Abstract
Background
Despite the use of statins, many patients with cardiovascular disease (CVD) have persistent residual risk. In a large Phase III trial (REDUCE-IT), icosapent ethyl (IPE) was shown to reduce the first occurrence of the primary composite endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina.
Methods
We conducted a cost-utility analysis comparing IPE to placebo in statin-treated patients with elevated triglycerides, from a publicly funded, Canadian healthcare payer perspective, using a time-dependent Markov transition model over a 20-year time horizon. We obtained efficacy and safety data from REDUCE-IT, and costs and utilities from provincial formularies and databases, manufacturer sources, and Canadian literature sources.
Results
In the probabilistic base-case analysis, IPE was associated with an incremental cost of $12,523 and an estimated 0.29 more quality-adjusted life years (QALYs), corresponding to an incremental cost-effectiveness ratio (ICER) of $42,797/QALY gained. At a willingness-to-pay of $50,000 and $100,000/QALY gained, there is a probability of 70.4% and 98.8%, respectively, that IPE is a cost-effective strategy over placebo. The deterministic model yielded similar results. In the deterministic sensitivity analyses, the ICER varied between $31,823-$70,427/QALY gained. Scenario analyses revealed that extending the timeframe of the model to a lifetime horizon resulted in an ICER of $32,925/QALY gained.
Conclusion
IPE represents an important new treatment for the reduction of ischemic CV events in statin-treated patients with elevated triglycerides. Based on the clinical trial evidence, we found that IPE could be a cost-effective strategy for treating these patients in Canada.
Keywords: cardiovascular diseases, icosapent ethyl, IPE, cost-effectiveness, cost per QALY
Introduction
In Canada, cardiovascular disease (CVD) is the second leading cause of death after cancer and a leading cause of hospitalization.1,2 Ischemic heart disease (IHD), the most common form of CVD, is the first cause of years of life lost and the second leading cause of disability-adjusted life years lost.3,4 Early detection and management of CVD risk factors have contributed to reducing the burden of CVD in the last few decades, but despite the widespread use of statins, many patients have persistent residual CV risk.5 Studies have shown that rates of CV events remain high even among patients who are receiving recommended treatments for CV prevention.6–10 In these patients, an elevated triglyceride level is believed to be an independent marker for an increased risk of ischemic events.11–15 Medications commonly used to reduce triglyceride levels are extended-release (ER) niacin and fibrates; however, these have not proven to be efficacious in reducing CV events.16–20
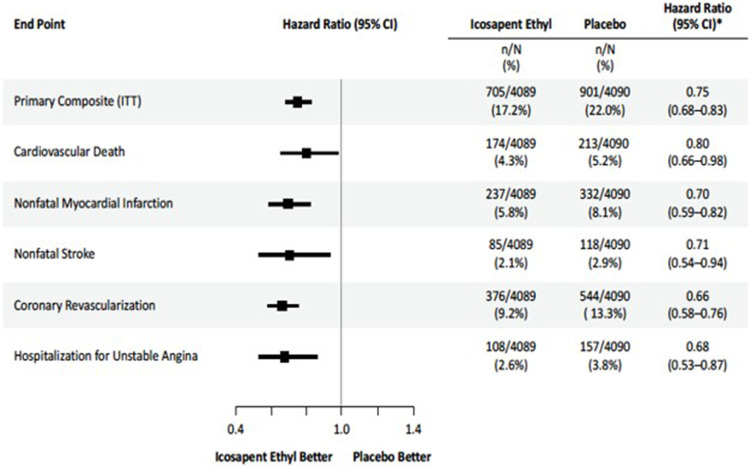
Icosapent ethyl (IPE) is a member of a new class of drugs that acts in multiple ways to reduce CV risk. Studies suggest that IPE may impact atherosclerotic processes, resulting in reduced development, slowed progression, improved endothelial functions, and increased stabilization of atherosclerotic plaque.21–23 It may also have anti-inflammatory, antioxidative, plaque-stabilizing, and membrane-stabilizing properties.24–26 In a randomized, placebo-controlled trial, the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT), the primary composite endpoint event (ie, cardiovascular death, nonfatal myocardial infarction (MI), nonfatal stroke, coronary revascularization, or unstable angina) occurred in 17.2% of the patients in the IPE group versus 22.0% in the placebo group (hazard ratio [HR], 0.75; 95% confidence interval [CI], 0.68 to 0.83; P<0.001).27 Additionally, in the Effect of VASCEPA® on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) trial, IPE demonstrated a regression of coronary plaque, suggesting an anti- atherosclerotic effect.23
Treatment advances are allowing many individuals who would have died of CVD in the past to now live longer with the disease.28 In Canada, in 2013, approximately 2.4 million Canadian adults lived with diagnosed heart disease and over 740,000 had a history of stroke.29,30 Although a reduction in mortality due to CVD has increased life expectancy for Canadians, a longer life lived in poor health is not necessarily indicative of improved health outcomes. Besides causing considerable difficulties for patients and affecting their quality-of-life (QoL), CVD also has a significant economic cost.31 Not only does CVD affect the health system, it also affects the overall economy through missed work and lower productivity. In Canada, total costs for CVDs were estimated to be $12 billion in 2008.31 The objective of the current study was to assess the economic impact of IPE in the reduction of ischemic CV events in Canada based on the results of REDUCE-IT, which evaluated the brand name IPE VASCEPA.27 VASCEPA is a new drug, approved in Canada to reduce the risk of CV events in statin-treated patients (primary and secondary prevention). Preventing or mitigating CV events can have a significant positive impact on healthcare costs and patient well-being.
Methods
Cost-Utility Analysis
We conducted a cost-utility analysis (CUA), from a Canadian publicly funded health care payer perspective, according to the most recent guidelines for the economic evaluation of Health technologies published by the Canadian Agency for Drugs and Technologies in Health (CADTH) in 2017.32 We expressed the results of the analysis as the cost per quality-adjusted life year (QALY) gained.33
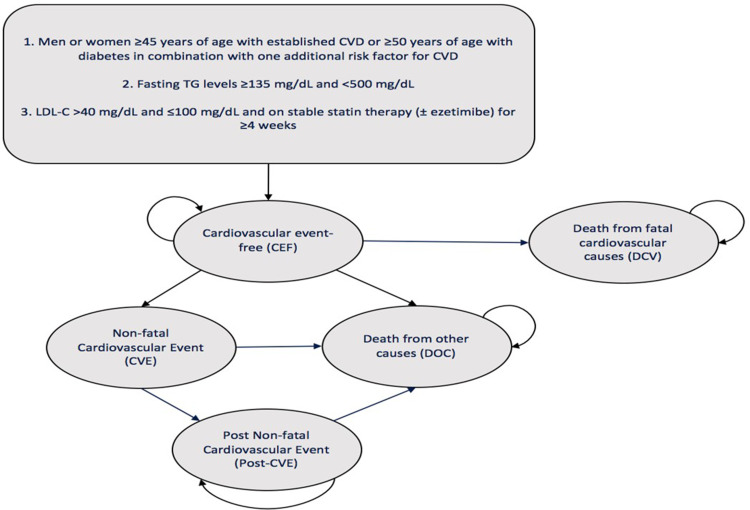
Our target population is in accordance with REDUCE-IT, which compared the effects of IPE 4 grams daily versus placebo in men and women with established CVD or with diabetes mellitus (DM) and other CVD risk factors, despite stable statin therapy and reasonably well-controlled levels of low-density lipoprotein cholesterol (LDL-C).27 All participants used a stable dose of a statin ± ezetimibe. The 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults provided guidance on the appropriate intensity of pharmacological treatment to reduce CVD, defining the intensity of statin therapy on the basis of the average expected LDL-C response to a specific statin and dose.34 Table 1 displays examples of high-, moderate-, and low-intensity statin therapy based on the 2013 ACC/AHA Guideline. In REDUCE-IT, the distribution of patients according to their intensity of statin therapy was high in 30.9%, moderate in 62.7%, and low in 6.4% of patients. The proportion of patients in the trial who used ezetimibe was 6.4%27 and we assumed that the distribution of specific statins within treatment intensity levels was evenly distributed between all treatments.
Table 1.
Examples of High-, Moderate-, and Low-Intensity Statin Therapy Based on the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults
| High-Intensity Statin Therapy | Moderate-Intensity Statin Therapy | Low-Intensity Statin Therapy |
|---|---|---|
| Atorvastatin 40–80mg | Atorvastatin 10–20mg | Fluvastatin 20–40mg |
| Rosuvastatin 20–40mg | Fluvastatin 40mg BID | Lovastatin 20mg |
| Fluvastatin XL 80mg | Pravastatin 10–20mg | |
| Lovastatin 40mg | Simvastatin 10 mg | |
| Pravastatin 40–80mg | ||
| Rosuvastatin 5–10mg |
Notes: Adapted from JAM Coll Cardiol, 68(1), Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, DePalma SM. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. 92–125, Copyright 2016, with permission from Elsevier.47 Only medications available in Canada are included.
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; BID, twice daily; mg, milligrams.
Consistent with the most recent Canadian HTA review of a CUA model for proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors by CADTH, we considered a 20-year time horizon in the base-case analysis.35 We discounted costs and effects incurred after one year at a 1.5% annual discounting rate, as per the most recent CADTH guidelines.32
We based the CUA on a probabilistic time-dependent Markov transition model, comparing IPE to placebo for the reduction of ischemic CV events. We cycled patients through the Markov model in one-year cycles to predict the long-term risk of major CV events through five different health states (Figure 1; Supplement 1). Patients entered the model in the CV event-free (CEF) state, where we assumed that they were at risk of non-fatal CV event (CVE), death from fatal CV causes (DCV), or death from other causes (DOC). Patients with a CVE remained in a post-non- fatal CV event (post-CVE) state, where we assumed that they were at risk of subsequent events. In terms of CVEs, we considered the incidence and distribution of each individual outcome included in the primary composite endpoint: CV death, nonfatal MI, nonfatal stroke, coronary revascularization, and unstable angina. We applied a half-cycle correction to more accurately reflect the continuous nature of the state transition (the assumption being that transitions occur, on average, half-way through each cycle instead of at the beginning of the cycle).
Figure 1.
Markov Model Structure.
Abbreviations: CEF, cardiovascular event-free; CVD, cardiovascular disease; CVE, non-fatal cardiovascular event; DCV, death from fatal cardiovascular causes; DOC, death from other causes; LDL-C, low-density lipoprotein cholesterol; mg/dL, milligrams per decilitre; Post-CVE, post non-fatal cardiovascular event; TG, triglyceride.
We obtained efficacy and safety outcomes from REDUCE-IT.27 We retrieved utility and disutility data from the literature, while we acquired costs, presented in 2019 Canadian dollars (CAD), from the manufacturer, provincial formularies and pharmacists’ databases, and Canadian literature sources.
Efficacy and Safety
We applied the individuals hazard ratios (HRs) of each event from REDUCE-IT (Figure 2) to reconstitute the Kaplan-Meier based on individual patient-level data (IPD), appraising the proportional hazards assumption for all HRs used in the model (Supplement 2). We extrapolated survival rates for the placebo group over the 5-year time horizon using parametric survival models, evaluating the best fit based on the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) statistics, using the Flexsurv for R package for time-to-event data and by visual fit to the Kaplan Meier curves (Supplement 3). In the absence of observed data after the initial 5-year period, we assumed subsequent event rates were equal to that of the placebo group (ie, a HR of 1), as opposed to a less conservative scenario where the benefit of IPE continued to accrue over time.
Figure 2.
Cumulative Incidence of the REDUCE-IT Individual First Primary Endpoint. From N Engl J Med, Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. 380:11–22. Copyright © 2019 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.27
Abbreviations: CI, confidence interval; ITT, intention-to-treat; n, number of patients in treatment group with an event; N, total of number of patients in treatment group.
The incidence of treatment-induced adverse effects (AEs) included in this analysis were those of peripheral edema, constipation, atrial fibrillation, and serious bleeding.27 We only took into account AEs that were statistically different (p <0.05) in disadvantage of IPE, with the exception of serious bleeding (p=0.06) as it is considered an important safety parameter.
Utilities and Disutilities
In this model, we applied multiplicatively acute CVE health state disutilities and post-event utilities to the baseline utility value (Table 2). The baseline utility represented the population under study, which comprised 70.7% patients with established CVD and 29.3% with DM and at least one additional CVD risk factor. In our model, patients experienced acute disutility in the year after their event, after which they would experience a chronic post‐ event utility. We also conducted a structured literature review to identify the disutility associated with each AE included in our model (Table 3).
Table 2.
Health State Utility Values Included in the Model
| Health State | Multiplier | Value | SE | References |
|---|---|---|---|---|
| Baseline (With existing CVD or DM) | 1.000 | 0.762 | 0.016 | J Stevanović et al (2016) and O’Reilly et al (2011)48,49 |
| Acute Nonfatal MI | 0.760 | 0.579 | 0.018 | |
| Acute Nonfatal Stroke | 0.628 | 0.479 | 0.040 | |
| Acute Coronary | ||||
| Revascularization | 0.808 | 0.616 | 0.038 | NICE clinical guideline |
| Acute Unstable Angina | 0.770 | 0.587 | 0.038 | CG181 (Lipid Modification)35,50 |
| Post Nonfatal MI | 0.808 | 0.616 | 0.018 | |
| Post Nonfatal Stroke | 0.683 | 0.520 | 0.040 | |
| Post Coronary | ||||
| Revascularization | 0.808 | 0.616 | 0.038 | |
| Post Unstable Angina | 0.808 | 0.616 | 0.018 | |
| CV Death | 0.000 | 0.000 | 0.000 | By Definition |
| Death | 0.000 | 0.000 | 0.000 | By Definition |
Abbreviations: CVD, cardiovascular disease; DM, diabetes mellitus; MI, myocardial infarction; NICE, National Institute for Health and Care Excellence; SE, standard error.
Table 3.
Disutility Values Included in the Model
| Adverse Event Disutility | Value | SE | References |
|---|---|---|---|
| Peripheral Edema | −0.005 | 0.0008 | Disutility derived from Sullivan et al (2016), with the assumption that this disutility lasts for 7 days51 |
| Constipation | −0.001 | 0.0009 | Disutility derived from Christensen et al (2016), with the assumption that this disutility lasts for 7 days52,53 |
| Atrial Fibrillation | −0.032 | 0.0071 | Disutility taken from Steg et al (2011), with the assumption that this disutility lasts for 0.5 years53,54 |
| Serious Bleeding | −0.104 | 0.0260 | Disutility taken from Tengs et al (2000), with the assumption that this disutility lasts for 1 month and IC bleeding will be associated with neurologic disability for 1 year55 |
Abbreviations: IC, intracranial; SE, standard error.
Costs
We used drug prices paid by the public payer (lower-cost alternatives) for IPE and all comparators. We obtained unit costs from the Ontario Drug Benefit Formulary/Comparative Drug Index, as of March 28, 2019 (Table 4).36 Given their respective recommended doses, the proportion of patients using ezetimibe (6.4%), and the distribution of different statins per treatment intensity, we estimated average annual costs of standard of care (SOC) + placebo and SOC + IPE to be $148.55 and $3728, respectively. Since we assumed after the trial period of 5- year CVE rates were equal in both groups, we also applied the annual costs of SOC + placebo to both groups.
Table 4.
Treatment Acquisition Costs of IPE and Other Lipid Lowering Therapies
| Drug | Strength | Statin Intensity | Recommended Dose | Dosage Form | Priceby Unit($) |
|---|---|---|---|---|---|
| IPE (VASCEPA®) | 1 g | – | 2g BID | Cap | $2.4500 |
| Ezetrol (Ezetimibe) | 10 mg | – | 10 mg daily | Tab | $0.1811 |
| Rosuvastatin calcium | 5 mg | – | 1- to 40 mg | Tab | $0.1284 |
| (CrestorTM and generics) | 10 mg | Moderate | Daily | Tab | $0.1354 |
| 20 mg | High | Tab | $0.1692 | ||
| 40 mg | High | Tab | $0.1990 | ||
| Atorvastatin calcium | 10 mg | Moderate | 10 to 80 mg at | Tab | $0.1743 |
| (LipitorTM and generics) | 20 mg | Moderate | Bedtime | Tab | $0.2179 |
| 40 mg | High | Tab | $0.2342 | ||
| 80 mg | High | Tab | $0.2342 | ||
| Fluvastatin sodium | 20 mg | Low | 20 to 40 mg at | Cap | $0.2202 |
| (LescolTM and generics) | 40 mg | Low | Bedtime | Cap | $0.3092 |
| Fluvastatin sodium | 80 mg | Moderate | 80 mg daily | Tab | $1.6225 |
| (Lescol XLTM) | |||||
| Lovastatin | 20 mg | Low | 20 to 40 mg at | Tab | $0.4919 |
| (MevacorTM and generics) | 40 mg | Moderate | Bedtime | Tab | $0.8985 |
| Pravastatin sodium | 10 mg | Low | 10 to 40 mg at | Tab | $0.2916 |
| (PravacholTM and generics) | 20 mg | Low | Bedtime | Tab | $0.3440 |
| 40 mg | Moderate | Tab | $0.4143 | ||
| Simvastatin | 5 mg | – | 10 to 80 mg at | Tab | $0.1023 |
| (ZocorTM and generics) | 10 mg | Low | Bedtime | Tab | $0.2023 |
| 20 mg | Moderate | Tab | $0.2501 | ||
| 40 mg | Moderate | Tab | $0.2501 | ||
| 80 mg | – | Tab | $0.2501 |
Abbreviations: BID, twice daily; g, gram; IPE, icosapent ethyl; mg, milligrams.
The annual costs related to CV events are depicted in Tables 5 (see Supplement 4 for more detailed cost data). We inflated the average annual per patient healthcare cost of complications to 2019 prices using the healthcare component of the consumer price index (CPI).37 In our model, we assumed that 75% of revascularizations were percutaneous coronary intervention (PCI) and 25% were coronary artery bypass grafting (CABG), based on the recommendation of clinical experts. According to Kaul et al, revascularization rates in the United States (US) were almost 3 times greater than in Canada.38 Therefore, for each coronary revascularization in the model, we only considered 35.7% to be performed in Canada. We estimated the subsequent years’ cost associated with coronary revascularization by using the proportional difference between the first year and subsequent year’s cost of CABG and PCI in a US study.39,40 Lastly, we counted acute care costs associated with episodes of hospitalizations at the initial onset of fatal MI and stroke, similarly to a published Canadian cost-utility analysis on hypertension.41
Table 5.
Annual Costs of Cardiovascular Events Included in the Model
| Health Care Cost | Value |
|---|---|
| Nonfatal MI - Initial Year | $12,392.82 |
| Nonfatal MI - Subsequent Years | $3278.32 |
| Nonfatal Stroke - Initial Year | $23,840.28 |
| Nonfatal Stroke - Subsequent Years | $4806.48 |
| Coronary Revascularization - Initial Yeara | $4676.47 |
| Coronary Revascularization - Subsequent Yearsa | $3411.53 |
| Unstable Angina - Initial Year | $5877.19 |
| Unstable Angina - Subsequent Years | $3463.99 |
| Cardiovascular Deathb | $7878.61 |
Notes: aThe cost of coronary revascularization was a weighted average of the costs of CABG and PCI, the distribution of which was based on expert opinion; bThe cost of CV death was calculated as the average of fatal MI and fatal stroke.
Abbreviations: CABG, coronary artery bypass grafting; CV, cardiovascular; MI, myocardial infarction; PCI, percutaneous coronary intervention.
The annual medical costs associated with follow-up and monitoring are presented in Table 6 (seeSupplement 4 for more detailed cost data), which were based on a number of assumptions (Supplement 5). We only considered the medical appointment to evaluate the response and adverse events, and the initial fasting lipid panel for the IPE group.
Table 6.
Annual Costs Associated with Follow-Up and Monitoring
| Unit Cost for Appointment and Laboratory | Value |
|---|---|
| Initial Year - IPE | $315.39 |
| Initial Year - Placebo | $203.36 |
| Subsequent Years - IPE | $203.36 |
| Subsequent Years - Placebo | $203.36 |
Notes: Assessment appointment for evaluating the response and adverse events, and the initial fasting lipid panel were only considered for the IPE group.
Abbreviation: IPE, icosapent ethyl.
We obtained the mean cost of adverse events (Table 7) from the Costing Analysis Tool of the Ontario Case Costing (OCC) database, which provides patient-level costs for inpatients and ambulatory care cases in Ontario for the years 2010–2018.39
Table 7.
Adverse Events Costs Included in the Model
| Adverse Events Probability | Value | SE | References |
|---|---|---|---|
| Peripheral Edema | $4663.00 | $313.61 | OCC (R.60.0)39 |
| Constipation | $3391.00 | $95.28 | OCC (K.59.0)39 |
| Atrial Fibrillation | $5985.00 | $118.99 | OCC (I.48.90)39 |
| Serious Bleeding | $5402.36 | $314.69 | OCC (K.92.2/K.62.5/I.62.0/R.31.0/R.04.0)39 |
Abbreviations: OCC, Ontario Case Costing; SE, standard error.
Base Case
Consistent with recent CADTH guidelines,32 we derived the base case analysis results from an analysis of uncertainty using a probabilistic model (see Supplement 5 for model assumptions). We generated probabilistic analyses (PAs) by simultaneously sampling from estimated probability distributions of model parameters (Table 8), performing a total of 5000 simulations. We generated descriptive statistics based on the simulated values for costs, QALYs, incremental costs, and incremental QALYs, and also constructed a cost-effectiveness acceptability curves (CEACs).
Table 8.
Parameters Varied in Probabilistic and Deterministic Sensitivity Analyses
| Parameter | Range Tested | Distribution |
|---|---|---|
| Starting Age | Range in REDUCE-IT trial | Normal |
| Proportion Males/Females | ± 25% | Beta α= 3.828 β= 1.518 |
| Statin Intensity | ± 25% | Dirichlet α= 0.627 β= 0.309 |
| Percentage of Ezetimibe Use | ± 25% | Beta α= 14.911 β= 217.830 |
| Percentage of PCI vs CABG | ± 25% | Beta α= 3.250 β= 1.083 |
| Revascularization rates in Canada vs United States | ± 25% | Beta α= 9.937 β= 17.923 |
| HR Primary Endpoint - First event | SE | Beta Individual parameter for each endpoint |
| Individual Component of the Primary Endpoint – First event | ± 25% | Beta Individual parameter for each endpoint |
| Individual Component of the Primary Endpoint – Subsequent event | ± 25% | Beta Individual parameter for each endpoint |
| Mortality Multiplier | SE | Gamma Individual parameter for each endpoint |
| Survival Parameters (Shape, Scale) | SE | Normal |
| Annual Drug Cost for Standard of Care | Lower and Higher Statin |
Gamma α= 1.099 β= 135.064 |
| Health Care Cost | SE | Gamma Individual parameter for each cost |
| Monitoring and Follow-up Frequency | Clinical Expert Range |
Gamma Individual parameter for each item |
| Monitoring and Follow-up Frequency | Difference between General Practitioner and Cardiologist |
Gamma |
| Adverse Event Incidence | ± 25% | Beta Individual parameter for each AE |
| Adverse event costs | SE | Gamma Individual parameter for each AE |
| Utility | SE | Beta Individual parameter for each health state |
Abbreviations: CABG, coronary artery bypass grafting; HR, hazard ratio; PCI, percutaneous coronary intervention; SE, standard error.
Uncertainty
We performed both deterministic sensitivity and probabilistic scenario analyses to assess the impact of each parameter on the base case results (see details in Supplement 6).
Model Outputs
We calculated incremental cost-effectiveness ratios (ICERs) as the incremental cost/QALY gained. As per the most recent CADTH guidelines, we presented the median probabilistic ICER.32 We evaluated the cost-effectiveness of IPE versus placebo based on the established willingness-to-pay (WTP) threshold of $50,000/QALY, which is a commonly accepted threshold in Canada.
Results
Base Case Analysis
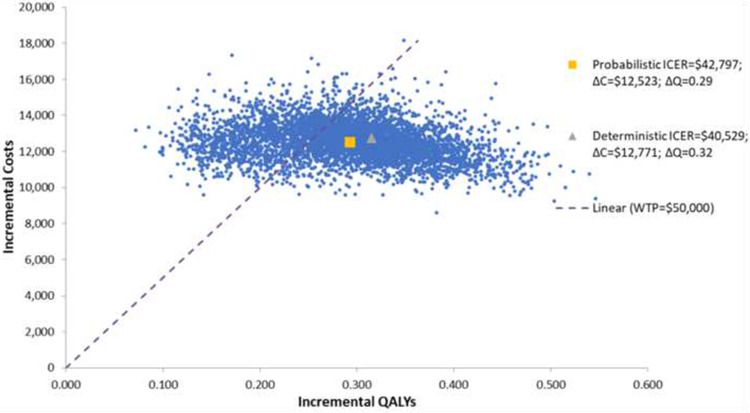
The results of the base case analysis are presented in Table 9. IPE was associated with an incremental cost of $12,523 and an additional 0.29 QALYs gained compared to placebo. The mean probabilistic ICER was $42,797/QALY gained.
Table 9.
Results of Probabilistic Analyses
| Probabilistic Model | IPE | Placebo |
|---|---|---|
| Total, Discounted | ||
| Costs, $ (SD) | $54,864 ($4483) | $42,341 ($4777) |
| QALYs (SD) | 9.88 (0.52) | 9.58 (0.49) |
| Difference (IPE vs Placebo) | ||
| Costs, $ (SD) | $12,523 ($1029) | |
| QALYs (SD) | 0.29 (0.07) | |
| ICER Probabilistic (IPE vs Placebo) | ||
| Costs ($)/QALY gained (SD) | $42,797 ($15,884) | |
Abbreviations: ICER, incremental cost-effectiveness ratio; IPE, icosapent ethyl; QALY, quality-adjusted life year; SD, standard deviation.
Expected discounted costs by treatment and cost categories are shown in Table 10. The majority of the total incremental costs is from the cost of IPE; however, the increase in medication costs is offset by the reduction in costs associated with the first and subsequent CV events.
Table 10.
Expected Discounted Costs, by Treatment and Cost Category
| Probabilistic Model | IPE | Placebo |
|---|---|---|
| Total Costs for 1 patient, Discounted ($) | ||
| Drug Cost (SD) | $18,807 ($843) | $2043 ($480) |
| Cardiovascular Disease Cost - First Event (SD) | $32,372 ($4283) | $36,448 ($4640) |
| Cardiovascular Disease Cost - Subsequent Event | $766 ($104) | $1388 ($188) |
| (SD) | ||
| Follow-Up (SD) | $1960 ($684) | $1760 ($610) |
| Adverse Events (SD) | $958 ($127) | $701 ($93) |
| Difference for 1 patient (IPE vs Placebo) | ||
| Drug Cost | $16,764 | |
| Cardiovascular Disease Cost - First Event | -$4075 | |
| Cardiovascular Disease Cost - Subsequent Event | -$622 | |
| Follow-Up | $200 | |
| Adverse Events | $257 | |
Abbreviations: IPE, icosapent ethyl; SD, standard deviation.
The gain in QALYs is driven largely by the decrease in CV events observed with IPE (Table 11). The disutility associated with AEs has only a minimal impact on the gain in QALYs.
Table 11.
Expected Discounted QALYs
| Probabilistic Model | IPE | Placebo |
|---|---|---|
| Total QALY for 1 patient, Discounted | ||
| Utility Associated with Cardiovascular Health States | 9.88 (0.52) | 9.59 (0.49) |
| (SD) | ||
| Disutility Associated with Treatment-related AEs (SD) | −0.005 (0.001) | −0.004 (0.001) |
| Difference for 1 patient (IPE vs Placebo) | ||
| Utility Associated with Cardiovascular Health States | 0.29 | |
| Disutility Associated with Treatment-related AEs | −0.001 | |
Abbreviations: AE, adverse event; IPE, icosapent ethyl; QALY, quality-adjusted life year; SD, standard deviation.
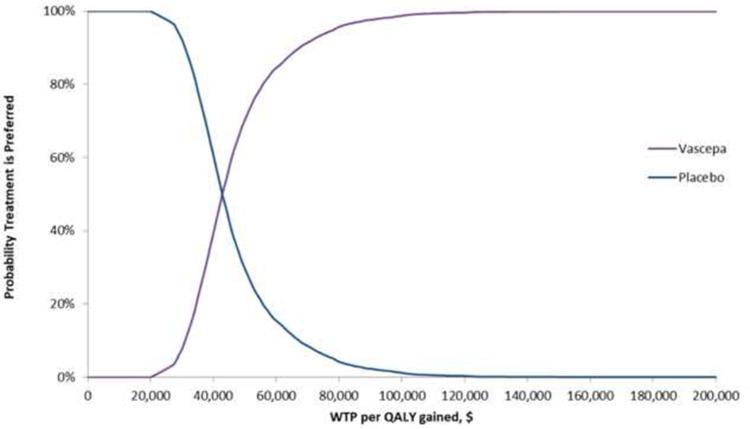
A scatter plot depicting the cost/QALY gained for each of the 5000 simulations is shown in Figure 3. The CEACs for WTP thresholds from $0 to $200,000 are presented in Figure 4. At a WTP of $50,000, there is a 70.4% probability that IPE is cost-effective relative to placebo. At a WTP of $100,000, there is a 98.8% probability that IPE is cost-effective relative to placebo.
Figure 3.
Scatter plot of Simulation of Cost-Effectiveness Plane for IPE vs Placebo.
Abbreviations: ∆C, incremental cost; ∆Q, incremental QALYs; ICER, incremental cost- effectiveness ratio; QALY, quality-adjustedlife year; WTP, willingness-to-pay.
Figure 4.
Cost-Effectiveness Acceptability Curves for IPE vs Placebo.
Abbreviations: QALY, quality-adjustedlife year; WTP, willingness-to-pay.
Uncertainty Analyses
The results of the deterministic sensitivity and probabilistic scenario analyses are presented in Supplement 6. The deterministic model yielded comparable results (ICER = $40,529/QALY gained) due to similar incremental costs and QALYs gained. From the deterministic sensitivity analyses, we found that the results were most sensitive to age, to the percentage of CV death among primary endpoints, and to the percentage of coronary revascularization in subsequent events. Based on the ranges tested, the ICERs varied between $31,823 and $70,427/QALY gained.
The results of the probabilistic scenario analyses indicated that the time horizon can have a large impact on the results, as extending the model to a lifetime horizon would result in an ICER of $32,925/QALY gained, while limiting the timeframe to 5 years would result in an ICER of $253,227/QALY gained. All other scenario analyses (ie, varying the discount rate, using a societal perspective, using the data from the Kaplan Meier from REDUCE-IT instead of the parametric survival function, and not using the half-cycle correction) yielded ICERs between $37,433 and $49,860/QALY gained.
Discussion
The objective of this study was to assess the economic impact of IPE in the reduction of ischemic CV events in Canadian statin-treated patients with CVD or DM+ RF with elevated triglycerides in Canada. Based on a CUA, from a publicly funded healthcare payer perspective over a 20-year time horizon, the probabilistic base-case analysis revealed that treatment with IPE was associated with an ICER of $42,797/QALY gained relative to placebo. At a WTP of $50,000 and $100,000/QALY gained, there is a probability of 70.4% and 98.8%, respectively, that IPE is a cost-effective strategy for this patient population. The largest driver of the increased costs seen with IPE is the cost of the medication itself; however, as IPE reduces the occurrence of CV events,27 it may not only reduce the costs associated with these events, but also improve the QALYs gained in these patients. Though IPE may increase the risk of certain AEs,27 the disutilities associated with these AEs are minimal.
This is the first economic evaluation specifically on IPE from a Canadian healthcare payer perspective; however, evaluations have recently been conducted on omega-3 fatty acid therapies in similar patient populations in other countries. Kodera et al (2018) performed a cost- effectiveness study comparing eicosapentaenoic acid (EPA), IPE is a highly purified form of EPA, plus statin therapy versus statin therapy alone in Japanese patients with hypercholesterolemia using data from the JELIS trial.42 The original study revealed that adding EPA to statin therapy significantly reduced the risk of major coronary events.43 In the cost- effectiveness analysis conducted over a 30-year period from a public healthcare funder perspective in Japan, the authors concluded that EPA plus statin therapy showed acceptable cost-effectiveness in secondary prevention (¥5.5 million/QALY gained or ~$68,669 CAD) but not primary prevention (¥29.6 million/QALY gained; ~369,564 CAD).42 In addition, Philip et al (2016) developed a cost-effectiveness model extrapolating the results of the same JELIS trial to a US population.44 In their analysis conducted over a 5-year time horizon, the authors reported both cost savings and improved utilities with EPA plus statin therapy versus statin monotherapy.44 Gao et al conducted a cost-effectiveness analysis in Australia with a 25- year time horizon, which also used data from REDUCE-IT.45 The authors also found that icosapent ethyl was associated with both higher costs and benefit, with an ICER of 59,036 Australian dollars (AUD)/QALY gained (~56,913 CAD), though this was not cost-effective according to their WTP threshold of 50,000 AUD/QALY.45 This study differs from our current analysis, not only because it was conducted from the perspective of another country with different healthcare cost prices and considerations, but also in terms of model inputs; however, of note, the key difference between the study by Gao et al and our current analysis was the difference in costs between IPE and placebo (16,805 AUD [~16,200 CAD] versus 12,523 CAD in our study), whereas the difference in QALYs between treatments was identical (0.29). This may be explained by the longer time horizon used in the study by Gao et al (25 versus 20 years in our study). In the US, Weintraub et al performed a cost-effectiveness analysis also based on the results of the REDUCE-IT clinical trial. They found that compared with standard care, IPE had an 89.4% probability of costing less than $50 000 per QALY gained when using SSR cost and a 72.5% probability of costing less than $50 000 per QALY gained when using WAC. These results are in line with what we found in our study.
Lastly, after completing a systematic review of cost-effectiveness studies on treatment strategies for the secondary prevention of CVD, Marquina et al concluded that omega-3 polyunsaturated fatty acids were cost-effective relative to standard care in most of the included studies, with ICERs ranging from 57,128 to 139,082 US dollars (~75,286 to 183,289 CAD).46
Some limitations are related to our model assumptions (Supplement 5). We assumed that the baseline characteristics obtained from REDUCE-IT (eg, age and gender distribution, the proportion of patients using ezetimibe, the distribution of statin treatment intensity levels, etc.) would be consistent with the target patient population in Canada. We also assumed event rates were equal in both treatment groups after the initial 5-year period, considering our 25-year time horizon, as we did not have follow-up data beyond this timepoint. Lastly, our assumptions on revascularization rates, the proportion of patients expected to receive a PCI versus a CABG, and the proportion of the patient’s management expected to be done by a primary care physician were based mainly on clinical expert consultation or limited data.
The estimated ICER was very sensible to the selected time horizon. Since it is a cost per QALY analysis, by limiting the time horizon for example to 5 years instead of 20 years, the long-term impact on the QALY gained with the treatment would be largely underestimated.
IPE represents an important new advanced treatment for the reduction of ischemic CV events in statin-treated patients with elevated triglycerides. Based on the clinical trial evidence, this CUA suggested that IPE could be a cost-effective strategy in Canada, based on the conventionally quoted threshold of $50,000/QALY gained.
Acknowledgments
The abstract of this paper was presented at the 2020 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) European Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value in Health 23, Supplement 2; December 2020: S496. https://www.ispor.org/heor-resources/presentations-database/presentation/euro2020-3282/108314
Funding Statement
This study was funded by HLS Therapeutics Inc.
Disclosure
JCG has received lecture fees and advisory board fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Sanofi, and Servier and advisory board fees from HLS Therapeutics. RAH has received consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, HLS Therapeutics, Novartis, Pfizer, Arrowhead Pharma, Amryt, Ultragenyx, Regeneron and Sanofi. LAL has received research funding from, has provided continuing medical education (CME) on behalf of, and/or has acted as an advisor to Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esperion, GSK, HLS Therapeutics, Janssen, Kowa, Lexicon, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier. The remaining authors declare no competing interests in this work.
References
- 1.Canadian Institute for Health Information. Inpatient hospitalizations, surgeries and childbirth indicators in 2013–2014. Ottawa (ON); 2019. Available from: https://secure.cihi.ca/free_products/CAD_Hospitalization%20and%20Childbirth_Infosheet_ENrev-web.pdf. Accessed March 14, 2023. [Google Scholar]
- 2.Statistics Canada. Leading causes of death, total population, by age group. Ottawa (ON); 2019. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401. Accessed March 14, 2023. [Google Scholar]
- 3.Kassebaum NJ, Arora M, Barber RM, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1603–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for. 249 causes of death, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fruchart JC, Davignon J, Hermans MP, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol. 2014;13:26. doi: 10.1186/1475-2840-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350–1357. doi: 10.1001/jama.2010.1322 [DOI] [PubMed] [Google Scholar]
- 7.Nambi V, Bhatt DL. Primary prevention of atherosclerosis: time to take a selfie? J Am Coll Cardiol. 2017;70:2992–2994. doi: 10.1016/j.jacc.2017.10.068 [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Venkataramani AS, Bhatt DL. Moving toward global primordial prevention in cardiovascular disease: the heart of the matter. J Am Coll Cardiol. 2015;66:1535–1537. doi: 10.1016/j.jacc.2015.08.027 [DOI] [PubMed] [Google Scholar]
- 9.Chait A, Ginsberg HN, Vaisar T, Heinecke JW, Goldberg IJ, Bornfeldt KE. Remnants of the triglyceride-rich lipoproteins, diabetes, and cardiovascular disease. Diabetes. 2020;69:508–516. doi: 10.2337/dbi19-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dash S, Leiter LA. Residual cardiovascular risk among people with diabetes. Diabetes Obes Metab. 2019;21(Suppl 1):28–38. doi: 10.1111/dom.13646 [DOI] [PubMed] [Google Scholar]
- 11.Klempfner R, Erez A, Sagit BZ, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the bezafibrate infarction prevention study and registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104 [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36:774–776. doi: 10.1093/eurheartj/ehu500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab. 2018;103:3019–3027. doi: 10.1210/jc.2018-00470 [DOI] [PubMed] [Google Scholar]
- 14.Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes Metab. 2019;21:366–371. doi: 10.1111/dom.13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth PP, Granowitz C, Hull M, Liassou D, Anderson A, Philip S. High triglycerides are associated with increased cardiovascular events, medical costs, and resource use: a real-world administrative claims analysis of statin-treated patients with high residual cardiovascular risk. J Am Heart Assoc. 2018;7:e008740. doi: 10.1161/JAHA.118.008740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72:330–343. doi: 10.1016/j.jacc.2018.04.061 [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegele RA, Gidding SS, Ginsberg HN, et al. Nonstatin low-density lipoprotein- lowering therapy and cardiovascular risk reduction-statement from ATVB council. Arterioscler Thromb Vasc Biol. 2015;35:2269–2280. doi: 10.1161/ATVBAHA.115.306442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Gregoire J, Pearson GJ, et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;2016(32):1263–1282. doi: 10.1016/j.cjca.2016.07.510 [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DL, Budoff MJ, Mason RP. A Revolution in Omega-3 Fatty Acid Research. J Am Coll Cardiol. 2020;76:2098–2101. doi: 10.1016/j.jacc.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 22.Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40:1135–1147. doi: 10.1161/ATVBAHA.119.313286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budoff MJ, Bhatt DL, Kinninger A, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41:3925–3932. doi: 10.1093/eurheartj/ehaa652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858:3131–3140. doi: 10.1016/j.bbamem.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 25.Nelson JR, Wani O, May HT, Budoff M. Potential benefits of eicosapentaenoic acid on atherosclerotic plaques. Vascul Pharmacol. 2017;91:1–9. doi: 10.1016/j.vph.2017.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Sherratt SCR, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by X-ray diffraction. Chem Phys Lipids. 2018;212:73–79. doi: 10.1016/j.chemphyslip.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 27.Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 28.The Conference Board of Canada. Mortality due to heart disease and stroke; 2015. Available from: https://www.conferenceboard.ca/hcp/provincial/health/heart.aspx?AspxAutoDetectCookieSupport=1. Accessed March 14, 2023.
- 29.Government of Canada. Stroke in Canada; 2016. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/stroke-in-canada.html. Accessed March 14, 2023.
- 30.Public Health Agency of Canada. Heart Disease in Canada. Public Health Agency of Canada; 2017. [Google Scholar]
- 31.Public Health Agency of Canada. Economic Burden of Ilness in Canada 2005–2008. Ottawa (ON): Public Health Agency of Canada; 2014. [Google Scholar]
- 32.CADTH. Guidelines for the Economic Evaluation of Health Technologies. 4th ed. Ottawa, Ontario (Canada): CADTH Methods and Guidelines; 2017. [Google Scholar]
- 33.McGregor M. Cost-utility analysis: use QALYs only with great caution. CMAJ. 2003;168:433–434. [PMC free article] [PubMed] [Google Scholar]
- 34.Stone Neil J, Robinson Jennifer G, Lichtenstein Alice H, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2013;2014(129):S1–S45. [DOI] [PubMed] [Google Scholar]
- 35.Canadian Agency for Drugs and Technologies in Health (CADTH). CDR Pharmacoeconomic review report for Praluent; 2016.
- 36.Ministry of Health and Long-Term Care of Ontario. Ontario drug benefit formulary/comparative drug index - formulary search; 2019. Available from: https://www.formulary.health.gov.on.ca/formulary/. Accessed March 14, 2023.
- 37.Statistics Canada. Consumer price index, health and personal care, by province (monthly); 2016. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000413. Accessed March 14, 2023.
- 38.Kaul P, Armstrong PW, Chang WC, et al. Long-term mortality of patients with acute myocardial infarction in the United States and Canada: comparison of patients enrolled in global utilization of streptokinase and t-PA for occluded coronary arteries (GUSTO)- I. Circulation. 2004;110:1754–1760. doi: 10.1161/01.CIR.0000142671.06167.91 [DOI] [PubMed] [Google Scholar]
- 39.Ministry of Health and Long Term Care. Ontario case costing - cost analysis tool, health data branch web portal; 2017. Available from: http://hsim.health.gov.on.ca/hdbportal/2015. Accessed March 14, 2023.
- 40.Henk HJ, Paoli CJ, Gandra SR. A retrospective study to examine healthcare costs related to cardiovascular events in individuals with hyperlipidemia. Adv Ther. 2015;32:1104–1116. doi: 10.1007/s12325-015-0264-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anis H, Sun H, Singh S, Woolcott J, Nosyk B, Brisson M. A cost-utility analysis of losartan versus atenolol in the treatment of hypertension with left ventricular hypertrophy. Pharmacoeconomics. 2006;24:387–400. doi: 10.2165/00019053-200624040-00008 [DOI] [PubMed] [Google Scholar]
- 42.Kodera S, Morita H, Kiyosue A, Ando J, Komuro I. Cost-effectiveness of statin plus eicosapentaenoic acid combination therapy for cardiovascular disease prevention in japanese patients with hypercholesterolemia - an analysis based on the Japan eicosapentaenoic acid lipid intervention study (JELIS). Circ J. 2018;82:1076–1082. doi: 10.1253/circj.CJ-17-0995 [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3 [DOI] [PubMed] [Google Scholar]
- 44.Philip S, Chowdhury S, Nelson JR, Benjamin everett P, Hulme-Lowe CK, Schmier JK. A novel cost-effectiveness model of prescription eicosapentaenoic acid extrapolated to secondary prevention of cardiovascular diseases in the United States. J Med Econ. 2016;19:1003–1010. doi: 10.1080/13696998.2016.1207652 [DOI] [PubMed] [Google Scholar]
- 45.Gao L, Moodie M, Li SC. The cost-effectiveness of omega-3 polyunsaturated fatty acids The Australian healthcare perspective. Eur J Intern Med. 2019;67:70–77. doi: 10.1016/j.ejim.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 46.Marquina C, Zomer E, Vargas-Torres S, et al. Novel treatment strategies for secondary prevention of cardiovascular disease: a systematic review of cost-effectiveness. Pharmacoeconomics. 2020;38:1095–1113. doi: 10.1007/s40273-020-00936-0 [DOI] [PubMed] [Google Scholar]
- 47.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, DePalma SM. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2016;68:92–125. doi: 10.1016/j.jacc.2016.03.519 [DOI] [PubMed] [Google Scholar]
- 48.O’Reilly DJ, Xie F, Pullenayegum E, et al. Estimation of the impact of diabetes-related complications on health utilities for patients with type 2 diabetes in Ontario, Canada. Qual Life Res. 2011;20:939–943. doi: 10.1007/s11136-010-9828-9 [DOI] [PubMed] [Google Scholar]
- 49.Stevanovic J, Pechlivanoglou P, Kampinga MA, Krabbe PF, Postma MJ. Multivariate meta-analysis of preference-based quality of life values in coronary heart disease. PLoS One. 2016;11:e0152030. doi: 10.1371/journal.pone.0152030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Clinical Guideline Centre. Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. London: National Clinical Guideline Centre; 2014. Available from: https://www.nice.org.uk/guidance/cg181/evidence/lipid‐modification‐update‐appendices‐243786638. Accessed March 14, 2023. [Google Scholar]
- 51.Sullivan PW, Ghushchyan VH. EQ-5D scores for diabetes-related comorbidities. Value Health. 2016;19:1002–1008. doi: 10.1016/j.jval.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 52.Christensen HN, Olsson U, From J, Breivik H. Opioid-induced constipation, use of laxatives, and health-related quality of life. Scand J Pain. 2016;11:104–110. doi: 10.1016/j.sjpain.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 53.National Institute for Health and care Excellence. Chronic obstructive pulmonaty disease in over 16s: diagnosis and management - Econominc model report”. NICE guideline [NG115]; 2018. [PubMed]
- 54.Steg PG, Alam S, Chiang CE, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98:195–201. doi: 10.1136/heartjnl-2011-300550 [DOI] [PubMed] [Google Scholar]
- 55.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004 [DOI] [PubMed] [Google Scholar]