It is hoped that the critical shortage of deceased human donor organs for clinical transplantation can be resolved by the transplantation of organs from pigs genetically engineered to protect their cells from the human immune response. There are compelling reasons why pig kidney transplantation might prove preferable in a first clinical trial.1 Although considerable progress has been made, it has been insufficient to date to warrant a formal clinical trial. However, encouraging progress has been made by (1) the availability of pigs with multiple genetic modifications, including deletion of the 3 identified carbohydrate xenoantigens (triple-knockout [TKO] pigs) (Table 1),2,3 and (2) the increasing availability of agents that block the CD40/CD154 costimulation pathway.4-6
TABLE 1.
Known carbohydrate xenoantigens expressed on pig cells
| Carbohydrate | Responsible enzyme | Gene-knockout pig |
|---|---|---|
| Gal | α-1,3-galactosyltransferase | GTKO |
| Neu5Gc | CMAH | CMAHKO |
| Sda | β-1,4 N-acetylgalactosaminyl transferase | β 4GalNT2KO |
CMAH, cytidine monophosphate N-acetylneuraminic acid hydroxylase; Gal, galactose-α1, 3-galactose; Neu5Gc, N-glycolylneuraminic acid.
The pig-to–nonhuman primate (NHP) model has proved indispensable in contributing to our progress during the past 35 y. As just a few examples, the model has enabled us to demonstrate that (1) a pig organ expressing 1 or more human complement-regulatory proteins survives longer than a wild-type (WT; ie, genetically unmodified) organ7; (2) an organ from a pig that does not express the important xenoantigen, Gal (a GTKO pig), survives longer than a WT organ8; (3) conventional immunosuppressive therapy is inefficient in suppressing the adaptive immune response,4,9 whereas (4) blockade of the CD40/CD154 costimulation pathway is much more successful,4,6,9 but blockade of the CD28/B7 pathway is less effective10,11; (5) an anti-CD154 agent may be preferable to an anti-CD40 agent12,13; (6) there is a prolonged systemic inflammatory response to a pig xenograft14; and (7) the results of pig organ transplantation are improved when the NHP recipient has low anti-pig antibody levels.15 It is also becoming clear that significant proteinuria occurs when rejection is developing but not when a rise in serum creatinine is related to dehydration.16 All of these important pieces of information, and several others, would have proved difficult or impossible to obtain if this animal model had not been developed.
With regard to determining the safety of pig organ transplantation relating to “infection,” the model has not proved as useful as might be hoped. For example, there has been no evidence of transmission of porcine endogenous retroviruses in an in vivo model in NHPs with pig organ grafts, and this may in part be associated with difficulties in transmitting the virus to NHPs.17 The potential risk of porcine endogenous retroviruses will, therefore, be unknown until clinical trials are initiated.18
However, we now face an experimental barrier that is proving very difficult to overcome (and, importantly, will not be seen in clinical pig organ xenotransplantation), and that is the NHP antibody response to what has been called the “fourth xenoantigen” (that seems to be exposed after deletion of expression of N-glycolylneuraminic acid [Neu5Gc] in the organ-source pig).2,19 Human antibody binding and serum cytotoxicity to cells from TKO pigs are often minimal or absent but, unfortunately, all Old World NHPs have antibodies against TKO pig cells, and these are associated with a very high level of serum cytotoxicity (Figure 1). This hurdle has proved very difficult to overcome, and the relevance of the pig-to-NHP model as a surrogate for future studies is now being questioned.
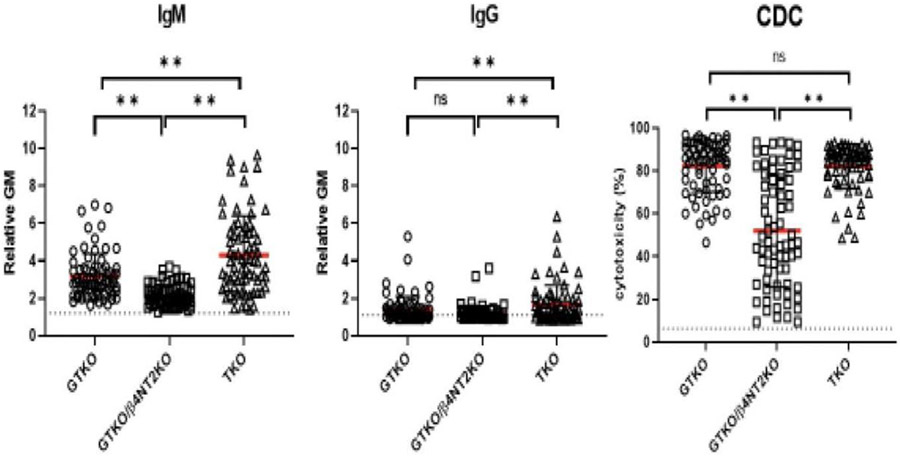
FIGURE 1.
IgM (left) and IgG (middle) antibody binding and CDC (right) of baboon sera to GTKO, GTKO/β-4GalNT2KO (DKO), and TKO pig PBMCs. IgM and IgG binding and serum cytotoxicity to TKO cells were higher or comparable with binding to GTKO cells. Although mean IgM and IgG binding and mean serum cytotoxicity to DKO cells were less than to TKO cells, many baboons had a high level of cytotoxicity to DKO cells (**P < 0.01). (Reproduced with permission from Yamamoto et al.21) CDC, complement-dependent cytotoxicity; IgG, immunoglobulin G; IgM, immunoglobulin M; PBMC, peripheral blood mononuclear cell; TKO, triple knockout.
Discussion continues on whether the additional expression in the TKO pig kidney of human “protective” proteins, for example, complement- or coagulation-regulatory or “anti-inflammatory” proteins, provides an increased likelihood of long-term graft survival. Although, under normal circumstances, there may be no human antibody binding to a TKO pig organ, additional transgenes would provide protection against immune activation associated with such conditions as ischemia-reperfusion injury or systemic infection.3,20
From the pioneering studies of the late David White and his colleagues in the early days of xenotransplantation research, it should be remembered that expression of a single human complement-regulatory protein, CD55 (DAF), in an otherwise WT pig organ provided considerable protection from early graft rejection and significantly extended graft survival.7 There is some more recent in vitro evidence that supports the benefit of expression of a human complement-regulatory or anti-inflammatory proteins in TKO cells,21 but this has not been conclusively confirmed by in vivo experiments. Although there are encouraging signs that this barrier can be overcome in some NHP recipients, particularly those receiving an anti-CD154 agent,6 consistent prolonged graft survival may remain difficult to achieve.
For many years, some of those in xenotransplantation research have given thought to identifying the carbohydrate structure of the “fourth xenoantigen” and then deleting it in the pig.22 This is clearly a scientific approach but would be time-consuming and may still not provide a solution to the problem. If a new xenoantigen is identified and then deleted, will yet another unknown xenoantigen be exposed? Furthermore, even when expression of Neu5Gc is not eliminated, but is retained, many baboons have high levels of serum cytotoxicity to the resulting GTKO/β4GalNT2KO pig cells (Figure 1), suggesting that there may be other factors involved rather than just absence of Neu5Gc expression. Even if the effort to identify and delete new xenoantigens solves the problem in pig-to-NHP organ transplantation, it will not directly impact clinical xenotransplantation.
The transplantation of pig kidneys in which expression of only the major xenoantigen, Gal, has been deleted (GTKO pigs; Table 1) has been associated with more consistently good results in NHPs than when TKO organs are transplanted (Figure 2).23 Some NHPs have low antibody binding to GTKO pig cells, although they may still have high serum cytotoxicity to these cells in vitro (Figure 1). Nevertheless, the results of GTKO pig-to-NHP kidney transplantation to some extent suggest that they can be considered comparable with TKO pig kidney transplants in humans. However, it is generally understood that the regulatory authorities would like the same source pig to be used in NHP studies as proposed for clinical trials. To my knowledge, however, the regulatory authorities have not been requested to come to a definitive decision on this important point. If the results of studies of GTKO pig kidney transplantation prove acceptable as a predictor of clinical trials of TKO pig kidneys, this would greatly facilitate progress toward the clinic. Nevertheless, it should be noted that the TKO barrier has been overcome in a small number of kidney transplants in NHPs6 (and in some heart transplants in NHPs, where the barrier seems possibly to be less restrictive.)
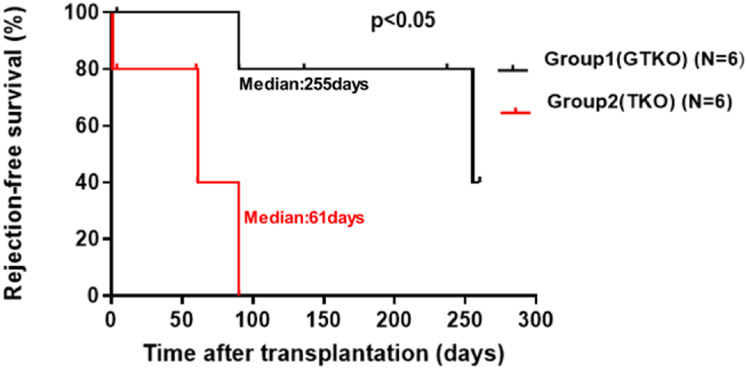
FIGURE 2.
The transplantation of kidneys from GTKO pigs (with added human transgenes) (group 1) in immunosuppressed baboons was significantly longer than that of TKO pig kidneys (with added human transgenes) (group 2). (Reproduced with permission from Iwase et al.23) TKO, triple knockout.
There are those who have suggested that the transplantation of genetically engineered pig kidneys into brain-dead human subjects might obviate the problem of the fourth xenoantigen (New York Times 10-20-21) and therefore negate the need for studies in NHPs.24 However, it is difficult to maintain a brain-dead subject in a hemodynamically and biochemically stable state for more than a few days, and so the information obtained would probably not add to that which we can confidently predict from the numerous in vitro studies that have been accumulated. Furthermore, brain death is associated with a systemic inflammatory response,25 as is xenotransplantation,14 which may be detrimental to xenograft survival. Therefore, successful function of the pig kidney xenograft may teach us little or nothing, but failure of the graft may result from reasons that will not be seen when the recipient is a living patient. Dysfunction or failure of the graft will need to be explained to the regulatory authorities, and so the study may be counterproductive to progress.
We are at a stage when we will learn much more and make more rapid progress if we initiate limited, carefully planned clinical trials26 rather than continue our studies in a laboratory model that clearly does not mimic the situation in humans (as evidenced by the wealth of in vitro data we have accumulated for several years).
If we wish to provide an alternative therapy to the thousands of patients on the waitlists for organ transplantation worldwide, now is surely the time to consider a different approach. Should we consider initiating a clinical trial involving a small number of patients (eg, 4, as suggested by Jagdale et al26)? Such a trial would provide us with data of immense importance and would advance our knowledge of xenotransplantation much more than if we persist with a model that has lost much of its relevance to the treatment of human patients.
If the kidney graft fails or an infection develops that cannot be readily controlled, the option of removing the kidney graft, discontinuing all immunosuppressive therapy, and returning to dialysis remains. (In my experience, most recipient NHPs that were necessarily euthanized when the pig kidney graft failed—because both native kidneys had been excised at the time of the transplant—could have been recovered, after excision of the rejected pig kidney, if dialysis had been available to sustain the recipient.) Furthermore, even if the patient becomes sensitized to pig antigens, the current evidence is that this would not be detrimental to subsequent kidney allotransplantation if a suitable kidney became available.27,28
Acknowledgments
Work in the author’s laboratory is funded by National Institutes of Health National Institute of Allergy and Infectious Diseases U19 grant AI090959 and Department of Defense grant WB1XWH-20-1-0559.
Footnotes
The opinions expressed in this article are his own and do not necessarily reflect those of eGenesis.
REFERENCES
- 1.Cooper DKC, Cleveland DC. The first clinical trial—kidney or heart? Xenotransplantation. 2021;28:e12644. [DOI] [PubMed] [Google Scholar]
- 2.Estrada JL, Martens G, Li P et al. Evaluation of human and nonhuman primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical organ xenotransplantation. Xenotransplantation. 2019;26:e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bühler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. [DOI] [PubMed] [Google Scholar]
- 5.Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO. hCD46Tg pig-to-baboon model. Xenotransplantation. 2014;21:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma D, Hirose T, Lassiter G, et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant. 2022;22:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzi E, Bhatti F, Schmoeckel M, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000;70:15–21. [PubMed] [Google Scholar]
- 8.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019;103:2090–2104. [DOI] [PubMed] [Google Scholar]
- 10.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heterotopic heart transplantation—exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ezzelarab MB, Ekser B, Isse K, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation. 2014;97:502–508. [DOI] [PubMed] [Google Scholar]
- 12.Shin JS, Kim JM, Kim JS, et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15:2837–2850. [DOI] [PubMed] [Google Scholar]
- 13.Shin JS, Kim JM, Min BH, et al. Pre-clinical results in pig-to-nonhuman primate islet xenotransplantation using anti-CD40 antibody (2C10R4)-based immunosuppression. Xenotransplantation. 2018;25:e12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwase H, Yamamoto T, Cooper DKC. Episodes of hypovolemia/dehydration in baboons with pig kidney transplants: a new syndrome of clinical importance? Xenotransplantation. 2019;26:e12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denner J. Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology. 2018;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishman JA. Prevention of infection in xenotransplantation: designated pathogen-free swine in the safety equation. Xenotransplantation. 2020;27:e12595. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Iwase H, Patel D, et al. Old World monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (triple-knockout). Sci Rep. 2020;10:9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto T, Hara H, Ayares D, et al. The problem of the ‘4th xenoantigen’ after pig organ transplantation in nonhuman primates may be overcome by expression of human ‘protective’ proteins. Xenotransplantation. 2020;28:e12658. [DOI] [PubMed] [Google Scholar]
- 22.Tector AJ, Adams AB, Lovasik B, et al. Anti-C5 antibody tesidolumab reduces early antibody-mediated rejection and prolongs survival in renal xenotransplantation. Ann Surg. 2021;274:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase H, Jagdale A, Yamamoto T, et al. Evidence suggesting that deletion of expression of N-glycolylneuraminic acid (Neu5Gc) in the organ-source pig is associated with increased antibody-mediated rejection of kidney transplants in baboons. Xenotransplantation. 2021;28:e12700. [DOI] [PubMed] [Google Scholar]
- 24.Cooper DKC. Genetically-engineered pig kidney transplantation in a brain-dead human subject. Xenotransplantation. 2021;28:e12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barklin A, Hvas CL, Toennesen E. The inflammatory response to brain death. In: The Brain-Dead Organ Donor: Pathophysiology and Management. Novitzky D, Cooper DKC eds. Springer; 2013:107–119. [Google Scholar]
- 26.Jagdale A, Kumar V, Anderson DJ, et al. Suggested patient selection criteria for initial clinical trials of pig kidney xenotransplantation in the United States. Transplantation. 2021;105:1904–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Hara H, Zhang Z, et al. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation. 2018;25:e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara H, Nguyen H, Wang ZY et al. Evidence that sensitization to triple-knockout pig cells will not be detrimental to subsequent allotransplantation. Xenotransplantation. 2021;28:e12701. [DOI] [PubMed] [Google Scholar]