Abstract
Charcot neuroarthropathy (CN) was first described over 150 years ago. Despite this there remains uncertanity around the factors that contribute to its development, and progression. This article will discuss the current controversies around the pathogenesis, epidemiology, diagnosis, assessment and management of the condition. The exact pathogenesis of CN is not fully understood, and it is likely to be multifactorial, with perhaps currently unknown mechanisms contributing to its development. Further studies are needed to examine opportunities to help screen for and diagnose CN. As a result of many of these factors, the true prevalence of CN is still largely unknown. Almost all of the recommendations for the assessment and treatment of CN are based on low-quality level III and IV evidence. Despite recommendations to offer people with CN nonremovable devices, currently only 40–50% people are treated with this type of device. Evidence is also lacking about the optimal duration of treatment; reported outcomes range from 3 months to more than a year. The reason for this variation is not entirely clear. A lack of standardised definitions for diagnosis, remission and relapse, heterogeneity of populations, different management approaches, monitoring techniques with unknown diagnostic precision and variation in follow-up times prevent meaningful comparison of outcome data. If people can be better supported to manage the emotional and physical consequences of CN, then this could improve people’s quality of life and well-being. Finally, we highlight the need for an internationally coordinated approach to research in CN.
Keywords: charcot neuroathropathy, controversies, diabetes
Charcot neuroarthropathy
Charcot neuroarthropathy (CN) is a relatively rare but serious complication that can affect people with peripheral neuropathy. It is most commonly diagnosed in people living in countries where diabetes is the most common cause of peripheral neuropathy. It is a progressive condition that affects the soft tissues, joints and bones. In the active phase there is uncontrolled inflammation, and the bones of the affected area become osteopenic which in turn can lead to fractures and joint dislocation. 1 Diabetic neuropathy is the leading cause of CN. 2 Other causes include infection, drugs, 3 autoimmune diseases, and trauma or tumours that damage the spinal cord. CN most frequently affects the foot and ankle; 4 however, it can also affect the knee, 5 hip, 5 spine 6 and the wrist.7–9 The underlying pathological process is the same regardless of the cause or site of the CN. Despite first being described over 150 years ago, CN still remains a poorly understood and frequently overlooked complication of diabetes. 10 This article will explore the controversies in the pathogenesis, epidemiology, management and outcomes of CN.
CN is known by several different terms which are all used interchangeably in the literature: neuroarthropathy, arthropathy, neuropathic osteoarthropathy and neuro-osteoarthropathy. These terms indicate a disease of joints that is associated with damage or disruption to the nervous system, in this case the peripheral nervous system. More recently, to acknowledge the role of soft tissue inflammation, expanding the term has been suggested. 11 This inflammation is a key feature of this disease and often the first clinical sign which alerts the clinician to this diagnosis. It has been proposed that ‘neuropathic inflammatory sarco-osteoarthropathy’ would be a better descriptive term for CN. 11
History of CN
The original description of CN has been attributed to French neurologist Jean-Martin Charcot. He described it as a complication of tabes dorsalis, a neurological manifestation of tertiary syphilis, which is now very rare. 12 Charcot was a physician who worked in Paris in 1800s and has several neurological conditions named after him. 13 He is considered the founder of modern neurology and is remembered as the first professor of neurology. Jordan 14 first described the condition in people with diabetes.
Controversies in the pathogenesis of CN
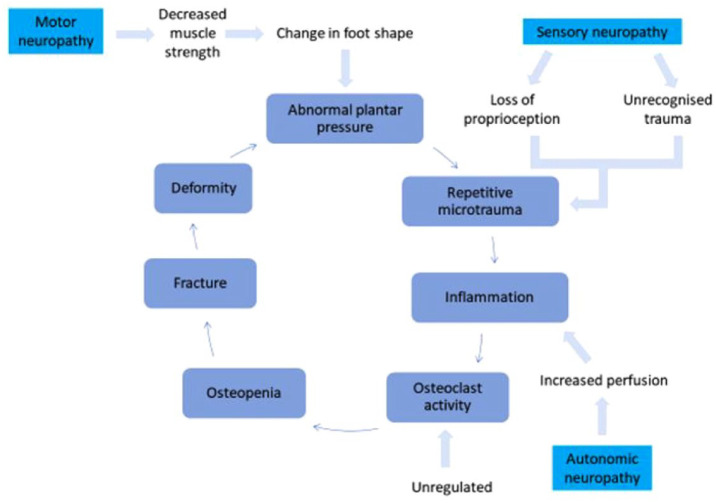
Historically, the pathogenesis of CN has been explained by two main theories: the neurotrophic/neurovascular and the neurotraumatic theories. The neurotrophic/neurovascular theory was proposed by Charcot. 12 He hypothesised that increased blood flow to the foot, now known to be associated with autonomic neuropathy, leads to bone resorption and bone weakness (osteopenia). The other hypothesis, the neurotraumatic theory, focuses on an insensate joint or bone being subjected to continued, repetitive pressure and trauma. This causes progressive damage to the affected bones and joints which then leads to fractures and deformity. The current controversy and theories surrounding the pathogenesis of CN including the role of RANKL and OPG have been detailed in a recent paper. 1 However, there remains a need for further studies to provided additional understanding on the mechanisms that can lead to some people with diabetes and neuropathy developing CN, while others do not. Figure 1 shows the schematic representation of the current theory of the relationship between neuropathy, trauma, inflammation and osteopenia which leads to CN.
Figure 1.
Schematic representation of the mechanisms involved in the pathogenesis of CN.
Source: Adapted from Kaynak et al. 15
The exact pathogenesis of CN is not fully understood, and it is likely that the causes are multifactorial, with perhaps currently unknown mechanisms contributing to the development of CN. It is not known how to prevent the development of CN, other than maintaining good diabetes control to reduce the risk of the development of neuropathy, which still does not completely eliminate the risk. Further high-quality studies are required to help elucidate the pathogenesis of CN.
Controversies in the epidemiology of CN
There is apparent variation in the reported incidence and prevalence rates for CN, and at this time we do not understand the reasons behind this. The majority of data comes from retrospective population-based case series. The estimated lifetime cumulative incidence for CN is 0.4–1.3% in people living with diabetes, rising to 13% in people who already attend diabetic foot specialist clinics. 16 In 2018 a regional survey of 205,033 people with diabetes in the East Midlands, UK, reported a point prevalence of 0.04%. 17 The largest epidemiological study was conducted in Denmark among 309,557 people with diabetes identified from hospital codes over a 23-year period (1995–2018). They reported an incidence rate of 7.4 per 10,000 person-years, and a prevalence of 0.56%. 18 These two studies are the only large epidemiological studies on CN.
The true incidence and prevalence of CN are still largely unknown. More recent studies suggest an increase in the numbers of people with CN, but this could be a result of increased awareness, rather than an actual increase in the incidence. Further studies are needed to confirm this. A national and/or international registry prospectively collecting data on the number of new cases of CN, and related patient characteristics may provide more accurate figures on the incidence of CN in people with diabetes and people with ‘at risk’ feet.
Controversies in the classification of CN
There is no universal agreed classification system for CN, but existing systems can be divided into systems which describe the progression of the destructive pathological process and those which describe the anatomical location of the affected bone or joints. The most commonly recognised classification systems which described the pathological process is the Eichenholtz system modified to include stage 0 the prodromal stage (Table 1).19,20 The two most common anatomical classifications systems are the Sanders and Frykberg, and second the Brodsky which was later modified (Table 2 ).21–23 The usefulness of the old and new classification systems in guiding treatment or predicting outcomes has not been evaluated. These classification systems show the progression of CN and perhaps are capable of predicting which people might benefit from surgery. However, a major evidence gap for the management of CN is the lack of a validated classification system that can identify when the CN has gone into remission.
Table 1.
| Stage | Radiographic finding | Clinical finding | |
|---|---|---|---|
| Stage 0 | Prodromal | Normal radiographs | Swelling, erythema, warmth |
| Stage I | Developmental | Osteopenia, osseous fragmentation, joint subluxation, or dislocation | Swelling, erythema, warmth, ligamentous laxity |
| Stage II | Coalescence | Absorption of debris, sclerosis, fusion of larger fragments | Decreased warmth, decreased swelling, decreased erythema |
| Stage III | Reconstruction | Consolidation of deformity, fibrous ankylosis, rounding and smoothing of bone fragments | Absence of warmth, absence of swelling, absence of erythema, fixed foot/ankle deformity |
Table 2.
A comparison of the two most common anatomical classification system for CN. Sanders and Frykberg and Brodsky classification systems to show the site and joints involved.21–23
| Brodsky | Sanders and Frykberg | Site | Joints involved |
|---|---|---|---|
| 1 | II | Midfoot | Lisfranc Tarsometatarsal joints |
| 2 | III | Hindfoot | Naviculocuneiform Talonavicular Calcaneocuboid |
| 3A | IV | Ankle | Ankle |
| 3B | V | Posterior calcaneum | Calcaneus |
| 4 | Not reported | Combined | |
| 5 | I | Forefoot | Interphalangeal joints |
The diagnosis and monitoring of CN
The diagnosis of CN is primarily based on clinical findings, supported by the results of radiological investigations. There is no universally agreed method or series of investigations to help diagnose CN in the early stages, Eichenholtz stage 0.
Current guidelines for healthcare professionals who provide treatment recommend baseline plain X-rays as the initial radiological investigation to diagnosis CN and, if this is inconclusive, magnetic resonance imaging (MRI). 24 In early CN at Eichenholtz stage 0, there may be no changes evident on plain X-ray. 20 In CN, blood chemical and haematological markers are usually normal or only marginally outside reference ranges, unless there is concurrent infection. 25
As the disease process of CN progresses, signs of inflammation usually resolve; however, the clinician faces the challenge of determining when complete remission has occurred. The presence of neuropathy means that subjective symptoms are often absent, and the signs of inflammation can be subtle, and this can make it difficult to assess when the foot has gone into remission. Guidelines recommend that offloading and immobilisation should be continued until the temperature difference between the affected and unaffected foot is less than 2°C (3.6°F), with no further radiological changes on X-ray.24,26 However, these recommendations are based on low-quality level III and IV evidence – that is, case series and expert opinion.
Controversies around the use of infrared thermometry
There is uncertainty over the diagnostic accuracy of infrared thermometry to monitor and diagnose disease remission in CN.
A temperature difference of greater than 2°C (3.6°F) between the affected and unaffected foot is indicative of inflammation, but this alone does not confirm the diagnosis. This diagnostic value is widely but not universally used in clinical practice, however when its origin is traced back to the original citation, the authors acknowledge that this cut-off was based on expert opinion. 27 The original recommendation of 2.2°C (4°F) has subsequently been rounded down and the majority of guidelines now state a temperature cut-off as 2°C. The impact of the difference from 2.2°C down to 2°C has not been investigated. However, it has been suggested that the cut off temperature difference of less than 2.0°C –2.2°C may be set too high. 28 This is because there is still a noticeable difference between the foot temperatures, which could indicate ongoing inflammation and could mean that the foot has not actually gone into remission. An over- or underestimation of the degree of inflammation could mean that treatment is continued for longer than necessary or discontinued prematurely.
There is uncertainty whether the infrared thermometry devices used in foot clinics are validated for the range of temperature measurements recorded on feet as opposed to core body temperatures which many of these devices were originally designed to measure.
Although the majority of foot clinics who have access to this technology use infrared thermometry as a guide to assess residual disease activity, there is inconsistency in the methods used. There is a lack of consensus on the period of acclimatisation after removing shoes and socks before the temperature readings are taken, the number of measurements taken and the sites on the foot used to measure the temperature.
Of course, comparing the skin temperature between the affected and unaffected foot assumes that the any inflammatory process is limited to just one foot. The presence of bilateral foot disease, revascularisation of one limb or the absence of a contra-lateral limb will invalidate the use of skin temperature measurement.
A cohort study with 32 people with CN, assessed the intra- and interrater reliability of using infrared thermometry to assess CN. 29 They reported good intra and interrater reliability of the test. However, this study did not address the uncertainties around the sensitivity and specificity of using infrared thermometry to monitor and diagnose remission in CN.
Controversies around the use of radiological investigations
There are a number of different radiological investigations that are used in clinical practice and research to diagnose remission in CN. However, the quality of evidence to support one technique over another is low. First, the diagnostic accuracy of individual radiological investigations used to diagnose remission is unknown. Second individual tests have limitations, which may render them inappropriate in specific circumstances for example people for whom MRI is contraindicated or not tolerated. Finally, there is no agreement on the ‘gold standard’ test or combination of tests to diagnose remission.
Plain X-rays as a technique to diagnose and monitor CN
Plain X-rays offer a relatively inexpensive, simple investigation, readily available in most hospitals. The main disadvantage of plain X-ray is the use of ionising radiation which can be harmful to the individual and the environment.
CN is a condition of inflammation characterised by oedema. However, plain X-rays demonstrate structural changes to the foot skeleton and not inflammation or oedema. At Eichenholtz stage 0, there will be no changes evident on plain X-ray. In CN, the use of plain X-ray can show initial deformity and the extent to which offloading and immobilisation has been effective in preventing the progression of bony foot deformity. Therefore, they are a measure of outcome rather than disease process.
Furthermore, studies have shown that plain X-rays have poor sensitivity to diagnose foot and ankle injuries. A small study comparing the diagnosis of sports inquires using plain X-rays and MRI scanning has shown that osteochondral fractures were frequently missed on plain X-ray, and only picked up on subsequent MRI. 30 Another study in people with sports injuries compared computer tomography (CT) to plain X-ray to diagnosis foot and ankle trauma. They reported a sensitivity of only 25–33% for midfoot fractures on plain X-rays. 31 In a case study review of misdiagnosis of traumatic cuneiform fractures, Olson et al. 32 discussed how the overlapping structure of the bones in the midfoot could make identifying abnormalities on plain X-ray more difficult.
MRI as a technique to diagnose and monitor CN
The use of MRI as a superior diagnostic tool in the diagnosis of CN is now well established. 24 In contrast to plain X-rays, MRI scans can show abnormalities which are not evident on plain X-rays. Bone oedema, one of the first sign of CN, is shown very clearly on MRI. MRI scans are also particularly useful at showing soft tissue structures such as muscle, ligaments and cartilage. The ability to see soft tissue structures is particularly important given more recent hypothesis that CN is a condition of soft tissue as well as bone. 11 The advantage of MRI scanning is that people are not subjected to radiation exposure as they are with plain X-rays. Having an MRI, however, involves lying flat and still in a noisy enclosed space, making it unsuitable for people with claustrophobia, or for those who cannot lie flat. MRI is contraindicated in people with some pacemakers, and some metals implants such as those who have had joint replacement surgery. MRI with contrast is contraindicated for people with significant kidney disease.
MRI scanners are expensive, and this has restricted access. With increasing investment, access to MRI is becoming more readily available and offers an opportunity to consider the potential benefits of expanding the use of MRI from purely diagnostic to include monitoring for remission in CN. However, this has not been validated. The usefulness of MRI in monitoring to identify disease remission as a replacement or adjunct to infrared thermometry and plain X-rays needs to be examined. A randomised feasibility study has explored the feasibility of using serial MRI without contrast in the monitoring of CN. This study has demonstrated that it is possible to recruit and retain participants and that the intervention serial MRI is achievable, safe and acceptable and shown that a definitive study to examine the effectiveness of serial MRI in diagnosing remission in CN is feasible. 33
Classification systems based on radiographical findings from X-rays and MRIs
Two classification system based on the assessment of X-rays have been proposed to evaluate the outcomes of CN. The first, developed by Bergin et al., 34 sought to establish a link between the level of destruction on X-ray and quality of life. The scale measured disruption, bone fragments, osteopenia, shape and loss of plantar arch. They did not assess any potential link to the degree of destruction seen on X-ray and clinical outcomes. A second case note study from one centre compared the effectiveness of well-recognised classification systems in predicting the need for surgical management. 35 They compared the Eichenholtz 18 and Schon et al. 36 systems with a new classification, the ‘D’ scoring systems which assesses density, distention/swelling, debris, disorganisation and dislocation/subluxation adapted from the system proposed by Yochum and Rowe. 37 They found statically significant difference between the scores of the nonsurgical and surgical groups for their newly proposed ‘D’ classification system but not for the Eichenholtz or Schon classification systems. They propose that the ‘D’ system could be used to support evidence-based decision-making on the need for nonsurgical or surgical management. This classification systems needs to be validated in a larger sample size.
With the emerging use of MRI as a diagnostic tool for CN, researchers and clinicians are developing systems which classify the stage and severity of CN based on MRI scanning.38,39 One such MRI classification (Table 3) describes the severity of CN according to the presence or absence of cortical fractures and whether the disease is active or inactive, based on the presence or absence of skeletal inflammation.
Table 3.
Clinical and MRI findings of CN.
| Stage | Grade | Clinical symptoms | MRI findings |
|---|---|---|---|
| Active | 0 | Mild inflammation No gross deformity |
Diffuse soft tissue and bone marrow oedema No cortical disruption Subchondral trabecula microfractures, ligament damage |
| Active | 1 | Severe inflammation Gross deformity |
Fractures with soft tissue and bone marrow oedema Osteoarthritis, cysts cartilage damage, osteochondrosis, joint effusion, fluid collection, bone erosion, bone lysis, debris, subluxation, ligament damage, tenosynovitis |
| Chronic | 0 | No inflammation No deformity |
No abnormal findings or minimal residual bone marrow oedema, subchondral sclerosis, bone cysts, osteoarthritis, ligament damage |
| Chronic | 1 | No inflammation Persistent gross deformity |
Residual bone marrow oedema, cortical callus, joint effusion, subchondral cysts, joint destruction, joint dislocation, fibrosis, osteophyte formation, bone remodelling, cartilage damage, ligament damage bone sclerosis, ankyloses, pseudoarthrosis |
Source: Adapted from Chantelau and Grützner. 39
CN, Charcot neuroarthropathy; MRI, magnetic resonance imaging.
A second team developed this further by quantifying the level of inflammation and damage; they assigned a score of between 0 and 2 for the presence of and degree of bone marrow oedema and fractures. They concluded that this semi-quantitative scoring method is a reliable way to assess the degree and severity of bony involvement in CN. 40
The ‘Balgrist Score’ is proposed as a new classification system developed following a case control study. 41 Serial MRIs in 65 feet of 56 patients who had previously been diagnosed and received offloading for CN were blinded assessed for soft tissue oedema, bone marrow oedema, joint destruction and fracture this was then linked to healing times. They concluded that the Balgrist score can be used in CN assessments to differentiate between CN that is likely to resolve in less than or greater than 90 days. The limitation of this score is this tool’s only having been validated for the mid- and hindfoot sites. Reviewing the inclusion and exclusion criteria for this study raises several potential issues. Only, half (35/56) of patients included in this review had diabetes. Nine patients were characterised as having peripheral artery disease which was not classified by severity. Finally, there were 11 patients classified jointly as having either kidney failure or transplantation. People who have received a transplant and are taking anti-rejection medications may have an altered inflammatory response influencing the characteristic oedema and destruction seen on MRI.
All these classification systems were developed using cohort or case studies in people with a confirmed diagnosis of CN, or research images from participant populations who were recruited to other trials. These proposed classification systems therefore need to be validated in a larger, more representative sample, which is matched for participant characteristics, treatment and outcomes; they need to include more than one centre to account for variations in approaches to nonsurgical and surgical management. Finally, the systems need to consider and allow for the presence of comorbidities that may affect the level of inflammatory response and how these comorbidities may influence treatment choices and outcomes.
Other imaging technologies used to diagnose and monitor CN
The value of newer imaging technologies, most of which use ionising radiation, such as three-phase bone scanning, computer tomography, positron emission tomography (PET) scan, and single-photon emission computed tomography (SPECT-CT) scan, magnetic resonance spectroscopy (MRS) and SPECT-CT, has yet to be established.28,42
Treatment of CN
Treatment of CN aims to stop the inflammatory process, relieve pain and maintain foot architecture. 43 The success of treatment depends on many factors including the Eichenholtz stage of CN at presentation, 20 the location of the bones and joints involved,21–23 the degree of deformity at presentation, and the presence of concurrent ulceration and or infection. 44
Conservative (nonsurgical) treatment of CN
Nonsurgical management of CN is to off-load and immobilise the foot by wearing a nonremovable device, either a cast or boot. 24 A nonremovable device immobilises the foot and minimises the potential for any further damage. 45
Cast or boots share the load and redistribute the pressure away from the affected bone/s or joint/s. Nonremovable devices are recommended as studies have shown that patient adherence to wearing removable devices is low, 46 which can result in longer healing times. A UK study showed that median time to healing and transfer into footwear was 9 months among people who received initial treatment with a nonremovable offloading device, compared with 12 months for those who did not. 47 Despite recommendations to offer people with CN nonremovable devices only 40–50% people receive a nonremovable device. 47 The reasons behind the choice of offloading device are not clear, but clinician preference, knowledge and skills and the choices of people with CN are all likely to contribute.
Alongside wearing the cast or boot, people are advised to reduce weightbearing and rest the foot as much as possible. Pinzur 48 noted that although specialist clinicians commonly advise people with CN to rest their foot and minimise weight bearing, in the belief that this further off-loads the foot and reduces deformity, there is no high-quality evidence that this reduces time to resolution or reduces deformity. Studies to examine and evaluate ‘safe’ activity levels in CN are needed. If people were able to be more physically active without detrimentally affecting the outcome of the CN, then this may reduce some of the physical limitations, reduce the emotional impact associated with the treatment, and reduce the associated cardiovascular risks of sedentary behaviour.
Pharmacological treatment of CN
The pathophysiology of CN is associated with increased bone resorption, leading to osteopenia. 49 Therefore, the use of pharmacological therapies to treat CN has focused on the re-establishment of the balance between bone formation and destruction. Seven published randomised controlled trials have evaluated different agents against placebo: calcitonin, 50 bisphosphonates51–53 and recombinant human parathyroid hormone. 54 Calcitonin, bisphosphonates and parathyroid hormone are designed to decrease bone breakdown. Another study compared high methylprednisolone, a high-dose steroid to reduce inflammation and zoledronate. 55 The evidence from these trials to support the use of pharmacological therapies is inconsistent. Some trials reported improvements in the markers of bone turnover and reduction in inflammation. However, all of these studies were underpowered, one was not blinded, 53 and two did not include relevant clinical outcomes such as time to healing or prevention of deformity.50,55 One further study compared denosumab, a specific anti-RANKL agent, against a population of historical controls. This study found that people who received treatment with denosumab had shorter fracture healing times, and time to cessation of total contact casting compared with those receiving usual care. 56 A UK audit showed that the prescription of bisphosphonates may increase treatment times, reporting a median time to remission of 12 months for those prescribed bisphosphonates compared with 10 months for those who did not receive them. 47 This was confirmed in a recent systematic review. 57 The poor quality of the evidence and inconsistency in findings have led to UK guidelines and international consensus documents, and a recent systematic review not recommending the use of pharmacological therapies to treat CN, unless being evaluated in a clinical trial.24,57,58 Further research is needed to examine and evaluate the effectiveness of pharmacological therapies in treating CN.
Surgical treatment of CN
Surgery is generally only considered when conservative treatment has failed to prevent the progression of foot deformity which could lead to ulceration and amputation. 58 Surgical intervention aims to correct any deformity and achieve a stable, flat foot. 59 A person-centred approach to surgical decision-making is essential. There are unanswered questions regarding when the optimal time is to operate in active versus chronic CN, 60 and secondly, when internal, external fixation, or a combination approach is most appropriate. Third, the likely time to rehabilitation and overall functional outcome with the persons own limb or a prosthetic device needs to be considered. There have been an increasing number of surgical case series published. A systematic review found that hindfoot and ankle are the most common sites require surgical treatment, due to joint instability. 60 This is because forefoot and midfoot CN can generally be successfully managed with conservative treatment of offloading and immobilisation, without the need for surgery. There is limited evidence to support the timing, patient selection and surgical techniques used in CN. 60 Nearly all the evidence on the surgical management of CN comes from North America. It could be argued that the healthcare insurance system in North America provides an incentive to operate, rather than in other countries such as the UK where a conservative approach is more likely.
Controversies in identifying remission of CN
Treatment is continued for active CN, until remission when there are no longer clinical signs of inflammation, and X-rays are stable with signs of healing. Different devices and techniques used in monitoring, and discrepancies around the definition of remission and whether this is based on clinical, thermometry or radiological measures individually or combined and decision thresholds for thermometry defined remission could also influence immobilisation time.
There is variation in the reported time to remission. Studies from the United Kingdom show a median time to remission of between 9 and 12 months.47,61,62 Studies from the United States demonstrate considerably shorter immobilisation times between 3 and 5 months.16,63–65 Results from studies conducted in Brazil, Germany and Denmark show remission times of 3–12 months, 3–6 months and 8.3 months, respectively.66–68
Individual patient and foot characteristics alongside different approaches to treatment may also contribute to this apparent variation in healing time: (1) the anatomical location of the CN, (2) the stage of CN when immobilisation is initiated, 69 (3) the use of nonremovable versus removable devices 47 and (4) the use of bisphosphates.47,57 There is a need to standardise the definition of remission, and reporting of CN studies to help understand the reason for these apparent variations.
Controversies in identifying relapse of CN
As with the diagnosis and monitoring for remission in CN there are very few ways except infrared thermometry and X-rays to check for signs of relapse. There is variation in the reported relapse rates. A study from Denmark reported relapse rates of 18%. 70 Studies from the United Kingdom reported longer treatment times with high rates of relapse between 33% and 35%.62,71 In contrast to the results from United Kingdom, studies from the United States found that shorter treatment times were associated with lower incidence of relapse within 1 months.16,63
Armstrong et al. 16 described a potential, but short-lived relapse of the CN following transfer from the offloading cast into footwear, with the offloading cast being reapplied for a period of 2.9 ± 1.2 weeks before temperatures were seen to stabilised again. The authors concluded that this may be a short-lived transient period of raised temperatures when people transfer from casts or boots into therapeutic foot. This may be considered by some to be part of the normal healing trajectory, but by others a relapse.
Comparison of the reported incidence relapse rates from different case series is challenging. There is no agreement about what constitutes a relapse. Variation exists between how to differentiate between a relapse and ‘new’ case, and whether the anatomical site for the relapse needs to be the same, adjacent to or in the same foot. Thus, there is a need for a consensus definition of relapse in CN.
Complications of CN
There is inconsistency in the reported frequency and severity of CN-related complications. Participant characteristics and length of follow-up can go some way to explain some of the variations. However, it is likely that different approaches to the treatment of CN and its complications may also contribute.
Controversies around morbidity in CN
Foot deformity and secondary ulceration are the most commonly reported complications of CN. Early treatment with immobilisation and offloading is known to prevent or limit the development of deformity and subsequent ulceration. In a case series in which magnetic resonance imaging was performed very early, 69% of patients with CN at stage 0 without a fracture at the time of immobilisation healed without deformities, in contrast to only 7% of participants with a delayed presentation at stage 1. 72 Observational studies have shown that early diagnosis, within 3 months of onset of symptoms, has also been shown to improve the functional outcomes and quality of life for the patient, possibly as a consequence of decreased treatment times and less deformity. 73
Observational studies have reported large variations in ulceration rates, between 11% and 75%.16,73–78 There are several reasons for these apparent variations: the stage and site of the CN when referred to specialist multidisciplinary foot clinics, treatment with a nonremovable versus removable device, adherence to treatment, and a conservative versus surgical approaches to management. A study by Fabrin et al. 79 suggested that the majority of ulcerations occur when the CN is in remission. The ulceration developed as a result of footwear design, delayed delivery of footwear or adherence with wearing the footwear. The incidence of ulceration increased to 31% once the foot was in remission and people had transferred into footwear. In one study, late presentation was associated with repeated episodes of ulceration, 74 and recurrent ulceration has been linked to amputation. 77
Amputation rates for CN vary from 2.7% to 28%.76,78,80–82 Patients with CN have an increased risk of major amputation, with rates as high as 28% if ulceration is present on the initial evaluation. 80 Major lower limb amputation is higher in patients who do not receive offloading 23% compared with 17% in those who did receive offloading. 78 People with CN and foot ulcers are between 8.5 and 12 times more likely to require a major amputation than patients with CN but without foot ulcers.77,83
Controversies around mortality in CN
There is variation in the reported mortality rates for CN, and we do not understand the reasons behind these apparent variations. Given the variations in follow-up time, different characteristics of the people (which are generally poorly reported), it is difficult to accurately quantify mortality rates after CN. CN does not happen in isolation, people may also have other microvascular complications of disease, that is, retinopathy and nephropathy which are independent risk factors of cardiovascular events. What is clear that people with CN have an increased mortality rate compared with the general population and those with diabetes but without foot complications. 84 In one study, a diagnosis of CN was found to increases mortality: average life expectancy was reduced by 14.4 years compared with the general UK population, with a mortality of 18.6% among 70 patients with CN after a median follow-up of 2.1 years. 85 After an average follow-up period of 8 years, Pakarinen et al. 73 reported a mortality rate of 29%; another study reported 5-year mortality rate of 33%. 74 A third study with a shorted follow-up period of 4 years reported a 1.7% mortality among 115 patients. 75 A more recent study compared people with diabetes with and without CN and reported that people with CN had a 2.7 increased risk of mortality (p = 0.003) when matched for confounders. 86 Finally, a study from Nottingham found no difference between mortality rates in people with CN, compared with those with neuropathic foot ulceration, and concluded that the neuropathy itself is associated with the increased mortality. 87 It is important to differentiate between people who have neuropathic and neuro-ischaemic foot complications. This is because people with neuro-ischaemic complications are more likely to be at increased risk of cardiovascular events. 88 A retrospective case series from Germany in 111 people with CN followed-up for 15 years found people with coronary heart disease had higher rates of mortality than those with CN but without coronary heart disease. 88
People’s experiences of living with CN
To date, there has been very little work exploring in any depth the most appropriate person-centred approach to support people living with CN. The small number of studies and heterogeneity of the study populations and treatments means it is difficult to understand the potential impact of CN on depression and health-related quality of life. A qualitative study exploring peoples experiences of living with and receiving treatment for CN found participants expressed frustration, with experiences of low mood, and low self-esteem. 89 These physical and emotional effects of CN on participants, their families, and relationships were reported to be substantial and sustained. Living with CN has ramifications that extend well beyond the physical limitations imposed directly by wearing the offloading device There are further physical, socioeconomic, and psychological consequences which people prioritise so as to actively manage their lives and their health.
The findings from both quantitative and qualitative research have shown that people with CN need to be able to access a wider range of support beyond their clinical team, to include psychological, and social care services. If we can understand how health and social care professionals and voluntary organisations can better support people to manage the emotional and physical consequences of CN, then this could improve their quality of life and well-being. This may in turn lead to better outcomes, with shorter time to remission, and a reduction in morbidity and mortality.
Discussion
This article has demonstrated the controversies around the diagnosis, assessment, and management of CN. These variations have arisen in the absence of a lack of robust evidence to guide healthcare professionals to diagnose, monitor and treat CN. Current guidance is primarily based on low-quality studies: level III evidence (case control studies) and level IV evidence (expert opinion) rather than high-quality meta-analyses, systematic reviews and randomised controlled trials (level I evidence). In the absence of definitive evidence to resolve variability between the approaches to management there is likely to be continued variatons in the reported time to healing and frequency of CN-related complications.
Given the rarity of CN and the challenges for making a diagnosis based on clinical examination and radiological imaging, there is a strong case for considering what other opportunities may exist to help screen for and diagnose CN.
To meaningfully compare outcome data, studies need to accurately describe the population at baseline and have access to agreed standard definitions for diagnosis, remission and relapse.
There is a lack of evidence about the sensitivity, specificity, cost-effectiveness, safety and patient acceptability for techniques used to diagnosis and monitor CN. 90 Furthermore, uncertainty continues about how monitoring techniques relate to treatment outcomes.
The main treatment for CN is conservative, with the use of a nonremovable cast or boot. It appears that the reported treatment times for CN vary, for not entirely clear reasons. Currently there is a lack of evidence to support the use of pharmacological therapies to treat CN, and surgery is primarily reserved for when conservative treatment has failed.
Given this lack of evidence to support the treatment and management of CN national and international health and care organisations and experts have identified CN as a research priority. In 2009 the US National Institute of Diabetes, Digestive and Kidney Diseases and the Office of Rare Diseases of the National Institutes of Health highlighted the need for a coordinated international approach to research into the pathophysiology, and management of CN. 91 While acknowledging the complexities of developing a randomised controlled trial, a review paper from the Lancet in 2015 emphasised the need for further research into CN including diagnosis, management, outcomes, and the impact on people’s health-related quality of life. 11 In the UK, National Institute for Health and Care Excellence (NICE) guidelines, ‘Diabetic foot problem: prevention and management’, have also included research into CN among its top five research priorities for the diabetic foot. 24
Despite these recommendations, a search of the four main clinical trial registries, only identified 17 trials on CN over the last 15 years, of which 10/17 (66.7%) were randomised controlled trials. Of these trials < 9/10 (90%) have investigated the use of pharmacological therapies to improve healing in CN. This review has shown that gaps remain in the evidence base for managing this condition.
The true prevalence of CN is still largely unknown. However, one study from Denmark estimated a 0 - 1 in every 2000 people will develop CN. 15 In the USA figures state that approximately one in every 2500 Americans will experience CN. 92 In the United Kingdom, a rare disease is defined as a condition which affects less than one, in 2000 people. 93 This means that CN may not fit the definition of a ‘rare disease’. However, there is definite overlap between the findings and recommendations of this review and the priorities of the UK Rare Disease Framework which are to:
Helping people get a final diagnosis faster.
Increasing awareness of rare diseases among healthcare professionals.
Better coordination of care.
Improving access to specialist care, treatments, and drugs. 93
This framework highlights the need for national and international collaboration to share knowledge and ideas, and complete high-quality research to ensure the best care and outcomes for people with rare diseases. For CN, this could mean developing a registry with internationally agreed definitions, of accurate up-to-date data on the incidence of CN, opportunities to examine and evaluate the effectiveness of current and new treatments for CN in larger populations, and to support researchers to identify people with CN likely to be willing to participate in research.
Conclusion
This article has shown the uncertainty about the pathogenesis, and true epidemiology of CN has still not been resolved. There is a lack of evidence on the sensitivity, specificity, cost-effectiveness, safety and patient acceptability of techniques used to diagnosis and monitor CN. There is a need to understand the different approaches to monitoring and treatment and identify whether and how these may contribute to the reported variation in time to healing, complications, and relapse rates. Finally, this indicates the need for an internationally coordinated approach to research in CN.
Acknowledgments
None.
Footnotes
ORCID iD: Catherine Gooday  https://orcid.org/0000-0001-5026-6788
https://orcid.org/0000-0001-5026-6788
Contributor Information
Catherine Gooday, Elsie Bertram Diabetes Centre, Norfolk & Norwich University Hospitals NHS Foundation Trust, Norwich NR4 7UY, UK.
Wendy Hardeman, Behavioural and Implementation Science Group, School of Health Sciences, Faculty of Medicine and Health Sciences, University of East Anglia, Norwich, UK.
Fiona Poland, Institute for Volunteering Research, Faculty of Medicine and Health Science, University of East Anglia, Norwich, UK.
Jim Woodburn, School of Health Sciences and Social Work, Griffith University, Southport, QLD, Australia.
Ketan Dhatariya, Elsie Bertram Diabetes Centre, Norfolk & Norwich University Hospitals NHS Foundation Trust, Norwich, UK; Norwich Medical School, University of East Anglia, Norwich, UK.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Catherine Gooday: Writing – original draft.
Wendy Hardeman: Supervision; Writing – review & editing.
Fiona Poland: Supervision; Writing – review & editing.
Jim Woodburn: Supervision; Writing – review & editing.
Ketan Dhatariya: Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: C.G., Clinical Doctoral Research Fellow (ICA-CDRF-2015-01-050) was funded by Health Education England (HEE)/National Institute for Health Research (NIHR) for this research project. The views expressed in this publication are those of the author(s) and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and material: Not applicable.
References
- 1.Jeffcoate W, Game F. The Charcot foot reflects a response to injury that is critically distorted by preexisting nerve damage: an imperfect storm. Diabetes Care 2022; 45: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 2.Sanders LJ. The charcot foot: historical perspective 1827-2003. Diabetes Metab Res Rev 2004; 20(Suppl. 1): S4–S8. [DOI] [PubMed] [Google Scholar]
- 3.Dhatariya K, Gooday C, Murchison R, et al. Pedal neuroarthropathy in a nondiabetic patient as a result of long-term amiodarone use. J Foot Ankle Surg 2009; 48: 362–364. [DOI] [PubMed] [Google Scholar]
- 4.Dardari D. An overview of Charcot’s neuroarthropathy. J Clin Transl Endocrinol 2020; 22: 100239–100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu V, Zhang J, Thahir A, et al. Charcot knee – presentation, diagnosis, management – a scoping review. Clin Rheumatol 2021; 40: 4445–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips S, Williams AL, Peters JR. Neuropathic arthropathy of the spine in diabetes. Diabetes Care 1995; 18: 867–869. [DOI] [PubMed] [Google Scholar]
- 7.Lambert AP, Close CF. Charcot neuroarthropathy of the wrist in type 1 diabetes. Diabetes Care 2005; 28: 984–985. [DOI] [PubMed] [Google Scholar]
- 8.Wilmot E, Jadoon K, Olczak S. Charcot’s neuroarthropathy of the wrist in type 2 diabetes Charcot’s. Pract Diab Int 2008; 25: 263. [Google Scholar]
- 9.Rastogi A, Prakash M, Bhansali A. Varied presentations and outcomes of Charcot neuroarthropathy in patients with diabetes mellitus. Int J Diabetes Dev Ctries 2019; 39: 513–522. [Google Scholar]
- 10.Donegan R, Sumpio B, Blume P. Charcot foot and ankle with osteomyelitis. Diabet Foot Ankle. 2013; 4: 21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffcoate W. Charcot foot syndrome. Diabet Med 2015; 32: 760–770. [DOI] [PubMed] [Google Scholar]
- 12.Charcot J. Sur quelques arthropathies qui paraissent dependre d’une lesion du cerveau ou de la moelle epiniere. Arch Des Physiol Norm et Path 1868; 1: 161–171. [Google Scholar]
- 13.Jean-Martin Charcot. Whonamedit? – a dictionary of medical eponyms, http://www.whonamedit.com/doctor.cfm/19.html
- 14.Jordan WR. Neuritic manifestations in diabetes mellitus. Arch Intern Med 1936; 57: 307–366. [Google Scholar]
- 15.Kaynak G, Birsel O, Güven M, et al. ‘An overview of the Charcot foot pathophysiology’. Diabet Foot Ankle 2013; 4: 21117. 10.3402/dfa.v4i0.21117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armstrong D, Todd W, Lavery L, et al. The natural history of acute Charcot’s arthropathy in a diabetic foot speciality clinic. Diabetic Medicine 1997; 14: 357–363. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf L, Musgrove M, Bentley J, et al. Prevalence of active Charcot disease in the East Midlands of England. Diabet Med 2018; 35: 1371–1374. [DOI] [PubMed] [Google Scholar]
- 18.Svendsen O, Rabe OO, Winther-Jensen M, et al. How common is the rare charcot foot in patients with diabetes? Diabetes Care 2021; 44: e62–e63. 10.2337/dc20-2590 [DOI] [PubMed] [Google Scholar]
- 19.Eichenholtz S. Charcot joints. Springfield, IL: C. Thomas, 1966, pp. 3–8. [Google Scholar]
- 20.Shibata T, Tada K, Hashizume C. The results of arthrodesis of the ankle for leprotic neuroarthropathy. J Bone Joint Surg 1990; 72: 749–756, http://www.ncbi.nlm.nih.gov/pubmed/2355038 [PubMed] [Google Scholar]
- 21.Sanders L, Frykberg R. Diabetic neuropathic osteoarthropathy: the Charcot foot. In: Frykberg RG.(ed.) The high risk foot in diabetes mellitus, New York, 1991, pp. 297–338. [Google Scholar]
- 22.Brodsky J. The diabetic foot. In: Mann R, Coughlin M. (eds) Surgery of the foot and ankle. St Louis, MO: Mosby, 1999, pp. 1385–1480. [Google Scholar]
- 23.Trepman E, Nihal A, Pinzur MS. Current topics review: Charcot neuroarthropathy of the foot and ankle. Foot Ankle Int 2005; 26: 46–63. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health Care Excellence. Diabetic foot problems: prevention and management. NG19, 2015, p. 1–49, https://www.nice.org.uk/guidance/ng19 (accessed 30 December 2021). [PubMed]
- 25.Petrova N, Edmonds M. Pathogenesis of Charcot neuroarthropathy and acute management. In: Boulton A, Rayman G, Wukich D. (eds) The foot in diabetes. 5th ed.Oxford: John Wiley, 2020, pp. 311–319. [Google Scholar]
- 26.Milne T, Rogers J, Kinnear E, et al. Developing an evidence-based clinical pathway for the assessment, diagnosis and management of acute Charcot neuro-arthropathy: a systematic review. J Foot Ankle Res 2013; 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong DG, Lavery LA. Monitoring healing of acute Charcot’s arthropathy with infrared dermal thermometry. J Rehabil Res Dev 1997; 34: 317–321. [PubMed] [Google Scholar]
- 28.Jeffcoate W. The causes and diagnosis of acute Charcot foot in diabetes. In: Hinchliffe R, Schaper N, Thompson M, et al. (eds) The diabetic foot. London: JP Medical Publishers, 2014, pp. 297–302. [Google Scholar]
- 29.Dallimore S, Puli N, Kim D, et al. Infrared dermal thermometry is highly reliable in the assessment of patients with Charcot neuroarthropathy. J Foot Ankle Res 2020; 13: 56, https://link.springer.com/10.1186/s13047-020-00421-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yammine K, Fathi Y. Ankle ‘sprains’ during sport activities with normal radiographs: incidence of associated bone and tendon injuries on MRI findings and its clinical impact. Foot (Edinb) 2011; 21: 176–178. [DOI] [PubMed] [Google Scholar]
- 31.Haapamaki VV, Kiuru MJ, Koskinen SK. Ankle and foot injuries: analysis of MDCT findings. AJR Am J Roentgenol 2004; 183: 615–622. [DOI] [PubMed] [Google Scholar]
- 32.Olson R, Mendicino S, Rockett M. Isolated medial cuneiform fractures: report of two cases and review of the literature. Foot Ankle Int 2000; 21: 150–154. [DOI] [PubMed] [Google Scholar]
- 33.Gooday C, Game F, Woodburn J, et al. A randomised feasibility study of serial magnetic resonance imaging to reduce treatment times in Charcot neuroarthropathy in people with diabetes (CADOM). J Foot Ankle Res 16(1). 10.1186/s13047-023-00601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergin S, Naidoo P, Williams C. A radiological severity scale to measure the impact of Charcot’s neuroarthropathy: an observational study. J Foot Ankle Res 2020; 13: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bijlani R, Lomasney L, Pinzur M, et al. ‘Examining the Potential Use of a Novel Radiographic Scoring System for Determining Surgical Intervention in Diabetic Charcot Arthropathy’, Foot Ankle Spec 2016; 10: 198–203. [DOI] [PubMed] [Google Scholar]
- 36.Schon LC, Weinfeld SB, Horton GA, et al. Radiographic and clinical classification of acquired midtarsus deformities. Foot Ankle Int 1998; 19: 394–404. [DOI] [PubMed] [Google Scholar]
- 37.Yochum T, Rowe L. Arthritic disorders. In: Yochum T, Rowe L. (eds) Essentials of skeletal radiology. 2nd ed.Baltimore, MD: Williams and Wilkins, 1996, pp. 842–850. [Google Scholar]
- 38.Zampa V, Bargellini I, Rizzo L, et al. Role of dynamic MRI in the follow-up of acute Charcot foot in patients with diabetes mellitus. Skeletal Radiol 2011; 40: 991–999, https://link.springer.com/content/pdf/10.1007%2Fs00256-010-1092-0.pdf [DOI] [PubMed] [Google Scholar]
- 39.Chantelau EA, Grützner G. Is the Eichenholtz classification still valid for the diabetic Charcot foot. Swiss Med Wkly 2014; 144: w13948. [DOI] [PubMed] [Google Scholar]
- 40.Meacock L, Petrova N, Donaldson A, et al. Novel semiquantitative bone marrow oedema score and fracture score for the magnetic resonance imaging assessment of the active charcot foot in diabetes. J Diabetes Res 2017; 2017: 8504137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berli M, Higashigaito K, Götschi T, et al. The ‘Balgrist score’ for evaluation of Charcot foot: a predictive value for duration of off-loading treatment. Skeletal Radiol 2021; 50: 311–320, https://link.springer.com/content/pdf/10.1007/s00256-020-03541-6.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolacchi F, Uccioli L, Masala S, et al. Proton magnetic resonance spectroscopy in the evaluation of patients with acute Charcot neuro-osteoarthropathy. Eur Radiol 2013; 23: 2807–2813. [DOI] [PubMed] [Google Scholar]
- 43.Frykberg R, Mendeszoon E. Management of the diabetic Charcot foot. Diabetes Metab Res Rev 2000; 16(Suppl1): S59–S65. [DOI] [PubMed] [Google Scholar]
- 44.Rogers L, Bevilacqua N. The diagnosis of charcot foot. Clin Podiatr Med Surg 2008; 25: 43–51. [DOI] [PubMed] [Google Scholar]
- 45.McGill M, Molyneaux L, Bolton T, et al. Response of Charcot’s arthropathy to contact casting: assessment by quantitative techniques. Diabetologia 2000; 43: 481–484. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong DG, Lavery LA, Kimbriel HR, et al. Activity patterns of patients with diabetic foot ulceration. Diabetes Care 2003; 26: 2595–2597. [DOI] [PubMed] [Google Scholar]
- 47.Game F, Catlow R, Jones G, et al. Audit of acute Charcot’s disease in the UK: the CDUK study. Diabetologia 2012; 55: 32–35. [DOI] [PubMed] [Google Scholar]
- 48.Pinzur MS. Current concepts review: Charcot arthropathy of the foot and ankle. Foot Ankle Int 2007; 28: 952–959. [DOI] [PubMed] [Google Scholar]
- 49.Jeffcoate W, Game F, Cavanagh P. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 2005; 366: 2058–2061. [DOI] [PubMed] [Google Scholar]
- 50.Bem R, Jirkovská A, Fejfarová V, et al. Intranasal calcitonin in the treatment of acute charcot neuroarthropathy. Diabetes Care 2006; 29: 1392–1394. [DOI] [PubMed] [Google Scholar]
- 51.Jude E, Selby P, Burgess J, et al. Bisphosphonates in the treatment of charcot neuroarthropathy: a double-blind randomised controlled trial. Diabetologia 2001; 44: 2032–2037. [DOI] [PubMed] [Google Scholar]
- 52.Pitocco D, Ruotolo V, Caputo S, et al. Six-month treatment with alendronate in acute charcot neuroarthropathy: a randomized controlled trial. Diabetes Care 2005; 28: 1214–1215. [DOI] [PubMed] [Google Scholar]
- 53.Pakarinen TK, Laine HJ, Mäenpää H, et al. The effect of zoledronic acid on the clinical resolution of charcot neuroarthropathy: a pilot randomized controlled trial. Diabetes Care 2011; 34: 1514–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrova N, Donaldson N, Bates M, et al. Effect of recombinant human parathyroid hormone (1-84) on resolution of active charcot neuro-osteoarthropathy in diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Care 2021; 44: 1613–1621, https://search.ebscohost.com/login.aspx?direct=true&scope=site&site=ehost-live&db=mdc&AN=34088701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Das L, Bhansali A, Prakash M, et al. Effect of methylprednisolone or zoledronic acid on resolution of active charcot neuroarthropathy in diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Care 2019; 42: e185–e186. [DOI] [PubMed] [Google Scholar]
- 56.Busch-Westbroek T, Delpeut K, Balm R, et al. Effect of single dose of RANKL antibody treatment on acute charcot neuro- osteoarthropathy of the foot. Diabetes Care 2018; 41: e21–e22, http://care.diabetesjournals.org/content/diacare/41/3/e21.full.pdf [DOI] [PubMed] [Google Scholar]
- 57.Rastogi A, Bhansali A, Jude EB. Efficacy of medical treatment for Charcot neuroarthropathy: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol 2021; 58: 687–696. [DOI] [PubMed] [Google Scholar]
- 58.Rogers L, Frykberg R, Armstrong D, et al. The Charcot foot in diabetes. Diabetes Care 2011; 34: 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wukich DK, Sung W. Charcot arthropathy of the foot and ankle: modern concepts and management review. J Diabetes Complications 2009; 23: 409–426. [DOI] [PubMed] [Google Scholar]
- 60.Schneekloth B, Lowery N, Wukich D. Charcot neuroarthropathy in patients with diabetes: an updated systematic review of surgical management. J Foot Ankle Surg 2016; 55: 586–590, http://www.ncbi.nlm.nih.gov/pubmed/26810129 [DOI] [PubMed] [Google Scholar]
- 61.Bates M, Petrova N, Edmonds M. How long does it take to progress from cast to shoes in the management of Charcot osteoarthropathy? Diabet Med 2006; 23(Suppl. 2): 27–100. [Google Scholar]
- 62.Stark C, Murray T, Gooday C, et al. 5 year retrospective follow-up of new cases of Charcot neuroarthropathy – a single centre experience. Foot Ankle Surg 2016; 22: 176–180, http://linkinghub.elsevier.com/retrieve/pii/S1268773115001253 [DOI] [PubMed] [Google Scholar]
- 63.Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications 1998; 12: 287–293. [DOI] [PubMed] [Google Scholar]
- 64.Pinzur M, Lio T, Posner M. Treatment of Eichenholtz stage 1 Charcot foot arthropathy with a weight-bearing total contact cast. Foot Ankle Int 2006; 27: 324–329. [DOI] [PubMed] [Google Scholar]
- 65.de Souza LJ. Charcot arthropathy and immobilization in a weight-bearing total contact cast. J Bone Joint Surg Am 2008; 90: 754–759. [DOI] [PubMed] [Google Scholar]
- 66.Moura-Neto A, Fernandes T, Zantut-Wittmann D, et al. Charcot foot: skin temperature as a good clinical parameter for predicting disease outcome. Diabetes Res Clin Pract 2012; 96: e11–e14. [DOI] [PubMed] [Google Scholar]
- 67.Kimmerle R, Chantelau E. Weight-bearing intensity produces Charcot deformity in injuryed neuropathic feet in diabetes. Exp Clin Endocrinol Diabetes 2007; 115: 360–364. [DOI] [PubMed] [Google Scholar]
- 68.Jansen RB, Christensen TM, Bülow J, et al. Markers of local inflammation and bone resorption in the acute diabetic charcot foot. J Diabetes Res 2018; 2018: 5647981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lavery L, Armstrong D, Walker S. Healing rates of diabetic foot ulcers assocaited with midfoot fracutres due to Charcot’s arthropathy. Diabet Med 2012; 14: 46–49. [DOI] [PubMed] [Google Scholar]
- 70.Jansen R, Jørgensen B, Holstein P, et al. Mortality and complications after treatment of acute diabetic Charcot foot. J Diabetes Complications 2018; 32: 1141–1147, https://go.openathens.net/redirector/nhs?url=https%3A%2F%2Fwww.clinicalkey.com%2Fcontent%2FplayBy%2Fdoi%2F%3Fv%3D10.1016%2Fj.jdiacomp.2018.09.013 [DOI] [PubMed] [Google Scholar]
- 71.Bates M, Petrova N, Edmonds M. How long does it take to progress from cast to shoes in the management of Charcot osteoarthropathy? Diabet Med 2006; 23(Suppl. 2): 27-A100. [Google Scholar]
- 72.Chantelau E. The perils of procrastination: effects of early vs. delayed detection and treatment of incipent Charcot fracture. Diabetic Med 2005; 22: 1707–1712. [DOI] [PubMed] [Google Scholar]
- 73.Pakarinen TK, Laine HJ, Mäenpää H, et al. Long-term outcome and quality of life in patients with Charcot foot. Foot Ankle Surg 2009; 15: 187–191. [DOI] [PubMed] [Google Scholar]
- 74.Leung H, Ho Y, Wong W. Charcot foot in a Hong Kong Chinese diabetic population. Hong Kong Med J 2009; 15: 191–195. [PubMed] [Google Scholar]
- 75.Fabrin J, Larsen K, Holstein P. Long-term follow-up in diabetic charcot feet with spontaneous onset. Diabetes Care 2000; 23: 796–800. [DOI] [PubMed] [Google Scholar]
- 76.Nilsen F, Molund M, Hvaal K. High incidence of recurrent ulceration and major amputations associated with Charcot foot. J Foot Ankle Surg 2018; 57: 301–304. [DOI] [PubMed] [Google Scholar]
- 77.Rahman NA, Fauzi AA, Chung TY, et al. Foot ulcers and their association with diabetic Charcot foot complications. Aust J Gen Pract 2020; 49: 48–53. [DOI] [PubMed] [Google Scholar]
- 78.O’Loughlin A, Kellegher E, McCusker C, et al. Diabetic charcot neuroarthropathy: prevalence, demographics and outcome in a regional referral centre. Ir J Med Sci 2016; 186: 151–156, https://link.springer.com/content/pdf/10.1007%2Fs11845-016-1508-5.pdf [DOI] [PubMed] [Google Scholar]
- 79.Fabrin J, Larsen K, Holstein P. Arthrodesis with external fixation in the unstable or misaligned Charcot ankle in patients with diabetes mellitus. Int J Low Extrem Wound 2007; 6: 102–107, http://journals.sagepub.com/doi/pdf/10.1177/1534734607302379 [DOI] [PubMed] [Google Scholar]
- 80.Saltzman C, Hagy M, Zimmerman B, et al. How effective is intensive nonoperative initial treatment of patients with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res 2005; 435: 185–190. [DOI] [PubMed] [Google Scholar]
- 81.Sinacore D, Withrington N. Recognition and management of acute neuropathic (Charcot) arthropathies of the foot and ankle. J Orthop Sport Phys Ther 1999; 29: 736–746, http://www.jospt.org/action/doSearch?AllField=charcot+foot+1999 [DOI] [PubMed] [Google Scholar]
- 82.Doria M, Viadé J, Palomera E, et al. Short-term foot complications in Charcot neuroarthropathy: a retrospective study in tertiary care centres in Spain. Endocrinología, Diabetes Nutrición (English ed) 2018; 65: 479–485. [DOI] [PubMed] [Google Scholar]
- 83.Sohn MW, Stuck RM, Pinzur M, et al. Lower-extremity amputation risk after Charcot arthropathy and diabetic foot ulcer. Diabetes Care 2010; 33: 98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sohn MW, Lee TA, Stuck RM, et al. Mortality risk of charcot arthropathy compared with that of diabetic foot ulcer and diabetes alone. Diabetes Care 2009; 32: 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Baal J, Hubbard R, Game F, et al. Mortality associated with acute charcot foot and neuropathic foot ulceration. Diabetes Care 2010; 33: 1086–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaudhary S, Bhansali A, Rastogi A. Mortality in Asian Indians with Charcot’s neuroarthropathy: a nested cohort prospective study. Acta Diabetol 2019; 56: 1259–1264. [DOI] [PubMed] [Google Scholar]
- 87.Gazis A, Pound N, Macfarlane R, et al. Mortality in patients with diabetic neuropathic osteoarthropathy (Charcot foot). Diabet Med 2004; 21: 1243–1246, https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1464-5491.2004.01215.x [DOI] [PubMed] [Google Scholar]
- 88.Bergis D, Bergis P, Hermanns N, et al. Coronary artery disease as an independent predictor of survival in patients with type 2 diabetes and Charcot neuro-osteoarthropathy. Acta Diabetol 2014; 51: 1041–1048, https://link.springer.com/content/pdf/10.1007%2Fs00592-014-0669-9.pdf [DOI] [PubMed] [Google Scholar]
- 89.Gooday C, Hardeman W, Game F, et al. A qualitative study to understand people’s experiences of living with Charcot neuroarthropathy. Diabet Med 2022; 39: e14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gooday C, Gray K, Game F, et al. Systematic review of techniques to monitor remission of acute Charcot neuroarthropathy in people with diabetes. Diabetes Metab Res Rev 2020; 36: e3328. [DOI] [PubMed] [Google Scholar]
- 91.Boulton A, Jeffcoate W, Jones T, et al. International collaborative research on Charcot’s disease. Lancet 2009; 373: 105–106. [DOI] [PubMed] [Google Scholar]
- 92.Amputation Prevention Centers of America. Is Charcot foot hereditary, 2022, https://www.apcofamerica.com/charcot-foot-hereditary/ (accessed 28 January 2022).
- 93.Department of Health Social Care. The UK rare diseases framework. Policy paper, 2021, https://www.gov.uk/government/publications/uk-rare-diseases-framework/the-uk-rare-diseases-framework (accessed 28 January 2022).