Abstract
An increasingly older population is one of the major social and medical challenges we currently face. Between 2010 and 2050, it is estimated that the proportion of adults over 65 years of age will double from 8% to 16% of the global population. A major concern associated with aging is the changes in health that can lead to various diseases such as cancer and neurogenerative diseases, which are major burdens on individuals and societies. Thus, it is imperative to better understand changes in sleep and circadian rhythms that accompany aging to improve the health of an older population and target diseases associated with aging. Circadian rhythms play a role in most physiological processes and can contribute to age-related diseases. Interestingly, there is a relationship between circadian rhythms and aging. For example, many older adults have a shift in chronotype, which is an individual’s natural inclination to sleep certain times of the day. As adults age, most people tend to go to sleep earlier while also waking up earlier. Numerous studies also suggest that disrupted circadian rhythms may be indicative of developing age-related diseases, like neurodegenerative disorders and cancer. Better understanding the relationship between circadian rhythms and aging may allow us to improve current treatments or develop novel ones that target diseases commonly associated with aging.
Keywords: circadian rhythms, aging, sleep, age-related diseases
Introduction
The General Role of Circadian Rhythms
Circadian rhythms are near 24-hour oscillations that exist in most biological processes such as sleep/wake cycles, hormone levels, attention, cognition, and mood across a 24-hour cycle.1 These endogenous rhythms are important for survival because they allow organisms to synchronize their internal biological processes with external environmental cues, such as the light-dark cycle. This synchronization allows organisms to anticipate and prepare for changes in their environment, and to optimize their physiological and behavioral responses accordingly.2 For example, circadian rhythms help organisms to adapt to changes in their environment, such as seasonal changes in light and temperature. Organisms can optimize their chances for survival and reproduction by adjusting their physiological and behavioral responses to changes. To maintain these rhythms, circadian clocks are found throughout the body and have a hierarchical timing system.3,4
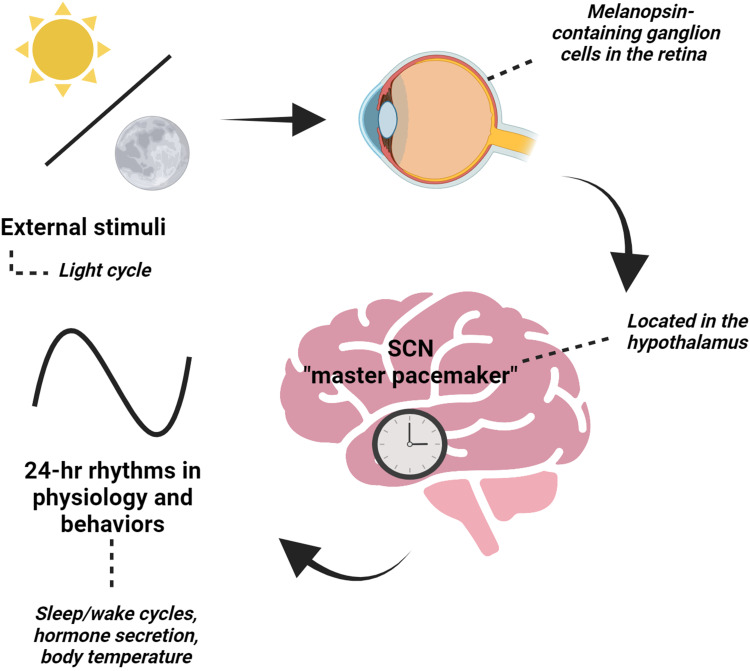
The suprachiasmatic nucleus (SCN), known as the master pacemaker, is in the dorsal hypothalamus and regulates behavioral and physiological rhythms (Figure 1).4–7 The SCN is unique in that it becomes entrained to the light cycles and receives direct projections from intrinsically photosensitive retinal ganglion cells (ipRGCs), which express the photoreceptor melanopsin.8–10 Melanopsin plays a key role in phototransduction functions such as circadian photoentrainment.11,12 Overall, the SCN receives light input, a potent timekeeper, to set the timing of rhythms by regulating neuronal activity, body temperature, and hormonal signals.13,14
Figure 1.
The suprachiasmatic nucleus (SCN), the “master” pacemaker, is located in the hypothalamus and is essential in setting 24-hr rhythms in behaviors and physiology based on external stimuli, like the light cycle. The SCN receives direct input from the retina of the eye through a specialized pathway known as the retinohypothalamic tract. When light enters the eye, it activates specialized photoreceptor cells called melanopsin-containing retinal ganglion cells. These cells then send signals to the SCN, which uses this information to adjust the body’s internal clock. Created with BioRender.com.
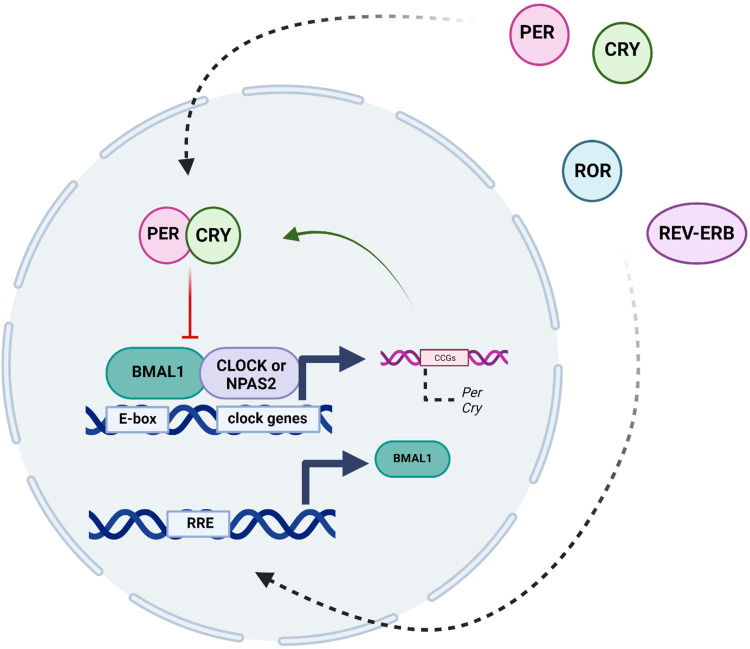
Individual cells have a molecular clock, which is an integral part of the circadian system (Figure 2). The molecular clock consists of a transcriptional/translational feedback loop that cycles over 24-hours in the absence of environmental stimuli.15–17 The major transcriptional activators consist of proteins Circadian Locomotor Output Cycles Kaput (CLOCK), or Neuronal PAS Domain Protein 2 (NPAS2), which bind to Brain and Muscle Arnt-like Protein 1 (BMAL1). These proteins heterodimerize to recognize E-box motifs and regulate the expression of thousands of genes.18 Of these clock-controlled genes, there are Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2) genes that act as repressors.19 Within the 24-hour cycle, PER and CRY proteins in turn are phosphorylated and feed back into the nucleus to inhibit the transcriptional activity of the CLOCK/NPAS2-BMAL1 complex, which inhibits their own expression and creates a negative feedback loop.20,21 Additionally, there are further key regulators of the circadian clock, such as the nuclear receptors REV-ERBα and REV-ERBβ, as well as the retinoic acid orphan receptor (ROR) (RORα, RORβ, and RORγ) establish another feedback loop.22,23 Specifically, while REV-ERBs act as transcriptional repressors of BMAL1 expression, RORs positively regulate the expression of BMAL1 by binding to sites Retinoic acid receptor-related Orphan Receptor Element (RORE) elements in the BMAL1 gene promoter. Overall, these transcriptional-translational feedback loops function in a cyclic fashion and are essential to the formation of biological rhythms.22
Figure 2.
The molecular clock consists of a transcriptional/translational feedback loop that cycles over 24-hours in the absence of environmental stimuli. The main transcription factors are Circadian Locomotor Output Cycles Kaput (CLOCK), or Neuronal PAS Domain Protein 2 (NPAS2), which bind to Brain and Muscle Arnt-like Protein 1 (BMAL1). These proteins heterodimerize and promote transcription of the Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2) genes. PER and CRY proteins are phosphorylated inhibit the CLOCK/NPAS2-BMAL1 complex, which inhibits their own expression and creates a negative feedback loop. There are additional key regulators of the circadian clock, such as the nuclear receptors REV-ERBα and REV-ERBβ, as well as the retinoic acid orphan receptor (ROR) (RORα, RORβ, and RORγ) establish another feedback loop. RORs positively regulate the expression of BMAL1 by binding to sites Retinoic acid receptor-related Orphan Receptor Element (RORE) elements in the BMAL1 gene promoter. Created with BioRender.com.
The circadian system plays an essential role in maintaining health as circadian disruptions may lead to the development of diseases.24,25 For instance, limited studies suggest that shift work may be a risk factor for cardiovascular disease.26 Furthermore, having later bedtimes can lead to an increase in the consumption of unhealthy foods, which cause an increase in body mass index.27 Circadian rhythms are, thus, pervasive throughout systems and a variety of diseases.
Moreover, circadian rhythms can vary within individuals and begin to develop in infancy and change throughout the life cycle.28,29 For example, older individuals typically wake up earlier and go to sleep earlier in comparison to younger individuals.30 Interestingly, aging can also lead to diseases such as cancer.31 Furthermore, changes in the circadian system and sleep patterns are associated with aging while circadian disruptions accelerate aging. There is, thus, a potential bidirectional relationship between the circadian system and aging. Here, we review the literature to better characterize this relationship between aging and the circadian system.
Normal Age-Related Changes in Circadian Changes and Sleep
Aging Impacts Circadian Rhythms
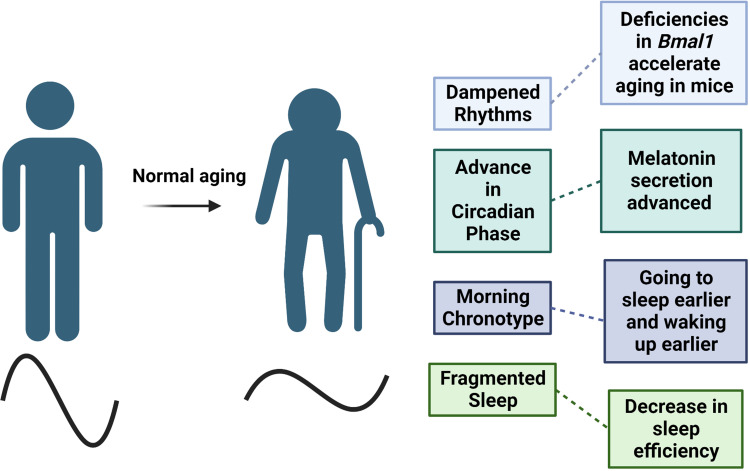
Numerous studies have suggested that aging is accompanied by dampened circadian rhythms and changes in sleep patterns (Figure 3). For example, it has been suggested that there is a decrease in amplitude and sleep becomes more fragmented with age.30 However, these changes have not been consistent across studies as there may be several confounds, such as the short length of the studies.
Figure 3.
Normal aging is characterized by the dampening of circadian rhythms, which leads to shifts in phase and decreases in amplitude. These changes can also contribute to sleep characteristics as total sleep time and quality decreases with age. Created with BioRender.com.
One consistent change in the majority of people is a shift in circadian phase as one ages. For example, there is a prominent shift in timing of body temperature and hormone secretion one hour earlier in older individuals.32,33 A specific example is the one-hour advance in melatonin secretion, which is associated with circadian rhythms and plays an essential role in sleep.34 The timing of melatonin secretion corresponds with the shifts in sleep-wake cycles. Moreover, previous studies suggested a decrease in melatonin levels exist in older adults; however, additional studies found that the levels of melatonin did not differ in healthy, older adults.35
Furthermore, these advances in phase also correspond with changes in chronotype with adults 60 and older typically waking up earlier and going to sleep earlier compared to younger adults.36 A self-comparison study in older adults found that the tendency to become a “morning person” increased with age.37 Additionally, a longitudinal study tracked the chronotype of 567 adult men in Finland for over 20 years and found participants became “mostly morning” types as they aged.38 Overall, biological shifts in circadian phase characterizes aging; however, aging also impairs one’s ability to adjust to environmental phase shifts.
As one ages, it becomes more difficult to adjust to schedule changes that may affect circadian phase. For example, chronic phase shifts caused adult rats to have longer periods of running rhythms in constant dark conditions while adolescent rats were able to adapt to chronic phase shifts.39 Additionally, older mice required more days to “re-entrain” after shifts in phases.40
Following phase advance, older adults had greater decreases in sleep efficiency, attention span, and body temperature amplitudes that persisted for a longer period of time in comparison to younger adults.41,42 In contrast, there were minimal differences between older and younger subjects following a phase delay.26 Thus, similar to younger individuals, phase delays may be better tolerated by an older population.
Age-Related Changes in the Circadian Clock
Aging is characterized by dampened oscillations of the circadian clock. It has been thought that the SCN, the master pacemaker, may change and play a role in aging. When fetal tissue SCN was graphed into aged animals, there was a restoration of dampened rhythms and these animals had an increase in longevity.43,44 Moreover, manipulating clock genes in the SCN lead to changes in physiology and behavior that match the changes seen in aging.45 Thus, the SCN plays a role within aging; however, the data vary on whether the SCN is vulnerable to age-related changes.
Age-related degradation exists at the network level in the SCN and the electrical output of the SCN is dampened with age.14,46 Additionally, it was found that the circadian amplitudes of neuronal firing were decreased in the SCN of older mice in comparison to younger ones.47 These data collectively support that there are age-related changes occurring in the SCN.
However, core molecular clocks within the SCN seem to remain robust.48–51 Further supporting this, a recent study found mRNA and protein levels of clock genes in the SCN remained unchanged in older nonhuman primates.52 Although clock genes remain robust in the SCN in older individuals, there are impacts from aging on the clock genes in the periphery.53 Aging has a negative effect on mRNA levels of Bmal1, Clock, Per1, and Per2 in mouse granulosa cells as the mRNA levels were decreased in older mice in comparison to younger.14,54 Additionally, clock genes in the ovaries had shifts in rhythms with age.54 Diurnal rhythms of Bmal1, Per1, and Per2 were also dampened in the mouse heart.55 However, not all cells/organs have clock genes affected by aging. For instance, no age-related variation was found in the clock genes of a rat kidney.56 In contrast, a recent study found age-related declines in clock genes in male mice in the hypothalamus, which contains the central SCN clock, and five other peripheral tissues, lung, kidney, skeletal muscle, heart, and adrenal gland.57 Thus, the effects of aging on clock genes may vary in different genes and throughout different cells and organs.
Interestingly, previous studies find that Bmal1 plays a key role in aging. For instance, deletion of Bmal1 leads to total loss of circadian rhythms and an increase in age-related diseases,58–60 and an additional study suggests that knocking out Bmal1 leads to accelerated aging.61 Supporting this, further research found that Bmal1 deficient cells had characteristics of aging,62 and mice with Bmal1 deficiencies exhibited early aging and age-related pathologies.58 Interestingly, mice with Bmal1 decencies that were given antioxidant treatments had an increase in average life span.59 It has also been found that mice with Bmal1 deficiencies exhibit an increase in the activity of mTORC1, a protein complex that plays a crucial role in regulating metabolic processes via the TOR pathway.63 This increase in mTORC1 activity led to accelerated aging in Bmal1 deficient mice, which supports that BMAL1 may be playing a role in aging via metabolic processes. Thus, normal aging is associated with potential changes in circadian genes, specifically Bmal1, which could lead to diseases later in life.
Aging Changes Sleep Patterns
One of the main regulators of sleep is the circadian timing system.64,65 Recent studies have shed light on the importance of sleep for the health and well-being of the older population.66 Moreover, studies suggest that sleep changes with aging. For example, older adults have a decrease in total sleep time67 and in sleep quality.68–70 Additionally, multiple studies support that deep sleep (slow wave sleep) decreases with age, and additional studies found decreases in slow wave sleep and changes in the brain in older individuals with no cognitive deficits.71,72
Overall, there are often changes in sleep that occur with aging, but these changes could plateau at 60 years old if one maintains a healthy lifestyle. However, recent studies suggest that having impaired sleep and rhythms contribute to age-related disorders.73,74 For instance, sleep-wake disruptions are potentially correlated with an increase in biomarkers associated with Alzheimer’s disease (AD).75 Thus, comorbidities can enhance normal age-related changes in sleep, which could be indicative of age-related diseases.
Circadian Rhythms and Sleep Changes in Age-Related Diseases
Aberrations in Circadian Rhythms and Sleep in Neurodegenerative Disorders
Alzheimer’s disease (AD) is a devastating disease that affects cognitive function and primarily occurs in older populations. Approximately 44 million people have Alzheimer’s or related dementia, and this is estimated to reach about 135 million by 2050.76,77 Circadian dysfunction and sleep disruptions are the most common characteristics in patients with AD.78 Previous survey results found that 45% of patients with Alzheimer’s disease (AD) have circadian disruptions and fragmented sleep.63 These disruptions can appear years prior to a medical diagnosis.79
There is also a decrease in rapid eye movement (REM) and non-REM sleep that are associated with tau pathology is the early stages of Alzheimer’s.79 Additionally, REM sleep bouts are decreased in patients with AD in comparison to age matched controls.80 Short and long sleep durations are also associated with worse outcomes for older adults, such as greater Aβ burden, greater depressive symptoms, higher body mass index, and cognitive decline.81 Interestingly, studies found decreases in slow wave sleep with aging occurred individuals with no cognitive deficits,58,59 but were also associated with the early stages of Alzheimer’s.61,62 Thus, it could be argued that sleep patterns changing with aging predict the risk of developing AD.
Furthermore, AD is characterized by dampened circadian rhythms. There is an overall decrease in amplitude and a delay in circadian phase.82,83 Additionally, a dampening and delay in rhythms increased the chances of developing AD.25 Supporting this, a recent study found that there was a weakening in rhythms and activity as older individuals went from cognitive impairment to AD.63 These studies collectively support that there may be an interaction between circadian disruptions and AD.
Moreover, initial studies found that there was degradation in the SCN in patients suffering from AD.29 Specifically, it was suggested that patients with AD had the loss of critical neurons in the SCN. In contrast, a more recent study found no significant differences in major neurons in the SCN in non-AD and AD patients.84 Although data varies on the role of the SCN in AD, there are potential changes in clock genes. For example, the rhythms of BMAL1, PER1 and CRY1 mRNA are lost in the pineal gland.85 In AD patients, there are also changes in BMAL1 mRNA expression that are complex, but the main differences are in the temporal phase relationship.30,86,87 Additionally, phosphorylated tau, a key pathological feature of AD, levels are increased in a mouse model of AD after chronic sleep deprivation and correlate to changes found in Bmal1 expression.88 These studies collectively support that the molecular clock is playing a role in AD.
In addition to AD, Parkinson’s disorder (PD), the 2nd most common neurodegenerative disorder, may also be associated with abnormalities in circadian rhythms and sleep. For example, a rat model of PD found there was decreased expression of clock genes that could be restored with melatonin.89 To follow up on this study, PD patients are treated with melatonin and measured the expression of BMAL1 and PER1.90 At baseline, BMAL1 was decreased and increased with melatonin while PER1 remained the same. Thus, PD patients have altered levels of clock genes that can restored with treatments that focus on circadian rhythms and sleep-wake cycles.
Additionally, there are variations in sleep characteristics in patients with PD. For example, male patients with Parkinson’s had an increase in daytime sleeping and were more active at night.91 Specifically, men with excessive daytime sleepiness combined with daytime napping were 3 times more likely to develop PD. Additionally, patients with PD are commonly diagnosed with insomnia and tend to have problems staying asleep.92 However, this could be due to motor issues that happen during the night.93
Furthermore, normal rhythms exist in rest-activity stages, but these rhythms are modified in patients with PD. Patients with mild to moderate PD have an overall decrease in motor activity but maintain diurnal rhythms. However, patients in the advanced stages of PD do not express diurnal rhythms in motor activity.94,95 Interestingly, a rat model of PD found overall activity was decreased, but there were no phase shifts and circadian rhythms were not disrupted.96 Supporting this, additional studies did not find circadian disruptions in patients with PD; however, patients being treated with dopaminergic treatments have later sleep onset, severe insomnia, and an advance in circadian phase.92,97–99 Moreover, a recent study utilized bright light therapy for one hour during the evening hours in PD patients receiving dopamine treatment and found an improvement in sleep problems.83
Thus, the phase shifts seen in patients with PD may be due to the dopaminergic treatments. Interestingly, male rats receiving a D1 dopamine agonist exhibit altered circadian genes in the SCN.100 When given a D1 dopamine agonist in the morning, the expression in the SCN of Per2 and Clock genes are increased and Per1 and Bmal1 are decreased in comparison to saline treatments. Receiving a D1 dopamine agonist in the evening had a decrease in the expression of Per2, Clock, Per1, and Bmal1 in the SCN. These alterations in clock genes in the SCN may underly the shifts in circadian phases in PD patients. Selecting the appropriate times in which dopaminergic treatments are received may improve symptoms. Furthermore, combining dopaminergic treatments with light therapies later in the evening may combat additional symptoms and help prevent additional phase shifting.
Disruptions in Circadian Rhythms and Sleep May Play a Role in Cancer
Cancer and aging are closely related as cancer frequency is higher in an older population in comparison to a younger one.101 In 2007, the International Agency for Research on Cancer (IARC) listed circadian disruptions as a probable carcinogen. Moreover, multiple studies propose that shift workers have a higher risk of developing cancer.102,103 For example, women who were nurses and did shift work for 30 years or more had a higher chance of developing breast cancer.104–106 A recent review examined about 26 studies focusing on shift work and breast cancer.102 Although the studies all varied, they collectively support that there is an increased risk of developing breast cancer after 20 years of shift work or after shorter periods with many consecutive nights of shift work.
Supporting these studies, research utilizing preclinical chronic jet lag models to disrupt circadian rhythms found that chronic jet lag may increase the chances of developing cancer. For example, chronic jet leg resulted in the deregulation of liver gene expression and metabolism that led to hepatocellular carcinoma.107 Chronic jet lag also accelerated the progression of Glasgow osteosarcoma in comparison to mice that were not disrupted.108 Recent studies found that chronic jet lag altered transcriptomes that have been linked to cancer-related pathways109 and changed the expression of genes related to glioma in several brain regions.110 Thus, chronic circadian disruptions could increase the chances of one developing cancer later in life.
Data varies on the relationship between cancer and the circadian clock. For instance, single-nucleotide polymorphisms (SNP) in CLOCK and/or BMAL1 genes are associated with an increase in susceptibility to develop prostate, breast, ovarian, and pancreatic cancer in humans.107,111,112 Additionally, suppression of CLOCK and BMAL1 promotes tumor progression.113,114 However, tissue from breast cancer patients expressed significantly higher CLOCK gene expression in comparison to healthy controls.115 CLOCK is also upregulated in glioma tissues, which pays a role in proliferation, survival, and migration of glioma cells.116,117 The differences from these studies in CLOCK expression and regulation may be due to the different types of tissues from different cancers. Thus, circadian genes may vary based on types of tissue and cancer.
Moreover, evidence also suggests that Cry and Per genes play a role in cancer. Mutations in these genes have been linked to a variety of cancers, including breast cancer, prostate cancer, and glioblastoma. For example, CRY1 was downregulated in human osteosarcoma cells.118 Additionally, Per and Cry genes may play a role in regulating cell proliferation, apoptosis, and DNA repair, which are all processes that can become dysregulated in cancer. For instance, mice with Per2 loss-of-function mutations exhibited a greater occurrence of tumors following exposure to γ-irradiation compared to mice with normal Per2 genes.119 Furthermore, the genes associated with cell proliferation, including c-myc, CyclinD1, and Gadd45, which are regulated by c-myc, were disrupted in Per2 mutants. These data, thus, support that multiple parts of the molecular clock may be playing a role in cancer.
However, other studies suggest that circadian genes may not play a role in cancer. For example, Clock gene mutant mice were not more tumor prone.120–122 Additionally, the TCGA database suggests that mutations in core circadian genes are low in cancer patients. Some studies suggest that circadian gene alterations are a shared feature of tumors.123 One thought could be that cancer is disrupting circadian rhythms, which leads to alterations in circadian genes that in turn enhance tumor progression. A recent study found that MYCN, a protein coding gene and hallmark of advanced neuroblastoma, disrupts the circadian clock.124 Specifically, MYCN was found to upregulate REV-ERBα and down regulate ROR and BMAL1. ROR activity was able to rescue the MYCN-mediated repression of BMAL1 expression. These data, thus, support that cancer may dysregulate the molecular clock. Overall, these data collectively support that there is a relationship between cancer and circadian rhythms and sleep.
The Role of Circadian Rhythms and Sleep in Cardiovascular Diseases
One of the major risk factors for heart failure and cardiovascular disease is aging.125 The aging process can negatively affect the health of the heart and the arterial system. Indeed, there are increases in atherosclerosis, hypertension, myocardial infarction, and stroke in the older population.126 Circadian rhythms also regulate the cardiovascular system and contribute to diseases. The core molecular clock is present in most cardiovascular cell types, which can function independently from the SCN.127–131
These clocks mediate fluctuations in cardiovascular processes. For example, heart rate fluctuates throughout the day.132 Research suggests this may be due to the cardiomyocyte circadian clock as cardiomyocyte-specific Clock mutant mice have decreases in heart rate.133 Additionally, circadian disruptions in animals can cause cardiomyopathy, cardiac fibrosis and systolic dysfunction, which can lead to cardiovascular death.134,135
Similar to cancer studies, shift workers were found to have a higher chance of developing coronary heart diseases.136 These data are consistent with a previous study that found a 23–24% increased risk of any coronary event in shift workers.137 Additionally, a recent study on shift work found that disruption of circadian rhythms exacerbates reperfusion injury in myocardial infarction.138 These compelling data support the potential relationship between circadian rhythms, aging and cardiovascular disease.
Conclusions
Bidirectional Relationship Between Aging and Circadian Rhythms
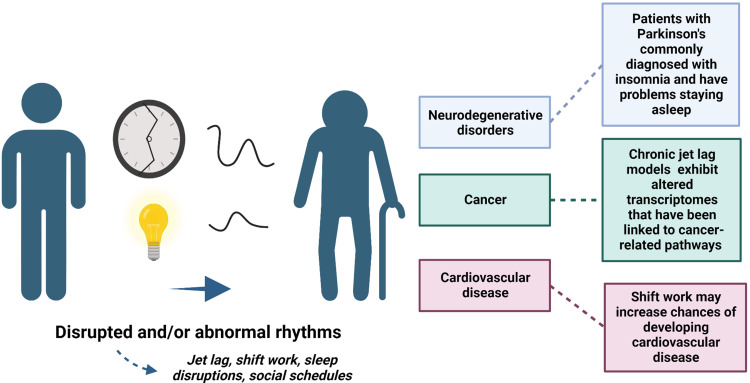
With our population having an increase in older adults, it is imperative to better characterize the bidirectional relationship between aging and circadian rhythms. There are changes in the circadian system and sleeping patterns that are commonly associated with aging and age-related diseases (Figure 4). Additionally, some changes may predict and contribute to age-related diseases such as neurodegenerative disorders and cancer.
Figure 4.
Disruptions in rhythms, like shift work, and abnormal rhythms can accelerate aging and lead to age-related diseases, like neurodegenerative disorders and cardiovascular disease. Differences in rhythms could also predict vulnerability to developing age-related diseases. Created with BioRender.com.
However, it is unclear whether circadian and sleep disruptions are characteristic of aging or if these disruptions lead to accelerated aging and age-related diseases. Most age-related diseases are characterized by circadian and sleep disruptions. For example, individuals suffering from neurodegenerative disorders, like AD, tend to have exasperated disruptions in rhythms.30 Moreover, sleeplessness can play a role in the immune system and heart diseases, which becomes worse in older populations. Better understanding whether changes in circadian rhythms and sleep problems are caused by aging or lead to age-related diseases may improve current treatments and ways to reduce the chances of developing these diseases.
As our older population grows, modern society is also characterized by chronic disruptions, like shift work, to circadian rhythms and sleep patterns. Several studies suggest that circadian rhythm and sleep disruptions can accelerate aging and exacerbate age-related diseases. For example, shift workers have a potential increased chance of developing cancer, and multiple chronic jet lag studies suggest circadian disruptions increase the risk of developing cancer. Thus, circadian disruptions may lead to age-related diseases.Individuals may be able to improve their circadian rhythms and sleep patterns by avoiding light exposure and could also utilize bright light therapy. Better understanding the relationship between circadian rhythms and aging will allow us to better treat the older population but will also allow us characterize types of disruptions to lead to age-related diseases.
Acknowledgments
Our lab has work that has been supported by R01 MH111601 (Colleen McClung) and NIH T32HL082610-15.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22:335–345. doi: 10.1016/S0893-133X(99)00145-1 [DOI] [PubMed] [Google Scholar]
- 2.Yerushalmi S, Green RM. Evidence for the adaptive significance of circadian rhythms. Ecol Lett. 2009;12:970–981. doi: 10.1111/j.1461-0248.2009.01343.x [DOI] [PubMed] [Google Scholar]
- 3.Patke A, Young MW, Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2 [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7 [DOI] [PubMed] [Google Scholar]
- 6.King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713 [DOI] [PubMed] [Google Scholar]
- 7.Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. [DOI] [PubMed] [Google Scholar]
- 8.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- 9.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dacey DM, Liao H-W, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387 [DOI] [PubMed] [Google Scholar]
- 11.Lucas RJ, Peirson SN, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hankins MW, Peirson SN, Foster RG. Melanopsin: an exciting photopigment. Trends Neurosci. 2008;31:27–36. doi: 10.1016/j.tins.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Hastings MH, Maywood ES, Brancaccio M. The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology. 2019;8(13):13. doi: 10.3390/biology8010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura TJ, Nakamura W, Yamazaki S, et al. Age-related decline in circadian output. J Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- 16.McClung CA, Rhythms C. the mesolimbic dopaminergic circuit, and drug addiction. Sci World J. 2007;7:194–202. doi: 10.1100/tsw.2007.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207 [DOI] [PubMed] [Google Scholar]
- 18.Koike N, Yoo S-H, Huang H-C, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aryal RP, Kwak PB, Tamayo AG, et al. Macromolecular assemblies of the mammalian circadian clock. Mol Cell. 2017;67:770–782.e6. doi: 10.1016/j.molcel.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu W, Nomura M, Ikeda M. Interactivating feedback loops within the mammalian clock: BMAL1 is negatively autoregulated and upregulated by CRY1, CRY2, and PER2. Biochem Biophys Res Commun. 2002;290:933–941. doi: 10.1006/bbrc.2001.6300 [DOI] [PubMed] [Google Scholar]
- 21.Takahashi JS. Molecular components of the circadian clock in mammals. Diabetes Obes Metab. 2015;17(Suppl 1):6–11. doi: 10.1111/dom.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fagiani F, Di Marino D, Romagnoli A, et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct Target Ther. 2022;7. doi: 10.1038/s41392-022-00899-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mormont M-C, Waterhouse J. Contribution of the rest–activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002;19:313–323. doi: 10.1081/CBI-120002606 [DOI] [PubMed] [Google Scholar]
- 25.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70:722–732. doi: 10.1002/ana.22468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esquirol Y, Perret B, Ruidavets JB, et al. Shift work and cardiovascular risk factors: new knowledge from the past decade. Arch Cardiovasc Dis. 2011;104:636–668. doi: 10.1016/j.acvd.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry Abingdon Engl. 2014;26:139–154. doi: 10.3109/09540261.2014.911149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20:49–65. doi: 10.1038/s41583-018-0088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner H, LeBourgeois MK, Geiger A, Jenni OG. Assessment of chronotype in four- to eleven-year-old children: reliability and validity of the children’s chronotype questionnaire (CCTQ). Chronobiol Int. 2009;26:992–1014. doi: 10.1080/07420520903044505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127:437–446. doi: 10.1172/JCI90328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694 [DOI] [PubMed] [Google Scholar]
- 32.Duffy JF, Dijk D-J, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1478–R1487. doi: 10.1152/ajpregu.1998.275.5.R1478 [DOI] [PubMed] [Google Scholar]
- 33.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-Y [DOI] [PubMed] [Google Scholar]
- 34.Duffy JF, Zeitzer JM, Rimmer DW, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001 [DOI] [PubMed] [Google Scholar]
- 35.Kin NY, Nair NMK, Schwartz NPV, Thavundayil G, Annable L. Secretion of melatonin in healthy elderly subjects: a longitudinal study. Ann N Y Acad Sci. 2004;1019:326–329. [DOI] [PubMed] [Google Scholar]
- 36.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 37.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11:429–438. doi: 10.1016/j.smrv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 38.Broms U, Pitkäniemi J, Bäckmand H, et al. Long-term consistency of diurnal-type preferences among men. Chronobiol Int. 2014;31:182–188. doi: 10.3109/07420528.2013.836534 [DOI] [PubMed] [Google Scholar]
- 39.Albert N, da Silva C, Díez-Noguera A, Cambras T. Different adaptation of the motor activity rhythm to chronic phase shifts between adolescent and adult rats. Behav Brain Res. 2013;252:347–355. [DOI] [PubMed] [Google Scholar]
- 40.Sellix MT, Evans JA, Leise TL, et al. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J Neurosci. 2012;32:16193–16202. doi: 10.1523/JNEUROSCI.3559-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monk TH, Buysse DJ, Reynolds CF, Kupfer DJ. Inducing jet lag in older people: adjusting to a 6-hour phase advance in routine. Exp Gerontol. 1993;28:119–133. doi: 10.1016/0531-5565(93)90002-U [DOI] [PubMed] [Google Scholar]
- 42.Monk TH, Kupfer DJ. Circadian rhythms in healthy aging—effects downstream from the pacemaker. Chronobiol Int. 2000;17:355–368. doi: 10.1081/CBI-100101051 [DOI] [PubMed] [Google Scholar]
- 43.Li H, Satinoff E. Fetal tissue containing the suprachiasmatic nucleus restores multiple circadian rhythms in old rats. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1735–R1744. doi: 10.1152/ajpregu.1998.275.6.R1735 [DOI] [PubMed] [Google Scholar]
- 44.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255 [DOI] [PubMed] [Google Scholar]
- 45.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2 [DOI] [PubMed] [Google Scholar]
- 46.Roozendaal B, van Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4 [DOI] [PubMed] [Google Scholar]
- 47.Nakamura TJ, Tokuda IT, Ishikawa T, et al. Age-related changes in the circadian system unmasked by constant conditions. eNeuro. 2015;2:64. doi: 10.1523/ENEURO.0064-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyse CA, Coogan AN, Selman C, Hazlerigg DG, Speakman JR. Association between mammalian lifespan and circadian free-running period: the circadian resonance hypothesis revisited. Biol Lett. 2010;6:696–698. doi: 10.1098/rsbl.2010.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolker DE, Vitaterna MH, Fruechte EM, Takahashi JS, Turek FW. Effects of age on circadian rhythms are similar in wild-type and heterozygous Clock mutant mice. Neurobiol Aging. 2004;25:517–523. doi: 10.1016/j.neurobiolaging.2003.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang H-C, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asai M, Yoshinobu Y, Kaneko S, et al. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010 [DOI] [PubMed] [Google Scholar]
- 52.Eghlidi DH, Luna SL, Brown DI, et al. Gene expression profiling of the SCN in young and old rhesus macaques. J Mol Endocrinol. 2018;61:57–67. doi: 10.1530/JME-18-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Liu Y, Escames G, et al. Deciphering clock genes as emerging targets against aging. Ageing Res Rev. 2022;81:101725. [DOI] [PubMed] [Google Scholar]
- 54.Jiang Z, Zou K, Liu X, et al. Aging attenuates the ovarian circadian rhythm. J Assist Reprod Genet. 2021;38:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonaconsa M, Malpeli G, Montaruli A, et al. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp Gerontol. 2014;55:70–79. doi: 10.1016/j.exger.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 56.Jagota A, Thummadi NB, Kukkemane K. Chapter 20 - circadian regulation of hormesis for health and longevity. In: Rattan SIS, Kyriazis M, editors. The Science of Hormesis in Health and Longevity. Academic Press; 2019:223–233. [Google Scholar]
- 57.Wolff CA, Gutierrez-Monreal MA, Meng L, et al. Defining the age-dependent and tissue-specific circadian transcriptome in male mice. Cell Rep. 2023;42:111982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kondratov RV, Vykhovanets O, Kondratova AA, Antoch MP. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging. 2009;1:979–987. doi: 10.18632/aging.100113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang G, Chen L, Grant GR, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chhunchha B, Kubo E, Singh DP. Clock protein bmal1 and Nrf2 cooperatively control aging or oxidative response and redox homeostasis by regulating rhythmic expression of Prdx6. Cells. 2020;9:E1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khapre RV, Kondratova AA, Patel S, et al. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging. 2014;6:48–57. doi: 10.18632/aging.100633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borbély AA. Sleep regulation: circadian rhythm and homeostasis. In: Sleep. editors, Ganten D, Pfaff D. Springer; 1982:83–103. doi: 10.1007/978-3-642-68333-6_3 [DOI] [Google Scholar]
- 65.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki K, Miyamoto M, Hirata K. Sleep disorders in the elderly: diagnosis and management. J Gen Fam Med. 2017;18:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- 68.Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- 69.Moraes W, Piovezan R, Poyares D, et al. Effects of aging on sleep structure throughout adulthood: a population-based study. Sleep Med. 2014;15:401–409. doi: 10.1016/j.sleep.2013.11.791 [DOI] [PubMed] [Google Scholar]
- 70.Schwarz JFA, Åkerstedt T, Lindberg E, et al. Age affects sleep microstructure more than sleep macrostructure. J Sleep Res. 2017;26:277–287. doi: 10.1111/jsr.12478 [DOI] [PubMed] [Google Scholar]
- 71.Varga AW, Wohlleber ME, Giménez S, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep. 2016;39:2041–2048. doi: 10.5665/sleep.6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baril -A-A, Beiser AS, Mysliwiec V, et al. Slow-wave sleep and MRI markers of brain aging in a community-based sample. Neurology. 2021;96:e1462–e1469. doi: 10.1212/WNL.0000000000011377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Vitiello MV, Gooneratne N. Sleep in normal aging. Sleep Med Clin. 2018;13:1–11. doi: 10.1016/j.jsmc.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ju Y-ES, Videnovic A, Vaughn BV. Comorbid sleep disturbances in neurologic disorders. Contin Lifelong Learn Neurol. 2017;23(1117):1117–1131. doi: 10.1212/CON.0000000000000501 [DOI] [PubMed] [Google Scholar]
- 75.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 76.Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet Lond Engl. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer Disease. Exp Mol Med. 2015;47:e148–e148. doi: 10.1038/emm.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uddin MS, Tewari D, Mamun AA, et al. Circadian and sleep dysfunction in Alzheimer’s disease. Ageing Res Rev. 2020;60(101046):101046. doi: 10.1016/j.arr.2020.101046 [DOI] [PubMed] [Google Scholar]
- 79.Lucey BP, McCullough A, Landsness EC, et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med. 2019;11:eaau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petit D, Gagnon J-F, Fantini ML, Ferini-Strambi L, Montplaisir J. Sleep and quantitative EEG in neurodegenerative disorders. J Psychosom Res. 2004;56:487–496. doi: 10.1016/j.jpsychores.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 81.Winer JR, Deters KD, Kennedy G, et al. Association of short and long sleep duration with amyloid-β burden and cognition in aging. JAMA Neurol. 2021;78:1–10. doi: 10.1001/jamaneurol.2021.2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilks H, Aschenbrenner AJ, Gordon BA, et al. Sharper in the morning: cognitive time of day effects revealed with high-frequency smartphone testing. J Clin Exp Neuropsychol. 2021;43:825–837. doi: 10.1080/13803395.2021.2009447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harper DG, Volicer L, Stopa EG, et al. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–368. doi: 10.1097/00019442-200505000-00004 [DOI] [PubMed] [Google Scholar]
- 84.Wang JL, Lim AS, Chiang W-Y, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol. 2015;78:317–322. doi: 10.1002/ana.24432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Y-H, Fischer DF, Kalsbeek A, et al. Pineal clock gene oscillation is disturbed in Alzheimer’s disease, due to functional disconnection from the ‘master clock’. FASEB J off Publ Fed Am Soc Exp Biol. 2006;20:1874–1876. [DOI] [PubMed] [Google Scholar]
- 86.Cermakian N, Waddington Lamont E, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer's disease patients and control subjects. J Biol Rhythms. 2011;26:160–170. doi: 10.1177/0748730410395732 [DOI] [PubMed] [Google Scholar]
- 87.Weissová K, Bartoš A, Sládek M, Nováková M, Moderate SA. Changes in the circadian system of Alzheimer's disease patients detected in their home environment. PLoS One. 2016;11(e0146200):e0146200. doi: 10.1371/journal.pone.0146200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niu L, Zhang F, Xu X, et al. Chronic sleep deprivation altered the expression of circadian clock genes and aggravated Alzheimer’s disease neuropathology. Brain Pathol. 2021;32(e13028). doi: 10.1111/bpa.13028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology. 2015;16:109–123. doi: 10.1007/s10522-014-9541-0 [DOI] [PubMed] [Google Scholar]
- 90.Delgado-Lara DL, González-Enríquez GV, Torres-Mendoza BM, et al. Effect of melatonin administration on the PER1 and BMAL1 clock genes in patients with Parkinson’s disease. Biomed Pharmacother Biomedecine Pharmacother. 2020;129:110485. [DOI] [PubMed] [Google Scholar]
- 91.Leng Y, Goldman SM, Cawthon PM, et al. Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson’s disease in older men. Int J Epidemiol. 2018;47:1679–1686. doi: 10.1093/ije/dyy098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu K, van Hilten JJ, Marinus J. The course of insomnia in Parkinson’s disease. Parkinsonism Relat Disord. 2016;33:51–57. doi: 10.1016/j.parkreldis.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 93.van Hilten JJ, Hoff JI, Middelkoop HA, Roos RA. The clinimetrics of hypokinesia in Parkinson’s disease: subjective versus objective assessment. J Neural Transm Park Dis Dement Sect. 1994;8:117–121. doi: 10.1007/BF02250922 [DOI] [PubMed] [Google Scholar]
- 94.Hilten J, Hoogland G, van der Velde EA, et al. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1993;56:874–877. doi: 10.1136/jnnp.56.8.874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Hilten JJ, van Dijk JG, Dunnewold RJ, et al. Diurnal variation of essential and physiological tremor. J Neurol Neurosurg Psychiatry. 1991;54:516–519. doi: 10.1136/jnnp.54.6.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baier PC, Branisa P, Koch R, et al. Circadian distribution of motor-activity in unilaterally 6-hydroxy-dopamine lesioned rats. Exp Brain Res. 2006;169:283–288. doi: 10.1007/s00221-005-0343-0 [DOI] [PubMed] [Google Scholar]
- 97.Bolitho SJ, Naismith SL, Rajaratnam SMW, et al. Disturbances in melatonin secretion and circadian sleep–wake regulation in Parkinson disease. Sleep Med. 2014;15:342–347. doi: 10.1016/j.sleep.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 98.Bordet R. Addiction, experimental models and neurobiological mechanisms. Therapie. 2015;70:133–146. doi: 10.2515/therapie/2014222 [DOI] [PubMed] [Google Scholar]
- 99.Breen DP, Vuono R, Nawarathna U, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–595. doi: 10.1001/jamaneurol.2014.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mesgar S, Jameie SB, Aliaghaei A, et al. Dopamine D1 receptor-mediated regulation of per1, per2, CLOCK, and BMAL1 expression in the suprachiasmatic nucleus in adult male rats. J Mol Neurosci. 2022;72:618–625. doi: 10.1007/s12031-021-01923-6 [DOI] [PubMed] [Google Scholar]
- 101.Berger NA, Savvides P, Koroukian SM, et al. Cancer in the elderly. Trans Am Clin Climatol Assoc. 2006;117:147–156. [PMC free article] [PubMed] [Google Scholar]
- 102.Hansen J. Night shift work and risk of breast cancer. Curr Environ Health Rep. 2017;4:325–339. doi: 10.1007/s40572-017-0155-y [DOI] [PubMed] [Google Scholar]
- 103.Zhou L, Zhang Z, Nice E, et al. Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J Hematol Oncol J Hematol Oncol. 2022;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lie J-AS, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control CCC. 2006;17:39–44. doi: 10.1007/s10552-005-3639-2 [DOI] [PubMed] [Google Scholar]
- 105.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563 [DOI] [PubMed] [Google Scholar]
- 106.Knutsson A, Alfredsson L, Karlsson B, et al. Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scand J Work Environ Health. 2013;39:170–177. doi: 10.5271/sjweh.3323 [DOI] [PubMed] [Google Scholar]
- 107.Kettner NM, Katchy CA, Fu L. Circadian gene variants in cancer. Ann Med. 2014;46:208–220. doi: 10.3109/07853890.2014.914808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filipski E, Delaunay F, King VM, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674 [DOI] [PubMed] [Google Scholar]
- 109.Khan S, Yong VW, Xue M. Circadian disruption in mice through chronic jet lag-like conditions modulates molecular profiles of cancer in nucleus accumbens and prefrontal cortex. Carcinogenesis. 2021;42:864–873. doi: 10.1093/carcin/bgab012 [DOI] [PubMed] [Google Scholar]
- 110.Khan S, Liu Y, Siddique R, et al. Impact of chronically alternating light-dark cycles on circadian clock mediated expression of cancer (glioma)-related genes in the brain. Int J Biol Sci. 2019;15:1816–1834. doi: 10.7150/ijbs.35520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angelousi A, Kassi E, Ansari-Nasiri N, et al. Clock genes and cancer development in particular in endocrine tissues. Endocr Relat Cancer. 2019;26:R305–R317. doi: 10.1530/ERC-19-0094 [DOI] [PubMed] [Google Scholar]
- 112.Cadenas C, van de Sandt L, Edlund K, et al. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle. 2014;13:3282–3291. doi: 10.4161/15384101.2014.954454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu Y, Hao J, Yuan G, et al. PER1 as a tumor suppressor attenuated in the malignant phenotypes of breast cancer cells. Int J Gen Med. 2021;14:7077–7087. doi: 10.2147/IJGM.S328184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang W, Zhao S, Jiang X, et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016;371:314–325. doi: 10.1016/j.canlet.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 115.Hoffman AE, Yi C-H, Zheng T, et al. CLOCK in breast tumorigenesis: genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 2010;70:1459–1468. doi: 10.1158/0008-5472.CAN-09-3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Z, Su G, Dai Z, et al. Circadian clock genes promote glioma progression by affecting tumour immune infiltration and tumour cell proliferation. Cell Prolif. 2021;54:e12988. doi: 10.1111/cpr.12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li A, Lin X, Tan X, et al. Circadian gene Clock contributes to cell proliferation and migration of glioma and is directly regulated by tumor-suppressive miR-124. FEBS Lett. 2013;587:2455–2460. doi: 10.1016/j.febslet.2013.06.018 [DOI] [PubMed] [Google Scholar]
- 118.Zhou L, Yu Y, Sun S, Zhang T, Wang M. Cry 1 regulates the clock gene network and promotes proliferation and migration via the Akt/P53/P21 pathway in human osteosarcoma cells. J Cancer. 2018;9:2480–2491. doi: 10.7150/jca.25213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fu L, Pelicano H, Liu J, Huang P, Lee CC. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/S0092-8674(02)00961-3 [DOI] [PubMed] [Google Scholar]
- 120.Antoch MP, Gorbacheva VY, Vykhovanets O, et al. Disruption of the circadian clock due to the Clock mutation has discrete effects on aging and carcinogenesis. Cell Cycle Georget Tex. 2008;7:1197–1204. doi: 10.4161/cc.7.9.5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Antoch MP, Toshkov I, Kuropatwinski KK, Jackson M. Deficiency in PER proteins has no effect on the rate of spontaneous and radiation-induced carcinogenesis. Cell Cycle Georget Tex. 2013;12:3673–3680. doi: 10.4161/cc.26614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science. 2021;371:eabb0738. doi: 10.1126/science.abb0738 [DOI] [PubMed] [Google Scholar]
- 123.Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 2019;5:475–494. doi: 10.1016/j.trecan.2019.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moreno-Smith M, Milazzo G, Tao L, et al. Restoration of the molecular clock is tumor suppressive in neuroblastoma. Nat Commun. 2021;12. doi: 10.1038/s41467-021-24196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li H, Hastings MH, Rhee J, et al. Targeting age-related pathways in heart failure. Circ Res. 2020;126:533–551. doi: 10.1161/CIRCRESAHA.119.315889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.CIR.0000048892.83521.58 [DOI] [PubMed] [Google Scholar]
- 127.Beesley S, Noguchi T, Welsh DK. Cardiomyocyte circadian oscillations are cell-autonomous, amplified by β-adrenergic signaling, and synchronized in cardiac ventricle tissue. PLoS One. 2016;11:e0159618. doi: 10.1371/journal.pone.0159618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maemura K, Takeda N, Nagai R. Circadian rhythms in the CNS and peripheral clock disorders: role of the biological clock in cardiovascular diseases. J Pharmacol Sci. 2007;103:134–138. doi: 10.1254/jphs.FMJ06003X2 [DOI] [PubMed] [Google Scholar]
- 129.Takeda N, Maemura K. Circadian clock and cardiovascular disease. J Cardiol. 2011;57:249–256. doi: 10.1016/j.jjcc.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 130.Lin C, Tang X, Zhu Z, et al. The rhythmic expression of clock genes attenuated in human plaque-derived vascular smooth muscle cells. Lipids Health Dis. 2014;13(14). doi: 10.1186/1476-511X-13-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Davidson AJ, London B, Block GD, Menaker M. Cardiovascular tissues contain independent circadian clocks. Clin Exp Hypertens N Y N. 2005;27:307–311. [PubMed] [Google Scholar]
- 132.Millar-Craig MW, Bishop CN, Raftery EB. Circadian variation of blood-pressure. Lancet Lond Engl. 1978;1:795–797. doi: 10.1016/S0140-6736(78)92998-7 [DOI] [PubMed] [Google Scholar]
- 133.Bray MS, Shaw CA, Moore MWS, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007 [DOI] [PubMed] [Google Scholar]
- 134.Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–H2337. doi: 10.1152/ajpheart.1998.275.6.H2334 [DOI] [PubMed] [Google Scholar]
- 135.Martino TA, Oudit GY, Herzenberg AM, et al. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1675–R1683. doi: 10.1152/ajpregu.00829.2007 [DOI] [PubMed] [Google Scholar]
- 136.Vetter C, Devore EE, Wegrzyn LR, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315:1726–1734. doi: 10.1001/jama.2016.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vyas MV, Garg AX, Iansavichus AV, et al. Shift work and vascular events: systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao Y, Lu X, Wan F, et al. Disruption of circadian rhythms by shift work exacerbates reperfusion injury in myocardial infarction. J Am Coll Cardiol. 2022;79:2097–2115. doi: 10.1016/j.jacc.2022.03.370 [DOI] [PMC free article] [PubMed] [Google Scholar]