Abstract
Purpose of Review
This focused report aims to discuss and summarize the use of conventional and emerging methods using cardiovascular magnetic resonance (CMR) imaging in cardiomyopathy patients with implanted cardiac devices to identify diffuse and focal inflammation and fibrosis.
Recent Findings
Many cardiomyopathy patients with diffuse and focal myocardial fibrosis have a unique need for cardiac imaging that is complicated by cardiovascular implantable electronic devices (CIEDs). CMR imaging can accurately image myocardial fibrosis and inflammation using T1 mapping and late gadolinium enhancement (LGE) imaging. CMR imaging in CIED patients, however, has been limited due to severe imaging artifacts associated with the devices. The emergence of wideband imaging variants of LGE and T1 mapping techniques can successfully reduce or eliminate CIED artifacts for the evaluation of myocardial substrate in cardiomyopathy patients.
Summary
Wideband imaging variants of LGE and T1 mapping techniques provide new tools for imaging focal and diffuse fibrosis and imaging in cardiomyopathy patients with implanted cardiac devices. These emerging techniques have the potential for great impact in clinical care of such patients as well as clinical research where imaging endpoints are desired.
Keywords: Cardiovascular magnetic resonance, Fibrosis imaging, Inflammation imaging, Cardiac devices, T1 mapping, Late gadolinium enhancement
Introduction
The number of patients with a cardiovascular implantable electronic device (CIED) such as pacemakers or implanted cardioverter devices (ICDs) is increasing worldwide [1, 2•], and so too are the indications for clinically indicated cardiovascular magnetic resonance (CMR) imaging [2•]. In the past, cardiomyopathy patients with CIEDs had limited access to CMR imaging due to the electromagnetic interactions between the strong magnetic field and particularly the components of these devices implanted in juxtaposition to the left or right clavicle [3–6]. Despite the usefulness of CMR in diagnosing cardiac diseases associated with inflammation and fibrosis in most patients [7], the evaluation of myocardial inflammation and fibrosis by CMR has remained limited until recently for cardiomyopathy patients with CIEDs.
Presently, many CIEDs have MRI conditional approval for safe scanning under certain conditions, and CMR can also be performed safely with low risk of complications in many patients with non-conditional devices [2•, 8]. The presence of a cardiac device may result in device-related artifact which can severely limit the diagnostic quality interpretation of the CMR images [6, 9, 10••, 11]. Late gadolinium enhancement (LGE) or parametric mapping sequences (T1, T2, extracellular volume) are widely used in identifying the presence of and quantifying the extent of myocardial inflammation and fibrosis in most patients and are critical to understanding non-ischemic myocardial inflammation [7]. These sequences, however, are severely degraded by image artifacts in patients with CIEDs thus making CMR an underutilized imaging methodology for identifying myocardial inflammation and fibrosis in cardiomyopathy patients. This focused review describes several imaging methods and recent advances that improve the capability of CMR to identify myocardial inflammation and scar in those with CIEDs. Furthermore, the review identifies areas where continued research and advancement is still needed to improve the clinical utility of CMR in cardiomyopathy patients with CIEDs.
Late Gadolinium Enhancement
Conventional Late Gadolinium Enhancement
The most common method for identifying myocardial injury, LGE, requires an extracellular gadolinium contrast agent that diffuses into tissues providing higher contrast in the extracellular volume and accumulates in areas of interstitial fibrosis while quickly washing out of healthy tissue. Areas with retained gadolinium have shorter T1 relaxation times, creating visible contrast with reference to normal, nulled myocardium. This imaging method is particularly useful for myocardial inflammation and focal fibrosis; however, diffuse fibrosis may not appear in conventional LGE imaging where there is no distinct healthy myocardium for reference [12].
In LGE acquisitions, the tissue goes through a single-point inversion and is allowed to recover longitudinal magnetization as modeled by Bloch Equations [13]. Tissues with retained gadolinium (focal reactive or replacement fibrosis) will have more recovered signal than normal tissue and will appear as a brighter signal intensity at the inversion time (TI) selected for image acquisition (Fig. 1) [14]. When the reference tissue is more subtle in contrast to tissue with diffuse fibrosis, the difference in relaxation recovery times, and thus signal intensity, may not be visually apparent. To identify acute myocarditis in patients, Abdel-Aty et al. found LGE had a 71% diagnostic accuracy out of 25 suspected patients and 23 healthy controls [15]. As a cornerstone of CMR imaging, LGE has been widely tested, researched, and optimized in the clinical setting; however, there are deficiencies that still plague the technique such as insufficient differentiation between diffuse and focal fibrosis and CIED-related artifacts. LGE has shown to be superior to other imaging methods when making a diagnostic decision or evaluating prognosis in cardiomyopathy patients, such as those with cardiac sarcoidosis who typically undergo fluorodeoxyglucose positron emission tomographic imaging for evaluation of disease [16, 17].
Fig. 1.
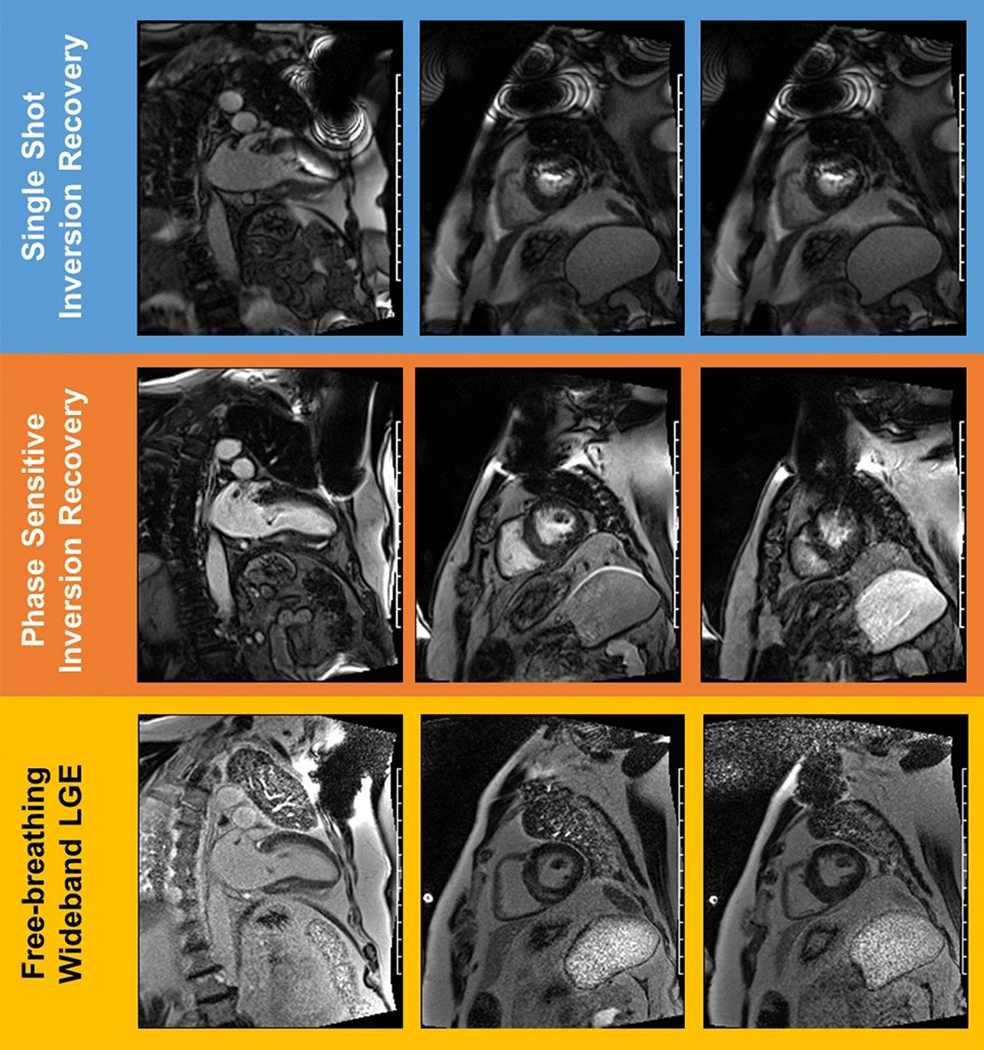
Examples of LGE sequence variants in device patients. Comparison of late gadolinium enhancement images in two chamber (left) and short axis (center, right) views for single-shot inversion recovery multi-slice acquisition (top), phase-sensitive inversion recovery (PSIR) single-slice acquisition (middle), and free-breathing, multi-slice wideband late gadolinium enhancement acquisition (bottom) where the blood pool is represented by white and myocardium are the grayer shades. The off-resonance banding artifacts are particularly apparent in the row of images near the anterior wall and towards the apex of the heart, impeding clinical diagnostic utility. For the PSIR acquisition, breathing artifacts and a smaller device artifact may be noted. These issues are resolved in the wideband acquisition where posterior and septal mid-wall enhancement may be noted in the basal regions
Although vital to most CMR imaging protocols of inflammation and fibrosis, conventional LGE with single-shot LGE or phase-sensitive inversion recovery (PSIR) is strongly susceptible to artifact caused by CIEDs. Ferromagnetic metals used in CIED generator boxes (and to a lesser degree, the leads) may induce metal artifacts in the image which may compromise the diagnostic ability of LGE CMR (Fig. 1). Artifacts resulting from CIEDs may completely or partially obstruct view of inflamed tissue as well as have off-resonant distortion in the image (Fig. 1). In a performance test of conventional LGE, Ranjan et al. reported 49% accuracy of myocardial scar detection in canines with phantom ICDs and radiofrequency ablations [18]. In a clinical CMR study by Bhuva et al., 32% of results were labeled as non-diagnostic using conventional LGE in patients with CIEDs such as permanent pacemakers, implantable loop recorders, and ICDs [10••]. In summary, conventional LGE performs poorly in patients with CIEDs, thus limiting the utility of CMR to evaluate scar and inflammation in cardiomyopathy populations.
Wideband LGE
Although conventional LGE provides high contrast between focal fibrosis and healthy myocardium, issues persist with CIEDs as mentioned previously such as metal artifacts and distortion. Recent innovative sequence development efforts aim to overcome the conventional LGE imaging limitations using a wideband (WB) LGE sequence also referred to as a high-frequency bandwidth LGE [19, 20]. The CIED artifact caused by multi-kilohertz frequency shifting of surrounding tissue outside of conventional LGE bandwidth can be eliminated when the bandwidth frequency for inversion and excitation pulses are increased from 1.1-kHz spectral bandwidth to the 2–6-kHz range to encompass the expected off resonance for CIED [20]. Using this frequency bandwidth shift in patients with CIED image artifacts for two-dimensional slices creates a wideband LGE sequence (WB LGE).
There are several approaches to WB LGE including a free-breathing motion-corrected (MOCO) approach. Bhuva et al. were able to remove artifacts from 19 out of 22 (87%) scans using the WB-MOCO free-breathing sequence [21]. These patients previously had conventional LGE imaging done which resulted in non-diagnostic images. Patients had devices such as implantable loop recorders, permanent pacemakers, and ICDs. In a different study of n = 136 CMR examinations, n = 43 exams (32%) that were classified as non-diagnostic with conventional LGE were of diagnostic quality with the WB LGE imaging method [10••]. In another breath-held WB LGE variant, Singh et al. demonstrated that 32 out of 41 (79%) images in their study using WB LGE were free from previous artifact in the conventional LGE images stemming from medical device artifact [22••]. This was done by applying a wideband offset at + 1500 Hz which resulted in the inversion pulse frequency increasing from 1.5 to 3.8 kHz [22••].
A study by Stevens et al., performed in 16 patients with ICDs with strong off-resonance artifacts on conventional LGE images, found that switching to a WB LGE successfully eliminated the artifact, resulting in 93.75% of scans with diagnostic quality [23]. In these patients, n = 10 had evident myocardial scar, while n = 5 had no evidence of myocardial scar [23]. In another study by Do et al., WB LGE was used with patients with non-conditional CIEDs (both pacemakers (PMs) and ICDs) and the specific absorption rate was kept between 0.07 and 0.1 W/kg [24]. Do et al. demonstrated that using a WB LGE pulse sequence resulted in 87% of 111 patients having no artifact-limiting interpretation of CMR [24]. Similarly, in patients with PMs and ICDs, Hilbert et al. showed comparable results wherein 86% of 28 patients had interpretable results from WB LGE CMR imaging [25]. In addition, Ibrahim et al. developed a wideband inversion recovery LGE sequence to reduce the CIED-induced metal artifact in myocardial fibrosis assessment [26]. By using a WB LGE sequence and directly comparing the results to conventional LGE, the study demonstrated that wideband inversion recovery LGE removes hyperintensity artifacts associated with CIEDs; however, geometric distortions and signal voids due to off-resonance effects still required localized shimming to resolve [26].
Not only does WB LGE present an opportunity to recover image quality for identification of myocardial inflammation and fibrosis in cardiomyopathy patients, but when diagnostic image quality can be achieved in patients with CIEDs, the clinical care and management of patients may be improved. In their effort to optimize detection of myocardial fibrosis in patients with CIEDs, Bhuva et al. utilized WB LGE to determine that CMR provided unexpected diagnoses in 22 patients and changed management in 113 out of 133 patients [10••]. When using WB LGE compared to conventional LGE, 100% of patient results were diagnostic and led to change in clinical management in 40 patients with defibrillators and pacemakers [10••].
These results demonstrate the successful implementation of WB LGE in patients with various CIEDs that cause diagnostic-limiting artifacts with conventional LGE methods and show that WB LGE may have a significant impact not only on the diagnostic quality of CMR scans but also in clinical management of patients with CIEDs after the successful identification of myocardial substrate. It should be noted that WB LGE only eliminates the off-resonant artifact caused by CIEDs that are common with conventional LGE and partial image voids may still exist in the myocardial wall due to lead placements. Motion artifacts, geometric distortions, and differentiating between diffuse fibrosis and healthy myocardium will still need to be explored to have meaningful images for certain patient populations. Finally, while WB LGE may identify focal LGE fibrosis, it may not identify diffuse myocardial fibrosis as with T1 mapping techniques.
T1 Mapping
Modified Look-Locker Inversion T1 Mapping
Limitations of visual qualitative assessments by LGE may be overcome by quantitative T1 mapping of the myocardium with or without gadolinium contrast, thereby allowing a more comprehensive assessment of myocardial inflammation and fibrosis. T1 parametric mapping is a now a cornerstone CMR tissue characterization technique to identify the presence of underlying myocardial pathology and provide quantitative voxel-by-voxel measurements of myocardial T1 relaxation [27–30]. The modified Look-Locker sequence (MOLLI) T1 mapping technique summates three Look-Locker inversion sets across a number of images at varying inversion recovery times into one dataset using a steady-state free procession (SSFP) readout. MOLLI sequences correct for motion artifact by increasing the signal-to-noise ratio (SNR), use a steady-state free precession acquisition, and have an image acquisition period less than 200 ms [31]. In a preliminary study, MOLLI was found to have high precision and reproducibility [12]. Although magnetic transfer (MT) affects the accuracy of MOLLI through underestimation of T1 values [32], MOLLI T1 mapping is useful for identifying myocardial T1 values outside the range of normality due to higher quality imaging [33].
In addition to the assessment of myocardial fibrosis, MOLLI native T1 values display a linear correlation to the percent amount of fibrosis pre-contrast while post-contrast show an inverse linear relationship with collagen volume fraction, which is a good indicator of the degree of fibrosis [34]. Using T1 mapping before and after contrast allows for the calculation of the extracellular volume (ECV) from parametric T1 maps [35]. The relationship between ECV and T1 MOLLI images is also closely correlated with interstitial fibrosis [34, 36–39].
One widely used version of MOLLI completes the image acquisition within a single breath-hold and seventeen heartbeats [40, 41]. A shortened modified Look-Locker sequence (shMOLLI) has been developed to address motion artifacts by decreasing the breath-hold time needed to a shorter breath-hold within only nine heartbeats [42]. Both MOLLI and shMOLLI T1 mapping studies found on average lower T1 values than phantom values in a myocardial range. This discrepancy is more prominent with higher T1 tissue values for both methods [43].
Although used widely in imaging for quality and artifact reduction, MOLLI-based methods have limitations including dependence on T2 values, MT, and inversion efficiency (Table 1) [43–45]. Importantly, conventional MOLLI with a SSFP readout is also susceptible to off-resonance error in T1 estimates which can translate to significant image quality and measurement accuracy issues with cardiomyopathy patients with CIEDs [44], thus limiting its utilization in this population.
Table 1.
Ranking of different sequences on various MRI factors. Cell color and symbols denote the underlying basis of sequence variant (see key at base of table). In general, high accuracy and precision are desired with low dependence on T2, magnetization transfer and heart rate
| Parameter | Low |
|
High | ||
|---|---|---|---|---|---|
| Accuracy | shMOLLI* | MOLLI* | WB/FLASH MOLLI* | SAPPHIRE‡ | SASHA† |
| Precision | SASHA† | SAPPHIRE‡ | shMOLLI* | WB/FLASH MOLLI* | MOLLI* |
| T2 Dependence | SASHA† | SAPPHIRE‡ | WB/FLASH MOLLI* | shMOLLI* | MOLLI* |
| MT Dependence | SASHA† | SAPPHIRE‡ | WB/FLASH MOLLI* | shMOLLI* | MOLLI* |
| Heart Rate Dependence | SAPPHIRE‡ | SASHA† | shMOLLI* | WB/FLASH MOLLI* | MOLLI* |
Abbreviations: FLASH = fast low angle shot; MOLLI = Modified Look Locker Inversion Recovery; MT= magnetization transfer; SAPPHIRE = Saturation Pulse Prepared Heart-Rate Independent Inversion Recovery; SASHA = Saturation recovery single shot-acquisition; shMOLLI = shortened MOLLI; WB = wideband
Inversion-recovery based method (red)
Saturation-recovery based method (blue)
Hybrid method (purple)
Saturation Recovery Single-Shot Acquisition T1 Mapping
A saturation recovery single-shot acquisition (SASHA) is another quantitative T1 mapping technique that is utilized to identify myocardial inflammation and fibrosis. Rather than using traditional inversion recovery methods, SASHA acquires 10 electrocardiogram-triggered single-shot balanced SSFP images in a breath-hold. After one data point is taken without magnetization, SASHA works by sequentially measuring 9 different TIs by pulsing the magnetization down with each heartbeat [46]. No correction is needed as the saturation recovery is not reliant on apparent T1 values thus increasing its accuracy [12].
In comparison studies with MOLLI and shMOLLI, SASHA has been shown to have higher T1 accuracy but lower precision [43]. Unlike MOLLI, SASHA has a similar performance across the range of T1 tissue values [47]. In phantom studies, however, SASHA performed lower in terms of observed image quality compared to Look-Locker inversion sequences like MOLLI and shMOLLI [48]. Saturation recovery methods such as SASHA are prone to noise artifact and may require further refinement [12]. Kellman et al. compared standard SASHA sequences to MOLLI and shMOLLI and demonstrated results agreeable to other studies showing that the saturation recovery method had better overall absolute accuracy to its MOLLI counterparts (Table 1). Overall, SASHA’s lower precision leads to variability of results but this sequence has consistent, accurate T1 values across a variety of studies. To our knowledge, SASHA’s compatibility with imaging cardiomyopathy patients with CIEDs has not been fully explored as an alternative to MOLLI and its derivatives in identifying myocardial inflammation and fibrosis and quantifying the extent of such pathologies.
Other Emerging Sequences
A few emerging CMR sequences for quantifying myocardial fibrosis and inflammation in patients may be particularly useful in cardiomyopathy patients with CIEDs. MOLLI and SASHA sequences can be modified by adding a fast, low-angle shot (FLASH) or gradient echo readouts to the current sequence to improve image quality. FLASH-MOLLI and wideband-FLASH-MOLLI are sequences of interest in which the FLASH is applied at the readout. In a study by Shao et al., patients with ICDs had significant artifact when scanned with FLASH-MOLLI which were subsequently eliminated by the use of the wideband-FLASH-MOLLI sequence [49]. The study validated their results by showing substantial artifacts were found in volunteers with an external ICD placed on the chest using FLASH-MOLLI which were again alleviated when using wideband-FLASH-MOLLI, essentially combining the benefits of quantitative T1 mapping with WB LGE where the wider bandwidth shifts the artifact away from the myocardium [49, 50].
Saturation-pulse prepared heart-rate independent inversion recovery (SAPPHIRE) is another T1 mapping sequence variant. SAPPHIRE is more comparable to SASHA as they both work by using saturation recovery methods. In simulations, the SAPPHIRE T1 mapping sequence was able to eliminate the ghosting artifact due to motion observed in phantom tests with a simulated arrhythmia, although a motion artifact was still observed in patient scans [51]. SAPPHIRE is also able to reduce scan time to 9 heartbeats and thus reduce heartrate dependence [51]. Table 1 shows an overall comparison of T1 mapping sequences regarding accuracy, precision, and dependency on factors. These are important imaging characteristics to balance when assessing myocardial inflammation and fibrosis in any patient, but particularly in cardiomyopathy patients with CIEDs.
Future Considerations
While much research has been done involving MOLLI and its derivative sequences on imaging patients with CIED to identify fibrotic tissue, SASHA and other saturation recovery techniques have yet to be thoroughly explored in this respect. Previous reports note that changes in flip angle and/or magnetization transfer may affect the variability of SASHA sequencing [48]. Wideband modifications to current T1 mapping sequences are emerging and require additional validation and study, particularly among cardiomyopathy patients with CIEDs who need to be evaluated for suspected myocarditis or myocardial fibrosis. Together with WB LGE sequences, WB mapping sequences will be extremely advantageous to CMR imaging of patients CIEDs for identifying both focal and diffuse myocardial fibrosis and inflammation. Prior studies are limited by the small sample sizes of patients and more research is needed to explore optimal CMR sequences to evaluate patients with CIEDs who may have diffuse fibrosis. While WB LGE sequences greatly improve the ability to identify focal fibrosis in patients with CIEDs, the utility of T1 mapping sequences to identify and quantify diffuse fibrosis in this population needs further development. This patient population may need to be serially followed with imaging to monitor progression of disease, and the emerging CMR sequences have the potential to open a whole new avenue of noninvasive care.
Conclusions
Wideband late gadolinium enhancement with CMR is an important diagnostic and analytic technique to identify focal inflammation and fibrosis in cardiomyopathy patients with implanted cardiac devices that inherently cause off-resonance artifacts in conventional LGE methods. T1 mapping techniques are widely used to identify and quantify focal and diffuse myocardial inflammation and fibrosis in CMR; however, the utility of these sequences is currently limited in patients with implanted cardiac devices. Several wideband variants of quantitative T1 mapping techniques are currently in development to address the unmet need for high diagnostic quality imaging to identify and quantify myocardial fibrosis in cardiomyopathy patients with implanted cardiac devices. Saturation recovery methods also show promise but require more research for the discussed populations.
Cardiovascular MRI has the capability to provide tissue-level definition that is often used as surrogate endpoints in clinical trials [52, 53]. Historically, many patients with implanted devices have been excluded from clinical trials with imaging endpoints due to the artifacts and image quality issues discussed here. This leads to the exclusion of a significant portion of patients who happen to be the most vulnerable and who may benefit the most from new therapies. The advances described herein will provide advances in clinical care as well as clinical research.
In conclusion, the recent availability of wideband CMR imaging modifications to late gadolinium enhancement and mapping sequences has greatly improved image quality and diagnostic capability for CMR to identify inflammation and fibrosis in cardiomyopathy patients with implanted cardiac devices and presents an area rich for further development.
Funding
This work is supported by an American Heart Association Collaborative Sciences Award 19CSL0134580004, and funding from the VCU Pauley Heart Center and the VCU Wright Center for Clinical & Translation Research (CCTR) Clinical and Translational Science Award (CTSA) UL1TR002649.
Abbreviations
- CIED
Cardiovascular implantable electronic device
- CMR
Cardiovascular magnetic resonance
- CVF
Collagen volume fraction
- FLASH
Fast, low-angle shot
- ICD
Implantable cardioverter defibrillator
- LGE
Late gadolinium enhancement
- MOCO
Motion corrected
- MOLLI
Modified Look-Locker inversion recovery
- MRI
Magnetic resonance imaging
- MT
Magnetization transfer
- PM
Pacemaker
- PSIR
Phase sensitive inversion recovery
- SAPPHIRE
Saturation-pulse prepared heart-rate independent inversion recovery
- SAR
Specific absorption rate
- SASHA
Saturation recovery single-shot acquisition
- shMOLLI
Shortened modified Look-Locker inversion recovery
- SR
Saturation recovery
- SSFP
Steady-state free precession
- TI
Inversion time
- WB LGE
Wideband late gadolinium enhancement
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors have no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Maass AH, Hemels MEW, Allaart CP. Magnetic resonance imaging in patients with cardiac implantable electronic devices. Neth Heart J. 2018;26:584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhuva AN, Moralee R, Moon JC, Manisty CH. Making MRI available for patients with cardiac implantable electronic devices: growing need and barriers to change. Eur Radiol. 2020;30:1378–84. •This article aims to enable healthcare providers to build MRI services urgently for cardiac device patients, so they may benefit from the same access to MRI as everyone else.
- 3.Baikoussis NG, Apostolakis E, Papakonstantinou NA, Sarantitis I, Dougenis D. Safety of magnetic resonance imaging in patients with implanted cardiac prostheses and metallic cardiovascular electronic devices. Ann Thorac Surg. 2011;91:2006–11. [DOI] [PubMed] [Google Scholar]
- 4.Camacho JC, Moreno CC, Shah AD, Mittal PK, Mengistu A, Lloyd MS, El-Chami MF, Lerakis S, Saindane AM. Safety and quality of 1.5-T MRI in patients with conventional and MRI-conditional cardiac implantable electronic devices after implementation of a standardized protocol. AJR Am J Roentgenol. 2016;207:599–604. [DOI] [PubMed] [Google Scholar]
- 5.Roguin A, Zviman MM, Meininger GR, Rodrigues ER, Dickfeld TM, Bluemke DA, Lardo A, Berger RD, Calkins H, Halperin HR. Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation. 2004;110:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyenhuis JA, Park SM, Kamondetdacha R, Amjad A, Shellock F, Rezai A. MRI and implanted medical devices: basic interactions with an emphasis on heating. IEEE Trans Device Mater Reliab. 2005;5:467–80. [Google Scholar]
- 7.Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 8.Shah AD, Morris MA, Hirsh DS, Warnock M, Huang Y, Mollerus M, Merchant FM, Patel AM, Delurgio DB, Patel AU, et al. Magnetic resonance imaging safety in nonconditional pacemaker and defibrillator recipients: a meta-analysis and systematic review. Heart Rhythm. 2018;15:1001–8. [DOI] [PubMed] [Google Scholar]
- 9.Nitz WR, Oppelt A, Renz W, Manke C, Lenhart M, Link J. On the heating of linear conductive structures as guide wires and catheters in interventional MRI. J Magn Reson Imaging. 2001;13:105–14. [DOI] [PubMed] [Google Scholar]
- 10. Bhuva AN, Kellman P, Graham A, Ramlall M, Boubertakh R, Feuchter P, Hawkins A, Lowe M, Lambiase PD, Sekhri N, et al. Clinical impact of cardiovascular magnetic resonance with optimized myocardial scar detection in patients with cardiac implantable devices. Int J Cardiol. 2019;279:72–8. ••This article demonstrates that the clinical yield from CMR using optimized LGE sequence in patients with cardiac implantable electronic devices is high, hence the need for device dependent LGE imaging strategy using wideband LGE to achieve clinical utility.
- 11.Pollo C, Villemure JG, Vingerhoets F, Ghika J, Maeder P, Meuli R. Magnetic resonance artifact induced by the electrode Activa 3389: an in vitro and in vivo study. Acta Neurochir (Wien). 2004;146:161–4. [DOI] [PubMed] [Google Scholar]
- 12.Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloch F The Principle of Nuclear Induction. Science. 1953;118:425–30. [DOI] [PubMed] [Google Scholar]
- 14.Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. [DOI] [PubMed] [Google Scholar]
- 16.Trivieri MG, Spagnolo P, Birnie D, Liu P, Drake W, Kovacic JC, Baughman R, Fayad ZA, Judson MA. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1878–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aitken M, Chan MV, Urzua Fresno C, Farrell A, Islam N, McInnes MD, Iwanochko M, Balter M, Moayedi Y, Thavendiranathan P, et al. Diagnostic accuracy of cardiac MRI versus FDG PET for cardiac sarcoidosis: a systematic review and meta-analysis. Radiology. 2022:213170. [DOI] [PubMed] [Google Scholar]
- 18.Ranjan R, McGann CJ, Jeong EK, Hong K, Kholmovski EG, Blauer J, Wilson BD, Marrouche NF, Kim D. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace. 2015;17:483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid S, Rapacchi S, Shivkumar K, Plotnik A, Finn JP, Hu P. Modified wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med. 2016;75:572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, Hu P. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhuva A, Ramlall M, Boubertakh R, Knott K, Feuchter P, Sekhri N, Schilling R, Kellman P, Moon J, Manisty C. (2017). Wideband free breathing MOCO LGE changes patient care in patients with implantable cardiac defibrillators. BMJ Publishing Group Ltd and British Cardiovascular Society. [Google Scholar]
- 22. Singh A, Kawaji K, Goyal N, Nazir NT, Beaser A, O’Keefe-Baker V, Addetia K, Tung R, Hu P, Mor-Avi V, et al. Feasibility of cardiac magnetic resonance wideband protocol in patients with implantable cardioverter defibrillators and its utility for defining scar. Am J Cardiol. 2019;123:1329–35. ••This article demonstrates that the assessment of standard LGE is markedly limited by artifact in patients with ICD and the use of wideband LGE significantly improves image quality and can accurately localize myocardial scar before ventricular tachycardia ablation.
- 23.Stevens SM, Tung R, Rashid S, Gima J, Cote S, Pavez G, Khan S, Ennis DB, Finn JP, Boyle N, et al. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter-defibrillators and ventricular tachycardia: late gadolinium enhancement correlation with electroanatomic mapping. Heart Rhythm. 2014;11:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do DH, Eyvazian V, Bayoneta AJ, Hu P, Finn JP, Bradfield JS, Shivkumar K, Boyle NG. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm. 2018;15:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilbert S, Weber A, Nehrke K, Börnert P, Schnackenburg B, Oebel S, Spampinato R, Rogge C, Richter S, Hindricks G, et al. Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. Europace. 2018;20:801–7. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim EH, Runge M, Stojanovska J, Agarwal P, Ghadimi-Mahani M, Attili A, Chenevert T, den Harder C, Bogun F. Optimized cardiac magnetic resonance imaging inversion recovery sequence for metal artifact reduction and accurate myocardial scar assessment in patients with cardiac implantable electronic devices. World J Radiol. 2018;10:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. 2014;16:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, Rolf A, Zitzmann S, Peker E, D’Angelo T, Pathan F. Native T1 and ECV of noninfarcted myocardium and outcome in patients with coronary artery disease. 2018;71:766–78. [DOI] [PubMed] [Google Scholar]
- 29.Nadjiri J, Nieberler H, Hendrich E, Greiser A, Will A, Martinoff S, Hadamitzky M. Performance of native and contrast-enhanced T1 mapping to detect myocardial damage in patients with suspected myocarditis: a head-to-head comparison of different cardiovascular magnetic resonance techniques. 2017;33:539–47. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG. T1 mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. 2013;6:1048–58. [DOI] [PubMed] [Google Scholar]
- 31.Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52:141–6. [DOI] [PubMed] [Google Scholar]
- 32.Robson MD, Piechnik SK, Tunnicliffe EM, Neubauer S. T1 measurements in the human myocardium: the effects of magnetization transfer on the SASHA and MOLLI sequences. Magn Reson Med. 2013;70:664–70. [DOI] [PubMed] [Google Scholar]
- 33.Vassiliou VS, Wassilew K, Cameron D, Heng EL, Nyktari E, Asimakopoulos G, de Souza A, Giri S, Pierce I, Jabbour A, et al. Identification of myocardial diffuse fibrosis by 11 heartbeat MOLLI T. MAGMA. 2018;31:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, Kaye DM, Taylor AJ. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80. [DOI] [PubMed] [Google Scholar]
- 35.Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, Taylor AJ. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibley CT, Noureldin RA, Gai N, Nacif MS, Liu S, Turkbey EB, Mudd JO, van der Geest RJ, Lima JA, Halushka MK, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamilton-Craig CR, Strudwick MW, Galloway GJ. Mapping for myocardial fibrosis by cardiac magnetic resonance relaxometry-a comprehensive technical review. Front Cardiovasc Med. 2016;3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao KY, Yang ZG, Xu HY, Liu X, Zhang Q, Shi K, Jiang L, Xie LJ, Wen LY, Guo YK. Histologic validation of myocardial fibrosis measured by T1 mapping: a systematic review and meta-analysis. J Cardiovasc Magn Reson. 2016;18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacif MS, Turkbey EB, Gai N, Nazarian S, van der Geest RJ, Noureldin RA, Sibley CT, Ugander M, Liu S, Arai AE, et al. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34:1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitts M, Breton E, Kholmovski EG, Dosdall DJ, Vijayakumar S, Hong KP, Ranjan R, Marrouche NF, Axel L, Kim D. Arrhythmia insensitive rapid cardiac T1 mapping pulse sequence. Magn Reson Med. 2013;70:1274–82. [DOI] [PubMed] [Google Scholar]
- 42.Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD. Shortened modified Look-Locker inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson 2010;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson RB, Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology. 2014;272:683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kellman P, Herzka DA, Arai AE, Hansen MS. Influence of Off-resonance in myocardial T1-mapping using SSFP based MOLLI method. J Cardiovasc Magn Reson. 2013;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellman P, Herzka DA, Hansen MS. Adiabatic inversion pulses for myocardial T1 mapping. Magn Reson Med. 2014;71:1428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow K, Flewitt JA, Green JD, Pagano JJ, Friedrich MG, Thompson RB. Saturation recovery single-shot acquisition (SASHA) for myocardial T(1) mapping. Magn Reson Med. 2014;71:2082–95. [DOI] [PubMed] [Google Scholar]
- 47.Heidenreich JF, Weng AM, Donhauser J, Greiser A, Chow K, Nordbeck P, Bley TA, Köstler H. T1- and ECV-mapping in clinical routine at 3 T: differences between MOLLI. ShMOLLI and SASHA BMC Med Imaging. 2019;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira T, Hafyane T, Stikov N, Akdeniz C, Greiser A, Friedrich MG. Comparison of different cardiovascular magnetic resonance sequences for native myocardial T1 mapping at 3T. J Cardiovasc Magn Reson. 2016;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med. 2017;77:1495–504. [DOI] [PubMed] [Google Scholar]
- 50.Shao J, Rapacchi S, Nguyen KL, Hu P. Myocardial T1 mapping at 3.0 tesla using an inversion recovery spoiled gradient echo readout and bloch equation simulation with slice profile correction (BLESSPC) T1 estimation algorithm. J Magn Reson Imaging. 2016;43:414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weingärtner S, Akçakaya M, Basha T, Kissinger KV, Goddu B, Berg S, Manning WJ, Nezafat R. Combined saturation/inversion recovery sequences for improved evaluation of scar and diffuse fibrosis in patients with arrhythmia or heart rate variability. Magn Reson Med. 2014;71:1024–34. [DOI] [PubMed] [Google Scholar]
- 52.Puntmann VO, Valbuena S, Hinojar R, Petersen SE, Greenwood JP, Kramer CM, Kwong RY, McCann GP, Berry C, Nagel E. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: part I-analytical validation and clinical qualification. J Cardiovasc Magn Reson. 2018;20:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ibanez B, Aletras AH, Arai AE, Arheden H, Bax J, Berry C, Bucciarelli-Ducci C, Croisille P, Dall’Armellina E, Dharmakumar R, Eitel I. Cardiac MRI endpoints in myocardial infarction experimental and clinical trials: JACC scientific expert panel. 2019;74:238–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


