Abstract
Background
Currently, there is still controversy about the differential changes in corneal endothelium function and morphology after phacoemulsification between Diabetes Mellitus (DM) and non-Diabetes Mellitus (non-DM) patients. In this study, we aimed to evaluate the influence of phacoemulsification on the corneal endothelium in DM and non-DM patients.
Methods
Databases of PubMed, Embase, Web of Science, and the Cochrane Library were searched for studies published between January 1, 2011 and December 25, 2021. The weighted mean difference and 95% confidence interval were used to estimate the outcomes of statistical analyses performed.
Results
Thirteen studies involving 1744 eyes were included in this meta-analysis. No significant difference was observed in the central corneal thickness (CCT), endothelial cell density (ECD), coefficients of variation (CV), or hexagonal cell percentage (HCP) between the DM and non-DM groups (CCT: P = 0.91; ECD: P = 0.07; CV: P = 0.06; HCP: P = 0.09) preoperatively. The CCT was significantly thicker in the DM group at 1 month (P = 0.003) and 3 months (P = 0.0009) postoperatively, and there was no significant difference at 6 months postoperatively (P = 0.26) than non-DM group. The CV was significantly higher and HCP was significantly lower in the DM group at 1 month (CV:P < 0.0001, HCP: P = 0.002), with no significant difference at 3 months (CV: P = 0.09, HCP: P = 0.36) and 6 months (CV: P = 0.32, HCP: P = 0.36) postoperatively than non-DM group. DM patients had lower ECD than non-DM patients at all postoperative time points (1 month, 3 months: P < 0.00001, 6 months: P < 0.0001).
Conclusions
The influence of phacoemulsification on corneal endothelial damage is greater in diabetic patients. Moreover, the recovery of corneal endothelial function and morphology is delayed in these patients. Clinicians should be more attentive to the corneal health of DM patients when considering phacoemulsification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-023-02924-2.
Keywords: Cornea, Phacoemulsification, Diabetic, Meta-analysis
Background
The global prevalence of diabetes mellitus (DM) is increasing and predicted to rise to 10.2% by 2030 and 10.9% by 2045 [1]. Poor blood glucose control, as well as advanced age, are the main risk factors for cataract development [2]. Cataracts are the main cause of visual impairment in individuals aged ≥ 50 years worldwide, accounting for approximately 45% of blindness cases [3]. The treatment for cataracts is mainly surgery, the most common surgical method being phacoemulsification combined with intraocular lens implantation. Although phacoemulsification is a well-established method with few complications, there is still a risk of damage to the corneal endothelium during the procedure. DM, in turn, is considered a risk factor for increased corneal endothelial damage after cataract surgery [4]. Corneal endothelial cells (CECs) of regular size and hexagonal shape form neatly arranged monolayers [5]. CECs rely on tight junctions and adherens junctions, Na+/K+-ATPase pump activity for paracellular fluid and ion transportation, and form an integral barrier function that plays a key role in regulating corneal hydration and maintaining corneal transparency [6, 7].
Clinically, the following four parameters are mainly used to evaluate the health status of the corneal endothelium: central corneal thickness (CCT), endothelial cell density (ECD), coefficients of variation (CV), and hexagonal cell percentage (HCP). The CCT is used as an index to measure corneal endothelial function. The extent of corneal swelling can be estimated by measuring its thickness, and this parameter can be an indicator of the degree of corneal damage that can even cause stromal edema [8]. In humans, where CECs have no regenerative ability, the ECD decreases with age and then tends to be stable. Any damage to CECs is mainly compensated by the expansion and movement of adjacent cells [9]. The CV is an index that reflects the size variability of the endothelial cell area. The HCP refers to the change in the shape of hexagonal cells. The CV and HCP can reflect the repair and healing process occurring upon endothelial cell damage; whenever CECs are damaged, the remaining cells expand and slide, showing an increase in cell size together with a decrease of hexagonal-shaped cells [10].
The health status of the cornea will affect the postoperative recovery of cataract surgery. DM can affect the health of the corneal endothelium [11]. It was suggested that the cornea of diabetic patients is more likely to be damaged after phacoemulsification [4]. In a previous study, researchers systematically analyzed corneal properties early after phacoemulsification (within 3 months) in diabetic and non-diabetic patients [12], although they did not conduct subsequent follow-up studies. Currently, there is still controversy about the long-term differential changes in corneal function and morphology after phacoemulsification between diabetic and non-diabetic patients. In this study, we aimed to evaluate the influence and potential risks of phacoemulsification on the cornea of diabetic and non-diabetic patients by reporting any changes in the CCT, ECD, CV, and HCP within 6 months after phacoemulsification. It is hoped to find the cause of corneal endothelium related complications in diabetic patients after phacoemulsification, which is helpful for clinical treatment.
Methods
Inclusion and exclusion criteria
The study included prospective studies. We included patients (1) with and without diabetes who underwent phacoemulsification and intraocular lens implantation, (2) whose outcomes included at least one data index of corneal properties (CCT, ECD, CV, and HCP), (3) with no other systemic diseases except DM, (4) whose blood glucose levels were stable, and (5) with no serious surgery-related complications. Patients with severe ocular and systemic complications caused by DM were excluded, such as those with proliferative diabetic retinopathy (PDR) and diabetic nephropathy. Those with mature cataracts (brown/white), cataract grade V, or other eye diseases were also excluded.
Search strategy and quality assessment
We selected relevant studies published between January 1, 2011 and December 25, 2021, by searching the databases PubMed, Embase, Web of Science, and the Cochrane Library (Trials Central). No language restrictions were applied. We used the following MeSH terms and Text Words: The complete search used for PubMed was: (((“Cataract”[Mesh]) OR (Cataracts [Title/Abstract])) OR (Lens Opacity* [Title/Abstract])) OR (Opaciti*, Lens [Title/ Abstract])) OR (Cataract*, Membranous [Title/Abstract])) OR (Membranous Cataract* [Title/Abstract])) OR (Pseudoaphakia [Title/Abstract])) OR (Phacoemulsification* [Title/Abstract]))) AND ((“Diabetes Mellitus”[Mesh]) OR (diabete* [Title /Abstract]) OR (diabetic* [Title/ Abstract])) AND ((“Cornea”[Mesh]) OR (Cornea* [Title/ Abstract])). Filters: from 2011/1/1 to 2021/12/25. Manual search was conducted on the reference lists of published key articles in English.
The quality of the selected studies was assessed using the Newcastle–Ottawa Scale (NOS) CASE CONTROL STUDIES, which includes three sections: selection (four items, four points), comparability (one item, two points), and exposure (three items, three points); a total of nine points is achievable, with scores ≥ 6 indicating good quality. Detailed items for the NOS are provided in Additional file 1.
Data extraction
Two independent investigators extracted the following information: first author and country, publication year, type of study, follow-up duration, patient age, number of eyes, ascertainment criteria for DM and cataracts, DM status (duration or fasting blood sugar or glycated hemoglobin [HbA1c]), presence of diabetic retinopathy, and literature quality assessment scores.
Statistical analysis
A forest plot was constructed and statistical and sensitivity analyses were performed using Review Manager 5.4.1. Sensitivity analysis was performed using the one-by-one exclusion method. The weighted mean difference (WMD) and 95% confidence interval (CI) were calculated based on selected outcomes. P < 0.05 was considered a statistically significant difference. I2 test and Cochran’s Q test were used to evaluate heterogeneity. No heterogeneity was indicated by I2 < 50% and P > 0.1, and the fixed-effect model was used to calculate pooled effect. If there was significant heterogeneity, a random effect model was used.
Publication bias estimate
Stata 14.0 was used for subgroup analysis and the publication bias test. The Egger’s test was used to estimate the publication bias. P < 0.05 was considered a statistically significant publication bias. The trim-and-fill method was used to evaluate the influence of publication bias on the interpretation of the results.
Results
Study selection
The literature selection process is shown in Fig. 1. In total, 1042 relevant studies (PubMed 132, EMbase 417, Web of Science 489, and Cochrane Library 4) were retrieved. Next, they were screened based on redundancy (801 studies remained), screening of topics (43 studies remained), and abstract information (22 studies remained). Nine studies were excluded after reading the full text: one with unknown glycemic control, one with an incomplete outcome index, two in which patients had serious DM complications (PDR surgery history, kidney disease dialysis history), two in which the basic information was not comprehensive, and three retrospective studies. Finally, 13 studies [13–25], including 1744 eyes (788 eyes in the DM group and 956 eyes in the non-DM group), were selected for this meta-analysis.
Fig. 1.
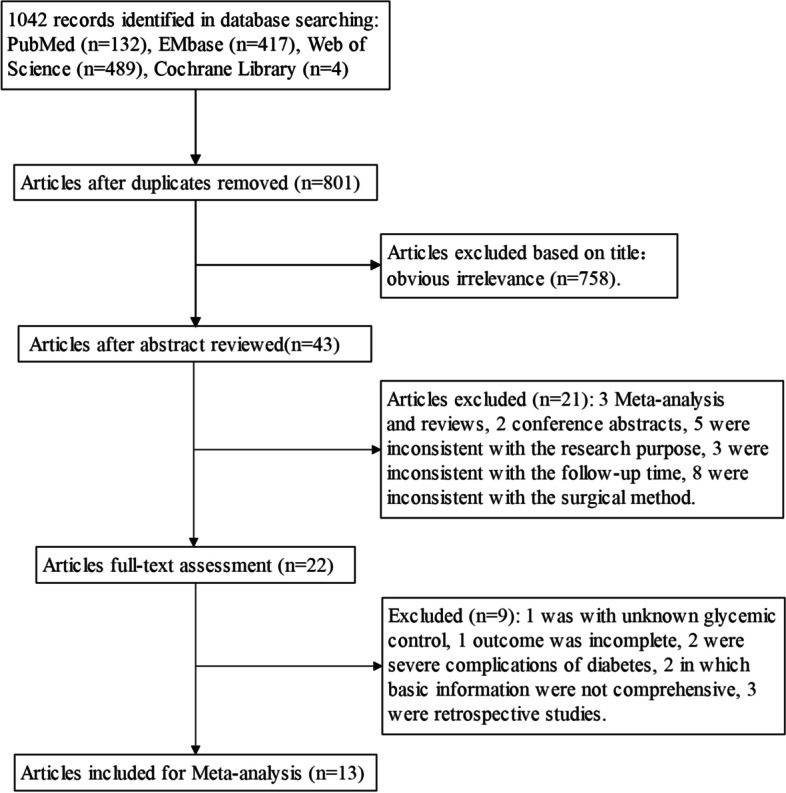
Workflow diagram of literature selection process
Quality assessment of the included literature
According to the NOS, eight studies scored 7, and five studies scored 8. All studies scored more than 6 points, indicating that the quality of the included studies was high. The characteristics of the included studies are provided in Table 1.
Table 1.
Characteristics of included studies
| Author year | Location | Type of study | Follow up duration(months) |
Age (year/SD, range) _______________ |
No.of eyes ______________ |
Ascertainment of diabetes | Classfication criteria of cataract | Diabetes condition | DR | NOS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM Group |
Non- DM Group |
DM Group |
Non- DM Group |
|||||||||
|
Hugod [18] 2011 |
Denmark |
Prospective controlled study |
3 | 75.4 ± 9.3 | 75.6 ± 8.6 | 30 | 30 | medical history | NA |
well control of blood sugar |
29 had no DR and 1 had mild NPDR | 7 |
|
Wang [24] 2013 |
China |
Prospective controlled study |
6 | 65.3 ± 11.0 | 69.2 ± 8.2 | 62 | 82 | NA | NA |
Fasting blood sugar < 8.0 mmol/L |
41 eyes without DR, 21 eyes with DR |
7 |
|
Zhao [25] 2013 |
China |
Prospective controlled study |
3 | 52 ~ 80 | 55 ~ 83 | 56 | 60 | NA | NA |
Fasting blood sugar < 8.0 mmol/L |
NA | 7 |
|
Li [13] 2016 |
China |
Prospective controlled study |
3 | 64.58 ± 12.46 | 65.12 ± 12.30 | 224 | 227 | Type 2 diabetes clinical diagnostic criteria published by WHO | NA | Duration(y) 1 ~ 3 | exclude DR | 7 |
|
Sahu [23] 2017 |
India |
Prospective controlled study |
3 | 63.38 ± 7.31 | 64.00 ± 8.32 | 60 | 60 |
American Diabetic Association (ADA 2007) |
LOCS III |
HbA1c(%) 6.87 ± 0.43 |
NA | 8 |
|
Chen [21](1) 2018 |
China |
Prospective controlled study |
1 | 63.56 ± 9.51 | 62.32 ± 8.37 | 44 | 48 | NA | NA |
Duration(y) 5 ~ 15 ( 8.38 ± 2. 59), HbA1c < 8% |
NA | 7 |
|
Chen [22](2) 2018 |
China |
Prospective controlled study |
6 | 62.8 ± 2.2 | 63.6 ± 2.4 | 60 | 60 | NA | NA | Duration(y) 4.4 ± 1.5, well control of blood sugar |
No serious diabetic complications |
7 |
|
Ganesan [15] 2019 |
India |
Prospective controlled study |
3 | 61.0 ± 6.3 | 58.7 ± 5.5 | 80 | 80 | NA | NA |
well control of blood sugar |
NA | 7 |
| Khokhar [16] 2019 | India | Prospective controlled study | 1 | 58.14 ± 11.96 | 58.74 ± 11.17 | 54 | 194 | NA | LOCS III | fasting blood sugar < 140 mg/dL and HbA1c < 7% | No DR or mild NPDR | 7 |
|
Fernández- Muñoz [19] 2019 |
Mexico | Prospective controlled study | 3 | 50 ~ 80 | 50 ~ 80 | 21 | 21 |
Type 2 diabetes clinical diagnostic criteria published by WHO |
LOCS II |
HbA1c < 6.5% in the previous 5 years |
exclude PDR | 8 |
|
Maadane [20] 2019 |
Maroc |
Prospective controlled study |
3 | 60.42 ± 6.48 | 62.0 ± 7.21 | 47 | 47 |
American Diabetic Association (ADA 2007) |
LOCS III | HbA1c < 7% | NA | 8 |
|
Budiman [17] 2020 |
Indonesia |
Prospective controlled study |
1 | 60.2 ± 9.4 | 61.6 ± 12.6 | 67 | 86 | diabetes history | NA | HbA1c < 10%(7.3 ± 1.08), and/or blood glucose < 200 mg/dL | NA | 8 |
|
Beato [14] 2021 |
Portugal |
Prospective controlled study |
6 | 72.7 ± 5.7 | 70.5 ± 6.3 | 45 | 43 | medical history, HbA1c level ≥ 6.5%, and/or current use of antidiabetic medication | NA | Duration(y) 9.1 ± 8.0; HbA1c levels (%): DM (6.8 ± 1.0), Non-DM (5.5 ± 0.4) | 6 eyes with mild to moderate NPDR | 8 |
DM Diabetes Mellitus, HbA1c Glycosylated Hemoglobin, DR Diabetic Retinopathy, PDR Proliferative Diabetic Retinopathy, NPDR Nonproliferative Diabetic Retinopathy, NOS The Newcastle– Ottawa quality assessment scale, LOCS II the Lens Opacities Classification System II, LOCS III the Lens Opacities Classification System III, NA Not Available
Meta-analysis outcomes
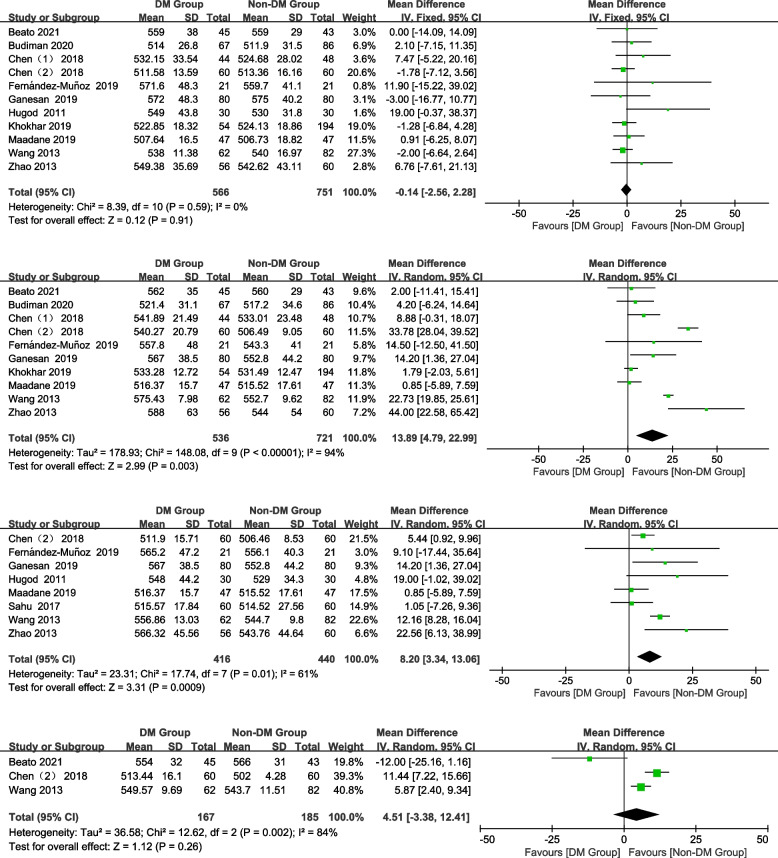
CCT
In total, 11, 10, 8, and 3 studies were included preoperatively and 1 month, 3 months, and 6 months postoperatively, respectively. No significant difference was observed in CCT between the groups preoperatively and 6 months postoperatively (Fig. 2; preoperative: WMD = -0.14, 95% CI: -2.51–2.28, Z = 0.12, P = 0.91; 6 months postoperatively: WMD = 4.51, 95% CI: -3.38–12.41, Z = 1.12, P = 0.26). However, the CCT in the DM group was significantly thicker than that in the non-DM group at 1 month and 3 months postoperatively (Fig. 2; 1 month postoperatively: WMD = 13.89, 95% CI: 4.79–22.99, Z = 2.99, P = 0.003; 3 months postoperatively: WMD = 8.20, 95% CI: 3.34–13.06, Z = 3.31, P = 0.0009).
Fig. 2.
Forest plot of CCT in DM group and non-DM group
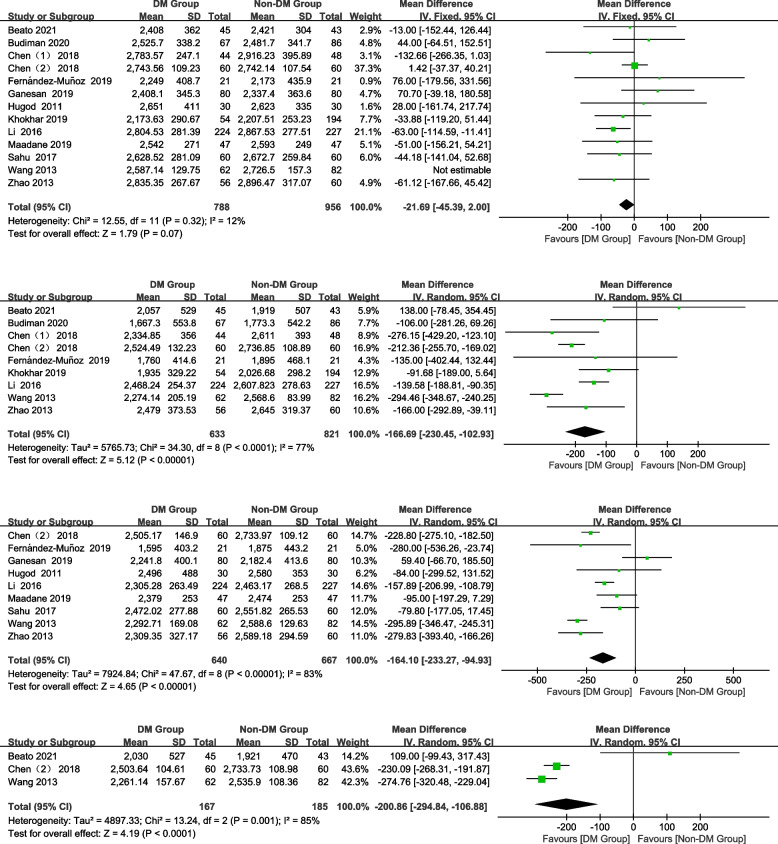
ECD
In total, 13, 9, 9, and 3 studies were included preoperatively and 1 month, 3 months, and 6 months postoperatively, respectively. There was no significant difference in the ECD between the DM group and non-DM group preoperatively (Fig. 3; WMD = -21.69, 95% CI: -45.39–2.00, Z = 1.79, P = 0.07). However, patients with DM had a significantly lower ECD than non-DM patients at all postoperative time points (Fig. 3; 1 month postoperatively: WMD = -166.69, 95% CI: -230.45–-102.93, Z = 5.12, P < 0.00001; 3 months postoperatively: WMD = -164.10, 95% CI:-233.27–-94.93, Z = 4.65, P < 0.00001; 6 months postoperatively: WMD = -200.86, 95% CI: -294.84–-106.88, Z = 4.19, P < 0.0001).
Fig. 3.
Forest plot of ECD in DM group and non-DM group
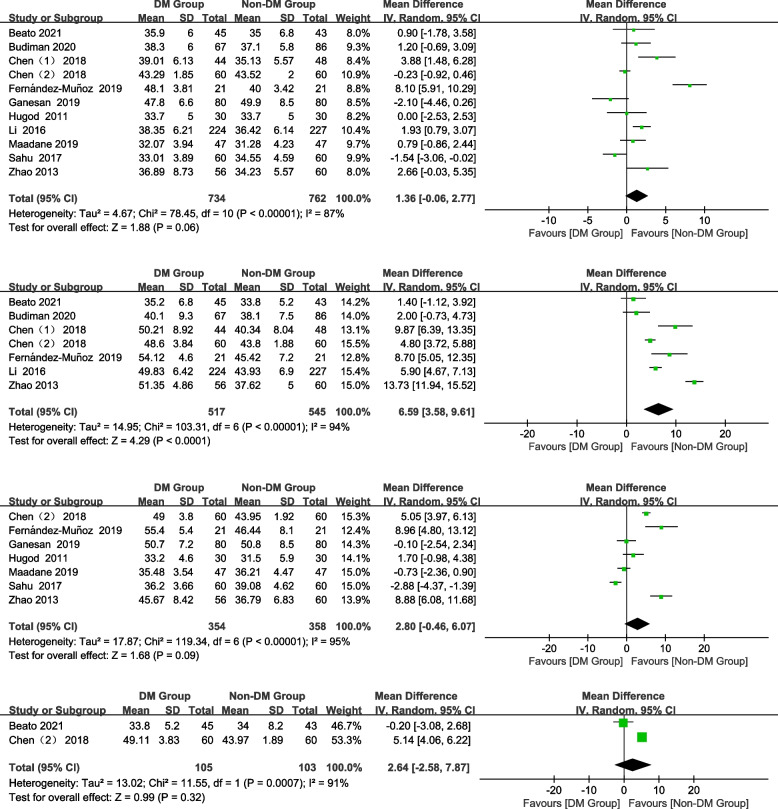
CV
In total, 11, 7, 7, and 2 studies were included preoperatively and 1 month, 3 months, and 6 months postoperatively, respectively. DM patients had a significantly higher CV at 1 month postoperatively than non-DM patients (Fig. 4; WMD = 6.59, 95% CI: 3.58–9.61, Z = 4.29, P < 0.0001). No significant difference was found preoperatively and 3 and 6 months postoperatively (Fig. 4; preoperative: WMD = 1.36, 95% CI: -0.06–2.77, Z = 1.88, P = 0.06; 3 months postoperatively: WMD = 2.80, 95% CI: -0.46–6.07, Z = 1.68, P = 0.09; 6 months postoperatively: WMD = 2.64, 95% CI: -2.58–7.87, Z = 0.99, P = 0.32).
Fig. 4.
Forest plot of CV in DM group and non-DM group
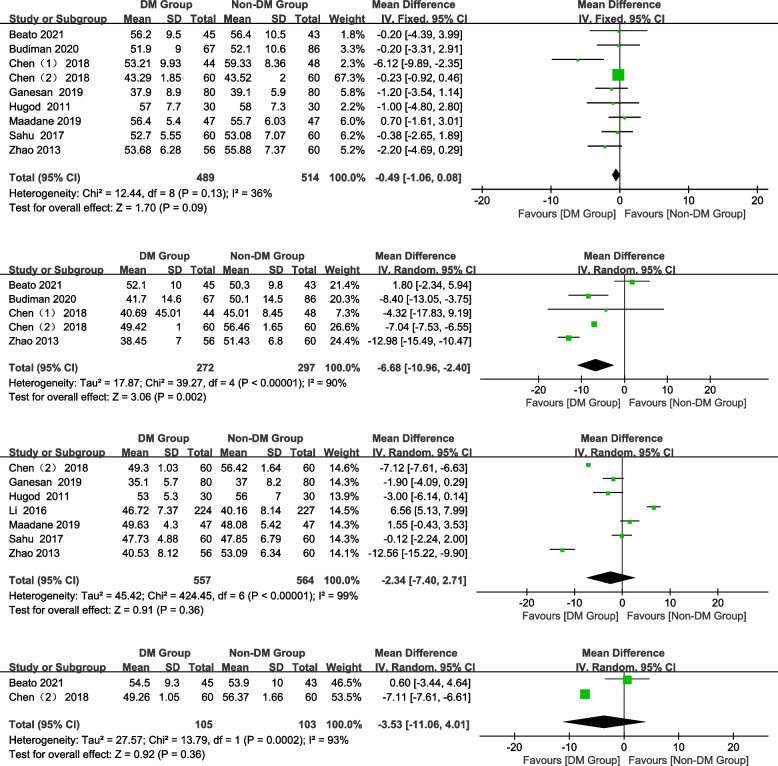
HCP
In total, 9, 5, 7, and 2 studies were included preoperatively and 1 month, 3 months, and 6 months postoperatively, respectively. The HCP of the DM group was significantly lower than that of the non-DM group at 1 month and postoperatively (Fig. 5; 1 month postoperatively: WMD = -6.68, 95% CI: -10.96–-2.4, Z = 3.06, P = 0.002). No significant differences were observed in the HCP between the groups preoperatively and at 3, 6 months postoperatively (Fig. 5; preoperative: WMD = -0.49, 95% CI: -1.06–0.08, Z = 1.70, P = 0.09; 3 months postoperatively: WMD = -2.34, 95% CI:-7.40–2.71, Z = 0.91, P = 0.36; 6 months postoperatively: WMD = -3.53, 95% CI: -11.06–4.01, Z = 0.92, P = 0.36).
Fig. 5.
Forest plot of HCP in DM group and non-DM group
Sensitivity
Sensitivity analysis publication bias analysis showed that the data of Li [13] were extremely unstable regarding the CV 3 months postoperatively and HCP preoperatively and 1 month and 3 months postoperatively; thus, these data were excluded from this analysis. Although partial results showed relatively large heterogeneity, the data were stable and reliable after sensitivity analysis. Sensitivity analysis was not performed at 6 months postoperatively owing to the small number of included studies.
Publication bias
The Egger’s test was used to estimate publication bias. Because of the low number of included studies for CV and HCP at 6 months postoperatively, no publication bias analysis was performed at this stage. There was no publication bias in the included studies, except for preoperative CCT (Table 2).
Table 2.
Publication bias
| Time | CCT | ECD | CV | HCP |
|---|---|---|---|---|
| preoperative | 0.012 | 0.656 | 0.468 | 0.662 |
| postoperative 1 month | 0.231 | 0.603 | 0.392 | 0.106 |
| postoperative 3 months | 0.719 | 0.577 | 0.342 | 0.069 |
| postoperative 6 months | 0.579 | 0.826 | NA | NA |
NA Not Available
The influence of preoperative CCT publication bias on the interpretation of the results was evaluated using the trim-and-fill method. The pooled effect sizes calculated by the fixed-effect model (pooled effect size: standard error of effect size) were 0.041 and 0.009, and the 95% CI was -0.071 to 0.152 and -0.099 to 0.116 before and after using the trim-and-fill method, respectively. No significant difference was found before and after using the trim-and-fill method (P = 0.478, P = 0.874, respectively). There was no asymmetry in the funnel plot after supplementing two studies (Supplemental studies are shown as “square” in Fig. 6). This showed that publication bias had little effect on the results, and the results were relatively stable (Fig. 6).
Fig. 6.
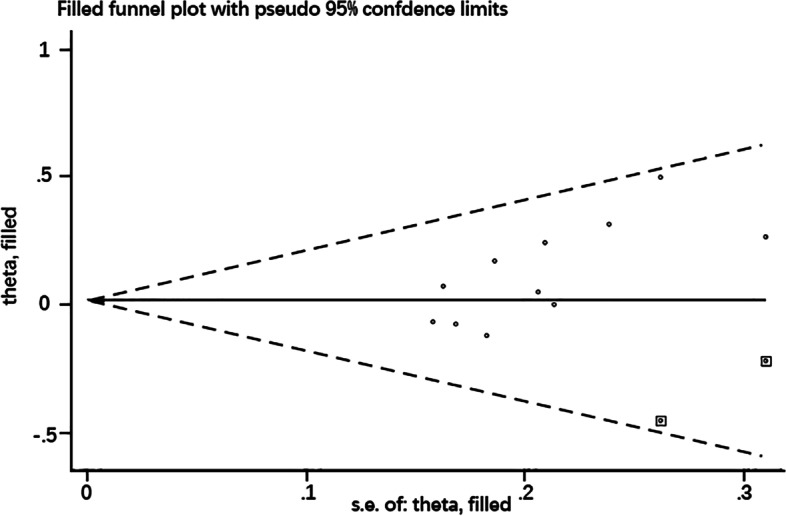
Funnel plot after using the trim-and-fill method
Discussion
CECs are reportedly lost at a rate of 2.5% per year within 10 years after cataract extraction [26], which is four times the normal physiological loss rate [11]. Patients of advanced age, with a long DM duration and poor blood sugar control, are at greater risk of CECs damage [27]. The mechanism of CEC-enhanced damage caused by DM is still unclear and may be related to the accumulation of advanced glycation end products in the CECs, leading to oxidative stress [28].
Oxidative stress decreases antioxidant levels and increases lipid peroxidation, resulting in CEC damage [29]. Corneal ultrastructural changes, mitochondrial swelling, and impaired function in patients with DM can lead to a decrease in ATP production and pump function in CECs [30]. Importantly, DM also reduces the activity of Na+/K+-ATP enzymes in endothelial cells [31], which is vital for maintaining endothelial cell function.
The ECD and HCP have been reported to be significantly lower, and the CV and CCT significantly higher in DM patients than in healthy controls [11, 27]. This was even more evident in patients with poor DM status, such as longer diabetes duration (≥ 10 years) and higher HbA1c levels (≥ 7%) [27]. In the present study, there were no significant differences in CCT, ECD, CV, and HCP preoperatively. These findings could be due to the age-specific cataract patients included in our study (50–80 years of age); non-cataract populations of other age groups were not included. Furthermore, we did not perform subgroup analysis on diabetes status (such as disease course and HbA1c level).
Decreased innervation, exposure to vitreous humor [32], increased hardness of lens nucleus [14], surgical trauma [33], intraoperative inflammatory response [15], and postoperative corneal edema [34] are important risk factors for CECs damage after phacoemulsification. However, the risk of CECs injury caused by the above factors increases in diabetic patients. The aim of modern cataract surgery is not only to improve vision but also to minimize the damage to CECs, especially in patients with cataracts and DM.
CCT
The hydration balance is regulated by the CEC pump in normal conditions. When the CEC pump is dysfunctional, the corneal stroma accumulates water, and swelling occurs, which is manifested by an increase in corneal thickness. However, persistent corneal edema and dysfunction do not occur unless the CEC number declines to < 500–1000 cells/mm [35].
The CCT of patients with DM has been found to be significantly higher than that of healthy individuals, and HbA1c is found to be positively correlated with CCT and CV and negatively correlated with ECD in patients with DM [35, 36]. The duration of DM has a significant impact on these parameters: the longer the DM duration, the higher the CCT and the lower the ECD [37]. In the present study, we found that CCT in the DM group was significantly higher than that in the non-DM group at the early postoperative period (3 months), suggesting that the impairment degree of corneal endothelial barrier function in the DM group was significantly higher than that in the non-DM group. From 3 to 6 months postoperatively, the difference in CCT between the two groups gradually decreased, indicating gradual recovery of the corneal endothelial function. Thus, the corneal endothelial barrier function was impaired at the early postoperative period and then gradually stabilized until 6 months postoperatively. This may be related to postoperative oxidative stress and inflammation response. DM itself [38] and surgical trauma [39] increase the oxidative stress level of CECs. Oxidative stress not only directly damages CECs [29] but also induces inflammation through multiple activation pathways [40]. Corneal edema alleviates with a decrease in inflammation, resulting in a lower CCT during the recovery process after phacoemulsification [15].
ECD
The percentage of endothelial cell loss (ECL%) in patients with DM was reported to be significantly higher than that in the control group after phacoemulsification [41, 42], and the damage was not restored to the preoperative state at 6 months postoperatively [14]. Joo et al. [43] found that the ECL in patients with DM was higher than that in non-DM patients 1 year after phacoemulsification, although not statistically significant. Furthermore, the duration of DM may affect postoperative ECD loss, with more ECD loss occurring when the duration is ≥ 10 years. Choi et al. [34] found that ECL% was about 2.06 ± 1.36% per year 10 years after phacoemulsification, and this persistent ECL may be related to corneal endothelial remodeling. Ganesan et al. [15] considered inflammation to be a risk factor for ECL in DM patients, whereas age and effective phacoemulsification time were the risk factors in non-DM patients after phacoemulsification.
Our results showed that there was no significant difference in ECD between the DM group and non-DM group preoperatively. However, the ECD in the DM group was significantly lower than that in the non-DM group and the ECL increased progressively compared with that in the non-DM group at 1–6 months postoperatively. This indicated that ECL was accelerated, which was unstable at 6 months postoperatively, and postoperative corneal recovery was delayed in patients with DM. Although the ECL% in patients with DM was higher than that in the control group after phacoemulsification, the intraoperative cumulative dissipated energy (CDE), fluid consumption, and operative time were not statistically significant [41]. The higher ECL postoperatively may be related to the advanced age of patients, increased cataract density, increased endothelial cell vulnerability in diabetic patients, increased trauma during cataract surgery, and grade of cataract [14, 16, 41].
CV and HCP
CV and HCP reflect the dynamic repair and healing process of CEC morphology after injury; the increase in CV indicates a large variability in cell size, and the decrease in HCP indicates an increase in pleomorphism. The remaining cells expand and slide after endothelial cell injury, which shows an increase in CV and a decrease in HCP. The morphology of CECs in patients with DM was unstable at 4 weeks after phacoemulsification [17]. The HCP of patients with DM decreased significantly 3 months postoperatively, whereas the CV showed no significant difference [18]. However, some studies reported that the CV of patients with DM was significantly higher than that of those without DM at 3 months postoperatively, although this difference did not affect the corneal function [19]. The HCP returned to its preoperative state 6 months postoperatively [14]. No significant change was found in the CV and HCP in either group at 1 year postoperatively [43].
Our results showed that the degree of morphological variation of CECs in diabetic patients was largest at 1 month postoperatively, which was significantly higher than that in non-diabetic patients, and subsequently, the degree of morphological variation of CECs gradually decreased. The corneal morphology of diabetic patients was more unstable in the early postoperative stage, indicating that the endothelial cells of diabetic patients have a weaker repair ability upon damage, and the repair process takes longer [20].
Despite the fact that there was no significant difference in the visual acuity between DM and non-DM patients after phacoemulsification was performed in controlled blood glucose levels [18], the impact of diabetes on corneal health cannot be ignored, as good control of blood glucose is frequently lost in DM. Compared with healthy individuals, the CECs of patients with DM have a lower tolerance to phacoemulsification, are more likely to be damaged, and take longer time to recover, which requires the surgeon to carefully protect the cornea in order to minimize corneal endothelial damage intraoperatively. Femtosecond laser-assisted cataract surgery (FLACS) is reported to cause less damage to the corneal endothelium in patients with DM and can reduce the ECL. This may be because the corneal endothelial injury caused by the small energy during FLACS is insufficient to cause significant damage [44]. Therefore, FLACS may be a better option for patients with DM than conventional phacoemulsification. Furthermore, it should not be ignored that age and DM status are important factors affecting corneal ECD. For DM patients who require cataract surgery, timely surgery is also important when blood glucose is well controlled.
Our study has some limitations. First, intraoperative CDE, fluid consumption, and operative time were not assessed, although most of the included studies showed no statistical differences between the two groups. Second, a randomized controlled trial could not be performed because of DM presence. Third, the cataract grade and expertise of operating surgeon cannot be standardized across all studies, which may be a confounding factor in this study. Finally, longer studies after 6 months, as well as subgroup analyses of DM status (such as DM duration and HbA1c level), were not performed because few studies were eligible for inclusion. In the future, we will continue to focus on the long-term dynamic changes in corneal properties after cataract surgery in patients with DM.
Conclusion
We conducted a longer dynamic and comprehensive analysis of the changes in corneal function and morphology in DM and non-DM patients after phacoemulsification and evaluated the repair process of corneal injury. Our study showed that the CCT and corneal endothelial morphology were greatly damaged in diabetes patients in the early period after phacoemulsification, but they gradually stabilized during the repair process from 1 to 6 months postoperatively. However, ECD was unstable at 6 months postoperatively in DM patients because the ECL in diabetic patients was still significantly higher than that in non-DM patients. This suggests that more than 6 months are required to recover corneal endothelial function and morphology in DM patients after phacoemulsification. This indicated that DM patients have a higher endothelial loss rate, delayed recovery time, and require a longer follow-up duration after phacoemulsification. Therefore, clinicians should be more attentive to the corneal health of DM patients when considering phacoemulsification.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- DM
Diabetes Mellitus
- non-DM
Non-Diabetes Mellitus
- CCT
Central corneal thickness
- ECD
Endothelial cell density
- CV
Coefficients of variation
- HCP
Hexagonal cell percentage
- CECs
Corneal endothelial cells
- WMD
Weighted mean difference
- CI
Confidence interval
- ECL
Endothelial cell loss
- CDE
Cumulative dissipated energy
- FLACS
Femtosecond laser-assisted cataract surgery
Authors’ contributions
All authors (YQY, HTC, ZXD, CYT, YSL, YHL and HL) contributed to the design for the study. YQY and HTC conducted literature search and statistical analysis. CYT, and YSL conducted data extraction, quality assessment. HTC and YHL conducted resolving the disputes. YQY completed the draft manuscript. ZXD conducted oversight on the process. HL contributed to interpret datas and revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
Supported by grants from the National Natural Science Foundation of China.
(Grant No.81960174).
Availability of data and materials
All data generated or analyzed during this study are included in relevant published articles.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.Drinkwater JJ, Davis WA, Davis TME. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. 2019;35:e3073. doi: 10.1002/dmrr.3073. [DOI] [PubMed] [Google Scholar]
- 3.GBD 2019 Blindness and Vision Impairment Collaborators. Vision Loss Expert Group of the Global Burden of Disease Study Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamdad S, Bolkheir A, Sedaghat MR, Motamed M. Changes in corneal thickness and corneal endothelial cell density after phacoemulsification cataract surgery: a double-blind randomized trial. Electron Physician. 2018;10:6616–6623. doi: 10.19082/6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the cornea: structure, function, and development. Prog Mol Biol Transl Sci. 2015;134:7–23. doi: 10.1016/bs.pmbts.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Sj T, Coster DJ. The corneal endothelium. Eye. 1990;4:389–391. doi: 10.1038/eye.1990.53. [DOI] [PubMed] [Google Scholar]
- 7.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishima S. Clinical investigations on the corneal endothelium–XXXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 1982;93:1–29. doi: 10.1016/0002-9394(82)90693-6. [DOI] [PubMed] [Google Scholar]
- 9.Gupta PK, Berdahl JP, Chan CC, et al. The corneal endothelium: clinical review of endothelial cell health and function. J Cataract Refract Surg. 2021;47:1218–1226. doi: 10.1097/j.jcrs.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 10.Carlson KH, Bourne WM, McLaren JW, et al. Variations in human corneal endothelial cell morphology and permeability to flfluorescein with age. Exp Eye Res. 1988;47:27–41. doi: 10.1016/0014-4835(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang K, Zhao L, Zhu C, et al. The effect of diabetes on corneal endothelium: a meta- analysis. BMC Ophthalmol. 2021;21:78. doi: 10.1186/s12886-020-01785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Chen X, Zhang X, Tang Q, Liu S, Yao K. Clinical evaluation of corneal changes aftr phacoemulsification in diabetic and non-diabetic cataract patients, a systematic review and meta-analysis. Sci Rep. 2017;7:14128. doi: 10.1038/s41598-017-14656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li MH, Fu XL, Yang WF. Effect and risk factors for corneal endothelial cells after phacoemulsification in diabetic cataract patients. Int Eye Sci. 2016;16:1048–1051. [Google Scholar]
- 14.Beato JN, Esteves-Leandro J, Reis D, et al. Corneal structure and endothelial morphological changes after uneventful phacoemulsification in type 2 diabetic and nondiabetic patients. Arq Bras Oftalmol. 2021;84:454–461. doi: 10.5935/0004-2749.20210071. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan N, Srinivasan R, Babu KR, Vallinayagam M. Risk factors for endothelial cell damage in diabetics after phacoemulsification. Oman J Ophthalmol. 2019;12:94–98. doi: 10.4103/ojo.OJO_200_2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khokhar S, Sen S, Dhull C. Active-fluidics-based torsional phacoemulsification in diabetic eyes: a prospective interventional study. Indian J Ophthalmol. 2019;67(5):619–624. doi: 10.4103/ijo.IJO_1146_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budiman B. Comparison of endothelial cell density, morphological changes and central corneal thickness after phacoemulsification between diabetic and non-diabetic patients. Open Ophthalmol J. 2020;14:15–20. doi: 10.2174/1874364102014010015. [DOI] [Google Scholar]
- 18.Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30:749–753. doi: 10.1097/ICO.0b013e31820142d9. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Muñoz E, Zamora-Ortiz R, Gonzalez-Salinas R. Endothelial cell density changes in diabetic and nondiabetic eyes undergoing phacoemulsification employing phaco-chop technique. Int Ophthalmol. 2019;39:1735–1741. doi: 10.1007/s10792-018-0995-y. [DOI] [PubMed] [Google Scholar]
- 20.Maadane A, Boutahar H, Bourakba S, Sekhsoukh R. Évaluation endothéliale cornéenne des patients diabétiques après une phacoémulsification [Corneal endothelial evaluation of diabetic patients after phacoemulsification] J Fr Ophtalmol. 2019;42:381–386. doi: 10.1016/j.jfo.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Li M. Effect of phacoemulsification on the corneal endothelium of diabetic patients with cataract. Int Eye Sci. 2018;18:1786–1791. [Google Scholar]
- 22.Chen Z, Song F, Sun L, et al. Corneal integrity and thickness of central fovea after phacoemulsification surgery in diabetic and nondiabetic cataract patients. Arch Med Sci. 2018;14:818–825. doi: 10.5114/aoms.2016.64036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahu PK, Das GK, Agrawal S, Kumar S. Comparative evaluation of corneal endothelium in patients with diabetes undergoing phacoemulsification. Middle East Afr J Ophthalmol. 2017;24:74–80. doi: 10.4103/meajo.MEAJO_242_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, Li JX, Wang YL, et al. Clinical effect analysis of phacoemulsification on cataract patients with diabetes mellitus. Int Eye Science. 2013;13:1163–1166. [Google Scholar]
- 25.Zhao C, Zhao GQ, Che CY, et al. Changes of corneal endothelium in diabetes patients after cataract phacoemulsification surgery by confocal microscopy. Int Eye Sci. 2013;13:876–879. [Google Scholar]
- 26.Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101:1014–1023. doi: 10.1016/S0161-6420(94)31224-3. [DOI] [PubMed] [Google Scholar]
- 27.Kim YJ, Kim TG. The effects of type 2 diabetes mellitus on the corneal endothelium and central corneal thickness. Sci Rep. 2021;11:8324. doi: 10.1038/s41598-021-87896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaji Y, Usui T, Oshika T, et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41:362–368. [PubMed] [Google Scholar]
- 29.Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Free Radic Biol Med. 2008;45:1047–1055. doi: 10.1016/j.freeradbiomed.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Aldrich BT, Schlotzer-Schrehardt U, Skeie JM, et al. Mitochondrial and morphologic alterations in native human corneal endothelial cells associated with diabetes mellitus. Invest Ophthalmol Vis Sci. 2017;58:2130–2138. doi: 10.1167/iovs.16-21094. [DOI] [PubMed] [Google Scholar]
- 31.Kaji Y, Amano S, Usui T, et al. Advanced glycation end products in Descemet's membrane and their effect on corneal endothelial cell. Curr Eye Res. 2001;23:469–477. doi: 10.1076/ceyr.23.6.469.6968. [DOI] [PubMed] [Google Scholar]
- 32.Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond) 2003;17:912–918. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 33.Gurnani B, Kaur K. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Pseudophakic Bullous Keratopathy. [PubMed] [Google Scholar]
- 34.Choi JY, Han YK. Long-term (≥10 years) results of corneal endothelial cell loss after cataract surgery. Can J Ophthalmol. 2019;54:438–444. doi: 10.1016/j.jcjo.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Storr-Paulsen A, Singh A, Jeppesen H, Norregaard JC, Thulesen J. Corneal endothelial morphology and central thickness in patients with type II diabetes mellitus. Acta Ophthalmol. 2014;92:158–160. doi: 10.1111/aos.12064. [DOI] [PubMed] [Google Scholar]
- 36.Chowdhury B, Bhadra S, Mittal P, Shyam K. Corneal endothelial morphology and central corneal thickness in type 2 diabetes mellitus patients. Indian J Ophthalmol. 2021;69:1718–1724. doi: 10.4103/ijo.IJO_3120_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvo-Maroto AM, Cerviño A, Perez-Cambrodí RJ, García-Lázaro S, Sanchis-Gimeno JA. Quantitative corneal anatomy: evaluation of the effect of diabetes duration on the endothelial cell density and corneal thickness. Ophthalmic Physiol Opt. 2015;35:293–298. doi: 10.1111/opo.12191. [DOI] [PubMed] [Google Scholar]
- 38.Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;201:1–30. doi: 10.1155/2017/8379327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsueh YJ, Meir YJ, Yeh LK, et al. Topical ascorbic acid ameliorates oxidative stress-induced corneal endothelial damage via suppression of apoptosis and autophagic flux blockage. Cells. 2020;9:943. doi: 10.3390/cells9040943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;12:1–9. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He X, Diakonis VF, Alavi Y, Yesilirmak N, Waren D, Donaldson K. Endothelial cell loss in diabetic and nondiabetic eyes after cataract surgery. Cornea. 2017;36:948–951. doi: 10.1097/ICO.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 42.Khalid M, Hanif MK, Islam QU, Mehboob MA. Change in corneal endothelial cell density after phacoemulsification in patients with type II diabetes mellitus. Pak J Med Sci. 2019;35:1366–1369. doi: 10.12669/pjms.35.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joo JH, Kim TG. Comparison of corneal endothelial cell changes after phacoemulsification between type 2 diabetic and nondiabetic patients. Medicine (Baltimore) 2021;100:e27141. doi: 10.1097/MD.0000000000027141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang KH, Song MY, Kim KY, Hwang KY, Kwon YA, Koh K. Corneal endothelial cell changes after femtosecond laser-assisted cataract surgery in diabetic and nondiabetic patients. Eye Contact Lens. 2021;47:664–669. doi: 10.1097/ICL.0000000000000823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in relevant published articles.