Abstract
There is a continuing need for donor hearts for infants with complex congenital heart defects. The transplantation of hearts from neonatal pigs would be an alternative to human organs, particularly if donor-specific immunological tolerance could be achieved. The great majority of infant humans do not make natural (preformed) antibodies against triple-knockout (TKO) pigs (that do not express any of the three known pig antigens against which humans have natural anti-pig antibodies). The transplantation of a heart from a TKO pig into an infant would therefore minimize any risk of early antibody-mediated rejection, and, with adequate immunosuppressive therapy, prolonged graft survival may well be achieved. Total host thymectomy (commonly carried out at the time of orthotopic heart transplantation in this age group) ± residual T-cell depletion and donor-specific pig thymus tissue transplantation might induce T-cell tolerance and allow immunosuppressive therapy to be discontinued (if there is in vitro evidence of T-cell and B-cell nonresponsiveness to donor-specific pig cells). Even if tolerance were not achieved, with continuing immunosuppressive therapy, the graft would likely “bridge” the patient until a suitable allograft became available or be associated with prolonged xenograft function.
Keywords: heart, infant, neonate, pig, tolerance, xenotransplantation
1 |. INTRODUCTION
A considerable experimental effort has been made to resolve the problem of the shortage of organs from human donors for clinical transplantation by the transplantation of organs from genetically engineered pigs into nonhuman primates (NHPs).1 Survival of genetically engineered pig heterotopic (non-life-supporting) hearts and life-supporting kidneys is now being measured in months or even years.2–4 These encouraging results have been obtained largely by two key genetic approaches–(a) deletion of pig antigens against which humans (and NHPs) have natural (preformed) antibodies5,6 and (b) the introduction into the pig of transgenes for human complement- and/or coagulation-regulatory proteins.1
There is a particular lack of deceased human donor hearts for transplantation in neonates and infants with life-threatening, complex congenital cardiac defects, for example, hypoplastic left heart syndrome, or congenital cardiomyopathy. Conventional management of these patients is less than ideal, and the outcome is certainly not as good as after heart allotransplantation.7 Furthermore, in this age group, mechanical assist devices are less than optimal.
With regard to heart transplantation, at Loma Linda University Children’s Hospital, heart transplants in neonates are associated with an almost 60% 25-years’ survival,7 and more recent data indicate patient survival at 1, 5, 10, and 15 years is currently 88%, 81%, 75%, and 69%, respectively (Bailey LL, and Fitts J, personal communication). Everitt et al8 reported 1, 5, and 10 years’ survivals of 79%, 77%, and 65% for unpalliated hypoplastic left heart syndrome, and the ISHLT reported a median survival of 22.3 years for all infants who underwent heart transplantation.9 Early mortality is probably associated with deterioration in the patient’s health status during the wait for a human donor heart (which is a mean of >2 months, but can extend to >1 year10). (This would be resolved if a heart was available immediately after birth.)
The difficulty in obtaining suitable donor hearts has resulted in a preference for staged palliation as primary therapy,11 which usually involves three operations during the first 3-6 years of life. The results remain suboptimal, with a 60%-70% 5-year survival, even though some patients may not have completed the course of palliative procedures. Even when staged palliation is complete, the quality of life of the patient may remain impaired.12 As the patient is left with a systemic right ventricle, heart transplantation may still be required, which may be complicated because some patients become allosensitized.13 At this stage, heart transplantation is associated with relatively poor 1-year survival.14 There is, therefore, a cumulative mortality and morbidity after each procedure.
The ready availability of a pig heart may allow the transplant to be carried out immediately after birth, thus avoiding any deterioration in health. The diagnosis of a complex cardiac abnormality can often be made during fetal life, and so the heart transplant can be planned in advance. Although we do not believe that the induction of tolerance is essential for the success of a cardiac xenograft, it would certainly be an advantage, particularly in infants and children in whom the graft would ideally need to function throughout life.
The concept of neonatal tolerance (in which the recipient’s immune system makes no effort to reject the graft) is not new, but the ability to achieve T-cell and B-cell tolerance to either an ABO-incompatible allograft or a genetically engineered pig xenograft has not been fully explored even in a clinically relevant neonatal NHP model. The extensive clinical studies by West and her colleagues have demonstrated that an ABO-incompatible heart graft in infants is associated with the development of B-cell tolerance to the incompatible A or B antigen.15–17 These specific antibodies are either no longer produced by the recipient or a state of “accommodation” is achieved.16,18 It is not known whether T-cell tolerance can also be achieved. The potential to develop donor-specific tolerance during the first few months of life would be of immense importance in heart transplantation in infants.
We suggest that the induction of tolerance should be explored by a combination of (a) genetically engineered pigs as the sources of the organs, (b) novel costimulation blockade-based immunosuppressive therapy, (c) thymectomy, and (d) donor-specific pig thymic transplantation.
2 |. DEVELOPMENT OF THE IMMUNE SYSTEM IN NEONATES
The immune system of neonatal humans and NHPs is “immature” and relatively easily “manipulated,”19 but it is not known whether B-cell and/or T-cell tolerance to a xenograft can be achieved.
Neonatal baboons do not hyperacutely reject pig heart grafts;20–22 this is associated with an absence of preformed “natural” antibodies in baboons (and humans) at that age.21–24 From previous studies by our group, infant baboons and humans begin to develop natural anti-AB blood group and anti-pig antibodies after approximately 3 months of age (Figure 1).24,25 In infant baboons, the mixed lymphocyte reaction (MLR) against baboon peripheral blood mononuclear cells (PBMCs) or pig PBMCs is developed, but relatively weak.25,26 There is, therefore, a “window of opportunity” during which organ transplantation could be carried out in the absence of preformed antibodies and in the presence of a “malleable” T-cell proliferative response.
FIGURE 1.
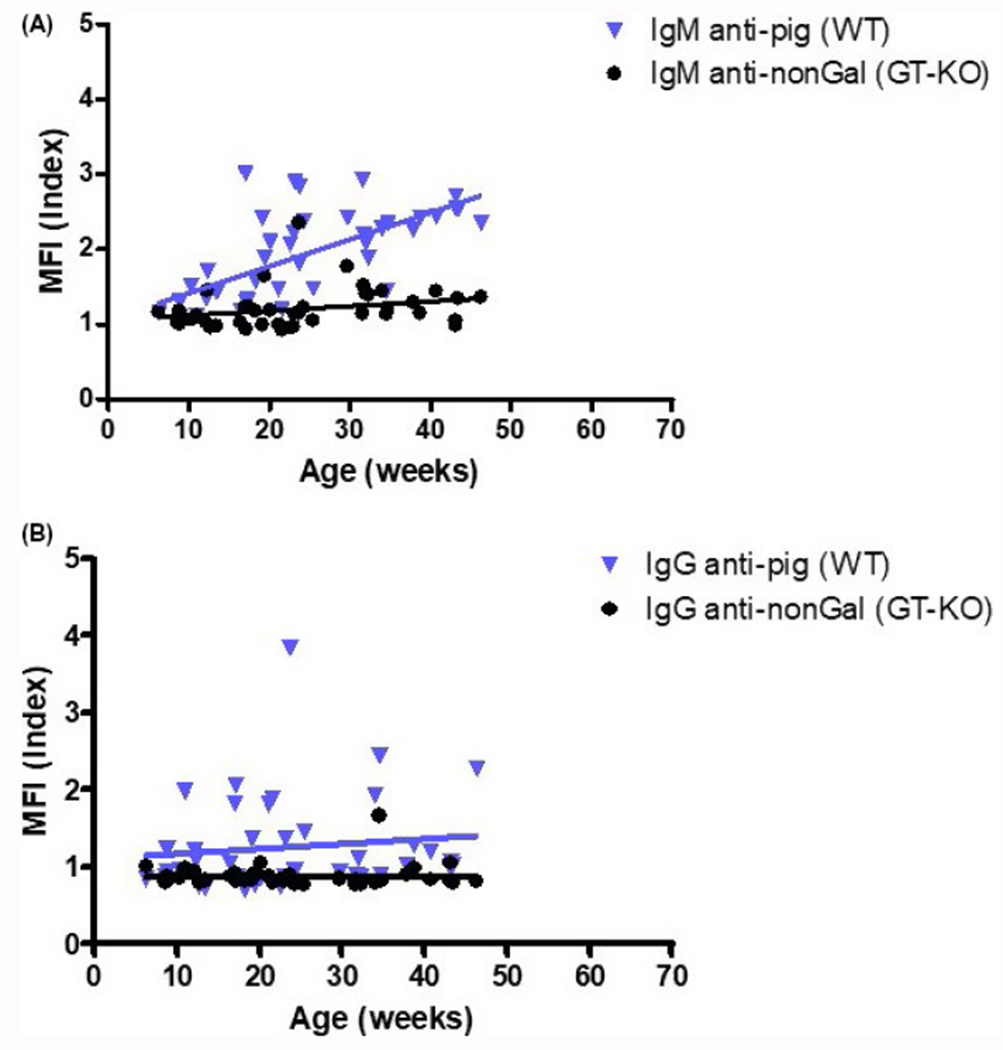
Binding of infant human (n = 42) serum antibodies to wild-type (WT, genetically unmodified) and GT-KO pig peripheral blood mononuclear cells (PBMC) (MFI index = mean fluorescence intensity of the serum sample divided by the MFI of the isotype control sample). A, Distribution of IgM reactivity against WT and GT-KO PBMC. Correlation of MFI index with age of each group is indicated by a line (vs WT, P = 0.073, r = 0.316; vs GT-KO, P = 0.129, r = 0.238). When sera from infants were tested, only 2 of 50 serum samples showed (very low) binding of IgM to TKO pig red blood cells (not shown, Hara, et al manuscript in preparation). B, Distribution of IgG reactivity against WT and GT-KO PBMC. Correlation of MFI index with age of each group is indicated by a line (vs WT, P = 0.381, r = −0.158; vs GT-KO, P = 0.021, r = 0.356). When sera from infants were tested, no IgG binding to TKO cells was detected in any of the 50 samples (not shown, Hara, et al manuscript in preparation). (Reproduced with permission from Rood et al Transplant Int 2007;20:1050-1058.)
However, if an AB-incompatible artery patch allograft or a pig xenograft is transplanted in a baboon at 3 months without immunosuppressive therapy, a donor-specific antibody response develops within 2 weeks.25 In contrast, when the graft is protected by a regimen of T-cell costimulation pathway blockade (anti-CD154 monoclonal antibody [mAb]) plus mycophenolate mofetil (MMF), no antibodies develop to the specific A or B or pig antigens expressed on the graft (or, of course, to self A or B antigens).25 One baboon with an AB-incompatible allograft developed neither anti-AB antibodies nor any increase on MLR during the 4 months’ period of follow-up after discontinuation of costimulation blockade therapy, suggesting that both B-cell and T-cell tolerance might have been induced.
Furthermore, when treatment with anti-CD154mAb alone was initiated at 1 month and continued for 6 months (in the absence of an allograft or xenograft), the baboon did not develop any anti-A or anti-B or anti-pig antibodies until 4 months after discontinuation of therapy, that is, at 11 months of age.
These data strongly suggest that (a) natural antibodies directed to carbohydrate antigens may be at least partly T cell-dependent, and (b) both T-cell and B-cell responses can remain suppressed by costimulation blockade therapy even in the presence of an AB-incompatible allograft or pig xenograft.
Anti-CD154mAbs may not be available for clinical transplantation within the near future in view of their thrombogenic properties, but an anti-CD40mAb may provide an alternative. An anti-CD40mAb (2C10R4) has proven effective in preventing T cell and elicited antibody responses to a genetically engineered pig organ xenograft in adult NHPs for months or even years.2,4,27 We believe that the effect of costimulation blockade on the indirect pathway of T-cell activation is particularly important.
Relatively recently, pigs have been produced that do not express the three known antigens against which humans have natural antibodies, that is, triple-knockout (TKO) pigs.5 These pigs do not express galactose-α1,3-galactose (Gal), N-glycolylneuraminic acid (Neu5Gc), or Sda (a product of β4GalNT2-KO) (Figure 2).5 We have measured antibody binding to TKO pig red blood cells (that do not express swine leukocyte antigens [SLA]) in the sera of 50 human neonates and infants, and determined that there is no IgM or IgG binding in the large majority of cases (48/50) (Hara, et al, unpublished data). As many adults do not have antibodies directed against TKO pig cells, in our study, we did not identify any maternally derived cross-placental IgG in these neonates. Furthermore, almost one-third of adult patients on the wait-list for a kidney allograft have no detectable anti-pig antibodies to TKO pig cells.6
FIGURE 2.
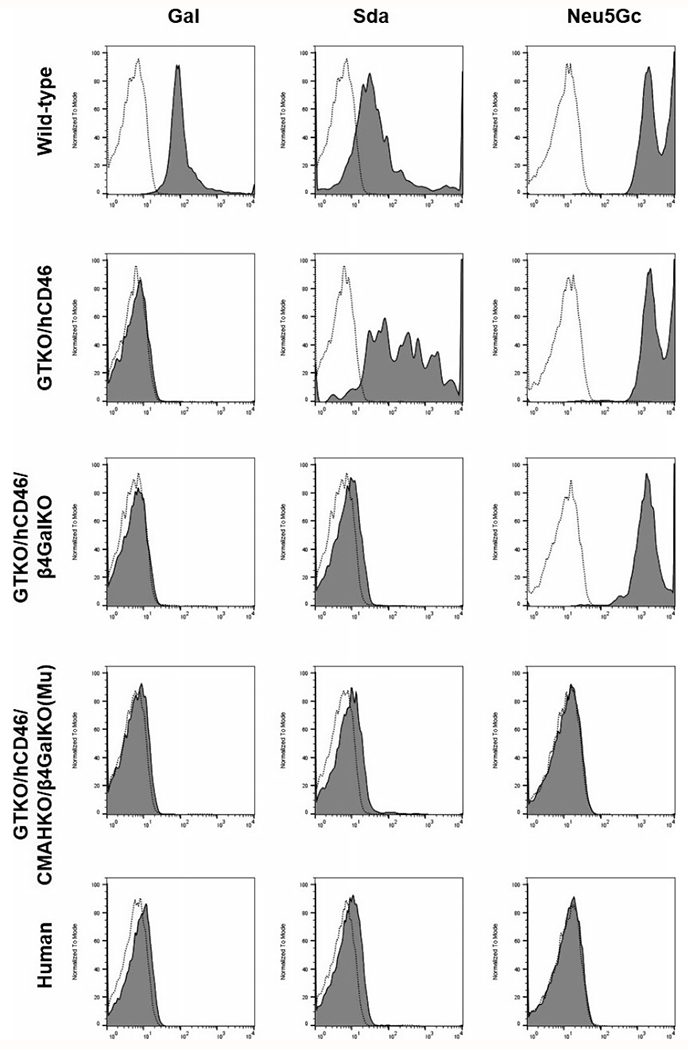
Expression of galactose-α1,3-galactose (Gal), N-glycolylneuraminic acid (Neu5Gc), and Sda (a product of β4GalNT2-KO) on red blood cells from (top to bottom) (a) a wild-type pig, (b) a GTKO/CD46 pig, (c) a GTKO/CD46/β4GalNT2-KO pig, (d) a triple-knockout (TKO) pig (in which all three pig glycan antigens have been deleted), and (e) a healthy human. Staining was for expression of Gal (isolectin BSI-B4), Sda (dolichos biflorus agglutinin, DBA), and Neu5Gc (chicken anti-Neu5Gc mAb). RBCs from GTKO/CD46 pigs expressed Sda and Neu5Gc, but not Gal. Neither Gal nor Sda was expressed on GTKO/CD46/β4GalNT2-KO pig RBCs. None of the 3 glycans was expressed on the TKO pig RBCs or on human RBCs
These data indicate that a TKO pig heart that expresses none of the known pig glycan xenoantigens could be transplanted into a neonatal patient with no natural (preformed) anti-pig antibodies. There would be no need to develop B-cell tolerance to the three known pig antigens (as is necessary when an ABO-incompatible allograft is transplanted) because the antigens are no longer expressed. However, other protein antigens, for example, SLA, will be expressed,6,28,29 and tolerance to these will have to be induced. This may be easier to achieve in neonates if memory cells have not yet developed. (If required, expression of SLA can be reduced by genetic engineering techniques that delete expression of SLA class I30 or reduce expression of SLA class II.31)
The important question, of course, is whether T-cell tolerance could be achieved. In a small number of adult patients with end-stage renal disease undergoing kidney allotransplantation, tolerance has been achieved by intensive pretransplant immunomodulation in combination with donor-specific hematopoietic progenitor cell transplantation, with the development of mixed hematopoietic cell chimerism.32 This therapy would not be successful when pigs are the source of hematopoietic cells33 and in any case would carry a significant risk in neonates and infants with severe cardiac insufficiency.
Based on the clinical studies of Markert and her colleagues, we propose an alternative approach that we believe would be preferable. We suggest that a combination of native thymectomy (which is almost routinely carried out in infants undergoing orthotopic heart transplantation to provide access for the surgical procedure and space for the donor heart) and donor-specific pig thymus transplantation might result in the induction of T-cell tolerance. Whether the thymectomy needs to be total or whether remnants of thymus will be detrimental (or even beneficial) to the development of tolerance is uncertain.
3 |. THYMUS TISSUE TRANSPLANTATION
Postnatal allogeneic HLA-nonmatched cultured thymus tissue allotransplants in patients with complete DiGeorge (immunodeficiency) syndrome have been carried out in children under the age of 2 years (median age 5 months; range 1.1-22.1 months) since 2003.34–36 Cultured postnatal donor-specific thymus tissue slices are transplanted at multiple sites into both quadriceps muscles of the recipient. Thymopoiesis has been observed in biopsies of the thymic tissue within 2 months, and naïve T cells have been monitored in the blood within 3-5 months.34,35 Importantly, parental parathyroid transplantation was successful when combined with third-party allogeneic thymus transplantation.36 Matching of the allogeneic thymus graft to the parathyroid donor HLA class II alleles that were unshared with the recipient appeared to be associated with the induction of tolerance toward the parathyroid graft.36
Neonates with complex congenital heart disease, but intact immune systems, are not comparable to patients with complete DiGeorge syndrome, as they have developed T cells during fetal life. After thymectomy (performed during the neonatal period), they become comparable to infants with incomplete DiGeorge syndrome, that is, they have some mature T cells. Nevertheless, we suggest that therapeutic depletion of existing T cells (eg, by antithymocyte globulin), followed by maintenance therapy with an anti-CD40mAb (which we have demonstrated in juvenile baboons is associated with very little recovery in the number of T cells4), may well induce a state of tolerance.
After depletion of T cells, the transplantation of pig thymus tissue (at the time of pig heart transplantation) may allow negative selection (and depletion) of developing antidonor T lymphocytes to enable T (and B)-cell tolerance to be achieved on a consistent basis. The pig thymus tissue would “reprogram” the recipient’s immune system to accept the pig graft as “self.”37 The thymus graft would be from the same pig source as the heart graft, that is, it would be donor-specific. The mechanisms by which thymus transplantation might induce a state of tolerance have been explored by Sykes and her colleagues in mouse and pig models.38–41
Thymus transplantation has been investigated as a means of inducing tolerance to an organ allograft or xenograft in adult animals. Work in pigs and NHPs (which is of most relevance to the present approach) has largely been by Yamada, et al.37,42,43 All of their studies have been carried out in juvenile or adult thymectomized pigs or baboons. Using various models of thymic tissue transplantation (eg, vascularized thymic lobes, composite thymokidneys, thymic implants into the omentum), tolerance was achieved to pig organ allografts, but not to pig organ xenografts in baboons, where anti-Gal antibody-mediated rejection developed despite some evidence of an effect on the T-cell response.37
After the transplantation into baboons of pig composite “thymokidneys” (ie, kidneys under the capsule of which autologous pig thymic tissue had been placed), Yamada’s group were able to demonstrate early baboon thymopoiesis in the porcine thymic tissue of these grafts.44 This was associated with in vitro evidence of donor-specific T-cell unresponsiveness, but unfortunately the baboons did not survive long enough to determine whether T-cell tolerance had been achieved.
Of relevance to the present suggested approach, Yamada’s studies demonstrated that corticosteroid therapy and old age of the recipient were detrimental to the induction of tolerance. (In the proposed approach, the recipient would obviously be young and corticosteroid therapy could be avoided.) The importance of the presence of viable donor thymic epithelium and the role of the thymus during the induction phase of tolerance, particularly during the first 1-2 weeks, were emphasized.
There are, however, important differences between Yamada’s studies and the present proposed approach, some of which have been mentioned above. (a) The recipients would be thymectomized neonates rather than thymectomized adults. (b) The thymic transplants would be carried out in the absence of any antibody to pig xenoantigens and at a time when recipient T-cell numbers and function are minimal. (c) The TKO pig donors will not express the three known carbohydrate antigens against which humans have natural antibodies and can develop elicited antibodies.5 (d) The immunosuppressive regimen has been fully proven to inhibit both antibody-mediated and cellular rejection in several xenotransplantation models.
The additional expression of one or more human complement-regulatory proteins, for example, CD46, CD55, CD59, and one or more human coagulation-regulatory proteins, for example, thrombomodulin, endothelial protein C receptor, would enhance xenograft survival by providing increased protection against the primate immune/inflammatory response.1 For example, a human complement-regulatory protein would protect against alternative pathway complement activation during ischemia-reperfusion injury or infection, whereas a human coagulation-regulatory protein would protect against the coagulation dysfunction seen between pigs and primates.45,46
4 |. IMMUNOSUPPRESSIVE AND ADJUNCTIVE THERAPY
For some years, the standard regimen in the pig-to-NHP models was based on anti-CD154mAb but, because of its thrombogenic properties, alternative costimulation blockade agents have been investigated.2,4,27 Anti-CD40mAb-based regimens have successfully prevented T cell–dependent immunity, that is, prevention of (a) an increase in the cellular proliferative response on mixed lymphocyte reaction (MLR),47 (b) a detectable elicited antibody response, and (c) T-cell infiltration of the graft. These regimens have been combined with induction T-cell depletion with antithymocyte globulin (ATG) and B-cell depletion with an anti-CD20mAb (rituximab).4 For maintenance therapy, the combination of costimulation blockade with either mycophenolate mofetil or rapamycin has been found effective with or without concomitant corticosteroid therapy.48
Using an anti-CD154mAb- or anti-CD40mAb-based regimen, features of thrombotic microangiopathy were documented in pig heart and kidney grafts in NHPs, and consumptive coagulopathy was problematic, but these complications have subsequently been eliminated by the transplantation of grafts from pigs expressing one or more human coagulation-regulatory proteins.2,4 It is likely that these complications develop as a result of activation of the vascular endothelium of the graft by natural (preformed) anti-pig antibody ± complement ± innate immune cells,49 combined with molecular incompatibilities in the coagulation systems between pigs and primates. We suggest that thrombotic microangiopathy and consumptive coagulopathy are much less likely to develop early after TKO pig organ transplantation in infants (if de novo antibody production is prevented), as there should be no binding of natural anticarbohydrate antibodies to the vascular endothelium of a TKO pig heart.
As mentioned above, other genetic modifications, for example, pigs expressing CTLA4-Ig,50 SLA Class I-KO pigs,30 or pigs with the MHC Class II mutation (CIITA-knockdown),31 that provide some protection from the T-cell response, could also be considered if the T-cell response proves stronger than anticipated (though reduced expression of SLA Class II might inhibit the induction of tolerance).
We have identified a substantial role for inflammation in the pig-to-NHP model, as evidenced, for example, by a sustained increase in C-reactive protein.51 The inflammatory response may be detrimental to the induction of tolerance52 and therefore needs to be prevented or minimized, either by drug therapy (eg, IL6-R blockade) or by further genetic engineering of the organ-source pig (eg, by expression of A20 or hemeoxygenase-1).53 We and others have hypothesized that the inflammatory and immune responses and coagulation disturbances are mutually potentiating each other.53
5 |. MONITORING FOR THE INDUCTION OF TOLERANCE
To determine whether donor-specific T-cell and B-cell tolerance has developed, immune monitoring would include measurement of anti-pig IgM and IgG antibodies by flow cytometry, complement-dependent serum cytotoxicity (using donor-specific pig PBMCs), T-cell and B-cell counts, and determination of T-cell and B-cell phenotypes and regulatory T cells. The ratio of regulatory T cells to memory T cells will help indicate whether tolerance has been achieved. Other assays will include functional assays of regulatory T-cell suppressor activity and CFSE-MLR (direct and indirect).
In vitro evidence of (a) an absence of anti-TKO pig antibodies (yet with, eg, the development of anti-Gal and/or anti-A/B blood group antibodies) and (b) no increase in the T-cell proliferative response to TKO pig cells on MLR (yet with an increase in the proliferative response to allogeneic cells) would strongly suggest that immunologic tolerance has been achieved. This might justify a gradual reduction in the immunosuppressive therapy, during which in vitro monitoring would continue, with eventual discontinuation.
6 |. COMMENT
Cardiac allotransplantation during the first few weeks of life is associated with the best outcome of any age group and offers perhaps the best opportunity of achieving immunological tolerance to the graft. The ability to transplant a genetically engineered pig heart would overcome the substantial barrier of obtaining a heart in a timely manner.
The excellent long-term outcomes of ABO-incompatible heart allotransplantation in this age group (and of pig heart xenotransplantation in older NHP recipients) provide encouragement that, with effective immunosuppressive therapy to prevent the cellular response, a pig graft would sustain life for a clinically relevant prolonged period of time. However, even though the induction of a state of immune tolerance in the recipient should prove easier than in older children and adults, it nevertheless provides significant challenges.
The patient would need to be monitored continuously for signs of infection, autoimmunity (eg, gastrointestinal disturbances, skin rash) and a potentially immunodeficient state (suggested by the immune assays). For example, in our previous study, after receiving immunosuppressive therapy for several months, four infant baboons developed collagenous colitis.54 However, when identified at an early stage, this condition was successfully treated with budesonide, suggesting that the baboons’ immune defensive mechanisms remained intact. Nevertheless, there is a potential heightened risk of infectious (or even neoplastic) complications in infants who have not yet developed memory T cells.
The effects of many aspects of the protocol we outline are uncertain, for example, the roles that might be played by thymectomy and/or thymic transplantation, and considerable experimental data need to be accumulated before a clinical trial should be considered. Furthermore, it is possible that 12 months’ immunosuppressive therapy will prove to be an insufficient period of time to enable tolerance to be achieved. On the basis of the immune assays, consideration would need to be given to prolonging immunosuppressive therapy for a longer period of time. However, even if T-cell tolerance is not achieved, prolonged survival of a genetically engineered pig heart in a recipient receiving immunosuppressive therapy would enable the heart to be employed either (a) as a short-term “bridge” to allotransplantation (probably without risk of allosensitization55) or (b) as long-term destination therapy. (If the initial intention of the pig heart transplant was to use it only as a “bridge,” then there would be no purpose in employing the tolerance-inducing regimen, eg, thymus transplantation, as this would not, of course, induce tolerance to a subsequent allograft. Furthermore, we would not begin to reduce immunosuppressive therapy while awaiting an allograft to become available.)
We do not believe that the field has advanced sufficiently to consider cardiac xenotransplantation today, though, with the current rate of advances in the field, it will not be long before clinical trials of organ xenotransplantation will become ethically justified. We do not necessarily believe that cardiac xenotransplantation in neonates needs to be delayed until (a) cardiac xenotransplantation has been carried out in adults or (b) tolerance has been achieved to AB-incompatible cardiac allografts in neonates.
In conclusion, we believe that the development of tolerance to a genetically engineered pig heart could be achieved in immunosuppressed neonates undergoing heart transplantation within the first few days of life if combined with thymectomy and donor-specific pig thymus tissue transplantation. The likelihood of success is greater in neonates than in any other age group, and a good case can be made that clinical cardiac xenotransplantation should first be carried out in the newborn.
ACKNOWLEDGMENTS
C.A.B. thanks the UAB School of Medicine and the Department of Surgery for funding that enabled him to participate in the preparation of this manuscript. Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959.
Abbreviations:
- mAb
monoclonal antibody
- MLR
mixed lymphocyte reaction
- NHP
non-human primate
- PBMC
peripheral blood mononuclear cells
- TKO
triple-knockout (ie, a pig that does not express the three known antigens against which humans have natural (preformed) antibodies)
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Cooper DKC, Ezzelarab MB, Hara H, et al. The pathobiology of pig-to-primate xenotransplantation: a historical review. Xenotransplantation. 2016;23:83–105. [DOI] [PubMed] [Google Scholar]
- 2.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO. hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun 2016;7:11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higginbotham L, Mathews D, Breeden CA, et al. Pre-transplant antibody screening and anti-cd154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwase H, Hara H, Ezzelarab M, et al. Immunologic and physiologic observations in baboons with life-supporting genetically-engineered pig kidney grafts. Xenotransplantation. 2017; 24:e12293. 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GALNT2 genes. Xenotransplantation. 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens GR, Reyes LM, Butler JR, et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA Class I knockout pigs. Transplantation. 2017;101(4):e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinnock RE, Bailey LL. Heart transplantation for congenital heart disease in the first year of life. Curr Cardiol Rev. 2011;7:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everitt MD, Boyle GJ, Schechtman KB, et al. Early survival after heart transplant in young infants is lowest after failed single-ventricle palliation: a multi- institutional study. J Heart Lung Transplant. 2012;31:509–516. [DOI] [PubMed] [Google Scholar]
- 9.ISHLT Registry slides. J Heart Lung Transplant. 2017;36:1037–1079.28779893 [Google Scholar]
- 10.Almond CS, Gauvreau K, Thiagarajan RR, et al. Impact of ABO-Incompatible listing on wait-list outcomes among infants listed for heart transplantation in the United States. Circulation. 2010;121:1926–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu DT, Lamour JM. Changing indications for pediatric heart transplant. Circulation. 2015;131:91–99. [DOI] [PubMed] [Google Scholar]
- 12.Newburger JW, Sleeper LA, Bellinger DC, et al. Pediatric Heart Network Investigators. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsoufi B, Deshpande S, McCracken C, et al. Results of heart transplantation following failed staged palliation of hypoplastic left heart syndrome and related single ventricle anomalies. Eur J Cardiothorac Surg. 2015;48:792–799. [DOI] [PubMed] [Google Scholar]
- 14.Simpson KE, Pruitt E, Kirklin JK, et al. Fontan patient survival after pediatric heart transplantation has improved in the current era. Ann Thorac Surg. 2017;103:1315–1320. [DOI] [PubMed] [Google Scholar]
- 15.West LJ, Pollock-Barziv SM, Dipchand AI, et al. ABO-incompatible heart transplantation in infants. N Engl J Med. 2001;344:793–800. [DOI] [PubMed] [Google Scholar]
- 16.West LJ. Targeting antibody-mediated rejection in the setting of ABO-incompatible infant heart transplantation: graft accommodation vs B cell tolerance. Curr Drug Targets Cardiovasc Haematol Disord 2005;5:223–232. [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Ang A, Pollock-Barziv SM, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10:1227–1233. [DOI] [PubMed] [Google Scholar]
- 18.Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008;13:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Platt JL, Cascalho M, West L. Lessons from cardiac transplantation in infancy. Pediatr Transplant. 2009;13:814–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplon RJ, Michler RE, Xu H, Kwiatkowski PA, Edwards NM, Platt JL. Absence of hyperacute rejection in newborn pig-to-baboon cardiac xenografts. Transplantation. 1995;59:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Xu H, Edwards NM, Chen JM, Dong X, Michler RE. Natural anti-pig xenoantibody is absent in neonatal human serum. J Heart Lung Transplant. 1995;14:749–754. [PubMed] [Google Scholar]
- 22.Minanov OP, Itescu S, Neethling FA, et al. Anti-Gal IgG antibodies in sera of newborn humans and baboons and its significance in pig xenotransplantation. Transplantation. 1997;63:182–186. [DOI] [PubMed] [Google Scholar]
- 23.Neethling FA, Cooper DKC, Xu H, Michler RE. Newborn baboon serum anti-αgalactosyl antibody levels and cytotoxicity to cultured pig kidney (PK15) cells. Transplantation. 1995;60:520–521. [DOI] [PubMed] [Google Scholar]
- 24.Rood PP, Tai HC, Hara H, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20:1050–1058. [DOI] [PubMed] [Google Scholar]
- 25.Dons EM, Montoya C, Long C, et al. T cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation. 2012;93:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan H, Gazarian A, Dubernard JM, Belot A, Michallet MC, Michallet M. Transplant tolerance induction in newborn infants: mechanisms, advantages, and potential strategies. Front Immunol. 2016;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladowski JM, Martens GR, Reyes LM, et al. Examining the biosynthesis and xenoantigenicity of class II swine leukocyte antigen protein. J Immunol. 2018;200(8):2957–2964. pII: ji1800022. 10.4049/jimmunol.1800022. (Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladowski JM, Reyes LM, Martens GR, et al. Swine leukocyte antigen class II is a xenoantigen. Transplantation. 2018;102:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reyes LM, Estrada JL, Wang ZY, et al. Creating class I MHC-null pigs using guide RNA and the Cas9 endonuclease. J Immunol. 2014;193:5751–5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara H, Witt W, Crossley T, et al. Human dominant-negative class II transactivator transgenic pigs – effect on the human anti-pig T cell immune response and immune status. Immunology. 2013;140:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawai T, Sachs DH, Sprangers B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng YL, Sachs DH, Cooper DKC. Porcine hematopoietic progenitor cell transplantation in nonhuman primates: a review of progress. Transplantation. 2005;79:1–9. [DOI] [PubMed] [Google Scholar]
- 34.Markert ML, Sarzotti M, Ozaki DA, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003;102:1121–1130. [DOI] [PubMed] [Google Scholar]
- 35.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinn IK, Olson JA, Skinner MA, et al. Mechanisms of tolerance to parental parathyroid tissue when combined with human allogeneic thymus transplantation. Allergy Clin Immunol. 2010;126:814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada K, Scalea J. Thymic transplantation in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol. 2012;885:191–212. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y, Sergio JI, Swenson K, et al. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol. 1997;159:2100–2107. [PubMed] [Google Scholar]
- 39.Zhao Y, Swenson K, Sergio JI, Sykes M. Pig MHC mediates positive selection of mouse CD4 + T cells with a mouse MHC-restricted TCR in pig thymus grafts. J Immunol. 1998;161:1320–1326. [PubMed] [Google Scholar]
- 40.Nikolic B, Gardner JP, Scadden DT, et al. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 41.Kalscheuer H, Onoe T, Dahmani A, et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol. 2014;192:3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vagefi PA, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature Swine: IV. The thymus is required during the induction phase, but not the maintenance phase, of renal allograft tolerance. Transplantation. 2004;77:979–985. [DOI] [PubMed] [Google Scholar]
- 43.Nobori S, Shimizu A, Okumi M, et al. Thymic rejuvenation and the induction of tolerance by adult thymic grafts. Proc Natl Acad Sci U S A. 2006;103:19081–19086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griesemer AD, Hirakata A, Shimizu A, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowan PJ, Robson SC, d’Apice AJ. Controlling coagulation dysregulation in xenotransplantation. Curr Opin Organ Transplant. 2011;16:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper DKC, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically-engineered pigs in xenotransplantation research. J Pathol. 2016;2016(238):288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee W, Hara H, Ezzelarab MB, et al. Initial in vitro studies on tissues and cells from GTKO/hCD46/NeuGcKO pigs. Xenotransplantation. 2016;23:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3- galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. [DOI] [PubMed] [Google Scholar]
- 49.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Phelps CJ, Ball SF, Vaught TD, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. [DOI] [PubMed] [Google Scholar]
- 51.Iwase H, Ekser B, Zhou H, et al. Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplantation. 2015;22:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chong AS, Alegre ML. Transplantation tolerance and its outcome during infections and inflammation. Immunol Rev. 2014;258:80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ezzelarab MB, Cooper DKC. Systemic inflammation in xenograft recipients (SIXR): a new paradigm in pig-to-primate xenotransplantation? Int J Surg. 2015;23(Pt B):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dons E, Echeverri GJ, Klein E, et al. Collagenous colitis-like condition in immunosuppressed infant baboons. Inflamm Bowel Dis. 2012;18:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q, Hara H, Breimer ME, Wang Y, Cooper DKC. Is sensitization to pig antigens detrimental to subsequent allotransplantation? Xenotransplantation. 2018;25(3):e12393. [DOI] [PMC free article] [PubMed] [Google Scholar]


