Abstract
Mucin MUC4 is an aberrantly expressed oncogene in pancreatic ductal adenocarcinoma (PDAC), yet no pharmacological inhibitors have been identified to target MUC4. Here, we adapted an in silico screening method using the Cancer Therapeutic Response Database (CTRD) to Identify Small Molecule Inhibitors against Mucins (SMIMs). We found that Bosutinib as a candidate drug to target oncogenic mucins among CTRD screening yielded 126 FDA-approved drugs. Functionally, Bosutinib treatment alone/and in combination with gemcitabine (Gem)/5’ fluorouracil (5FU) reduced viability, migration, colony formation, in vivo xenograft growth, and human PDAC organoid proliferation and growth in multiple PDAC cell lines. Further, biochemical and molecular analyses showed that Bosutinib exhibited these functional effects by downregulating MUC4 mucin at both transcript and translation levels in a dose- and time-dependent manner. Mechanistically, global transcriptome analysis in PDAC cells upon treatment with Bosutinib revealed disruption of the Src-ERK/AKT-FosL1 pathway, leading to decreased expression of MUC4 and MUC5AC mucins. Taken together, Bosutinib is a promising, novel, and highly potent SIMMs to target MUC4/MUC5AC mucins. This mucin-targeting effect of Bosutinib can be exploited in the future with cytotoxic agents to treat mucinous tumors.
Keywords: Bosutinib, Pancreatic ductal adenocarcinoma, SRC/ERK/FosL1, MUC4, MUC5AC
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal disease with a five-year survival of 11.5%, one of the lowest among major cancers [1]. Multiple factors specific to PDAC contribute to this dismal prognosis, including its late symptomatic presentation and lack of early detection methods leading to delayed diagnosis, which result in only 20% of patients being eligible for surgical resection at the time of clinical diagnosis. For the other 80% of patients with borderline resectable, locally advanced, or metastatic tumors, their outcome largely depends on their response to systemic therapy. Unfortunately, currently available chemotherapy regimens have limited therapeutic efficacy in PDAC, largely due to its complex tumor microenvironment (TME), which comprises a dense stroma with cancer-associated fibroblasts and immune cells and a hypervascularity state, together creating a physical and biological barrier to chemotherapies. Recent advances in multi-agent chemotherapy regimens, such as FOLFIRINOX (Oxaliplatin, Irinotecan, Leucovorin, and Fluorouracil), have improved the therapeutic response but are associated with high toxicity, limiting their widespread and prolonged usage. As a result, there has been a rising interest in identifying small molecule inhibitors of MUCINS (SMIM)/ targeted therapies in PDAC. However, many had promising preclinical results but were not found to improve disease-free survival (DFS) or overall survival (OS) in phase II and III clinical trials, such as Vandetanib (EGFR, RET, VEGFR2 inhibitor) in the ViP trial and Selumetinib and MK-2206 (MEK and PI3K/AKT inhibitors) [2–5]. One important reason proposed for such disappointing clinical results was that these trials did not identify patient subgroups with specific biomarkers that would predict the response to these targeted therapies.
MUCINs (MUCs) are major tumor antigens and are less explored therapeutically in PDAC. Among MUCs, MUC4 is a large transmembrane glycoprotein, undetectable in normal pancreas, but its expression progressively increases from early to late stages of pancreatic intraepithelial neoplasms (PanINs) to PDAC and eventually to metastatic cancer. We and others have previously demonstrated that MUC4 is an important oncogene in PDAC, playing a critical role in tumor initiation, progression, metastasis, tumor interaction with TME, and drug resistance [6–10]. Correspondingly, downregulation of MUC4 using shRNA resulted in a significant decrease in PDAC tumor growth, metastasis, and chemotherapy resistance in in vitro studies and in vivo mouse models [11–13]. The mechanisms of MUC4-mediated changes have also been gradually elucidated, one of which is via the interaction of its three EGF domains with HER2 and HER3 receptors, which lead to the activation of AKT-NFkB, FAK/SRC-JNK, and PI3K-ERK-cMYC pathways, resulting in PDAC tumor proliferation, invasion, and metastasis [14–16]. In addition, the promoter sequence of MUC4 mucin has also been well categorized, along with a dozen of transcription factors that regulate the expression of this protein [17, 18]. Earlier and recent studies made a plot demonstrating the expression of MUC4 mucin and its negative regulation of nucleoside transporters and gemcitabine resistance [13, 19]. Sagar et al. demonstrated that MUC4 knockout (KO) using the CRISPR/Cas9 system enhanced gemcitabine sensitivity by regulating high human equilibrative nucleoside transporter 1 (hENT1) and human concentrative nucleoside transporter 3 (hCNT3) levels [19]. Supportively, the ectopic introduction of MUC4 cDNA in PDAC cells showed decreased gemcitabine sensitivity [10]. Similar to MUC4, MUC5AC is another mucin found to be newly expressed in PanINs and PDAC. As a secreted protein, MUC5AC has been long recognized as a valuable tumor marker for early PDAC detection and has also recently been found to play important roles in PDAC, especially in tumor metastasis [20]. Multiple transcription factors for MUC5AC have also been discovered, many of which overlap with those of MUC4 mucin [21].
This study tested the pre-clinical characterization of SMIMs targeting MUC4 and/or MUC5AC mucins in combination with traditional chemotherapeutic agents. We identified an USFDA-approved SMIM, Bosutinib, specifically docks MUC4 and MUC5AC mucins through unbiased computational analysis. We then demonstrated that Bosutinib inhibits PDAC tumor initiation, proliferation, and metastasis both in vitro and in vivo by downregulating MUC4 and MUC5AC and improves the therapeutic efficacy of existing chemotherapies such as Gemcitabine and 5FU. Transcriptomic analysis on PDAC cells treated with Bosutinib revealed downregulation of the SRC-AKT/ERK-FosL1 pathway. Our findings maximize the utility of Bosutinib to be included in PDAC clinical trials especially targeting high MUC4 and MUC5AC expressing patients, and is a potentially valuable addition to the current PDAC treatment regimen.
2. Materials and Methods
2.1. Identification of small-molecule inhibitors specific to MUC4.
To identify specific small molecule inhibitors affecting the MUC4 mucin, MUC4 was queried against the drug signatures in the connectivity map, the cancer therapeutics response database, and the pharmacogenomics transcriptional signatures database using the iLINCS (http://www.ilincs.org/ilincs/) web-server. The hence identified drugs were further assessed for the z-score of the connections between MUC4 and the drug signatures with the FDA approval identifying 5 top target drugs, which were further assessed in PDAC cell lines.
2.2. Cell culture and reagents
T3M4, Colo 357, CD18/HPAF PDAC cell lines, normal immortalized human pancreatic epithelial nestin-positive cells (HPNE), cholangiocarcinoma cell line (KMCH), NCI-H1437 and A549 non-small-cell-lung cancer (NSCLC), human breast epithelial MDA-MB231 cells were obtained from ATCC and were cultured in high glucose DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin. Luciferase-labeled Colo 357 cell line was generated using Firefly Luciferase Lentivirus via stable transfection. Colo 357 MUC4-knockdown cell line was generated using shRNA targeting MUC4 mucin (MUC4 shRNA sequence list in Table S1). Bosutinib was purchased from Selleckchem.com (cat#: S1014).
2.3. Cell viability assay
Cell viability assays were performed to determine IC50 values of Bosutinib, Regorafenib, Dasatinib, Sildenafil, Bexarotene, and Gemcitabine using the Calcein AM cell permanent dye from Invitrogen (cat#: C1430). IC50 values for each drug were calculated using GraphPad Prism 9.0 software.
2.4. Western blot, immunofluorescence, and immunohistochemistry analysis
Western blot analysis was performed as described previously. Primary and secondary antibodies incubated in PVDF membrane were exposed to chemiluminescent-based detection reagents (Pierce™ ECL western blotting substrate, Catalog # 32209), followed by visualization of protein bands using X-ray film or high-resolution digital cameras attached to iBright Imaging system (Thermo Fisher Scientific) [22, 23]. For immunofluorescence analysis, PDAC cells were seeded in sterile glass coverslip placed in 12 well plates, and confocal analysis of xenograft tissues was processed by rehydration (xylene and alcohol series), blocking with horse serum, incubation with primary and fluorescent-labeled secondary antibodies, DAPI staining, mounting with anti-fade mounting reagent followed by visualization using Zeiss 710 CLSM and 800 CLSM with Airyscan microscopes [22]. Immunohistochemistry (IHC) was performed in a pancreatic tissue array (PA 1003, US Biomax) containing 25 cases of PDAC in quadruple cores. The TMA was stained with antibodies specific for MUC4 (8G7 (in-house generated, 1 mg/mL), MUC5AC (1: 200, Abcam, #AB3649), FOSL1 (1:100, Cell signaling technology, # 5281), and WNT7B (1:200, Invitrogen, # PA5–103480). Similarly, the xenograft tissues exposed to Bosutinib, Gemcitabine, Bosutinib, and Gemcitabine and control were stained with Ki67 (1:300, Cell signaling technology, # 9027) (proliferation marker) and cleaved Caspases-3 (1:200, Cell signaling technology, # 9661) (apoptosis marker). IHC procedures were adapted from our earlier publications [22, 23].
2.5. RNA isolation, quantitative real-time PCR (qRT-PCR), and digital droplet PCR (dd-PCR)
Total RNA isolation, RNA quantification, cDNA conversion, qPCR, and dd-PCR were performed following the methodology published previously [18, 22]. PCR primers are listed in table S2.
2.6. Human PDAC Organoid generation, maintenance, and drug treatment
Human PDAC organoid was generated from fresh tumor tissue of patients who underwent PDAC resection. We used human PDAC tumor samples for the establishment of pancreatic organoids according to the previously described protocol [24, 25]. In brief, tumor samples were chopped and digested enzymatically using digestion media containing collagenase II (Millipore Sigma, C7657), Dispase (Thermo Fisher, 17105041), and 1% FBS. After digestion, the cells were embedded in matrigel (Corning, 356255) and incubated in the incubator for 15 to 20 mins before adding organoid-specific media [22]. Three independent replicates of organoids were treated with Gemcitabine or 5FU alone or in combination with Bosutinib. Real-time images of the organoids were captured every 3 hours for 72 hours using the IncuCyte-S5 live-cell imaging system (Sartorius). The kinetic data (organoid average growth) were analyzed and graphically represented using IncuCyte software (Sartorius).
2.7. In vivo xenograft mouse model and treatment strategy
Mice experiments were performed per the ARRIVE, University of Nebraska Medical Center Institutional Animal Care and Use Committee, and the United States Public Health Service guidelines. Mice were housed under 12 hr light/dark cycle at 22±1°C and 50±10% humidity with ad libitum access to food and water. Six to eight weeks old athymic mice underwent orthotopic implantation with Luciferase-labeled Colo 357 cells between ages 6–8 weeks. Initial IVIS imaging was performed on post-op day 14, and animals were randomized into 4 groups: control, Bosutinib, Gemcitabine, and Bosutinib+Gemcitabine combination. During the first 12 days of treatment, animals received daily PBS IP injection, daily Bosutinib 30mg/kg IP injection, biweekly Gemcitabine 12.5mg/kg IP injection, or daily Bosutinib 30mg/kg IP injection with biweekly Gemcitabine 12.5mg/kg IP injection respectively. Due to the limited treatment effect observed, the drug dosage was increased to Bosutinib 50mg/kg and Gemcitabine 25mg/kg on treatment day 13 and continued till the end of the treatment schedule. Weekly IVIS imaging was performed to assess treatment response. All animals were sacrificed after 3 weeks of treatment, with primary tumors as well as other pertinent organs sent for formalin tissue fixation, H&E staining, and immunofluorescent staining for confocal microscopy.
2.8. RNA seq and in silico analysis
Global RNA sequencing was performed on total RNA isolated (Qiagen RNA easy RNA isolation kit, Qiagen, Valencia, CA, USA) from PDAC cells treated with Bosutinib or DMSO vehicle control or MUC4 specific shRNA transfected cells. The isolated total RNA was quantified using a Nanodrop spectrophotometer (NanoDrop ND100, Wilmington, DE, USA), and a sample of 2000 ng was provided for RNA sequencing analysis at the UNMC genome core facility. We followed all the procedures such as cDNA library preparation (RNA-seq library kit, Illumina), ligation, cDNA synthesis, linearization, cDNA amplification (HiSeq2500), and sequencing data were analyzed using the R-Bioconductor and processed using the human genome GRCH38 version. Differentially expressed genes between control, drug treatment, and MUC4 knockdown were analyzed using the DESeq2 package within R-Bioconductor followed by functional annotation using Ingenuity Pathway analysis™, as described previously [23, 26]. For gene correlation analysis in human PDAC patients, the transcriptome data across the TCGA-PAAD cohort were downloaded, and gene expression in each patient’s sample was analyzed for a correlation between AKT1, FOSL1, WNT7B, MUC4, and MUC5AC. The scatter plot representing the correlations (spearman correlation) between mucins and analyzed markers was reported [23].
2.9. Statistical analysis
Student t-tests were used for between-group comparisons. All statistical analyses were performed using GraphPad Prism 9.0 software. For PDAC tissue IHC analysis, Spearman correlations were used to determine association markers measured in the same cases. Composite scores were compared by demographic and clinical information using the Wilcoxon rank sum test. SAS software version 9.4 was used for analysis (SAS Institute Inc., Cary, NC).
3. Results
3.1. In silico computational analysis through connectivity mapping identified potential small molecule inhibitors against MUCINS
Computational analysis of the connectivity mapping through the cancer therapeutics response database (CTRS) yielded 126 targets as potential Small Molecule Inhibitors against Mucins (SMIMs) against MUC4 mucin. These drugs were further assessed for availability and FDA approval status, yielding five potential targets for further analysis, including Regorafenib, Dasatinib, Bosutinib, Sildenafil, and Bexarotene (Fig. 1A and Table S3).
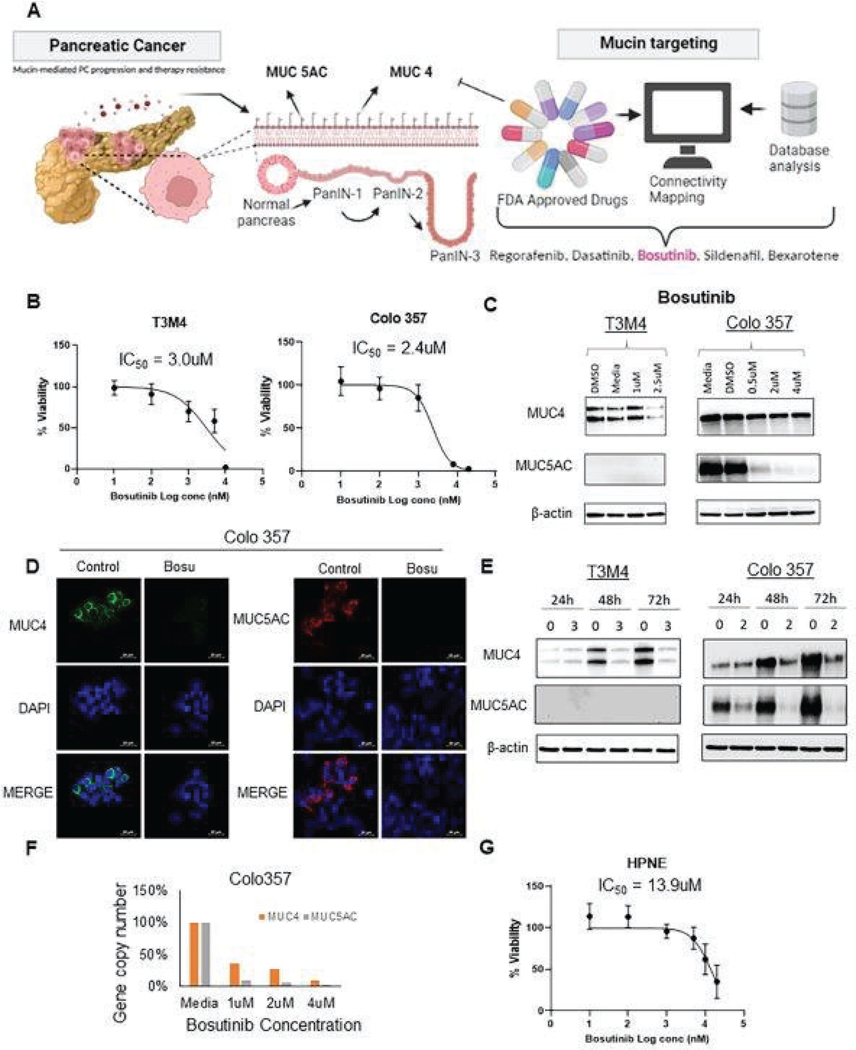
Fig. 1. Identification of Bosutinib as a potential MUC4 specific targeting SMIM.
(A) Schematic illustration of computational analysis using iLINCS to identify potential MUC4-specific small-molecule inhibitors through connectivity mapping and drug database. The five potential candidate drugs identified were: Regorafenib, Dasatinib, Bosutinib, Sildenafil, and Bexarotene. (B) PDAC cells T3M4 and Colo 357 were treated with Bosutinib concentrations ranging from 1 nM to 20 μM, and dose-dependent toxicity was measured using Calcein AM dye. Live cells will uptake non-fluorescent Calcein AM cell-permeant dye and convert it into green fluorescent Calcein by the action of esterase present endogenous within the cancer cell. Based on the green fluorescence viability and IC50 of each drug was calculated. (C) PDAC cells were treated with Bosutinib for 48 hr, and the dose-dependent treatment effect of Bosutinib in PDAC cells and its effect on MUC4 and MUC5AC protein expression by Western blots analysis. (D) Representative immunofluorescent images of Colo 357 cell line and its impact on MUC4 and MUC5AC expression upon Bosutinib treatment. Scale Bar denotes 20μm. (E) Time-dependent treatment effects of Bosutinib in PDAC cells and its effect on MUC4 and MUC5AC protein expression as detected by western blot analysis. (F) Digital Droplet PCR (ddPCR) detects MUC4 and MUC5AC at the transcription level upon Bosutinib exposure in PDAC cells. Specifically, each sample was fractionated into 20,000 droplets, and PCR amplification of the template molecules (MUC4, MUC5AC) occurred in each individual droplet. The final gene copy number was counted and normalized to the β-actin copy number. (G) Viability of the normal immortalized pancreatic epithelial nestin-positive cell line, HPNE, after Bosutinib treatment.
3.2. Small molecule inhibitor of mucins and Gemcitabine affects PDAC cell proliferation and growth.
PDAC cell lines T3M4, CD18/HPAF, and Colo 357 were treated with different doses of the five potential SMIMs for 48 hours. Calcein AM cytotoxicity assay demonstrated that Bosutinib reduces the viability of PDAC cells in a dose-dependent (0–20uM) manner (Fig. 1B). We observed that the IC50 of Bosutinib was 3.0uM in T3M4 and 2.4uM in Colo 357 PDAC cells. Similarly, Regorafenib and Dasatinib demonstrated dose-dependent cytotoxicity with IC50 of 1.4uM and 1.6uM, respectively (Fig. S1A and S1B). Sildenafil and Bexarotene did not demonstrate any observable cytotoxicity on the PDAC cell lines tested (Fig. S1C and S1D). In addition to the molecularly targeted agent, we also tested the viability and response against Gemcitabine in T3M4 and Colo 357 PDAC cells. Our results demonstrated that Gemcitabine inhibits the growth of T3M4 (IC50 of 797 nM) and Colo 357 (IC50 of 134 nM) PDAC cells (Fig. S1E and S1F).
3.3. Bosutinib and other in silico identified SMIMs inhibit mucin expression in a dose- and time-dependent manner in PDAC and other MUCs expressing cancer cells.
To evaluate the effect of individual drugs on mucin expression, the protein lysates were analyzed using western blot and confocal analyses. Bosutinib treatment resulted in a dose-dependent reduction in MUC4 protein expression in both T3M4 and Colo 357 cells and MUC5AC protein expression in Colo 357 cell line (Fig. 1C, 1D and, Fig. S2A). In addition, the time-dependent Bosutinib treatment in PDAC cells downregulated MUC4 and MUC5AC transcription by QPCR analysis (Fig. S2B). Sildenafil reduced MUC4 expression in both CD18/HPAF and T3M4 cell lines, whereas Bexarotene reduced MUC4 expression in the T3M4 cell line alone (Fig. S3). In contrast, Regorafenib and Dasatinib did not show any observable reduction in MUC4 protein expression (Fig. S3A, S3B, S3C, and S3D). On the other hand, Bexarotene did not affect MUC5AC protein in Colo 357 cell line and Sildenafil in CD18/HPAF and T3M4. Further, Dasatinib treatment significantly increased MUC5AC protein emphasizing the compensatory activation of Mucins. We could not detect any alteration of MUC5AC protein in response to Regorafenib, Bosutinib, Sildenafil, Dasatinib, and Bexarotene in T3M4 PDAC cells due to undetectable endogenous levels. Taking into consideration of both the cytotoxicity on PDAC cells and the significant downregulation of the expression of multiple mucins, Bosutinib was selected as the main drug of interest for this study. Supportive to PDAC cells, Cholangiocarcinoma (KMCH), NSCLC (H1437, A549), and breast cancer cells (MDA MB 231), treatment with Bosutinib showed a reduction in MUC4 (KMCH and H1437) and MUC5AC (KMCH, A549, MDA MB 231) protein expression. Similar to MUC5AC in PDAC cells, we could not detect alteration in MUC4 mucin in A549 and MDA MB 231 cells due to a lack of endogenous MUC4 (Fig. S4).
Further investigation in Bosutinib showed that the reductions in mucin protein expression were time-dependent (Fig. 1E) and occurred at the transcription level, as demonstrated by the digital droplet PCR assay (Fig. 1F and Fig. S3E). In addition, Bosutinib resulted in a persistent reduction in mucin expression in combination therapy with Gemcitabine (Fig. S5). Finally, Bosutinib showed low cytotoxicity in a normal immortalized pancreatic nestin-positive epithelial cell line (HPNE) with IC50 of 13.9uM (Fig. 1G), likely due to the absence of MUCINS (MUC4 and MUC5AC) in these cells.
3.4. Bosutinib treatment results in global transcriptome variations in PDAC cells
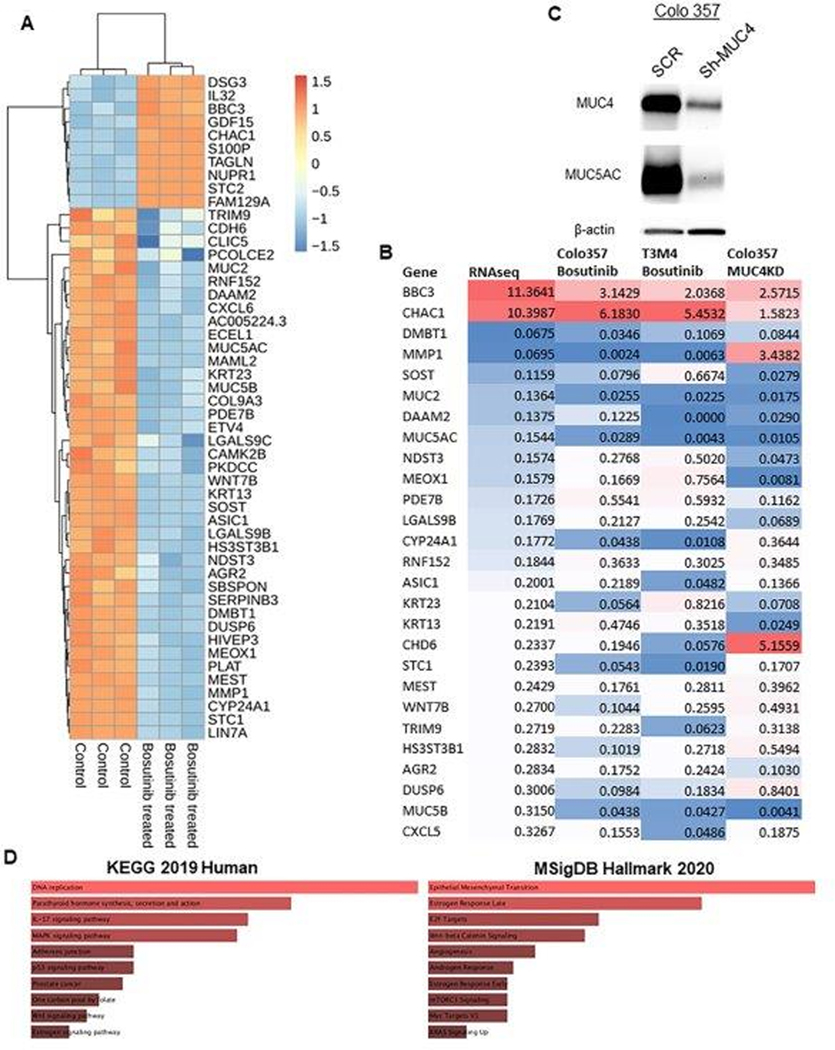
Colo 357 cells treated with/without Bosutinib were subject to RNA sequencing analyses. After Bosutinib treatment, 3882 genes were significantly downregulated, and 3885 genes were significantly upregulated (both p<0.05). A summary of the top 10 upregulated and top 25 downregulated genes (ranked by log2 fold change) were shown in the heatmap (Fig. 2A), and these results were validated using qRT-PCR in Colo 357 and T3M4 PDAC cells treated with Bosutinib (Fig. 2C). Examples of the top downregulated genes included DMBT1, WNT7B, AGR2, DUSP6, and CXCL5. In addition, a Colo 357 MUC4-knockdown cell line was generated with MUC4 shRNA (Fig. 2B). The qRT-PCR analysis demonstrated that most transcriptome variations observed in Bosutinib-treated cells were also observed in MUC4-KD cells, with the exceptions of MMP1 and CHD6 (Fig. 2C). Lastly, pathway analysis using KEGG 2019 Human database and MSigDB Hallmark 2020 database demonstrated that multiple key oncologic pathways were downregulated, including DNA replication, IL-17 signaling, MAPK signaling, Wnt-beta Catenin signaling, Epithelial-Mesenchymal Transition (EMT), Angiogenesis, Myc pathway, as well as KRAS signaling (Fig. 2D). Further, ingenuity pathway analysis (IPA) on significantly downregulated genes (Log2Fold change <−1) upon Bosutinib treatment revealed that biological functions such as tumor cell migration and invasion, formation of cellular protrusions, cellular transformation, cytoskeleton organization, and colorectal cancer metastasis signaling were affected. IPA analysis on the cancer network demonstrated several molecules associated with cancer cell signaling and metastasis, such as Ras, Rac, Rho-GTP, Rho-GDP, Rho-GDI-Rho-GDP, RHOBTB1, GPCR, JNK, PDGF, CREB, CaMKII, and phosphatase (PTPase) were affected upon Bosutinib treatment. Specifically, IPA revealed the impact of Bosutinib on cellular growth and proliferation (Fig. S6), suggesting that Bosutinib elicits a comprehensive signaling regulation via downregulating MUC4 and MUC5AC MUCs in PDAC.
Fig. 2. Global transcriptomic analysis in PDAC cell lines after Bosutinib treatment.
(A) Global RNA sequencing was performed on total RNA isolated from PDAC cells treated with Bosutinib for 48 hr. In total, 7,767 genes were significantly differentially expressed (p<0.05). A representative of the top 10 upregulated and top 25 downregulated genes are shown in the heat map. The scale bar represents row scales. (B) Colo 357 PDAC cells were stably transfected with shRNA-specifically targeting MUC4 and non-specific scramble vector control. Total cell lysates were prepared from both shRNA and scramble vector introduced PDAC cells and analyzed by western blot for knockdown of MUC4 and MUC5AC mucins. (C) Quantitative RT-PCR validation of RNA sequencing data in Colo 357, T3M4, and Colo 357 MUC4 KD cell lines. (D) Bar plots represent pathway enrichment analysis of significantly downregulated genes obtained from RNA sequencing upon Bosutinib treatment in PDAC cells, as analyzed using KEGG and MsigDB Hallmark database.
3.5. Bosutinib in combination with gemcitabine/5FU inhibits PDAC cell proliferation and colony growth in vitro
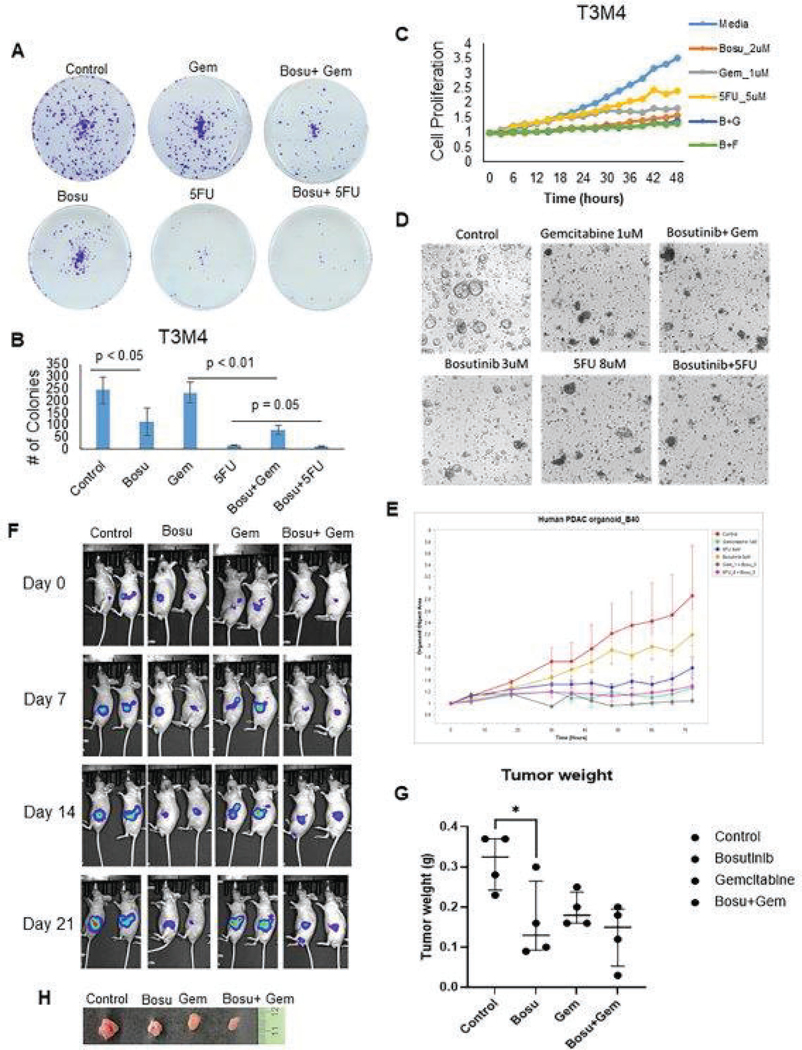
Bosutinib reduced PDAC cell growth potential through the colony formation assay and reduced their proliferation, as reflected by the proliferation assay using Incucyte® live imaging system (Fig. 3A, 3B, and 3C). When Bosutinib was combined with either Gemcitabine or 5FU, an additional reduction in tumorigenesis and proliferation was observed, as compared to gemcitabine or 5FU treatment alone (Fig. 3A, 3B, and 3C). Similar inhibition in growth and proliferation was observed in the human PDAC organoids treated with Bosutinib alone and in combination with Gemcitabine or 5FU (Fig. 3D, 3E, Fig. S7A, and S7B). Thus, functional analyses revealed Bosutinib consistently inhibited PDAC cell growth and proliferation across multiple 2D PDAC cell lines and 3D preclinical models.
Fig. 3. Bosutinib treatment reduces PDAC cell, tumoroid, and xenograft tumor proliferation, growth, and migration.
(A and B) Analysis of treatment effects of Bosutinib monotherapy and its combination effects with Gemcitabine or 5FU using Colony formation assay. Representative images of colony inhibition in response to Bosutinib ±gemcitabine or 5FU (A). The bar graph represents the average number of colonies formed or responded to drug treatment in the T3M4 cells (N=3) (B). (C) Proliferation inhibition of T3M4 PDAC cells after Bosutinib monotherapy and combination therapies of Bosutinib + Gemcitabine or 5FU. The line graph shows decreased cellular proliferation after treatment with Bosutinib, Gemcitabine, and 5FU alone, and it further decreased T3M4 cell proliferation in combination treatment for up to 48 hr. (D and E) Human PDAC organoids were treated with Bosutinib alone and in combination with Gemcitabine or 5FU. Representative light microscope images showing the effect of Bosutinib alone and in combination with Gemcitabine or 5FU on PDAC organoid growth (D). The organoid growth rate was monitored using the IncuCyte® sx5 live imaging system. The line graph shows the reduction in organoid growth against Bosutinib as a single agent and/or with gemcitabine or 5FU (E). (F-H) In vivo Colo 357 xenograft model demonstrating reduced tumor growth and weight after Bosutinib monotherapy and combination therapy with Gemcitabine. Representative bioluminescence images of athymic nude mice after orthotopic implantation of luciferase-tagged Colo 357 PDAC cells implanted in the pancreas followed by treatment with Bosutinib (oral gavage) and /or gemcitabine (intraperitoneal route (i.p)) or 5 FU (i.p). Images were acquired at weekly intervals on days 0, 7, 14, and 21 after the start of drug treatment. Tumor responses against drug treatment were evaluated based on bioluminescent images acquired by i.p injection of luciferin to athymic nude mice, followed by imaging using an IVIS imaging system (F). The dot plot shows the tumor weight of individual xenografts from each cohort at necropsy (N=4) (G). Representative images of Colo 357 xenograft tumor excised from PBS control, Bosutinib, Gemcitabine, and its combination-treated mice (H).
3.6. Bosutinib treatment significantly decreased tumor growth and metastasis
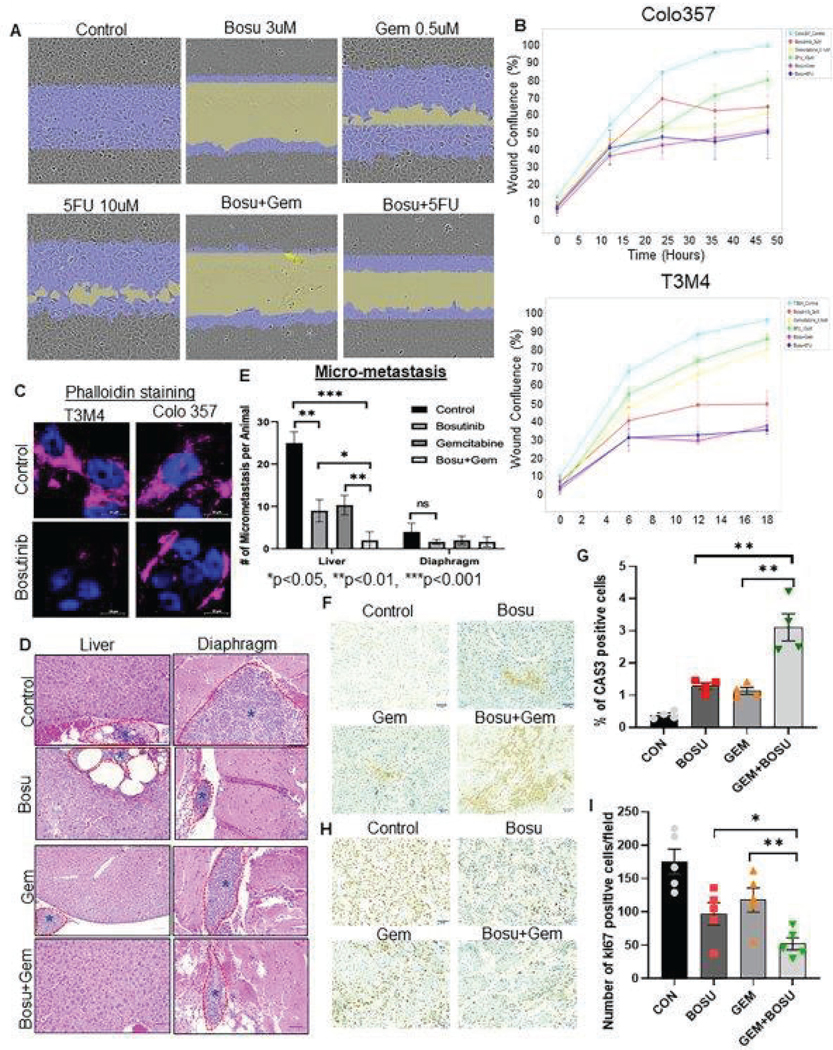
Lastly, in vivo xenograft experiments in athymic mice were performed by undergoing orthotopic implantation of luciferase-labeled Colo 357 cells in mice pancreas. Two weeks after tumor implantation, mice were randomized into four groups: control, Bosutinib, Gemcitabine, and Bosutinib+Gemcitabine combination. Bosutinib monotherapy resulted in significant tumor weight reduction compared to Gemcitabine and control, and the combination group showed a trend toward lower tumor growth and weight compared to gemcitabine monotherapy (Fig. 3F, 3G, and 3H). In addition, Bosutinib monotherapy reduced T3M4 and Colo 357 cells’ wound healing potential, and the effect was further augmented in combination therapy with either Gemcitabine or 5FU (Fig 4A, 4B, and Fig. S8A). Similarly, another SMIMs targeting agent, Sildenafil, also inhibited the motility and wound healing capacity of MUC4 expressing T3M4 and Capan-1 PDAC cells (Fig. S8B). Furthermore, immunofluorescent analysis of phalloidin staining demonstrated reduced actin filament in T3M4 and Colo 357 cells treated with Bosutinib, suggesting that Bosutinib could decrease cellular cytoskeleton dynamics and stability (Fig. 4C). Lastly, in in vivo xenograft experiment, mice treated with Bosutinib developed fewer liver (p<0.01) and diaphragmatic micro-metastases compared to control and fewer liver micro-metastases in the Bosutinib+gemcitabine combination therapy group compared to control (p<0.001) or Gemcitabine alone (p<0.01) (Fig. 4D and 4E). Positively, tissue staining analysis of xenograft tissues treated with Bosutinib and/or Gemcitabine revealed an increase in positive cytoplasmic staining for cleaved caspases-3 and a decrease in positive nuclear staining for the proliferation marker Ki67 in combination drug treatment groups compared to single (Bosu or Gem) agents. These findings imply that Bosutinib inhibits xenograft growth by increasing apoptosis and decreasing the proliferation index of PC cells (Fig. 4F, 4G, 4H, and 4I).
Fig. 4. Bosutinib reduces PDAC tumor cell growth, migration, and metastasis.
(A and B) Representative micrographs show the wound healing effects of Bosutinib alone and combined with Gemcitabine and 5FU in PDAC cells. The IncuCyte® sx5 live imaging system was used to monitor the wound healing capacity of PDAC cells upon Bosutinib and/or Gemcitabine alone and in combination treatment, and the system was programmed to capture images every 6 hours for up to 48 hr (A). The line graph demonstrates the cell migration inhibitory potential of Bosutinib ±gemcitabine or 5FU in T3M4 and Colo 357 cell lines (B). (C) Representative immunofluorescent images demonstrate reduced phalloidin staining after Bosutinib treatment in T3M4 and Colo 357 PDAC cells. The scale bar denotes 10μm. (D) Representative Hematoxylin and Eosin stained sections of micrometastases PDAC tumor to liver and diaphragm in vivo xenograft model. Dotted lines show reduced liver and diaphragmatic metastasis after Bosutinib monotherapy and combination therapy with Gemcitabine. Light microscopy images were taken at 200X magnification (10X (eyepiece) x 20X objective lens), and a scale bar was drawn using the Leica LAS EZ software. (E) The bar graph shows the number of micrometastases observed with drug treatment in the in vivo xenograft model. Bosutinib and Gemcitabine monotherapy significantly reduced the number of liver metastases; combination treatment of Bosutinib and Gemcitabine resulted in further reduction in liver metastases compared to Gemcitabine alone (p<0.05). (F- I) The effect of Bosutinib alone and in combination with Gemcitabine was assessed using expression analysis of apoptosis marker cleaved caspase-3 and proliferation marker Ki67 in PDAC xenograft tissues. (F) Representative micrographs showing cytoplasmic staining for cleaved Caspases 3 antibody in primary pancreatic xenograft tissues of all the treatment groups. (G) The bar combined with the scatter plot shows the quantification of cleaved caspase-3 positive cells per field in each group (** P<0.01). (H) Representative images of Ki67 nuclear staining in xenograft tissues excised from mice exposed to Bosutinib and/or Gemcitabine. (I) Bar graph with scatter plot demonstrated the difference in Ki67 nuclear positive expression between the drug-treated mice xenograft tissue (*P<0.05, **P<0.01). Statistical significance between groups was determined using a student t-test. The scale bars for F and H denote 6 mm as measured through Image J software.
3.7. Bosutinib downregulates mucin expression via Src-AKT/ERK-FosL1 pathway
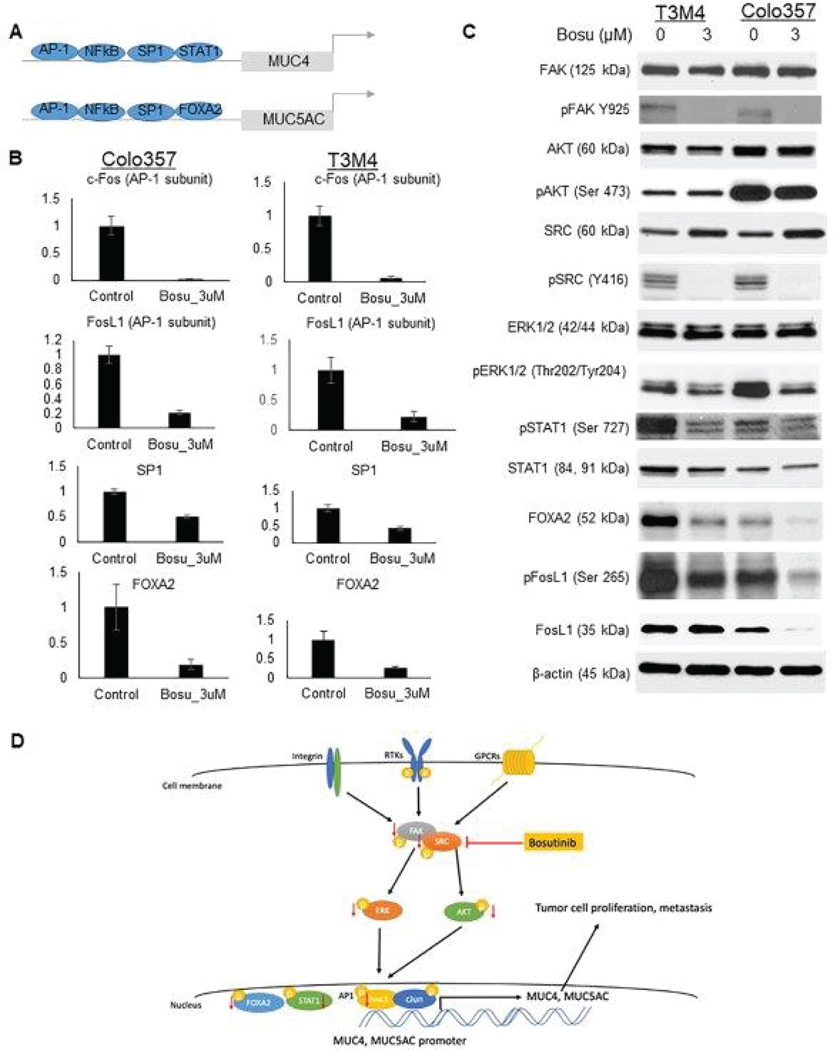
It has been previously established that Bosutinib is a dual inhibitor of BCR-ABL and src tyrosine kinases [27]. Apart from mucins, several downstream oncogenic and migration-associated signaling pathways, including the AKT and ERK, were also affected due to Bosutinib treatment. Our western blot validated the downregulation of phospho-Src after Bosutinib treatment and phospho-FAK, a component in the src multiprotein complex. In addition, multiple transcription factors related to MUC4 and MUC5AC were found to be downregulated in PDAC cells treated with Bosutinib, including c-Fos, FosL1, SP1, FOXA2, STAT1, and RelA/p65 (Fig. 5A, 5B, 5C and Fig. S9). FosL1 is a common transcription factor of MUC4 and MUC5AC and was significantly downregulated in T3M4 and Colo 357 cell lines. Figure 5D summarizes the schematic representation of the proposed mechanism of Bosutinib in PDAC cells, impacting reduced expression of MUC4 and MUC5AC mucins expression and signaling.
Fig. 5. Mechanism of action of Bosutinib impacting expression of MUC4 and MUC5AC.
(A) Schematic representation of MUC4 and MUC5AC promoters and transcription factors regulating MUC4 and MUC5AC mucin expression. (B) q-RTPCR analysis of expression of common transcription factors of MUC4 and MUC5AC in Colo 357 and T3M4 PDAC cell lines after Bosutinib treatment. Data are shown as fold change (log2) + SD (N = 3). (C) PDAC cells (T3M4, Colo 357) were treated with the indicated concentration of Bosutinib for 48 hr, and cell lysates were harvested. Proteins were analyzed by western blot analysis using antibodies specific for total and phosphorylated forms of FAK, AKT, ERK, SRC, and common transcription factors specific for MUC4 and MUC5AC (STAT1, FOXA2, FosL1). (D) Schematic representation of the proposed mechanism of action of Bosutinib impacting MUC4 and MUC5AC expression in PDAC. Western blotting results show that Bosutinib inhibits SRC/FAK multiprotein complex and downregulates ERK and AKT pathways, leading to reduced transcription factors such as FosL1, FOXA2, and STAT1, resulting in reduced expression of MUC4 and MUC5AC.
3.8. Bosutinib inhibits pancreatic cancer cell activities via the Wnt pathway
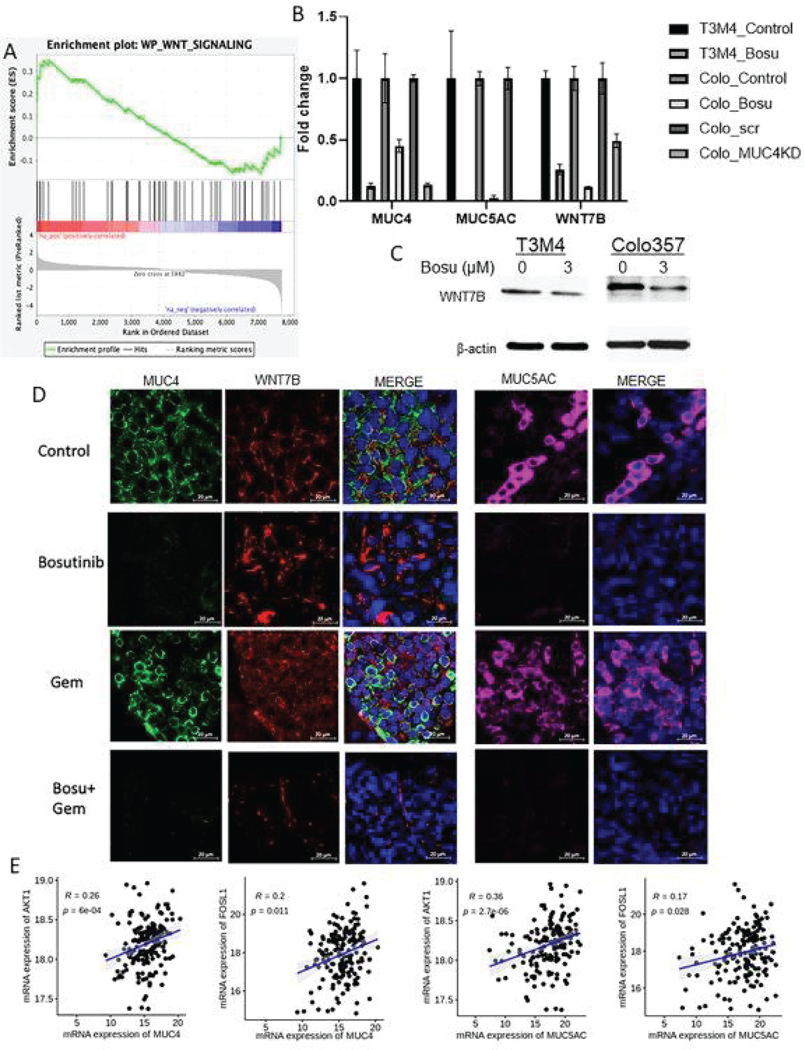
To further investigate the downstream effect of Bosutinib after the downregulation of mucins, enrichment pathway analysis was performed using the RNA sequencing results. Compared with pancreatic cancer cells treated with Bosutinib, the Wnt signaling pathway was significantly enriched in the control cells (Fig. 6A). Genes involved in the Wnt pathway were significantly downregulated after Bosutinib treatment, including SOST, DAAM2, and WNT7B. In particular, WNT7B was significantly downregulated in Bosutinib-treated T3M4 and Colo 357 cells and Colo 357 MUC4-KD cells, suggesting WNT7B is a downstream molecule in MUC4 signaling (Fig. 6B and 6C). Lastly, athymic mice treated with Bosutinib or a combination of Bosutinib and Gemcitabine showed significant downregulation of MUC4, MUC5AC, and WNT7b expression (Fig. 6D). Our results confirm that mucin signaling disruption, specifically MUC4 mucin downregulation, can affect WNT signaling.
Fig. 6. Impacts on PDAC cellular function by affecting WNT signaling and its upstream and downstream regulator’s clinical correlation.
(A) Wiki pathway gene set enrichment analysis was performed using RNA sequencing results obtained from Colo357 PDAC cells treated with or without Bosutinib. The enrichment analysis demonstrates downregulations of genes associated with the WNT signaling pathway after Bosutinib treatment, including WNT7b, SOST, DAAM2, and CAMK2B. (B) q-RTPCR analysis of MUC4, MUC5AC, and WNT7B gene expression in T3M4, Colo 357 after 48 hr of Bosutinib treatment, and Colo 357 MUC4KD and scramble control cell lines. Data are shown as fold change (log2) + SD (N = 3). (C) Western blots of WNT7B protein expression in T3M4 and Colo 357 cell lines after 48 hr of Bosutinib treatment. (D) Representative immunofluorescent images of MUC4, WNT7B, and MUC5AC protein expression and membranous localization in xenograft tumor tissues after Bosutinib monotherapy or combination therapy of Bosutinib and Gemcitabine. The scale bar denotes 20μm. (E) We extracted the transcriptome data from TCGA-PAAD and analyzed for clinical correlation between the mRNA expression of MUC4 and MUC5AC with AKT1, and FOSL1. Scatter plots showing a positive Spearman correlation between MUC4 and AKT1 (r=0.26, p=0.0006), MUC4 and FOSL1 (r = 0.2, p=0.011), MUC5AC and AKT1 (r = 0.36), p<0.001), and MUC5AC and FOSL1 (r = 0.17), p=0.028) in the TCGA dataset.
3.9. MUC4 and MUC5AC expression correlated with AKT, FOSL1, and WNT7B
Though Bosutinib treatment in PDAC cells downregulates AKT, ERK, FOSL1, and WNT signaling to reduce mucin expression, clinical correlation among these mucin-regulating transcription factors or regulators remains largely unexplored. Hence, through TCGA-PAAD global transcriptomic data, we analyzed the correlation between mucins and AKT, FOSL1, and WNT7B. We found a statistically significant correlation between AKT1 and MUC4 (Spearman correlation (r = 0.26), p=0.0006), FOSL1, and MUC4 (Spearman correlation (r = 0.2), p=0.011), and WNT7B and MUC4 (Spearman correlation (r = 0.41), p<0.001) co-expression (Fig. 6E and Fig. S10A). Similarly, we also observed a similar trend and positive correlation between AKT versus MUC5AC (Spearman correlation (r = 0.36), p<0.001), FOSL1 versus MUC5AC (Spearman correlation (r = 0.17), p=0.028), and WNT7B versus MUC5AC (Spearman correlation (r = 0.097), p=0.21) in the same TCGA patient cohort (Fig. 6E and Fig. S10A). Further, to obtain the correlation of mucins protein expression and FOSL1 and WNT7B in consecutive cases, we performed IHC analysis on serial sections of PDAC patient tissues. We found that both MUC5AC and WNT7B expressions were observed in serial sections of the same PDAC cases (Patinet#1, 2, 3), but there is no association or correlation between MUC5AC and WNT7B (ρ= −0.274, p=0.19) (Table S4). Similarly, FOSL1 and MUC4 composite scores were not significantly correlated with each other (ρ= −0.011, p=0.96) ((Table S5 and Fig. S10B). This may be due to the low number of cases analyzed. However, the expression of WNT7B (Wilcoxon rank sum test, grade (p=0.020), and stage 2 versus 3 or4 (p=0.026)) and FOSL1 (Wilcoxon rank sum test, stage 2 versus 3 or4 (p=0.032)) showed a significant correlation with tumor grade and stage. These results suggest that Bosutinib will elicit its inhibitory action on PDAC cell proliferation and metastasis by targeting widely expressed WNT7B or FOSL1 signaling regulators that eventually downregulate pathogenic mucins (MUC4 or MUC5AC).
4. Discussion
PDAC remains one of the deadliest cancers among major malignancies. One main contributing reason is the lack of effective therapy, resulting in drug resistance and disease recurrence in the majority of patients. In recent years, investigations in targeted therapy have yielded some promising therapeutic candidates, such as Erlotinib (EGFR inhibitor) in locally advanced or metastatic PDAC [28] and Olaparib (PARP inhibitor) in a minority of PDAC patients who harbor BRCA mutations [29]. Another therapeutic target that is less explored is mucin, specifically MUC4 and MUC5AC mucins. It has been demonstrated that MUC4 is important in PDAC tumor initiation, progression, metastasis, tumor interaction with TME, and drug resistance, and MUC5AC is important in PDAC metastasis, drug resistance, and escaping host response.[20] Therefore, this study aims to identify potential SMIMs targeting MUC4 mucin using bioinformatics and to analyze the therapeutic efficacy of identified MUC4 SMIMs using PDAC cell line models as well as preclinical 3D tumoroid and in vivo orthotopic mouse models.
Out of the 126 potential candidates SMIMs identified using the Cancer Therapeutic Response Database, we selected five candidates based on their existing literature and FDA approval status. Of these five drugs, Bosutinib is the most promising one as it inhibits the proliferation of PDAC cell lines and significantly downregulates the expression of MUC4 and MUC5AC mucins in multiple PDAC cell lines. Bosutinib is a dual inhibitor of BCR-ABL and SRC tyrosine kinases, affecting PARP activation for sensitization of cytotoxic agents [27, 30]. It has been FDA-approved for treating chronic myelogenous leukemia (CML) and is well-tolerated in clinical use. Additionally, other studies identified several cancer-related signaling target effects of Bosutinib, which contribute to its anti-cancer property, including inhibition of MAPK and Myc pathways [31], and the PARP DNA repair pathway [30]. Consistent with the existing literature, we observed global transcriptomic variations involving 7737 genes. Many of the downregulated genes are oncogenic targets involved in pathways such as DNA replication, IL-17 signaling, MAPK signaling, Wnt signaling, EMT process, and Myc pathways. While our study’s primary goal was to identify a MUC4 mucin targeting inhibitor, these anti-cancer off-targets of Bosutinib suggest a potentially superior therapeutic efficacy in clinical usage.
In this study, Bosutinib significantly reduced PDAC tumorigenesis and proliferation in PDAC cell line models and preclinical models, including organoid and mouse xenograft models. In particular, we generated the organoids from human PDAC surgical specimens, a model that has been shown in previous studies to provide a better resemblance of patient therapy response than traditional 2D cell culture [24, 25]. These results are consistent with the previously discussed functions of MUC4 mucin, which include tumor initiation and progression; by reducing MUC4 expression, Bosutinib effectively inhibits these cancer cell mechanisms [10, 12, 14–16, 32]. In addition, when combined with traditional chemotherapies such as Gemcitabine, Bosutinib demonstrated a persistent reduction in MUC4 and MUC5AC expression and functionally resulted in further reductions in tumorigenesis and proliferation in all in vitro and in vivo models. This finding aligns with previous reports on the role of MUC4 and MUC5AC in chemoresistance; by inhibiting these mucins, the therapeutic efficacy of traditional chemotherapies could be augmented [10, 13, 16, 20].
Early metastasis is a major challenge in PDAC management, as over 50% of PDAC patients have distant metastasis at initial presentation [1]. The exact mechanism of PDAC metastasis has yet to be elucidated. Nonetheless, several drivers have been identified to play important roles in this process, including a network of transcription factors and miRNAs regulating the EMT process, cancer stem cells (CSC), as well as mucins [33]. In particular, MUC4 has been shown to modulate the PDAC tumor microenvironment by interacting with basement membrane (BM) protein fibulin-2 and disrupting BM integrity [32], inhibiting integrin-based interaction of tumor cells with ECM proteins [12], as well as binding to circulating galectin-3 which exposes surface adhesion molecules for endothelial cell interaction to facilitate metastasis [8]. Similarly, MUC5AC facilitates distant metastasis by promoting the expression of α3 and β3 integrins, MMP3, and VEGF-α, activating the ERK and VEGFR1 pathways and disrupting E-Cadherin/ β-Catenin axis by Krüppel-like zinc-finger protein GLI1 [34, 35]. In this study, Bosutinib treatment resulted in a significant reduction in the expression of MUC4 and MUC5AC in PDAC cell lines and functionally reduced tumor cell migration potential in vitro as well as metastasis in vivo, including Bosutinib monotherapy and Bosutinib + gemcitabine combination compared to control or Gemcitabine alone, respectively. These findings are consistent with the existing understanding of the important tumor-promoting role of MUC4 and MUC5AC mucins on PDAC metastasis.
Mechanistically, our results suggest that Bosutinib regulates the expression of MUC4 and MUC5AC via the Src-AKT/ERK-FosL1 pathway. This result is supported by previously published work by Vultur et al., which demonstrated the central function of Bosutinib in inhibiting the Src/FAK multiprotein complex and impacting downstream events [36]. Several other MUC4 and MUC5AC transcription factors were also significantly downregulated in this study, such as pSTAT1, FOXA2, SP1, and RelA/p65, suggesting other pathways that regulate the expression of these mucins were also impacted by Bosutinib treatment and remained to be elucidated. Lastly, enrichment analysis and RNA and protein validations suggest that the WNT signaling pathway, especially WNT7B, is likely one of the mediators between the downregulated MUC4/MUC5AC mucins and the reduced tumor cell functions observed above. It has been previously reported that WNT7B promotes cell-autonomous Wnt/β-Catenin activation and in vitro and in vivo tumorigenesis, stemness, and chemoresistance in PDAC high expression of WNT7B in patients has been associated with reduced overall survival [37, 38]. Therefore, our results suggest Bosutinib reduces the expression of MUC4 and MUC5AC mucins, which in turn inhibits WNT7B and the WNT pathway, leading to decreased PDAC tumorigenesis and progression.
In conclusion, Bosutinib treatment reduced MUC4 and MUC5AC mucin expression in PDAC tumor cells and inhibited PDAC tumorigenesis, proliferation, and metastasis in vitro and in vivo. Combination therapy of Bosutinib and Gemcitabine further reduced PDAC tumor proliferation and metastasis. These preclinical findings should be validated in clinical trials to evaluate the potential role of Bosutinib as an adjunct to the current PDAC treatment regimen. In fact, Bosutinib has been tested in phase I clinical trials of advanced solid tumors either as a monotherapy or in combination with capecitabine but did not yield significant benefits in overall survival. However, with the findings from this study, these clinical trials could be improved by specifically selecting patients with high MUC4/MUC5AC expression who might derive the most benefit from this targeted therapy. It has been suggested for clinical trials of other targeted therapies such as Erlotinib that without selecting participants with the targeted mutation, the clinical impact of a small molecule inhibitor might be masked by the genetic heterogeneity of study participants [39, 40]. Therefore, we recommend that future targeted clinical trials of Bosutinib should start with patients with high MUC4/ MUC5AC expression to evaluate its therapeutic efficacy and to truly fulfill the goals of personalized medicine.
Supplementary Material
Both T3M4 and Colo 357 PDAC cells were treated with Bosutinib, and images were acquired at 0 hr (before drug treatment) and after 24 hr (initiation of treatment). PDAC cells were analyzed for their decreased migratory effect in response to Bosutinib alone. Representative images of Bosutinib treatment at 0 and 24 hr, respectively, in PDAC cells (A). MUC4 expressing T3M4 and Capan-1 cells were treated with Sildenafil, and representative images acquired at 0 and 24 hr were presented (B).
Regorafenib (A), Dasatinib (B), Sildenafil (C), Bexarotene (D), and Gemcitabine (E) affect PDAC cell proliferation and growth. PDAC cells were treated with the indicated concentration of drugs followed by Calcein AM dye treatment. Viable cells were measured by green fluorescence/luminescence emitted by the dye using a luminometer and IC50 for each drug was calculated based on the dose-response curve generated using Graphpad Prism 8 software.
PDAC cells were seeded in 12 well plates with a sterile coverslip (10, 000 cells/well) for confocal analysis and in a 60 mm culture dish (1×106) for RNA analysis. Bosutinib was treated with indicated concentration (3μM) for 48 hrs and stained for MUC4 (8G7) antibody followed by fluorescently conjugated secondary (Alexa Flour) antibody exposure. (A) Representative confocal microscopy images of T3M4 cells after Bosutinib treatment followed by staining with MUC4 (8G7) antibody counterstained with DAPI. Scale Bar denotes 20μm. (B) T3M4 PDAC cells were treated with the indicated concentration of Bosutinib for 48 and 72 hr. total RNA was extracted, and cDNA was converted, followed by detection using qRTPCR primers specific for MUC4 and MUC5AC. The bar graph shows mRNA expression of MUC4 and MUC5AC upon Bosutinib treatment relative to untreated controls. MUC4 and MUC5AC transcripts were normalized using loading controls (β-action or GAPDH).
(A-D) the in silico identified SMIMs were tested for their potency to affect mucin expression. T3M4 and Colo 357 PDAC cells were treated with an increasing dose of Regorafenib (A), Sildenafil (B), Dasatinib (C), and Bexarotene (D). Doses for each drug were decided based on viability assay. Cell lysates were prepared, and mucin expression was analyzed by western blot against antibodies against MUC4 and MUC5AC mucins. (E) Absolute copy number alteration of MUC4 and MUC5AC in response to various indicated concentrations of Bosutinib in Colo357 PDAC cells. Positive droplets were indicated by blue dots, and negative droplets were specified as grey dots.
Cholangiocarcinoma cells (KMCH), NSCLC (A549), and breast cancer (MDA MB 231) cells were treated with the indicated concentration of Bosutinib and analyzed for MUC4 and MUC5AC protein expression using 2% SDS-agarose electrophoresis and PVDF membranes transferred mucins were visualized by probing with antibodies against MUC4 and MUC5AC. Beta-actin was used as an internal loading control.
Detection of MUC4 and MUC5AC protein expression using western blot analysis against specified Bosutinib monotherapy and combination treatment with Bosutinib and Gemcitabine in PDAC cells.
A list of 35 genes was recognized by IPA from the input of significantly downregulated genes (log2Fold changes <−1) upon Bosutinib treatment to construct a connectivity network related to cancer-associated genes. Bosutinib-mediated downregulated genes are demonstrated as a graphical summary of significantly downregulated genes and their association with cell growth and proliferation.
(A and B) Human PDAC organoids were treated with Bosutinib and/or Gemcitabine, or 5FU with indicated concentration, and its effect was observed up to 72 hr in the IncuCyte® sx5 live imaging system. Representative light microscope images show the relative proliferation of human organoids in response to Bosutinib+Gemcitabine or 5FU (A). The area occupied by each organoid and their proliferation rate was demonstrated as a line graph under each drug treatment.
PDAC cells were treated with the indicated concentration of Bosutinib, and cells were harvested, RNA was isolated, and qRT-PCR was conducted to examine the relative fold change of MUCs specific transcription factors STAT1 and RelA/p65 (NFκB subunit).
Mucin and other transcripts were analyzed in the TCGA data set, and protein expression was analyzed in the serial section of PDAC patients for their correlative and mutual coexpression in the same patients. (A) Scatter plot demonstrating a statistically significant correlation between MUC4 and WNT7B (left) (Spearman correlation, r=0.41, p<0.0001). The scatter plot illustrates a non-significant association between MUC5AC and WNT7B (Spearman correlation, r=0.097, p=0.21) in the TCGA-PAAD dataset. (B) Representative light microscope images of PDAC patient tissues (#1, 2, and 3) exhibit positive staining for MUC5AC, WNT7B, MUC4, and FOSL1.
Acknowledgments and funding
This research work was supported by funding, in parts, by the following grants from the National Institutes of Health/National Cancer Institute (NIH/NCI) Grants R01CA263575, R01CA256973, R01CA273349, R01 CA183459, R01 CA195586, R01 CA206444, R01 CA210637, R01 CA228524, U01 CA200466, U01 CA210240, and P01 CA217798. Schematic figures were generated using BioRender.com.
Abbreviations:
- PDAC
Pancreatic ductal adenocarcinoma
- MUC4
Mucin 4
- MUC5AC
Mucin 5AC
- SIMMs
Small Molecule Inhibitors against Mucins
- CSC
cancer stem cells
Footnotes
Declaration of competing interest
SKB is a founding member of Sanguine Diagnostics and Therapeutics, Inc. All other authors declare no potential conflicts of interest.
References
- [1].SEER Cancer Stat Facts: Pancreatic Cancer.
- [2].Hammel P, Huguet F, van Laethem JL, Goldstein D, Glimelius B, Artru P, Borbath I, Bouche O, Shannon J, Andre T, Mineur L, Chibaudel B, Bonnetain F, Louvet C, Group LAPT, Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial, JAMA, 315 (2016) 1844–1853. [DOI] [PubMed] [Google Scholar]
- [3].Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L, Tejani MA, Seery TE, Dy IA, Al Baghdadi T, Hendifar AE, Doyle LA, Lowy AM, Guthrie KA, Blanke CD, Hochster HS, Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial, JAMA Oncol, 3 (2017) 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW, Propper D, Wasan H, Falk S, Cunningham D, Coxon F, Ross P, Madhusudan S, Wadd N, Corrie P, Hickish T, Costello E, Campbell F, Rawcliffe C, Neoptolemos JP, Vandetanib plus Gemcitabine versus placebo plus Gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial, Lancet Oncol, 18 (2017) 486–499. [DOI] [PubMed] [Google Scholar]
- [5].Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grutzmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dorken B, Oettle H, Riess H, CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial, J Clin Oncol, 35 (2017) 3330–3337. [DOI] [PubMed] [Google Scholar]
- [6].Bafna S, Singh AP, Moniaux N, Eudy JD, Meza JL, Batra SK, MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells, Cancer Res, 68 (2008) 9231–9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chaturvedi P, Singh AP, Batra SK, Structure, evolution, and biology of the MUC4 mucin, FASEB J, 22 (2008) 966–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK, Novel INTeraction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer, Clin Cancer Res, 17 (2011) 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bafna S, Kaur S, Momi N, Batra SK, Pancreatic cancer cells resistance to Gemcitabine: the role of MUC4 mucin, Br J Cancer, 101 (2009) 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mimeault M, Johansson SL, Senapati S, Momi N, Chakraborty S, Batra SK, MUC4 down-regulation reverses chemoresistance of pancreatic cancer stem/progenitor cells and their progenies, Cancer Lett, 295 (2010) 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK, Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis, Cancer Res, 64 (2004) 622–630. [DOI] [PubMed] [Google Scholar]
- [12].Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK, MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins, Mol Cancer Res, 5 (2007) 309–320. [DOI] [PubMed] [Google Scholar]
- [13].Skrypek N, Duchene B, Hebbar M, Leteurtre E, van Seuningen I, Jonckheere N, The MUC4 mucin mediates gemcitabine resistance of human pancreatic cancer cells via the Concentrative Nucleoside Transporter family, Oncogene, 32 (2013) 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chaturvedi P, Singh AP, Chakraborty S, Chauhan SC, Bafna S, Meza JL, Singh PK, Hollingsworth MA, Mehta PP, Batra SK, MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells, Cancer Res, 68 (2008) 2065–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ponnusamy MP, Singh AP, Jain M, Chakraborty S, Moniaux N, Batra SK, MUC4 activates HER2 signalling and enhances the motility of human ovarian cancer cells, Br J Cancer, 99 (2008) 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW, Mahapatra S, Batra SK, Jain M, MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma, Expert Opin Ther Targets, 21 (2017) 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perrais M, Pigny P, Ducourouble MP, Petitprez D, Porchet N, Aubert JP, Van Seuningen I, Characterization of human mucin gene MUC4 promoter: importance of growth factors and proinflammatory cytokines for its regulation in pancreatic cancer cells, J Biol Chem, 276 (2001) 30923–30933. [DOI] [PubMed] [Google Scholar]
- [18].Kumar S, Das S, Rachagani S, Kaur S, Joshi S, Johansson SL, Ponnusamy MP, Jain M, Batra SK, NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer, Oncogene, 34 (2015) 4879–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sagar S, Leiphrakpam PD, Thomas D, McAndrews KL, Caffrey TC, Swanson BJ, Clausen H, Wandall HH, Hollingsworth MA, Radhakrishnan P, MUC4 enhances gemcitabine resistance and malignant behaviour in pancreatic cancer cells expressing cancer-associated short O-glycans, Cancer Lett, 503 (2021) 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Manne A, Esnakula A, Abushahin L, Tsung A, Understanding the Clinical Impact of MUC5AC Expression on Pancreatic Ductal Adenocarcinoma, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krishn SR, Ganguly K, Kaur S, Batra SK, Ramifications of secreted mucin MUC5AC in malignant journey: a holistic view, Carcinogenesis, 39 (2018) 633–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaushik G, Seshacharyulu P, Rauth S, Nallasamy P, Rachagani S, Nimmakayala RK, Vengoji R, Mallya K, Chirravuri-Venkata R, Singh AB, Foster JM, Ly QP, Smith LM, Lele SM, Malafa MP, Jain M, Ponnusamy MP, Batra SK, Selective inhibition of stemness through EGFR/FOXA2/SOX9 axis reduces pancreatic cancer metastasis, Oncogene, 40 (2021) 848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seshacharyulu P, Halder S, Nimmakayala R, Rachagani S, Chaudhary S, Atri P, Chirravuri-Venkata R, Ouellette MM, Carmicheal J, Gautam SK, Vengoji R, Wang S, Li S, Smith L, Talmon GA, Klute K, Ly Q, Reames BN, Grem JL, Berim L, Padussis JC, Kaur S, Kumar S, Ponnusamy MP, Jain M, Lin C, Batra SK, Disruption of FDPS/Rac1 axis radiosensitizes pancreatic ductal adenocarcinoma by attenuating DNA damage response and immunosuppressive signalling, EBioMedicine, 75 (2022) 103772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaushik G, Ponnusamy MP, Batra SK, Concise Review: Current Status of Three-Dimensional Organoids as Preclinical Models, Stem Cells, 36 (2018) 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rauth S, Karmakar S, Batra SK, Ponnusamy MP, Recent advances in organoid development and applications in disease modeling, Biochim Biophys Acta Rev Cancer, 1875 (2021) 188527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Karmakar S, Rauth S, Nallasamy P, Perumal N, Nimmakayala RK, Leon F, Gupta R, Barkeer S, Venkata RC, Raman V, Rachagani S, Ponnusamy MP, Batra SK, RNA Polymerase II-Associated Factor 1 Regulates Stem Cell Features of Pancreatic Cancer Cells, Independently of the PAF1 Complex, via Interactions With PHF5A and DDX3, Gastroenterology, 159 (2020) 1898–1915 e1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Keller G, Schafhausen P, Brummendorf TH, Bosutinib: a dual SRC/ABL kinase inhibitor for the treatment of chronic myeloid leukemia, Expert Rev Hematol, 2 (2009) 489–497. [DOI] [PubMed] [Google Scholar]
- [28].Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National G. Cancer Institute of Canada Clinical Trials, Erlotinib plus Gemcitabine compared with Gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group, J Clin Oncol, 25 (2007) 1960–1966. [DOI] [PubMed] [Google Scholar]
- [29].Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algul H, O’Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL, Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer, N Engl J Med, 381 (2019) 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kirmizibayrak PB, Ilhan R, Yilmaz S, Gunal S, Tepedelen BE, A Src/Abl kinase inhibitor, bosutinib, downregulates and inhibits PARP enzyme and sensitizes cells to the DNA damaging agents, Turkish Journal of Biochemistry, 43 (2018) 101–109. [Google Scholar]
- [31].Winter GE, Rix U, Carlson SM, Gleixner KV, Grebien F, Gridling M, Muller AC, Breitwieser FP, Bilban M, Colinge J, Valent P, Bennett KL, White FM, Superti-Furga G, Systems-pharmacology dissection of a drug synergy in imatinib-resistant CML, Nat Chem Biol, 8 (2012) 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Senapati S, Gnanapragassam VS, Moniaux N, Momi N, Batra SK, Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells, Oncogene, 31 (2012) 3346–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, Schnurr M, Lauber K, Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches, Radiat Oncol, 14 (2019) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Inaguma S, Kasai K, Ikeda H, GLI1 facilitates the migration and invasion of pancreatic cancer cells through MUC5AC-mediated attenuation of E-cadherin, Oncogene, 30 (2011) 714–723. [DOI] [PubMed] [Google Scholar]
- [35].Yamazoe S, Tanaka H, Sawada T, Amano R, Yamada N, Ohira M, Hirakawa K, RNA interference suppression of mucin 5AC (MUC5AC) reduces the adhesive and invasive capacity of human pancreatic cancer cells, J Exp Clin Cancer Res, 29 (2010) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vultur A, Buettner R, Kowolik C, Liang W, Smith D, Boschelli F, Jove R, SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells, Mol Cancer Ther, 7 (2008) 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arensman MD, Kovochich AN, Kulikauskas RM, Lay AR, Yang PT, Li X, Donahue T, Major MB, Moon RT, Chien AJ, Dawson DW, WNT7B mediates autocrine Wnt/beta-catenin signaling and anchorage-independent growth in pancreatic adenocarcinoma, Oncogene, 33 (2014) 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang Z, Xu Y, Zhao C, Fzd7/Wnt7b signaling contributes to stemness and chemoresistance in pancreatic cancer, Cancer Med, 10 (2021) 3332–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoyer K, Hablesreiter R, Inoue Y, Yoshida K, Briest F, Christen F, Kakiuchi N, Yoshizato T, Shiozawa Y, Shiraishi Y, Striefler JK, Bischoff S, Lohneis P, Putter H, Blau O, Keilholz U, Bullinger L, Pelzer U, Hummel M, Riess H, Ogawa S, Sinn M, Damm F, A genetically defined signature of responsiveness to erlotinib in early-stage pancreatic cancer patients: Results from the CONKO-005 trial, EBioMedicine, 66 (2021) 103327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Renouf DJ, Tang PA, Hedley D, Chen E, Kamel-Reid S, Tsao MS, Tran-Thanh D, Gill S, Dhani N, Au HJ, Wang L, Moore MJ, A phase II study of erlotinib in gemcitabine refractory advanced pancreatic cancer, Eur J Cancer, 50 (2014) 1909–1915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Both T3M4 and Colo 357 PDAC cells were treated with Bosutinib, and images were acquired at 0 hr (before drug treatment) and after 24 hr (initiation of treatment). PDAC cells were analyzed for their decreased migratory effect in response to Bosutinib alone. Representative images of Bosutinib treatment at 0 and 24 hr, respectively, in PDAC cells (A). MUC4 expressing T3M4 and Capan-1 cells were treated with Sildenafil, and representative images acquired at 0 and 24 hr were presented (B).
Regorafenib (A), Dasatinib (B), Sildenafil (C), Bexarotene (D), and Gemcitabine (E) affect PDAC cell proliferation and growth. PDAC cells were treated with the indicated concentration of drugs followed by Calcein AM dye treatment. Viable cells were measured by green fluorescence/luminescence emitted by the dye using a luminometer and IC50 for each drug was calculated based on the dose-response curve generated using Graphpad Prism 8 software.
PDAC cells were seeded in 12 well plates with a sterile coverslip (10, 000 cells/well) for confocal analysis and in a 60 mm culture dish (1×106) for RNA analysis. Bosutinib was treated with indicated concentration (3μM) for 48 hrs and stained for MUC4 (8G7) antibody followed by fluorescently conjugated secondary (Alexa Flour) antibody exposure. (A) Representative confocal microscopy images of T3M4 cells after Bosutinib treatment followed by staining with MUC4 (8G7) antibody counterstained with DAPI. Scale Bar denotes 20μm. (B) T3M4 PDAC cells were treated with the indicated concentration of Bosutinib for 48 and 72 hr. total RNA was extracted, and cDNA was converted, followed by detection using qRTPCR primers specific for MUC4 and MUC5AC. The bar graph shows mRNA expression of MUC4 and MUC5AC upon Bosutinib treatment relative to untreated controls. MUC4 and MUC5AC transcripts were normalized using loading controls (β-action or GAPDH).
(A-D) the in silico identified SMIMs were tested for their potency to affect mucin expression. T3M4 and Colo 357 PDAC cells were treated with an increasing dose of Regorafenib (A), Sildenafil (B), Dasatinib (C), and Bexarotene (D). Doses for each drug were decided based on viability assay. Cell lysates were prepared, and mucin expression was analyzed by western blot against antibodies against MUC4 and MUC5AC mucins. (E) Absolute copy number alteration of MUC4 and MUC5AC in response to various indicated concentrations of Bosutinib in Colo357 PDAC cells. Positive droplets were indicated by blue dots, and negative droplets were specified as grey dots.
Cholangiocarcinoma cells (KMCH), NSCLC (A549), and breast cancer (MDA MB 231) cells were treated with the indicated concentration of Bosutinib and analyzed for MUC4 and MUC5AC protein expression using 2% SDS-agarose electrophoresis and PVDF membranes transferred mucins were visualized by probing with antibodies against MUC4 and MUC5AC. Beta-actin was used as an internal loading control.
Detection of MUC4 and MUC5AC protein expression using western blot analysis against specified Bosutinib monotherapy and combination treatment with Bosutinib and Gemcitabine in PDAC cells.
A list of 35 genes was recognized by IPA from the input of significantly downregulated genes (log2Fold changes <−1) upon Bosutinib treatment to construct a connectivity network related to cancer-associated genes. Bosutinib-mediated downregulated genes are demonstrated as a graphical summary of significantly downregulated genes and their association with cell growth and proliferation.
(A and B) Human PDAC organoids were treated with Bosutinib and/or Gemcitabine, or 5FU with indicated concentration, and its effect was observed up to 72 hr in the IncuCyte® sx5 live imaging system. Representative light microscope images show the relative proliferation of human organoids in response to Bosutinib+Gemcitabine or 5FU (A). The area occupied by each organoid and their proliferation rate was demonstrated as a line graph under each drug treatment.
PDAC cells were treated with the indicated concentration of Bosutinib, and cells were harvested, RNA was isolated, and qRT-PCR was conducted to examine the relative fold change of MUCs specific transcription factors STAT1 and RelA/p65 (NFκB subunit).
Mucin and other transcripts were analyzed in the TCGA data set, and protein expression was analyzed in the serial section of PDAC patients for their correlative and mutual coexpression in the same patients. (A) Scatter plot demonstrating a statistically significant correlation between MUC4 and WNT7B (left) (Spearman correlation, r=0.41, p<0.0001). The scatter plot illustrates a non-significant association between MUC5AC and WNT7B (Spearman correlation, r=0.097, p=0.21) in the TCGA-PAAD dataset. (B) Representative light microscope images of PDAC patient tissues (#1, 2, and 3) exhibit positive staining for MUC5AC, WNT7B, MUC4, and FOSL1.