Abstract
Introduction/Objectives:
Sleep disorders affect around 50 to 70 million Americans, with chronic insomnia being the most common, especially in the elderly population. With an 11-fold increase in the US office visits due to insomnia, from 0.8 to 9.4 million, between 1993 and 2015, it is imperative to identify the modifiable risk factors. The aim of our study was to examine the association of risk factors and comorbid medical conditions with insomnia in patients 65 years, and older.
Methods:
We performed a retrospective electronic medical record review of the patients aged 65 years and older, who visited our suburban internal medicine office between July 1, 2020 and June 30, 2021. Patients were divided into insomnia group, and the group without insomnia. The associated variables were compared.
Results:
Among 2431 patients, 247 patients (10.2%) had insomnia. Mean ages of the patients in the insomnia group and the group without insomnia were comparable (77 ± 8.1 year vs 76 ± 7.5 year; P = .211). There was a significantly greater frequency of women in the insomnia group compared to the group without insomnia (63.2% vs 55.5%; P = .022). In the insomnia group, there were significantly higher frequencies of association of certain comorbidities compared to the group without insomnia, such as dementia (6.5% vs 3.4%; P = .015), depression (30.8% vs 14.9%; P < 0.001), anxiety disorder (34.4% vs 17.4%; P < .001), atrial fibrillation (19.4% vs 13.4%; P = .01), and chronic pain disorders (32.8% vs 18.9%; P < .001). Logistic regression analysis showed significantly greater odds of insomnia in patients who had depression (OR = 1.860, 95% CI 1.342–2.576; P < .001), anxiety (OR = 1.845, 95% CI 1.342-2.537; P < .001), and chronic pain disorders (OR = 1.901, 95% CI 1.417-2.549; P < .001).
Conclusions:
Female sex, dementia, depression, anxiety, chronic pain disorders, and atrial fibrillation are associated with insomnia in the elderly patients. Presence of depression, anxiety, and chronic pain disorders are associated with greater odds of having insomnia in the elderly patients.
Keywords: insomnia, elderly population, risk factors of insomnia, insomnia in elderly, sleep disorder
Introduction
Sleep disorders affect around 50 to 70 million Americans, with insomnia being the most common. 1 Insomnia can be divided into short term and chronic. The International Classification for Sleep Disorders, Version 3 (ICSD-3) states that in order to be diagnosed with chronic insomnia, one needs to have one of the following issues for a minimum of 3 days a week for at least 3 months: difficulty initiating sleep, difficulty maintaining sleep, waking earlier than desired, resistance to going to bed on appropriate schedule, or difficulty sleeping without a parent or caregiver. 2 About 10% of the US population have been found to have chronic insomnia. 1 Short term insomnia follows the same criteria as chronic insomnia but for less than 3 months. 3
Insomnia has an involved pathophysiology consisting of psychological cognitive arousal, altered circadian rhythm, decreased function of the sleep-wake switch, and homeostatic mechanisms. 4 In patients who are 65 years and older, these factors play a role more commonly than the younger population. For example, it has been illustrated that sleep efficiency decreases after the age of 60 as this population spends less time in REM and slow wave sleep. 5
Chronic insomnia has been linked to many other disorders, whether it be medical, neurological, or mental. There are several non-modifiable factors that have been shown to contribute to insomnia such as genetics and personality traits. 4 Additionally, there has been an association of insomnia identified in several groups, such as military personnel and veterans, those with traumatic brain injury (TBI), depression, anxiety, substance abuse, and cardiovascular disorders.3,4 The increase in the diagnosis of insomnia has led to an 11-fold increase, from 0.8 million to 9.4 million, in the US outpatient office visits from 1993 to 2015. This has led to the risk factors being investigated that have a relationship with the diagnosis of insomnia. 5 Additionally, insomnia is common in the older population with about 30% to 48% having insomnia symptoms when compared to the 12% to 20% incidence in the general population. 5
Guidelines to manage chronic insomnia recommend starting with Cognitive Behavioral Therapy for Insomnia (CBTI).4,5 Subsequent options are usually pharmacologic, where one can use many drugs such as suvorexant, eszopiclone, and zaleplon. 6 However, when graded on outcome and appropriateness of use in the strategy for best patient care, all pharmacologic agents were given the grade of being “weak.” 7 In the attempt to manage insomnia one can prioritize treating comorbid medical conditions that may exhibit a bidirectional relationship with insomnia. 5
There is a lack of agreement among various risk factors and comorbid medical conditions and their associations with insomnia.2,8Managing these risk factors appropriately may lead to better management of insomnia as well as the underlying medical conditions. In this study, our aim was to study the association of risk factors and comorbid medical conditions that were associated with insomnia in the elderly population.
Materials and Methods
Study Design and Setting
Our study was a retrospective non-matched case-control study of the entire population of our patients who were 65 years of age, or older, who visited our suburban internal medicine office between July 1, 2020 and June 30, 2021, which involved a convenience sampling of the existing electronic medical records.
Participants
Patients aged 65 years or older who visited our internal medicine office between July 1, 2020 and June 30, 2021 were included. We excluded patients who were younger than 65 years of age; and patients 65 years of age or older who visited our internal medicine office before July 1, 2020 and after June 30, 2021.
Variables
We collected the following data on each patient: age, sex, race, alcohol use, tobacco use, recreational drug use; comorbid medical conditions, such as hypertension, diabetes mellitus (DM), cerebrovascular accident, seizure disorder, traumatic brain injury, dementia, Parkinson’s disease, depression, bipolar disorder, anxiety disorder, schizophrenia, coronary artery disease (CAD), congestive heart failure (CHF), atrial fibrillation, other cardiac arrhythmia, chronic obstructive pulmonary disease (COPD), asthma, obstructive sleep apnea (OSA), gastroesophageal reflux disorder (GERD), liver cirrhosis, chronic kidney disease (CKD), anemia, cancer, hypothyroidism, hyperthyroidism, osteoarthritis, other rheumatologic diseases, peripheral neuropathy, chronic pain disorders, frail (assessed by Katz Index of risk assessment of activities of daily living, in which a score of 6 was interpreted as independent or not-frail, while a score of 0-5 was interpreted as non-independent or frail), and insomnia. We also collected the data of systolic and diastolic blood pressures, body mass index (BMI), total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and triglyceride level, vitamin B12, vitamin D, estimated glomerular filtration rate (eGFR), hemoglobin, thyroid stimulating hormone (TSH), folate level, aspartate aminotransferase (AST), alanine aminotransferase (AST), mortality; use of medications, such as antihypertensive medication, oral hypoglycemic agent (OHA), statin, antidepressants, benzodiazepines, non-benzodiazepines sleeping medication, anticoagulants, anti-epileptic medications, long-acting beta-agonist (LABA), short-acting beta-agonist (SABA), proton pump inhibitor (PPI), central nervous system stimulants, and histamine receptor antagonist. We recorded the collected data into a Microsoft Excel (2016, Redmond, Washington, USA) spreadsheet.
Data Source and Access
This study was reviewed and approved by the Institutional Review Board of the Cooper University Healthcare (CUHC). Permission was granted to use materials that were collected solely for research study purposes as per the) requirements, and the informed consent waivers were granted by the Institutional Review Board. This study was fully compliant with the ethical standards set forth by the CUHC. All investigators had full access to the data available only in the electronic medical records of the list of patients approved by the medical informatics of the CUHC, who were selected based on the selection criteria of the study.
Bias
To address the potential for unmeasured confounding associated with insomnia, we excluded patients who had known comorbidity that could have been associated with insomnia, such as shift work insomnia, narcolepsy, terminally ill patients under hospice care, patients with periodic limb movement disorder, frequent international travelers experiencing jet lag, and patients with metabolic encephalopathy.
Study Size
The entire population of our 2431 patients who were 65 years of age, or older, who visited our internal medicine office between July 1, 2020 and June 30, 2021.
Statistical Methods
Statistical analysis was computed by using SPSS (Statistical Package for the Social Sciences, version 15.01, IBM, Armonk, New York, USA) software. The patients were divided into 2 groups: first group represented the patients who suffered from insomnia, and the second group consisted of patients who did not have insomnia. For the continuous variables, data distribution was tested by using a test of skewness. Those with a value between 1 and −1 were normally distributed and an independent t-test was used for analysis. Those with skewness that were greater than 1 or less than −1 illustrated that the data was non-parametric and Mann-Whitney U-test was used for the analysis. Categorical variables were analyzed using either chi-square tests or Fisher exact tests. A decision was made between which one based on the expected value of the data. Logistic regression was used to model the outcome of insomnia. Independent variables within the model were determined by using the gradient boosting model through a function that determines the most important data elements that predict insomnia. In this study, significance was defined as a P < .05.
Results
A total of 2431 patients were included in this study. Two hundred forty seven (10.2%) patients had insomnia and 2184 (89.8%) did not have insomnia (Table 1). There was no statistically significant difference in the mean ages between the patients in the insomnia group and the group without insomnia (77 ± 8.1 year vs 76 ± 7.5 year; P = .211; Table 1). There was significantly greater frequency of women in the insomnia group compared to the group without insomnia (63.2% vs 55.5%; P = .022; Table 1). The majority of patients in the insomnia group and in the group without insomnia were of White race (79.4% vs 77.2%), followed by of Black (16.1% vs 18.8%) and other races (4.5% vs 4.0%). There was no significant difference in the race distribution between the 2 groups. Analysis of social factors, such as tobacco use, alcohol use, and recreational drug use, did not show significant differences between the patients with insomnia and patients without insomnia (Table 1). Similarly, there was no statically significant difference in the systolic and diastolic blood pressures, BMI, blood levels of total cholesterol, LDL-C, HDL-C, triglyceride, vitamin B12, vitamin D, hemoglobin, TSH, folate, AST, AST, and eGFR between the 2 groups (Table 1).
Table 1.
Baseline Characteristics and Laboratory Parameters.
| Variable | Variable | Patients with insomnia (n = 247) | Patients without insomnia (n = 2184) | P |
|---|---|---|---|---|
| Age | Years, mean (SD) | 77.3 (8.1) | 76.7 (7.5) | .211 |
| Sex | Male, n (%) | 91(36.8) | 971 (44.5) | .022 |
| Female, n (%) | 156 (63.2) | 1213 (55.5) | ||
| Race | White, n (%) | 196 (79.4) | 1686 (77.2) | .449 |
| Black, n (%) | 40 (16.1) | 411 (18.8) | ||
| Other, n (%) | 11 (4.5) | 87 (4.0) | ||
| Social | Tobacco use, n (%) | 100 (40.5) | 960 (44.0) | .297 |
| Alcohol use, n (%) | 130 (52.6) | 1132 (51.8) | .811 | |
| Recreational drug use, n (%) | 7 (2.8) | 53 (2.4) | .698 | |
| Vitals | Systolic BP (mmHg), mean (SD) | 126 (17) | 129 (36) | .453 |
| Diastolic BP (mmHg), mean (SD) | 74 (9) | 74 (10) | .764 | |
| Weight | BMI (kg/m2), mean (SD) | 27.5 (5.5) | 28.1 (6.0) | .201 |
| Lab values | Total Cholesterol (mg/dL), mean (SD) | 172.2 (43.5) | 169.6 (59.1) | .500 |
| LDL (mg/dL), mean (SD) | 94.9 (34.5) | 91.9 (35.1) | .209 | |
| HDL (mg/dL), mean (SD) | 55.8 (15.9) | 56.8 (41.5) | .704 | |
| TG (mg/dL), mean (SD) | 102.7 (54.1) | 112.0 (64.2) | .006 | |
| Vit B12 (pg/mL), mean (SD) | 753.1 (536.3) | 765.9 (601.7) | .792 | |
| Vit D (ng/mL), mean (SD) | 37.7 (13.2) | 39.4 (17.5) | .204 | |
| eGFR (mL/min/1.73 m2), mean (SD) | 61.6 (15.8) | 62.1 (16.9) | .699 | |
| Hb (g/dL), mean (SD) | 12.9 (1.9) | 12.9 (2.7) | .938 | |
| TSH (mIU/L) (median, 25th-75th) | 2.00 (1.20-3.08) | 2.01 (1.31-3.00) | .639 | |
| Folate (ng/mL) (median, 25th-75th) | 16.8 (12.5-20.0) | 17.0 (11.7-20.0) | .807 | |
| ALT (U/L) (median, 25th-75th) | 17 (13-22) | 17 (13-23) | .389 | |
| AST (U/L) (median, 25th-75th) | 20 (17-25) | 21 (17-25) | .764 |
Abbreviations: n, number of patients; SD, standard deviation; BP, blood pressure; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; Vit B12, Vitamin B12; Vit D, Vitamin D; eGFR, estimated glomerular filtration rate; Hb, Hemoglobin; TSH, thyroid stimulating hormone; ALT, alanine transaminase; AST, aspartate aminotransferase.
We found significantly higher frequencies of association of several co-morbid medical conditions in the group of patients with insomnia compared to the group of patients without insomnia, such as dementia (6.5% vs 3.4%; P = .015), depression (30.8% vs 14.9%; P < .001), anxiety disorder (34.4% vs 17.4%; P < .001), atrial fibrillation (19.4% vs 13.4%; P = .01), and chronic pain disorders (32.8% vs 18.9%; P < .001; Figure 1). There were no statistically significant differences between the 2 groups in the frequencies of the comorbidities, such as hypertension, diabetes mellitus, cerebrovascular accident, seizure disorder, traumatic brain injury, Parkinson’s disease, bipolar disorder, schizophrenia, coronary artery disease, congestive heart failure, other cardiac arrhythmias excluding atrial fibrillation, chronic obstructive pulmonary disease, asthma, obstructive sleep apnea, gastroesophageal reflux disease, liver cirrhosis, chronic kidney disease, anemia, cancer, hypothyroidism, hyperthyroidism, osteoarthritis, other rheumatological diseases, peripheral neuropathy, and frailty; and mortality (Table 2).
Figure 1.
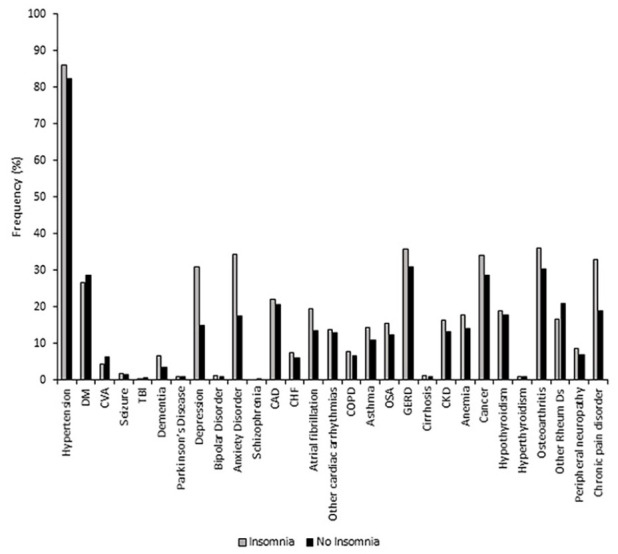
Frequencies of associated medical diagnoses.
Table 2.
Comparative Association of Comorbid Medical Conditions and Mortality.
| Variable | Variable | Patients with insomnia (n = 247) | Patients without insomnia (n = 2184) | P |
|---|---|---|---|---|
| Comorbidities | Hypertension, n (%) | 212 (85.9) | 1797 (82.3) | .619 |
| DM, n (%) | 66 (26.7) | 623 (28.5) | .551 | |
| CVA, n (%) | 88 (4.4) | 137 (6.3) | .388 | |
| Seizure, n (%) | 4 (1.6) | 33 (1.5) | .786 | |
| TBI, n (%) | 1 (0.4) | 11 (0.5) | 1.000 | |
| Dementia, n (%) | 16 (6.5) | 74 (3.4) | .015 | |
| Parkinson’s Ds, n (%) | 2 (0.8) | 19 (0.9) | 1.000 | |
| Depression, n (%) | 76 (30.8) | 326 (14.9) | <.001 | |
| Bipolar Ds, n (%) | 3 (1.2) | 21 (1.0) | .729 | |
| Anxiety Dis, n (%) | 85 (34.4) | 380 (17.4) | <.001 | |
| Schizophrenia, n (%) | 0 (0.0) | 7 (0.3) | 1.000 | |
| CAD, n (%) | 54 (21.9) | 448 (20.5) | .619 | |
| CHF, n (%) | 18 (7.3) | 134 (6.1) | .478 | |
| Atrial fibrillation, n (%) | 48 (19.4) | 293 (13.4) | .010 | |
| Other cardiac arrhythmias, n (%) | 34 (13.8) | 279 (12.8) | .660 | |
| COPD, n (%) | 19 (7.7) | 142 (6.5) | .499 | |
| Asthma, n (%) | 35 (14.2) | 237 (10.9) | .117 | |
| OSA, n (%) | 38 (15.4) | 269 (12.3) | .169 | |
| GERD, n (%) | 88 (35.6) | 675 (30.9) | .130 | |
| Cirrhosis, n (%) | 3 (1.2) | 22 (1.0) | .736 | |
| CKD, n (%) | 40 (16.2) | 287 (13.1) | .183 | |
| Anemia, n (%) | 44 (17.8) | 306 (14.0) | .107 | |
| Cancer, n (%) | 84 (34.0) | 623 (28.5) | .072 | |
| Hypothyroidism, n (%) | 47 (19.0) | 386 (17.7) | .600 | |
| Hyperthyroidism, n (%) | 2 (0.8) | 21 (1.0) | 1.000 | |
| Osteoarthritis, n (%) | 89 (36.0) | 659 (30.2) | .059 | |
| Other Rheum Ds, n (%) | 41 (16.6) | 458 (21.0) | .107 | |
| Peripheral neuropathy, n (%) | 21 (8.5) | 153 (7.0) | .388 | |
| Chronic pain dis, n (%) | 81 (32.8) | 412 (18.9) | <.001 | |
| Frail, n (%) | 13 (5.1) | 118 (5.4) | .694 | |
| Mortality | Dead, n (%) | 16 (6.5) | 95 (4.3) | .129 |
Abbreviations: n, number of patients; DM, diabetes mellitus; CVA, cerebrovascular accident; TBI, traumatic brain injury; Parkinson’s Ds, Parkinson’s disease; Bipolar Ds, bipolar disorder; Anxiety Dis, Anxiety disorder; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disorder; CKD, chronic kidney disease; Other Rheum Ds, other rheumatological disorders; Chronic pain dis, chronic pain disorder.
Analysis of medication usage showed that the group with insomnia had a significantly higher usage of some medications compared to the group without insomnia, such as antidepressants (39.7% vs 21.4%; P < .001), benzodiazepines (24.3% vs 12.2%; P < .001), non-benzodiazepines prescription sleeping medications (21.9% vs 3.2%; P < .001), and anticoagulants (26.7% vs 20.8%; P = .033). There were no significant differences in the use of antihypertensives, oral hypoglycemics, statins, antiepileptics, long-acting beta agonists, short acting beta agonists, central nervous system stimulants, and antihistamines between the 2 groups (Table 3).
Table 3.
Comparison of Medication Use Between the Two Groups.
| Medications | Patients with insomnia (n = 247) | Patients without insomnia (n = 2184) | P |
|---|---|---|---|
| Antihypertensive med, n (%) | 177 (72.0) | 1496 (68.6) | .281 |
| OHA, n (%) | 43 (17.4) | 451 (20.7) | .229 |
| Statin, n (%) | 151 (61.1) | 1374 (62.9) | .578 |
| Antidepressants, n (%) | 98 (39.7) | 468 (21.4) | <.001 |
| Benzodiazepines, n (%) | 60 (24.3) | 266 (12.2) | <.001 |
| Non-benz sleep med, n (%) | 54 (21.9) | 69 (3.2) | <.001 |
| Anticoagulants, n (%) | 66 (26.7) | 455 (20.8) | .033 |
| Antiepileptic med, n (%) | 29 (11.7) | 181 (8.3) | .067 |
| LABA, n (%) | 9 (3.6) | 110 (5.0) | .336 |
| SABA, n (%) | 43 (17.4) | 294 (13.5) | .089 |
| PPI, n (%) | 82 (33.2) | 628 (28.8) | .147 |
| CNS stimulants, n (%) | 6 (2.4) | 33 (1.5) | .279 |
| Antihistamines, n (%) | 56 (22.7) | 502 (23.0) | .909 |
Abbreviatios: n, number of patients; OHA, oral hypoglycemic agent; Non-benz sleep med, non-benzodiazepine sleeping medication; LABA, long acting beta2 agonist; SABA, short acting beta2 agent; PPI, proton pump inhibitor; CNS, central nervous system.
Logistic regression analysis showed significantly greater odds of insomnia in patients who had depression (OR = 1.860, 95% CI 1.342-2.576; P < .001), anxiety (OR = 1.845, 95% CI 1.342–2.537; P < .001), and chronic pain disorders (OR = 1.901, 95% CI 1.417–2.549; P < .001; Table 4).
Table 4.
Influence of Risk Factors and Comorbidities on Insomnia.
| Variable | B | P | Exp(B) | 95% CI for Exp(B) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age (years) | 0.012 | .178 | 1.013 | 0.994 | 1.031 |
| Sex male | −0.122 | .395 | 0.885 | 0.667 | 1.173 |
| Race White | 0.063 | .712 | 1.065 | 0.761 | 1.491 |
| Depression | 0.620 | <.001 | 1.860 | 1.342 | 2.576 |
| Anxiety | 0.613 | <.001 | 1.845 | 1.342 | 2.537 |
| Dementia | 0.526 | .08 | 1.692 | 0.939 | 3.049 |
| Chronic pain disorders | 0.642 | <.001 | 1.901 | 1.417 | 2.549 |
Discussion
We found that in the elderly population, female sex, dementia, depression, anxiety, chronic pain disorder, and atrial fibrillation were associated with insomnia. A meta-analysis of sex differences in insomnia found that females were more at risk for insomnia than males, and that it was progressive with increasing age. 9 During the peri-menopausal phase, menopausal phase, and some years after the menopause, changes in the estrogen and progesterone levels adversely influence, both the initiation and maintenance of sleep which results into insomnia.10–12 However, after the cessation of the menopausal hormonal changes, the pathogenesis of subsequent insomnia is not well understood. In our study of elderly population, we believe that our female patients were way beyond their menopausal phase. Additionally, in the elderly population, the issues that contribute to insomnia occur in both genders, such as mood disorders, medical comorbidities, modifiable lifestyle factors, and aging. 12 Our finding of higher association of insomnia in elderly females align with the available literature.
In our study patients with dementia had a significantly increased risk for having insomnia. A systematic review and meta-analysis evaluated the risk of incident all-cause dementia in individuals with insomnia, and concluded that insomnia was associated with increased risk of all causes of dementia (RR = 1.53 95% CI 1.07–2.18, z = 2.36, P = .02). 13 Another study investigated a longitudinal relationship between insomnia and dementia in the US adults aged 65 years or older. The subjects were followed over a 10-year period to determine if their insomnia scores at that time were associated with dementia. It was concluded that those who had increased severity in insomnia symptoms had a 45% to 58% increased risk of having dementia. 14 One can argue that dementia has a bidirectional relationship with insomnia which can be illustrated by different neurobiological mechanisms. Patients with dementia of Alzheimer’s type, can have abnormal melatonin secretion patterns leading to abnormal sleep and insomnia. 15 This has been confirmed in those with the clinical diagnosis of dementia of Alzheimer’s type and postmortem analysis. 16 This is likely due to the dysfunctional connection between the suprachiasmatic nucleus and pineal gland which leads to a change in the circadian rhythm by disrupting normal melatonin secretion. 17 The role of insomnia in dementia has been well established in patients with Parkinson’s disease. The pathogenesis involves brainstem degeneration causing dementia. The disruptions in the brainstem leads to alterations in the REM phase of sleep which causes insomnia. 18 The cumulative pathogenesis of all types of dementia happens to be brain damage which leads to disruptions of the neural networks in the brain causing insomnia. 19
The bidirectional association of insomnia leading to the development of dementia was demonstrated in a study that observed the effect of sleep deprivation in the brains of mice. The authors found that sleep deprivation for 3 weeks lead to accumulation of Amyloid-beta, a hallmark of Alzheimer’s dementia, in the brain extracellular space. 20 Additionally, looking closer to various subtypes of dementia can provide further information in the possibility of how the interaction between insomnia and dementia may occur and when insomnia presents in its course. In Alzheimer’s dementia, symptoms of insomnia tend to precede cognitive dysfunction. In patients with Parkinson’s disease, insomnia tends to happen more than 10 years later after the initial presentation. 18 This could very well explain why we found no significant difference in the association of Parkinson’s disease in those with and without insomnia. In these patients, insomnia might not had manifested at the time of our data collection. It is fair to state that the relationship between dementia and insomnia is complex. Looking into what caused dementia could help prevent insomnia and offer proper management. Some types of of dementia might precede insomnia and others might happen after. Additionally, certain medication management could influence insomnia in those with certain types of dementia. For example, when treating Alzheimer’s dementia, donepezil can be associated with increased risk of insomnia. 21 In those with insomnia receiving anticholinergic medications, it can worsen Alzheimer’s dementia. 18,22
We found a significantly increased association of depression with insomnia. Our finding aligns with the findings of the study conducted by Bei and associates, in which they reported that symptoms of insomnia were seen in 80% to 90% of patients who had major depressive disorder. 23 In the prospective National Institute of Mental Health Epidemiologic Catchment Area study, 7954 participants were questioned about sleep complaints and psychiatric symptoms using the Diagnostic Interview Schedule, at baseline and 1 year later, and found that 14% of the participants with persistent insomnia had depression compared to less than 1% who had no sleep complaints (P < .001). 24 Another prospective study reported that the chronic sleep issues were associated with increased risk of developing depression. 25 There are several possible mechanisms how insomnia and depression could be inter-related. In individuals with depression, there happens to be an increase in the REM density, and a decrease in the time spent in short wave sleep (SWS). 26 Decreased SWS is also seen in patients diagnosed with insomnia. Additionally, in genetically predisposed individuals, abnormalities in the CLOCK (circadian locomotor output cycles kaput) gene results in aberrations in the suprachiasmatic nuclei pacemakers which leads to insomnia. 26 In patients with depression who had a genetic variant of the CLOCK gene, there was an increased risk of insomnia in their lifetime.26, 27 An overactive hypothalamus has been noted to play a role in both insomnia and depression as well.26,28,29 It is important to note that in many cases, the symptoms of insomnia tend to precede the diagnosis of depression, hence the knowledge of this association may play an important role in the management. For example, in the National Institute of Mental Health Epidemiologic Catchment Area study, the participants with persistent insomnia had a higher chance of developing depression (OR = 39.8; 95% CI: 19.8-80.0) compared to those whose had resolved insomnia (OR = 1.6; 95% CI: 0.5–5.3). 24 In another study, in participants with depression, insomnia was seen to occur before or at the same time of the making the diagnosis of depression. 30 Insomnia is associated with a greater severity of depression and those with insomnia also have a greater risk to relapse with depression. 23 Hence, an early identification of association of insomnia with depression may offer better management opportunities, especially in the elderly population.
In our study, patients with insomnia had a significantly greater association with anxiety. This has been observed in other studies, as well. In a community based study of 772 participants, individuals with insomnia had 17.4 times greater likelihood of having clinically significant anxiety when compared to those without insomnia. 29 In another epidemiologic study, insomnia had been shown to affect about 50% of those with anxiety. 31 How anxiety and insomnia affect each other at the neurobiological level is unclear, however there are multiple proposed mechanisms. One of the mechanisms is thought to be an increased sleep reactivity. 32 Increased sleep reactivity due to stress interrupts sleep by causing difficulty in falling, or staying asleep. It has been found to be linked with anxiety and insomnia. 32 If the brain has neural circuits that make it prone to increased sleep reactivity, there happens to be a higher risk of insomnia and anxiety. In many cases anxiety first comes about at the same time, or after insomnia in those who have both conditions. 30 The knowledge of this association may help the course of management for those with insomnia. Additionally, those with anxiety tend to have a higher amount of sleep threat monitoring, whereby their anxiety overwhelms their thinking about their sleep. An example of this is focusing too much on certain aspects of their sleep such as their heart rate and other bodily functions. Cognitive behavioral therapy has been shown to help suppress these thoughts that could delay sleep and heighten arousal. 33
Our finding of significantly high association of insomnia in patients with chronic pain disorder aligns with the existing scientific literature. It is estimated that the 50% to 80% of those with chronic pain disorder have insomnia. 34 The potential neurobiological mechanism involving the association of insomnia and chronic pain stem from similar pathways in the brain which help us understand how these 2 conditions likely predispose each other, such as the dopaminergic and serotonergic pathways play a role in how pain is tolerated and how sleep is regulated. 35 A potential consequence of this association can explain how an increase in insomnia duration and severity can cause an increase in the amount of perceived pain. 36 One of the mechanisms happens to be an increase in the pro-inflammatory cytokines that stems from 1 having abnormal sleep, which leads to an increased pain perception and decreased pain tolerance. 34 A study that asked questions about insomnia, somatic complaints, and pain to volunteers concluded that those with habitual insomnia had a greater reactivity to pain. It was also seen that those who had a bad night sleep had worsened pain compared to those with a good night sleep. Also, those with more than usual pain in a certain day had worse sleep compared to those with less than usual pain. 37 These findings can help illustrate the bidirectional relationship between sleep and pain. Another study showed that those with chronic pain have worse sleep characterized by delayed sleep onset, increased nighttime awakening, and worse sleep quality compared to those without chronic pain. 38 Knowing this information about the neurobiological mechanisms and the way insomnia and chronic pain can affect each other can lead to more appropriate management of patients who present with both of these conditions. In individuals with chronic pain who have comorbid insomnia, taking steps to improve sleep has been shown to improve short and long term pain. 39 In others with insomnia and comorbid chronic pain, improving pain symptoms through pharmacological or non-pharmacological methods, have been shown to improve insomnia. 36
In our study patients with insomnia had a significantly greater association of atrial fibrillation. A population based retrospective study found that those with insomnia had a higher association of having atrial fibrillation (HR = 1.08, 95% CI 1.01–1.14). 40 Another study looked into the sleep and risk of arrhythmias, and found that those with a healthy sleep pattern had a lower risk of atrial fibrillation (HR comparing extreme categories: 0.71, 95% CI 0.64–0.80) and bradyarrhythmias (HR comparing extreme categories: 0.65, 95% CI 0.54–0.77), but not ventricular arrhythmias. 41 The finding of ventricular arrhythmias not having a relation to insomnia can also help explain why in our study other cardiac arrhythmias, excluding atrial fibrillation, did not show significant association with insomnia.
We also found that use of antidepressants, benzodiazepines, non-benzodiazepines sleeping medications, and anticoagulants were significantly higher in patients with insomnia. We believe this was most likely due to underlying diagnoses for which these groups of medications were prescribed.
Our study had a few limitations. Being a retrospective chart review study, we had to rely solely on the documentation entered in the electronic medical record by the patient care teams, hence certain items, such as the exact time of onset of insomnia in relation to the onset of comorbidities, could not be reliably ascertained. The major strength of our study was having a large sample size from 1 primary care office in which the study subjects received care from a small group of primary care physicians, and were followed by their specific physicians for a long time which allowed them to chronologically document associated comorbidities and the emerging disorders over a long time period.
Conclusion
We conclude that female sex, dementia, depression, anxiety, chronic pain disorder, and atrial fibrillation are associated with insomnia in the elderly patients. Presence of depression, anxiety, and chronic pain disorders are associated with greater odds of having insomnia in the elderly patients. Further studies at different settings are needed to identify similar, or additional risk factors.
Acknowledgments
The authors thank Christine Rickette, RN (study coordinator) for her contribution to this study.
Footnotes
Author Contributions: NM and SR made substantial contributions to the study design, drafting, data acquisition and analysis, and manuscript writing. All authors contributed in data collection and manuscript writing and review. KH analyzed the data. SR contributed in revising the manuscript critically for improved intellectual content, and final approval for the version to be published.
Data Availability: The authors declare that data supporting the findings of this study are available within the article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics: This study was reviewed and approved by the Institutional Review Board of the Cooper University Health Care (CUHC), Camden, New Jersey, USA (IRB:22-083). Permission was granted to use materials that were collected solely for research study purposes as per the Health Insurance Portability and Accountability Act (HIPPA) requirements, and the informed consent waivers were granted by the Institutional Review Board. This study was fully compliant with the ethical standards set forth by the CUHC institutional review board. All investigators had full access to the data available only in the electronic medical records of the list of patients approved by the medical informatics of the CUHC, who were selected based on the selection criteria of the study.
Informed Consent: Not applicable. Being a retrospective chart review study the Institutional Review Board waived the need for informed consent.
ORCID iDs: Roshni Gandhi  https://orcid.org/0000-0002-0517-7118
https://orcid.org/0000-0002-0517-7118
Ian Millstein  https://orcid.org/0000-0002-8253-7412
https://orcid.org/0000-0002-8253-7412
Satyajeet Roy  https://orcid.org/0000-0002-1536-3678
https://orcid.org/0000-0002-1536-3678
References
- 1.Altevogt BM, Colten HR. (eds.). Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. National Academies Press; 2006. [PubMed] [Google Scholar]
- 2.Kay-Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ. 2016;354:i2123. [DOI] [PubMed] [Google Scholar]
- 3.Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5): 547–558. [DOI] [PubMed] [Google Scholar]
- 4.Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020;26(4 Suppl):S76–S84. [DOI] [PubMed] [Google Scholar]
- 5.Patel D, Steinberg J, Patel P. Insomnia in the elderly: a review. J Clin Sleep Med. 2018;14(6):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moloney ME, Ciciurkaite G, Brown RL. The medicalization of sleeplessness: results of U.S. office visit outcomes, 2008-2015. SSM Popul Health. 2019;8:100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto R, Shinzawa M, Isaka Y, Yamakoshi E, Imai E, Ohashi Y, Hishida A; CKD-JAC Investigators. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin J Am Soc Nephrol. 2018; 13(12):1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. [DOI] [PubMed] [Google Scholar]
- 10.Umeda M, Kim Y. Gender differences in the prevalence of chronic pain and leisure time physical activity among US adults: a NHANES study. Int J Environ Res Public Health. 2019;16(6):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krystal AD, Edinger J, Wohlgemuth W, Marsh GR. Sleep in peri-menopausal and post-menopausal women. Sleep Med Rev. 1998;2(4):243–253. [DOI] [PubMed] [Google Scholar]
- 12.Seib C, Anderson D, Lee K. Prevalence and correlates of sleep disturbance in postmenopausal women: the Australian Healthy Aging of Women (HOW) Study. J Womens Health. 2014;23(2):151–158. [DOI] [PubMed] [Google Scholar]
- 13.de Almondes KM, Costa MV, Malloy-Diniz LF, Diniz BS. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiatr Res. 2016;77:109–115. [DOI] [PubMed] [Google Scholar]
- 14.Beydoun HA, Beydoun MA, Weiss J, Hossain S, Huang S, Alemu BT, Zonderman AB. Insomnia as a predictor of diagnosed memory problems: 2006-2016 Health and Retirement Study. Sleep Med. 2021;80:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohashi Y, Okamoto N, Uchida K, Iyo M, Mori N, Morita Y. Daily rhythm of serum melatonin levels and effect of light exposure in patients with dementia of the Alzheimer’s type. Biol Psychiatry. 1999;45(12):1646–1652. [DOI] [PubMed] [Google Scholar]
- 16.Mishima K, Tozawa T, Satoh K, Matsumoto Y, Hishikawa Y, Okawa M. Melatonin secretion rhythm disorders in patients with senile dementia of Alzheimer’s type with disturbed sleep-waking. Biol Psychiatry. 1999;45(4):417–421. [DOI] [PubMed] [Google Scholar]
- 17.Wu YH, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s disease. Sleep Med. 2007;8(6):623–636. [DOI] [PubMed] [Google Scholar]
- 18.Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65–74. [DOI] [PubMed] [Google Scholar]
- 19.Deschenes CL, McCurry SM. Current treatments for sleep disturbances in individuals with dementia. Curr Psychiatry Rep. 2009;11(1):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns A, Rossor M, Hecker J, et al. The effects of donepezil in Alzheimer’s disease - results from a multinational trial. Dement Geriatr Cogn Disord. 1999;10(3):237–244. [DOI] [PubMed] [Google Scholar]
- 22.Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15(10):777–796. [DOI] [PubMed] [Google Scholar]
- 23.Bei B, Asarnow LD, Krystal A, Edinger JD, Buysse DJ, Manber R. Treating insomnia in depression: Insomnia related factors predict long-term depression trajectories. J Consult Clin Psychol. 2018;86(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 25.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157(1):81–88. [DOI] [PubMed] [Google Scholar]
- 26.Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 27.Serretti A, Benedetti F, Mandelli L, Lorenzi C, Pirovano A, Colombo C, Smeraldi E. Genetic dissection of psychopathological symptoms: insomnia in mood disorders and CLOCK gene polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;121B(1):35–38. [DOI] [PubMed] [Google Scholar]
- 28.Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57(2):531–553. [DOI] [PubMed] [Google Scholar]
- 29.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15. [DOI] [PubMed] [Google Scholar]
- 31.Chellappa SL, Aeschbach D. Sleep and anxiety: From mechanisms to interventions. Sleep Med Rev. 2022;61:101583. [DOI] [PubMed] [Google Scholar]
- 32.Kalmbach DA, Anderson JR, Drake CL. The impact of stress on sleep: pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J Sleep Res. 2018;27(6): e12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gosling JA, Batterham P, Ritterband L, et al. Online insomnia treatment and the reduction of anxiety symptoms as a secondary outcome in a randomised controlled trial: the role of cognitive-behavioural factors. Aust N Z J Psychiatry. 2018;52(12):1183–1193. [DOI] [PubMed] [Google Scholar]
- 34.Cheatle MD, Foster S, Pinkett A, Lesneski M, Qu D, Dhingra L. Assessing and managing sleep disturbance in patients with chronic pain. Sleep Med Clin. 2016;11(4):531–541. [DOI] [PubMed] [Google Scholar]
- 35.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostovar-Kermani T, Arnaud D, Almaguer A, et al. Painful sleep: insomnia in patients with chronic pain syndrome and its consequences. Folia Med. 2020;62(4):645–654. [DOI] [PubMed] [Google Scholar]
- 37.Wei Y, Blanken TF, Van Someren EJW. Insomnia really hurts: effect of a bad night’s sleep on pain increases with insomnia severity. Front Psychiatry. 2018;9:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18(2):77–83. [DOI] [PubMed] [Google Scholar]
- 39.Vitiello MV, Zhu W, Von Korff M, et al. Long-term improvements in sleep, pain, depression, and fatigue in older adults with comorbid osteoarthritis pain and insomnia. Sleep. 2022; 45(2):zsab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee HH, Chen YC, Chen JJ, Lo SH, Guo YL, Hu HY. Insomnia and the risk of atrial fibrillation: a population-based cohort study. Acta Cardiol Sin. 2017;33(2):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Zhou T, Ma H, et al. Healthy sleep patterns and risk of incident arrhythmias. J Am Coll Cardiol. 2021;78(12): 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]


