ABSTRACT
Introduction: Freezing cold injuries (FCI) are a common risk in extreme cold weather operations. Although the risks have long been recognised, injury occurrences tend to be sparse and geographically distributed, with relatively few cases to study in a systematic way. The first challenge to improve FCI medical management is to develop a common nomenclature for FCI classification. This is critical for the development of meaningful epidemiological reports on the magnitude and severity of FCI, for the standardisation of patient inclusion criteria for treatment studies, and for the development of clinical diagnosis and treatment algorithms.
Methodology: A scoping review of the literature using PubMed and cross-checked with Google Scholar, using search terms related to freezing cold injury and frostbite, highlighted a paucity of published clinical papers and little agreement on classification schemes.
Results: A total of 74 papers were identified, and 28 were included in the review. Published reports and studies can be generally grouped into four different classification schemes that are based on (1) injury morphology; (2) signs and symptoms; (3) pathophysiology; and (4) clinical outcome. The nomenclature in the different classification systems is not coherent and the discrete classification limits are not evidence based.
Conclusions: All the classification systems are necessary and relevant to FCI medical management for sustainment of soldier health and performance in cold weather operations and winter warfare. Future FCI reports should clearly characterise the nature of the FCI into existing classification schemes for surveillance (morphology, symptoms, and appearance), identifying risk-factors, clinical guidelines, and agreed inclusion/exclusion criteria for a future treatment trial.
KEYWORDS: Cold injury classification, extreme cold weather, cold injury, cold ischaemia, peripheral injuries, freezing cold injury, Frostbite, FCI
Introduction
Injuries caused by or related to cold weather have claimed many human lives and resulted in various debilitating injuries for civilian and military personnel [1–7]. A simple solution to lowering medical risks in cold weather would be to avoid cold exposure but, for some professions, including the military, this is an assumed risk. An increasing proportion of cold weather novices are facing injury risks as interest in extreme cold regions grows. This includes the austere and severe Arctic environment because of current patterns of climate change, commercial interests, and increasing security threats [8,9].
Military activity requires soldiers to master cold weather conditions to operate successfully, as cold weather has the potential to render a potent fighting force useless [5,6]. Neglecting the challenges of operating in cold weather conditions may negatively influence operational effectiveness and pose a risk both to the mission and the larger force. For the military, effective prevention and management of cold weather injuries is vital to sustaining the force.
Freezing cold injury, also labelled frostbite, represents a key debilitating injury with a risk of tissue loss. To better prevent and treat these injuries, a consistent nomenclature to describe the severity and extent of injury is needed. This is important for injury surveillance, to determine associated injury risk factors, and for treatment algorithms, ensuring the most effective salvage of injured tissue according to the proper classification of the nature of the injury.
The North Atlantic Treaty Organization (NATO) is a political and military alliance that includes specific interests in military operations in cold weather regions [10,11]. Within NATO, the Science and Technology Organization (STO) [12] sponsors research task groups under specialised panels. The Human Factors and Medicine Panel (HFM) appointed a Research Task Group (RTG 310) to provide the scientific basis for the sustainment of soldier health and performance in cold weather operations and identify critical research gaps.
The national representatives of the HFM-RTG-310 constitute a broad range of scientists and clinicians. Among the priorities of this research group, a common nomenclature for FCI is urgently needed to track medical issues and to properly classify injuries for treatment or experimental interventions.
Most Cold Weather Injury (CWI) publications deal with acral portions of the body that are most susceptible to freezing cold injury (FCI) (i.e. ears, nose, fingers, hands, toes, and feet). However, an initial PubMed review of this body of literature revealed significant discrepancies in the description and assessment of FCI and even what defines an FCI. Even where aspects of pathophysiology, treatment, and outcome were thoroughly outlined, the NATO panel did not find any common agreement for military activities in nomenclature, nor any common diagnostic criteria for FCI.
One important task for the RTG 310 is to inform NATO leadership on the existing strategies that have been reported for FCI classification. From this literature, the subcommittee summarised and reported here the classification strategies that may be useful for FCI monitoring and management.
The aim of this paper is to present a position paper regarding classification systems for FCI, from the perspective of military activities.
Method
A subcommittee of the HFM 310 workgroup conducted a scoping review of existing literature on FCI classification schemes. The literature scan was performed using PubMed and augmented with Google Scholar. Search terms included relevant PubMed MeSH headings and related terms including “cold injuries”, “freezing cold”, “cold ischaemia”, “extreme cold exposure” along with “injury classification”, “diagnostic criteria”, and “prognostic criteria”.
The reference lists of relevant articles were further cross-referenced until reaching redundancy with no further new relevant articles emerging.
In total 74 papers were identified with the given search terms and in the cross-references. Papers without abstract (n = 9), in other languages than English (n = 19), or with content other than classification (n = 18) were excluded. The scoping review was based on the remaining 28 papers.
Results and discussion
Existing systems of FCI classification
FCI has numerous clinical pictures ranging from injuries with discrete and minor signs that resolve completely, to injuries that result in major limb amputation [10]. Patients with FCI frequently also present with multisystem injuries [11]. The symptoms and signs from FCI vary greatly; the long-term sequelae [4,10,12–16] have been little explored [17], and the timeline for spontaneous recovery can take several weeks and months [18].
This paper has identified the following systems of FCI classification according to:
morphology of the injury
symptoms and signs
pathophysiology
outcome of FCI
Classification according to morphology (degree/level) of injury
Traditionally, frostbite has been classified into different degrees/levels analogous to the classification of burn injuries [10]. If only the dermal layer is involved in an FCI, the injury has traditionally been regarded a superficial FCI, subdivided into 1st degree and 2nd degree. A freezing process involving the subcutaneous layers and/or deeper into muscles, tendons, and bones is classified as a deep FCI, subdivided into 3rd degree and 4th degree (Table 1) [1,7,10,19–21].
Table 1.
Classification of FCI according to morphology.
| Morphology/level | ||
|---|---|---|
| Superficial frostbite | 1stdegree | Partial intradermal frostbite |
| 2nd degree | Complete dermal frostbite | |
| Deep frostbite | 3rd degree | Injury down into the subcutaneous tissue |
| 4th degree | Injury to deeper structures (i.e. muscles and bone) | |
If only the outer layer of the skin is frozen (intradermal lesion), the injury is regarded as a 1st degree FCI [12]. In a 2nd degree FCI, the total level of dermis is affected involving dermal fluid areas resulting in blisters, superficial erosions, and possibly skin necrosis. If the FCI develops into the subcutaneous level, a 3rd degree FCI develops involving subcutaneous vessels resulting in haemorrhagic blister formation, and dermal necrosis. At 2nd degree, and particularly at 3rd degree, the nerves and pain receptors in the subcutis are involved and pain is gradually reduced. If the injury develops deeper beneath the subcutaneous layer a 4th degree FCI will occur with full- thickness oedema and subsequent freezing of all the deeper anatomical structures and no pain [10].
An alternate 2-tiered morphological classification into superficial and deep has been proposed to simplify classification for field use, particularly before diagnostic imaging and hospital assessment. This classification differs between superficial FCI (= no or minimal anticipated tissue loss) and deep FCI (= anticipated tissue loss) [22–24].
Classification of FCI according to symptoms and signs
Symptoms and signs of FCI in superficial/mild frostbite are challenging as the symptoms fluctuate, making an exact early diagnosis difficult. For deep/severe frostbite, somewhat clearer clinical criteria are provided [20]. Table 2 presents some major clinical symptoms and signs in FCI.
Table 2.
Major symptoms and signs at different levels of freezing cold injuries.
| Mild | Moderate | Severe | Full thickness | |
|---|---|---|---|---|
| Colour | Reddish skin, white patches (“frostnip”) | Pronounced hyperemia | bluish-white discoloration | bluish-black discoloration |
| Blister | No blister | Blisters with white or clear fluid | Haemorrhagic or black blisters | No/erupted blisters |
| Lesions | No lesions | Superficial erosions | Skin-necrosis | Necrosis of deeper tissue |
| Pain | Intense stinging pain | Gradually reduced pain | Significantly reduced pain or still pain in cyanotic injury | No pain sensation |
Frostnip is a white patch on the skin that diminishes or disappears immediately if the skin is warmed up in time and is regarded as a mild form of FCI [11]. Frostnip rewarmed within 30 min of appearance has been found to resolve completely [25]. Frostnip is considered distinct from the more serious frostbite but may precede it [7]. The understanding of a frostnip being totally clinically reversible [25] has been challenged as sequelae also is reported from FCI grade 1 [1]. When frostnip lasts for more than 30 min, it might represent a greater risk of sustaining a new/further FCI, especially in the following weeks.
The scientific literature indicates that the classification of FCI according to symptoms and signs is a mixture of morphological signature, and clinical symptoms and signs [10].
Classification according to pathophysiology
The aetiology of FCI is complex as multiple pathophysiological processes are involved [17] with FCI classified at different stages of its pathophysiology [10,22,25]. These stages overlap and the changes depend on the freezing rate, the duration of freezing, and the extent of injury and thawing. The distinction and naming of the phases are not totally agreed upon in the literature.
Stage 1 is called the pre-freezing stage occurring before ice crystal formation in the tissue [12]. Skin sensation is lost at skin temperature around 8°C, and further cooling involves microvascular constriction and the arteriovenous anastomoses (AVA) shunting distal blood flow [26]. The vasoconstriction is usually followed by cold-induced vasodilatation (CIVD), also named the “‘hunting response’”, to protect the extremities from cold injury, unless the core body temperature is low [27], or the fact that the CIVD fatigues and thus likely is transient.
Stage 2 is the formation of extra-cellular ice crystals when the temperature in the human tissue has reached a level of −0.55°C [2,19,28,29]. The electrolyte content of the human tissue lowers the freezing point of skin slightly below the freezing point of water (0°C). Stage 2 has also been labelled the freeze–thaw phase, often recognised when temperatures fluctuate around 0°C. The risk of FCI increases with lower tissue temperatures and the probability of FCI is about 95% at a tissue temperature of −4.4°C [30]. The rate and duration of the cooling process in the human tissue might alter due to skin-moisture [31,32], peripheral vasoconstriction, contact with supercooled liquids (f.i. petrol) [5] conductive heat-loss (f.i. very cold metal surfaces) [33], and convective heat-loss [34]. One important thermal convection factor is the wind chill temperature (WCT) [35] (Figure 1).
Figure 1.
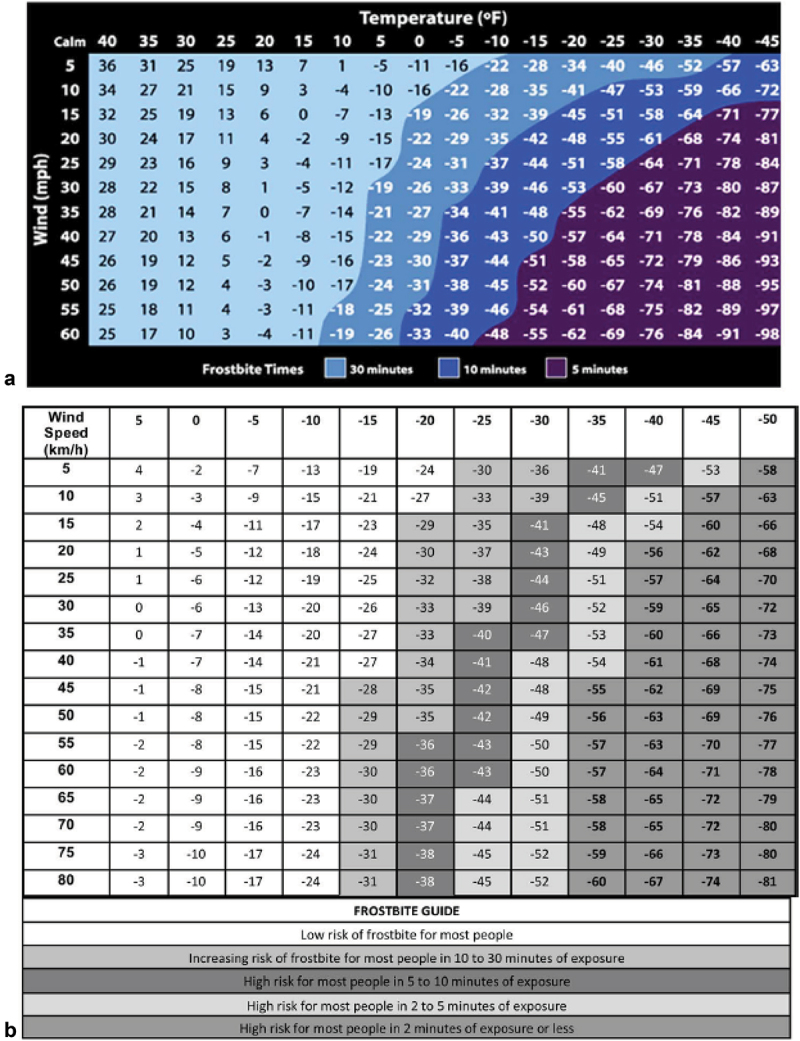
WCT Index and frostbite time indicated on both charts is the risk of cheek frostbite in the most susceptible 5% of the population [23,35,36].
The WCT is dependent on direct skin exposure to the wind that causes tissue temperatures to be reached sooner. WCT is also related to clothing insulation capacity, the duration of exposure, body area [37], maintenance of core body temperature [23,36], and possibly also personal susceptibility. Whether exposure to non-freezing temperature situations for long periods, underlying vascular or neurologic disease, or multisystem injuries affect FCI pathophysiology, is still unknown [25].
Stage 3 is, for pragmatic treatment reasons, regarded as the vascular phase with more pronounced pathophysiology including coagulation within the vessels, plasma leakage, and shunting. Stage 3 also refers to the time between rewarming and advanced hospital management (warm ischaemia time), regarded as a critical window in the treatment of FCI regarding injury outcome [38,39].
Stage 4 is the final pathophysiological status in the injury development including a late ischaemic phase during thawing involving oxygen-free radicals, neutrophil activation, and numerous inflammatory changes, and platelet aggregation leading to thrombosis, ischaemia, and gangrene [22,28] (Table 3).
Table 3.
Major pathophysiologic factors in different phases in freezing cold injuries.
| Staging | Description | Pathophysiology factors |
|---|---|---|
| Stage 1 | Pre-freezing Phase | Tissue cooling with accompanying vasoconstriction and ischaemia; no ice crystal formation |
| Stage 2 | Freeze-thaw phase | Ice crystal formation start extracellularly (slow in-freeze) or direct intracellularly (rapid in-freeze) |
| Stage 3 | Vascular stasis phase | Reperfusion injury, coagulation within the vessels, thrombosis, plasma leakage and shunting |
| Stage 4 | Ischaemic phase | Progressive tissue ischaemia and infarction, inflammation |
FCI has also been sub-divided according to whether the pathophysiology affects cells and extracellular fluids (direct effects) or the process involves the function of the organised tissue and the integrity of the circulation (indirect effects) [11]. Most common in FCI, the ice crystals first form in the extracellular fluid spaces, increasing osmotic pressure, causing intracellular dehydration and hyperosmolality and damage to cell membranes followed by ultrastructural capillary damage, loss of mitochondria in muscle cells, and other intracellular damage [40]. Reperfusion injury related to the thawing-phase involves oxygen-free radicals, neutrophil activation, numerous inflammatory changes, platelet aggregation, and thrombosis ending with reperfusion-ischaemia.
A simplified scheme of FCI-pathology classification with only two pathophysiological approaches has also been proposed; the cooling – supercooling–freezing stage; and a vascular stage that includes thawing (rewarming) and post-thaw [40]. Another dichotomised pathophysiological classification is divided into acute injury and different post-injury symptoms. In many ways, the long-term sequelae with vasomotor disorders and neuropathy might lead to major functional limitations several years after FCI [7,17].
Classification according to outcome of FCI
An FCI grading system based on the clinical outcome (tissue loss and risk of amputation) and radiological examination has also been proposed by Cauchy et al. [41]. Seventy patients with FCI were evaluated after a rapid (1 h) rewarming of the tissue in warm (38°C) water, where the final outcome was tissue loss and the risk of amputation [42]. This retrospective clinical reinterpretation was combined with data obtained from early isotopic bone scans. Based on the clinical appearance and anatomical extension of the frostbite lesion, a grading classification was proposed.
1st grade is categorised by the absence of initial lesion. 2nd grade was associated with the initial lesion on distal phalanx. The 3rd grade was attributed to an initial lesion on intermediate or proximal phalanx, while the 4th grade included an initial carpal/tarsal lesion (Figure 2). This classification is used in clinical hospital practice, as the initial radiological findings correlate well with clinical outcomes regarding amputation level [22].
Figure 2.
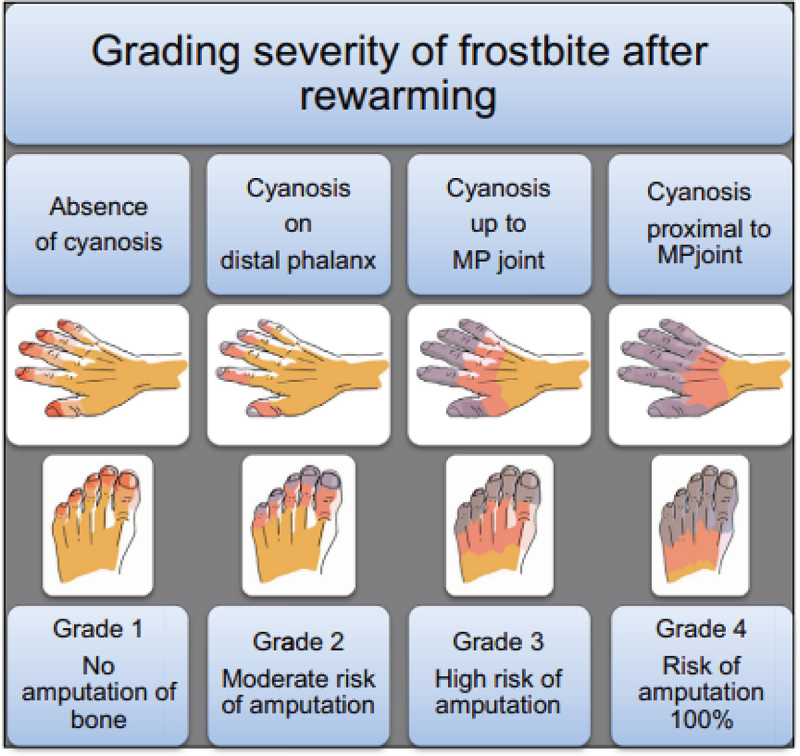
Grading severity of frostbite and bone amputation risk after rewarming [41].
When the human tissue is frozen, the patient is in a cold ischaemia situation. Injured tissue could, to some extent, be saved with advanced hospital care, although the level and duration of the in-freeze situation, among other factors is decisive [38,39,43]. Surgical amputation decisions are determined in part by altitude as the most relevant amputation risk factor derived from a stepwise regression model [44]. The Yukon Frostbite Protocol relies on the Cauchy system of frostbite grading to determine treatment [39]. The Helsinki frostbite management protocol for severe frostbite cases also classifies FCI according to outcome [43]. The treatment protocols again refer to the Hennepin Score to quantify salvage rates in a standardised manner [38].
Discussion
The existing classification systems seem to be developed for different purposes and for different parts in the chain of treatment. Nomenclature used in a field care evaluation may neither be particularly relevant for acute advanced hospital care nor for the assessment of long-term sequelae of FCI. The summarised characteristics of the different classification systems based on military training and warfare are presented in Table 4.
Table 4.
The characteristics of the different classification systems.
| CLASSIFICATION SYSTEM | Characteristics |
|---|---|
| Morphology of injury | Most common used system of FCI classification Difficult to interpret in early assessment of FCI |
| Symptoms and signs | Important for acute/first aid/field care FCI assessment Assessment is challenged by fluctuation of symptoms and signs |
| Pathophysiology | Important for the understanding of the FCI development Useful for treatment principles, particularly the FCI-immunology |
| Outcome of FCI | Important for prognosis and advanced FCI hospital treatment Less useful in early field-diagnosis outside the hospital but may be useful post rewarming for evacuation decisions. |
FCI described by its clinical presentation is often quite difficult in terms of assessing the extent of frostbite injury [1,10]. The discoloration of the skin changes and fluctuates according to several environmental factors and duration of the cold exposure [22]. As soon as the rewarming or thawing phase starts, the colour will change accordingly. It can take weeks before the full extent of damage is apparent [2]. The diagnostic value of the skin colour is also highly dependent on the clinical team’s experience and diagnostic awareness.
Blisters are important clinical features as they are rather objective markers of FCI severity. If no blisters are present, the scope of the FCI might be regarded as mild. As soon as a blister is recognised, the colour of the fluid content determines the severity of the FCI. In the most serious FCI symptoms and signs other than blisters will be the marked clinical signs. There seems to be a universal agreement in the scientific literature that an FCI with a blister is regarded as a serious injury in need of proper care.
A nomenclature able to foresee the outcome of the FCI appears to be a very useful classification for both the doctor and patient [19]. It allows accurate determination at a very early stage and the likely extent of subsequent tissue loss [10]. On the other hand, FCI classification based on retrospective methodology might challenge the prospective application in acute situations to predict the prognosis for the patient [41,42,45]. The waiting period (weeks or months), necessary to determine the amputation level, is likely to cause mental anguish for the patient. FCI symptoms such as numbness and coldness of the injured part have been regarded as secondary clinical signs and symptoms, after vasoconstriction and ischaemia, because they are usually of little help for predicting prognosis before rewarming [44].
Finally, the definition of outcome/sequel of FCI has also been questioned, according to knowledge from off-label and experimental research and treatment protocols [17,39,46–48]. The outcome-classification system starts at day 0 (just after rewarming) and is, at least in part, dependent on early bone scan results.
FCI nomenclature in wilderness medicine may rely on other evaluation limits or systems than advanced trauma care in hospital. Hospital-based diagnostic techniques are challenging in early field care assessment [41] regarding early diagnosis and classification [45]. However, advances in new technology, including mobile hand-held equipment, may help this process [49]. FCI has also been invasively treated according to Cauchy grade 2 or 3 in an ambulatory setting (outpatient therapy) [39].
Further, it has been postulated that amputation from FCI almost never happens at ambient air temperatures above −10°C [2]. The majority of FCIs with an amputation outcome most often occur at air temperatures below −20°C [39]. Altitude induces a temperature drop of approximately 1°C every 150 metres of ascent (about 6.5°C/1000 m) making altitude a relevant risk factor for amputation [44]. An FCI can also occur in liquid seawater due to a higher human tissue in-freeze temperature, as seawater freezes at−1.9°C [23]. We therefore find that the ambient air temperature for FCI classification purposes might be of limited value.
Conclusions
The existing classification systems were developed for different purposes, and different FCI nomenclature is attributed to different clinical situations
Hospital-based classification systems might be challenging to use in field care situations
Field care evaluation and FCI classification may not be particularly relevant for advanced hospital care nor the assessment of long-term sequelae of FCI
The nomenclature in the different classification systems is not coherent, and the discrete classification limits are challenged
Even though further research and science into classification strategies is necessary, the existing classification system for FCI classification for monitoring and management are relevant for military activities
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Norheim AJ, Borud E.. Frostbite in the Norwegian armed forces. Tidsskr Nor Laegeforen. 2018;138(14). DOI: 10.4045/tidsskr.17.1070 [DOI] [PubMed] [Google Scholar]
- [2].Heil K, Thomas R, Robertson G, et al. Freezing and non-freezing cold weather injuries: a systematic review. Br Med Bull. 2016;117(1):79–8. [DOI] [PubMed] [Google Scholar]
- [3].Guly H. History of accidental hypothermia. Resuscitation. 2011;82(1):122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Golden FS, Francis TJ, Gallimore D, et al. Lessons from history: morbidity of cold injury in the Royal Marines during the Falklands Conflict of 1982. Extrem Physiol Med. 2013;2(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DeGroot DW, Castellani JW, Williams JO, et al. Epidemiology of U.S. Army cold weather injuries, 1980-1999. Aviat Space Environ Med. 2003;74(5):564–570. [PubMed] [Google Scholar]
- [6].Armed Forces Health Surveillance C . Cold weather injuries, active and reserve components, U.S. Armed Forces, July 2008-June 2013. MSMR. 2013;20(10):12–17. discussion 16-17. [PubMed] [Google Scholar]
- [7].Ikaheimo TM, Hassi J. Frostbites in circumpolar areas. Glob Health Action. 2011;4:8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hassi J, Rytkönen M, Kotaniemi J, et al. Impacts of cold climate on human heat balance, performance and health in circumpolar areas. Int J Circumpolar Health. 2005;64(5):459–467. [DOI] [PubMed] [Google Scholar]
- [9].Rossati A. Global warming and its health impact. Int J Occup Environ Med (The IJOEM). 2017;8(1 January):963–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Imray C, Grieve A, Dhillon S, et al. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85(1007):481–488. [DOI] [PubMed] [Google Scholar]
- [11].Long WB 3rd, Edlich RF, Winters KL, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15(1):67–78. [DOI] [PubMed] [Google Scholar]
- [12].Arvesen A, Rosen L, Eltvik LP, et al. Skin microcirculation in patients with sequelae from local cold injuries. Int J Microcirc Clin Exp. 1994;14(6):335–342. [DOI] [PubMed] [Google Scholar]
- [13].Carlsson D, Burström L, Heldestad Lilliesköld V, et al. Neurosensory sequelae assessed by thermal and vibrotactile perception thresholds after local cold injury. Int J Circumpolar Health. 2014;73(1):23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carlsson D, Pettersson H, Burström L, et al. Neurosensory and vascular function after 14 months of military training comprising cold winter conditions. Scand J Work Environ Health. 2016;42(1):61–70. [DOI] [PubMed] [Google Scholar]
- [15].Ervasti O, Hassi J, Rintamaki H, et al. Sequelae of moderate finger frostbite as assessed by subjective sensations, clinical signs, and thermophysiological responses. Int J Circumpolar Health. 2000;59(2):137–145. [PubMed] [Google Scholar]
- [16].Ingram BJ, Raymond TJ. Recognition and treatment of freezing and nonfreezing cold injuries. Curr Sports Med Rep. 2013;12(2):125–130. [DOI] [PubMed] [Google Scholar]
- [17].Regli IB, Strapazzon G, Falla M, et al. Long-Term Sequelae of Frostbite-A Scoping Review. Int J Environ Res Public Health. 2021;18(18):9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murphy JV, Banwell PE, Roberts AH, et al. Frostbite: pathogenesis and treatment. J Trauma Acute Care Surg. 2000;48(1):171. [DOI] [PubMed] [Google Scholar]
- [19].Handford C, Buxton P, Russell K, et al. Frostbite: a practical approach to hospital management. Extrem Physiol Med. 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sachs C, Lehnhardt M, Daigeler A, et al. The triaging and treatment of cold-induced injuries. Dtsch Arztebl Int. 2015;112(44):741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hall A, Sexton J, Lynch B, et al. Frostbite and immersion foot care. Mil Med. 2018;183(suppl_2):168–171. [DOI] [PubMed] [Google Scholar]
- [22].McIntosh SE, Freer L, Grissom CK, et al. Wilderness medical society clinical practice guidelines for the prevention and treatment of frostbite: 2019 update. Wilderness Environ Med. 2019;30(4S):S19–32. [DOI] [PubMed] [Google Scholar]
- [23].Castellani JW, Eglin CM, Ikäheimo TM, et al. ACSM expert consensus statement: injury prevention and exercise performance during cold-weather exercise. Curr Sports Med Rep. 2021;20(11):594–607. [DOI] [PubMed] [Google Scholar]
- [24].Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168(3):305–311. [PMC free article] [PubMed] [Google Scholar]
- [25].Imray CH, Oakley EH. Cold still kills: cold-related illnesses in military practice freezing and non-freezing cold injury. J R Army Med Corps. 2005;151(4):218–222. [DOI] [PubMed] [Google Scholar]
- [26].Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature. 2016;3(1):92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Daanen H. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89(5):411–426. [DOI] [PubMed] [Google Scholar]
- [28].Heggers JP, Robson MC, Manavalen K, et al. Experimental and clinical observations on frostbite. Ann Emerg Med. 1987;16(9):1056–1062. [DOI] [PubMed] [Google Scholar]
- [29].Hallam MJ, Cubison T, Dheansa B, et al. Managing frostbite. BMJ. 2010;341:c5864. [DOI] [PubMed] [Google Scholar]
- [30].Danielsson U. Windchill and the risk of tissue freezing. J Appl Physiol. 1996;81(6):2666–2673. [DOI] [PubMed] [Google Scholar]
- [31].Wilson O, Goldman RF, Molnar GW. Freezing temperature of finger skin. J Appl Physiol. 1976;41(4):551–558. [DOI] [PubMed] [Google Scholar]
- [32].Molnar G, Hughes A, Wilson O, et al. Effect of skin wetting on finger cooling and freezing. J Appl Physiol. 1973;35(2):205–207. [DOI] [PubMed] [Google Scholar]
- [33].Geng Q, Holmer I, Hartog DE, et al. Temperature limit values for touching cold surfaces with the fingertip. Ann Work Expo Health. 2006;50(8):851–862. [DOI] [PubMed] [Google Scholar]
- [34].Castellani JW, Young AJ, Ducharme MB, et al. Prevention of cold injuries during exercise. 2006. [DOI] [PubMed]
- [35].Lankford HV, Fox LR. The wind-chill index. Wilderness Environ Med. 2021;32(3):392–399. [DOI] [PubMed] [Google Scholar]
- [36].Qian X, Fan J. Prediction of clothing thermal insulation and moisture vapour resistance of the clothed body walking in wind. Ann Work Expo Health. 2006;50(8):833–842. [DOI] [PubMed] [Google Scholar]
- [37].Tikuisis P. Finger cooling during cold air exposure. Bull Am Meteorol Soc. 2004;85(5):717–724. [Google Scholar]
- [38].Nygaard RM, Whitley AB, Fey RM, et al. The Hennepin score: quantification of frostbite management efficacy. J Burn Care Res. 2016;37(4):e317–322. [DOI] [PubMed] [Google Scholar]
- [39].Poole A, Gauthier J, MacLennan M. Management of severe frostbite with iloprost, alteplase and heparin: a Yukon case series. CMAJ Open. 2021;9(2):E585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mills W. Clinical aspects of freezing cold injury. Textbooks Mil Med: Med Aspects Harsh Environ. 2002;1:429–466. [Google Scholar]
- [41].Cauchy E, Davis CB, Pasquier M, et al. A new proposal for management of severe frostbite in the austere environment. Wilderness Environ Med. 2016;27(1):92–99. [DOI] [PubMed] [Google Scholar]
- [42].Cauchy E, Chetaille E, Marchand V, et al. Retrospective study of 70 cases of severe frostbite lesions: a proposed new classification scheme. Wilderness Environ Med. 2001;12(4):248–255. [DOI] [PubMed] [Google Scholar]
- [43].Lindford A, Valtonen J, Hult M, et al. The evolution of the Helsinki frostbite management protocol. Burns. 2017;43(7):1455–1463. [DOI] [PubMed] [Google Scholar]
- [44].Carceller A, Javierre C, Ríos M, et al. Amputation risk factors in severely frostbitten patients. Int J Environ Res Public Health. 2019;16(8):1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cauchy E, Chetaille E, Lefevre M, et al. The role of bone scanning in severe frostbite of the extremities: a retrospective study of 88 cases. Eur J Nucl Med. 2000;27(5):497–502. [DOI] [PubMed] [Google Scholar]
- [46].Norheim AJ, Mercer J, Musial F, et al. A new treatment for frostbite sequelae; Botulinum toxin. Int J Circumpolar Health. 2017;76(1):1273677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Taylor MS. Lumbar epidural sympathectomy for frostbite injuries of the feet. Mil Med. 1999;164(8):566–567. [PubMed] [Google Scholar]
- [48].Weum S, de Weerd L. Ultrasound-guided sympathetic block of the radial artery with botulinum toxin to treat vasospasm. Plast Reconstr Surg Glob Open. 2018;6(7):e1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kaspar M, Partovi S, Aschwanden M, et al. Assessment of microcirculation by contrast-enhanced ultrasound: a new approach in vascular medicine. Swiss Med Wkly. 2015;145:0304. [DOI] [PubMed] [Google Scholar]


