Abstract
Background
Bariatric surgery is the most effective treatment for sustained weight reduction and obesity-related comorbidities. The development of gallstones as a result of rapid weight loss is a well-known consequence of bariatric procedures. It remains unclear, if there is an increased risk of these gallstones becoming symptomatic.
Methods
A retrospective analysis of 505 consecutive patients submitted to either Roux-en-Y Gastric Bypass or Sleeve Gastrectomy between January and December 2019 was performed. The aim of our study was to determine the incidence of symptomatic cholelithiasis in asymptomatic patients with their gallbladder in situ after bariatric surgery and to identify potential risk factors for its development.
Results
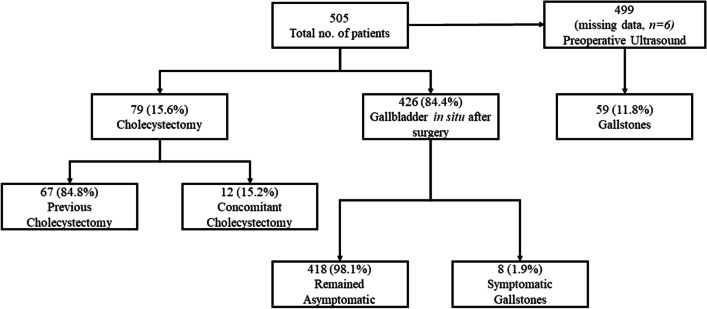
Of the 505 patients included, 79 (15.6%) underwent either previous cholecystectomy.
(n = 67, 84.8%) or concomitant cholecystectomy during bariatric surgery (n = 12, 15.2%). Among the remaining 426 (84.4%) patients, only 8 (1.9%) became symptomatic during the 12-month follow-up period. When compared with patients who remained asymptomatic, they had a higher median preoperative BMI (47.0 vs. 42.8, p = 0.046) and prevalence of cholelithiasis on preoperative ultrasound (62.5% vs. 10.7%, p = 0.001). Multivariate analysis revealed preoperative BMI and cholelithiasis on preoperative ultrasound as independent risk factors for symptomatic biliary disease (OR 1.187, 95%CI 1.025–1.376, p = 0.022 and OR 10.720, 95%CI 1.613–71.246, p = 0.014, respectively).
Conclusion
Considering a low incidence of symptomatic gallstones after bariatric surgery, concomitant cholecystectomy should only be performed in symptomatic patients undergoing bariatric surgery. Preoperative factors, such as a higher BMI and positive ultrasound for cholelithiasis, may be related to the development of symptomatic gallstones.
Keywords: Bariatric surgery, Gallstones, Symptomatic, Cholecystectomy
Introduction
The prevalence of overweight and obesity is increasing and some reports estimate that 57.8% of world population will have overweight or obesity by 2030 [1, 2].
Bariatric surgery is the most effective treatment for sustained weight reduction and for obesity-related comorbidities, especially for patients with clinically severe obesity [3]. The two most common bariatric surgeries worldwide are Roux-en-Y Gastric Bypass (RYGB) and Sleeve Gastrectomy (SG), being laparoscopy the gold-standard approach [4].
Rapid weight loss as a result of bariatric surgery can, however, predispose to the development of gallstones [5]. During early weight loss after the procedure, patients are at increased risk for gallstones due to an increased bile cholesterol saturation and poor gallbladder emptying [6, 7]. It has been advocated that the prophylactic use of ursodeoxycholic acid (UDCA) decreases cholesterol saturation and therefore gallstone incidence during weight loss [8, 9].
Doubts emerge over this increased incidence of gallstones and its influence on developing symptomatic gallbladder disease. A population-based cohort study in Sweden demonstrated a fivefold increased risk of symptomatic gallstone disease after bariatric surgery compared with that in the general population [10]. On the other hand, rates of symptomatic gallstones postbariatric surgery as low as 0.9% have been reported, despite a high incidence of new onset gallstones [11].
Distinct approaches to this issue have been proposed. While, currently, conservative options, such as the use of UDCA or performing cholecystectomy concomitantly with bariatric surgery only in the presence of symptomatic gallstones, appear to be superior [12, 13], others advocate routine prophylactic cholecystectomy [14, 15]. Consequently, to date there is no definitive answer as to what approach to prevent symptomatic cholelithiasis should be used.
The aim of this study was to determine the incidence of symptomatic cholelithiasis after SG and RYGB and identify potential risk factors for developing symptomatic cholelithiasis after bariatric surgery. Perhaps there is no clear answer to the dilemma of performing or not performing a cholecystectomy concomitantly with bariatric surgery. Most importantly may be stratifying patients by their risk factors for symptomatic cholelithiasis and, individually, decide for a more aggressive or conservative approach.
Methods
From January 2019 to December 2019, 520 patients were submitted to a bariatric procedure in our institution. Among them, 15 patients underwent a bariatric procedure other than RYGB or SG and were excluded. Therefore, we performed a retrospective analysis of 505 consecutive patients who underwent either RYGB or SG. The study was approved by the Institutional Review Board (CES 46–21).
Patients were considered eligible for bariatric surgery according to the National Institutes of Health Consensus Conference guidelines (body mass index (BMI) ≥ 35 kg/m2 associated with obesity-related co-morbidities or a BMI ≥ 40 kg/m2). The choice of bariatric procedure was made individually regarding patient characteristics, comorbidities, previous treatments, preference and surgeon recommendation. In the context of preoperative evaluation, an ultrasonography was conducted for evaluation of hepatobiliary abnormalities, including the presence of fatty liver disease and gallstones.
Concurrent cholecystectomy was only performed in patients who had symptoms consistent with biliary disease. Data concerning demographic and laboratory evaluations were obtained by consultation of medical records. No gallstone-lowering prophylaxis was used for those with gallbladder in situ. In our institution postoperative visits are done at 1, 6, 12 and 18 months and annually thereafter. Due to the COVID-19 pandemic when face-to-face consultation was not possible, patients were interviewed by telephone.
Our primary aim was to define the risk of symptomatic gallstones after SG and RYGB. For this purpose, patients with previous cholecystectomy or with concomitant cholecystectomy during bariatric surgery, despite having their characteristics analyzed at the time of bariatric surgery, were not considered for subsequent analysis. Only asymptomatic patients with their gallbladder in situ remained, and the occurrence of symptomatic biliary disease was sought.
Symptomatic cholelithiasis is defined as gallbladder pain in the presence of gallstones and, when uncomplicated (i.e., biliary colic), is frequently vague or mild and may be limited to nausea or gastric reflux symptoms [16]. In order to minimize missing cases, a comprehensive definition was used, and symptomatic cholelithiasis after bariatric procedures was defined by the presence of biliary symptoms (typical biliary colic, nonspecific upper quadrant abdominal pain, nausea and vomiting) in the follow-up or emergency department visits, the necessity of any hospital admission for biliary complications (acute cholecystitis, biliary pancreatitis, cholangitis or symptomatic choledocholithiasis) or the performance of cholecystectomy for such complications.
Our secondary objective was to identify potential risk factors for developing symptomatic gallstones after bariatric surgery. With that in mind, we compared patients who developed symptomatic cholelithiasis with those with gallbladder in situ who remained asymptomatic during the follow-up period. Preoperative characteristics such as age, gender, preoperative BMI, gallstones or severe steatosis on preoperative ultrasound, and obesity related disorders namely diabetes and dyslipidemia, as well as factors related to the bariatric procedure, such as the type of surgery and its effectiveness (i.e., %EWL at 6 and 12 months) were considered for comparison. We further compared asymptomatic patients at the time of bariatric surgery with those requiring concurrent cholecystectomy to find additional factors to stratify the risk for symptomatic cholelithiasis.
Statistical analysis was performed using SPSS® 26.0 for Mac (IBM Co., Armonk, NY,
USA). Significance was assumed for p-values inferior to 0.05. Non-parametric Mann–Whitney.
U-test was used to compare the distribution of continuous variables. Chi-square or Fisher's exact test were used to compare proportions, as appropriate. Considering statistically significant variables on univariate analysis and clinically relevant variables, a multivariate analysis was performed. Logistic regression was used for multivariate analysis.
Results
From January 2019 to December 2019, 505 patients underwent either RYGB (n = 340, 67.3%) or SG (n = 165, 32.7%). Among them, median age was 45 years (range 20–67), 422 (83.6%) were female and median preoperative BMI was 42.7 kg/m2 (IQR 39.5–46.1). Only 1 (0.02%) patient was submitted to an open procedure for concomitant intestinal transit reconstruction. One-hundred and three (20.4%) patients were submitted to RYGB or SG as a revisional surgery (mostly after laparoscopic adjustable gastric banding (LAGB)). There was no post-operative mortality. A follow-up period of 12 months was possible for all patients. Baseline cohort demographic parameters are shown in Table 1.
Table 1.
Population baseline parameters
| All n = 505 | |
|---|---|
| Age at surgery (years), median (range) | 45 (20–67) |
| Female, n (%) | 42.7 (39.5–46.1) |
| DM, n (%) | 131 (25.9) |
| Dyslipidemia, n (%) | 233 (46.1) |
| RYGB, n (%) | 340 (67.3) |
| SG, n (%) | 165 (32.7) |
| Revisional surgery, n (%) | 103 (20.4) |
BMI: body mass index, IQR: interquartile range, DM: diabetes mellitus, RYGB: Roux-en-Y gastric bypass, SG: sleeve gastrectomy
Sixty-seven (13.3%) patients had had prior cholecystectomy, and 12 (2.4%) patients with symptomatic cholelithiasis underwent concomitant cholecystectomy during bariatric procedure. Therefore, 426 (84.4%) patients remained with their gallbladders in situ. Of these patients, 8 (1.9%) eventually developed symptomatic gallstones during the 12-month follow-up (Fig. 1). Complicated symptomatic cholelithiasis was found in only 1 (12.5%) patient who presented with biliary pancreatitis.
Fig. 1.
Flow chart of patients who underwent RYGB or SG
Considering the existence of gallstones in the preoperative ultrasound (n = 59) and those who underwent previous (n = 67) or concomitant cholecystectomy (n = 12), preoperative gallbladder disease was found in 128 (25.3%) patients.
Regarding patients with preoperative asymptomatic gallstones versus patients with no evidence of preoperative gallstones (Table 2), previously bariatric surgery was the only risk factor identified for having gallstones (30.6% vs 14.8%, p = 0.013).
Table 2.
Comparison between patients with and without preoperative evidence of gallstones
| Preoperative asymptomatic gallstones N = 49 |
No evidence of preoperative gallstones N = 371 |
P value | |
|---|---|---|---|
| Age (years), median (range) | 45 (33–67) | 45 (20–67) | 0.392 |
| Female, n (%) | 41 (83.7) | 300 (88.0) | 0.846 |
| Type of surgery (%) | 0.338 | ||
| RYGB, n (%) | 29 (59.2) | 247 (66.6) | |
| SG, n (%) | 20 (40.8) | 124 (33.4) | |
| Revisional surgery, n (%) | 15 (30.6) | 55 (14.8) | 0.013 |
| Preoperative BMI (kg/m2), median (IQR) | 42.3 (39.2 – 46.8) | 42.9 (39.4 – 46.2) | 0.791 |
| DM, n (%) | 11 (22.4) | 91 (24.5) | 0.860 |
| Glucose (mg/dL), median (IQR) | 97 (84.5 – 113) | 95 (87 – 108.25) | 0.575 |
| HbA1c (%), median (IQR) | 5.5 (5.3–5.9) | 5.6 (5.3 – 6.0) | 0.661 |
| Dyslipidemia, n (%) | 21 (42.9) | 157 (42.3) | 1.000 |
| Total cholesterol (mg/dL), median (IQR) | 187 (164–220) | 184 (161–209) | 0.407 |
| Severe steatosis, n (%) | 10 (23.2) | 88 (25.7) | 0.853 |
RYGB Roux-en-Y gastric bypass, SG sleeve gastrectomy, BMI body mass index, IQR interquartile range, DM diabetes mellitus, HbA1c hemoglobin A1c
Univariate analysis revealed that patients who developed symptomatic gallstones had a higher median preoperative BMI (47.0 vs. 42.8 kg/m2, p = 0.046) and prevalence of gallstones on preoperative abdominal ultrasound (62.5% vs. 10.7%, p = 0.001) when compared with patients who remained asymptomatic during follow-up (Table 3). Multivariate analysis was then performed and denoted preoperative BMI (OR 1.187, 95% CI 1.025–1.376) and gallstones on preoperative ultrasound (OR 10.720, 95% CI 1.613–71.246) as independent risk factors for developing symptomatic biliary disease after bariatric surgery (Table 4).
Table 3.
Comparison between asymptomatic and symptomatic patients who remained with their gallbladder in situ after bariatric surgery
| Asymptomatic gallbladder in situ N = 418 | Symptomatic gallstones N = 8 | P value | |
|---|---|---|---|
| Age (years), median (range) | 45 (20–67) | 37.5 (29–61) | 0.062 |
| Female, n (%) | 340 (81.3) | 7 (87.5) | 1.000 |
| Type of surgery (%) | 1.000 | ||
| RYGB, n (%) | 276 (66) | 5 (62.5) | |
| SG, n (%) | 142 (34) | 3 (37.5) | |
| Revisional surgery, n (%) | 75 (17.9) | 1 (12.5) | 1.000 |
| Preoperative BMI (kg/m2), median (IQR) | 42.8 (39.5–46.1) | 47.0 (42.4–53.3) | 0.046 |
| Gallstones on preoperative abdominal US, n (%) | 44 (10.7) | 5 (62.5) | 0.001 |
| DM, n (%) | 102 (24.4) | 2 (25) | 1.000 |
| Glucose (mg/dL), median (IQR) | 95 (86.3–109) | 93.5 (81.3–107.8) | 0.760 |
| HbA1c (%), median (IQR) | 5.6 (5.3–6.0) | 5.8 (5.5–5.9) | 0.366 |
| Dyslipidemia, n (%) | 197 (47.1) | 3 (37.5) | 0.728 |
| Total cholesterol (mg/dL), median (IQR) | 184 (162–212) | 186 (158.3–223.5) | 0.823 |
| LDL-c (mg/dL), median (IQR) | 111 (91–137) | 112.5 (82–148.8) | 0.970 |
| HDL-c (mg/dL), median (IQR) | 48 (41–55) | 54 (45–59) | 0.312 |
| Triglycerides (mg/dL), median (IQR) | 111 (82–146) | 100 (78.8–211.8) | 0.941 |
| Severe steatosis, n (%) | 96 (25.2) | 2 (28.6) | 1.000 |
| Postoperative, 6 m %EWL, median (IQR) | 66.7 (55.6–81.3) | 55.4 (46.3–64.3) | 0.055 |
| Postoperative, 12 m | |||
| BMI (kg/m2), median (IQR) | 27.9 (25.4–30.6) | 29.7 (23.3–36.2) | 0.493 |
| %EWL, median (IQR) | 84.6 (70.2–97.8) | 79.3 (61.7–105.9) | 0.638 |
RYGB Roux-en-Y gastric bypass, SG sleeve gastrectomy, BMI body mass index, IQR interquartile range, DM diabetes mellitus, HbA1c hemoglobin A1c, LDL-c low density lipoprotein cholesterol, HDL-c high density lipoprotein cholesterol, %EWL percentage of excess weight loss
Table 4.
Multivariate analysis for factors related to symptomatic gallstones
| OR (95% CI) | P value | |
|---|---|---|
| Age | 0.931 (0.846–1.024) | 0.142 |
| Gender | 0.999 (0.093–10.721) | 0.999 |
| Type of bariatric surgery | 0.720 (0.098–5.309) | 0.747 |
| Preoperative BMI | 1.187 (1.025–1.376) | 0.022 |
| Gallstones on preoperative abdominal US | 10.720 (1.613–71.246) | 0.014 |
| Dyslipidemia | 1.621 (0.267–9.847) | 0.600 |
| %EWL, 6 m | 0.995 (0.976–1.015) | 0.639 |
OR odds ratio, CI confidence interval, BMI body mass index, %EWL percentage of excess weight loss
At the time of bariatric surgery, symptomatic patients who required concomitant cholecystectomy (n = 12) were not significantly different from those who were asymptomatic (n = 440) (Table 5).
Table 5.
Comparison between asymptomatic patients and patients who underwent concomitant cholecystectomy at the time of bariatric surgery
| Asymptomatic gallbladder in situ N = 426 | Concomitant cholecystectomy N = 12 | P value | |
|---|---|---|---|
| Age, (years), median (range) | 45 (20– 67) | 44 (26–62) | 0.726 |
| Female, n (%) | 347 (81.5%) | 12 (100) | 0.136 |
| Preoperative BMI (kg/m2), median (IQR) | 42,9 (39.5–46.2) | 39.2 (37.4–48.8) | 0.537 |
| DM, n (%) | 104 (24.4) | 3 (25) | 1.000 |
| Glucose (mg/dL), median (IQR) | 95 (86.3–109) | 101 (84–110) | 0.481 |
| HbA1c (%), median (IQR) | 5.6 (5.3–6.0) | 5.6 (5.2–6.0) | 0.913 |
| Dyslipidemia, n (%) | 200 (46.9%) | 5 (41.7) | 0.718 |
| Total cholesterol (mg/dL), median (IQR) | 184 (162–213) | 182 (160–198.5) | 0.672 |
| LDL-c (mg/dL), median (IQR) | 111 (91–137) | 114 (86–120.5) | 0.659 |
| HDL-c (mg/dL), median (IQR) | 48 (41–55) | 48 (46–55) | 0.470 |
| Triglycerides (mg/dL), median (IQR) | 111 (82–146) | 110 (78.5–136) | 0.809 |
| Severe steatosis, n (%) | 101 (25.3) | 3 (25) | 1.000 |
BMI body mass index, IQR interquartile range, DM diabetes mellitus, HbA1c hemoglobin A1c, LDL-c low density lipoprotein cholesterol, HDL-c high density lipoprotein cholesterol
Discussion
Obesity is a well-known risk factor for cholelithiasis [17]. In our cohort we found a prevalence of preoperative gallbladder disease of 25.3%. Similar results were found in other studies, with a prevalence of cholelithiasis in bariatric population being estimated at 20.8 to 36,7% [15, 18–23].
Early after bariatric surgery there is a greater incidence of gallstones. An increased bile saturation of cholesterol and crystallization-promoting proteins, such as mucin [24], along with defective gallbladder emptying, result in cholesterol crystal formation and gallstone development [6, 7, 24]. Some authors suggest that rapid weight loss is the main element leading to gallstone formation after either RYGB or SG [25] and that gallstone formation during active weight loss seems to increase in an exponential fashion [26]. Nonetheless, the type of bariatric procedure may also play an important role in the overall risk for symptomatic cholelithiasis. Sneineh et al. reported that a change in BMI after bariatric surgery could not explain the significantly increased incidence of symptomatic cholelithiasis following RYGB (14.5%) compared to SG (4.4%), LAGB (4.1%) and one anastomosis gastric bypass (OAGB) (7.5%). The lack of interference with the biliary contraction mechanisms and enterohepatic circulation in SG and LAGB was in part considered responsible for this discrepancy [27].
In our cohort we found an incidence of symptomatic cholelithiasis after bariatric surgery of 1.9% during a 12-month follow-up period, with 87.5% of patients presenting with uncomplicated gallbladder disease (i.e., biliary colic). Several studies with a similar study design, in which no gallstone-lowering prophylaxis was used, reported an incidence of 0.9% to 6.2% [11, 21–23, 27]. When focusing on the need for subsequent cholecystectomy, Swartz et al. reported that 14.7% of patients with prior RYGB required later cholecystectomy [28], while Altieri et al. found that 9.7% of patients underwent cholecystectomy after RYGB and 10.1% after SG [29].
A nationwide Swedish multiregister study found that, while prior to RYGB patients with obesity were already at an increased risk for cholecystectomy, this risk was even higher after. RYGB, and only returning to baseline after 36 months [30]. Alternatively, a cohort study by Chen et al. reported that, even though the bariatric surgery group had a significantly higher rate of gallstone disease than the general population (2.89% vs. 1.15%, p < 0.001), there were no significant differences between the bariatric surgery group when compared with patients with obesity that did not underwent a bariatric procedure. Hence, they concluded that the risk of symptomatic cholelithiasis did not increase after bariatric surgery, despite being higher than the general population [31]. On the other hand, our results on the incidence of symptomatic cholelithiasis (1.9%) are comparable to the risk of symptomatic gallstones in the general population with asymptomatic gallstones, which is 1 to 4% per year [32]. In the general population, treatment of asymptomatic gallstone patients is not routinely recommended, and most patients are treated by elective laparoscopic cholecystectomy when they become symptomatic [33]. Considering the low incidence of gallstone disease after bariatric procedures, the same expectant approach appears appropriate [13, 22, 28].
In order to find particular patients where a more aggressive approach could be used, we sought risk factors for symptomatic cholelithiasis. In our series we noted that patients who developed symptomatic gallstones had higher preoperative BMI than patients that remained asymptomatic (47.0 vs. 42.8 kg/m2, p = 0.046), and higher rates of gallstones on preoperative abdominal ultrasound (62.5% vs. 10.7%, p = 0.001). These two risk factors remained as independent risk factors for developing symptomatic gallstones after multivariate analysis (OR 1.187, 95% CI 1.025–1.376; OR 10.720, 95% CI 1.613–71.246; respectively). These results may indicate that preoperative characteristics, namely high BMI and cholelithiasis, are probably more important for the development of symptomatic cholelithiasis than factors more intrinsically related to bariatric surgery, such as the type of surgery used or its effectiveness (i.e., %EWL). Prospective studies are needed in order to validate these findings and to accurately identify patients which might benefit to be submitted to cholecystectomy in same surgical time.
Age, gender and obesity related disorders, notably diabetes, dyslipidemia and severe steatosis were not considered significantly different between the two groups in our population. We further compare those who had concomitant cholecystectomy with patients that left the operating room with their gallbladders in situ, but did not find any significant difference between them.
Other studies indicated that a weight loss of more than 25% of original weight [20], rapid weight loss [21], restrictive procedures [31], female gender [21, 31] and increased age [34] were associated with an increased risk of symptomatic gallstones. Noteworthy, a recent study reported that patients who had another bariatric procedure in the past were at an increased risk for gallstone formation and cholecystectomy [27]. Conversely, some studies failed to find any predictors of symptomatic cholelithiasis [22, 35]. As new studies emerge some of these risk factors may be further supported and others identified.
Postoperative administration of UDCA, for a period of 6 months, was considered effective for reducing the rate of gallstone formations after bariatric surgery [36, 37] and reduced the need for urgent cholecystectomy [37]. Nonetheless, its cost-effectiveness [38] and compliance [28] are questionable. Therefore, in our institution, we do not routinely use UDCA for gallstone prevention. To clarify the role of UDCA, prospective trials regarding this issue should provide better evidence on this matter.
Despite a clear shift towards a more conservative approach, with several studies concluding that concomitant cholecystectomy should only be performed in symptomatic patients [13, 22, 23, 28, 35, 39, 40], there is still evidence supporting prophylactic cholecystectomy [14, 41]. In fact, a large study by Weiss et al. reported that in comparison with RYGB alone, patients who underwent concomitant cholecystectomy had improved survival and long-term outcomes. Additionally, cholecystectomy patients who had underwent prior RYGB had higher risk of conversion to open cholecystectomy, post-operative complications, and death, when compared with cholecystectomy patients without any prior bariatric procedure [41].
Limitations of our work include its retrospective nature. This study was performed in a single institution; nonetheless, this assures homogeneity in treatment plan, because all patients were evaluated and treated by the same multidisciplinary team. The rate of symptomatic cholelithiasis after bariatric surgery may have been underestimated for two reasons. Firstly, due to the COVID-19 pandemic, several follow-up consultations were accomplished remotely by telephone, and that may have resulted in omitted symptoms. Secondly, despite our comprehensive definition for symptomatic cholelithiasis, unspecific or vague upper abdominal pain and nausea from gallstones, most likely, have been attributed to another cause than the other way around. As postoperative abdominal ultrasound is not routinely performed after bariatric surgery, we could not identify what proportion of patients developed asymptomatic gallstones.
Further studies, multicentric and prospective in nature, with longer follow-up period should be the key to definitively establish risk factors for development of symptomatic gallstones after bariatric surgeries and identify patients who might benefit the most with concomitant cholecystectomy.
Conclusion
In line with recent available evidence, our results on the incidence of symptomatic gallstones after bariatric surgery support the performance of cholecystectomy concomitantly with bariatric surgery only in symptomatic patients. Higher BMI and preoperative ultrasound for cholelithiasis were independent risk factors for development of symptomatic gallstones after SG and RYGB. Further prospective studies are needed in order to accurately clarify the role of these risk factors. In the future, predictive modeling taking in consideration BMI and preoperative ultrasound may assist in the decision-making process of identifying patients that could benefit from concomitant cholecystectomy.
Acknowledgements
The authors would like to acknowledge all members of the Obesity Integrated Responsibility Unit (CRI-O) group.
Author’s contributions
JN, HSS, MR - manuscript writing; JN, MR, FC, AP - data collection; JN, HSS, BSP - statistical analysis; HSS, FR, ACP, JP, ELC, SC - patient orientation; All authors reviewed the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data Availability
All the data supporting the findings of this study are available from the authors upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Institutional Review Board (CES 46–21).
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jorge Nogueiro and Hugo Santos-Sousa are First co-authors contributed equally.
Eduardo Lima-da-Costa and Silvestre Carneiro are Seniors co-authors contributed equally.
References
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Picot J, et al (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13(41):1–190 215–357, iii-iv [DOI] [PubMed]
- 4.Angrisani L, et al. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–1832. doi: 10.1007/s11695-015-1657-z. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman ML, et al. Gallstone formation after rapid weight loss: a prospective study in patients undergoing gastric bypass surgery for treatment of morbid obesity. Am J Gastroenterol. 1991;86(8):1000–1005. [PubMed] [Google Scholar]
- 6.Al-Jiffry BO, et al. Changes in gallbladder motility and gallstone formation following laparoscopic gastric banding for morbid obestity. Can J Gastroenterol. 2003;17(3):169–174. doi: 10.1155/2003/392719. [DOI] [PubMed] [Google Scholar]
- 7.Gebhard RL, et al. The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology. 1996;24(3):544–548. doi: 10.1002/hep.510240313. [DOI] [PubMed] [Google Scholar]
- 8.Broomfield PH, et al. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med. 1988;319(24):1567–1572. doi: 10.1056/NEJM198812153192403. [DOI] [PubMed] [Google Scholar]
- 9.Mazzella G, et al (1991) Comparative evaluation of chenodeoxycholic and ursodeoxycholic acids in obese patients. Effects on biliary lipid metabolism during weight maintenance and weight reduction. Gastroenterology 101(2):490–6 [DOI] [PubMed]
- 10.Jonas E, et al. Incidence of postoperative gallstone disease after antiobesity surgery: population-based study from Sweden. Surg Obes Relat Dis. 2010;6(1):54–58. doi: 10.1016/j.soard.2009.03.221. [DOI] [PubMed] [Google Scholar]
- 11.Hasan MY, et al. Gallstone Disease After Laparoscopic Sleeve Gastrectomy in an Asian Population-What Proportion of Gallstones Actually Becomes Symptomatic? Obes Surg. 2017;27(9):2419–2423. doi: 10.1007/s11695-017-2657-y. [DOI] [PubMed] [Google Scholar]
- 12.Doulamis IP, et al. Concomitant cholecystectomy during bariatric surgery: The jury is still out. Am J Surg. 2019;218(2):401–410. doi: 10.1016/j.amjsurg.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Warschkow R, et al. Concomitant cholecystectomy during laparoscopic Roux-en-Y gastric bypass in obese patients is not justified: a meta-analysis. Obes Surg. 2013;23(3):397–407. doi: 10.1007/s11695-012-0852-4. [DOI] [PubMed] [Google Scholar]
- 14.Amstutz S, et al. Potential Benefits of Prophylactic Cholecystectomy in Patients Undergoing Bariatric Bypass Surgery. Obes Surg. 2015;25(11):2054–2060. doi: 10.1007/s11695-015-1650-6. [DOI] [PubMed] [Google Scholar]
- 15.Nougou A, Suter M. Almost routine prophylactic cholecystectomy during laparoscopic gastric bypass is safe. Obes Surg. 2008;18(5):535–539. doi: 10.1007/s11695-007-9368-8. [DOI] [PubMed] [Google Scholar]
- 16.Elwood DR (2008) Cholecystitis. Surg Clin North Am 88(6):1241–52, viii [DOI] [PubMed]
- 17.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20(6):981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Guzman HM, et al. Incidence and Risk Factors for Cholelithiasis After Bariatric Surgery. Obes Surg. 2019;29(7):2110–2114. doi: 10.1007/s11695-019-03760-4. [DOI] [PubMed] [Google Scholar]
- 19.Iglezias Brandao de Oliveira CE (2003) Adami Chaim, and B.B. da Silva, Impact of rapid weight reduction on risk of cholelithiasis after bariatric surgery. Obes Surg 13(4): 625–8 [DOI] [PubMed]
- 20.Li VK, et al. Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc. 2009;23(7):1640–1644. doi: 10.1007/s00464-008-0204-6. [DOI] [PubMed] [Google Scholar]
- 21.Melmer A, et al. Incidence of Gallstone Formation and Cholecystectomy 10 Years After Bariatric Surgery. Obes Surg. 2015;25(7):1171–1176. doi: 10.1007/s11695-014-1529-y. [DOI] [PubMed] [Google Scholar]
- 22.Morais M, et al. Gallstones and Bariatric Surgery: To Treat or Not to Treat? World J Surg. 2016;40(12):2904–2910. doi: 10.1007/s00268-016-3639-2. [DOI] [PubMed] [Google Scholar]
- 23.Pineda O, et al. A Prospective Study of the Conservative Management of Asymptomatic Preoperative and Postoperative Gallbladder Disease in Bariatric Surgery. Obes Surg. 2017;27(1):148–153. doi: 10.1007/s11695-016-2264-3. [DOI] [PubMed] [Google Scholar]
- 24.Gustafsson U, et al. Changes in gallbladder bile composition and crystal detection time in morbidly obese subjects after bariatric surgery. Hepatology. 2005;41(6):1322–1328. doi: 10.1002/hep.20686. [DOI] [PubMed] [Google Scholar]
- 25.Coupaye M, et al. Comparison of the incidence of cholelithiasis after sleeve gastrectomy and Roux-en-Y gastric bypass in obese patients: a prospective study. Surg Obes Relat Dis. 2015;11(4):779–784. doi: 10.1016/j.soard.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Weinsier RL, Wilson LJ, Lee J. Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med. 1995;98(2):115–117. doi: 10.1016/S0002-9343(99)80394-5. [DOI] [PubMed] [Google Scholar]
- 27.Sneineh MA, et al. Increased Incidence of Symptomatic Cholelithiasis After Bariatric Roux-En-Y Gastric Bypass and Previous Bariatric Surgery: a Single Center Experience. Obes Surg. 2020;30(3):846–850. doi: 10.1007/s11695-019-04366-6. [DOI] [PubMed] [Google Scholar]
- 28.Swartz DE, Felix EL. Elective cholecystectomy after Roux-en-Y gastric bypass: why should asymptomatic gallstones be treated differently in morbidly obese patients? Surg Obes Relat Dis. 2005;1(6):555–560. doi: 10.1016/j.soard.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Altieri MS, et al. Incidence of cholecystectomy after bariatric surgery. Surg Obes Relat Dis. 2018;14(7):992–996. doi: 10.1016/j.soard.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Wanjura V, et al. Cholecystectomy after gastric bypass-incidence and complications. Surg Obes Relat Dis. 2017;13(6):979–987. doi: 10.1016/j.soard.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Chen JH, et al. Bariatric Surgery Did Not Increase the Risk of Gallstone Disease in Obese Patients: a Comprehensive Cohort Study. Obes Surg. 2019;29(2):464–473. doi: 10.1007/s11695-018-3532-1. [DOI] [PubMed] [Google Scholar]
- 32.Friedman GD. Natural history of asymptomatic and symptomatic gallstones. Am J Surg. 1993;165(4):399–404. doi: 10.1016/S0002-9610(05)80930-4. [DOI] [PubMed] [Google Scholar]
- 33.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368(9531):230–239. doi: 10.1016/S0140-6736(06)69044-2. [DOI] [PubMed] [Google Scholar]
- 34.Chang J, et al. Predictive factors of biliary complications after bariatric surgery. Surg Obes Relat Dis. 2016;12(9):1706–1710. doi: 10.1016/j.soard.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Yardimci S, et al. Is Concomitant Cholecystectomy Necessary for Asymptomatic Cholelithiasis During Laparoscopic Sleeve Gastrectomy? Obes Surg. 2018;28(2):469–473. doi: 10.1007/s11695-017-2867-3. [DOI] [PubMed] [Google Scholar]
- 36.Adams LB, et al. Randomized, Prospective Comparison of Ursodeoxycholic Acid for the Prevention of Gallstones after Sleeve Gastrectomy. Obes Surg. 2016;26(5):990–994. doi: 10.1007/s11695-015-1858-5. [DOI] [PubMed] [Google Scholar]
- 37.Magouliotis DE, et al. Ursodeoxycholic Acid in the Prevention of Gallstone Formation After Bariatric Surgery: an Updated Systematic Review and Meta-analysis. Obes Surg. 2017;27(11):3021–3030. doi: 10.1007/s11695-017-2924-y. [DOI] [PubMed] [Google Scholar]
- 38.Benarroch-Gampel J, et al. Cost-effectiveness analysis of cholecystectomy during Roux-en-Y gastric bypass for morbid obesity. Surgery. 2012;152(3):363–375. doi: 10.1016/j.surg.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellner SJ, et al. Routine cholecystectomy is not mandatory during morbid obesity surgery. Surg Obes Relat Dis. 2007;3(4):456–460. doi: 10.1016/j.soard.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Tustumi F, et al. Cholecystectomy in Patients Submitted to Bariatric Procedure: A Systematic Review and Meta-analysis. Obes Surg. 2018;28(10):3312–3320. doi: 10.1007/s11695-018-3443-1. [DOI] [PubMed] [Google Scholar]
- 41.Weiss AC, et al. Concomitant cholecystectomy should be routinely performed with laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2015;29(11):3106–3111. doi: 10.1007/s00464-014-4033-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the findings of this study are available from the authors upon reasonable request.