Abstract
Background
Endometrial cancer generally presents at an early stage affording a high rate of surgical cure. Early in the Covid-19 pandemic it was suggested that treatment of tumors with favorable pathologic features might safely be delayed.
Objective
We hypothesized that disruption to health care services during the pandemic would impact the stage at presentation for these patients. Here we compare the stage at presentation of Endometrial Cancer in the months immediately preceding the pandemic to the stage at presentation during the pandemic.
Study design
Charts of patients presenting with Endometrial Cancer between January 1, 2018 and April 30, 2022 were reviewed. March 1, 2020 separated the “pre-pandemic” era from the “pandemic” era. Data was collected regarding patient age, body mass index (BMI), tumor stage, histology, grade, size, and depth of invasion.
Results
322 of 374 (86.09%) of surgically staged patients presented with FIGO stage I disease “pre-pandemic” compared to 263 of 329 (71.73%) of surgically staged patients in the “pandemic” cohort (p =.029). 2.08% pre-pandemic compared to 5.48% during the pandemic presented with FIGO stage IV (p =.015).
Conclusion
We found a significant difference in the stage at presentation in endometrial cancer patients, a statistic which may be attributed to the disruption of healthcare services caused by the Covid-19 pandemic.
Keywords: Endometrial cancer, Covid-19, Stage at diagnosis, Stage migration
1. Introduction
Endometrial cancer is the most common gynecological cancer and the fourth most common cancer in women in the United States. Over 60,000 cases have been diagnosed annually in the US in recent years, with 65,950 anticipated for 2022 (Cancer Facts & Figures, 2022). The annual incidence is increasing at least partly as a result of an aging population, an increase in the proportion of women still at risk, and an increase in obesity (Cancer Facts & Figures, 2022). As such a common cancer, it is possible that its identification and treatment might have been affected by a significant disrupter of healthcare delivery.
In March 2020, Covid-19, caused by the novel coronavirus SARS-CoV-2, was declared a worldwide pandemic. Traditional healthcare services were abruptly disrupted, as stay at home orders limited office visits, and resources were diverted to the acute care of the infected patients and the prevention of contagious spread. This, in turn, forced deviation from the then standard of care, as the healthcare industry pivoted to address the needs created by the pandemic. Professional organizations scrambled to provide guidance for care of patients under these new circumstances (Uwins et al., 2020, ESMO, 2022). In the case of Endometrial Cancer, Society of Gynecologic Oncologists (SGO) offered recommendations regarding which patients could safely be offered delayed surgical intervention, and which ones needed to be prioritized for more immediate surgical treatment (Gyn Onc, 2022). Concerns were raised as to how the anticipated decrease in screening mammography, colonoscopy, or pap smears might impact the stage at diagnosis for breast, colon, and cervical cancers, and the potential impact on prognosis was projected (Patt et al., 2020, Guven et al., 2021). It was postulated that endometrial cancer diagnosis might not be impacted in the same way, however, because the majority are Type 1 tumors which usually present at an early stage and have a good prognosis (Hamilton et al., 2021). These low grade endometrial cancers are considered slow-growing and were therefore assigned lower priority for surgical intervention (Uwins et al., 2020); because the disease is generally brought to medical attention by symptoms rather than screening studies, it was felt that lack of access to diagnostic studies might not impact the disease trajectory as much as the altered access to surgery (Uwins et al., 2020). By the end of the third month of the pandemic however, Kaiser Permanente was already noting a 41% decrease in weekly new endometrial cancer diagnoses in its Northern California practices, as well as a 33% decrease in calls regarding abnormal vaginal bleeding (Suh-Burgmann et al., 2020), suggesting that the impact of the pandemic on endometrial cancer diagnosis might be substantial. As highlighted by Kaiser experience, even early in the pandemic there appears to have been an impact on patients’ seeking care, let alone presenting for evaluation or biopsy or being triaged to surgery.
The purpose of this study is to determine the effects of the Covid-19 pandemic on stage at presentation for endometrial cancer by reviewing the cases seen in Southern Oregon the years immediately preceding the pandemic and comparing them to those seen since the declaration of Oregon’s stay at home policy on March 14, 2020.
2. Methods
We obtained IRB approval through Western IRB (20217156) for this retrospective chart review. We queried Willamette Valley Cancer Center billing records for unique endometrial cancer patients seen between January 1, 2018, and April 30, 2022. The Willamette Valley Cancer Center serves a population of at least 1 million people, covering the greater part of Southern Oregon and the edge of Northern California.
January 1, 2018 to February 29, 2020 represents the “pre-pandemic” cohort, and March 1, 2020 to January 31, 2022 represents the “pandemic” cohort. This community formally went into “lockdown” on March 14, 2020, shutting schools and limiting hospital services, but because the impact of the looming international crisis was already anticipated by early March, we designated March 1, 2020 as the dividing point between the two cohorts.
We identified unique patients presenting as “New Patients” with an endometrial cancer diagnosis. The newly diagnosed endometrial cancer patients’ charts were queried for age, BMI, stage, histology, tumor grade, depth of myometrial invasion, tumor size, surgical approach, and pre-operative CA-125. The data was tabulated using Google Sheets and comparisons were made between the cohorts using chi-squared and z tests of two proportions.
We compared the age and BMI of the patients in each cohort. We tabulated the percentage of patients presenting in FIGO stage I, II, III, and IV and compared the percentages in each stage between cohorts. Because the majority of these patients present in stage I, we further compared those presenting in stage IA and IB and then further subdivided the stage IA patients to compare the number of patients presenting with stage IA grade 1 non-invasive cancer between cohorts. An attempt was made to quantify length of time from initiation of bleeding to appointment, biopsy, and surgery, but as these were inconsistently documented in the medical record, we felt they were not sufficiently reliable data to report or draw conclusions from.
Statistical significance was determined using the online tool “Social Science Statistics” available at “Socscistatistics.com.”.
3. Results
835 unique patient records were identified with a “New Patient” visit and a new endometrial cancer billing code (C54.1) between January 1, 2018 and April 30, 2022. 423 of these patients were seen between January 1, 2018 and February 29, 2020; these were designated our “pre-pandemic” cohort. 412 patients were seen between March 1, 2020 and April 30, 2022, and were designated as “pandemic” cohort. The difference between the number of unique patients seen in each cohort was not statistically significant (p >.05) (chi-squared = 0.07245).
After reviewing the charts and removing the records of patients who were presenting with recurrent disease or who had relocated and were now presenting as a “transfer of care” for ongoing surveillance, we identified 384 unique new endometrial cancer patients in the “pre-pandemic” cohort and 347 in the “pandemic” cohort. During the “pre-pandemic” 26 months we were seeing an average of 14.77 new endometrial cancer patients per month, whereas during the “pandemic” 26 months we saw 13.35 patients per month, a 9.6% decrease. This was not a statistically significant decrease.
The age at presentation was similar between the cohorts. The mean age in the “pre-pandemic” cohort was 65.5 +/− 10.8 (range of 30–95) compared to the mean age in the “pandemic” cohort of 64.6 +/− 11.3 (range of 32–90) (p =.26). The BMI of the patients in the two cohorts were also not statistically significantly different; 35.16 +/− 9.70 (range 16–65) compared to 36.03 +/− 9.86 (range 17–71) (p =.25). See Table 1.
Table 1.
Patient and tumor characteristics before and during the pandemic.
| Pre-pandemic | Pandemic-era | P-value | |
|---|---|---|---|
| Age (years) | 65.5 ± 10.8 | 64.6 ± 11.3 | p >.05 |
| BMI (kg/m2) | 35.2 ± 9.7 | 36.0 ± 9.9 | p >.05 |
| Endometrioid histology | 80.2% | 81.9% | p >.05 |
| Serous histology | 11.1% | 11.7% | p >.05 |
| Other Histology | 8.7% | 6.4% | p >.05 |
| Grade 1 | 57.0% | 55.8% | p >.05 |
| Grade 2 | 17.1% | 15.2% | p >.05 |
| Grade 3 | 26.0% | 29.1% | p >.05 |
| Robotic | 91.4% | 88.8% | p >.05 |
There was no difference in the percentage of patients in each cohort with endometrioid, serous, clear cell, carcinosarcoma, or undifferentiated histology. In the “pre-pandemic” cohort, 304 of 387 patients with documented histology (79.2%) had endometrioid histology, 42 (10.9%) had serous histology, 14 (3.6%) clear cell histology, 11 had carcinosarcoma, 7 had undifferentiated histology, and one had a glassy cell variant. In the “pandemic” cohort, 288 of 347 with documented histology (83.0%) had endometrioid histology, 40 (11.5%) had serous histology, 8 had clear cell histology, 8 had carcinosarcoma, 5 were undifferentiated, and one had a neuroendocrine tumor. (Chi-square statistic = 1.3399 with p =.512). There was no difference between the cohorts in patients presenting with grade 1, 2, or 3 tumors. In the “pre-pandemic” cohort, 217 (56.5%) were grade 1, 65 (16.8%) were grade 2, and 99 (25.8%) were grade 3. In the “pandemic” cohort, 184 (53.0%) were grade 1, 50 (14.4%) were grade 2, and 96 (27.7%) were grade 3. (Chi-square statistic is 1.0656 with p =.587). The majority of those treated surgically had a robotic assisted minimally invasive surgical approach, both “pre-pandemic” (91.4%) and during the pandemic (88.8%). In the “pandemic” cohort we found that more patients declined surgery in favor of “holistic therapies” (3 compared to none in the “pre-pandemic” group), equal numbers declined surgery to pursue fertility (2 in each cohort), and more were treated with an open approach in the “pre-pandemic” group (often open surgery combined with a complex hernia repair): 14 (3.6%) before the pandemic versus 6 (1.7%) during the pandemic. Also, while more patients were deemed not to be surgical candidates in the “pandemic” cohort (5.2% which represented 18 patients, two of whom died of Covid between being diagnosed with endometrial cancer and their scheduled surgery) compared to 11 (2.7%) in the pre-pandemic cohort, this did not reach statistical significance.
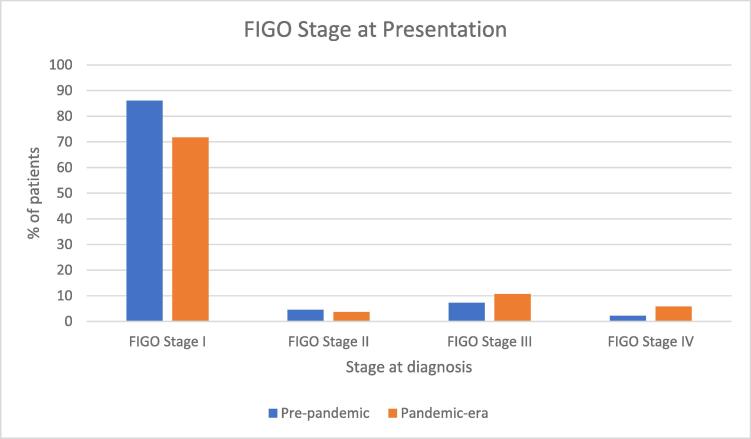
There was statistical significance in the FIGO stage at presentation. In the “pre-pandemic” cohort, 322 of 374 surgically staged patients (86.09%) patients were diagnosed with FIGO stage I disease whereas in the “pandemic” cohort, 263 of 329 surgically staged patients (71.73%) patients presented with FIGO stage I disease, p =.029. The percentages of patients with FIGO stage II and III disease were not statistically different between the two cohorts. In the “pre-pandemic” cohort, 17 of 374 (4.54%) presented with FIGO stage II disease compared to 12 of 329 (3.65%) in the “pandemic” cohort, p =.55). In the “pre-pandemic” cohort, 27 of 374 (7.22%) presented with FIGO stage III disease compared with 35 of 329 (10.64%) in the “pandemic” cohort, p =.11). The difference in those presenting with FIGO stage IV was statistically significant with p =.012; in the “pre-pandemic” cohort, 8 of 374 (2.14%) presented with FIGO stage IV disease compared with 19 of 329 (5.78%) in the “pandemic” cohort. See Fig. 1 and Table 2.
Fig. 1.
FIGO stage at diagnosis for endometrial cancer patients diagnosed in the 2 years prior to the pandemic compared to stage at diagnosis in the 2 years following declaration of the COVID-19 pandemic.
Table 2.
Stage at diagnosis.
| FIGO Stage I | FIGO Stage II | FIGO Stage III | FIGO Stage IV | |
|---|---|---|---|---|
| Pre-pandemic | 86.1% | 4.5% | 7.2% | 2.1% |
| Pandemic-era | 71.7% | 3.7% | 10.6% | 5.8% |
In a subgroup analysis of patients with FIGO stage IA (stage I but with <50% invasion), there was no statistical significance between the cohorts, (229 of 374, 61.23% compared to 218 of 329, 66.26%, p =.17) nor was there a difference when comparing the group that might represent the very earliest form of the disease, Stage IA grade 1 non-invasive endometrioid endometrial cancer. 97 of 374 (25.94%) compared to 83 of 329 (25.23%) presented with noninvasive grade one disease, p =.83. The statistically significant difference in patients presenting with stage I disease between the pre-pandemic and pandemic groups was concentrated in the difference in those presenting with FIGO stage IB disease: pre-pandemic 24.9% (93 of 374) compared to 13.7% (45 of 329) during the pandemic, p =.00019.
We also identified a significant difference in stage at presentation when combining patients with “local disease” (FIGO stages I and II) and comparing them to those with “advanced disease” (FIGO stages III-IV). For this comparator, p =.005. Looking at stage I disease as “early” and stages II-IV as “advanced” (or those for whom adjuvant therapy is usually warranted), we also saw a statistically significant shift p =.029.
4. Discussion
4.1. Summary of main results
We found a statistically significant difference in the FIGO stage at presentation of Endometrial Cancer patients diagnosed prior to the pandemic compared to those diagnosed during the pandemic. In the “pre-pandemic” cohort, 322 of 374 staged patients (86.09%) were diagnosed with FIGO stage I disease whereas in the “pandemic” cohort, 263 of 329 staged (71.73%) patients presented with FIGO stage I disease (p =.0029). There was also a statistically significant difference in those presenting with distant metastatic disease (FIGO stage IV) during this time frame: 8 of 374 (2.14%) in the pre-pandemic time frame compared with 19 of 329 (5.78%) in the “pandemic” cohort (p =.012). More patients presented with FIGO stage III disease during the pandemic, but this did not reach statistical significance (p =.11). The significance of the shift in stage at presentation could be interpreted to differentiate between those whose disease broadly warrants adjuvant therapy and those for whom surveillance suffices. This may herald significant differences in overall cost of care and prognosis.
4.2. Results in the context of published literature
To date, there is no data published on the impact of the Covid-19 pandemic specifically on the stage at diagnosis of endometrial cancer. Wylie et al described a decrease in endometrial cancer and endometrial hyperplasia diagnoses in Ireland during the first year of the pandemic,(Wylie et al., 2021) and Drescher et al reported similar findings in their exploration of cancer case trends in the American Pacific Northwest following the onset of the pandemic. (Drescher et al., 2021) This is consistent with our data in that we also demonstrated a decrease in the number of endometrial cancer cases diagnosed after the onset of the pandemic although our numbers did not reach statistical significance. Our data further enhances the granularity of the available information by demonstrating a concerning shift towards more advanced disease in those who were diagnosed during this era. Early in the pandemic, concerns were raised regarding the impact that a halt or delay in screening might have on subsequent cervical cancer diagnoses,(Patt et al., 2020) and while that seems to have been born out, the data regarding possible worsened cervical cancer mortality due to the pandemic has not yet matured sufficiently to reach publication, perhaps because the ongoing stressors of the pandemic have yet to calm sufficiently to allow proper academic assessment. Akin to the concerns raised for cervical cancer diagnoses, Kaiser Permanente’s early identification of a decrease in calls concerning postmenopausal bleeding and then a decrease in endometrial cancer diagnosis in their patients (Suh-Burgmann et al., 2020) may indicate that Endometrial Cancer diagnosis and staging will prove to have been impacted just as cervical cancer has been.
4.3. Strengths and weaknesses
A strength of this study is that our practice has long provided the majority of gynecologic oncologic care in our region. Despite this data’s being generated out of a single practice, it represents care delivered at multiple sites in South and Central Oregon in the United States, as we represent the only full time gynecologic oncologic practice in this geographic region. Therefore, we feel this work accurately captures the trends in diagnosis of a large catchment area and is thus epidemiologically representative of the impact of the Covid related disruption in healthcare access on Endometrial Cancer stage at diagnosis.
Despite the pandemic related travel restrictions and infection concerns, patients continued to travel to our center for this care. Smaller outlying hospitals in this geographic region had even more significant contraction of services than our tertiary care center; in order to gain expedited access to minimally invasive endometrial cancer surgical care, patients traveled. It is possible that some patients with very early endometrial cancers were treated locally, or were never referred even for a virtual consultation, and therefore were not included in our analysis, but we feel that is unlikely, and was rare if it did occur.
A weakness of our study is that it represents a less urban population in the American Pacific Northwest, and the findings may not be generalizable. We would like to compare our numbers to those for all of Oregon, but the most recent Oregon Stage Cancer Registry data is from 2020, and therefore we do not yet have a statewide picture of the impact of the Covid-19 pandemic on cancer indices. Furthermore, the state registry does not provide publicly available granular details on stage and grade, and therefore might not allow immediate analysis of stage migration in this setting.
4.4. Implications for practice and future research
We have described stage migration, but we have not been able to define a cause for this shift in stage at diagnosis. It could have been due to slower presentation for evaluation for vaginal bleeding, a decrease in available clinic appointments, increased time to referral, delay in being seen by a gynecologic oncologist, or a delay in surgical management due to decreased availability of operating room time. We tried to quantify the impact of these potential sources of delay in cancer care and attribute the shift in stage at presentation to one of these potential causes, but found that identifying how long a patient had been bleeding, or how long the clinic visit was delayed was inconsistently documented in the chart and therefore not a reliable or reproducible data point. The Kaiser Permanente health care system may be uniquely suited to address these specifics as they track patient phone calls, reasons for phone calls, (Suh-Burgmann et al., 2020) and have control over a patient’s entire cancer journey in a way that is not always reproducible in the fractured health care delivery available in the community setting. Also, although we identified evidence of “stage migration,” we do not have data as to whether this impacted endometrial cancer mortality; such data is unlikely to be available for several years. Because Covid-19 related stage migration has already been linked to higher early mortality in several malignancies (Guven et al., 2021), it remains possible that this will prove to be the case in endometrial cancer as well.
The Covid-19 pandemic has disrupted health care in the United States and worldwide for three years now and promises continued disruption. Successive waves of the virus have been met with responses tailored to the immediate challenge. The first wave of the pandemic elicited strict stay at home orders, as the virus, its virulence, and its transmission patterns were yet unknown; the effect of these orders was an acute contraction of routine health care services. It was postulated that this contraction would negatively impact diagnostic and therapeutic services for different disease entities differently. Early in the pandemic, concerns were raised regarding the downstream effects of diverting healthcare resources away from cancer screening, and as the first wave of the pandemic passed, public health attention turned towards tending to the backlog of patients who had missed screening opportunities. A subsequent wave of preventable cancers was anticipated. We experienced a 10% decrease in new endometrial cancer patients during the two years since the pandemic was declared, suggesting that perhaps there are even more patients with endometrial cancer that have avoided medical care during the pandemic, and who may present in the future with more advanced disease.
For Gynecologic Oncology, attention turned to risk mitigation, and professional organizations around the world rapidly published guidelines for the treatment of gynecologic malignancies in a time of public health uncertainty and limited resources. Virtual services replaced traditional office-based appointments. Only the most urgent patient needs were addressed in person, and each interaction was weighed in terms of potential Covid-19 viral risk and need for personal protective equipment. In addition to recommendations for strict social distancing in the clinical setting, surgical prioritization moved to the forefront as a mechanism to decrease use of PPE and preserve valuable hospital bed space. Elective surgeries were canceled or delayed, and patients were triaged according to their need for immediate surgery; priority was given to those with immediate life threatening conditions, and then to high grade tumors and other conditions for which a delay in surgery would predict a negative outcome. (Uwins et al., 2020) Specifically, low grade early stage endometrial cancers were identified as a cohort which could safely be deferred for several months or treated temporarily with hormonal methods such as a levonorgestrel IUD or megestrol (Uwins et al., 2020).
5. Conclusion
We present here data that identifies “stage migration” in our population of endometrial cancer patients, with significantly fewer patients being diagnosed at FIGO stage I, and significantly more being diagnosed with distant metastatic disease, FIGO stage IV, in the pandemic-affected months compared to the months immediately preceding the pandemic.
We present these data regarding endometrial cancer stage at diagnosis in the setting of the Covid-19 public health crisis to raise awareness of the possibility of “stage migration” even in a cancer that presents overwhelmingly at an early stage and with low histologic grade. Over three years since the first death from Sars-Cov-2 in the United States, we are still experiencing disrupted healthcare delivery due to new viral strains, supply chain challenges, and staffing shortages (Manchanda et al., 2021). It will be important not only to mark these shifts in stage at diagnosis, but also to explore causes of these shifts, specific breakdowns in care delivery or delay in diagnosis. We hope this data and these questions can help to inform future public health decisions regarding endometrial cancer care.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cancer Facts & Figures, 2022. American Cancer Society. www.cancer.org. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html.
- Drescher C.W., Bograd A.J., Chang S.C., et al. Cancer case trends following the onset of the COVID-19 pandemic: A community-based observational study with extended follow-up. Cancer. 2021 doi: 10.1002/cncr.34067. 1;128(7):1475–1482. [DOI] [PubMed] [Google Scholar]
- ESMO, 2022. ESMO management and treatment adapted recommendations in the COVID-19 era: Endometrial cancer. www.esmo.org. Accessed June 7, 2022. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic/gynaecological-malignancies-endometrial-cancer-in-the-covid-19-era.
- Guven D.C., Sahin T.K., Yildirim H.C., et al. Newly diagnosed cancer and the COVID-19 pandemic: tumour stage migration and higher early mortality. BMJ Supportive & Palliative Care. 2021 doi: 10.1136/bmjspcare-2021-003301. Published online October 28, bmjspcare-2021-003301. [DOI] [PubMed] [Google Scholar]
- Gyn Onc Considerations During COVID-19. Society of Gynecologic Oncology. Accessed June 8, 2022. https://www.sgo.org/resources/gyn-onc-considerations-during-covid-19/.
- Hamilton C.A., Pothuri B., Arend R.C., et al. Endometrial cancer: A society of gynecologic oncology evidence-based review and recommendations. Gynecol. Oncol. 2021;160(3):817–826. doi: 10.1016/j.ygyno.2020.12.021. [DOI] [PubMed] [Google Scholar]
- Manchanda R., Oxley S., Ghaem-Maghami S., Sundar S. COVID-19 and the impact on gynecologic cancer care. Int. J. Gynecol. Obstetrics. 2021;155(S1):94–101. doi: 10.1002/ijgo.13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt D., Gordan L., Diaz M., et al. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020;4:1059–1071. doi: 10.1200/cci.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh-Burgmann E.J., Alavi M., Schmittdiel J. Endometrial Cancer Detection During the Coronavirus Disease 2019 (COVID-19) Pandemic. Obstetrics Gynecol. 2020;136(4):842–843. doi: 10.1097/aog.0000000000004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uwins C., Bhandoria G.P., Shylasree T., et al. COVID-19 and gynecological cancer: a review of the published guidelines. Int. J. Gynecol. Cancer. 2020;30:1424–1433. doi: 10.1136/ijgc-2020-001634. ijgc-2020-001634. [DOI] [PubMed] [Google Scholar]
- Wylie J., Quinn D., Donnelly D.W., et al. The impact of the COVID-19 pandemic on endometrial cancer and endometrial hyperplasia diagnoses: a population-based study. Am. J. Obstetrics Gynecol. 2021;226(5):737–739.e2. doi: 10.1016/j.ajog.2021.12.259. [DOI] [PMC free article] [PubMed] [Google Scholar]