Abstract
Increased immune cell infiltration into tumors is associated with improved patient survival and predicts response to immune therapies. Thus, identification of factors that determine the extent of immune infiltration is crucial, so that methods to intervene on these targets can be developed. T cells enter tumor tissues through the vasculature, and under control of interactions between homing receptors on the T cells and homing receptor ligands (HRLs) expressed by tumor vascular endothelium and tumor cell nests. HRLs are often deficient in tumors, and there also may be active barriers to infiltration. These remain understudied but may be crucial for enhancing immune-mediated cancer control. Multiple intratumoral and systemic therapeutic approaches show promise to enhance T cell infiltration, including both approved therapies and experimental therapies. This review highlights the intracellular and extracellular determinants of immune cell infiltration into tumors, barriers to infiltration, and approaches for intervention to enhance infiltration and response to immune therapies.
Keywords: lymphocytes, tumor-infiltrating; tumor microenvironment; cytotoxicity, immunologic; CD8-positive T-lymphocytes
Introduction
The density and spatial distributions of immune infiltrates in tumors, prior to treatment, are associated with patient survival and responses to immune therapy, for melanoma and other cancers.1–6 The extent of intratumoral immune cell infiltrate at any time reflects dynamic processes including immune cell extravasation from the vasculature and distribution among tumor cells, proliferation, survival, and retention in the tumor microenvironment (TME). In this review, we will address mechanisms that mediate T cell homing to tumors from the vasculature (section I), barriers to T cell infiltration, localization, and retention within the tumor mass (section II), and potential therapies to enhance homing to tumors (section III).
Barriers to immune cell extravasation and infiltration include multiple mechanisms such as vascular limitations, absence of critical T cell homing chemokines, extracellular matrix barriers, and cell-associated barriers. Understanding barriers to intratumoral immune infiltrates can identify better targets to improve efficacy of therapies.
Mechanisms of T cell homing
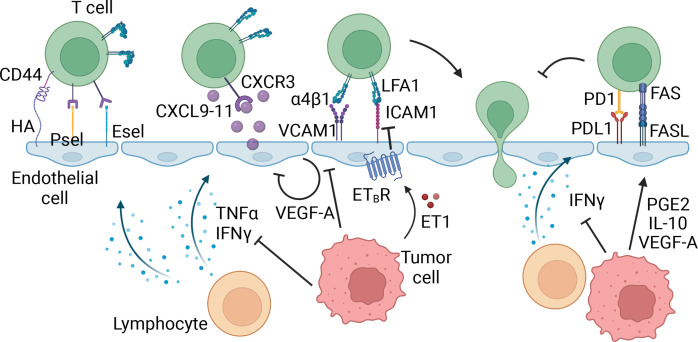
Tumors may be infiltrated by naïve, effector, or effector-memory cells.7–9 To enter peripheral tissues, including tumors, T cells must extravasate through the vasculature via a multistep process, which has been described in detail by Ley et al, and is summarized in figure 1 and table 1.10 The specific repertoire of homing receptors (HRs) and homing receptor ligands (HRLs) required for T cell entry depends on T cell activation status and anatomical location of the target tissue.8 10 11 Endothelial HRLs are differentially expressed in a tissue-specific manner and are further influenced by inflammation. In murine subcutaneous tumor models, ligand-receptor interactions required for infiltration of effector and effector-memory T cells include vascular cell adhesion molecule 1 (VCAM-1)-integrin α4β1, intracellular adhesion molecule 1 (ICAM-1)-lymphocyte function-associated antigen-1 (LFA1), E-selectin ligand (ESL)-E-selectin, and hyaluronic acid (HA)-CD44, as well as chemokine signaling through CXCR3 ligation of CXCL9-11 (table 1, figure 1).11 In these tumor models and human solid tumors, endothelial expression VCAM-1, ICAM-1, and CXCL9-11 is regulated by inflammatory cytokines tumor necrosis factor (TNF)α, interferon (IFN)γ, and chemokines, including CCL5.12 Tumors can thus interfere with T cell homing directly by suppressing production of TNFα, IFNγ, CCL5, or CXCL9-11, or indirectly by recruiting suppressive cell types. One way to enhance T cell infiltration may be to deliver these cytokines or chemokines to the TME or to increase their expression.
Figure 1.
Effector T cell homing in tumors. The first step in effector T cell extravasation into peripheral tissues involves tethering and rolling on the endothelial cells, mediated by homing receptors CD44, P-selectin ligand (PSL), and E-selectin ligand (ESL) on the T cell and homing receptor ligands, hyaluronic acid (HA), P-selectin (Psel), and E-selectin (Esel) on the vasculature. Inflammatory molecules interferon (IFN)γ and tumor necrosis factor (TNF)α expressed in the tumor microenvironment (TME) can upregulate these homing receptor ligands, thereby facilitating the recruitment of T cells. Next, inflammatory cytokines CXCL9-11 expressed by endothelial cells, and other cells in the TME, bind to CXCR3, activating integrins α4β1 and lymphocyte function-associated antigen 1 (LFA1). These integrins subsequently bind to vascular cell adhesion molecule 1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1) on the vasculature, allowing firm adhesion and arrest of the T cell, followed by extravasation through the endothelial cell layer into the tissue. In this process, tumor cells can inhibit expression of inflammatory cytokines and chemokines in the TME, thereby indirectly suppressing the expression of homing ligands. Additionally, tumor cells express factors that can directly signal within the endothelial cells to downregulate homing receptor ligands, or upregulate T cell suppression molecules directly on the vasculature. Made with Biorender.com.
Table 1.
Homing receptors and ligands on vasculature
| Ligand on vasculature | Receptor on T cell | Effect of interaction |
| Naïve T cells | ||
| PNAd | CD62L | Rolling/Tethering |
| CCL19 | CCR7 | Integrin activation |
| CCL21 | CCR7 | Integrin activation |
| ICAM-1 | LFA1 | Firm adhesion/arrest |
| Effector T cells | ||
| PSGL-1 | P-selectin | Rolling/Tethering |
| ESL | E-selectin | Rolling/Tethering |
| HA | CD44 | Rolling/Tethering |
| CXCL9-11 | CXCR3 | Integrin activation |
| VCAM-1 | α4β1 | Firm adhesion/arrest |
| ICAM-1 | LFA1 | Firm adhesion/arrest |
| MAdCAM1 | α4β7 | Firm adhesion/arrest |
ESL, E-selectin ligand; HA, hyaluronic acid; ICAM-1, intracellular adhesion molecule 1; PNAd, peripheral node addressin; VCAM-1, vascular cell adhesion molecule 1.
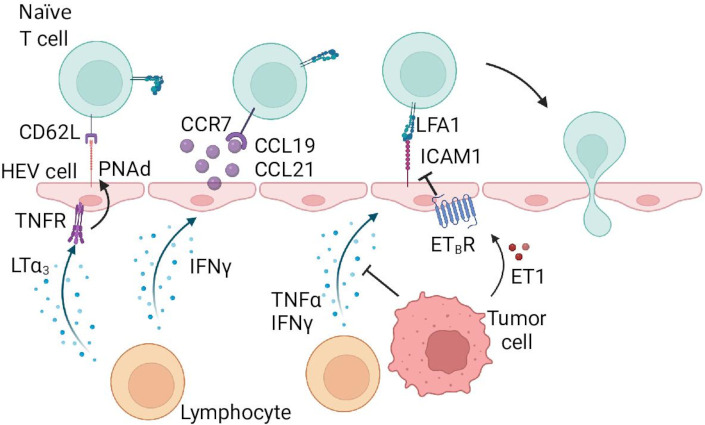
Naïve T cells can home to tumors directly and can be activated in situ.7 This process is mediated by specialized vasculature, called high-endothelial venules (HEV). HEV express peripheral node addressin (PNAd), CCL19, CCL21, and ICAM-1 which interact with CD62L, CCR7, and LFA1 on naïve T cells (figure 2, table 1).13 14 These interactions initiate rolling/tethering on the HEV, activation, and subsequent firm adhesion and cell arrest on the vasculature. HEV are present in normal secondary lymphoid organs (eg, lymph nodes), but not in uninflamed peripheral tissues.15 Interestingly, though, chronic inflammation induces lymphoid neogenesis in peripheral tissues, including human solid tumors, resulting in tertiary lymphoid structures (TLS), a key feature of which is HEV-like PNAd+ vasculature.15 16 T cells and B cells extravasate through HEVs and organize in TLS, mediated by fibroblasts and perpetuated by B and T cells.17 TLS in tumors have been associated with improved survival and clinical response to checkpoint blockade therapy.18–20 Interestingly, in a meta-analysis of checkpoint blockade therapy response among seven different cancer types, CXCL13, a well-known marker for TLS, was significantly and selectively expressed in responding patients.21 Much remains unclear on how TLS can support intratumoral immune responses and whether they enhance infiltration of in situ activated T cells into the tumor parenchyma.
Figure 2.
Naïve T cell homing in tumors. Similar to effector T cells, naïve T cells require multiple signals to extravasate through the vasculature. Contrastingly, naïve T cells require specialized vasculature called high-endothelial venules (HEV), which are capable of expressing the proper receptors and chemokines. Tethering and rolling is initiated by peripheral node addressin (PNAd) binding to CD62L on the T cell. Chemokines CCL19 and CCL21 activate lymphocyte function-associated antigen-1 (LFA1) integrin, and facilitate firm adhesion and arrest by binding of activated LFA1 to intracellular adhesion molecule 1 (ICAM-1). PNAd, CCL19, and CCL21 get upregulated by factors expressed in the tissue microenvironment, LTα3 induces PNAd expression through TNFR signaling, and interferon ((IFN)γ induces CCL19 and CCL21 expression. Tumor cells can inhibit expression of these cytokines, as well as directly downregulate ICAM-1 expression through endothelin-1-endothelin receptor B (ETBR) signaling. Made with Biorender.com.
Distorted organization of the tumor vasculature can impair T cell infiltration. Because tumors often grow rapidly, new blood vessels are not formed quickly enough to provide sufficient nutrients and oxygen, leading to marked tissue hypoxia.22 Hypoxia induces hypoxia-inducible factor 1α expression in tumor cells and endothelial cells (ECs), which upregulate expression of pro-angiogenic genes, including vascular endothelial growth factor (VEGF)-A. They induce rapid neovascularization that results in irregularly shaped, dilated, and tortuous vessels, which are leaky and poorly covered by pericytes.23 These structural defects in tumor vasculature alone present hurdles for T cell infiltration, likely due to uneven blood flow, disrupted endothelial junctions, and cytoskeletal alterations in the ECs preventing proper transmigration.24 In addition, the ECs generated in rapid neovascularization often respond inefficiently to inflammatory signals, failing to upregulate HRL sufficiently, even when proper inflammatory cytokines and chemokines are present.25 26 VEGF-A and fibroblast growth factor (FGF)-2 also can directly interfere with TNF-induced adhesion molecule expression on ECs (figure 1).27 28 Other molecules expressed in the TME such as nitric oxide and epidermal growth factor like domain multiple 7 can also directly suppress EC activation.27 29 30
Furthermore, ECs themselves can express molecules that create barriers to T cell infiltration, such as endothelin receptor B (ETBR), indoleamine 2,3-dioxygenase (IDO)-1, programmed-death ligand (PD-L)1, and Fas ligand (FasL) (figure 1). When ETBR is expressed by tumor vascular ECs, T cell homing is reduced through poorly understood mechanisms, but inhibiting ETBR enhances T cell homing and augments immunotherapies.31 Similarly, IDO-1 and PD-L1 can inhibit T cell function.32 FasL expression on tumor-associated vasculature decreases the influx of CD8+ T cells due to selective killing of the T cells.33 These mechanisms are more biological than physical barriers, yet, they do restrict functional T cell infiltration into tumors and are important mechanisms to address when developing strategies to enhance T cell presence.
Barriers to T cell infiltration, localization, and retention
To control cancers, infiltrating T cells must be retained there and traffic among and interact with tumor cells.6 Patients with diffuse immune cell infiltration among tumor cells have a better prognosis than patients whose infiltrates are confined to perivascular spaces.1 Therefore, tumors may evade immune recognition by inducing barriers to diffuse immune cell infiltration and motility among tumor cells.
T cell movement is driven by both intrinsic motile capacity and extrinsic environmental organization and cues, including matrix proteins that provide a physical ‘roadmap’ for T cells and chemical guidance cues in the form of chemokines (figure 3A).34 This makes T cell motility highly dependent on activation status, antigen density, chemokine gradients, and target tissue type. Separately, in tissues with dense extracellular matrix (ECM), inflammatory signals such as TNFα, IFNγ and transforming growth factor (TGF)β can induce secretion of proteases to loosen the ECM matrix and to allow integrin-mediated T cell motility within the tissue.35–37 In viral infections, T cell motility along ECM proteins is driven by integrins α1β1 (CD49a) and α2β1 (CD49b).38–40 These all are crucial to T cell motility and localization within tissues and provide targets for immune evasion for the tumor. Tumor cells can overexpress cell-cell adhesion proteins, possibly forming physical barriers to T cell motility among tumor cells.41 42 Each component of these barriers to T cell infiltration and motility will be explained in greater detail in this section, “Barriers to T cell infiltration, localization, and retention.”
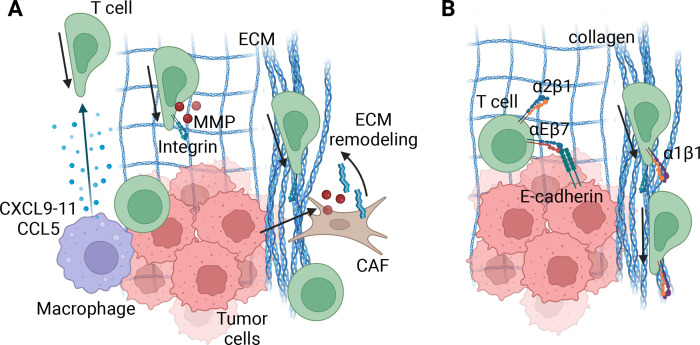
Figure 3.
T cell migration in the tumor microenvironment (TME). (A). Speed and direction of T cells in tumors relies on various factors, including, but likely not limited to, chemokine gradients, extracellular matrix (ECM) organization and integrin expression and migratory capacity of the T cell. Tumor cells or myeloid cells in close proximity to tumor cells can express CXCL9-11 and/or CCL5, which directs effector T cells to the tumor cells directly. When the ECM is organized in a relaxed, diffuse meshwork of ECM molecules, similar to normal epithelial tissues, T cells may migrate along them in an integrin-dependent manner. Local expression of matrix metalloproteinases (MMPs) will furthermore guide their migration. However, cancer-associated fibroblasts (CAFs) often remodel the ECM into a dense and disorganized matrix, rendering T cells unable to leave and get ‘stuck’ in stromal areas of the tumor. Due to high variability from tumor to tumor, the exact specifics of the organization and structure likely determine the ultimate effect the ECM has on T cell motility and function, and requires further investigation. (B) Retention of T cells into a tissue microenvironment relies on ECM and cell-binding integrins. These include, but are likely not limited to, α1β1 and α2β1 binding to collagen and αEβ7 binding to E-cadherin on epithelial and/or tumor cells. Additionally, adhesion of α1β1 to collagen increases T cell motility, providing both a retention and migration mechanism. In tumors with localized, dense collagen this may lead to rapid motility, thereby effectively distracting the T cell from forming long-lasting engagement with target cells. Made with Biorender.com.
Leukocyte migration within tissues
Mechanisms of T cell migration within tissues have been reviewed.43 Amoeboid migration enables T cells to move rapidly with undiminished migration velocities, such that they generate fast, low-affinity, and directional crawling.43 Alternatively, T cells can adapt a mesenchymal-like motility phenotype, characterized by adhesive spreading and non-directional slow motility, often in an integrin-dependent manner.44 In healthy and tumor tissues, T cell motility is a dynamic balance between these two phenotypes, described as amoeboid-mesenchymal plasticity,43 45 which allows T cells to overcome ECM barriers through adaptability and shape change.46 47 Additionally, single-cell movement by T cells is linearly related to collagen pore size and inversely related to collagen or surface stiffness or concentration.44 48 In vivo, migration is further shaped by chemokines, adhesive ligands, and the degree of T cell confinement in the tissue.43 In tumors specifically, it remains unclear whether and how T cell migration patterns are distorted compared with migration in normal or inflamed peripheral tissues, and how much they rely on chemokine-driven, integrin-dependent, and/or integrin-independent migration. Interestingly, when chemically forcing an amoeboid migration state, T cells showed enhanced speed and migratory distance in three-dimensional collagen matrices or murine pancreatic tumors, whereas a forced mesenchymal state reduced speed and directionality.44 Future studies will have to determine whether increased speed and directionality correlates to improved effector T cell function and tumor cell killing.
Chemokine barriers
Chemokine gradients are important for recruitment of T cells through endothelium, and for migration within the tissues after extravasation from the vasculature. High intratumoral levels of CCL2-5, CXCL9, and CXCL10 strongly correlate with greater intratumoral T cell infiltrates in treatment-naïve solid tumors, suggesting these chemokines may support both recruitment and dispersion of T cells within tumors.12 49 50 In infection models and solid tumors, myeloid-derived CXCL9 recruits effector T cells specifically to target cells, through CXCR3 expressed by activated T cells (figure 3A)12 51 52 and that CXCL9-mediated recruitment of CD8+ T cells depends on IFNγ for induction.12 51 52 In murine tumors, the intratumoral CXCL9-CXCR3 chemokine axis is crucial in the response to PD-1 blockade.53 Furthermore, tumor cells themselves can produce CXCL9-11 in the presence of IFNγ and toll-like receptor (TLR) ligands.54 55 Suppression of IFNγ might thus provide a distinct mechanism of immune evasion through downregulation of CXCL9-11 expression in myeloid and tumor cells. CCL5 also can be expressed by tumor cells and correlates with diffuse T cell infiltration in treatment-naïve advanced ovarian tumors.12 Epigenetic silencing of CCL5 has been correlated with a lack of T cell infiltration and is suggested to be another immune evasion mechanism tumors employ.12
Extracellular matrix barriers
The ECM is composed of collagen, fibronectin, elastin, glycosaminoglycans, and proteoglycans, many of which are produced and organized by fibroblasts and macrophages.56 ECM is a major determinant of tissue architecture, and ECM organization itself determines the direction and efficiency of T cell movement.57 Most normal epithelial tissues are in a tensional homeostatic state, which leads to a relaxed meshwork of collagens and other ECM components, likely allowing optimal lymphocyte motility. In tumors, however, activated cancer-associated fibroblasts (CAFs), inflammation, high interstitial pressure, and increased expression of collagen-processing lysyl oxidases can increase collagen deposition, cross-linking, and distorted organization.56 58 Additionally, tumors often express higher levels of matrix metalloproteinases (MMPs) than normal epithelial tissue, leading to increased remodeling of ECM fibers.59 In solid tumors, collagen alignment, length, width, density, and straightness is altered compared with adjacent normal tissue.60 Tumors can limit T cell motility and efficient ‘serial killing’ of tumor cells by modulating ECM organization, manipulating expression of ECM components, MMPs, and/or lysyl oxidases (figure 3A).56 61 Tumor cells mainly manipulate these components through recruitment and activation of CAFs with TGFα, TGFβ, platelet-derived growth factor, epidermal growth factor, and FGF-2.62 63 Critical questions are how to target CAF activation most efficiently to improve T cell motility and tumor cell killing.
Collagen structure in tumors
A major problem to understanding the roles of differential collagen organization on T cell function in tumors is that no standardized visualization method exists to characterize ECM components, especially collagen.60 Currently used methods range from conventional to two-photon microscopic techniques visualizing collagen structures with varying levels of sensitivity, making it difficult to compare among studies. Regardless, anecdotal studies can teach us about different collagen structures and how they may affect T cell localization, motility, and function. Recently, new tools have been developed for quantitative and automated measurement of a broad range of features in ECM organization, which will allow for more standardized and objective studies in the future.64 65
A common collagen organization in tumors involves dense, aligned collagen fibers outside tumor cell clusters. T cells are often confined within these collagen-dense regions and are unable to interact with tumor cells.66 Collagenase treatment, but not integrin blockade, can liberate T cells, suggesting that in this type of ECM organization, the collagen fibers form a physical barrier or restraint.66 In other collagen-rich tumors, including breast carcinoma models,67 collagen is deposited more evenly throughout the tumor. However, even when among tumor cells, these fibers are often still dense, linear and highly aligned. T cells, similar to many other cell types, can use these aligned collagen fibers as highways along which to migrate rapidly.58 68 To kill a tumor cell, a CD8+ T cells must arrest and engage with its target for an extended time.51 In vitro, CD8+ effector T cells can effectively kill a target cell in 5 min, although in vivo it appears to require interactions of 30 min to 2 hours.69 70 Separately, CD4+ T cells can provide help in the form of inflammatory cytokines, as well as directly kill tumor cells through ligation of FasL and TNF-related apoptosis-inducing ligand (TRAIL).71 Direct killing by CD4+ T cells likely requires arrest and lasting engagement similar to CD8+ T cells. For its helper function, however, a CD4+ T cell may not need to arrest and engage specifically, but it would need to reach close proximity to tumor cells. When trafficking along collagen fibers at high velocity, it can be envisioned that the T cell is unable to engage as efficiently and durably as when it moves more slowly. Thus, tumors can have distinct forms of collagen deposition and create structural barriers, preventing T cells from leaving stromal regions, or collagen-highways that distract T cells from engaging with target cells. Both forms of collagen deposition can be mechanisms to hijack the ECM in the TME to prevent optimal T cell recognition, although in contrasting ways.
In addition to forming structural barriers, collagens can also act as ligands for the immune inhibitory receptor leukocyte associated Ig-like receptor-1 (LAIR-1).72 73 On binding collagens, LAIR-1 signaling inhibits T and NK cell function in vitro.74 75 Furthermore, LAIR-1 signaling promotes generation of exhausted T cells and renders murine lung tumors resistant to anti-PD-1/PD-L1 therapy.76 This suggests that collagens can also directly inhibit T cell function, even if they do not create a physical barrier.
Matrix metalloproteinases
MMPs are enzymes that degrade collagens and other ECM components and, thus, can enable tumor invasion or immune cell infiltration. Activated T cells express MMP RNA, but there is minimal MMP expression at the cell surface.46 Interestingly, upregulation of MMP expression in CD8+ T cells enhanced T cell migration through collagen in vitro, while addition of an MMP inhibitor in vitro and in vivo reduced this invasive advantage.77 This suggests that increased MMP activity can enhance immune cell migration in tumors. Furthermore, collagen fragments resulting from MMP cleavage can activate integrin-dependent T cell motility, suggesting collagen components can create chemotactic gradients toward intratumoral regions with high collagen remodeling.78 Whether this MMP-driven chemotactic motility distracts T cells from interacting with tumor cells, or whether it recruits them to tumor cell-dense areas likely depends on the collagen deposition in individual tumors, and remains to be comprehensively studied.
Overall, more comprehensive analyses of T cell migration patterns in relation to different ECM components, and their organization, through live imaging of patient-derived tumor slices66 could provide important insights into T cell mobility, localization, and its relation to cytotoxic function in tumors.
Cell-associated barriers
Cell-cell adhesions
Cell-cell junctions include adherens junctions, desmosomes, hemidesmosomes, tight junctions, and gap junctions, which mediate barrier functions and intercellular communication. Several genes encoding cell-cell junction proteins have been identified in treatment-naïve melanoma, ovarian cancer, and non-small cell lung cancer: filaggrin, dystonin, junction plakoglobin, plakophilin-3, desmoplakin, desmocollin-3, periplakin, and trop-2, upregulation of which is associated with immune cell exclusion.42 79 In univariate analyses, overall survival was significantly shorter for melanomas overexpressing filaggrin or trop-2, and ovarian cancers overexpressing filaggrin.42 Questions remain about whether these associations are causative or whether the concordant overexpression of these barrier molecule genes reflects an upstream signal that limits T cell infiltration. In a murine melanoma model, T cell infiltrates were not altered by knockout of junction plakoglobin or plakophilin-3 individually, or by filaggrin and dystonin together, although tumor-promoting effects were observed for some of them.41 Additional investigation is warranted to evaluate the impact of modulating all genes concurrently or blocking upstream signals that induce them.
Oncogenic pathways
The oncogenic WNT/β-catenin pathway is commonly activated in human cancers, and its activation has also been associated with lack of T cell immune signatures, explained by insufficient recruitment of BATF3+ dendritic cells and low production of critical T cell homing chemokines.80 Interestingly, WNT/β-catenin activation, ETBR expression, and overexpression of barrier molecule genes have non-overlapping associations with lack of T cell immune signatures.42 Additional studies are needed to understand the role of oncogenic pathways in these other barriers.
Retention mechanisms
Mechanisms for retaining T cells in peripheral tissues may involve integrins and chemokine receptors.
Integrin function
Integrins are heterodimer adhesion molecules comprising alpha and beta subunits. On T cells, they enable binding ECM components or receptors specifically expressed by tissue resident cells, to drive motility or long-lasting arrest and engagement.40 67 81 α1β1 and α2β1 in particular are involved in T cell binding to collagens in epithelial tissues,82 while integrin αEβ7 binds E-cadherin, often expressed on the cell surface of tumor cells from epithelial origin.83 Tumors vary widely in their expression and organization of ECM components, as well as their level of surface E-cadherin. Such variation may impact the role these integrins play in defining spatial localization and retention of T cells within tumors. Additionally, the integrins are expressed at varying levels on tumor infiltrating T cells, and those expression levels are likely affected by differential expression of cytokines and growth factors in the TME.67 84 85 Each collagen-binding integrin appears to affect T cell motility and function separately. For example, α1β1 drives T cell motility in lung infection and tumor models.40 67 Depending on the collagen deposition, the increased motility can distract T cells from engaging with tumor cells, essentially creating retention and T cell dysfunction simultaneously (figure 3B).67 Overall, α1β1 expression on tumor-specific T cells after vaccination with tumor-specific peptides is associated with improved survival in patients with melanoma.86 This suggests that despite the distraction from engagement with tumor cells, α1β1 may enhance overall tumor control. On the other hand, α2β1 neither drives T cell motility nor blocks engagement with tumor cells, suggesting a different role for α2β1 ligation to collagen ligands (figure 3B). αEβ7 may allow retention of tissue resident memory T cells in peripheral tissues, and to contribute directly to lasting engagements of T cells with tumor cells and to mediate their specific killing (figure 3B).81 87 Thus, integrin function overall appears more complicated than simply ‘retention’. Integrins affect motility, localization, and/or long-lasting engagement with target cells and serve different, individual purposes with opposing results in terms of T cell function. It is important to understand the individual function of each integrin in T cell motility, retention, and adhesion both in steady-state peripheral tissues and tumors.
Egress mechanisms
Lymphocytes with long-term residence in peripheral tissues are tissue-resident memory T cells (TRM): they upregulate CD69, whose expression and signaling downregulates sphingosine-1-phosphate receptor (S1P)1.88 Due to the importance of S1P1 for the migration of T cells to lymphatics, CD69 blocks egress of T cells from the tissue.89 In solid tumors, whether treatment-naïve or treated with immune therapies, infiltrating T cells often express high levels of CD69, suggesting a similar mechanism may limit their egress.84 Chemokines and other molecules may also play roles in the return of T cells to the circulation. Egress of naïve T cells from peripheral tissues depends on CCL21-CCR7 ligation, both in steady state and in acute inflammation.90 91 However, in chronic inflammation and tumors, T cells seem to egress to the lymph node via a CCR7-independent, yet unknown, mechanism.92 93 It is not well understood whether egress mechanisms drain functional T cells from tumors, or whether egress may be a necessary and beneficial process to reinvigorate suppressed and/or exhausted cells in the lymph node or to allow recirculation of naïve T cells. Recently developed photoactivation strategies and advanced imaging94 can provide insights into the beneficial or detrimental effects of T cell egress from tumors for overall function. Furthermore, these in vivo models of T cell migration can answer key questions on which mechanisms and receptors are responsible for T cell egress from tumors, specifically, and how they may be targetable in patients.
Potential therapies to enhance homing toward and migration within tumors
Intratumoral immune stimulatory molecules
Clinical experience supports therapeutic impact of intratumoral therapies with cytokines, toll-like receptor agonists, and other molecules that enhance local inflammation. Their mechanisms are understudied, but may involve enhancement of T cell homing. The TLR7 agonist imiquimod is Food and Drug Administration (FDA) approved for treatment of superficial basal cell and squamous cell cancers, and has been used for primary and metastatic melanomas with anecdotal successes. It enhanced expression of E-selectin on tumor vasculature of squamous cell cancers, increased CXCL10 and other critical T cell homing ligands in basal cell cancers and melanomas, and increased T cell infiltration in those cancers.95–97 Injection of interleukin-2 (IL-2) directly into metastases of melanoma can induce local regression in a high proportion of patients, but does not have abscopal effects, and the ability of that intervention to overcome T cell homing barriers is understudied.98 Direct injection of IFNγ into tumors of patients with melanoma, concurrent with a melanoma vaccine, increased CXCL10 expression in the tumor, but did not result in improved total T cell infiltration97; however, there was selective increase in vaccine-specific T cells after IFNγ injection.99 Even those encouraging findings are disappointing in that a median of only 2% of the total tumor-infiltrating lymphocytes were vaccine-induced.99 Thus, low clinical response rates of immunogenic melanoma vaccines100 are likely due to insufficient T cell homing signals within established human melanoma metastases, but intratumoral therapies with immune activating agents can overcome some of the tumor-associated barriers to infiltration. Similar opportunities may be worth exploring to enhance therapeutic effectiveness of chimeric antigen receptor (CAR)-T cells for solid tumors.
A recent preclinical study showed that local delivery of CXCL9-11 plasmids in the TME, through nanoparticles, increased CD8+ T cell infiltration and tumor control in a lung cancer model.101 This shows the potential of inducing direct expression of T cell-recruiting molecules in the TME and should be further investigated.
Potential impact of current approved therapies on immune infiltrates
Systemic PD-1 and cytotoxic T-lymphocyte associated protein-4 antibody blockade can increase immune cells within tumors that respond to therapy, especially memory CD8+ T cells.102 103 Presumably, PD-1 blockade re-invigorates T cells already in the tumors, which secrete IFNγ and thus increase expression of IFN-inducible chemokines (CXCL9-11) and other HRLs, thus, recruiting more T cells. Inhibition of mutated BRAF in advanced melanoma also increased T cell infiltrates within about 2 weeks of starting therapy, and concurrent with clinical tumor regression. That infiltration typically is reduced when the tumors subsequently progress again. The mechanism for this transient increase in T cells is unclear, but may be due to lysis of tumor cells and disruption of the tumor-associated barriers to infiltration Short-term blockade of mutated BRAF has been combined with adoptive T cell therapy for advanced melanoma, with an encouraging 75% objective response rate, but median progression-free survival of <6 months.104 There is rationale for combining immune therapies with targeted therapy or cytotoxic chemotherapies that may overcome tumor-associated barriers to immune infiltration. Such combinations will be enhanced by a deeper understanding of the barriers and how to overcome them on a long-term basis.
Vascular normalization
Targeting VEGF signaling, through blocking antibodies or small-molecule inhibitors, is a promising treatment to improve both the structure of blood vessels in tumors and their expression of HRLs and chemokines, with promise to improve patient outcomes. Several murine and human studies support this concept, and VEGF blockade further improves patient survival when combined with checkpoint inhibitors.105 Another strategy to normalize the vasculature involves treatment with TNF superfamily cytokine LIGHT. In preclinical models for solid tumors, LIGHT induced vessel normalization and enhanced VCAM-1 and ICAM-1 expression.106 Furthermore, LIGHT induced the formation of PNAd+ HEV and TLS in these tumor models, which increased infiltration and in situ activation of naïve T cells.106 107 Several other potential therapies to normalize tumor vasculature are currently being studied in preclinical settings,108 although their effect on T cell infiltration remains to be elucidated.
Oncolytic viruses
Oncolytic viruses target and replicate in tumor cells, causing tumor cell lysis, which in turn releases danger-associated molecular patterns and pathogen-associated molecular patterns into the TME. This can induce type I IFN expression and subsequent expression of inflammatory cytokines and chemokines.109 Additionally, oncolytic viruses can be engineered to deliver genes to tumor cells, thereby forcing expression of inflammatory molecules in the TME. Talimogene Laherparepvec (T-vec) is a granulocyte-macrophage colony-stimulating factor (GM-CSF)-encoding oncolytic virus, which can induce durable control of regional melanoma metastases110 and is now FDA approved for intratumoral therapy. However, combining it with PD-1 blockade did not improve clinical outcomes over PD-1 blockade alone.111 Studies in murine tumor models have shown that oncolytic viruses armed with CXCL11 significantly enhanced CD8+ T cell infiltration, and strongly increased efficacy of CAR-T cell therapy, suggesting that induced expression of chemokines by tumor cells potentiates T cell responses.112–114 However, whether this is due to increased homing or directed T cell motility remains to be elucidated. Other experimental oncolytic viruses deliver vasculature-targeted molecules to the TME, such as anti-VEGF antibodies.115 Thus, in addition to targeting tumor cells directly, oncolytic viruses convert the TME into a more inflammatory environment, which may enhance T cell recruitment and function.
Epigenetic targeting
Epigenetic regulation plays an important role in physiological immune responses, as well as in immunoediting by cancer cells. Therefore, histone deacetylase inhibitors (HDACi) and DNA methyltransferase inhibitors (DNMTi) have shown the potential to promote immune-mediated tumor destruction.116 One of the many immune response-related targets of DNMTi and HDACi is induced expression of CXCL9-11 and CCL5 by human and murine tumor cells, which corresponds with increased migration of CD8+ T cells in vitro and in murine solid tumor models.116 117 Both DNMTi and HDACi have furthermore been shown to augment benefit of checkpoint blockade therapies across multiple murine tumor models.116
Collagen and ECM remodeling
Multiple integrins (including, but likely not limited to, α1β1, α2β1, αVβ1, αVβ3, α4β1, α4β7, αEβ7 and αLβ2) can affect T cell infiltration, motility, and engagement with tumor cells through adhesion to or motility along ECM matrix or cells in the TME.10 43 T cell activation status, chemokines, and other factors in the TME determine integrin expression patterns on CD8+ T cells.67 82 85 With a deeper understanding of these factors, it may be possible to modulate expression of selected integrins for most optimal T cell motility and tumor cell engagement.67 This would create opportunities to adjust vaccination and adoptive transfer strategies to activate T cells with a favorable integrin and homing receptor repertoire so that activated T cells would not simply respond to their antigens, but could also infiltrate and engage with tumor cells.
Enhancing T cell migration through the ECM will also depend on enhanced understanding of how ECM affects T cell motility in normal and distorted tissues and how to alter collagen organization. For example, treatment with recombinant hyaluronan and proteoglycan link protein 1 in a melanoma model promoted a more ‘basket-weave’ ECM structure, more closely resembling normal epithelial tissue.118 This correlated with improved T cell infiltration; however, whether it ultimately led to more frequent interactions with tumor cells is unknown. Alternatively, MMP inhibitors have been shown to increase T cell function in tumors, suggesting that inhibition of ECM remodeling could improve T cell motility. It remains to be established how these therapies affect T cell motility in different ECM organizations. Whether they are beneficial or detrimental to T cell function likely depends on their capacity to normalize specific ECM structures.
CAF-targeted therapies
CAFs are responsible for the majority of ECM components in tumors. Furthermore, they express ECM remodeling elements, such as MMPs, thereby contributing in multiple ways to disorganized and rigid ECM barriers.56 Extensive characterization of stromal cells in different TMEs by single cell RNA sequencing has revealed that CAFs are not defined by a few functional subpopulations. Instead, there is a spectrum of heterogeneity, with the overall balance either shifting toward a protumorigenic or antitumorigenic outcome.119–121 Thus, the definition of a CAF is variable, and successful strategies for targeted therapy likely depend on the range of CAF ‘phenotypes’ of a specific tumor. For example, preclinical studies have indicated that deletion of alpha-smooth muscle actin (αSMA)+ myofibroblast CAFs in a model for pancreatic cancer fails to reduce tumor growth.122 Desmoplastic melanoma, characterized specifically by high numbers of fibroblasts and dense fibrous stroma, frequently contain intratumoral TLS embedded in the desmoplastic tumors, and desmoplastic melanomas are more responsive to immune therapies than non-desmoplastic melanomas.123 124 Similarly, in desmoplastic pancreatic adenocarcinoma, collagen I and αSMA+ fibroblasts were correlated with T cell positioning in close proximity to tumor cells.6 These studies suggest that, in a minority of tumors, CAFs may provide a favorable environment for an immune response. A recent framework for CAFs has been proposed and can guide efforts to study and to target CAF subpopulations in future studies.125 In general, two main branches of CAFs are described: matrix-producing contractile cells (myoCAFs) and cells with an immunomodulatory secretome (iCAFs). iCAF and myoCAF polarization depends on their respective proximity to tumor cells, as well as their ability to respond to IL-1βR and TGFβR signaling.126 127 In a mouse model for rectal cancer, it has been shown that presence of iCAFs correlates with a higher ECM deposition, and treatment with an IL-1βR antagonist in combination with radiotherapy resulted in increased CD8+ T cell infiltration and reduced tumor growth.127 However, the distinct subtypes need to be investigated in greater depth to understand their individual role in immune modulation and tumor growth in different tumor types.125 Current clinical trials targeting CAFs thus focus on limiting CAF activation (inhibitors of FGF-receptor, Hedgehog, and TGFβ), limiting CAF function (targeting CXCR4, LoxL, focal adhesion kinase, Rho-associated protein kinase, connective tissue growth factor, and fibroblast activation protein) and CAF normalization (targeting vitamin A and vitamin D pathways).125 However, fully characterizing CAF heterogeneity and their generation in tumor subtypes, such as the thorough study done by Ogawa et al,128 remains crucial in further developing these different targeting strategies in the clinic. Furthermore, future studies will have to elucidate how each of these targeting strategies may affect ECM distribution and T cell infiltration. Preclinical tumor models for lung and colorectal cancer, when co-injected with CAFs, showed intratumoral normalization of CAFs after NADPH oxidase (NOX)4 inhibition, a downstream factor of TGFβ1. This normalization coincided with increased T cell infiltration, suggesting a promising effect of the treatment for barrier reduction.129
Focused ultrasound
An emerging approach for enhancing effector T cell homing into tumors is focused ultrasound (FUS), which enables non-invasive, non-ionizing acoustic disruption of tumors through thermal (T-FUS) or mechanical (M-FUS) means. T-FUS can stimulate release of inflammatory cytokines including IL-12, IFNγ, and TNFα.130–133 In normal tissues, M-FUS has upregulated ICAM-1 and VCAM-1, improving mesenchymal stem cell homing to muscle134–136 and also upregulated E-selectin, P-selectin, and ICAM-1.137 138 In murine melanoma and breast cancer models, M-FUS has transiently upregulated expression of ICAM-1, VCAM-1, and multiple pro-inflammatory cytokines, but not in a manner sufficient to yield robust T cell infiltration into these tumors.139 140 Findings on the impact of M-FUS for blood-brain barrier disruption in brain tumors have been mixed, with some results lending to no changes in adhesion molecule expression or homing of activated T cells141 and others suggesting modulable increases in ICAM levels.142 T cell-mediated protection has been achieved through rational deployment of FUS in combination with immune adjuvants including TLR agonists, chemotherapy, and checkpoint blockade.132 143–146
FUS holds promise as a non-invasive allied strategy for mediating HRL expression and T cell infiltration. Its role will be further crystallized by future efforts to understand (1) how acoustic exposure conditions impact vascular integrity and sustain HRL expression and (2) if and how immunomodulatory effects are conserved across diverse naïve and malignant tissue settings.
Summary
The presence and distribution of T cells in tumors is determined by complex interactions among ECs, tumor cells, stromal cells, immune cells, and ECM and the subsequent molecular and cellular composition of the TME. Therefore, every tumor has a unique TME, and cellular and molecular components are present in different quantities and phenotypes. The individual roles of several components have been evaluated, however, how they interact and influence each other in different situations and environments need to be dissected further. Newly developed techniques, such as intravital imaging, live tissue imaging of human tumor slices, spatial transcriptomics and single cell RNA sequencing provide opportunities to analyze the components in greater depth and can shine some necessary light on the interplay between cellular and molecular components involved in T cell presence and distribution in tumors. By elucidating the involved mechanisms and how they influence one another, the field can establish targets to improve T cell presence and tumor cell killing in tumors, and in a patient-specific manner.
Acknowledgments
We thank Anna Dimberg at Uppsala University for the Biorender laboratory license to assemble the figures.
Footnotes
Twitter: @ndsheybani
Contributors: MMM, NDS, KML, and CLS drafted and/or substantially revised the manuscript. MMM assembled the figures and table. All authors read and approved the final manuscript.
Funding: Support was provided by United States Public Health Services Research grants T32 CA163177 (KML), Cancer Center Support Grant P30 CA44579, and Cancer Research Institute Clinical Laboratory Integration Project (CLS, KML). MMM was the recipient of the Rebecca Clary Harris Fellowship from the University of Virginia and the postdoctoral grant from Barncancerfonden (Sweden, TJ2022-0016). NDS was supported by an NIH Director’s Early Independence Award DP5 (DP5OD031846) and NCI F99/K00 Predoctoral to Postdoctoral Fellow Transition Award (K00CA234954).
Competing interests: The following disclosures apply to CLS, but are not related to this work: research support to the University of Virginia from Celldex (funding, drug), GlaxoSmithKline (funding), Merck (funding, drug), 3M (drug), Theraclion (device staff support), Polynoma (PI of clinical trial of melanoma vaccine); funding to the University of Virginia for Scientific Advisory Boards for Immatics and CureVac. Also CLS receives license fee payments through the UVA Licensing and Ventures Group for patents for peptides used in cancer vaccines. No potential conflicts of interest were disclosed by MMM, NDS, and KML.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Erdag G, Schaefer JT, Smolkin ME, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 2012;72:1070–80. 10.1158/0008-5472.CAN-11-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogunovic D, O’Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 2009;106:20429–34. 10.1073/pnas.0905139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mihm MCJ, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest 1996;74:43–7. [PubMed] [Google Scholar]
- 4. Wu X, Li J, Connolly EM, et al. Combined anti-VEGF and anti-CTLA-4 therapy elicits humoral immunity to galectin-1 which is associated with favorable clinical outcomes. Cancer Immunol Res 2017;5:446–54. 10.1158/2326-6066.CIR-16-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011;9:204. 10.1186/1479-5876-9-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carstens JL, Correa de Sampaio P, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun 2017;8:15095. 10.1038/ncomms15095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson ED, Enriquez HL, Fu YX, et al. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med 2010;207:1791–804. 10.1084/jem.20092454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brinkman CC, Peske JD, Engelhard VH. Peripheral tissue homing receptor control of naïve, effector, and memory CD8 T cell localization in lymphoid and non-lymphoid tissues. Front Immunol 2013;4:241. 10.3389/fimmu.2013.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peske JD, Woods AB, Engelhard VH. Control of CD8 T-cell infiltration into tumors by vasculature and microenvironment. Adv Cancer Res 2015;128:263–307. 10.1016/bs.acr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ley K, Laudanna C, Cybulsky MI, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 2007;7:678–89. 10.1038/nri2156 [DOI] [PubMed] [Google Scholar]
- 11. Woods AN, Wilson AL, Srivinisan N, et al. Differential expression of homing receptor ligands on tumor-associated vasculature that control CD8 effector T-cell entry. Cancer Immunol Res 2017;5:1062–73. 10.1158/2326-6066.CIR-17-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dangaj D, Bruand M, Grimm AJ, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell 2019;35:885–900. 10.1016/j.ccell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peske JD, Thompson ED, Gemta L, et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 2015;6:1–15. 10.1038/ncomms8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engelhard VH, Rodriguez AB, Mauldin IS, et al. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. J Immunol 2018;200:432–42. 10.4049/jimmunol.1701269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307–25. 10.1038/s41568-019-0144-6 [DOI] [PubMed] [Google Scholar]
- 16. van de Walle T, Vaccaro A, Ramachandran M, et al. Tertiary lymphoid structures in the central nervous system: implications for glioblastoma. Front Immunol 2021;12:724739. 10.3389/fimmu.2021.724739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez AB, Peske JD, Woods AN, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep 2021;36:109422. 10.1016/j.celrep.2021.109422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–55. 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020;577:561–5. 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 20. Lynch KT, Young SJ, Meneveau MO, et al. Heterogeneity in tertiary lymphoid structure B-cells correlates with patient survival in metastatic melanoma. J Immunother Cancer 2021;9:e002273. 10.1136/jitc-2020-002273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Litchfield K, Reading JL, Puttick C, et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021;184:596–614. 10.1016/j.cell.2021.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Georganaki M, van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol 2018;9:3081. 10.3389/fimmu.2018.03081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003;3:401–10. 10.1038/nrc1093 [DOI] [PubMed] [Google Scholar]
- 24. Zhao Y, Li J, Ting KK, et al. The VE-cadherin/β-catenin signalling axis regulates immune cell infiltration into tumours. Cancer Lett 2021;496:1–15. 10.1016/j.canlet.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 25. Griffioen AW, Damen CA, Blijham GH, et al. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 1996;88:667–73. [PubMed] [Google Scholar]
- 26. Castermans K, Griffioen AW. Tumor blood vessels, a difficult hurdle for infiltrating leukocytes. Biochim Biophys Acta 2007;1776:160–74. 10.1016/j.bbcan.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 27. Huang H, Langenkamp E, Georganaki M, et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-κB-induced endothelial activation. FASEB J 2015;29:227–38. 10.1096/fj.14-250985 [DOI] [PubMed] [Google Scholar]
- 28. Dirkx AEM, Oude Egbrink MGA, Kuijpers MJE, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res 2003;63:2322–9. [PubMed] [Google Scholar]
- 29. Delfortrie S, Pinte S, Mattot V, et al. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res 2011;71:7176–86. 10.1158/0008-5472.CAN-11-1301 [DOI] [PubMed] [Google Scholar]
- 30. Gehad AE, Lichtman MK, Schmults CD, et al. Nitric oxide-producing myeloid-derived suppressor cells inhibit vascular E-selectin expression in human squamous cell carcinomas. J Invest Dermatol 2012;132:2642–51. 10.1038/jid.2012.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buckanovich RJ, Facciabene A, Kim S, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008;14:28–36. 10.1038/nm1699 [DOI] [PubMed] [Google Scholar]
- 32. Georganaki M, Ramachandran M, Tuit S, et al. Tumor endothelial cell up-regulation of IDO1 is an immunosuppressive feed-back mechanism that reduces the response to CD40-stimulating immunotherapy. Oncoimmunology 2020;9:1730538. 10.1080/2162402X.2020.1730538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Motz GT, Santoro SP, Wang L-P, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med 2014;20:607–15. 10.1038/nm.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krummel MF, Bartumeus F, Gérard A. T cell migration, search strategies and mechanisms. Nat Rev Immunol 2016;16:193–201. 10.1038/nri.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol 2010;10:712–23. 10.1038/nri2852 [DOI] [PubMed] [Google Scholar]
- 36. Overstreet MG, Gaylo A, Angermann BR, et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat Immunol 2013;14:949–58. 10.1038/ni.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Espinosa-Carrasco G, Le Saout C, Fontanaud P, et al. Integrin β1 optimizes diabetogenic T cell migration and function in the pancreas. Front Immunol 2018;9:1156. 10.3389/fimmu.2018.01156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 2004;20:167–79. 10.1016/s1074-7613(04)00021-4 [DOI] [PubMed] [Google Scholar]
- 39. Richter M, Ray SJ, Chapman TJ, et al. Collagen distribution and expression of collagen-binding alpha1beta1 (VLA-1) and alpha2beta1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J Immunol 2007;178:4506–16. 10.4049/jimmunol.178.7.4506 [DOI] [PubMed] [Google Scholar]
- 40. Reilly EC, Lambert Emo K, Buckley PM, et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc Natl Acad Sci U S A 2020;117:12306–14. 10.1073/pnas.1915681117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leick KM, Rodriguez AB, Melssen MM, et al. The barrier molecules junction plakoglobin, filaggrin, and dystonin play roles in melanoma growth and angiogenesis. Ann Surg 2019;270:712–22. 10.1097/SLA.0000000000003522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salerno EP, Bedognetti D, Mauldin IS, et al. Human melanomas and ovarian cancers overexpressing mechanical barrier molecule genes lack immune signatures and have increased patient mortality risk. Oncoimmunology 2016;5:e1240857. 10.1080/2162402X.2016.1240857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fowell DJ, Kim M. The spatio-temporal control of effector T cell migration. Nat Rev Immunol 2021;21:582–96. 10.1038/s41577-021-00507-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabdanov ED, Rodríguez-Merced NJ, Cartagena-Rivera AX, et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat Commun 2021;12:1–17. 10.1038/s41467-021-22985-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Talkenberger K, Cavalcanti-Adam EA, Voss-Böhme A, et al. Amoeboid-mesenchymal migration plasticity promotes invasion only in complex heterogeneous microenvironments. Sci Rep 2017;7:1–12. 10.1038/s41598-017-09300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp 2003:277–85. 10.1042/bss0700277 [DOI] [PubMed] [Google Scholar]
- 47. Wolf K, Müller R, Borgmann S, et al. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 2003;102:3262–9. 10.1182/blood-2002-12-3791 [DOI] [PubMed] [Google Scholar]
- 48. Wolf K, Te Lindert M, Krause M, et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 2013;201:1069–84. 10.1083/jcb.201210152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harlin H, Meng Y, Peterson AC, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69:3077–85. 10.1158/0008-5472.CAN-08-2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity 2021;54:859–74. 10.1016/j.immuni.2021.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ariotti S, Beltman JB, Borsje R, et al. Subtle CXCR3-dependent chemotaxis of CTLs within infected tissue allows efficient target localization. J Immunol 2015;195:5285–95. 10.4049/jimmunol.1500853 [DOI] [PubMed] [Google Scholar]
- 52. Hickman HD, Reynoso GV, Ngudiankama BF, et al. CXCR3 chemokine receptor enables local CD8+ T cell migration for the destruction of virus-infected cells. Immunity 2015;42:524–37. 10.1016/j.immuni.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chow MT, Ozga AJ, Servis RL, et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 2019;50:1498–1512. 10.1016/j.immuni.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dengel LT, Norrod AG, Gregory BL, et al. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. J Immunother 2010;33:965–74. 10.1097/CJI.0b013e3181fb045d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mauldin IS, Wang E, Deacon DH, et al. TLR2/6 agonists and interferon-gamma induce human melanoma cells to produce CXCL10. Int J Cancer 2015;137:1386–96. 10.1002/ijc.29515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Henke E, Nandigama R, Ergün S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front Mol Biosci 2019;6:160. 10.3389/fmolb.2019.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hallmann R, Zhang X, Di Russo J, et al. The regulation of immune cell trafficking by the extracellular matrix. Curr Opin Cell Biol 2015;36:54–61. 10.1016/j.ceb.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 58. Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol 2010;22:697–706. 10.1016/j.ceb.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nissen NI, Karsdal M, Willumsen N. Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. J Exp Clin Cancer Res 2019;38:115. 10.1186/s13046-019-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zunder SM, Gelderblom H, Tollenaar RA, et al. The significance of stromal collagen organization in cancer tissue: an in-depth discussion of literature. Crit Rev Oncol Hematol 2020;151:102907. 10.1016/j.critrevonc.2020.102907 [DOI] [PubMed] [Google Scholar]
- 61. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016;16:582–98. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 62. Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 2001;264:169–84. 10.1006/excr.2000.5133 [DOI] [PubMed] [Google Scholar]
- 63. Heneberg P. Paracrine tumor signaling induces transdifferentiation of surrounding fibroblasts. Crit Rev Oncol Hematol 2016;97:303–11. 10.1016/j.critrevonc.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 64. Liu Y, Eliceiri KW. Quantifying fibrillar collagen organization with curvelet transform-based tools. J Vis Exp 2020:1–23. 10.3791/61931 [DOI] [PubMed] [Google Scholar]
- 65. Wershof E, Park D, Barry DJ, et al. A Fiji macro for quantifying pattern in extracellular matrix. Life Sci Alliance 2021;4:1–11. 10.26508/lsa.202000880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 2012;122:899–910. 10.1172/JCI45817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Melssen MM, Lindsay RS, Stasiak K, et al. Differential expression of CD49a and CD49b determines localization and function of tumor-infiltrating CD8+ T cells. Cancer Immunol Res 2021;9:583–97. 10.1158/2326-6066.CIR-20-0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pruitt HC, Lewis D, Ciccaglione M, et al. Collagen fiber structure guides 3D motility of cytotoxic T lymphocytes. Matrix Biol 2020;85–86:147–59. 10.1016/j.matbio.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Breart B, Lemaître F, Celli S, et al. Two-Photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest 2008;118:1390–7. 10.1172/JCI34388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Coppieters K, Amirian N, von Herrath M. Intravital imaging of CTLs killing islet cells in diabetic mice. J Clin Invest 2012;122:119–31. 10.1172/JCI59285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Melssen M, Slingluff CLJ. Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol 2017;47:85–92. 10.1016/j.coi.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyaard L, Hurenkamp J, Clevers H, et al. Leukocyte-associated Ig-like receptor-1 functions as an inhibitory receptor on cytotoxic T cells. J Immunol 1999;162:5800–4. [PubMed] [Google Scholar]
- 73. Saverino D, Fabbi M, Merlo A, et al. Surface density expression of the leukocyte-associated Ig-like receptor-1 is directly related to inhibition of human T-cell functions. Hum Immunol 2002;63:534–46. 10.1016/s0198-8859(02)00409-3 [DOI] [PubMed] [Google Scholar]
- 74. Rygiel TP, Stolte EH, de Ruiter T, et al. Tumor-expressed collagens can modulate immune cell function through the inhibitory collagen receptor LAIR-1. Mol Immunol 2011;49:402–6. 10.1016/j.molimm.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 75. Meyaard L, Adema GJ, Chang C, et al. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997;7:283–90. 10.1016/s1074-7613(00)80530-0 [DOI] [PubMed] [Google Scholar]
- 76. Peng DH, Rodriguez BL, Diao L, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8+ T cell exhaustion. Nat Commun 2020;11. 10.1038/s41467-020-18298-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ahrends T, Spanjaard A, Pilzecker B, et al. CD4+ T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity 2017;47:848–861. 10.1016/j.immuni.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 78. Fields GB. The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells 2019;8:984. 10.3390/cells8090984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chae YK, Choi WM, Bae WH, et al. Overexpression of adhesion molecules and barrier molecules is associated with differential infiltration of immune cells in non-small cell lung cancer. Sci Rep 2018;8. 10.1038/s41598-018-19454-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Spranger S, Dai D, Horton B, et al. Tumor-residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell 2017;31:711–723. 10.1016/j.ccell.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Le Floc’h A, Jalil A, Franciszkiewicz K, et al. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-fc molecule triggers lytic granule polarization via a phospholipase cgamma-dependent pathway. Cancer Res 2011;71:328–38. 10.1158/0008-5472.CAN-10-2457 [DOI] [PubMed] [Google Scholar]
- 82. Hogg N, Laschinger M, Giles K, et al. T-cell integrins: more than just sticking points. J Cell Sci 2003;116:4695–705. 10.1242/jcs.00876 [DOI] [PubMed] [Google Scholar]
- 83. Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 1994;372:190–3. 10.1038/372190a0 [DOI] [PubMed] [Google Scholar]
- 84. Salerno EP, Olson WC, McSkimming C, et al. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int J Cancer 2014;134:563–74. 10.1002/ijc.28391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Melssen MM, Olson W, Wages NA, et al. Formation and phenotypic characterization of CD49a, CD49b and CD103 expressing CD8 T cell populations in human metastatic melanoma. Oncoimmunology 2018;7:e1490855. 10.1080/2162402X.2018.1490855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murray T, Fuertes Marraco SA, Baumgaertner P, et al. Very late antigen-1 marks functional tumor-resident CD8 T cells and correlates with survival of melanoma patients. Front Immunol 2016;7:573. 10.3389/fimmu.2016.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Franciszkiewicz K, Le Floc’h A, Boutet M, et al. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res 2013;73:617–28. 10.1158/0008-5472.CAN-12-2569 [DOI] [PubMed] [Google Scholar]
- 88. Mackay LK, Braun A, Macleod BL, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol 2015;194:2059–63. 10.4049/jimmunol.1402256 [DOI] [PubMed] [Google Scholar]
- 89. Skon CN, Lee JY, Anderson KG, et al. Transcriptional downregulation of S1PR1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 2013;14:1285–93. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ohl L, Mohaupt M, Czeloth N, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 2004;21:279–88. 10.1016/j.immuni.2004.06.014 [DOI] [PubMed] [Google Scholar]
- 91. Steele MM, Lund AW. Afferent lymphatic transport and peripheral tissue immunity. J Immunol 2021;206:264–72. 10.4049/jimmunol.2001060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Brown MN, Fintushel SR, Lee MH, et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J Immunol 2010;185:4873–82. 10.4049/jimmunol.1000676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Torcellan T, Hampton HR, Bailey J, et al. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc Natl Acad Sci U S A 2017;114:5677–82. 10.1073/pnas.1618446114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Steele MM, Churchill MJ, Breazeale AP, et al. Quantifying leukocyte egress via lymphatic vessels from murine skin and tumors. J Vis Exp 2019. 10.3791/58704 [DOI] [PubMed] [Google Scholar]
- 95. Panelli MC, Stashower ME, Slade HB, et al. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol 2007;8:R8. 10.1186/gb-2007-8-1-r8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med 2008;205:2221–34. 10.1084/jem.20071190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mauldin IS, Wages NA, Stowman AM, et al. Intratumoral interferon-gamma increases chemokine production but fails to increase T cell infiltration of human melanoma metastases. Cancer Immunol Immunother 2016;65:1189–99. 10.1007/s00262-016-1881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Weide B, Derhovanessian E, Pflugfelder A, et al. High response rate after intratumoral treatment with interleukin-2: results from a phase 2 study in 51 patients with metastasized melanoma. Cancer 2010;116:4139–46. 10.1002/cncr.25156 [DOI] [PubMed] [Google Scholar]
- 99. Tran CA, Lynch KT, Meneveau MO, et al. Intratumoral IFN-γ or topical TLR7 agonist promotes infiltration of melanoma metastases by T lymphocytes expanded in the blood after cancer vaccine. J Immunother Cancer 2023;11:e005952. 10.1136/jitc-2022-005952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005;175:6169–76. 10.4049/jimmunol.175.9.6169 [DOI] [PubMed] [Google Scholar]
- 101. Ma Y, Liu Y, Zhi Y, et al. Delivery of CXCL9/10/11 plasmid DNAs promotes the tumor-infiltration of T cells and synergizes with PD1 antibody for treating lung cancer. Cancer Nano 2022;13:1–13. 10.1186/s12645-022-00116-z [DOI] [Google Scholar]
- 102. Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells. Cancer Immunol Res 2016;4:194–203. 10.1158/2326-6066.CIR-15-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Huang RR, Jalil J, Economou JS, et al. CTLA4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res 2011;17:4101–9. 10.1158/1078-0432.CCR-11-0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Borch TH, Harbst K, Rana AH, et al. Clinical efficacy of T-cell therapy after short-term BRAF-inhibitor priming in patients with checkpoint inhibitor-resistant metastatic melanoma. J Immunother Cancer 2021;9:e002703. 10.1136/jitc-2021-002703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol 2020;11:2813. 10.3389/fimmu.2020.598877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johansson-Percival A, Ganss R. Therapeutic induction of tertiary lymphoid structures in cancer through stromal remodeling. Front Immunol 2021;12:674375. 10.3389/fimmu.2021.674375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yu P, Lee Y, Liu W, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol 2004;5:141–9. 10.1038/ni1029 [DOI] [PubMed] [Google Scholar]
- 108. Yang T, Xiao H, Liu X, et al. Vascular normalization: a new window opened for cancer therapies. Front Oncol 2021;11:719836. 10.3389/fonc.2021.719836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xie Y, He L, Lugano R, et al. Key molecular alterations in endothelial cells in human glioblastoma uncovered through single-cell RNA sequencing. JCI Insight 2021;6:e150861. 10.1172/jci.insight.150861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of optim: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer 2019;7:145. 10.1186/s40425-019-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chesney JA, Ribas A, Long GV, et al. Randomized, double-blind, placebo-controlled, global phase III trial of talimogene laherparepvec combined with pembrolizumab for advanced melanoma. J Clin Oncol 2023;41:528–40. 10.1200/JCO.22.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu Z, Ravindranathan R, Li J, et al. CXCL11-armed oncolytic poxvirus elicits potent antitumor immunity and shows enhanced therapeutic efficacy. Oncoimmunology 2016;5:e1091554. 10.1080/2162402X.2015.1091554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moon EK, Wang L-CS, Bekdache K, et al. Intra-tumoral delivery of CXCL11 via a vaccinia virus, but not by modified T cells, enhances the efficacy of adoptive T cell therapy and vaccines. Oncoimmunology 2018;7:e1395997. 10.1080/2162402X.2017.1395997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang G, Zhang Z, Zhong K, et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol Ther 2023;31:134–53. 10.1016/j.ymthe.2022.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang L, Chard Dunmall LS, Cheng Z, et al. Remodeling the tumor microenvironment by oncolytic viruses: beyond oncolysis of tumor cells for cancer treatment. J Immunother Cancer 2022;10:e004167. 10.1136/jitc-2021-004167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Topper MJ, Vaz M, Marrone KA, et al. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol 2020;17:75–90. 10.1038/s41571-019-0266-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Qin Y, Vasilatos SN, Chen L, et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene 2019;38:390–405. 10.1038/s41388-018-0451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kaur A, Ecker BL, Douglass SM, et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov 2019;9:64–81. 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pelon F, Bourachot B, Kieffer Y, et al. Cancer-associated fibroblast heterogeneity in axillary lymph nodes drives metastases in breast cancer through complementary mechanisms. Nat Commun 2020;11:404. 10.1038/s41467-019-14134-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kanzaki R, Pietras K. Heterogeneity of cancer-associated fibroblasts: opportunities for precision medicine. Cancer Sci 2020;111:2708–17. 10.1111/cas.14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Qian J, Olbrecht S, Boeckx B, et al. A pan-cancer blueprint of the heterogeneous tumor microenvironment revealed by single-cell profiling. Cell Res 2020;30:745–62. 10.1038/s41422-020-0355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–34. 10.1016/j.ccr.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stowman AM, Hickman AW, Mauldin IS, et al. Lymphoid aggregates in desmoplastic melanoma have features of tertiary lymphoid structures. Melanoma Res 2018;28:237–45. 10.1097/CMR.0000000000000439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Eroglu Z, Zaretsky JM, Hu-Lieskovan S, et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature 2018;553:347–50. 10.1038/nature25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sahai E, Astsaturov I, Cukierman E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020;20:174–86. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Biffi G, Oni TE, Spielman B, et al. Il1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 2019;9:282–301. 10.1158/2159-8290.CD-18-0710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nicolas AM, Pesic M, Engel E, et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell 2022;40:168–84. 10.1016/j.ccell.2022.01.004 [DOI] [PubMed] [Google Scholar]
- 128. Ogawa Y, Masugi Y, Abe T, et al. Three distinct stroma types in human pancreatic cancer identified by image analysis of fibroblast subpopulations and collagen. Clin Cancer Res 2021;27:107–19. 10.1158/1078-0432.CCR-20-2298 [DOI] [PubMed] [Google Scholar]
- 129. Ford K, Hanley CJ, Mellone M, et al. NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Res 2020;80:1846–60. 10.1158/0008-5472.CAN-19-3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kramer G, Steiner GE, Gröbl M, et al. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate 2004;58:109–20. 10.1002/pros.10314 [DOI] [PubMed] [Google Scholar]
- 131. Hu Z, Yang XY, Liu Y, et al. Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCS. Biochem Biophys Res Commun 2005;335:124–31. 10.1016/j.bbrc.2005.07.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sheybani ND, Witter AR, Thim EA, et al. Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J Immunother Cancer 2020;8:e001008. 10.1136/jitc-2020-001008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Razavi M, Zheng F, Telichko A, et al. Effect of pulsed focused ultrasound on the native pancreas. Ultrasound Med Biol 2020;46:630–8. 10.1016/j.ultrasmedbio.2019.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lee J, Chang WS, Shin J, et al. Non-invasively enhanced intracranial transplantation of mesenchymal stem cells using focused ultrasound mediated by overexpression of cell-adhesion molecules. Stem Cell Res 2020;43:101726. 10.1016/j.scr.2020.101726 [DOI] [PubMed] [Google Scholar]
- 135. Burks SR, Ziadloo A, Hancock HA, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One 2011;6:e24730. 10.1371/journal.pone.0024730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jang KW, Tu T-W, Rosenblatt RB, et al. MR-guided pulsed focused ultrasound improves mesenchymal stromal cell homing to the myocardium. J Cell Mol Med 2020;24:13278–88. 10.1111/jcmm.15944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. McMahon D, Hynynen K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 2017;7:3989–4000. 10.7150/thno.21630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Mathew AS, Gorick CM, Thim EA, et al. Transcriptomic response of brain tissue to focused ultrasound-mediated blood-brain barrier disruption depends strongly on anesthesia. Bioeng Transl Med 2021;6:e10198. 10.1002/btm2.10198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cohen G, Chandran P, Lorsung RM, et al. The impact of focused ultrasound in two tumor models: temporal alterations in the natural history on tumor microenvironment and immune cell response. Cancers (Basel) 2020;12:350. 10.3390/cancers12020350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Aydin O, Chandran P, Lorsung RR, et al. The proteomic effects of pulsed focused ultrasound on tumor microenvironments of murine melanoma and breast cancer models. Ultrasound Med Biol 2019;45:3232–45. 10.1016/j.ultrasmedbio.2019.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Curley CT, Stevens AD, Mathew AS, et al. Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound. Theranostics 2020;10:8821–33. 10.7150/thno.47983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Lee H, Guo Y, Ross JL, et al. Spatially targeted brain cancer immunotherapy with closed-loop controlled focused ultrasound and immune checkpoint blockade. Sci Adv 2022;8:eadd2288. 10.1126/sciadv.add2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Silvestrini MT, Ingham ES, Mahakian LM, et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2017;2:e90521. 10.1172/jci.insight.90521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kheirolomoom A, Ingham ES, Mahakian LM, et al. CPG expedites regression of local and systemic tumors when combined with activatable nanodelivery. J Control Release 2015;220:253–64. 10.1016/j.jconrel.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Qu S, Worlikar T, Felsted AE, et al. Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 2020;8:e000200. 10.1136/jitc-2019-000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Eranki A, Srinivasan P, Ries M, et al. High-intensity focused ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin Cancer Res 2020;26:1152–61. 10.1158/1078-0432.CCR-19-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]