Abstract
The clinical course of the first patient to receive a gene-edited pig heart transplant was recently reported by the University of Maryland team. Although the pig heart functioned well for >40 days, serum anti-pig antibodies then increased, and the patient sadly died after 60 days. Because of his debilitated pre-transplant state, the patient never thrived despite excellent graft function for several weeks, and the cause of his demise continues to be uncertain. A few days before an increase in anti-pig antibodies was observed, the patient had received intravenous human immunoglobulin (IVIg), and whether this played a role in his cardiac deterioration has been discussed. Furthermore, mcfDNA testing indicated an increase in pig cytomegalovirus (CMV), and its possible role in the development of cardiac dysfunction has also been considered. On the basis of the limited data provided in the publication and on our previous investigations into whether IVIg contains anti-TKO pig antibodies and therefore might be deleterious to TKO pig organ xenografts, we suggest that the steady rise in anti-pig antibody titer was more consistent with the failure of the immunosuppressive regimen to prevent elicited anti-TKO pig antibody production, rather than from the passive transfusion of IVIg or the presence of pig CMV in the graft. Although the outcome of the Maryland experience was disappointing, valuable lessons were learned. Our attention was drawn to the potential risks of heart transplantation in a “deconditioned” patient, the administration of IVIg, the transmission of pig CMV, and of the difficulties in interpreting myocardial biopsy findings.
Keywords: antibody-mediated, clinical, cytomegalovirus, genetically modified, heart, IVIg, pig, rejection, xenotransplantation
1 ∣. INTRODUCTION
The first clinical pig heart transplant, carried out by our colleagues at the University of Maryland at Baltimore on January 7, 2022,1 attracted considerable public attention and stimulated interest in the potential of xenotransplantation as a therapeutic option for patients with terminal organ failure.
The Maryland team had obtained encouraging results from pig orthotopic heart transplantation in baboons.2-4 Using essentially the same genetically-engineered pigs (with 10 genetic modifications) and immunosuppressive regimen (based on blockade of the CD40/CD154 co-stimulation pathway), one would have anticipated an equally encouraging result from their first clinical effort, particularly as the high prevalence of a positive cross-match against “triple-knock-out” (TKO) pigs, likely associated with a putative “4th xenoantigen” recognized by nonhuman primates (NHP),5-8 is not observed in humans. However, despite the excellent early function of the pig heart, the final outcome was disappointing. Based on the admittedly limited data provided in the recent report,1 what factors do we believe contributed to the failure of this patient to thrive, and to his ultimate demise?
1.1 ∣. General considerations
One major difference between the experimental studies and the clinical experience was that the recipients in the laboratory were healthy baboons, whereas the Maryland patient was in a very debilitated state before the transplant was undertaken. We agree with the authors’ conclusion that his severely-debilitated state played a major role in his failure to benefit as anticipated from the replacement of his failing heart with a healthy pig heart. Although details were not given, before the transplant he was reported to have adrenal insufficiency, and had suffered episodes of gastrointestinal bleeding, bacteremia, and drug-induced leukopenia. In addition, pre-transplant he had been bed-bound, with cardiac cachexia, refractory ventricular ectopy, and requiring veno-arterial extracorporeal membrane oxygenation (ECMO) support for 46 days and had been non-ambulatory through much of this time, thus inevitably reducing his physiological resilience.
Frailty is among the best predictors for poor outcome after surgery, even in the absence of postoperative requirement for immunosuppression, as is necessary following a transplant. Under such circumstances, few centers would have considered allotransplantation to be a viable therapeutic option in this patient due to secondary immunologic compromise from malnutrition and low probability of recovery.
In regard to pioneering efforts in medicine, it is not uncommon for the first few patients to be in extremis, or for a clinical experiment to be undertaken in a patient who is less than optimal for the study. For example, the first human heart allotransplant carried out by the major pioneer in the field, Norman Shumway, was in a patient who developed multiple complications before dying 2 weeks later.9 In retrospect, he was arguably a patient with too many comorbidities and/or fragility to recover from such a major surgical procedure. The Maryland experience reminds us that taking on a profoundly debilitated patient compromises our ability to define the therapeutic potential of the heart xenograft procedure, and to distinguish between host- and graft-associated effects on this transplant’s outcome.
1.2 ∣. Post-transplant clinical course of the Maryland patient
The Maryland patient suffered numerous complications, which we suggest were largely attributable to his debilitated state. During the transplant procedure, when the aortic cross-clamp was removed, it was found that the clamp had caused an extensive dissection of the aorta. This is an uncommon complication associated with open-heart surgery, but probably reflects the poor underlying quality of the patient’s tissues associated with advanced cardiovascular disease additionally complicated by malnutrition and a prolonged period of decreased aortic wall tensioning on veno-arterial ECMO support. Although successfully repaired, this complication appears to have been associated with acute renal failure that required regular dialysis throughout the two months that the patient remained alive (despite insertion of a renal artery stent).
On postoperative days 12 and 49, laparotomies were undertaken for suspected abdominal complications, and revealed evidence of prior “bowel ischemia,” though no conclusive pathology was identified. These surgical procedures almost certainly set back his recovery. A short video of the patient (watching the US National Football League [NFL] super-bowl on television) in the Intensive Care Unit one month after the transplant showed a man who appeared slightly jaundiced, slightly breathless, and struggling to concentrate on what people were saying to him, all features of his debilitated state. At that interval, the pig heart was functioning well and, under the usual post-cardiac transplant circumstances where the recipient is physiologically intact before surgery, one would have expected the patient to be fully ambulatory and at home.
His debilitated state was also demonstrated by continuing low white blood cell and platelet counts, and low immunoglobulin levels. These must have made it difficult for the medical team to judge what level of immunosuppressive therapy he required. The patient’s body weight fell by 25% during his 2-month postoperative stay in hospital. It was the low plasma immunoglobulin level and concern for diminished protective immunity that apparently spurred the medical team to administer intravenous immunoglobulin (IVIg), initially on post-transplant day 43. This has raised the question of whether the IVIg, which could have contained anti-pig antibodies, might have contributed to the graft hypertrophy, diastolic dysfunction, and presumed humoral rejection of the heart graft that developed soon after IVIg administration.
1.3 ∣. Intravenous immunoglobulin (IVIg) therapy
IVIgs are purified IgG products prepared from a pool of 5000-10 000 blood donors, and typically contain >95% unmodified IgG, and only trace amounts of IgA and IgM.10 Our group previously identified 10 different brands of commercially available IVIg in the United States.11
IVIg has been used for >3 decades in the prevention or treatment of antibody-mediated rejection in HLA-sensitized patients undergoing organ allotransplantation and in those receiving ABO-incompatible kidney allografts,10,12-19 usually as an adjuvant intended to suppress rebound of alloantibody. IVIg is also used in the treatment of autoimmune diseases, such as Kawasaki disease, idiopathic thrombocytopenic purpura, and myasthenia gravis20,21 and treatment of severe infection.22
The effect of IVIg in experimental xenotransplantation remains controversial, with some groups reporting a benefit,23-29 but others reporting no benefit30 or even harm.31 IVIg has been demonstrated to contain antibodies to galactose-α1,3-galactose (Gal) and to N-glycolylneuraminic acid (Neu5Gc),32,33 which are not expressed on a TKO pig heart and thus are unlikely to have affected the outcome in the Maryland case. IVIg can affect both innate and adaptive immunity,19,21 and has been reported to (i) delay rejection of guinea pig-to-rat heart xenotransplants (in which both species express galactose-α1,3-galactose [Gal] antigens) through anti-complement activity and/or anti-idiotypic antibodies,34 (ii) delay rejection of wild-type pig hearts in NHPs,23 and (iii) prolong survival of wild-type pig kidneys perfused with human blood ex vivo.35
The mechanisms by which IVIg has been postulated to have a beneficial effect in xenotransplantation include (i) the presence of anti-idiotypic antibodies against xenoreactive antibodies,34 or (ii) by inhibiting complement activation,21 even though IVIg does not inhibit IgM binding to pig cells (which mediates complement activation).
The Maryland team therefore faced a dilemma as to whether the benefits of IVIg therapy outweighed the potential risks. Anti-pig antibodies, if present in the IVIg preparation administered, could be harmful to the graft. However, the deteriorating general state of the patient, with falling levels of plasma proteins, convinced them that the risk should be accepted.
It is important to note that the reason for administering IVIg to the Maryland patient did not appear to be to prevent or reverse rejection because no rejection had been seen at this time (day 43). It was administered because of the patient’s hypogammaglobulinemia (total IgG level 185 mg/day) and concern with regard to potential infection, e.g., pig cytomegalovirus (CMV), as a noticeable increase in CMV mcfDNA had occurred from day 20). Retrospective quantitative PCR performed on a spleen sample from the donor pig was positive for pig CMV, confirming the pig as the source of the virus. CMV was also demonstrated by PCR in peripheral blood mononuclear cells from the patient, demonstrating viremia if not cross-species infection.
Triple-knockout (TKO) pigs are considered by most in the field as being ideal sources of organs for clinical xenotransplantation because many humans have no preformed antibody to TKO pig cells. Our study in 2020 investigated in vitro whether IVIg contains anti-TKO pig antibodies that are cytotoxic to pig cells.11 Undiluted pooled human serum and five different commercial preparations of IVIg were tested for IgM and IgG binding to red blood cells (RBCs) from wild-type, α1,3-galactosyltransferase gene-knockout (GTKO), and TKO pigs by flow cytometry. Complement-dependent lysis of IVIg against these pig (p) RBCs was measured by hemolytic assay.
In this study, pooled human serum and four of five commercial IVIg preparations contained anti-pig IgG that bound to wild-type and GTKO pRBCs and/or pig aortic endothelial cells, but this was not associated with cytotoxicity to either cell type in vitro. Most preparations did not contain anti-TKO pig IgM and were not cytotoxic to TKO pig cells. One preparation of IVIg contained antibodies that bound to TKO pRBCs, but there was no cytotoxicity to TKO pRBCs. Indeed, when rabbit complement (i.e., exogenous complement) was added in each of these conditions, cytotoxicity remained negative in the complement-dependent cytotoxicity assay.
Our results suggested that IVIg administration to human recipients of TKO pig grafts should be safe. However, anti-pig antibody levels in IVIg vary considerably depending on the brand or lot number.11,33 Therefore, if IVIg is to be used in xenotransplantation, it is logical that an IVIg with a low anti-pig antibody level should be selected by screening the IVIg before its administration. It is not known whether this was done by the Maryland group.
In summary, since IVIg was not associated with any cytotoxicity in vitro, even when wild-type pRBCs were the target, we would conclude that IVIg likely had no significant direct complement activation effect against TKO cells in the Maryland case. Our study suggested that most preparations of IVIg do not contain IgG or IgM directed to TKO pig cells. Therefore, it should be safe to administer to recipients of a TKO pig organ. However, the specific preparation of IVIg would need to be screened before its administration.
1.4 ∣. Rejection of the pig heart
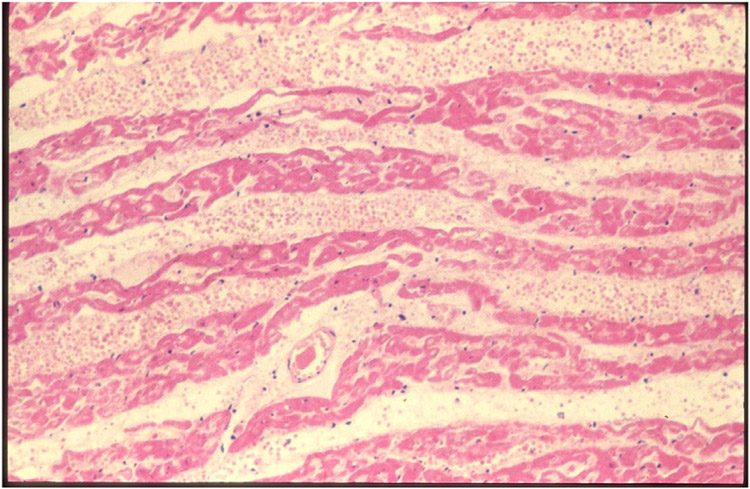
The Maryland patient underwent several endomyocardial biopsies to monitor for rejection of the graft. The first biopsy (day 34) showed no evidence of antibody-mediated or cellular rejection. Based on the International Society for Heart and Lung Transplantation (ISHLT) histopathological criteria, the second biopsy (day 50) was reported as not showing antibody-mediated or cellular rejection, and yet there was “focal capillary damage with extravasated erythrocytes and edema.”1 The ISHLT criteria, however, are based on the histopathological features seen in allotransplantation, and may not be relevant to xenotransplantation. (Indeed, interstitial hemorrhage and edema were prominent features of hyperacute rejection reported in the very early days of xenotransplantation research when wild-type pig hearts were transplanted into immunosuppressed or non-immunosuppressed baboons [Figure 1].36-38)
FIGURE 1.
Photomicrograph of a wild-type pig heart that ceased functiong within an hour of being continually perfused by baboon ABO-compatible blood (in an experiment carried out in 1985). Interstitial hemorrhage and edema are promineny features of hyperacurte rejection (Hematoxylin and eosin, x150).The appearances are similar to those shown in Figure 4, panel B, of the Maryland report (Griffith et al., 2022, reference 1)
In the Maryland pig heart, antibody staining indicated IgG and IgM deposition in the capillaries, though staining for C4d was negative. Minimal features of myocardial ischemia were evident, and there was no cellular infiltrate. The presence of interstitial hemorrhage and edema and bound antibody strongly suggests antibody-mediated rejection. Furthermore, an increase in anti-pig antibodies was observed on day 48, with features of significant myocardial dysfunction at the same time. Serum xenograft-specific IgM and IgG levels subsequently continued to rise. The fact that the patient needed ECMO support again on day 49 indicates his deterioration during the previous few days. Support for the diagnosis of rejection also comes from the observations that (i) troponin I levels were increasing, and (ii) levels of xenograft-derived cell-free DNA were increasing. Under the circumstances of this clinical experiment, what other realistic cause than rejection for the occurrence of these changes can be suggested?
It is noteworthy that the first description of this histopathological picture came on day 50, just a few days after the first infusion of IVIg on day 43, and detection of anti-pig antibodies in serum on day 48. Furthermore, between days 43 and 50, several other features suggesting antibody-mediated rejection had developed. Despite the comprehensive and detailed in vitro data in the laboratory suggesting that IVIg, particularly if screened for the absence of anti-TKO antibodies, may be safe to administer to patients with TKO pig grafts, the close temporal association of rejection with IVIg administration raised suspicions regarding a possible causative effect of the IVIg.
In our estimation, however, the steady rise in anti-pig antibody titer demonstrated in this patient is more consistent with failure of the immunosuppressive regimen to block elicited anti-TKO pig antibody production, rather than from the passive transfusion of IVIg. Balancing the provision of sufficient immunosuppressive therapy to prevent rejection but not to allow infection to develop would have been particularly difficult in this patient. Whether IVIg might have any effect in blocking the cytotoxicity associated with elicited anti-pig antibody (that may have been produced by the recipient) cannot be ascertained from the available data.
Various steps were taken by the Maryland team to try to reverse the presumed rejection episode, including plasmapheresis followed by IVIg infusion, which may have been counterproductive if IVIg were pathogenic. Because this treatment was combined with complement inhibition with a C1-esterase inhibitor and eculizumab, administered to reduce complement-dependent cytotoxicity, absence of C4d staining in the heart does not exclude a role for complement-independent antibody-mediated graft injury. As there was no cellular infiltration in the graft, however, it is unlikely that injury was associated with antibody-dependent cellular cytotoxicity, unless infiltrating NK cells or macrophages underwent necrosis or were cleared by other treatments given to this patient.
In an effort to reverse antibody-mediated rejection, the anti-CD20mAb, Rituximab, was administered. It is not clear from the report whether this was administered before or after the complement inhibitors. If given after complement had been blocked, the anti-CD20mAb may not have efficiently killed the B cells. In addition, it does not have an immediate effect on depleting antibody-producing plasma cells.39,40 Of note, in our experience, corticosteroid or anti-CD20mAb treatment have been uniformly unsuccessful in interrupting antibody elaboration or reversing antibody-mediated graft injury in pig-to-NHP xenotransplant models.
By day 56, a myocardial biopsy showed extensive myocardial necrosis secondary to rejection. Although 40% of the myocardial cells were deemed to be necrotic, the left ventricular ejection fraction was reported to be 70%, which we consider to be an artifact of the greatly reduced chamber size associated with marked graft hypertrophy. At autopsy on day 60, the pig heart showed extensive features consistent with antibody-mediated rejection and had almost doubled in weight.
1.5 ∣. Could pig cytomegalovirus (pCMV) have played a role in cardiac xenograft failure?
There has been much conjecture of whether the evidence for the presence of pCMV in the recipient was sufficient to associate pCMV with failure of the graft. As early as 2002, Mueller and Fishman began their extensive studies on CMV in baboons with pig organ grafts.41-46 They demonstrated that (i) piglets could remain CMV-negative if they were weaned from the sow within the first week after birth, and (ii) the presence of pig CMV in the recipient could result in the development of consumptive coagulopathy in the recipient and thrombotic microangiopathy in the graft. These findings were later confirmed by others.47 However, the evidence available to us from the Maryland experience suggests that anti-pig antibody elaborated by the patient (or just possibly passively acquired through the repeated administration of IVIg) is more likely to have been the primary precipitating factor in the development of graft failure.
1.6 ∣. Comment
We fully recognize the immense effort put into the care of this patient by the Maryland team, and we applaud the willingness of the patient to undergo such an experimental procedure. All of us in the field of xenotransplantation research have learned a great deal from this experience. Fully recognizing that it is much easier to suggest alternative approaches retrospectively, we suggest that the major lessons learned from the Maryland experience include:
Selection of the patient is critical. In our judgment, only patients with a realistic chance of recovery from the planned operative procedure, and whose survival is not likely to be limited by other patient conditions or characteristics, should be offered pig heart transplantation at this stage of the development of clinical xenotransplantation. Patients in an advanced state of debility or “deconditioning” should not be included in the first clinical trials. (If such patients are purposely or inadvertently included, treatment with a thyroid hormone, e.g., triiodothyronine, which has been demonstrated to stimulate mitochondrial function and thus replace energy stores, might result in improvement in the patient’s metabolic status.48,49
A decision to administer IVIg to a patient with a pig organ graft should be made only with extreme caution. The in vitro evidence is weak that IVIg can stimulate antibody-mediated rejection, and thus a mechanism remains uncertain. While the adsorption of anti-pig antibodies from IVIg before transfusion should reduce the risk of injury, this approach may not prevent immune injury of the graft by endogenous antibody or other mechanisms.
More sensitive methods of monitoring the potential organ-source pig for the presence of pig CMV, and potentially for other microorganisms, need to be employed. As illustrated by the Maryland experience, knowing that the pig had been raised and housed in a biosecure pig facility proved to be insufficient to prevent inadvertent transmission of pCMV to the first heart recipient. pCMV viral replication in the pig heart may have contributed to the patient’s demise.
Other factors that need further investigation include (i) the level and, particularly, location, e.g., vascular endothelium, of expression of the human protective proteins in the graft, and (ii) the efficacy of the humanized anti-CD40 mAb (KPL-404)50 to suppress the immune response to a pig xenograft, which does not appear to have been tested previously. Both factors could have influenced the susceptibility of the graft to rejection. In this regard, a clinical trial in kidney allotransplantation of a similar humanized anti-CD40mAb (CFZ533, Iscalimab) appears to have been discontinued for lack of efficacy (https://www.reuters.com/business/healthcare-pharmaceuticals/novartis-halts-study-iscalimab-in-kidney-transplant-patients-2021-09-03/).
It should be noted that, in pig-to-NHP models of heart transplantation, the longest survivals reported to date have been less than 9 months,4,51 with these grafts being lost through antibody-mediated rejection. Although, in the absence of the problems associated with the 4th xenoantigen,5,11 the results in humans may be superior to those in NHPs, the outcome for a patient with a pig heart graft remains uncertain. In the case of the Maryland patient, if the graft failed, there was no alternative therapy available, e.g., he was not considered to be eligible for an allograft or for the insertion of a mechanical assist device. Perhaps the initial patients in a clinical trial of pig heart transplantation should be those who, although not candidates for mechanical device support, could be successfully bridged to allotransplantation by a xenograft. These might include adults with a restrictive cardiomyopathy or infants with complex congenital heart disease.51-55 Successful bridging would provide experience that would subsequently enable destination therapy to be pursued.
One final comment, somewhat related to the above discussion, is that at this very early stage in the introduction of xenotransplantation into the clinic, it would seem wise to offer pig organ transplantation only to those patients who have proven themselves to be compliant with medical recommendations, instructions, and treatment. As the potential risk of transfer of a pig infection from the patient to the community is a cause for concern, we suggest that any patient with a known history of noncompliance sufficient to preclude his/her acceptance for allotransplantation should also be precluded from being a candidate for xenotransplantation. In the Maryland case, although their team planned to oversee the patient’s post-transplant care very closely, a noncompliant patient who developed an infectious complication could put others at risk.
Although the outcome of the Maryland experience was disappointing, valuable lessons were learned. If the transplant had been carried out in a brain-dead subject and followed for 3 days, it would have been considered a great success, but little new information would have come from this exercise. We would not have been made aware of the potential risks of factors such as (i) heart transplantation in a “deconditioned” patient, (ii) the administration of IVIg, (iii) the transmission of pig CMV, and (iv) the difficulties in interpreting the biopsy findings.
We should remind ourselves that the Maryland patient, the first to receive a gene-edited pig heart, survived considerably longer than the first patient to receive a human heart transplant in 1967. That patient survived for only 18 days.56 This should encourage us to persevere until clinical pig heart transplantation becomes a routinely successful procedure.
ACKNOWLEDGMENTS
Work on xenotransplantation in the authors’ laboratories is supported in part by NIH NIAID U19 grant AI090959 and UO1 grant AI153612, a Department of Defense grant W81XWH2010559, and by provision of genetically modified pigs from Revivicor and eGenesis.
Abbreviations:
- CMV
cytomegalovirus
- GTKO
α1,3-galactosyltransferase gene-knockout
- IVIg
intravenous immunoglobulin
- NHP
nonhuman primate
- p
pig
- RBCs
red blood cells
- TKO
triple (gene) knockout
Footnotes
CONFLICT OF INTEREST
DKCC is a consultant to eGenesis Bio of Cambridge, MA, but the opinions expressed in this article are his own and do not necessarily reflect those of eGenesis. No other author has a conflict of interest.
REFERENCES
- 1.Griffith BP, Goerlich CE, Singh AK, et al. Genetically-modified porcine = to-human cardiac xenotransplantation. N Engl J Med 2022. doi: 10.1056/NEJMoa2201422 Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goerlich CE, Griffith B, Hanna P, et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J Thorac Cardiovasc Surg. 2021;S0022-5223(21)01261-7, doi: 10.1016/j.jtcs.2012.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goerlich CE, Griffith B, Singh AK, et al. Blood cardioplegia induction, perfusion storage and graft dysfunction in cardiac xenotransplantation. Front Immunol. 2021; 12:667093. doi: 10.3389/fimmu 2021.667093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohiuddin MM, Goerlich CE, Singh AK, et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 2022;29(3):e12744. doi: 10.1111/xen.12744 Epub 2022 Mar 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes.Xenotransplantation 2015;22:194–202, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto T, Iwase H, Patel D, et al. Old World monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (triple-knockout). Sci Rep. 2020. Jun 17;10:9771. doi: 10.1038/s41598-020-66311-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foote JB, Jagdale A, Yamamoto T, et al. Histopathology of pig kidney grafts with/without expression of the carbohydrate Neu5Gc in immunosuppressed baboons. Xenotransplantation. 2021;28(6):e12715. doi: 10.1111/xen.12715 Epub 2021 Oct 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwase H, Jagdale A, Yamamoto T, et al. Evidence suggesting that deletion of expression of N-glycolylneuraminicacid (Neu5Gc) in the organ-source pig is associated with increased antibody-mediated rejection of kidney transplants in baboons. Xenotransplantation. 2021;28(4):e1700. doi: 10.1111/xen.12700 [DOI] [PubMed] [Google Scholar]
- 9.Stinson EB, Dong E, Schroeder JS, Harrison DC, Shumway NE Initial clinical experience with heart transplantation. Am J Cardiol 1968;22:791–803. [DOI] [PubMed] [Google Scholar]
- 10.Jordan SC, Toyoda M, Kahwaji J, Vo AA Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am J Transplant 2011;11:196–202. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Cui Y, Patel D, et al. Effect of intravenous immunoglobulin (IVIg) on primate complement-dependent cytotoxicity of genetically-engineered pig cells: relevance to clinical xenotransplantation. Sci Rep. 2020;10(1):11747. doi: 10.1038/s41598-020-68505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyan DB, Li VA, Czer L, Trento A, Jordan SC Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation 1994;57:553–562. [PubMed] [Google Scholar]
- 13.Jordan SC, Quartel AW, Czer LSC, et al. Posttransplant therapy using high-dose human immunoglobulin (intravenous gammaglobulin) to control acute humoral rejection in renal and cardiac allograft recipients and potential mechanism of action. Transplantation 1998;66:800–805 [DOI] [PubMed] [Google Scholar]
- 14.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull 2015;114:113–125. [DOI] [PubMed] [Google Scholar]
- 15.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med;2008;359:242–251. [DOI] [PubMed] [Google Scholar]
- 16.Dhanda R, Shah Y, Bardapure M, Bhattacharjya S, Sharma AK Excellent renal allograft survival in donor-specific antibody transplant patients-role of intravenous immunoglobulin and rabbit antithymocyte globulin. (Letter) Transplantation 2009;88:444. [DOI] [PubMed] [Google Scholar]
- 17.Kessler L, Parissiadis A, Bayle F, et al. Evidence for humoral rejection of a pancreatic islet graft and rescue with rituximab and IV immunoglobulin therapy. Am J Transplant 2009;9:1961–1966. [DOI] [PubMed] [Google Scholar]
- 18.Bachler K, Amico P, Honger G, et al. Efficacy of induction therapy with ATG and intravenous immunoglobulins in patients with low-level donor-specific HLA-antibodies. Am J Transplant 2010;10:1254–1262. [DOI] [PubMed] [Google Scholar]
- 19.Tedla FM, Roche-Recinos A, Brar A. Intravenous immunoglobulin in kidney transplantation. Curr Opin Organ Transplant 2015;20:630–637. [DOI] [PubMed] [Google Scholar]
- 20.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 2001;345:747–755. [DOI] [PubMed] [Google Scholar]
- 21.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation 2009;88:1–6. [DOI] [PubMed] [Google Scholar]
- 22.Van Gent R, Metselaar HJ, Kwekkeboom J. Immunomodulation by hyperimmunoglobulins after solid organ transplantation: Beyond prevention of viral infection. Transplant Rev (Orlando) 2017;31:78–86. [DOI] [PubMed] [Google Scholar]
- 23.Magee JC, Collins BH, Harland RC, et al. Immunoglobulin prevents complement-mediated hyperacute rejection in swine-to-primate xenotransplantation. J Clin Invest 1995;96:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautreau C, Kojima T, Woimant G, Cardoso J, Devillier P, Houssin D Use of intravenous immunoglobulin to delay xenogeneic hyperacute rejection. An in vivo and in vitro evaluation. Transplantation 1995;60:903–907. [PubMed] [Google Scholar]
- 25.Latremouille CH, Genevaz D, Hu MC, et al. Normal human immunoglobulins for intravenous use (IVIg) delay hyperacute xenograft rejection through F(ab’)2-mediated anti-complement activity. Clin Exp Immunol 1997;110:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbani L, Cardoso J, Soubrane O, Houssin D, Gautreau C Fab fragments from intravenous immunoglobulin prevent hyperacute rejection in the guinea pig-to-rat combination without reducing hemolytic complement activity in rat serum. Transplant Proc 2000;32:2707–2709. [DOI] [PubMed] [Google Scholar]
- 27.Roos A, Rieben R, Faber-Krol MC, Daha MR IgM-enriched human introvenous immunoglobulin strongly inhibits complement-dependent porcine cell cytotoxicity mediated by human xenoreactive antibodies. Xenotransplantation 2003;10:596–605. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Kim DH, Choi HJ, et al. Anti-CD40 antibody-mediated costimulation blockade promotes long-term survival of deep-lamellar porcine corneal grafts in non-human primates. Xenotransplantation 2017;24. doi: 10.1111/xen.12298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Choi SeH, Lee HJu, et al. Comparative efficacy of anti-CD40 antibody-mediated costimulation blockade on long-term survival of full-thickness porcine corneal grafts in nonhuman primates. Am J Transplant2018;18:2330–2341. [DOI] [PubMed] [Google Scholar]
- 30.Buhler L, Pidwell D, Dowling RD, Newman D, Awwad M, Cooper DKC Different responses of human anti-HLA and anti-alphagal antibody to long-term intravenous immunoglobulin therapy.Xenotransplantation 1999;6:181–186 [DOI] [PubMed] [Google Scholar]
- 31.Adams AB, Kim SC, Martens GR, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. AnnSurg 2018;268:564–573, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreau N, Blancho G, Boulet C, et al. Natural anti-Gal antibodies constitute 0.2% of intravenous immunoglobulin and are equally retained on a synthetic disaccharide column or on an immobilized natural glycoprotein. Transplant Proc 2000;32:882–883. [DOI] [PubMed] [Google Scholar]
- 33.Lu Q, Padler-Karavani V, Yu H, et al. LC-MS analysis of polyclonal human anti-Neu5Gc xeno-autoantibodies immunoglobulin G Sub-class and partial sequence using multistep intravenous immunoglobulin affinity purification and multienzymatic digestion. Anal Chem 2012;84:2761–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schussler O, Genevaz D, Latremouille C, Goussev N, Kaveri S, Glotz D Intravenous immunoglobulins for therapeutic use contain anti-idiotypes against xenophile antibodies and prolong discordant graft survival. Clin Immunol Immunopathol 1998;86:183–191. [DOI] [PubMed] [Google Scholar]
- 35.Fiane AE. Videm V, Mellbye OJ, et al. Immunoglobulin prolongs survival of pig kidneys perfused ex vivo with human blood. Scand J Immunol 1998;47:568–574. [DOI] [PubMed] [Google Scholar]
- 36.Lexer G, Cooper DKC, Rose AG, et al. Hyperacute rejection in a discordant (pig to baboon) cardiac xenograft model. J Heart Transplant 1986;5:411–418. [PubMed] [Google Scholar]
- 37.Rose AG, Cooper DKC, Human PA, et al. Histopathology of hyperacute rejection of the heart: experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant 1991;10:223–234. [PubMed] [Google Scholar]
- 38.Rose AG, Cooper DKC. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J Heart Lung Transplant 1996;15:804–817. [PubMed] [Google Scholar]
- 39.Alwayn IPJ, Xu Y, Basker M, et al. Effects of specific antiB and/or antiplasma cell immunotherapy on antibody production in baboons: depletion of CD20- and CD22-positive B cells does not result in significantly decreased production of antiaGal antibody.Xenotransplantation 2001;8:157–171. [DOI] [PubMed] [Google Scholar]
- 40.Alwayn IPJ, Basker M, Buhler L, Cooper DKC The problem of anti-pig antibodies in pig-to-primatexenografting: current and novel methods of depletion and/or suppression of production of anti-pig antibodies. Xenotransplantation 1999;6:157–168. [DOI] [PubMed] [Google Scholar]
- 41.Mueller NJ, Barth RN, Yamamoto S, et al. , Fishman JA Activation of cytomegalovirus in pig-to-primate organ transplantation. J Virol 2002;76:4734–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mueller NJ, Sulling K, Gollackner B, et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am. J. Transplant 2003;3:1057–1064. [DOI] [PubMed] [Google Scholar]
- 43.Mueller NJ, Kuwaki K, Dor F, et al. , Fishman JA Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation 2004;78:1449–1453. [DOI] [PubMed] [Google Scholar]
- 44.Mueller NJ, Livingston C, Knosalla C, et al. , Yamada K, Cooper D, Fishman JA Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in pig-to-baboon xenotransplantation. J Infect Dis 2004;189:1628–1633. [DOI] [PubMed] [Google Scholar]
- 45.Mueller NJ, Ezzelarab M, Buhler L, et al. Monitoring of porcine and baboon cytomegalovirus infection in xenotransplantation. Xenotransplantation 2009:16:535–536. [DOI] [PubMed] [Google Scholar]
- 46.Gollackner B, Mueller NJ, Houser S, et al. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation 2003;75:1841–1847. [DOI] [PubMed] [Google Scholar]
- 47.Yamada K, Tasaki M, Sekijima M, et al. Porcine cytomegalovirus infection is associated with early rejection of kidney grafts in a pig to baboon xenotransplantation model. Transplantation 2014;98:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harper M-E, Seifert EL. Thyroid hormone effects on mitochondrial energetics.Thyroid 2008;18:145–156. [DOI] [PubMed] [Google Scholar]
- 49.Novitzky D, Cooper DKC. Thyroid hormone treatment in heart surgery and heart transplantation. In Thyroid and Heart. (lervasi G, et al. , eds). Springer, Switzerland, 2020. pp.409–436. [Google Scholar]
- 50.Muralidharan S, Njenga M, Garron T, Bondensgaard K, Paolini JF Preclinical immunopharmacologic assessment of KPL-404, a novel, humanized, non-depleting antagonistic anti-CD40 monoclonal antibody. J Pharmacol Exp Ther. 2022;381:12–21. doi: 10.1124/jpet.121.000881 [DOI] [PubMed] [Google Scholar]
- 51.Cleveland DC, Jagdale A, Carlo WF, et al. The genetically engineered heart as a bridge to allotransplantation in infants: just around the corner? Ann Thorac Surg. 2021;50003-4975(21)00982-6. doi: 10.1016/j.athoracsur.2021.05.0025. Online ahead of print [DOI] [PubMed] [Google Scholar]
- 52.Cleveland D, Adam Banks C, Hara H, Carlo WF, Mauchley DC, Cooper DKC The case for cardiac xenotransplantation in neonates: is now the time to reconsider xenotransplantation for hypoplastic left heart syndrome? Pediatr Cardiol. 2018. doi: 10.1007/s00246-018-1998-1 (Pedaitr Cardiol. 2019 Feb:40(2):437-444.) [DOI] [PubMed] [Google Scholar]
- 53.Pierson RN, Burdorf L, Madsen JC, Lewis GD, d’Alessandro A Pig-to-human heart transplantation: Who goes first? Am J Transplant 2020;20:2669–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierson RN, Allan JS, Cooper DKC, et al. Pig heart and lung xenotransplantation: present status. J Heart Lung Transplant. 2022;S1053-2498(22)01914-3. doi: 10.1016/j.healun.2022.04.010 Online ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaban R, Cooper DKC, Pierson RN III. Pig heart and lung xenotransplantation: present status. J Heart Lung Transplant. 2022;S1053-2498(22)01914-3. doi: 10.1016/j.healun.2022.04.010 Online ahead of printv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barnard CN. The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. S Afr Med J. 1967;41:1271–1274. [PubMed] [Google Scholar]