Abstract
In March 2020 the South African COVID-19 Modelling Consortium was formed to support government planning for COVID-19 cases and related healthcare. Models were developed jointly by local disease modelling groups to estimate cases, resource needs and deaths due to COVID-19. The National COVID-19 Epi Model (NCEM) while initially developed as a deterministic compartmental model of SARS-Cov-2 transmission in the nine provinces of South Africa, was adapted several times over the course of the first wave of infection in response to emerging local data and changing needs of government. By the end of the first wave, the NCEM had developed into a stochastic, spatially-explicit compartmental transmission model to estimate the total and reported incidence of COVID-19 across the 52 districts of South Africa. The model adopted a generalised Susceptible-Exposed-Infectious-Removed structure that accounted for the clinical profile of SARS-COV-2 (asymptomatic, mild, severe and critical cases) and avenues of treatment access (outpatient, and hospitalisation in non-ICU and ICU wards). Between end-March and early September 2020, the model was updated 11 times with four key releases to generate new sets of projections and scenario analyses to be shared with planners in the national and provincial Departments of Health, the National Treasury and other partners. Updates to model structure included finer spatial granularity, limited access to treatment, and the inclusion of behavioural heterogeneity in relation to the adoption of Public Health and Social Measures. These updates were made in response to local data and knowledge and the changing needs of the planners. The NCEM attempted to incorporate a high level of local data to contextualise the model appropriately to address South Africa’s population and health system characteristics that played a vital role in producing and updating estimates of resource needs, demonstrating the importance of harnessing and developing local modelling capacity.
Background
On 31 December 2019, the World Health Organization (WHO) reported a cluster of pneumonia cases in Wuhan City, China known as COVID-19, the infectious disease caused by ‘Severe Acute Respiratory Syndrome Coronavirus 2’ (SARS-CoV-2) [1]. By November 2020, 216 countries and territories had reported over 47,000,000 cases and 1,200,000 deaths [2]. At the time in South Africa, at the end of the first wave of infection, COVID-19 cases were detected in all nine provinces reaching over 725,000 reported cases in the public and private sectors combined [3].
Aimed at understanding the spread of the disease and the impacts of different interventions, a number of mathematical models of the burden and cost of the COVID-19 epidemic have been developed over the past two years across low- and middle-income countries (LMIC). Mathematical models provide a valuable framework for analysing the transmission and impact of infectious diseases. The application of disease transmission models and costing tools provide a platform to turn the surveillance data collected by national control programmes into strategic information to support policy makers in programme and funding decisions.
In South Africa, the South African COVID-19 Modelling Consortium (SACMC), convened by the National Institute for Communicable Diseases (NICD) on behalf of the National Department of Health (NDOH), coordinated mathematical modelling efforts. This group of researchers from academic, non-profit, and government institutions was formed to provide, assess and validate model projections to support planning by the South African government. Two models were developed: the National COVID-19 Epi Model (NCEM) developed jointly by disease modelling groups at the University of Cape Town, Stellenbosch University, and the University of the Witwatersrand, and the National COVID-19 Cost Model (NCCM) developed by the group at the University of the Witwatersrand. These models have been used since the end of March 2020 to project cases, resource needs and deaths and assess the extent to which these were impacted by emerging variants [4–13].
From the start of the pandemic, uncertainty existed in almost all central aspects of SARS-CoV-2, including its prevalence, transmission, the proportion of infected people who remain asymptomatic, the role of seasonality, whether cross-immunity to the virus from other infections exists, and the extent to which immunity to the virus itself persists—as well as the impact of any of the implemented non-pharmaceutical interventions (NPIs) such as social distancing and mask wearing. Since the emergence of variants in December 2020 and introduction of vaccination, additional uncertainty arose with respect to variant characteristics and vaccine effectiveness. Despite this uncertainty, governments around the world have had to draft and update policy in response to the changing global threat.
South Africa is in the unusual position among LMIC [14] in that it has access to existing public health infrastructure such as surveillance networks and data collection systems, as well as local capacity for infectious disease modelling and economic analysis. The COVID-19 pandemic has brought to light the similarities in and differences between country approaches to epidemic decision-making and response. LMIC in particular have had to balance higher baseline disease burden from other causes with the potential for economic contraction and existing demands on the healthcare system. From a modelling perspective, the availability of data, demand for projections and the use of scenarios in determining health policy may vary between LMIC and HIC.
This paper presents the first versions of the NCEM and its features used to estimate cases, deaths and hospitalisations over the first wave of the epidemic in South Africa. A particular focus is placed on demonstrating its rapid evolution in response to local data and knowledge. The NCEM was updated several times over the course of the first wave in South Africa as local data became available and the need for projections at different stages of the treatment pathway and at different spatial scales changed. In this period from March 2020 to October 2020, the primary use of the NCEM was for projection of the timecourse of case numbers and hospitalisations, as well as cumulative indicators such as the number of expected COVID-19-associated deaths. The model was further adapted to incorporate the impact of variants and vaccines in subsequent waves of COVID-19 through a scenario-analysis approach, though the description of these models and their application are beyond the scope of this manuscript.
The first wave of COVID-19 represented a time where modelling was required to support decision-making despite uncertainty in knowledge of the disease and limitations in local data availability. Therefore this manuscript presents the technical modelling framework along with decisions on data and adjustments to the framework, to generate a model used for projection, the consideration of behavioural factors in updating the framework and its application at a district level for the first wave of COVID-19 in South Africa. The modelling process, model evolution and a reflection on modelling during this time and in this context that could highlight best practices and lessons learned to assist LMIC with modelling for pandemic preparedness is presented in Silal, Pulliam et al [15].
Methodology
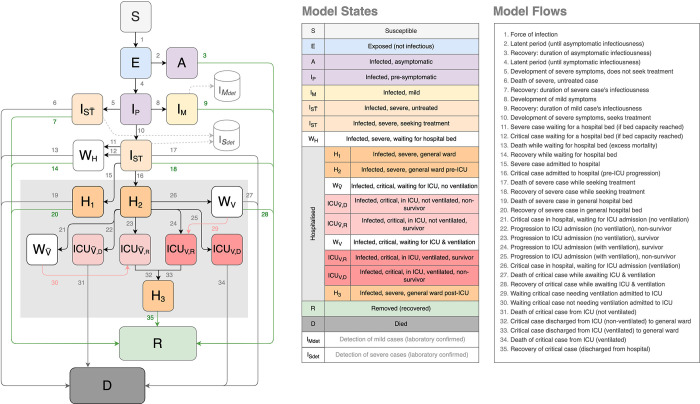
The National COVID-19 Epi Model (NCEM) was initially developed as a deterministic compartmental model of SARS-Cov-2 transmission in the nine provinces of South Africa. Case and death trends, and model performance were observed for a few months at the start of the pandemic. Following this, and in line with requests for decision-making support at a finer spatial granularity, the NCEM underlying structure, assumptions and geographic scope were updated to a stochastic, compartmental, spatially-explicit transmission model to estimate the total and reported incidence of COVID-19 across the 52 districts of South Africa (structural updated model as at 5 September 2020). The updated model followed a generalised Susceptible-Exposed-Infectious-Removed (SEIR) structure that incorporated disease severity (asymptomatic, mild, severe and critical cases) and access to treatment in and out of hospital (outpatients, Intensive Care Unit (ICU) and non-ICU care) (Fig 1).
Fig 1. The National COVID-19 Epi Model structure (updated) [7].
Key features of the updated NCEM include:
Hospital treatment pathway The pathway in hospital was modelled such that patients may be treated in a non-ICU (general medical) or an ICU ward, with or without support by mechanical ventilation in ICU. Informed by admissions data, the pathway was divided into a series of compartments to allow for different times to recovery and death. To incorporate limited capacity, a series of ‘waiting’ compartments was modelled to simulate individuals who, due to capacity constraints, are in need of a hospital or ICU bed but unable to occupy one. As beds become available, patients in these waiting compartments may transition to general hospital/ ICU compartments.
Treatment seeking behaviour and health access These were modelled such that not all severely and critically ill individuals are assumed to access hospital-level care. Individuals in these compartments experience higher mortality rates than those who receive the appropriate level of care (e.g., patients who require but do not receive mechanical ventilation are assumed to have 100% mortality).
Detection Given that many infected individuals are asymptomatic or mildly symptomatic and are not likely to seek out a diagnostic test, and additionally, owing to the severe laboratory capacity constraints in South Africa, not all infected individuals will be diagnosed. Changing priorities in testing of hospitalised patients and non-hospitalised mild cases over the course of the epidemic were captured in the model.
Spatial scale The scale of the model is at the district level (subnational administrative level two), reflecting the population size and connectivity of each of the 52 districts in South Africa. Baseline movement between districts is estimated based on aggregate cell phone mobility data from 1 March 2020. District-to-district connectivity matrices were constructed based on the proportion of mobile phone events that occur in each district outside the home district. The home district is defined as the place where a mobile device is located between 10 pm and 4 am. Separate matrices were constructed for each lockdown restriction to reflect the average levels of movement between districts for each period.
Behavioural heterogeneity The behavioural heterogeneity was incorporated through an adjustment to the force of infection. This captures the phenomenon that differences exist in contact patterns among individuals where some individuals experience different risks and exhibit different behavioural patterns, with highly connected individuals becoming infected earlier in the epidemic and infecting more contacts. The force of infection (transmission function) was altered to decrease as immunity builds up in the most connected individuals early on in the epidemic [16].
The model was developed in R and C++, with the deterministic differential equations solved using the deSolve package in R [17, 18], and stochastic differential equations solved using the diffeqr package in Julia [19]. A parallel implementation is also available in the code.
The update to the NCEM was informed by a scenario analysis that considered alternate behavioural and epidemiological assumptions. The assumptions are described with the analysis presented in detail in the Results section and Supplementary file.
Data
Case data were obtained from NICD’s Notifiable Medical Conditions database and hospital admissions and deaths data were obtained from the NICD’s Data for COVID (DATCOV), the sentinel hospital surveillance database.
While contact rates are known to be affected by social, economic, demographic and other structures, locally representative contact data did not exist at a provincial or district level to incorporate into the model. Further, no representative usage data existed on Non-pharmaceutical interventions (NPI). Therefore in the absence of data, contact rates for the population were based on analyses on the reproductive number over time [20, 21] and Google mobility data from Google COVID-19 Community Mobility Reports [22]. At the district level, population data for 2020 were obtained from Statistics South Africa [23] while spatial influence was estimated from reflect time-dependent mobility between districts. Mobility between districts over time was estimated from mobile phone event data from Vodacom South Africa. Here daily event data (excluding static devices) with district source and destination information were normalised to establish the proportion of daily events per source in each destination district. This was used to generate average proportion of daily events matrices over the periods of restrictions. The model estimates both the deaths due to COVID-19 in hospital and all COVID-19 deaths, regardless of whether the patient had been hospitalised at the time of death. Total deaths due to COVID-19 were estimated from the South African Medical Research Council’s Weekly Excess Deaths reports [24, 25]. As the spatiotemporal patterns of excess deaths, confirmed COVID-19 case reports and officially reported COVID-19 deaths suggest that the majority of estimated excess deaths are due to COVID-19, total projected deaths due to COVID-19 in the model were calibrated to 80% of estimated excess mortality by province. This is in line with the South African Medical Research Council’s estimate of excess deaths attributable to COVID-19 [26].
Disaster-related restrictions in South Africa took the form of a series of alert levels that were gradually relaxed over the course of the epidemic [27]. These alert levels, generally implemented uniformly across all districts, enforced social distancing, restricted movement between districts, and reduced contacts among individuals, but also limited trade in different sectors over the course of the epidemic [28]. As the restrictions affected business sectors differentially, and representative local-level data on adherence to and impact of NPIs did not exist at the time, general measures were used to account for the impact of the alert levels on reducing average contacts in the population over time. Changes in average contacts were estimated from the effective reproductive number over time based on analyses by the NICD for early periods of restrictions (alert levels 5 and 4) when the impact of a build-up in population immunity was low [21]. For alert levels 3, 2 and 1 thereafter, changes in contact rates at the provincial level were updated based on reductions in time spent in places of residence as determined by Google Mobility Trend Data [22].
Results
Between March and September 2020 the NCEM was updated 11 times with four key releases. The principal findings of a subset of key releases are presented in this section.
Original model performance
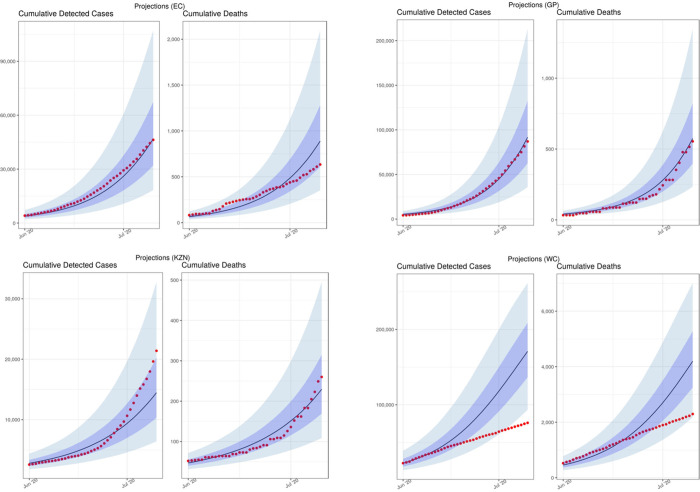
On 12 June 2020, we published a set of short-term projections to estimate cases and deaths for June and July [6]. We first estimated cumulative detected cases and cumulative deaths from 21 March 2020 to 15 July 2020 (Fig 2). These projections show that the original NCEM model closely estimated the reported cumulative detected cases observed for the projection period. It was during this period that daily deaths and admissions began to flatten in the Western Cape province, for reasons that were not well understood at the time. These results were strongly caveated in the original report as not incorporating potentially important elements such as heterogeneity and behavioural response to the epidemic. Given that infectious disease models such as the NCEM are mechanistic models driven by the underlying biology of the virus, population-level behaviour characteristics, and the care pathways, it was not possible to quantitatively predict the deceleration in daily cases and deaths that would result, without prior knowledge of how these factors would play out.
Fig 2. Model performance: Previously projected (12 June) vs observed cumulative detected cases and cumulative deaths (selected provinces: Eastern Cape (EC), Gauteng (GP), KwaZulu-Natal (KZN) and Western Cape (WC)) [median projected = black lines; observed data = red dots].
The deviation of the model from the observed trends in detected cases and reported deaths lead to additional uncertainty and confusion amongst policymakers and the public as to the likely end of the wave and the implication for provinces where cases were still low. This also had a negative impact on the perceived credibility of the model where models tended to be viewed as forecasts, rather than tools generating projections under a set of assumptions. We responded by performing a number of scenario analyses to interrogate the impact that four distinct factors could have had in explaining the difference between the NCEM projections from June and reported case and death data in the Western Cape.
These were:
A lower than assumed population attack rate, possibly due to different levels of susceptibility in different population groups (including children) or the presence of existing T-cell derived-immunity after prior exposure to other coronaviruses. This is modelled by allowing a proportion of individuals to be immune throughout the course of the epidemic.
Behaviour change in response to an increased local death rate. takes into account a potential impact of public awareness of the increasing deaths and the looming threat of overwhelmed healthcare facilities in the Western Cape, which, combined with communication campaigns, may have resulted in better adherence to NPIs (e.g. masks, hand washing and physical distancing) and in those most at risk for severe COVID-19 disease taking additional precautions to isolate themselves. This is modelled by allowing the population in each district to reduce interactions when district death rates are high and increase interactions when death rates are low.
Better adherence to non-pharmaceutical interventions regardless of death rate is incorporated to reflect the population’s will to adhere to NPI, as they were known at the time, regardless of a national directive to do so, or at a time when restrictions are being relaxed. This is modelled by assuming that the level of adherence to NPIs in Level 4 (measured by population contact rate) does not increase when restrictions were relaxed to Level 3 and beyond.
Behavioural heterogeneity acknowledges that some members of society experience different risks and exhibit different behavioural patterns, introducing substantial variation in the number of people that different people infect, with highly connected individuals becoming infected earlier in the epidemic and infecting more contacts. This is modelled through adjusting the transmission function (force of infection) to be inflated at the start of the epidemic, but decrease as immunity builds up in the most connected individuals early on.
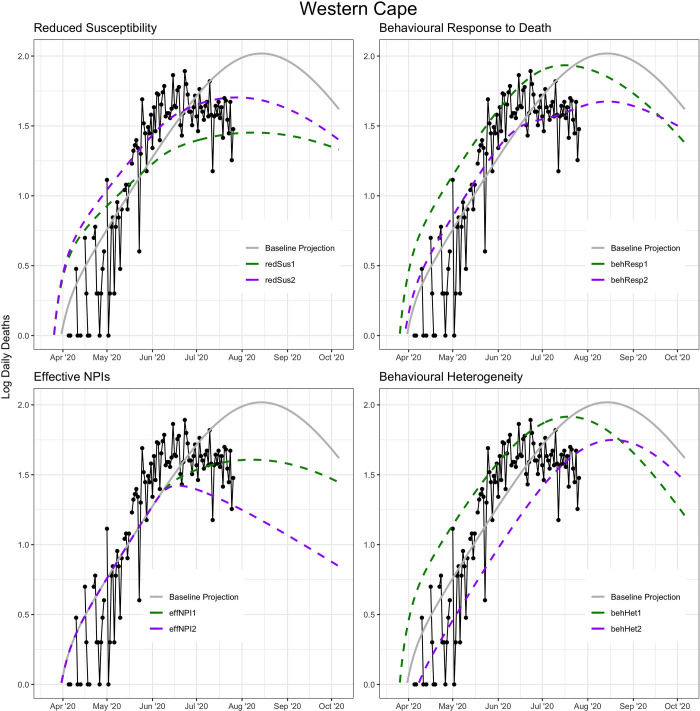
It is probable that the explanation for the earlier-than-projected plateauing of admissions and deaths at the time in the Western Cape was a combination of these factors, and there was not as yet enough evidence in the international literature or local data for any of these factors (Fig 3). Nonetheless, we ran a number of scenario analyses to determine how well these factors would explain the early plateau in the Western Cape, and what the impact of similar phenomena in the three provinces (EC, GP, KZN) with the most progressed epidemics over the next months would be, bearing in mind that the purpose of the analysis is to demonstrate how each one of these phenomena may be a possible explanation for the observed trends in the Western Cape, rather than attempting to find a best fitting parameter set for each phenomenon (Table A in S1 Text).
Fig 3. Impact of alternative scenarios on current and projected deaths in the Western Cape.
When applying each scenario to the three provinces with the next most advanced epidemics—Eastern Cape, Gauteng and KwaZulu-Natal—we noticed that each scenario in turn led to either later or lower peaking of cases than our original projections. The exception was the behaviour response to high mortality scenario in which the behavioural response threshold is assumed to be 110 deaths per day, which peaked at roughly the same level but shifted the peak forward slightly in all three provinces (Fig A in S1 Text). Based on these analyses, we chose to include the notion of behavioural heterogeneity as the most plausible explanation as it is a known infectious disease phenomenon that is broadly true of all social contact networks. The model was updated accordingly in the subsequent version.
NCEM updated model projections
In the original model, we projected the need for hospitalisation based on severity rates, as a means of quantifying the likely burden on the hospital facilities. However with challenges in access to care, and growing estimates of excess deaths, a different approach was considered. Two scenarios were simulated for planning purposes: (1) Use, where admissions to hospital reflect reported use of services with access to care for COVID-19 patients calibrated to 80% of estimated weekly excess deaths, and (2) Need, where all those with COVID-19 in need of hospital-level care access and receive it (similar to the original model).
Data-based estimates on length of hospital stay and transitions between stages along the hospital pathways were derived from DATCOV [29]. As 100% of private hospitals but only 53% of public hospitals were participating in DATCOV at the time of the analysis, public sector admissions were underrepresented in this dataset. To adjust for this, we calculated a province-specific inflation factor for the number of general and ICU admissions based on the total number of hospital beds available in both sectors versus the number of beds in hospitals represented in the DATCOV dataset. This inflation factor was applied to the DATCOV admissions data and used for calibration. Both sets of admissions data are presented in the results below, with the inflated data referred to as “adjusted DATCOV data”. This includes the length of hospital stay and the proportion of patients in general versus. ICU wards.
Given the challenges in accurately identifying and recording confirmed COVID-19 deaths, excess deaths provide a more robust measure of mortality. Total projected deaths due to COVID-19 in the model were calibrated to estimates of excess mortality by province from 6 May to 28 August 2020 where 80% of excess deaths were assumed to be due to COVID-19 infection [26].
Due to the limited and underrepresented district-level admissions data available at the time, model calibration to hospital admissions and deaths was performed at the provincial level even though the model is implemented at the district level with district-specific populations and spatial influence. While all provinces were individually calibrated, provinces with smaller numbers of confirmed cases, hospitalisations and deaths (Free State, Limpopo, Mpumalanga, North West, and Northern Cape) were less easily calibrated. The model code is available under https://sacovid19mc.github.io/, with a full set of provincial model output in the supplementary file. Additionally, district level model output for the ‘needs scenario’ has been made available to decision-makers in a dashboard [30].
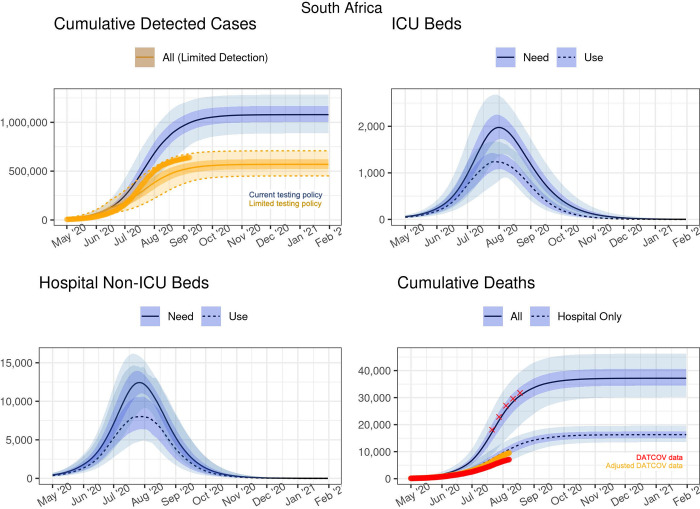
As testing guidelines and practices changed between provinces and over time as best practices on testing emerged with the limited supply of test kits and testing backlogs, cumulative detected cases were estimated under two scenarios i) moderate testing coverage as implemented in May and June, and ii) a more limited testing coverage policy implemented from mid-June that prioritised testing in hospitalised cases and in healthcare workers. Fig 4 summarises the projected cumulative detected cases at the national level assuming the current testing policy (blue) and a limited policy of detecting only hospitalised cases (orange). Importantly, a change in the testing policy only affects the number of detected cases, not any of the other projections. Table 1 gives an overview of the projections at select dates.
Fig 4. Projected cases and inpatient bed need and use at the national level.
The red crosses in the bottom right-hand panel represents 80% of the excess deaths found in the SAMRC analysis.
Table 1. Projections of national cases, deaths and admissions needed at select dates.
| Table for SA | ||||||
|---|---|---|---|---|---|---|
| Cumulative Incidence | Active Cases | Cumulative Detected | ||||
| Date | Total | Symptomatic | All | Symptomatic | Limited Detection | |
| 2020-09-01 | 15,201,000 (13,138,000–17,023,000) | 3,697,800 (2,835,700–4,521,500) | 462,600 (267,000–817,800) | 85,260 (46,810–151,500) | 517,000 (372,000–668,300) | |
| 2020-10-01 | 15,840,000 (14,112,000–17,488,000) | 3,914,700 (3,172,100–4,688,100) | 102,300 (54,020–238,000) | 19,320 (9,730–47,560) | 557,800 (433,500–700,400) | |
| 2020-11-01 | 15,977,000 (14,415,000–17,581,000) | 3,961,300 (3,246,400–4,720,300) | 20,840 (10,210–52,710) | 3,950 (1,840–10,690) | 567,500 (447,800–707,100) | |
| 2020-12-01 | 16,004,000 (14,480,000–17,596,000) | 3,970,400 (3,260,300–4,727,800) | 4,510 (2,050–11,990) | 860 (370–2,500) | 569,300 (450,400–708,600) | |
| 2021-01-01 | 16,010,000 (14,499,000–17,599,000) | 3,972,000 (3,263,400–4,729,700) | 950 (390–2,700) | 180 (70–570) | 569,800 (451,000–708,900) | |
| 2021-02-01 | 16,011,000 (14,501,000–17,600,000) | 3,972,400 (3,264,000–4,730,000) | 210 (70–650) | 40 (10–140) | 569,900 (451,200–709,000) | |
| Cumulative Admissions | Hospital Beds in Use | Cumulative Deaths | ||||
| Date | General | ICU | Non-ICU | ICU | Hospital | All |
| 2020-09-01 | 97,500 (73,290–121,400) | 11,940 (9,010–15,060) | 3,830 (1,720–8,410) | 720 (400–1,090) | 14,250 (10,300–17,760) | 33,710 (24,020–43,010) |
| 2020-10-01 | 105,700 (85,730–127,400) | 12,950 (10,550–15,860) | 950 (360–2,810) | 200 (90–430) | 15,740 (12,680–19,210) | 36,330 (28,640–45,600) |
| 2020-11-01 | 107,800 (88,740–128,900) | 13,190 (10,900–16,110) | 190 (70–680) | 40 (10–130) | 16,170 (13,320–19,510) | 37,000 (29,850–46,140) |
| 2020-12-01 | 108,200 (89,310–129,300) | 13,240 (10,970–16,150) | 40 (10–160) | <10 (<10–40) | 16,260 (13,460–19,570) | 37,140 (30,120–46,230) |
| 2021-01-01 | 108,300 (89,420–129,400) | 13,250 10,980–16,160) | <10 (<10–40) | <10 (<10 - <10) | 16,290 (13,490–19,580) | 37,160 (30,170–46,260) |
| 2021-02-01 | 108,300 (89,450–129,400) | 13,260 (10,980–16,160) | <10 (<10 - <10) | <10 (<10 - <10) | 16,300 (13,500–19,580) | 37,170 (30,180–46,260) |
Under the moderate testing scenario, cumulative detected cases were projected to continue to grow up to 1.2 million in early November 2020, and only marginally so thereafter, whereas only approximately 567,500 cases (447,800–707,100) were estimated to be detected under limited testing. By September, the reported number of detected cases had already surpassed the median of the limited testing scenario and over 725,000 detections by November 2020.
The COVID-19 epidemic was estimated to have peaked nationally in mid-July with approximately 16,000,000 infections by December 2020, representing 26.8% (24.3% - 29.5%) of the population. Total deaths were estimated to continue to increase until early November when the cumulative number of all deaths would reach 37,000 (of which 16,000 will have been in hospital); thereafter the growth rate was estimated to be very low (Fig 4).
For the estimation of hospital bed requirements, scenarios of both the estimated need and the actual use of ICU and non-ICU beds are depicted. The peak number of general hospital (i.e. non-ICU) beds in use was estimated to be reached in early-August, at around 8,000 beds (when around 12,500 beds were estimated to have been needed). The peak number of ICU beds in use was estimated to be reached around the same time, with around 1,100 beds (when more than 2,000 beds would have been needed) (Fig 4).
DATCOV dataAdjusted DATCOV data
At a provincial level, considerable variation was observed and projected in the timing and height of peak infection between the provinces (Fig 5). This allowed the strain on healthcare resources to be spread out, potentially allowing for more healthcare capacity based on how well resources such as beds, oxygen, staff and test kits and reagents could be shifted between and within provinces. Within provinces, patients needing hospitalisation could be moved between under-and better resourced districts.
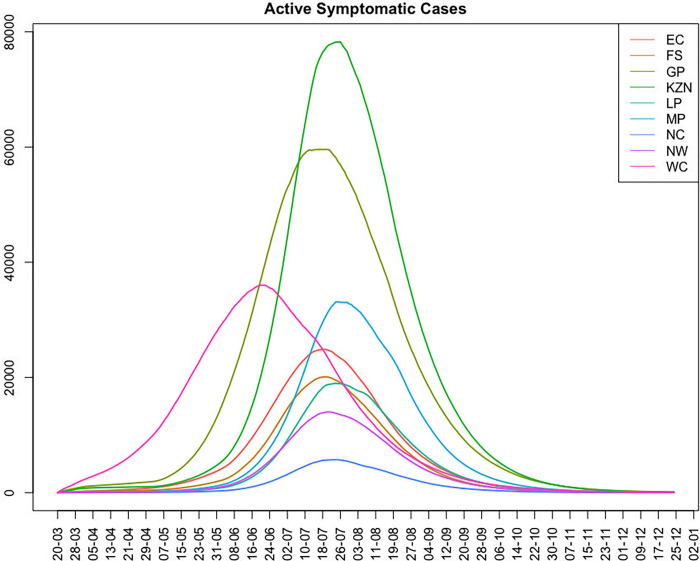
Fig 5. Estimated daily active symptomatic cases by province from week beginning 20 March 2020 to 25 December 2020.
For all provinces, projections of deaths and cases requiring hospitalisation are presented in Figs B-J and Tables D-L in S1 Text. Across provinces, estimates of all COVID-19 related deaths were projected to be almost double those of the reported COVID-19 related deaths occurring in hospital (which the DATCOV hospital surveillance dataset aims to capture). For example, in the Eastern Cape Province (Fig B in S1 Text), adjusting for hospital representativeness in the DATCOV database, hospital-based deaths were projected to reach 2500 by October 2020, while total deaths were estimated at more than 7,500 at the same time- assuming 80% of excess deaths were due to COVID-19. This differential is additionally reflected in the difference in the projected need for and use of hospital services such that use of hospital beds was estimated to peak at 100 and 750 median ICU and non-ICU beds respectively whereas if all patients who needed care received it, the estimated need for hospital beds would be 200 and 1500 median ICU and non-ICU beds respectively.
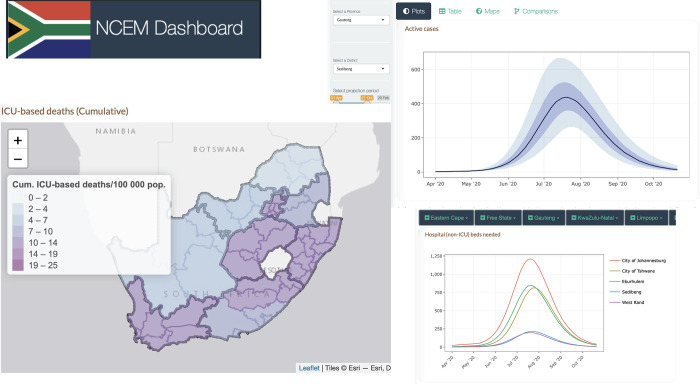
The NCEM dashboard provides users with the ability to interact with projections of cases, hospitalisations and deaths for the first wave of the epidemic across the 52 districts in South Africa [30]. Fig 6 shows a sample of outputs such as maps, comparison line plots, tables and reports at the district level, where the district-level waves show additional variation within provinces. The interactive map was created in R using the leaflet package [31] with base layer shapefile obtained from GADM, the Database of Global Administrative Areas (https://gadm.org/download_country40.html).
Fig 6. NCEM dashboard sample output: Interactive dashboard for district level projections of cases, admissions and deaths as at 11 September 2020 [30].
The interactive map was created in R using the leaflet package [31] with base layer shapefile obtained from GADM, the Database of Global Administrative Areas (https://gadm.org/download_country40.html).
Discussion
The purpose of this paper is to describe the NCEM and its implementation during the first wave of the COVID-19 epidemic in South Africa. A reflection on modelling methodology and assumptions during a period of uncertainty, where rapid evolution of models is required, is beneficial for modellers and model-users alike. Mathematical modelling plays a role in improving both the understanding of a disease, and planning to make better decisions and reduce disease impact. The model presented here has been developed using data that is subject to a high degree of uncertainty. All models are simplifications of reality that are designed to describe and predict system behaviour and are a product of the assumptions and data with which they are developed. As such, the model used to support planning in South Africa was refined regularly throughout the South African COVID-19 epidemic [15].
COVID-19 modelling groups around the world had various levels of impact on a country or countries’ policy choices. In South Africa, given our consortium’s early mandate from the National Department of Health, the government department tasked with coordinating the country’s COVID-19 response, we were able to work closely with decision makers and planners at all levels. This includes two of the three Ministerial Advisory Committees on COVID-19 advising the Minister of Health to National Treasury through to the individual teams tasked with planning facility readiness and, from 2021 on, the vaccine programme. Examples of uses of model projections included the estimation of drug quantities for in-patient and out-patient care at facilities; the quantification of additional mortuary and burial spaces; the cost of ventilation equipment for all hospitals; estimating the required supply of oxygen, planning of district facility space, scenario planning of interventions, and supporting the analysis of the macroeconomic impact of the epidemic under different scenarios by the Reserve Bank. Modelling reports were additionally published on the NICD website along with daily case, admissions and surveillance reports [32].
Publicly-accessible dashboards visualising the main results of NCEM outputs for the first wave [30] and our resurgence monitoring metrics and 2-week forecasts [33] additionally allowed us to make central model results available to the public. Continuously updating model assumptions, parameters and projections over the course of the epidemic and communicating changes through an established communication pipeline to planners across the different levels of government, led to increased credibility and reliance on model outputs as a signal for planning. This was further demonstrated across the subsequent three waves of infections where model projections were routinely requested to assist with planning.
The NCEM attempted to incorporate a high level of local data to contextualise the model appropriately to address South Africa’s population and health system characteristics. The data however, were not always complete or representative of the full population. For example, in many countries, reported SARS-CoV-2 related deaths were only a fraction of the total excess deaths seen during the last two years. South Africa is one of the few African countries with a vital statistics reporting system that allows the estimation of excess deaths. This put the country in the unusual situation for a LMIC to be able to estimate SARS CoV-2 mortality more precisely. COVID-19-specific mortality reports in South Africa were largely limited to within-hospital deaths, with public sector hospitals dependent on manual capturing of confirmed COVID-19 deaths. Under-reporting of COVID-19 mortality arose from individuals not having been tested for SARS-CoV-2 before dying, especially when dying outside of hospitals or care facilities, and incomplete reporting of in-hospital deaths. Pressure on data-capturing systems during the peaks of waves additionally likely resulted in under-reporting of both confirmed COVID-19 admissions and deaths. Furthermore, access to health services in South Africa is variable. Less than 60% of poor households have available, affordable and acceptable access to health services, with rural communities having lower access than urban communities [34]. Less than half of influenza deaths are medically attended each year [35].
Our projections at the district level did not capture low-level clustering of cases. The population level model made assumptions simplifying how contacts between infectious and uninfected people occur through grouping individuals at the district level. It does not also capture the effects of specific events on local transmission. However contact rates in each district were adjusted uniformly based on national policy changes, and stochastic simulation allowed for variation at each time step for each state transition. Additionally, the 6-month projections during the first wave of infections failed to take into account the possibility of new variants at the time (as variants of SARS-CoV-2 had not yet been detected), which ultimately have a substantial impact on the trajectory of the epidemic a few months after the end of the first wave.
The role of population behaviour in determining the trajectory and scale of the epidemic is more influential in COVID-19 due to the absence of a cure or vaccine at the time of developing the model. It is not possible to precisely capture human emotion and behaviour that varies on a fine spatial scale in an equation, least of all in an unprecedented situation such as the current pandemic. Accounting for these unknowns requires that models are run stochastically and estimates are presented with uncertainty bands reflective of variation in the parameters driving the model and the model process itself.
The NCEM is currently being adapted and implemented to assess the impact of vaccine and infection-derived immunity on the likely severity of future infection. However, the future of the spread of SARS-CoV-2 and the impact of COVID-19 on health and health resources depends on many unknowns, including the duration of infection- and vaccine-derived immunity. Depending on the nature of immunity from either past infection or vaccination, the future of SARS-CoV-2 could be regular annual epidemics caused by novel variants, seasonal epidemics, epidemics occurring every few years or even sporadic, unpredictable epidemics. It is therefore important to continue to monitor the epidemic and remain vigilant to detect localised outbreaks as and when they occur.
Conclusion
The NCEM has been developed as a tool to project cases and deaths due to COVID-19. Its features allow for transmission to be modelled at a fine spatial scale and population behaviour to be captured through mobility, contact patterns and adherence to non-pharmaceutical and pharmaceutical interventions. The model presented in this manuscript has demonstrated the need to adapt models rapidly to changing local data and knowledge to support government planning needs. The model has since been adapted to include variants and vaccines and calibrated to additional waves of COVID-19.
The pandemic presented the opportunity for infectious disease modellers around the world to rapidly develop tools. Pandemic preparedness endeavours need to incorporate provisions for increasing modelling capacity and training of future modellers, particularly in LMIC. These should be prioritised by local governments and donors alike.
Supporting information
(DOCX)
Acknowledgments
The authors acknowledge the support of the National Department of Health and all planners, stakeholders and decision-makers who made use of these data systems and analyses to respond to the COVID-19 epidemic in South Africa. A large number of colleagues within the National Institute of Communicable Diseases, the Ministerial Advisory Committee on COVID-19, the Clinton Health Access Initiative, provincial and national Departments of Health, and National Treasury alerted us to policy-relevant questions, assisted the development of our models by providing data, discussing details of model structure, and reviewing assumptions and results. We are especially grateful to decision makers and planners at all levels of the health system who continuously used our outputs in the management of COVID-19.
Data Availability
All model code available under https://sacovid19mc.github.io/.
Funding Statement
SPS, RAH, JN, SM and FK and the development of the SACMC dashboards are funded by the Wellcome Trust [GN: 2114236/Z/18Z] and the Clinton Health Access Initiative. This research was funded in whole, or in part, by the Wellcome Trust [GN: 2114236/Z/18Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. JRCP is supported by the Department of Science and Innovation and the National Research Foundation. Any opinion, finding, and conclusion or recommendation expressed in this material is that of the authors, and the NRF does not accept any liability in this regard. The work of GMR and LJ on the SACMC has been made possible by the generous support of the American People and the President’s Emergency Plan for AIDS Relief (PEPFAR) through the United States Agency for International Development (USAID) under the terms of Cooperative Agreement 72067419CA00004 to HE2RO. The contents are the responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID or the United States Government. https://www.state.gov/pepfar/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.National Institute for Communicable Diseases. Novel Coronavirus Outbreak In Wuhan City, China: National Institute for Communicable Diseases; 2020 [Available from: https://www.nicd.ac.za/novel-coronavirus-outbreak-in-wuhan-city-hubei-province-of-china/.
- 2.Worldometer. Countries where COVID-19 has spread 2020 [Available from: https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/.
- 3.National Department of Health South Africa. Update on Covid-19 (01st November 2020) 2020 [Available from: [https://sacoronavirus.co.za/2020/11/01/update-on-covid-19-01st-november-2020/].
- 4.Silal S, Pulliam J, Meyer-Rath G, Nichols B, Jamieson L, Moultrie H. Estimating cases for COVID-19 in South Africa: Long term Provincial projections. National Institute for Communicable Diseases. 2020. [Google Scholar]
- 5.Silal S, Pulliam J, Meyer-Rath G, Nichols B, Jamieson L, Moultrie H. Estimating cases for COVID-19 in South Africa: Long term projections. National Institute for Communicable Diseases. 2020. [Google Scholar]
- 6.Silal S, Pulliam J, Meyer-Rath G, Nichols B, Jamieson L, Moultrie H. Estimating cases for COVID-19 in South Africa: Short Term projections: June 2020. National Institute for Communicable Diseases. 2020. [Google Scholar]
- 7.Silal S, Pulliam J, Meyer-Rath G, Nichols B, Jamieson L, Moultrie H. Estimating cases for COVID-19 in South Africa: Assessment of alternate scenarios. National Institute for Communicable Diseases. 2020. [Google Scholar]
- 8.Silal S, Pulliam J, Meyer-Rath G, Nichols B, Jamieson L, Kimmie Z, et al. Estimating cases for COVID-19 in South Africa Update: 19 May 2020. National Institute for Communicable Diseases. 2020. [Google Scholar]
- 9.Silal S, Pulliam J, Meyer-Rath G, Jamieson L, Moultrie H. COVID-19 modelling update: Considerations for a potential third wave. National Institute for Communicable Diseases. 2021. [Google Scholar]
- 10.Silal S, Pulliam J, Meyer-Rath G, Jamieson L, Moultrie H. COVID-19 modelling update: Considerations for the third wave, including the impact of the delta variant of concern (July 2021). National Institute for Communicable Diseases. 2021. [Google Scholar]
- 11.Silal S, Pulliam J, Meyer-Rath G, Jamieson L, Moultrie H. COVID-19 modelling update: Considerations for a potential fourth wave (November 2021). National Institute for Communicable Diseases. 2021. [Google Scholar]
- 12.Nichols BE, Jamieson L, Zhang SR, Rao GA, Silal S, Pulliam JR, et al. The role of remdesivir in South Africa: preventing COVID-19 deaths through increasing intensive care unit capacity. Clinical infectious diseases. 2021;72(9):1642–4. doi: 10.1093/cid/ciaa937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo Y, Jamieson L, Edoka I, Long L, Silal S, Pulliam JR, et al., editors. Cost-effectiveness of remdesivir and dexamethasone for COVID-19 treatment in South Africa. Open Forum Infectious Diseases; 2021: Oxford University Press; US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organisation for Economic Cooperation and Development (OECD). DAC List of ODA Recipients: OECD.org; 2022 [Available from: https://www.oecd.org/dac/financing-sustainable-development/development-finance-standards/daclist.htm.
- 15.Silal SP, Pulliam J, Jamieson L, Nichols B, Moultrie H, Meyer-Rath M. The role of modelling in planning and budgeting for South Africa’s COVID-19 response. In: Govender K, George G, Padarath A, Moeti T, editors. South African Health Review 2021. Durban: Health Systems Trust; 2021. URL: https://www.hst.org.za/publications/South%20African%20Health%20Reviews/Chapter2_SAHR21_04022022_OD.pdf. [Google Scholar]
- 16.Weitz JS, Park SW, Eksin C, Dushoff J. Awareness-driven behavior changes can shift the shape of epidemics away from peaks and toward plateaus, shoulders, and oscillations. Proceedings of the National Academy of Sciences. 2020;117(51):32764–71. doi: 10.1073/pnas.2009911117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soetaert K, Petzoldt T, Setzer RW. Solving Differential Equations in R: Package deSolve. Journal of Statistical Software. 2010;33(9):1–25.20808728 [Google Scholar]
- 18.Soetaert K, Petzoldt T, Setzer R. deSolve: General solvers for initial value problems of ordinary differential equations (oDE), partial differential equations (pDE), differential algebraic equations (dAE) and delay differential equations (dDE). R-Forge. 2016. [Google Scholar]
- 19.Rackauckas C, Nie Q. Differentialequations. jl–a performant and feature-rich ecosystem for solving differential equations in julia. Journal of Open Research Software. 2017;5(1). [Google Scholar]
- 20.National Institute for Communicable Diseases. The Daily COVID-19 Effective Reproductive Number (R) in South Africa–week 33 2020. 2020.
- 21.National Institute for Communicable Diseases. The Initial and Daily COVID-19 Effective Reproductive Number (R) in South Africa 10 June 2020. 2020.
- 22.Google LLC. Google COVID-19 Community Mobility Reports 2020 [Available from: https://www.google.com/covid19/mobility.
- 23.Statistics South Africa. Mid-year District Population Projections. (unpublished)2020.
- 24.Moultrie T, Dorrington R, Laubscher R, Groenewald P, Bradshaw D. Excess Deaths: Additional Measures And Approaches To Understanding Covid-19 Related Mortality In South Africa. South African Medical Research Council. 2020. [Google Scholar]
- 25.Bradshaw D, Laubscher R, Dorrington R, Groenewald P, Moultrie T. Report on weekly deaths in South Africa, 1 January -18 August 2020 (Week 33). South African Medical Research Council. 2020. [Google Scholar]
- 26.Moultrie T, Dorrington R, Laubscher R, Groenewald P, Bradshaw D. Correlation of Excess Natural Deaths with Other Measures of the COVID-19 Pandemic in South Africa. South African Medical Research Council. 2020. [Google Scholar]
- 27.National Department of Health South Africa. About the alert system 2020 [Available from: https://www.gov.za/covid-19/about/about-alert-system.
- 28.National Department of Health South Africa. COVID-19 Risk Adjusted Strategy 2020 [Available from: https://sacoronavirus.co.za/covid-19-risk-adjusted-strategy/.
- 29.Jamieson L, Van Schalkwyk C, Nichols BE, Meyer-Rath G, Silal S, Pulliam J, et al. Differential in-hospital mortality and intensive care treatment over time: Informing hospital pathways for modelling COVID-19 in South Africa. PLOS Global Health. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.South African COVID-19 Modelling Consortium. NCEM Dashboard. The Modelling and Simulation Hub, Africa (MASHA); 2020.
- 31.Graul C. leafletR: Interactive Web-Maps Based on the Leaflet JavaScript Library. R package version 0.4–0; 2016. [Google Scholar]
- 32.National Institute for Communicable Diseases. Modelling Consortium Projections NICD; 2022 [Available from: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/modelling-consortium-projections/.
- 33.South African COVID-19 Modelling Consortium. The SACMC Epidemic Explorer. The Modelling and Simulation Hub, Africa (MASHA); 2020.
- 34.Burger R, Christian C. Access to health care in post-apartheid South Africa: availability, affordability, acceptability. Health Economics, Policy and Law. 2020;15(1):43–55. doi: 10.1017/S1744133118000300 [DOI] [PubMed] [Google Scholar]
- 35.Tempia S, Walaza S, Moyes J, Cohen AL, McMorrow ML, Treurnicht FK, et al. Quantifying how different clinical presentations, levels of severity, and healthcare attendance shape the burden of influenza-associated illness: a modeling study from South Africa. Clinical Infectious Diseases. 2019;69(6):1036–48. doi: 10.1093/cid/ciy1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All model code available under https://sacovid19mc.github.io/.