Abstract
Purpose
Globally, the incidence of herpes zoster (HZ) is increasing, and the resulting zoster-associated pain (ZAP) severely affects the quality of life of patients. Therefore, active treatment of ZAP and prevention of postherpetic neuralgia (PHN) are very important for patients in the early stage of the disease. This retrospective observational study aimed to evaluate the effect of CT-guided pulsed radiofrequency (PRF) combined with ozone injection on zoster-associated pain.
Patients and Methods
From 2018 to 2020, 84 patients with AHN (n=28), SHN (n=32), or PHN (n=24) underwent PRF combined with ozone injection treatment after pharmacologic and conservative therapies failed. The visual analogue scale (VAS), the Pittsburgh Sleep Quality Index (PSQI), and pregabalin consumption were recorded at baseline, post-PRF, and at 1, 3, 6, and 12 months after treatment. The number of remediations performed and adverse reactions were recorded, and treatment inefficiency was calculated using a VAS score greater than 3 as the criterion.
Results
The pooled results demonstrated statistically significant decreases in VAS scores, PSQI scores and consumption of pregabalin post-PRF and at 1, 3, 6, and 12 months follow-up (P<0.05). Compared with the PHN group, both the AHN and SHN groups showed clinical and statistical improvement in VAS scores and PSQI scores and in consumption of pregabalin (P< 0.05). At 1 year after the operation, the PHN group had a significantly greater number of remediation events and greater treatment inefficiency than the other two groups. No serious adverse events were observed during the procedure or during the follow-up period.
Conclusion
CT-guided PRF combined with ozone injection is safe and effective for individuals with ZAP, and its short-term and long-term effects are significant. In a sense, early PRF combined with ozone injection is more effective.
Keywords: herpes zoster, zoster-associated pain, postherpetic neuralgia, radiofrequency, ozone
Introduction
Herpes zoster (HZ) is caused by the reactivation of dormant varicella zoster virus (VZV), which lurks in sensory ganglia such as the dorsal root ganglia (DRG) and the trigeminal ganglion. Following VZV infection, an inflammatory reaction of the ganglion and peripheral nerves occurs, and corresponding tissue injury occurs along the descending sensory nerve.1,2 The incidence of HZ is age-related, ranging from 1.2 to 3.4 per 1000 persons per year among younger adults and from 3.9 to 11.8 per 1000 persons per year in elderly patients.3 Postherpetic neuralgia (PHN) is one of the most common and debilitating complications of HZ, greatly decreasing the quality of life of patients and bringing serious burdens to medical systems worldwide.4 PHN is associated with long-lasting neurohypersensitivity that can have undesirable mental consequences such as depression, fatigue and sleep disorders.5 Therefore, improving therapeutic effectiveness, preventing the occurrence of PHN, and achieving rapid pain relief for patients with zoster-associated pain (ZAP) have been a focus of attention for pain physicians.
Currently, multiple drugs are available for the treatment of ZAP; however, the therapeutic effectiveness of these drugs is not compelling, and their side effects are sometimes intolerable.6 Especially for elderly patients and those with digestive system dysfunction, large skin lesions, or underlying disease, oral medication has a poor effect, and these patients are more prone to develop long-term PHN.7 Alternatively, interventional procedures are appropriate for patients who are suffering from unsatisfactory drug curative effects or intolerable side effects.8 Pulsed radiofrequency (PRF) is a minimally invasive and repeatable technique in which a transient PRF current of 300–500 kHz is applied together with an intermittent PRF current of 480 milliseconds to provide sufficient heat dissipation time, ensuring that the treated tissue does not reach a temperature higher than 42°C and ensuring the complete preservation of the structure and function of nerve fibres. Thus, complications such as skin sensation, skin numbness, and motor nerve damage rarely occur.9 The DRG are considered latent infection sites of VZV since they contain primary sensory afferent neurons. When the immune system is weakened, the latent virus in the DRG proliferates and destroys axons, resulting in demyelination and changes in transmembrane ion channels.10 Thus, abnormal electrical impulses are produced by the damaged sensory nerve, inducing hyperalgesia when they are transmitted to the spinal cord.11 Positive therapeutic effects of PRF treatment of the DRG have been achieved in managing ZAP, and application of pulsed radiofrequency to the DRG has become an attractive treatment option for individuals with ZAP.12,13
Ozone is believed to be the strongest naturally occurring oxidant. Various studies have proven the favourable effects of ozone in the treatment of chronic viral infections and circulatory diseases.14 Ozone at concentrations of approximately 30 µg/mL improves oxygen supply and reduces the inflammatory reaction to pain-producing mediators at the disease site, thus reducing ischaemia and oedema of the nerve root.15–17 Recent studies have shown that ozone treatment can effectively relieve acute and chronic pain through its anti-inflammatory and immune modulating effects.18 In particular, injection of ozone into the intervertebral foramen (IVF) as a treatment for chronic intractable PHN has resulted in excellent outcomes, with significant reduction in spontaneous pain and mechanical allodynia as well as improved quality of life.19
Despite the use of multiple therapies in the treatment of ZAP to date, no therapy has been proven to be fully effective. Thus, more efficient therapeutic approaches, including combination therapy, should be considered. However, the effects of administering a combination of PRF and ozone injections in patients suffering from ZAP have not yet been studied. We performed a retrospective observational study in which we assessed the therapeutic effects of the use of a combination of PRF and ozone injections near the DRG in patients suffering from ZAP. These results will be helpful for ZAP treatment.
Methods
Patients
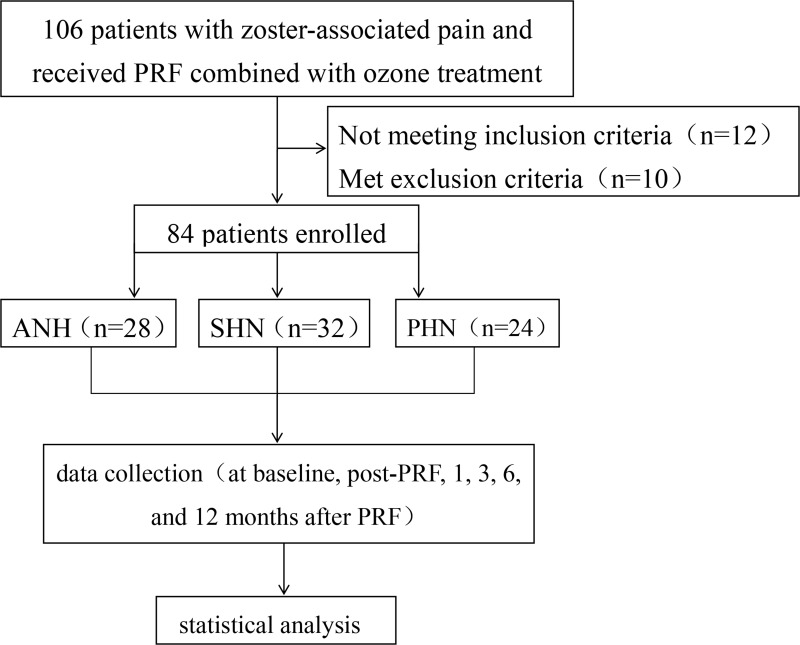
Data in our study were prospectively collected and retrospectively analysed. The study was reviewed and approved by the Ethics Committee of Shanxi Bethune Hospital (YXLL-2022-119) and conducted in accordance with the Declaration of Helsinki. A total of 106 patients with zoster-associated pain (ZAP), including 42 males and 64 females 35–84 years of age, were selected at the Pain Department of the Shanxi Bethune Hospital from January 2018 to January 2020, and 84 patients met the inclusion criteria (Figure 1). The patients’ baseline data were collected through the electronic medical record system of the hospital, and the questionnaire data were collected from the patients at various time points via telephone, WeChat and outpatient follow-up visits. The patients were divided into three groups according to the duration of their disease: acute herpetic neuralgia (AHN, <30 days), subacute herpetic neuralgia (SHN, 30–90 days) and postherpetic neuralgia (PHN, >90 days). Treatment of all patients with pulsed radiofrequency and ozone injection was performed by the same physician.
Figure 1.
Flow diagram presents study design and patient flow.
Abbreviations: PRF, pulsed radiofrequency; AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia.
Inclusion and Exclusion
The inclusion criteria were as follows: (1) clinically diagnosed with zoster-associated pain (Figure 2) that affected sleep; (2) less than 84 and more than 35 years of age; (3) the region affected by herpes zoster involved only the unilateral somatic nerve (Figure 3); (4) preoperative oral administration of pregabalin and other conservative methods did not relieve the pain; (5) underwent PRF combined with ozone injection under CT guidance and able to objectively evaluate the perceived pain and complete the follow-up.
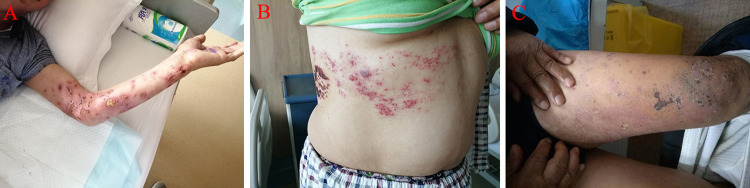
Figure 2.
Herpes zoster in different distribution areas.
Notes: (A) Herpes zoster of left upper limb; (B) herpes zoster on right front chest and back; (C) herpes zoster of left lower limb.
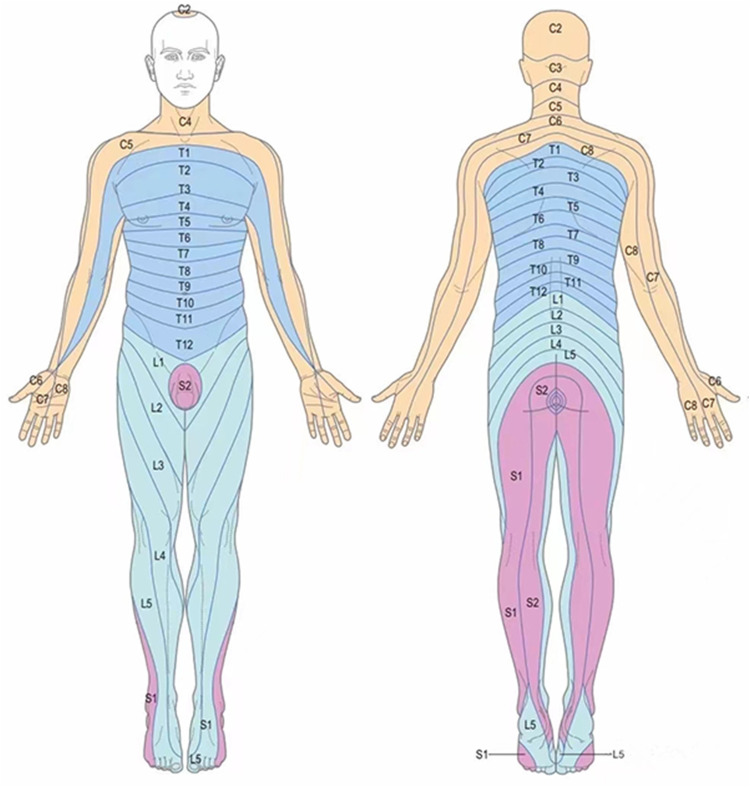
Figure 3.
The picture shows the distribution of the skin nodes in the innervated area, and the distribution of the nerve skin nodes involving C2-S1 in herpes zoster patients is located in one limb.
The exclusion criteria were as follows: (1) ZAP involving the trigeminal nerve; (2) other minimally invasive treatments such as spinal cord stimulation and secondary PRF administered during the one-year follow-up period; (3) severe endocrine system disease or long-term use of hormone or immunosuppressive therapy; (4) severe organ dysfunction or organic disease.
Procedure
All patients received oral pregabalin before treatment and were treated according to the consensus of Chinese experts on HZ, but their pain remained moderate to severe. For patients who underwent routine drug therapy who experienced temporary pain relief, the DRG at the relevant spinal level were treated with PRF combined with ozone injection. The patient lay on the bed of the CT scanner in a lateral position with the operated side facing up. During the entire operation, continuous nasal oxygen inhalation and ECG monitoring were performed. The positioning grid was fixed with adhesive tape to the affected herpes side. PRF of the DRG within three segments was performed; the treatment centred on the segment that experienced the most severe pain and extended to the adjacent top and bottom segments. CT scanning was used to locate the upper 1/3 of the DRG outside the intervertebral foramen as the target; based on this, the needle entry angle and the needle entry point on the skin were calculated, and the needle entry point was marked. Following routine skin sterilization, draping, and local anaesthesia with 1% lidocaine, the RF trocar (20 G, length 100 mm, active end length 10 mm) was slowly introduced and advanced under CT guidance. Finally, the needle tip was positioned in the upper quadrant of the vertebral foramen, and the location of the puncture needle was verified by two-dimensional (Figure 4) and three-dimensional reconstruction (Figure 5).
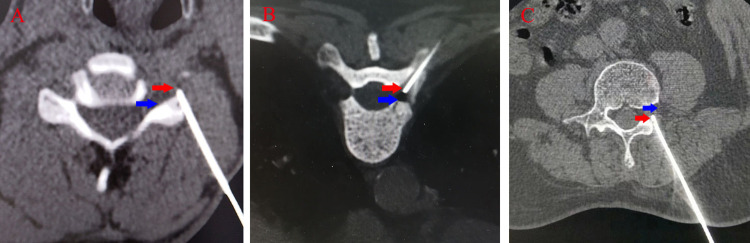
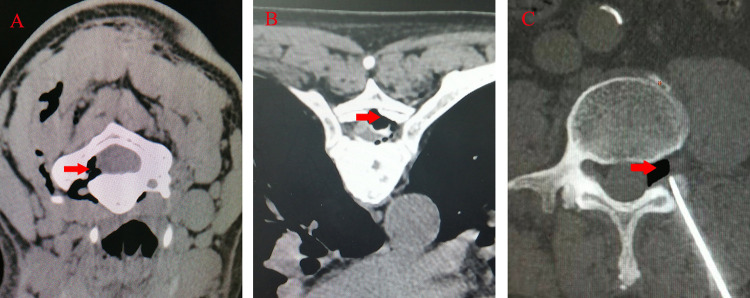
Figure 4.
CT guided neck (A), chest (B) and lumbar (C) puncture (two-dimensional display shows that the needle tip is located outside the intervertebral foramen).
Note: The red arrow indicates the current position reached by the puncture needle, and the blue arrow indicates the intervertebral foramen.
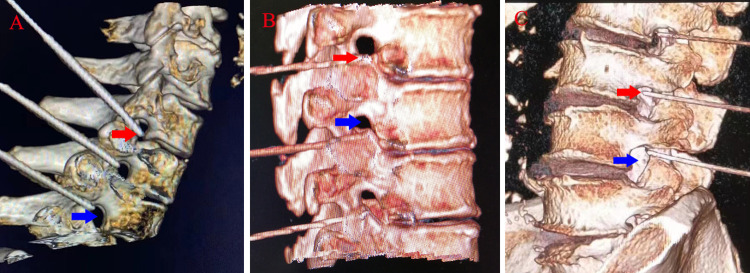
Figure 5.
CT guided neck (A), chest (B) and lumbar (C) puncture (three-dimensional imaging shows that the needle tip is located outside the intervertebral foramen).
Note: The red arrow indicates the current position reached by the puncture needle, and the blue arrow indicates the intervertebral foramen.
The core of the radiofrequency puncture needle was removed and drawn back without removing blood, gas or liquid. The sensory and exercise tests were conducted at voltages of 0.1–1.0 V; a high-frequency current of 50 Hz and a low-frequency current of 2 Hz were used in the sensory and exercise tests, respectively. Symptoms such as soreness, swelling, numbness or tingling in the original pain area can be induced by the sensory test, and the exercise test may cause fibrillation and pulsation of the trunk muscle fibres in relevant segments. The temperature, time, pulse width, and frequency were set at 42 °C, 360 s, 20 ms, and 2 Hz, respectively.
When PRF had been completed, the electrode core was pulled out. There was no blood, gas or liquid in the puncture needle. A therapeutic solution (40 mg of 2% lidocaine hydrochloride and 1 mL of mecobalamin diluted into 0.9% normal saline; total volume 9 mL) was injected into each section. Following injection of the therapeutic solution, 3 mL of 30 µg/mL ozone was administered to each segment, and its diffusion was verified by CT (Figure 6). Finally, the needle was pulled out while compressing the puncture point. The patient was returned to the ward after 20 minutes of observation.
Figure 6.
CT showed that ozone diffused well in ipsilateral epidural and intervertebral foramen; (A) ozone diffused well around the dorsal root ganglion of the neck; (B) ozone diffused well around thoracic dorsal root ganglion; (C) ozone diffused well around the lumbar dorsal root ganglion.
Note: The red arrow indicates the ozone position.
Patients who still had pain after surgery and were unable to tolerate oral analgesics were treated by subcutaneous injection of drugs into the damaged area. The specific drug used for this purpose was a combination of triamcinolone acetonide (10 mg) and 0.5% lidocaine (20 mL). This was injected subcutaneously at the site at which the patient was experiencing the most pain (2 mL for each part and up to 10 parts to cover the entire pain area).
Efficacy Assessment and Follow-Up
Baseline demographic characteristics were collected, including age, sex, side on which the pain was distributed, nerve branches affected, disease duration, and concomitant disease. The VAS (visual analogue scale) was used to measure the severity of pain (0 = no pain and 10 = intolerable pain). The patients were evaluated at baseline (T0), post-PRF (T1) and at 1 month (T2), 3 months (T3), 6 months (T4), and 12 months (T5) after PRF. The quality of the patients’ sleep was assessed using the Pittsburgh Sleep Quality Index (PSQI), which consists of 19 individual items weighted on a 0-to-3 interval scale; this index yields scores ranging from 0 to 20, and lower scores denote better sleep quality. The items on the PSQI were summed to create a total score that measured overall sleep quality.
The dose of pregabalin and the total number of subcutaneous injections were observed and recorded at each observation time point. The total ineffective treatment rate was defined as a pain score ≥ 3/10 with the need for continuous analgesia or other remedies. All adverse events, including numbness, haemorrhage, infection, pneumothorax, and spinal injury, were recorded. A physician who was blinded to patient status was responsible for data collection.
Statistical Analysis
SPSS 26 (IBM Corporation, Armonk, NY, USA) was used to perform statistical analysis of the demographic and clinical data. The Shapiro‒Wilk (SW) test was performed to analyse the normality of the measurement data. Normally distributed measurement data are presented as mean±SD. The differences between the two groups were compared using the t-test, and the differences between more than two groups were analysed by repeated ANOVA. Counting data are expressed as frequencies and rates, and categorical variables were tested using the chi-square test or Fisher’s test. Measurement data that show a nonnormal distribution are presented as the median (quartile) [M (Q25, Q75)], and the Kruskal‒Wallis rank sum test was applied. Multiple comparisons were made using the Bonferroni test. This study is a retrospective analysis. All statistical analyses and charts were prepared using GraphPad Prism 8.0 and Adobe Photoshop CS6. P<0.05 was considered to indicate statistical significance.
Results
General Characteristics of Patients
Eighty-four patients were included in this retrospective study. The patients were divided into 3 groups (AHN: 13.860±3.923, SHN: 54.940±6.550, and PHN: 102.710±6.524) according to the disease phase. The patients’ age, gender, sex, dermatome involved dermatome, VAS scores, comorbidities, and duration of pain are summarized in Table 1. All patients successfully underwent PRF combined with ozone injection. The key difference across the 3 groups was the duration of pain. One patient in the SHN group and one in the PHN group were lost to follow-up 6 months after surgery.
Table 1.
Baseline Characteristics of the Study Patients
| Demographic Information | AHN Group | SHN Group | PHN Group | P value |
|---|---|---|---|---|
| Age, years, mean±SD | 61.960±12.300 | 61.060±12.187 | 62.630±14.598 | 0.902 |
| Gender, n, male/female | 8/20 | 14/18 | 9/15 | 0.476 |
| Pain duration, days, mean±SD | 13.860±3.923 | 54.940±6.550 | 102.710±6.524 | <0.001 |
| Left/right, n | 14/14 | 15/17 | 11/13 | 0.951 |
| Involved dermatome, n | 0.168 | |||
| Cervical, n | 4 (14.3%) | 3 (9.4%) | 2 (8.3%) | |
| Thoracic, n | 15 (53.6%) | 18 (56.2%) | 7 (29.2%) | |
| Lumbosacral, n | 9 (32.1%) | 11 (34.4%) | 15 (62.5%) | |
| Pain VAS score, mean±SD | 6.73±0.94 | 6.68±1.11 | 6.47±1.32 | 0.682 |
| Comorbidities (n,%) Hypertension | 3(10.7%) | 3(9.4%) | 4(16.7%) | 0.687 |
| Comorbidities (n,%) Diabetes | 5(17.9%) | 6(18.8%) | 7(29.2%) | 0.548 |
| Comorbidities (n,%) Tumour | 3(10.7%) | 3(9.4%) | 4(16.7%) | 0.687 |
Note: Numeric values are expressed as numbers (%).
Abbreviations: AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia.
VAS Scores
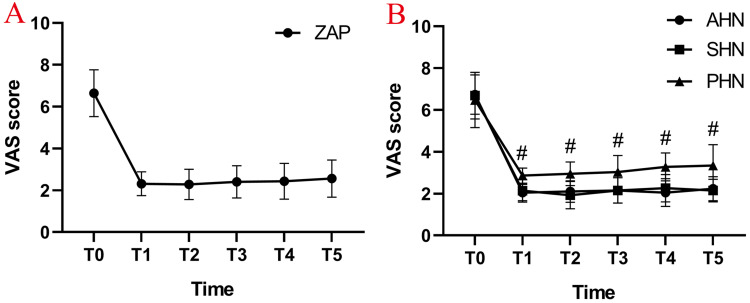
Compared with the preoperative VAS, the postoperative VAS in all three groups decreased significantly (P < 0.05) (Figure 7A). This difference was maintained for up to one year. Compared with the PHN group, the VAS scores of the AHN group and the SHN group were lower at each time point after the operation, and the difference was statistically significant (#P < 0.05) (Figure 7B). There was no significant difference in VAS score between the AHN group and the SHN group (P > 0.05) (Figure 7B).
Figure 7.
Change in visual analogue scale(VAS). (A) The average VAS score for all patients with ZAP decreased significantly after PRF combined with ozone injection treatment; (B) the mean VAS scores in the AHN and SHN groups dropped significantly compared with the VAS score of the PHN group at the time of post-PRF combined with ozone injection treatment. T0: at baseline; T1: post-PRF; T2: 1 months after PRF; T3: 3 months after PRF, T4: 6 months after PRF; T5: 12 months after PRF; #P < 0.05.
Abbreviations: ZAP, zoster-associated pain; AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia; VAS, visual analogue scale.
Pittsburgh Sleep Quality Index (PSQI) Assessment
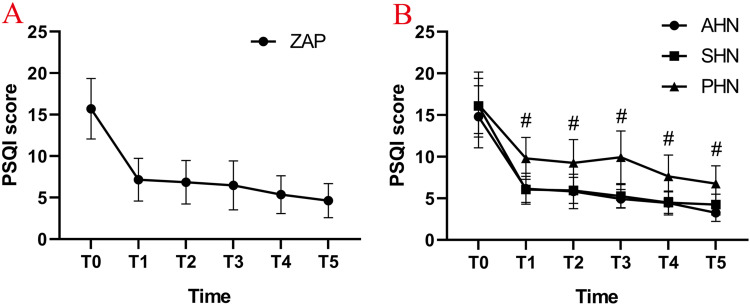
Compared with the PSQI scores before the operation, the PSQI scores of the patients in all three groups were decreased, and sleep quality was significantly improved (P < 0.05) (Figure 8A). There was no significant difference between the AHN group and the SHN group in PSQI score on the day after the operation (P > 0.05) (Figure 8B). Compared with the PSQI scores of the AHN and SHN groups, the PSQI scores of the patients in the PHN group increased at each time point after the operation, and the difference was statistically significant (#P < 0.05) (Figure 8B).
Figure 8.
Change in Pittsburgh Sleep Quality Index (PSQI). (A) The average PSQI scores reduced significantly after PRF combined with ozone injection treatment; (B) the PSQI scores in the AHN and SHN groups decreased more significantly compared with the score in the PHN group at any time point after PRF combined with ozone injection treatment. T0: at baseline; T1: post-PRF; T2: 1 months after PRF; T3: 3 months after PRF; T4: 6 months after PRF; T5: 12 months after PRF; #P < 0.05.
Abbreviations: ZAP, zoster-associated pain; AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia; PSQI, Pittsburgh Sleep Quality Index.
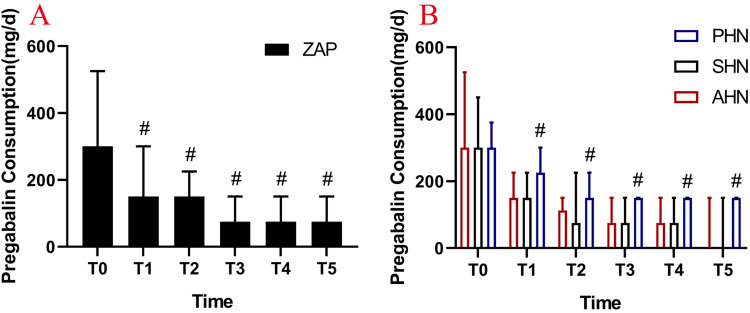
Consumption of Pregabalin and Remediation Events
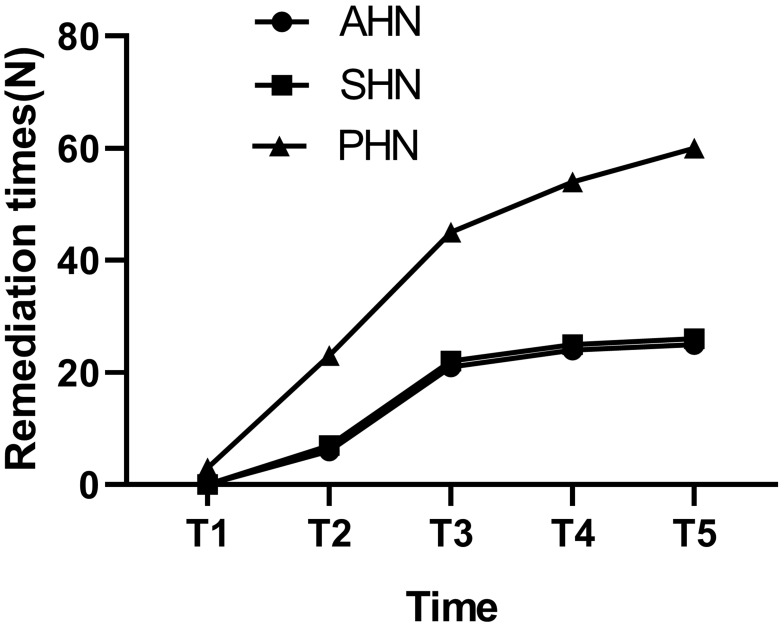
The data (Figure 9A) showed that the daily dosage of oral pregabalin in the three groups gradually decreased with the passage of time. However, compared with the AHN group and the SHN group, the daily dosage of oral pregabalin was higher in the PHN group during postoperative follow-up (#P < 0.05) (Figure 9B). The results showed that three individuals in the PHN group received rescue treatment on the second day after the operation, while the patients in the AHN and SHN groups received no subcutaneous injections (Figure 10). The follow-up results showed that the numbers of remediations performed in the patients in the AHN group at 1 month, 3 months, 6 months and 12 months after surgery were 6, 21, 24 and 25, respectively. In the SHN group, the numbers of remediations were 7, 22, 25 and 26 at 1 month, 3 months, 6 months and 12 months, respectively. In the PHN group, we observed that the number of remediations was significantly increased to 23, 45, 54 and 60 at 1 month, 3 months, 6 months, and 12 months after surgery, respectively (Figure 10).
Figure 9.
Consumption of pregabalin doses before and after treatment. (A) The dosage of pregabalin was significantly reduced in patients with ZAP after operation compared with that before operation; (B) the daily oral dose of pregabalin was higher in the PHN group during postoperative follow-up than in the AHN and SHN groups. T0: at baseline; T1: post-PRF; T2: 1 months after PRF; T3: 3 months after PRF; T4: 6 months after PRF; T5: 12 months after PRF; #P < 0.05.
Abbreviations: ZAP, zoster-associated pain; AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia.
Figure 10.
Comparison of remedy times.
Abbreviations: AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia.
Ineffective Therapeutic Rate
The total ineffective therapeutic rate was calculated based on patient pain VAS score >3/10 and a need for ongoing medical remedy or subcutaneous injection. The total ineffective therapeutic rates in the AHN group were 0% (0/28), 7.14% (2/28), 17.85% (5/28), 17.85% (5/28), and 14.28% (4/28) post-PRF and at 1, 3, 6, and 12 months of follow-up, respectively. In the SHN group, the rates were 0% (0/32), 12.50% (4/32), 18.75% (6/32), 19.35% (6/31), and 16.13% (5/31) at the same follow-up points, and in the PHN group they were 8.33% (2/24), 20.8% (5/24), 33.33% (8/24), 30.43% (7/23), and 30.43% (7/23) (Table 2).
Table 2.
Occurrence of Ineffective Therapeutic Rate at Various Time Points
| Ineffective Therapeutic Rate (%) | Group | ||
|---|---|---|---|
| AHN Group | SHN Group | PHN Group | |
| Post-PRF | 0/28, 0% | 0/32, 0% | 2/24, 8.33% |
| 1 month | 2/28, 7.14% | 4/32, 12.50% | 5/24, 20.8% |
| 3 month | 5/28, 17.85% | 6/32, 18.75% | 8/24, 33.33% |
| 6 month | 5/28, 17.85% | 6/31, 19.35% | 7/23, 30.43% |
| 12 month | 4/28, 14.28% | 5/31, 16.13% | 7/23, 30.43% |
Note: Numeric values are expressed as numbers (%).
Abbreviations: AHN, acute herpetic neuralgia; SHN, subacute herpetic neuralgia; PHN, postherpetic neuralgia.
Side Effects
No serious adverse events were observed during the procedure or during the follow-up period. Potential serious adverse events included leakage of cerebrospinal fluid, numbness, haemorrhage, infection, pneumothorax, and spinal injury. Only one patient experienced a minor complication during treatment. This patient, who underwent ozone injection near the DRG at C5, experienced dizziness, nausea, vomiting, pale face and abrupt haemodynamic deterioration. This deterioration manifested as a decrease in blood pressure (BP) from 135/82 mmHg to 80/55 mmHg and a decrease in heart rate (HR) from 75 bpm to 53 bpm. The puncture needle was immediately removed from the patient’s neck, he was immediately returned to a supine position, and the intravenous infusion rate and the oxygen uptake concentration were increased. After approximately 5 minutes, the patient’s HR and BP returned to the initial level, and the above symptoms disappeared. After 20 minutes of observation, the patient was returned to the ward safely without further adverse reactions.
Discussion
ZAP, neuropathic pain associated with HZ in patients during and after HZ healing, causes a certain burden to families and to the medical system and affects the quality of life of sufferers for a prolonged period of time.20 Therefore, it is particularly important to implement minimally invasive interventional therapy as early as possible in patients for whom conventional drug treatment and conservative treatment are ineffective.
The retrospective study reported here included 84 patients with ZAP who received PRF combined with ozone injection in our hospital. The patients were divided into 3 groups according to the duration of their illness; there were 28 cases in the AHN group, 32 cases in the SHN group, and 24 cases in the PHN group. It was found that the VAS and PSQI scores of all patients were lower at each observation point after the operation than they had been before the operation, indicating that this operation has a good therapeutic effect on patients with ZAP. The VAS and PSQI scores of the patients in the AHN and SHN groups were lower than those of the patients in the PHN group post-PRF and at 1, 3, 6, and 12 months after the operation. The results show that application of this minimally invasive surgical method greatly improves the quality of life and the sleep quality of patients with ZAP and results in a shorter course of the disease.
The DRG, which contain neurons with many receptor channels, are sensory ganglia. The DRG are also the location at which latent VZV lies dormant. As a consequence, they are primary targets for relieving ZAP.21 Individual DRG were selected as the target of minimally invasive interventional PRF therapy in this study. Ozone injection around the DRG should be accurate. It is also desirable that part of the ozone injection enter the lateral dural cavity, where it can act on the posterior horn of the spinal cord. Therefore, the accuracy of tip positioning is very important. CT guidance of tip positioning is safe and accurate. CT two-dimensional imaging and three-dimensional localization can enable the practitioner to accurately find the target, thereby minimizing complications and benefitting patients.
A recent retrospective study found that it may be difficult to ensure long-term efficacy of early adjuvant analgesic drugs in the treatment of ZAP.22 Clinically, some patients with ZAP find that they must reduce or stop medication because of the side effects of the drugs used. For such patients, nondrug therapy is very important. Therefore, we selected patients with poor response to drug therapy and other conservative treatments and treated them with PRF combined with ozone injection therapy. We found that the shorter the course of the patients’ disease was, the higher the effective rate and the lower the number of remediations applied. This indicates that early surgical intervention is very important for patients with ZAP.
PRF therapy for ZAP achieves its analgesic effects through neural regulation.23,24 The mechanism of these effects may be related to generation of the nerve thermal disconnection effect, to changes in synaptic transmission, cell morphology or c-fos gene expression in the superficial layer of the spinal dorsal horn, to weakening of microglial activation, and/or to reverse blocking of abnormal impulse transmission by small unmyelinated nerve fibres. The effects may also be related to an increase in norepinephrine and 5-hydroxytryptamine, neurotransmitters that activate the spinal descending inhibitory system, regulate the level of central pain mediators, and affect the expression of neuronal genes.8,25,26 Therefore, in this study, the DRG at the cervical intervertebral foramen, the thoracic intervertebral foramen and the lumbar intervertebral foramen were treated with PRF. During the operation, the abnormal sensation in the original area in which the patient experienced ZAP was replicated accurately, and radiofrequency treatment of three consecutive ganglia was performed at the same time to ensure coverage of the entire pain area and achieve good surgical results.
Ozone has been comprehensively used in the treatment of chronic pain because of its strong oxidizing activity. Local injection of ozone can quickly relieve pain, nerve congestion and oedema, and it also reduces the local tissue temperature and increases the supply of oxygen to the tissue.27,28 Ozone kills bacteria, inactivates viruses and spreads easily.29 In this study, ozone was injected around the spinal nerve. This can effectively eliminate pain-inducing factors such as substance P, bradykinin, and interleukins around the spinal nerve root and the DRG, reduce the production of local inflammatory mediators, improve the local oxygen state, and promote repair of the injured nervous system tissue.30,31 The use of ozone near the DRG is very safe, and it can be injected around the intervertebral foramen. Ozone destroys the cell membranes of bacteria by oxidizing phospholipids and lipoproteins; it also inhibits the growth of fungi, destroys viral capsids, and disrupts the viral reproductive cycle by destroying the oxidative contact between viruses and cells.32–34 Our follow-up results showed that the VAS scores of the patients in the three groups were decreased both on the day after the operation and at 1, 3, 6, and 12 months after the operation, and their daily dosage of pregabalin was reduced, indicating that PRF combined with ozone injection has good clinical efficacy in the treatment of ZAP.
Interestingly, a 12-month retrospective analysis of patients with different courses of disease found that the VAS scores of patients in the AHN and SHN groups decreased more significantly than those of patients in the PHN group at each time point after the operation and that pregabalin dosage was lower in the AHN and SHN groups. In that study, the sleep quality of the PHN treatment group was worse than that of the other two groups after the operation, indicating that early implementation of PRF combined with ozone injection can significantly improve the sleep status of ZAP sufferers and improve their quality of life. Therefore, early application of PRF combined with ozone injection may result in earlier repair of peripheral nerve fibres, alleviate neurogenic inflammatory reactions, reduce peripheral and central nerve sensitization, inhibit plastic changes in the nervous system, and block progression to chronic pain.
For patients who experienced poor surgical effects and were unable to tolerate oral drug treatment, we used local injections of lidocaine and triamcinolone acetonide to remedy the damaged skin. Lidocaine works by partially inhibiting the voltage-gated sodium channels of damaged or dysfunctional unmyelinated C fibres and small myelinated A fibres.35 Corticosteroids have a potent anti-inflammatory effect that might alleviate nerve damage and thereby relieve HZ pain and prevent PHN.36 In comparison, subcutaneous injection of lidocaine and triamcinolone is a simple and safe method that blocks abnormal impulse transmission by the injured peripheral nerve and reduces inflammation and pain. After 1 year, the total number of remediations performed in the three groups were 25, 26, and 60, indicating that subcutaneous injection was relatively effective at relieving pain in the early stage of ZAP.
In terms of complications, one patient with HZ in the upper limbs had an adverse reaction after ozone injection during the operation. This may be related to stimulation of the carotid sinus after ozone injection, injury to the vertebral artery and its branches, or to the rapid rate at which the ozone was injected, which may have caused the epidural pressure to increase, resulting in increased intracranial pressure. This finding prompts us to pay attention to the following in future operations: (1) the ozone should be injected slowly; (2) contrast media should be used before ozone injection to further predict the distribution of the injected drug and reduce the risk of its entering the blood and damaging other surrounding tissues; (3) during the operation, ozone should be injected intermittently, and the patients’ feelings should be monitored constantly so that adverse reactions can be identified and handled in time; and (4) when a high spinal cord segment is being treated, the amount of ozone injected can be reduced appropriately according to the size of the spinal canal and the intervertebral foramen.
Limitations
Our study has several limitations. First, it has a retrospective design with a small sample size and a long follow-up interval. There may be some uncontrolled confounding factors in data collection. More high-quality prospective clinical trials with large sample sizes and long observation times are needed for further research and exploration. Second, we only included patients with HZ located in the cervical to lumbosacral areas. The effect of PRF combined with ozone injection on patients with facial HZ remains unknown. Third, this study cannot provide standardized guidelines regarding the use of PRF, such as treatment cycle, set frequency or accurate intervention time.
Conclusions
CT-guided PRF combined with ozone injection is safe and effective for ZAP patients, and its short-term and long-term effects are significant. In a sense, early PRF combined with ozone injection is more effective.
Abbreviations
AHN, acute herpetic neuralgia; BP, blood pressure; DRG, dorsal root ganglia; HR, heart rate; HZ, Herpes zoster; IVF, intervertebral foramen; PHN, postherpetic neuralgia; PRF, pulsed radiofrequency; PSQI, Pittsburgh Sleep Quality Index; SHN, subacute herpetic neuralgia; T0, at baseline; T1, post-PRF; T2, at 1 month after PRF; T3, at 3 months after PRF; T4, at 6 months after PRF; T5, at 12 months after PRF; VAS, visual analogue scale; VZV, varicella zoster virus; ZAP, zoster-associated pain.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lee SH, Lee JY, Yeon H, Rho MC, Bae J, Park HJ. Pain changes and new neurologic sign in post-herpetic neuralgia: a clue in the diagnosis of malignancy-a case report. Ann Palliat Med. 2022;11:2773–2777. doi: 10.21037/apm-21-2567 [DOI] [PubMed] [Google Scholar]
- 2.Ding YY, Li HX, Hong T, Zhao RJ, Yao P, Zhao GY. Efficacy and safety of computed tomography-guided pulsed radiofrequency modulation of thoracic dorsal root ganglion on herpes zoster neuralgia. Neuromodulation. 2019;22:108–114. doi: 10.1111/ner.12858 [DOI] [PubMed] [Google Scholar]
- 3.Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14:192. doi: 10.3390/v14020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theresa MS, Brett S, Jeannine MB. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454. doi: 10.2147/JMDH.S106340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo SQ, Shen MX, Zhang LL, et al. The effect of interventional pain management on treating postherpetic neuralgia. Indian J Dermatol. 2019;64:251. doi: 10.4103/ijd.IJD_130_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X, Wei LL, Zeng Q, Lin K, Zhang J. The treatment of topical drugs for postherpetic neuralgia: a network Meta-analysis. Pain Phys. 2020;23:541–551. [PubMed] [Google Scholar]
- 7.Haiou Z, Zhiguang W, Haifei J, et al. A systematic review and meta-analysis of independent risk factors for postherpetic neuralgia. Ann Palliat Med. 2021;10(12):12181–12189. doi: 10.21037/apm-21-3028 [DOI] [PubMed] [Google Scholar]
- 8.Zhang JF, Williams JP, Zhao QN, Liu H, An JX. Combined high-voltage pulsed radiofrequency and ozone therapy versus ozone therapy alone in treating postherpetic neuralgia: a retrospective comparison. Med Gas Res. 2023;13:15–22. doi: 10.4103/2045-9912.352660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kagan T, Berker C, Ahmet GG, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72:496–500. doi: 10.1016/j.surneu.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EW, Michael BY, Zhang MD, et al. Varicella-zoster virus early infection but not complete replication is required for the induction of chronic hypersensitivity in rat models of postherpetic neuralgia. PLoS Pathog. 2021;17:e1009689. doi: 10.1371/journal.ppat.1009689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen EJ. Commentary on herpes zoster and postherpetic neuralgia. Clin Infect Dis. 2021;73:e3218–e3219. doi: 10.1093/cid/ciaa1192 [DOI] [PubMed] [Google Scholar]
- 12.Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS One. 2017;12:e0183559. doi: 10.1371/journal.pone.0183559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Phys. 2017;20:E411–E418. doi: 10.36076/ppj.2017.E418 [DOI] [PubMed] [Google Scholar]
- 14.Vlassi E, Vlachos P, Kornaros M. Effect of ozonation on table grapes preservation in cold storage. J Food Sci Technol. 2018;55:2031–2038. doi: 10.1007/s13197-018-3117-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautam S, Rastogi V, Jain A, Singh AP. Comparative evaluation of oxygen-ozone therapy and combined use of oxygen-ozone therapy with percutaneous intradiscal radiofrequency thermocoagulation for the treatment of lumbar disc herniation. Pain Pract. 2011;11:160–166. doi: 10.1111/j.1533-2500.2010.00409.x [DOI] [PubMed] [Google Scholar]
- 16.Bernardino C, Norberto SR, Dominga G, et al. Long-term improvement in refractory headache following ozone therapy. J Altern Complement Med. 2013;19:453–458. doi: 10.1089/acm.2012.0273 [DOI] [PubMed] [Google Scholar]
- 17.Murphy K, Elias G, Steppan J, et al. Percutaneous treatment of herniated lumbar discs with ozone: investigation of the mechanisms of action. J Vasc Interv Radiol. 2016;27:1242–1250. doi: 10.1016/j.jvir.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 18.Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther. 2015;9:2677–2685. doi: 10.2147/DDDT.S74518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JS, Wei X, Bao JJ, et al. A combined ozone interventional therapy for intractable post herpetic neuralgia-a five-year follow up study. Chin J Pain Med. 2016;22:34–40. [Google Scholar]
- 20.Ricardo NW, Kamran G. Herpes zoster-prevention, diagnosis, and treatment. Hautarzt. 2022;73:442–451. doi: 10.1007/s00105-022-04992-9 [DOI] [PubMed] [Google Scholar]
- 21.Alexandra ER, Eellan S, Chen YA. Case report: dorsal root ganglion (DRG) stimulation for acute neuropathic pain from acute herpes zoster infection. SAGE Open Med Case Rep. 2021;9:2050313X211062297. doi: 10.1177/2050313X211062297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing X, Sun K, Yan M. Delayed initiation of supplemental pain management is associated with postherpetic neuralgia: a retrospective study. Pain Phys. 2020;23:65–72. [PubMed] [Google Scholar]
- 23.Iulia P, Alexandras B, Kenneth DC, Nebojsa NK. Overcoming clinical challenges of refractory neuropathic pain. Expert Rev Neurother. 2022;22:595–622. doi: 10.1080/14737175.2022.2105206 [DOI] [PubMed] [Google Scholar]
- 24.Sluijter ME, Cosman E, Rittman W, et al. The effects of pulsed radiofrequency field applied to dorsal root ganglion: a preliminary report. Pain Clin. 1998;11:109–117. [Google Scholar]
- 25.Mila P, Ognjen V, Tanja V, Frederick M, Alaa AE. Safety of conventional and pulsed radiofrequency lesions of the dorsal root entry zone complex (DREZC) for interventional pain management: a systematic review. Pain Ther. 2022;11:411–445. doi: 10.1007/s40122-022-00378-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan S, Michael C, Sachin S, Frederick M, Alaa AE, Ognjen V. Pulsed radiofrequency in interventional pain management: cellular and molecular mechanisms of action - an update and review. Pain Phys. 2021;24:525–532. [PubMed] [Google Scholar]
- 27.Reyhaneh P, Hamid RF, Sana K, Leila Sadat MJ. Comparative effectiveness of paravertebral ozone injection and caudal epidural steroid-hyaluronidase injection in lumbosacral spinal stenosis. Br J Neurosurg. 2021;8:1–6. doi: 10.1080/02688697.2021.1885626 [DOI] [PubMed] [Google Scholar]
- 28.Lin SY, Zhang SZ, An JX, et al. The effect of ultrasound-guided percutaneous ozone injection around cervical dorsal root ganglion in zoster-associated pain: a retrospective study. J Pain Res. 2018;11:2179–2188. doi: 10.2147/JPR.S163340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Jaziri AA, Mahmoodi SM. Painkilling effect of ozone-oxygen injection on spine and joint osteoarthritis. Saudi Med J. 2008;29:553–557. [PubMed] [Google Scholar]
- 30.Luo WJ, Yang F, Yang F, et al. Intervertebral foramen injection of ozone relieves mechanical allodynia and enhances analgesic effect of gabapentin in animal model of neuropathic pain. Pain Phys. 2017;20:E673–E685. [PubMed] [Google Scholar]
- 31.Cristiano S, Giacomo B, Stefano R, et al. Ultrasound-guided periradicular oxygen-ozone injections as a treatment option for low back pain associated with sciatica. Int Orthop. 2021;45:1239–1246. doi: 10.1007/s00264-021-04975-w [DOI] [PubMed] [Google Scholar]
- 32.Đuričić D, Valpotić H, Samardžija M. Prophylaxis and therapeutic potential of ozone in buiatrics: current knowledge. Anim Reprod Sci. 2015;159:1–7. doi: 10.1016/j.anireprosci.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 33.Artis AS, Aydogan S, Sahin MG. The effects of colorectally insufflated oxygen-ozone on red blood cell rheology in rabbits. Clin Hemorheol Microcirc. 2010;45:329–336. doi: 10.3233/CH-2010-1316 [DOI] [PubMed] [Google Scholar]
- 34.Aydos TR, Başar MM, Kul O, et al. Effects of ozone therapy and taurine on ischemia/reperfusion-induced testicular injury in a rat testicular torsion model. Turk J Med Sci. 2014;44:749–755. doi: 10.3906/sag-1308-20 [DOI] [PubMed] [Google Scholar]
- 35.Navez ML, Monella C, Bosl I, Sommer D, Delorme C. 5% lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4:1–15. doi: 10.1007/s40122-015-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y, Zhang JJ, Chen N, He L, Zhou MK, Zhu CR. Corticosteroids for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2013;28:CD005582. doi: 10.1002/14651858.CD005582.pub4 [DOI] [PubMed] [Google Scholar]