussions between clinical and laboratory specialties are encouraged to promote awareness of amyloidosis subtypes.
Available Therapies and Current Standard of Care for AL and ATTR Amyloidoses
Treatment of amyloidoses can limit further production of amyloid proteins and help prevent further tissue damage. Management strategies must target the underlying cause of amyloidoses; therefore, accurate subtyping is essential for appropriate treatment selection.
AL Amyloidosis
The goals of treatment in AL amyloidosis are to substantially reduce the supply of the monoclonal immunoglobulin light chain (hematologic response) and to stabilize or reduce existing deposits of amyloid and preserve or improve organ function (organ response). Amyloid deposits elicit little or no local immunologic response, but the intermediate FLC dimers and oligomers are very toxic to cells. Therefore, hematologists aim to reduce this burden of proteotoxic precursors as quickly as possible. Therapeutic interventions may aim to change the aberrant amyloid precursor protein, target the cell producing the abnormal FLCs (plasma cells) to decrease the precursor, disrupt stabilization to keep proteins in a more solubilized form and reduce deposits in organs, or clear amyloid that has already been deposited. 5 New therapies are under investigation in these areas, including CAEL-101, an amyloid fibril–targeted therapy. 43
Response to treatment is measured by both hematologic response and organ response. Given rapid disease progression, frequent assessment and follow-up are critical to ensure patients receive optimal therapy.
Hematologic response
Hematologic response is categorized as complete response (CR), very good partial response (VGPR), partial response (PR), no response (NR), or progression.5,44 Optimally, treatment will achieve CR, defined as normalization of FLC levels and kappa/lambda ratio and negative serum and urine immunofixation. For patients with moderate-to-severe kidney failure, hematologists may also be satisfied with a VGPR, where the dFLC is <40 mg/L. 26 Anything less than VGPR is considered inadequate and requires a change in treatment.
As management of AL amyloidosis evolves, definitions of acceptable response also change. Emergent literature shows additional OS benefit in patients achieving deeper suppression of FLC (involved free light chains [iFLC] <0.2 g/L and dFLC <0.1 g/L). 45 This finding has prompted some centers, including the Mayo Clinic, to move to the use of mass spectrometry to increase the sensitivity threshold for monitoring of serum and urine protein.
Time to hematologic response is also a key consideration for treatment. Hematologic response to therapy should occur within the first 3 months of treatment; if not, a change in treatment is warranted. 44 Overall survival data following treatment with frontline bortezomib demonstrate the substantial impact of an early response on AL amyloidosis outcomes: patients who achieved CR or VGPR at 1 or 3 months have better OS outcomes than those who achieve a similar response at 6 months. 45
Multidisciplinary Perspective.
The role of kidney transplant is evolving as newer treatments lead to deeper, more complete hematologic responses and sustained quality-of-life improvements. Hematologic CR predicts positive outcomes following kidney transplant, including OS and longer time to graft loss.46,47 As the field moves away from kidney transplant up front, there may be a role for ACEi/ARBs as well as SGLT2 inhibitors in patients without cardiac involvement.
Organ response
Organ response and progression criteria in AL amyloidosis have been defined for the heart, kidney, liver, and peripheral nervous system, but expectations for the timing of these responses are still a clinical challenge.44,48 Organ responses lag behind hematologic response and organ function may continue to improve more than a year after hematologic complete response, lending support to a direct cytotoxic effect of sFLC in addition to damage caused by deposition of amyloid fibrils. 15,48,49 Depth of organ response is associated with improved survival; therefore, physicians should closely consider the efficacy of AL therapy and make changes in patients who achieve suboptimal organ responses. 50
Multidisciplinary Perspective.
Early identification of disease progression should be an effort of the multidisciplinary team. Chemotherapy should be resumed urgently at the first sign of organ progression or the re-emergence of a clone; teams should not wait for patients to meet to the clinical trial parameter for defining progression of disease (100% increase in FLC) before retreatment. 51
Chemotherapy and immunotherapy
Conventional and emerging systemic therapies for AL amyloidosis include alkylating agents (melphalan, cyclophosphamide), steroids (dexamethasone), proteasome inhibitors (bortezomib, ixazomib), immunomodulators (thalidomide, pomalidomide), and immunotherapy (daratumumab, a monoclonal antibody targeting CD38 on the surface of plasma cells). 10
The cyclophosphamide-bortezomib-dexamethasone (CyBorD) regimen is considered standard of care for management of AL amyloidosis. 52 A prospective evaluation of the first-line use of the CyBorD regimen showed real-world response rates of 60% and overall projected 5-year survival of about 55%. Treatment was associated with severe adverse events in about 10% of patients. Response rates were poorest in patients with advanced cardiac disease, but even in this group of high-risk patients, hematologic response to CyBorD therapy prevented further worsening of cardiac damage. 53 In Canada, these therapies are available through compassionate access programs. The dose of dexamethasone may be attenuated based on factors including predicted tolerability of steroids, existent neuropathy, age, and frailty.
In the recent ANDROMEDA study, the addition of daratumumab to the CyBorD regimen improved deep hematologic response as well as organ responses and led to meaningful improvements in survival from major organ deterioration and hematologic progression compared with CyBorD alone.52,54 This new treatment paradigm, which became available in the last year, may revolutionize the treatment of AL amyloidosis in selected patients.
Therapy that can remove existing amyloid deposits may be the key for improved outcomes, particularly in patients with advanced cardiac disease who have limited response to hematologic treatment. The anti-fibrillary antibody CAEL-101 binds to either kappa or lambda light chains deposited in organs and leads to removal of existing amyloid deposits. Early results from CAEL-101 clinical trials show that even patients with partial hematologic response can have substantial organ improvements. 55 A phase 3 trial investigating CyBorD plus or minus daratumumab in combination with CAEL-101 is currently recruiting patients (NCT04512235).
Multidisciplinary Perspective.
Confirmation of suspected amyloidosis is important even in patients with multiple myeloma already receiving CyBorD therapy. The natural history, treatment requirements, and treatment-related morbidity and mortality are very different for multiple myeloma and AL amyloidosis; therefore, it is important for physicians to understand whether they are managing one or both disorders.
A multidisciplinary approach to active management of AL amyloidosis should include frequent monitoring from cardiologists, nephrologists, and hematologists to see patients through the difficulties of chemotherapy. Attention from a diverse care team will ensure choice of an optimal monitoring strategy and timely assessment of treatment response. It can be challenging for patients to separate disease progression from the side effects of medications; therefore, multidisciplinary care should also seek to manage patient expectations and to encourage patients to continue therapy.
Collaborative support should also extend to patient education and supportive therapy. Considerations for multidisciplinary care in patients with AL amyloidosis include fluid and weight management (which may be exacerbated by dexamethasone), neuropathic pain management, GI and hepatic involvement, and management of bleeding risk. Nutrition is an important and often overlooked consideration in patients with AL amyloidosis.
Autologous stem cell transplant
Autologous stem cell transplant (ASCT) is an effective therapy for selected patients with AL amyloidosis. Early use of ASCT therapy in AL amyloidosis illustrated the importance of careful patient selection, use of experienced reference centers for transplantation, and support from a multidisciplinary team. 56 Contemporary long-term data show that one-third of selected low-risk patients with predominant kidney or early cardiac involvement can be expected to survive ≥15 years post-transplant. 57
The clinical challenge is refining who is eligible for ASCT and in whom the procedure might be too risky. Clinical experience from the Mayo Clinic in Minnesota and from Princess Margaret Hospital in Toronto showed that early hematologic CR is a positive prognostic factor for OS following transplant, while treatment-related mortality is increased in patients with elevated cardiac biomarkers. 58 Other variables that exclude patients from ASCT include low ejection fraction, high New York Heart Association (NYHA) heart failure class, number of organs involved, and age. 59
Patients already on dialysis are rarely considered for ASCT because of the increased rate of complications. For patients with moderate kidney dysfunction, the choice to undergo ASCT can be challenging. Although ASCT may lead to hematologic CR which could improve survival and prevention of cardiac death, patients are likely to reach ESKD more quickly due to substantial organ strain at the time of peri-engraftment.
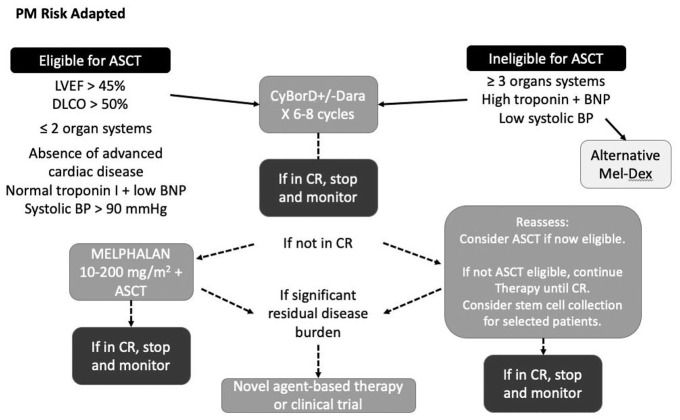
Autologous stem cell transplant is considered a second-line therapy in AL amyloidosis. All patients should receive initial treatment with CyBorD (plus daratumumab if available and appropriate) for an average of 6 to 8 cycles. In some cases, patients who are initially ineligible for transplant because of unacceptable risk for treatment-related mortality may become transplant-eligible following systemic chemotherapy. A proposed risk-adapted approach to ASCT is illustrated in Figure 3. Patients are considered for ASCT only if they have not achieved a hematologic CR to avoid unnecessary exposure to the treatment-related morbidity and mortality of transplant.
Figure 3.
Risk-adapted approach to autologous stem cell transplant in light chain amyloidosis.
Figure courtesy of Dr Donna Reece, University Health Network, University of Toronto.
ATTR Amyloidosis
Cardiac amyloidosis is managed through 2 parallel management pathways: use of disease-modifying therapy (DMT) to prevent further amyloid deposition and symptomatic management of cardiac sequelae. 3
Symptomatic management
Symptomatic management of cardiac amyloidosis addresses heart failure and other cardiovascular complications including arrythmias. The CCS/CHFS 2020 Society Joint Position Statement on cardiac amyloidosis describes the following management considerations: 3
Diuresis using loop diuretics, mineralocorticoid receptor antagonists, and thiazide diuretics are preferred for heart failure supportive management. β-blockers, calcium channel blockers, ACEi/ARBs, and digoxin should be avoided or used cautiously because they are often poorly tolerated by patients with cardiac amyloidosis
Anticoagulation for atrial fibrillation/flutter
Pacemaker implantation for symptomatic bradycardia
Defibrillator implantation for secondary prevention in appropriate patients
Consideration of heart transplantation for highly selected patients
Disease-modifying therapy
Treatment of ATTR amyloidosis has changed substantially in recent years. Available DMTs for ATTR amyloidosis now include “silencers” that block specific mRNA sequences coding for pathogenic proteins in the liver (inotersen, patisiran) and “stabilizers” that bind to the center of the TTR protein to prevent misfolding (tafamidis).60-64 Treatment with intravenous patisiran or subcutaneous inotersen is recommended for patients with hATTR amyloidosis with ambulatory polyneuropathy. 3 Tafamidis (oral) is recommended for patients with ATTR cardiac amyloidosis and NYHA class I-III symptoms. 3 Symposium presenters noted that in their clinical cardiology experience, tafamidis is generally well tolerated, while gene silencers have more variable results; many patients remain stable or do better, and some do worse. Investigational approaches in clinical trials include other TTR protein stabilizers and antibody-mediated removal of amyloid deposits. 22
Multidisciplinary Perspective.
The CCS/CHFS Joint Position Statement recommends the interdisciplinary management of cardiac amyloidosis. 3 Monitoring by cardiologists and nephrologists may be useful for the management of treatment adverse events. Drugs used for the management of AL amyloidosis can have deleterious effects on the heart, particularly in patients who have underlying cardiac disease or heart dysfunction. 65 Meanwhile, inotersen has been associated with anti-neutrophil cytoplasmic autoantibody (ANCA) kidney vasculitis, and patients may require surveillance from a nephrologist. 62
Limitations
This conference featured multidisciplinary discussion of cases, and learning points reflect the assessments by the involved experts/authors.
Implications
Early diagnosis and treatment of amyloidoses are essential to improve outcomes, both of which require a multidisciplinary approach and a low threshold to suspect amyloidosis and expedite diagnostic workup.
Appropriate treatment decisions require precise disease subtyping and increased awareness of different types of amyloidosis, particularly when assessing kidney involvement. Tools such as mass spectrometry can be helpful in clarifying equivocal diagnoses and are the standard tool for amyloid subtyping.
Treatment options are increasing for common forms of cardiac and kidney amyloidoses. Emerging therapies and refined definitions of treatment response hold promise for further improvement in outcomes.
Acknowledgments
The authors wish to thank liV Agency Inc. for medical writing support (Iris Boraschi, Janice Carr Meisner, Paul Heron, Lisa Kellenberger).
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Abhijat Kitchlu: No relevant conflicts of interest to disclose. Christopher T. Chan: No relevant conflicts of interest to disclose. Kenar D. Jhaveri: Consultancy agreements with Secretome, George Clinicals, PMV pharmaceuticals, GSK, and Calliditas. K.D.J. reports honoraria from the American Society of Nephrology and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. Diego Delgado: Consulting fees and speakers’ honoraria from Akcea, Alnylam, Pfizer. Paul Tam: No relevant conflicts of interest to disclose
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Abhijat Kitchlu  https://orcid.org/0000-0002-4340-5046
https://orcid.org/0000-0002-4340-5046
References
- 1.Picken MM.The pathology of amyloidosis in classification: a review. Acta Haematol. 2020;143(4):322-334. [DOI] [PubMed] [Google Scholar]
- 2.Ash S, Shorer E, Ramgobin D, et al. Cardiac amyloidosis-A review of current literature for the practicing physician. Clin Cardiol. 2021;44(3):322-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fine NM, Davis MK, Anderson K, et al. Canadian Cardiovascular Society/Canadian Heart Failure Society Joint Position statement on the evaluation and management of patients with cardiac amyloidosis. Can J Cardiol. 2020;36(3):322-334. [DOI] [PubMed] [Google Scholar]
- 4.Merlini G, Bellotti V.Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583-596. [DOI] [PubMed] [Google Scholar]
- 5.Merlini G, Seldin DC, Gertz MA.Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29:192420110411-192420111933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson MD, Buxbaum JN, Eisenberg DS, et al. Amyloid nomenclature 2020: update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2020;27(4):217-222. [DOI] [PubMed] [Google Scholar]
- 7.Ravichandran S, Lachmann HJ, Wechalekar AD.Epidemiologic and survival trends in amyloidosis, 1987–2019. New Eng J Med. 2020;382:1567-1568. [DOI] [PubMed] [Google Scholar]
- 8.Falk RH, Alexander KM, Liao R, et al. AL (Light-Chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol. 2016;68:1323-1341. [DOI] [PubMed] [Google Scholar]
- 9.Schentrup D.Diagnosing multiple myeloma in primary care. Clinician Reviews. 2018;28:16-21. [Google Scholar]
- 10.Gertz MA.Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am J Hematol. 2020;95(7):848-860. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005;79(4):319-328. [DOI] [PubMed] [Google Scholar]
- 12.Deshpande PP, Jhaveri KD.The role of the kidney in AL amyloidosis. Nephrol Dial Transplant. 2021;36:1597-1599. [DOI] [PubMed] [Google Scholar]
- 13.Benson MD. The hereditary amyloidoses. Best Practice Res Clin Rheumatol 17(6):909-927. [DOI] [PubMed] [Google Scholar]
- 14.Rowczenio DM, Noor I, Gillmore JD, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. 2014;35(9):E2. [DOI] [PubMed] [Google Scholar]
- 15.Dember LM.Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458-3471. [DOI] [PubMed] [Google Scholar]
- 16.Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD.Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1(2):e000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson MD, Berk JL, Dispenzieri A, et al. Tissue biopsy for the diagnosis of amyloidosis: experience from some centres. Amyloid. 2022;29(1):8-13. [DOI] [PubMed] [Google Scholar]
- 18.Gertz MA, Rajkumar SV.Amyloidosis: Diagnosis and Treatment. Louisville, KY: Humana; 2010. [Google Scholar]
- 19.Phull P, Sanchorawala V, Connors LH, et al. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid. 2018;25(1):62-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubrey SW, Hawkins PN, Falk RH.Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97(1):75-84. [DOI] [PubMed] [Google Scholar]
- 21.Castaño A, Drachman BM, Judge D, Maurer MS.Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruberg FL, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34(7):520-528. [DOI] [PubMed] [Google Scholar]
- 24.Lobato L, Rocha A.Transthyretin amyloidosis and the kidney. Clin J Am Soc Nephrol. 2012;7:133720120426-133720121346. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza A, Osman K, Costa Chase C, et al. The hematologist’s role in amyloidosis management: disease awareness, diagnostic workup, and practice patterns. Blood. 2020;136:28-29. [Google Scholar]
- 26.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:232520140812-232520142332. DOI: 10.1182/blood-2014-04-570010 [DOI] [PubMed] [Google Scholar]
- 27.Dispenzieri A.Renal risk and response in amyloidosis. Blood. 2014;124:2315-2316. DOI: 10.1182/blood-2014-08-596338 [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Dispenzieri A, Lacy MQ, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:98920120213-98920120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilleness B, Ruberg FL, Mussinelli R, et al. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133:21520181017-21520181223. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN.Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171-174. [DOI] [PubMed] [Google Scholar]
- 31.Suhr OB, Lundgren E, Westermark P.One mutation, two distinct disease variants: unravelling the impact of transthyretin amyloid fibril composition. J Intern Med. 2017;281(4):337-347. [DOI] [PubMed] [Google Scholar]
- 32.Ferraro PM, D’Ambrosio V, Di Paolantonio A, et al. Renal involvement in hereditary transthyretin amyloidosis: an italian single-centre experience. Brain Sci. 2021;11:98020210724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solignac J, Delmont E, Fortanier E, et al. Kidney involvement in hereditary transthyretin amyloidosis: a cohort study of 103 patients. Clin Kidney J. 2022;15(9):1747-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swan N, Skinner M, O’Hara CJ.Bone marrow core biopsy specimens in AL (primary) amyloidosis. Am J Clin Pathol. 2003;120(4):610-616. [DOI] [PubMed] [Google Scholar]
- 35.Lapman S, Whittier WL, Parikh R, et al. Immune checkpoint inhibitor–associated renal amyloid A amyloidosis: a case series and review of the literature. J Onco Nephrol. 2020;4:52-58. [Google Scholar]
- 36.Wanchoo R, Karam S, Uppal NN, et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45(2):160-169. [DOI] [PubMed] [Google Scholar]
- 37.Herrmann SM, Perazella MA.Immune Checkpoint inhibitors and immune-related adverse renal events. Kidney Int Rep. 2020;5:113920200429-113920201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamlouk O, Selamet U, Machado S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:220190106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitchlu A, Jhaveri KD, Wadhwani S, et al. A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep. 2021;6(1):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman J, Dogan A.Fibrinogen alpha amyloidosis: insights from proteomics. Expert Rev Proteomics. 2019;16(9):783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prokaeva T, Spencer B, Kaut M, et al. Soft tissue, joint, and bone manifestations of AL amyloidosis: clinical presentation, molecular features, and survival. Arthritis Rheum. 2007;56(11):3858-3868. [DOI] [PubMed] [Google Scholar]
- 42.Rapezzi C, Lorenzini M, Longhi S, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20(2):117-124. [DOI] [PubMed] [Google Scholar]
- 43.Mayo C.Clinical Trials. https://www.mayo.edu/research/clinical-trials/diseases-conditions/amyloidosis (2022)
- 44.Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26(11):2317-2325. [DOI] [PubMed] [Google Scholar]
- 45.Ravichandran S, Cohen OC, Law S, et al. Impact of early response on outcomes in AL amyloidosis following treatment with frontline Bortezomib. Blood Cancer J. 2021;11:11820210621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Law S, Cohen O, Lachmann HJ, et al. Renal transplant outcomes in amyloidosis. Nephrol Dial Transplant. 2021;36:355-365. [DOI] [PubMed] [Google Scholar]
- 47.Angel-Korman A, Stern L, Sarosiek S, et al. Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 2019;95:40520181221-40520181411. [DOI] [PubMed] [Google Scholar]
- 48.Kaufman GP, Dispenzieri A, Gertz MA, et al. Kinetics of organ response and survival following normalization of the serum free light chain ratio in AL amyloidosis. Am J Hematol. 2015;90(3):181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi J, Guan J, Jiang B, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107:418820100211-418820104193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muchtar E, Dispenzieri A, Leung N, et al. Depth of organ response in AL amyloidosis is associated with improved survival: grading the organ response criteria. Leukemia. 2018;32(10):2240-2249. [DOI] [PubMed] [Google Scholar]
- 51.Palladini G, Merlini G.When should treatment of AL amyloidosis start at relapse? Early, to prevent organ progression. Blood Adv. 2019;3:212-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:61220150518-61220150615. [DOI] [PubMed] [Google Scholar]
- 54.Kastritis E, Palladini G, Minnema MC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385:46-58. [DOI] [PubMed] [Google Scholar]
- 55.Khouri J, Anwer F, Samaras CJ, et al. Safety, tolerability and efficacy of Cael-101 in AL amyloidosis patients treated on a phase 2, open-label, dose selection study to evaluate the safety and tolerability of Cael-101 in patients with AL amyloidosis. Blood. 2020;136:21. [Google Scholar]
- 56.Jaccard A, Moreau P, Leblond V, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083-1093. [DOI] [PubMed] [Google Scholar]
- 57.Sidana S, Sidiqi MH, Dispenzieri A, et al. Fifteen year overall survival rates after autologous stem cell transplantation for AL amyloidosis. Am J Hematol. 2019;94(9):1020-1026. [DOI] [PubMed] [Google Scholar]
- 58.Jimenez-Zepeda VH, Franke N, et al. Autologous stem cell transplant is an effective therapy for carefully selected patients with AL amyloidosis: experience of a single institution. Br J Haematol. 2014;164(5):722-728. [DOI] [PubMed] [Google Scholar]
- 59.Schönland SO, Dreger P, de Witte T, Hegenbart U.Current status of hematopoietic cell transplantation in the treatment of systemic amyloid light-chain amyloidosis. Bone Marrow Transplant. 2012;47(7):895-905. [DOI] [PubMed] [Google Scholar]
- 60.Maurer MS, Elliott P, Merlini G, et al. Design and rationale of the phase 3 ATTR-ACT clinical trial (tafamidis in transthyretin cardiomyopathy clinical trial). Circ Heart Fail. 2017;10(6):003815. [DOI] [PubMed] [Google Scholar]
- 61.Bulawa CE, Connelly S, Devit M, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012;109:962920120529-962920129634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benson MD, Waddington-Cruz M, Berk JL, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22-31. [DOI] [PubMed] [Google Scholar]
- 63.Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11-21. [DOI] [PubMed] [Google Scholar]
- 64.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:100720180827-100720181016. [DOI] [PubMed] [Google Scholar]
- 65.Plummer C, Driessen C, Szabo Z, et al. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J. 2019;9:2620190226. [DOI] [PMC free article] [PubMed] [Google Scholar]