Abstract
ObjectiveS:
This study examined prescription NSAIDs as one of the leading predictors of incident depression and assessed the direction of the association among older cancer survivors with osteoarthritis.
Methods:
This study used a retrospective cohort (N = 14, 992) of older adults with incident cancer (breast, prostate, colorectal cancers, or non-Hodgkin’s lymphoma) and osteoarthritis. We used the longitudinal data from the linked Surveillance, Epidemiology, and End Results -Medicare data for the study period from 2006 through 2016, with a 12-month baseline and 12-month follow-up period. Cumulative NSAIDs days was assessed during the baseline period and incident depression was assessed during the follow-up period. An eXtreme Gradient Boosting (XGBoost) model was built with 10-fold repeated stratified cross-validation and hyperparameter tuning using the training dataset. The final model selected from the training data demonstrated high performance (Accuracy: 0.82, Recall: 0.75, Precision: 0.75) when applied to the test data. SHapley Additive exPlanations (SHAP) was used to interpret the output from the XGBoost model.
Results:
Over 50% of the study cohort had at least one prescption of NSAIDs. Nearly 13% of the cohort were diagnosed with incident depression, with the rates ranging between 7.4% for prostate cancer and 17.0% for colorectal cancer. The highest incident depression rate of 25% was observed at 90 and 120 cumulative NSAIDs days thresholds. Cumulative NSAIDs days was the sixth leading predictor of incident depression among older adults with OA and cancer. Age, education, care fragmentation, polypharmacy, and zip code level poverty were the top 5 predictors of incident depression.
Conclusion:
Overall, 1 in 8 older adults with cancer and OA were diagnosed with incident depression. Cumulative NSAIDs days was the sixth leading predictor with an overall positive association with incident depression. However, the association was complex and varied by the cumulative NSAIDs days.
Keywords: Osteoarthritis, NSAIDs, depression, cancer, inflammation, machine learning, older adults
Introduction
The presence of depression in older adults has received considerable attention because of its high prevalence and potential risk for neurocognitive disorders.1-4 A meta-analysis of the elderly population aged 75 years or older reported that 17.1% suffered from depressive disorders. 5 A systematic review of longitudinal studies of depression among older adults (age ⩾ 65 years) suggested heterogeneous results of protective factors and risk factors for depression. 6 Although many protective and risk factors have been identified, given the failure of antidepressants7,8 and the increased prevalence of treatment-resistant depression,9,10 research has been directed toward understanding the pathophysiology of depression and discovering alternative therapeutic agents.
While the pathophysiology of depression is still evolving, the role of inflammation has been widely recognized. Studies have explained the relationship between inflammation and depression through mechanistic pathways.11,12 Researchers who consider depression as an inflammatory disease use supporting evidence such as high levels of inflammatory biomarkers in patients with depression,13,14 high prevalence of depression in inflammatory chronic conditions,15,16 and cytokine-induced depressive symptoms in individuals. 17 For example, a meta-analysis concluded that several inflammatory biomarkers were positively associated with depression. 18 Individuals with inflammatory conditions such as diabetes or cancer are 3 times as likely to develop depression as the general population. 15 In addition, individuals with overexpressed inflammatory markers were more likely to have treatment-resistant depression. 19 Other researchers may not consider depression an inflammatory condition. 20 Such assertion is supported by observations that only a subset of patients with depression (ranging from 25% to 50%) have increased inflammation. 21 A prospective study reported that inflammation predicted incident depression only in men and not in women, 22 although women experience higher rates of depression than men across all cultures. 23 Increased treatment response in individuals with depression and high levels of inflammatory biomarkers is also used as evidence against the assertion that depression is an inflammatory condition.21,24
Furthermore, there may be a bidirectional relationship between chronic inflammatory diseases and depression through low-grade chronic inflammation.15,16 The high rates of depression found in individuals with osteoarthritis (OA) may be an indication of this bidirectional relationship. 25 Regardless of the mixed results, inflammation plays a significant role in depression. For example, factors such as psychosocial stressors, smoking, poor sleep, and other factors that increase the risk for incident depression are also associated with systemic inflammation. 26
Thus, the association of anti-inflammatory drugs with depression onset and depression treatment has been of particular research interest.27,28 Specifically, the association of non-steroidal anti-inflammatory drugs (NSAIDs), the most commonly prescribed anti-inflammatory drugs, with depression has been studied.29-31 These studies suggest that inhibition of COX-2 through the use of NSAIDs can lead to antidepressant effects and may help reduce depressive symptoms. Although some clinical trials reported higher antidepressant effects with NSAIDs as compared to placebo, others observed no statistically significant difference. In a randomized controlled trial of 2528 older adults (70 years and older), patients did not observe an improvement in their depressive symptoms from the use of NSAIDs over time. 32
NSAIDs were found to be associated with lower depression scores in patients with OA. 33 However, a cross-sectional study on the association of NSAIDs with the prevalence of depression among those with inflammatory conditions did not find an association. 34 A recent study conducted in Sweden reported that aspirin reduced the risk of depression and other psychiatric illnesses in cancer patients, however, the risk was found to be high with other NSAIDs. 35 Thus, the association of NSAIDs with depression incidence is not clear.
Therefore, this study examined the association of NSAIDs with incident depression using a retrospective cohort of older adults with cancer and pre-existing OA. Cancer and OA were selected to study the association of NSAIDs with depression for several reasons. First, NSAIDs is the treatment of choice for OA because of inflammation and pain symptom.36-38 Second, NSAIDs are also used in cancer patients for relieving pain. 39 Third, current research indicate that patients with cancer are among one of the most high-risk population to develop depression. Studies have shown that cancer patients are 3 to 5 times more likely to be diagnosed with major depression.40,41 Fourth, there is limited literature on the incidence of depression among patients with cancer and OA. Fifth, the relationship between OA, cancer, and depression is even more complicated due to the presence of inflammation and the subsequent chronic use of NSAIDs. NSAIDs are known to affect cancer as well as depression due to underlying chronic inflammation.
The objective of this study is to assess whether prescription NSAIDs is one of the leading predictors of incident depression and assess the direction of the association of prescription NSAIDs with incident depression among older adults with OA and incident cancer (breast, prostate, colorectal cancers, and non-Hodgkin’s lymphoma (NHL)) in the United States using interpretable machine learning approaches.
Methods
Study design
A retrospective cohort study design was adopted using data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry linked with fee-for-service Medicare claims with a study period from 2006 through 2016. The study cohort included older adults (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing OA. Cancer patients were identified using the site of cancer from SEER data during the patient identification period. For identifying OA, International Classification of Diseases, 9th and 10th edition Clinical Modification (ICD-9-CM [715.xx], ICD-10-CM [M15.x, M16.x, M17.x, M18.x, M19.x]) diagnosis codes were used. 42 Patients with one inpatient or 2 outpatient claims 30-day apart were included as OA patients. The index date was used to anchor the 12-month baseline and 12-month follow-up period. The index date was defined as the date of the incident cancer diagnosis. The use of NSAIDs was assessed during the baseline period. We retrospectively identified OA 24 to 12 months before the index date. We followed the patients for 12 months after the index date to identify incident depression.
Data source
Data were leveraged from multiple sources. The SEER data provided information on all newly diagnosed cancer patients including date of cancer diagnosis, site of cancer, stage of cancer, tumor size, histology, initial cancer treatment, and other clinical variables. Because of SEER’s high-quality population-based data which is collected through 17 cancer-based registries, 43 it is extensively used by the researcher for studying cancer incidence and their related outcomes. 44 Medicare data provided healthcare claims of the beneficiaries such as inpatient and outpatient visits, hospital stays, screening and evaluation tests, laboratory service, etc., and was linked to SEER by encrypted patient ID. SEER also provides Census Bureau data on income and education at the census tract and zip code level. These files were matched to patient-level data using the zip code of the patient’s residence. For obtaining the county-level information such as the availability of providers and healthcare facilities, the Area health resource files (AHRF) were used. 45 AHRF was linked to SEER-Medicare using the state and county Federal Information Processing Standards (FIPS) code.
Study cohort
The cohort consisted of older adults who have been diagnosed with incident primary cancer between 2008 and 2015 and OA before cancer diagnosis. Additionally, Medicare fee-for-service beneficiaries who were continuously enrolled in part A and B for the study period and Part D for the baseline period and follow-up period were included in the study. Patients diagnosed with cancer through death certificates or autopsy were excluded. Patients who had a history of cancer diagnosis and unknown/missing cancer stage information and Health Maintenance Organizations (HMO) enrollment any time during the study period were excluded (see Appendix 1 for number of individuals retained based on the inclusion and exclusion criteria).
Appendix 1.
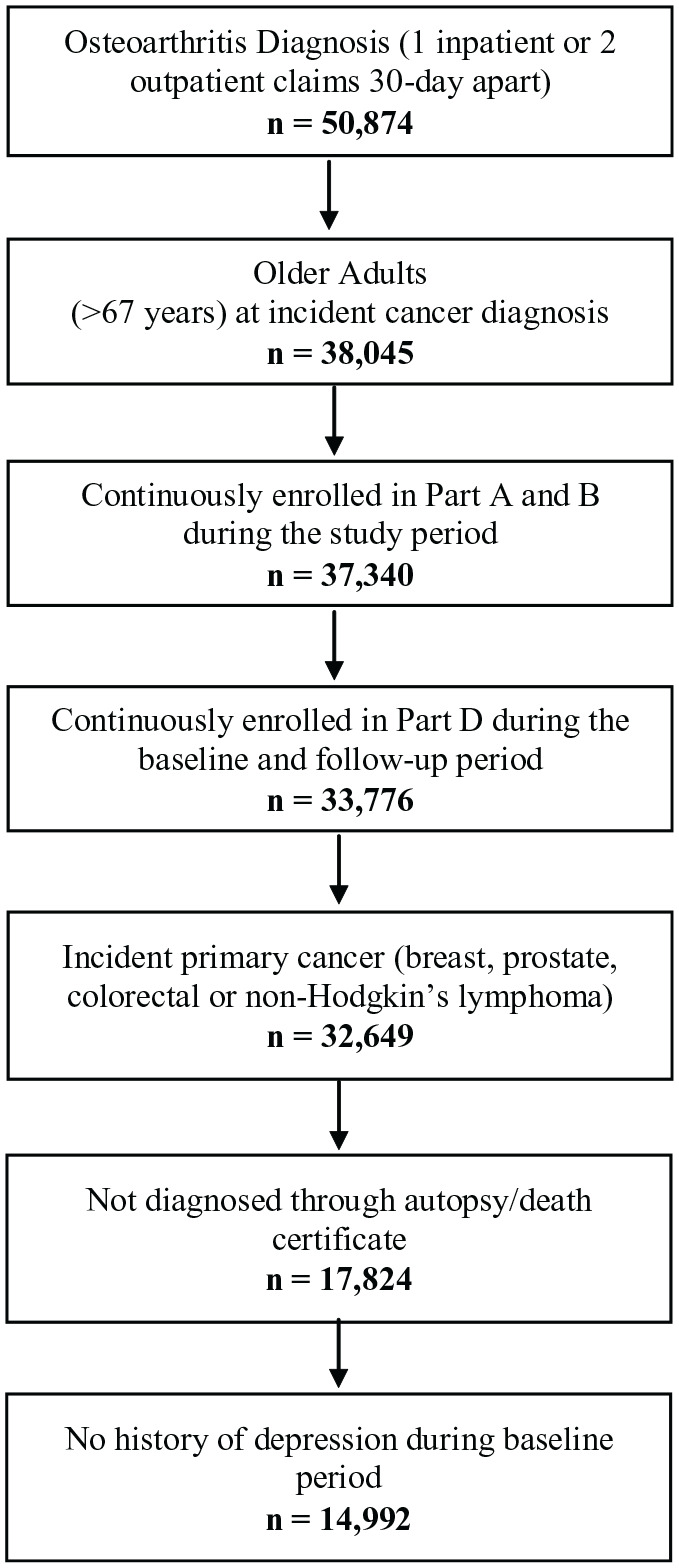
Patient attrition flow chart for fee-for-service medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with cancer and osteoarthritis in linked SEER Cancer Registry and Medicare Claims files, 2006 to 2016.
Target variable—incident depression
A binary incident depression variable was created. Depression was identified using ICD-9-CM (396.xx, 311) and ICD-10-CM (F06.xx, F30.xx, F32.xx, F33.xx, F34.xx, F39) codes. 46 To identify incident depression, we required cancer survivors to be free of diagnosed depression during the baseline period. To ensure the availability of prescription NSAIDs data from Part D claims, incident depression was determined during the years 2008 and 2015.
Features
To include a comprehensive list of features that can influence the association between cumulative NSAIDs use and incident depression, we used the conceptual framework by Park et al. 47 We selected 106 features, and feature reduction was conducted using the permutation feature importance score to select the top 60 features for training the model.
Sex (male, female), race and ethnicity (White, African American, Hispanic/Latino, and other races), age, education level (zip-code level less than or greater than high school diploma), median income (median household income in zip-code level), and region of residence (Northeast, South, Northcentral, and West), supplemental insurance coverage (Medicaid, private, and others), inpatient healthcare use and type of provider visit (eg, primary care provider visit, pain specialist, etc.), and counties with or without screening centers, oncology centers, mental health centers, and psychiatric ERs were included as features.
Medical conditions included indicator variables for any painful condition (arthritis (except OA) and joint pain conditions, headache, migraine, back or neck pain, and neuropathic pain), asthma, cardiac arrhythmias, chronic obstructive pulmonary disease (COPD), dementia, anxiety, diabetes, hyperlipidemia, and hypertension. Furthermore, drug, tobacco and alcohol abuse were also included as features. All conditions were identified using ICD-9-CM and ICD-10-CM codes during the baseline period.
Medications that could influence incident depression were identified from the Part D file using the generic drug name dispensed during the baseline period. These consisted of NSAIDs, opioids, corticosteroids, statins, etc. NSAIDs were measured as cumulative continuous days which was constructed using days supplied and filled date. In this algorithm, prescription fills with more than 15 days gap triggered a reset in the calculation of cumulative NSAIDs days to ensure continuous NSAIDs use. Both selective and non-selective NSAIDs were captured. All other prescription drugs included in the study were measured as binary variables.
The fragmentation of care index (FCI) was calculated from the modified version of a previously validated continuity of care index. 48 FCI is based on the total number of healthcare encounters, the number of different providers, and the proportion of encounters with each of the providers. The value of FCI ranged between 0 and 100, where 0 represents no fragmentation of care and 100 represents all fragmented care. For each interpretation, we divided the FCI by 10 so that each point represents a 10-percentage point increase in FCI, thus the FCI for our study ranged from 0 to 10.
Predictive and interpretable machine learning (ML) analyses
For converting categorical variables into a usable machine learning format, we used the one-hot encoding process in the data preparation step. 49 We split the dataset into training and test data sets where 70% of the data were used for training and 30% of the data were kept as a hold-out for the testing phase. Our study cohort contained an imbalance classification (ie, the number of incident depression cases and non-depression controls were not equal). The imbalanced nature of the data poses a challenge for predictive modeling because most of the ML algorithms for classification were developed for balanced data with an equal number of cases and controls. 50 A comprehensive analysis of methods for addressing the class imbalance data problem suggests several approaches such as data-level, algorithm-level, and a hybrid approach that combines both. 51 The data-level method includes over-sampling the minority class and under-sampling the majority class. The algorithm-level method retains the training data distribution but uses penalties, weights, and decision thresholds. A study comparing different data-level methods to address class imbalance concluded that there is no single best-performing method as it is highly dependent on the data distribution. 52 Therefore, we applied several methods including Synthetic Minority Over-sampling Technique (SMOTE), random under-sampling of controls (i.e., no depression), and random oversampling of cases (i.e., incident depression), weights (scale_pos_weight) and decision thresholds through iterative process. In the preliminary analysis, we observed that random under-sampling combined with random over-sampling and weighting performed best in reducing class imbalance.
We used XGBoost as the predictive model because of its higher prediction accuracy and increased emphasis on the reduction of estimation variance or bias. XGBoost combines decision trees, where one model learns from the prediction error made by the previous model, and thereby makes a stronger model in each subsequent step. 53 XGBoost also controls the overfitting of the data using regularization and gains faster processing speed through parallelization. We used repeated stratified 10-fold cross-validation along with hyperparameter tuning. Cross-validation provides insights into how well the model is realizing the hypothesized relationship between features and its potential performance with unseen data while hyperparameter tuning runs multiple trials in the training data with pre-specified hyperparameters and makes necessary adjustments from the errors found in the previous step. 54 Grid search was used to identify the best performing hyperparameters for the model.
Model performance metrics such as accuracy, precision, recall, F1 score, and area under the receiver operating characteristic (AUROC) were derived from the hold-out test data based on the final model built during the training phase. The final model with the highest precision and recall is used to evaluate the model performance.
We leveraged SHapley Additive exPlanations (SHAP), a novel model-agnostic interpretable ML method to interpret the output from the XGBoost model. SHAP provides global and local interpretations. Despite being a relatively new technique, SHAP has been widely accepted and implemented in predictive modeling including healthcare research. 55 We also created a simplified relationships plot based on SHAP values to identify the top 15 predictors and their overall directionality in their effect on incident depression. However, it is difficult to interpret the marginal contribution of each feature and the presence of heterogeneity from the summary plot, SHAP dependence plots were used for interpretation. 56 Top interactions from the final model were identified using Xgbfir package. 57 SHAP interaction values break down the feature contributions into their main and interaction effects. These values were used to highlight and visualize interactions in data through SHAP interaction plots. We implemented predictive modeling using XGBoost package version 1.5.0 in Python version 3.8.8. 53 We used TreeSHAP for getting SHAP values, and related SHAP features with Python 3.8.8 and the SHAP package (0.40). 55
Results
The analytical cohort consisted of 14,992 older adults with pre-existing OA and an incident cancer diagnosed between 2008 and 2015. A majority of the cohort was non-Hispanic White (72.8%), female (63.3%) with a mean age of 77.43 years (Appendix 2). More than 40% of the cohort resided in the Western region and 45.7% were married. In addition to Medicare insurance, 14.3% had Medicaid insurance.
Appendix 2.
Sample Characteristics for Fee-for-Service Medicare Beneficiaries (Age ⩾ 67 Years at Incident Cancer Diagnosis) With Cancer and Osteoarthritis Linked SEER Cancer Registry and Medicare Claims Files, 2006 to 2016.
| N | % | |
|---|---|---|
| ALL | 14 992 | 100 |
| Cancer type | ||
| Breast cancer | 6674 | 44.5 |
| Prostate cancer | 4189 | 27.9 |
| Colorectal cancer | 2795 | 18.6 |
| Non-Hodgkin’s lymphoma | 1334 | 8.9 |
| Year of cancer diagnosis | ||
| 2008 | 1658 | 11.1 |
| 2009 | 1669 | 11.1 |
| 2010 | 1686 | 11.2 |
| 2011 | 1749 | 11.7 |
| 2012 | 1825 | 12.2 |
| 2013 | 2065 | 13.8 |
| 2014 | 2108 | 14.1 |
| 2015 | 2232 | 14.9 |
| Sex | ||
| Female | 9487 | 63.3 |
| Male | 5505 | 36.7 |
| Race | ||
| White | 10 918 | 72.8 |
| African American | 1736 | 11.6 |
| Hispanic | 1192 | 8.0 |
| Other race | 937 | 6.3 |
| Marital status | ||
| Married | 6847 | 45.7 |
| Not married | 8145 | 54.3 |
| SEER region | ||
| Northeast | 3303 | 22.0 |
| South | 3758 | 25.1 |
| North Central | 1716 | 11.4 |
| West | 6215 | 41.5 |
| Medicaid | ||
| Yes | 2149 | 14.3 |
| No | 12 843 | 85.7 |
| Multimorbidity | ||
| Yes | 13 879 | 92.6 |
| No | 1113 | 7.4 |
| Polypharmacy | ||
| Yes | 5481 | 36.6 |
| No | 9511 | 63.4 |
| County-level mental health centers | ||
| Yes | 7555 | 50.4 |
| No | 7423 | 49.5 |
| County-level psychiatric emergency department | ||
| Yes | 11 050 | 73.7 |
| No | 3928 | 26.2 |
| Continuous variables | Mean | SD |
| Zip-level income in dollars* | ||
| 41 353.4 | 24 775.4 | |
| Zip-level poverty status* | ||
| 12.5 | 10.0 | |
| Zip-level education* | ||
| 24.7 | 16.8 | |
| Age in years* | ||
| 77.4 | 6.9 | |
| Care fragmentation index* | ||
| 0.7 | 0.2 | |
| Cumulative NSAIDs use days* | ||
| 117.1 | 121.5 | |
Based on 14,992 older fee-for-service Medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing Osteoarthritis who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period. See methods section for other inclusion criteria and definitions of study, baseline-, and follow-up periods.
Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; SEER, Surveillance, Epidemiology, and End Results Cancer Registry.
Continuous variables for which mean and standard deviation is reported.
Nearly half of the study cohort (50.6%) had at least 1 prescription for NSAIDs and 27.1% were using it for longer than 60 days. Table 1 presents the prevalence rate of NSAID use by selected characteristics of the study cohort. Significant subgroup differences in the prevalence of NSAID use were observed. The prevalence of NSAID use was highest in breast cancer (54.3%) and lowest in colorectal cancer (45.4%). Females had a higher prevalence of NSAID use as compared to males (52.3% vs 47.8%). African Americans (58.7%) and Hispanics (58.6%) were more likely to be NSAID users compared to Whites (48.6%). A higher percentage of older cancer survivors living in southern regions used NSAIDs compared to patients living in the northeast region (60.1% vs 39.4%). A higher percentage of cancer survivors with polypharmacy used NSAIDs compared to those without polypharmacy (61.4% vs 44.4%).
Table 1.
Sample Characteristics by NSAIDs Use Among Fee-for-Service Medicare Beneficiaries (Age ⩾ 67 Years at Incident Cancer Diagnosis) with Cancer and Osteoarthritis Linked SEER Cancer Registry and Medicare Claims Files, 2006 to 2016.
| NSAIDs use |
No NSAIDs use |
Chi-square | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| ALL | 7591 | 100 | 7401 | 100 | |
| Cancer type | <0.001 | ||||
| Breast cancer | 3622 | 54.3 | 3052 | 45.7 | |
| Prostate cancer | 2033 | 48.5 | 2156 | 51.5 | |
| Colorectal cancer | 1268 | 45.4 | 1527 | 54.6 | |
| Non-Hodgkin’s Lymphoma | 668 | 50.1 | 666 | 49.9 | |
| Year of cancer diagnosis | <0.001 | ||||
| 2008 | 874 | 52.7 | 784 | 47.3 | |
| 2009 | 866 | 51.9 | 803 | 48.1 | |
| 2010 | 871 | 51.7 | 815 | 48.3 | |
| 2011 | 951 | 54.4 | 798 | 45.6 | |
| 2012 | 922 | 50.5 | 903 | 49.5 | |
| 2013 | 996 | 48.2 | 1069 | 51.8 | |
| 2014 | 1069 | 50.7 | 1039 | 49.3 | |
| 2015 | 1042 | 46.7 | 1190 | 53.3 | |
| Sex | <0.001 | ||||
| Female | 4957 | 52.3 | 4530 | 47.7 | |
| Male | 2634 | 47.8 | 2871 | 52.2 | |
| Race | <0.001 | ||||
| White | 5303 | 48.6 | 5615 | 51.4 | |
| African American | 1019 | 58.7 | 717 | 41.3 | |
| Hispanic | 699 | 58.6 | 493 | 41.4 | |
| Other race | 475 | 50.7 | 462 | 49.3 | |
| Marital status | 0.050 | ||||
| Married | 3407 | 49.8 | 3440 | 50.2 | |
| Not married | 4184 | 51.4 | 3961 | 48.6 | |
| SEER region | <0.001 | ||||
| Northeast | 1302 | 39.4 | 2001 | 60.6 | |
| South | 2260 | 60.1 | 1498 | 39.9 | |
| North Central | 863 | 50.3 | 853 | 49.7 | |
| West | 3166 | 50.9 | 3049 | 49.1 | |
| Medicaid | <0.001 | ||||
| Yes | 1390 | 64.7 | 759 | 35.3 | |
| No | 6201 | 48.3 | 6642 | 51.7 | |
| County-level Mental Health Centers | <0.001 | ||||
| Yes | 3673 | 48.6 | 3882 | 51.4 | |
| No | 3913 | 52.7 | 3510 | 47.3 | |
| County-level Psychiatric Emergency Department | <0.001 | ||||
| Yes | 5399 | 48.9 | 5651 | 51.1 | |
| No | 2187 | 55.7 | 1741 | 44.3 | |
| Multimorbidity (⩾2 chronic conditions) | <0.001 | ||||
| Yes | 7123 | 51.3 | 6756 | 48.7 | |
| No | 468 | 42.0 | 645 | 58.0 | |
| Polypharmacy | <0.001 | ||||
| Yes | 3366 | 61.4 | 2115 | 38.6 | |
| No | 4225 | 44.4 | 5286 | 55.6 | |
Based on 14,992 older adults (age ⩾ 67 years at incident cancer diagnosis) with incident primary cancer and OA, who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period; Median income: Zip-level median income from Census Bureau file; Poverty: percent zip-level persons living below poverty from Census Bureau file; Education: zip-level percent persons with less than high school diploma from Census Bureau file.
Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; SEER, Surveillance, Epidemiology, and End Results Cancer Registry.
Overall, 12.8% of the cohort were diagnosed with incident depression. Table 2 summarizes selected group differences of the study cohort by incident depression. The incidence of depression was highest among older adults with colorectal cancer (17.0%) and was lowest among those with prostate cancer (7.4%). The incident depression rates for those with NHL and breast cancers were 16.0% and 13.9%. Females had a significantly higher incidence of depression as compared to males (14.9% vs 9.2%). The highest incidence of depression was observed among Hispanic older adults (14.9%), closely followed by White older adults (13.7%). Patients with polypharmacy had double the incidence of depression as compared to those without polypharmacy (18.0% vs 9.9%).
Table 2.
Sample Characteristics by Incident Depression Among Fee-for-Service Medicare Beneficiaries (Age ⩾ 67 Years at Incident Cancer Diagnosis) With Cancer and Osteoarthritis Linked SEER Cancer Registry and Medicare Claims Files, 2006 to 2016.
| Depression |
No depression |
Chi-square |
|||
|---|---|---|---|---|---|
| N | % | N | % | ||
| ALL | 1926 | 100 | 13 066 | 100 | |
| Cancer type | <0.001 | ||||
| Breast cancer | 929 | 13.9 | 5745 | 86.1 | |
| Prostate cancer | 309 | 7.4 | 3880 | 92.6 | |
| Colorectal cancer | 474 | 17.0 | 2321 | 83.0 | |
| Non-Hodgkin’s lymphoma | 214 | 16.0 | 1120 | 84.0 | |
| Year of cancer diagnosis | 0.811 | ||||
| 2008 | 195 | 11.8 | 1463 | 88.2 | |
| 2009 | 207 | 12.4 | 1462 | 87.6 | |
| 2010 | 215 | 12.8 | 1471 | 87.2 | |
| 2011 | 229 | 13.1 | 1520 | 86.9 | |
| 2012 | 234 | 12.8 | 1591 | 87.2 | |
| 2013 | 262 | 12.7 | 1803 | 87.3 | |
| 2014 | 287 | 13.6 | 1821 | 86.4 | |
| 2015 | 297 | 13.3 | 1935 | 86.7 | |
| Sex | <0.001 | ||||
| Female | 1418 | 14.9 | 8069 | 85.1 | |
| Male | 508 | 9.2 | 4997 | 90.8 | |
| Race | <0.001 | ||||
| White | 1497 | 13.7 | 9421 | 86.3 | |
| African American | 170 | 9.8 | 1566 | 90.2 | |
| Hispanic | 178 | 14.9 | 1014 | 85.1 | |
| Other race | 67 | 7.2 | 870 | 92.8 | |
| Marital status | <0.001 | ||||
| Married | 763 | 11.1 | 6084 | 88.9 | |
| Not married | 1163 | 14.3 | 6982 | 85.7 | |
| SEER region | 0.597 | ||||
| Northeast | 412 | 12.5 | 2891 | 87.5 | |
| South | 506 | 13.5 | 3252 | 86.5 | |
| North Central | 221 | 12.9 | 1495 | 87.1 | |
| West | 787 | 12.7 | 5428 | 87.3 | |
| Medicaid | <0.001 | ||||
| Yes | 378 | 17.6 | 1771 | 82.4 | |
| No | 1548 | 12.1 | 11 295 | 87.9 | |
| Mental Health Centers | 0.018 | ||||
| Yes | 1019 | 13.5 | 6536 | 86.5 | |
| No | 905 | 12.2 | 6518 | 87.8 | |
| Psychiatric Emergency Department | 0.595 | ||||
| Yes | 1429 | 12.9 | 9621 | 87.1 | |
| No | 495 | 12.6 | 3433 | 87.4 | |
| Multimorbidity | <0.001 | ||||
| Yes | 1844 | 13.3 | 12 035 | 86.7 | |
| No | 82 | 7.4 | 1031 | 92.6 | |
| Polypharmacy | <0.001 | ||||
| Yes | 985 | 18.0 | 4496 | 82.0 | |
| No | 941 | 9.9 | 8570 | 90.1 | |
Based on 14,992 older fee-for-service Medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing osteoarthritis who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period. See methods section for other inclusion criteria and definitions of study, baseline-, and follow-up periods.
Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; SEER, Surveillance, Epidemiology, and End Results Cancer Registry.
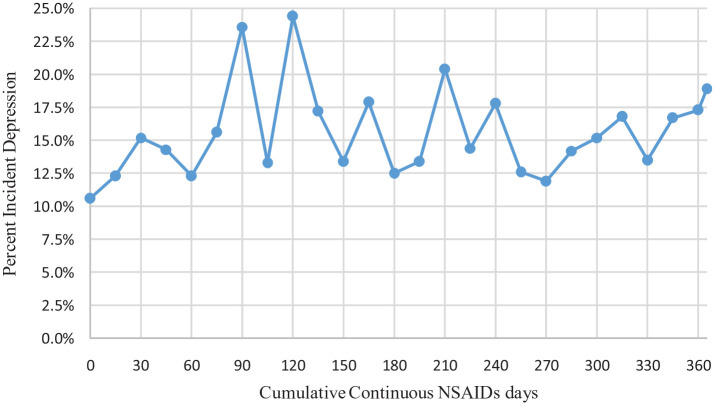
Figure 1 demonstrates incident depression rates with cumulative continuous NSAIDs use days. The plot represents changes in the rates of incident depression with each increase in cumulative NSAIDs days by 15 days. The incident rates of depression ranged from 10% to 25% by cumulative continuous NSAIDs use days. Older cancer survivors using NSAIDs between 0 and 30 days had higher rates of incident depression followed by a decrease between 30 and 60 days. The highest rate of incident depression observed was 25%, at 90 and 120 days thresholds.
Figure 1.
Incident depression rates by cumulative continuous NSAIDs days among fee-for-service medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with cancer and osteoarthritis in linked SEER Cancer Registry and Medicare Claims files, 2006 to 2016.
Based on 14,992 older adults (age ⩾ 67 years at incident cancer diagnosis) with cancer and OA, who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period. Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; SEER, Surveillance, Epidemiology, and End Results Cancer Registry.
The incident depression model built using the XGBoost classifier demonstrated high performance. Model accuracy from the test data was 0.82, precision score was 0.75, recall score was 0.75, F1 score was 0.75, and AUROC score was 0.80.
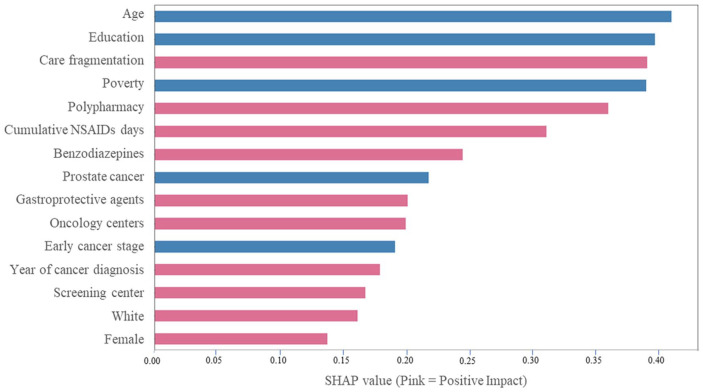
Figure 2 represents the top 15 predictors and their simplified relationships with incident depression using SHAP values. The plot is ranked according to the feature importance and provides information on simplified relationships between these predictors and incident depression, where pink represents a positive impact meaning increase in depression incidence. Age, zip-code level education, care fragmentation, zip-code level poverty, and polypharmacy are the top 5 predictors of incident depression. Cumulative NSAIDs days was the sixth leading predictor of incident depression. Regarding the overall direction of the associations, female sex, care fragmentation, polypharmacy, and cumulative continuous NSAIDs days increased the risk of incident depression. However, older age, zip-code level education, zip-code level poverty, prostate cancer, and early-stage cancer diagnosis predicted a lower risk of depression.
Figure 2.
Simplified relationships between top predictors and incident depression among fee-for-service medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with cancer and osteoarthritis in linked SEER Cancer Registry and Medicare Claims files, 2006 to 2016. The x-axis represents the marginal contribution of a feature to the change in the predicted probability of incident depression diagnosis; pink color represent increase and blue color represents decrease in the incidence of depression.
Based on 14,992 older fee-for-service Medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing OA and who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period.
Care fragmentation = Bice-Boxerman continuity of care index to calculate care fragmentation during the 12-month baseline period (See Methods). Age: age at incident cancer diagnosis; Median income: Zip-level median income from Census Bureau file; Poverty: percent zip-level persons living below poverty from Census Bureau file linkage; Education level: zip-level percent persons with less than high school diploma from Census Bureau file linkage; Oncology and screening centers: area-level information from area health resource file linkage. Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; OA, osteoarthritis; SEER, Surveillance, Epidemiology, and End Results Cancer Registry; SHAP, Shapley Additive exPlanations.
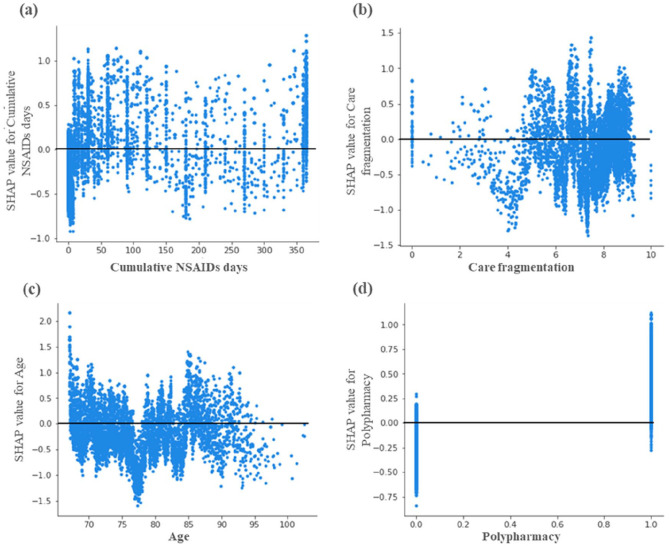
Further examination of the predictors suggested individual-level heterogeneity in the relationships among predictors and the target variables (Figure 3). The individual SHAP dependence plot of cumulative NSAIDs days (Figure 3a) suggested a complex relationship, despite having a positive association. We observed that the relationship between NSAIDs use and incident depression was dependent upon the number of cumulative NSAIDs days.
Figure 3.
Heterogeneity in relationships between predictors and incident depression. SHAP dependence plots among fee-for-service medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with cancer and osteoarthritis in linked SEER Cancer Registry and Medicare Claims files, 2006-2016. The figures represent the SHAP log-odd values by cumulative continuous NSAIDs days (a), care fragmentation (b), age (c), and polypharmacy (d). In all the figures the x-axis represents the numeric value of each feature, and the y-axis represents the marginal contribution of the feature to the change in the predicted probability of incident depression diagnosis for each individual in the dataset. Based on 14 992 older fee-for-service Medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing Osteoarthritis and who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period. Abbreviations: NSAIDs, non-steroidal anti-inflammatory drugs; SEER, Surveillance, Epidemiology, and End Results Cancer Registry; SHAP, Shapley Additive exPlanations.
For care fragmentation Figure 3b, a lower incidence of depression was observed between the FCI values of 3 and 5 and a higher incidence after the FCI value crosses 6. The individual dependence plot for age shows a sharp decrease in the incident depression between the ages of 75 and 80 years. After the age of 85 years, the relationship between incident depression and age was weak and dispersed. Polypharmacy was among the top predictors of incident cancer and shows a clear association with an increased incidence of depression among patients with polypharmacy.
Key feature interactions and their effect on incident depression are listed in Table 3. Gain, F1 score, and weighted F1 score are provided along with their rank in the model. Age and education were the highest observed interaction with the second highest gain (666.4) in the model. Age also interacted with care fragmentation and poverty leading to a gain of 638.0 and 493.2, respectively. Prostate cancer and polypharmacy were among the highest interacting features with an F1 score of 4 and gain ranked sixth.
Table 3.
Key Feature Interactions in the Incident Depression Model Among Fee-for-Service Medicare Beneficiaries (Age ⩾ 67 Years at Incident Cancer Diagnosis) with Cancer and Osteoarthritis Linked SEER Cancer Registry and Medicare Claims Files, 2006 to 2016.
| Feature interactions | Gain | F1 score | Weighted F1 score | Gain rank | F1 score rank | Weighted F1 score rank |
|---|---|---|---|---|---|---|
| Age and education | 666.4 | 96 | 9.9 | 2 | 6 | 8 |
| Care fragmentation and age | 637.9 | 106 | 8.3 | 5 | 4 | 9 |
| Prostate cancer and polypharmacy | 614.5 | 4 | 2.2 | 6 | 84 | 30 |
| Poverty and FCI | 590.6 | 94 | 11.9 | 7 | 8 | 6 |
| FCI and zip-code level education | 577.3 | 93 | 3.5 | 8 | 9 | 19 |
| Age and zip-code level poverty | 493.2 | 95 | 14.8 | 10 | 7 | 5 |
Based on 14,992 older fee-for-service Medicare beneficiaries (age ⩾ 67 years at incident cancer diagnosis) with incident cancer and pre-existing Osteoarthritis who were continuously enrolled in Medicare Part A, and Part B during the study period, and Part D during baseline period and follow-up period. See methods section for other inclusion criteria and definitions of study, baseline-, and follow-up periods.
Abbreviations: FCI, fragmentation care index; SEER, Surveillance, Epidemiology, and End Results Cancer Registry.
Discussion
In this first US population-based study of older cancer survivors with pre-existing OA, 1 in 8 adults were diagnosed with incident depression. There are no studies on OA and cancer population and therefore, we compared our findings among cancer survivors. One published study reported 3.3% of incident major depressive disorder (MDD) among older adults with incident cancer. 58 The incident depression in this study is higher because the study cohort included older adults with cancer and OA, where OA patients are known to be 2 to 3 times more likely to be diagnosed with depression.59,60
The study findings suggest the need for depression screening and treatment. The overall high rates of incident depression among cancer survivors and age being the top predictor of incident depression have implications for depression screening for depression in older adults. Routine depression screening can identify older adults who may be at high risk for depression and may also help prevent the onset of other chronic conditions. 61 Robust evidence suggests that depression in older adults is a risk factor for dementia, 62 and remains an under-recognized target for the prevention of dementia. 63 The U.S. Preventive Services Task Force (USPSTF) currently recommends depression screening among older adults. 64 However, barriers to screening exist.65,66 Thus, the current study findings reinforce the need for depression screening among older adults.
Although the study findings suggest the need for depression treatment among older adults with cancer, the practitioners may need to consider collaborative care for depression instead of fragmented care practices. For example, it has been reported that collaborative depression care is more effective in reducing depressive symptoms than usual care in numerous randomized controlled trials (RCTs). The SMaRT Oncology—2 trial was an implementation of an integrated collaborative care model in cancer patients. 67 The trial reported lower depression scores along with less anxiety, pain, fatigue, and improved quality of life and physical functioning and provided evidence of the utility of collaborative care in multimorbid populations including cancer. 67
This study cohort also fits the definition of multimorbidity with having at least 2 conditions (cancer and OA). 68 There is robust evidence on the challenges to care management of adults with multimorbidity including care fragmentation. 69 In our study, care fragmentation was the third leading predictor of incident depression. Patients with high care fragmentation were more likely to have incident depression in the model. Taken together, the findings suggest that individuals with cancer, OA, and incident depression may need a holistic and patient-centered approach for effective treatment of all 3 conditions. A collaborative care approach, in which care is managed by a team consisting of a case manager, primary care physician, and mental health specialist may be warranted. 70
In this study, a majority (50.6%) of older adults received prescription NSAIDs consistent with published studies.71,72 In the ML model of incident depression, cumulative NSAIDs days was the sixth leading predictor of incident depression with the overall effect of increasing the risk of depression. Moreover, in the bivariate analysis, we observed that older adults without any NSAIDs use had a lower incidence of depression as compared to those with any NSAIDS use. This observation can be interpreted as a positive association between NSAIDs use and incident depression. However, an examination of the individual-level predictions revealed a complex relationship between variations in incident depression predictions and the number of cumulative continuous NSAIDs days. The individual SHAP dependence plot of cumulative NSAIDs days (Figure 3a) indicated that for most cancer survivors with no NSAID use, the risk of incident depression was lower. However, for a small subset of cancer survivors with no NSAID use, a higher risk of incident depression was observed. These findings taken together suggest that no NSAIDs use is beneficial among older cancer survivors. A closer examination of the dependence plot revealed that the association of cumulative NSAID days with incident depression varied by levels of NSAIDs use. For example, between >0 and 50 NSAID days, SHAP values increased sharply suggesting an increased risk of incident depression with short-term NSAIDs use. At higher values, between 150 and 200 NSAIDs days, the relationship between NSAIDs days and incident depression is mixed. At very high levels of NSAIDs days, the risk of incident depression increases sharply, although a subset of cancer survivors with high levels of NSAIDs use (>200 cumulative days) had a lower risk of incident depression. Overall, the relationship of NSAIDs days and incident depression is very complex. Future studies need to further ascertain the heterogeneity of treatment effects using methods such as double machine learning, single learner, and X-learner, and identify thresholds of NSAID days that may reduce the risk of depression. 73 Future prospective research studies are needed to confirm these findings and identify a subgroup of older adults with OA and cancer who may benefit from treatment with NSAIDs. 29
A number of other variables (eg, age, education, care fragmentation, poverty, and polypharmacy) predicted incident depression, consistent with bivariate findings. Polypharmacy was the fourth leading predictor of incident depression and led to an increase in the incidence of depression. Multiple studies and meta-analyses have identified a positive association between the number of medications or polypharmacy and depression.74-76 These findings suggest that medication management therapy (MTM) services may be essential. Under the recent rules from the Centers for Medicare and Medicaid Services (CMS), Medicare Part D insurers are required to offer free MTM services for complex patients such as those with 3 or more chronic conditions. However, the implementation of MTM programs has faced many challenges, 77 despite the potential to improve outcomes. Future studies need to identify MTM strategies that can overcome the barriers and optimize the outcomes for cancer survivors with polypharmacy and multimorbidity.
Our study has several strengths. To date, this is the first study to assess the incidence of depression among older adults with incident cancer and pre-existing OA. We also identified the top predictors and their associations to incident depression using interpretable machine learning methods. Our study assessed the heterogeneity in the effects of NSAIDs treatment with incident depression by measuring NSAIDs use with cumulative continuous NSAIDs days rather than a simple dichotomous measure. Linked cancer-registry and Medicare claims data made it possible to control for cancer-related factors such as the stage of cancer and capture care across all settings. This study also has some limitations. The severity of OA in terms of symptoms, pain, and mobility may also affect depression onset and our study did not have information on the severity of OA or pain. However, we used pain conditions as a proxy. We included only prescription NSAIDs data because data on over-the-counter NSAIDs were not available. We included 4 different cancers, including sex-specific cancers, in our study and combined them within one model. Although we controlled for cancer type in the model, it is possible that the association of NSAIDs to depression incidence may depend on the type of cancer. Although we corrected for class imbalance using data-level (random under-sampling and over-sampling) and algorithm level methods (weighting), these methods may not perfectly adjust for class imbalance.
Conclusion
In this first study among older adults with OA and incident cancer, 1 in 8 adults developed incident depression. Older adults with colorectal cancer had the highest incidence of depression, followed by patients with NHL. Cumulative NSAIDs days was the sixth leading predictor of incident depression but their association remains complex, driven by the length of NSAID use . Several sociodemographic and economic factors were the leading predictors of incident depression. Future studies with prospective study designs are recommended to further confirm the assoication of NSAIDs and depression.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was, in part, supported by the National Institutes of Health (NIH) Agreement No. 1OT2OD032581-01 (Usha Sambamoorthi) and the National Institute on Minority Health and Health Disparities through the Texas Center for Health Disparities (NIMHD), 5U54MD006882-10 (Usha Sambamoorthi). The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH. We thank the IMS personnel for helping us with data acquisition.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NFS and US contributed to the study conception and design, data analysis and interpretation, manuscript writing, and final approval of the manuscript. CS, TL, ND, and AL, contributed to the data interpretation, discussion, revision to the manuscript, and final approval of the manuscript. US aquired data for the study
Data Availability: The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer registry data and the Centers for Medicare & Medicaid Services’ (CMS) Medicare data linkage were utilized in the present study (https://healthcaredelivery.cancer.gov/seermedicare/).
References
- 1.Zenebe Y, Akele B, W/Selassie M, Necho M.Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry. 2021;20:1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiske A, Wetherell JL, Gatz M.Depression in older adults. Annu Rev Clin Psychol. 2009;5:363-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail Z, Elbayoumi H, Fischer CE, et al. Prevalence of depression in patients with mild cognitive impairment: A systematic review and meta-analysis. JAMA Psychiatr. 2017;74:58-67. [DOI] [PubMed] [Google Scholar]
- 4.Sáiz-Vázquez O, Gracia-García P, Ubillos-Landa S, et al. Depression as a risk factor for Alzheimer’s disease: a systematic review of longitudinal meta-analyses. J Clin Med. 2021;10:1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luppa M, Sikorski C, Luck T, et al. Age- and gender-specific prevalence of depression in latest-life – systematic review and meta-analysis. J Affect Disord. 2012;136:212-221. [DOI] [PubMed] [Google Scholar]
- 6.Maier A, Riedel-Heller SG, Pabst A, Luppa M.Risk factors and protective factors of depression in older people 65+. A systematic review. PLoS One. 2021;16:e0251326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rink L, Braun C, Bschor T, Henssler J, Franklin J, Baethge C.Dose increase versus unchanged continuation of antidepressants after initial antidepressant treatment failure in patients with major depressive disorder: a systematic review and meta-analysis of randomized, double-blind trials. J Clin Psychiatry. 2018;79:17r11693. [DOI] [PubMed] [Google Scholar]
- 8.Mallery L, MacLeod T, Allen M, et al. Systematic review and meta-analysis of second-generation antidepressants for the treatment of older adults with depression: questionable benefit and considerations for frailty. BMC Geriatr. 2019;19:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82:29169. [DOI] [PubMed] [Google Scholar]
- 10.Johnston KM, Powell LC, Anderson IM, Szabo S, Cline S.The burden of treatment-resistant depression: a systematic review of the economic and quality of life literature. J Affect Disord. 2019;242:195-210. [DOI] [PubMed] [Google Scholar]
- 11.Slavich GM, Irwin MR.From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnadas R, Cavanagh J.Depression: an inflammatory illness?: Figure 1. J Neurol Neurosurg Psychiatry. 2012;83:495-502. [DOI] [PubMed] [Google Scholar]
- 13.Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD.Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:901-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E.Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front Neurosci. 2018;12:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voinov B, Richie WD, Bailey RK.Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. Prim Care Companion CNS Disord. 2013;15:26226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blume J, Douglas SD, Evans DL.Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anisman H, Hayley S, Turrin N, Merali Z.Cytokines as a stressor: implications for depressive illness. Int J Neuropsychopharmacol. 2002;5:357-373. [DOI] [PubMed] [Google Scholar]
- 18.Howren MB, Lamkin DM, Suls J.Associations of depression with c-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171-186. [DOI] [PubMed] [Google Scholar]
- 19.Strawbridge R, Hodsoll J, Powell TR, et al. Inflammatory profiles of severe treatment-resistant depression. J Affect Disord. 2019;246:42-51. [DOI] [PubMed] [Google Scholar]
- 20.Inserro A. Depression: not an inflammatory disease, but inflammation plays a huge role. Am J Manag Care. [Google Scholar]
- 21.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM.Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. 2019;49:1958-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ernst M, Brähler E, Otten D, et al. Inflammation predicts new onset of depression in men, but not in women within a prospective, representative community cohort. Sci Rep. 2021;11:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derry HM, Padin AC, Kuo JL, Hughes S, Kiecolt-Glaser JK.Sex differences in depression: does inflammation play a role? Curr Psychiatry Rep. 2015;17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. Arch Gen Psychiatry. 2013;70:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veronese N, Stubbs B, Solmi M, et al. Association between lower limb osteoarthritis and incidence of depressive symptoms: data from the osteoarthritis initiative. Age Ageing. 2017;46:470-476. [DOI] [PubMed] [Google Scholar]
- 26.Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler-Forsberg O, N. Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME.Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404-419. [DOI] [PubMed] [Google Scholar]
- 28.Kohler O, Krogh J, Mors O, Eriksen Benros M.Inflammation in depression and the potential for anti-inflammatory treatment. Curr Neuropharmacol. 2016;14:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai S, Guo W, Feng Y, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91:21-32. [DOI] [PubMed] [Google Scholar]
- 30.Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatr. 2014;71:1381-1391. [DOI] [PubMed] [Google Scholar]
- 31.Na KS, Lee KJ, Lee JS, Cho YS, Jung HY.Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:79-85. [DOI] [PubMed] [Google Scholar]
- 32.Fields C, Drye L, Vaidya V, Lyketsos C.Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: findings from a randomized controlled trial. Am J Geriatr Psychiatry. 2012;20:505-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iyengar RL, Gandhi S, Aneja A, et al. NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med. 2013;126:1017.e11-1017.e18. [DOI] [PubMed] [Google Scholar]
- 34.Shaikh NF, Sambamoorthi U.Prescription non-steroidal anti-inflammatory drugs (NSAIDs) and depression among adults with inflammatory chronic conditions in the United States. Psychiatr Q. 2020;91:209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu K, Sjölander A, Lu D, et al. Aspirin and other non-steroidal anti-inflammatory drugs and depression, anxiety, and stress-related disorders following a cancer diagnosis: a nationwide register-based cohort study. BMC Med. 2020;18:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidelines for Osteoarthritis Treatments. Arthritis Foundation. 2019. Accessed February 11, 2022. https://www.arthritis.org/diseases/more-about/guidelines-for-osteoarthritis-treatments
- 38.Buelt A, Narducci DM.Osteoarthritis Management: updated guidelines from the American College of Rheumatology and Arthritis Foundation. Am Fam Physician. 2021;103:120-121. [PubMed] [Google Scholar]
- 39.Prommer EE.Pharmacological management of cancer-related pain. Cancer Control. 2015;22:412-425. [DOI] [PubMed] [Google Scholar]
- 40.Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. [DOI] [PubMed] [Google Scholar]
- 41.Linden W, Vodermaier A, MacKenzie R, Greig D.Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343-351. [DOI] [PubMed] [Google Scholar]
- 42.Shrestha S, Dave AJ, Losina E, Katz JN.Diagnostic accuracy of administrative data algorithms in the diagnosis of osteoarthritis: a systematic review. BMC Med Inform Decis Mak. 2016;16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SEER-Medicare Linked Database | Division of Cancer Control and Population Sciences. National Cancer Institute. 2022. Accessed March 30, 2022. https://healthcaredelivery.cancer.gov/seermedicare/ [Google Scholar]
- 44.Enewold L, Parsons H, Zhao L, et al. Updated overview of the SEER-Medicare data: Enhanced Content and Applications. J Natl Cancer Inst Monographs. 2019;2020:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Area Health Resources Files. Health Resources and Services Administration. 2022. Accessed March 30, 2022. https://data.hrsa.gov/topics/health-workforce/ahrf
- 46.Fiest KM, Jette N, Quan H, et al. Systematic review and assessment of validated case definitions for depression in administrative data. BMC Psychiatry. 2014;14:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park K. Park’s Textbook of Preventive and Social Medicine-Banarsidas Bhanot | Ayurveda | Medicine. 2015. Accessed April 21, 2018. https://www.scribd.com/document/326461178/K-Park-Park-s-Textbook-of-Preventive-and-Social-Medicine-Banarsidas-Bhanot-2015-1
- 48.Liu S, Yeung PC.Measuring fragmentation of ambulatory care in a tripartite healthcare system. BMC Health Serv Res. 2013;13:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Towards Data Science. Workflow of a Machine Learning project | by Ayush Pant | Towards Data Science. 2019. Accessed March 30, 2022. https://towardsdatascience.com/workflow-of-a-machine-learning-project-ec1dba419b94
- 50.Kaur H, Pannu HS, Malhi AK.A systematic review on imbalanced data challenges in machine learning. ACM Comput Surv. 2020;52:1-36. [Google Scholar]
- 51.Johnson JM, Khoshgoftaar TM.Survey on deep learning with class imbalance. J Big Data. 2019;6:27. [Google Scholar]
- 52.Van Hulse J, Khoshgoftaar TM., Napolitano A. Experimental perspectives on learning from imbalanced data. In: Proceedings of the 24th International Conference on Machine Learning, 2007:935-942. [Google Scholar]
- 53.The Sphinx Developers. XGBoost Documentation — xgboost 1.5.2 documentation. 2021. Accessed February 10, 2022. https://xgboost.readthedocs.io/en/stable/
- 54.Little MA, Varoquaux G, Saeb S, et al. Using and understanding cross-validation strategies. Perspectives on Saeb et al. GigaScience. 2017;6:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lundberg SM, Allen PG, Lee SI.A unified approach to interpreting model predictions. Paper presented at: 31st Conference on Neural Information Processing Systems; December 4–-9, 2017; Long Beach, CA. [Google Scholar]
- 56.Towards Data Science. Explain Your Model with the SHAP Values | by Dr. Dataman | Towards Data Science. 2019. Accessed March 30, 2022. https://towardsdatascience.com/explain-your-model-with-the-shap-values-bc36aac4de3d
- 57.GitHub - limexp/xgbfir: XGBoost Feature Interactions Reshaped. Accessed March 30, 2022. https://github.com/limexp/xgbfir
- 58.Alwhaibi M, Sambamoorthi U, Madhavan S, Bias T, Kelly K, Walkup J.Cancer type and risk of newly diagnosed depression among elderly medicare beneficiaries with incident breast, colorectal, and prostate cancers. J Natl Compr Canc Netw. 2017;15:46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambamoorthi U, Shah D, Zhao X.Healthcare burden of depression in adults with arthritis. Expert Rev Pharmacoecon Outcomes Res. 2017;17:53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stubbs B, Aluko Y, Myint PK, Smith TO.Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing. 2016;45:228-235. [DOI] [PubMed] [Google Scholar]
- 61.Vieira ER, Brown E, Raue P.Depression in older adults: screening and referral. J Geriatr Phys Ther. 2014;37:24-30. [DOI] [PubMed] [Google Scholar]
- 62.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D.Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dafsari FS, Jessen F.Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl Psychiatry. 2020;10:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US preventive services task force recommendation statement. JAMA. 2016;315:380-387. [DOI] [PubMed] [Google Scholar]
- 65.Smith R, Meeks S.Screening older adults for depression: barriers across clinical discipline training. Innov Aging. 2019;3:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Colligan E, Cross-Barnet C, Lloyd J, McNeely J.Barriers and facilitators to depression screening in older adults: a qualitative study. Aging Ment Health. 2018;2:509-348. [DOI] [PubMed] [Google Scholar]
- 67.Sharpe M, Walker J, Hansen CH, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet. 2014;384:1099-1108. [DOI] [PubMed] [Google Scholar]
- 68.Johnston MC, Crilly M, Black C, Prescott GJ, Mercer SW.Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29:182-189. [DOI] [PubMed] [Google Scholar]
- 69.Pearson-Stuttard J, Ezzati M, Gregg EW.Multimorbidity—a defining challenge for health systems. Lancet Public Health. 2019;4:e599-e600. [DOI] [PubMed] [Google Scholar]
- 70.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. New Engl J Med. 2010;363:2611-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marcum ZA, Hanlon JT.Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care. 2010;18:24. [PMC free article] [PubMed] [Google Scholar]
- 72.Davis JS, Lee HY, Kim J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart. 2017;4:e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ling YM, Upadhyaya P, Chen LM, Jiang X, Kim Y.Emulate randomized clinical trials using heterogeneous treatment effect estimation for personalized treatments: methodology review and benchmark. J Biomed Inform. 2023;137:104256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ersoy S, Engin VS.Risk factors for polypharmacy in older adults in a primary care setting: a cross-sectional study. Clin Interv Aging. 2018;13:2003-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ho RCM, Fu EHY, Chua ANC, Cheak AAC, Mak A. Clinical and psychosocial factors associated with depression and anxiety in Singaporean patients with rheumatoid arthritis. Int J Rheum Dis. 2011;14:37-47. [DOI] [PubMed] [Google Scholar]
- 76.Palapinyo S, Methaneethorn J, Leelakanok N.Association between polypharmacy and depression: a systematic review and meta-analysis. J Pharm Pract Res. 2021;51:280-299. [Google Scholar]
- 77.Ferreri SP, Hughes TD, Snyder ME.Medication therapy management: current challenges. Integr Pharm Res Pract. 2020;9:71-81. [DOI] [PMC free article] [PubMed] [Google Scholar]