Abstract
The COVID-19 pandemic necessitated remote cancer care delivery via the internet and telephone, rapidly accelerating an already growing care delivery model and associated research. This scoping review of reviews characterised the peer-reviewed literature reviews on digital health and telehealth interventions in cancer published from database inception up to May 1, 2022, from PubMed, Cumulated Index to Nursing and Allied Health Literature, PsycINFO, Cochrane Reviews, and Web of Science. Eligible reviews conducted a systematic literature search. Data were extracted in duplicate via a pre-defined online survey. Following screening, 134 reviews met the eligibility criteria. 77 of those reviews were published since 2020. 128 reviews summarised interventions intended for patients, 18 addressed family caregivers, and five addressed health-care providers. 56 reviews did not target a specific phase of the cancer continuum, whereas 48 reviews tended to address the active treatment phase. 29 reviews included a meta-analysis, with results showing positive effects on quality of life, psychological outcomes, and screening behaviours. 83 reviews did not report intervention implementation outcomes but when reported, 36 reported acceptability, 32 feasibility, and 29 fidelity outcomes. Several notable gaps were identified in these literature reviews on digital health and telehealth in cancer care. No reviews specifically addressed older adults, bereavement, or sustainability of interventions and only two reviews focused on comparing telehealth to in-person interventions. Addressing these gaps with rigorous systematic reviews might help guide continued innovation in remote cancer care, particularly for older adults and bereaved families, and integrate and sustain these interventions within oncology.
Introduction
Before the COVID-19 pandemic, digital health and telehealth were uncommon in standard, global oncology care. Within clinical research, there has been over a decade of interest on leveraging technology to increase care accessibility, scalability, and cost-effectiveness. Delivering interventions by telephone and internet—via phone calls, videoconferencing, mobile applications, and web pages—can reduce the time burdens of cancer treatment1 and extend access to evidence-based interventions for cancer prevention and control, surveillance, supportive care, treatment decision making, and more. As research in remote cancer-care delivery has grown rapidly over the past decade, with an abrupt acceleration during the pandemic, we sought to characterise the current state of the evidence by synthesising and summarising existing literature reviews in this field.
There are multiple formal definitions of both digital health and telehealth that often overlap. Digital health is defined as using “digital technologies for health, such as the internet of things”,2 and “computing platforms, connectivity, software, and sensors for health care and related uses”.3 Telehealth has been defined by the US Health Resources and Services Administration as “the use of electronic information and telecommunications technologies to support long-distance clinical health care, patient and professional health-related education, public health, and health administration”.4 Although this definition is inclusive of digital health, telehealth is often used more narrowly to describe synchronous interactions between health-care providers and patients (eg, an appointment done via encrypted videoconferencing). In this Review, the terms digital health and telehealth are used together to represent the full spectrum of remotely delivered, technology-supported, health-care interventions.
For a comprehensive summary of the science on remote cancer care, this scoping review extends the previous findings from an overview of reviews on telehealth interventions for post-treatment cancer survivors5 in three ways. First, the previous overview of reviews suggests that future research specifically addresses cancer prevention and health promotion. This scoping review addresses this need by summarising review literature on the use of telehealth across the cancer trajectory, from cancer prevention to end of life and bereavement. Second, this scoping review also provides the first compilation of reviews of digital health in cancer, which is important given the expansion of reimbursement coverage to include asynchronous electronic communication (ie, via patient portals) during the pandemic.6 Moreover, digital health practices are rapidly evolving and hold considerable promise for scaling interventions to be more accessible and cost-effective by reducing reliance on health-care personnel. The third extension of previous reviews is that this scoping review includes interventions for cancer health-care providers and family cancer caregivers. Digital health and telehealth practices hold promise for facilitating more collaborative care across health-care disciplines, and for overcoming many of the barriers family caregivers face to accessing their own health care.
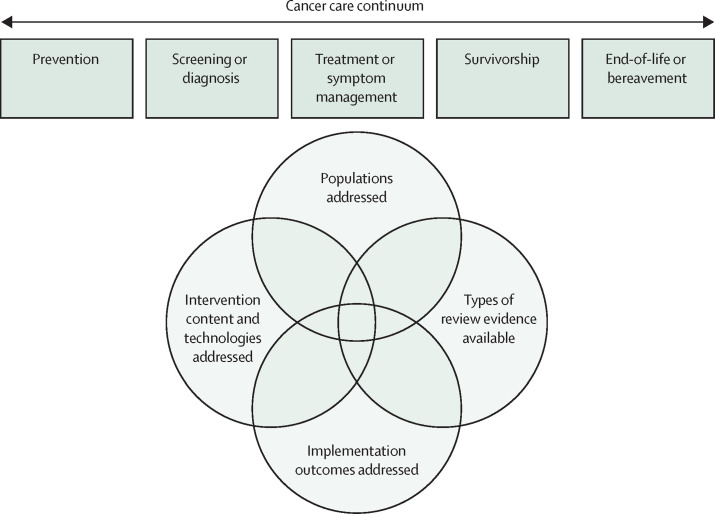
The extensions we include in this scoping review are necessary to understand the broader science on digital health and telehealth practices across cancer care. Identifying whether the summarised primary literature is inadequate or out of date is important to direct where research is most needed to improve cancer care delivered by digital health and telehealth. Describing trends in, and the available evidence for, digital and telehealth interventions and practices in cancer care can also help direct third-party payment for empirically supported services. Therefore, we identify and summarise systematic reviews of digital health and telehealth across the cancer care continuum to detail the state of this science, and to identify important gaps to guide future reviews (figure 1 ).
Figure 1.
Framework of the scoping review
Methods
A comprehensive electronic literature search was conducted for peer-reviewed systematic reviews published from database inception to May 1, 2022. The protocol was registered (INPLASY protocol 3635)7 and we adhered to PRISMA for Scoping Reviews guidelines.8 No funding source specifically supported this Review.
Eligibility criteria
The included reviews were required to meet a priori eligibility criteria: English-based or English-translated literature, published in a peer-reviewed journal, and met the population, intervention, comparison, outcome, and study design criteria as detailed.9 When possible, these criteria were built into the search strategies for each database, but all studies were reviewed manually to establish eligibility. There were no exclusion criteria based on geographical locations, participant sex or ages (ie, studies with children, adolescents, and young adults were eligible for inclusion), or reported outcomes.
For the population criteria, the exposure of interest was cancer, whether as an individual at risk for cancer, a patient undergoing cancer care, a cancer survivor, a family cancer caregiver, or a health-care provider involved in cancer care delivery. Reviews that included any studies focused on health conditions other than cancer were excluded.
For the intervention criteria, reviews were included if all studies evaluated a digital health or telehealth intervention or health-care practice, as defined by the US Health Resources and Services Administration.4 Reviews that focused on technologies that collect and transmit health data (ie, remote patient monitoring) without an explicit tie-in to intervention or improving health-care outcomes were excluded. For comparison and outcome criteria, there were no restrictions (eg, reviews including single-arm pilot trials were eligible for inclusion).
For the study design criteria, all included records were literature reviews that used a systematic search method. In addition to formally identified systematic reviews labelled as scoping, narrative, or integrative were systematic reviews otherwise labelled, which were eligible for inclusion if they specified a systematic literature search of electronic databases. We considered limiting our search to only systematic reviews adhering to PRISMA guidelines; however, to identify reviews summarising pertinent literature more broadly to digital health and telehealth in cancer care, we chose to include all reviews specifying a systematic search strategy.
Search strategy and selection criteria
The authors created search strategies with a medical librarian (CJ) to identify published systematic reviews on the topic of digital health, telehealth, and cancer. By use of a combination of medical subject headings, text phrases, keywords, and other database-specific terminologies, strategies were developed to find relevant review articles. The databases searched were PubMed, Cumulative Index to Nursing and Allied Health Literature, American Psychological Association PsycINFO, Cochrane Reviews, and Web of Science. Reference lists of related literature were also reviewed for pertinent records. Review articles selected for the initial screening included a title or abstract with at least one subject heading, phrase, or keyword related to cancer and to digital health and telehealth. Unpublished and grey literature were not pursued. Literature was initially searched on Sept 1, 2021, with an update on May 2, 2022, to include published reports before this date. As part of the peer review process, a phrase (“intervention” and “telephone”, “online”, “digital”, “mobile”, “Internet”, “technology”, “application”, or “text”) was added to the search strategy. The final search with this updated strategy was done on Sept 19, 2022, with additional search terms to identify any remaining pertinent records published up to May 1, 2022. Complete search strategies, including demarcation for the search strategy update, and database information are reported in appendix 1 (pp 1–3).
Selection and screening process
Unique records were compared with the eligibility criteria with Rayyan,10 an online review tool. Coders were not masked to journals or authors during screening. Before the first screening round, a screening guide was created to train all reviewing authors on the eligibility criteria. In the first round, study titles and abstracts were reviewed by two of three coders (KMS, RU, or JVG). Discrepancies between coders were resolved by consensus. All citations that initially met the criteria were included in a second round of full-text article screening. Full-text articles were reviewed by two of eight authors (KMS, KLT, CS, BDG, RU, JVG, RJF, or CAL), with discrepancies resolved by KMS or RU. Reasons for exclusion during the full-text review stage were recorded.
Data extraction and summary
Data were extracted from the included records with a standardised, predefined form with Qualtrics, an online survey tool, which was pilot tested by coders. Articles were not blinded during data extraction. Data were extracted independently by two of eight authors (KMS, KLT, CS, BDG, RU, JVG, RJF, CAL), with discrepancies resolved by KMS, RU, or JVG. Coders extracted review metadata and data about the eligible population, intervention characteristics, and study design (appendix pp 4–7). Extraction and categorisation of implementation outcomes according to the terminology used by Proctor and colleagues11 was completed post-hoc by consensus of KMS, KLT, and CS. Article selection, screening, and data extraction were completed by Oct 4, 2022. Data were tabled and summarised, and gaps in the review literature were identified.
Results
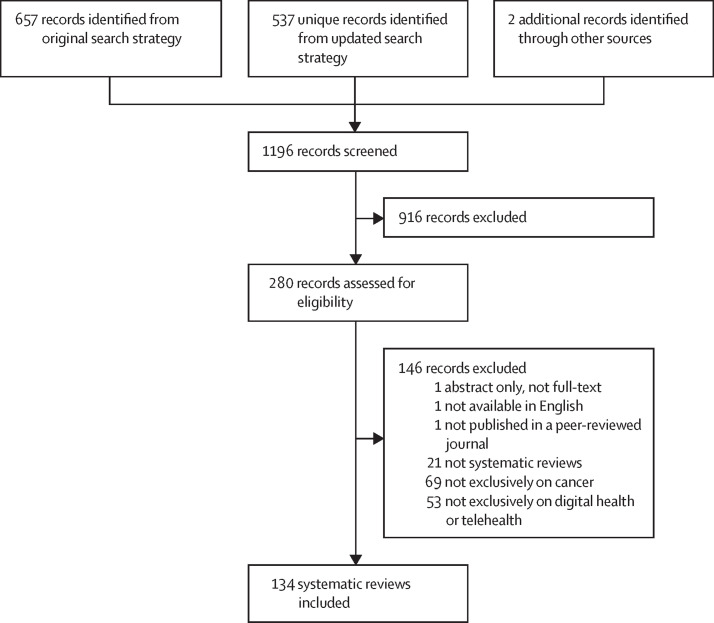
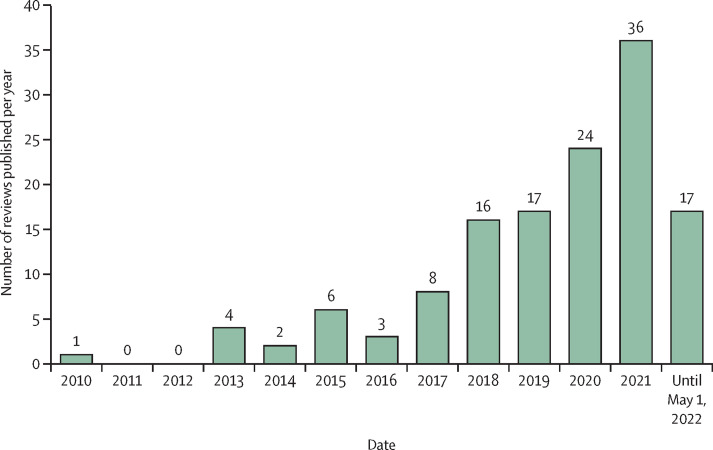
In total, 1196 unique records were identified, of which 280 full texts were assessed for eligibility (figure 2 ). Of these, 146 were excluded (see appendix 2 on screening results for a full list of excluded full text reviews with exclusion reasons). The remaining 134 reviews met eligibility criteria and were included in the synthesis.12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145 Study information is presented in the table and appendix (pp 8–46). Included reviews were published as early as 2010, with a notable increase beginning in 2018 (figure 3 ). The median number of studies included in the reviews was 16 (range: 0–121). We found no evidence to indicate that any of the reviews were industry sponsored.
Figure 2.
PRISMA flowchart
Table.
Descriptions of 134 included reviews from 2010 to 2022
| Reviews, n (%) | ||
|---|---|---|
| Population data* | ||
| Targeted individuals | ||
| Patients | 128 (95·5%) | |
| Family | 18 (13·4%) | |
| Health-care personnel | 5 (3·7%) | |
| Cancer types | ||
| Brain | 1 (0·7%) | |
| Breast | 17 (12·7%) | |
| Colorectal | 5 (3·7%) | |
| Gynecological | 7 (5·2%) | |
| Haematological | 4 (3·0%) | |
| Lung | 3 (2·2%) | |
| Prostate | 4 (3·0%) | |
| Skin | 6 (4·5%) | |
| Multiple or not specified | 95 (70·9%) | |
| Cancer care continuum | ||
| Prevention | 5 (3·7%) | |
| Screening or diagnosis | 12 (9·0%) | |
| Treatment or symptom management | 48 (35·8%) | |
| Survivorship | 29 (21·6%) | |
| End-of-life or bereavement | 2 (1·5%) | |
| Multiple or not specified | 56 (41·8%) | |
| Intervention data† | ||
| Telehealth components | ||
| Synchronous telehealth | 78 (58·2%) | |
| Text messaging or short messaging service | 37 (27·6%) | |
| Email or secure messaging | 39 (29·1%) | |
| eHealth | 87 (64·9%) | |
| Mobile application | 78 (58·2%) | |
| Types of intervention or care | ||
| Health behaviour change | 24 (17·9%) | |
| Psychosocial support or distress management | 18 (13·4%) | |
| Disease detection or management | 14 (10·4%) | |
| Medical decision making | 2 (1·5%) | |
| Multiple or not specified | 73 (54·5%) | |
| Provider involvement | ||
| Health-care professional | 9 (6·7%) | |
| Fully automated or self-guided | 1 (0·7%) | |
| Multiple or not specified | 124 (92·5%) | |
| Review design data | ||
| Meta-analysis | ||
| Yes | 29 (21·6%) | |
| No | 105 (78·4%) | |
| Comparator (if required) | ||
| Usual care | 11 (8·2%) | |
| In-person | 2 (1·5%) | |
| Any | 18 (13·4%) | |
| Other telehealth modality | 0 (0%) | |
| None required | 103 (76·9%) | |
| Quality or bias assessment | ||
| Yes | 90 (67·2%) | |
| No | 44 (32·8%) | |
| Implementation outcome data (Proctor et al. classification)† | ||
| None | 83 (61·9%) | |
| Acceptability | 36 (26·9%) | |
| Adoption | 8 (6·0%) | |
| Appropriateness | 6 (4·5%) | |
| Feasibility | 32 (23·9%) | |
| Fidelity | 29 (21·6%) | |
| Cost | 8 (6·0%) | |
The median number of studies included per review was 16 with a range from 0–121. Albino de Queiroz and colleagues55 included two reviews within the single publication (N1=121 and N2=15). Each sample was included separately for the computation of these statistics.
Reviews might be reflected in more than one category (eg, can include interventions for both patients and family members, or report more than one kind of implementation outcome; except for multiple or not specified, or none).
Figure 3.
Number of reviews published per year on digital health and telehealth in cancer
All extracted data are also available in sortable worksheets available at the Open Science Framework. These worksheets serve as comprehensive and complete evidence maps, which readers are encouraged to use to further examine how populations, intervention content and technologies, types of review evidence, and implementation outcomes addressed by reviews differ across the cancer-care continuum, among other questions of interest.
Populations
128 (95·5%) of 134 included reviews addressed patient-level intervention studies. 18 (13·4%) of 134 reviews included caregiver-level intervention studies and only five focused exclusively on caregivers. Five reviews (3·7%) addressed provider-level interventions that aimed to improve patient cancer care experiences through provider continued education or enhanced intra-provider collaboration.
95 reviews (70·9%) did not restrict study inclusion on the basis of cancer type. Of reviews that addressed one or more specific cancer types, 17 were on breast cancer, seven were gynaecological, six were on skin cancer, five were on colorectal cancer, four were on prostate cancer, four were haematological, three were on lung cancer, and one was on brain cancer. Ten reviews (7·5%) focused on paediatric, adolescent, or young adult patients with cancer or their caregivers. None of the reviews focused on older adult patients with cancer or caregivers.
56 (41·8%) of 134 studies did not restrict inclusion (or did not specify) on the basis of the stage of the care continuum, although they tended to focus on interventions delivered during active treatment through survivorship. Among the reviews that focused on one or more specific stages of the continuum, 48 (35·8%) addressed the active treatment phase. Two reviews addressed studies of care for patients at the end of life, but none addressed caregiver bereavement.
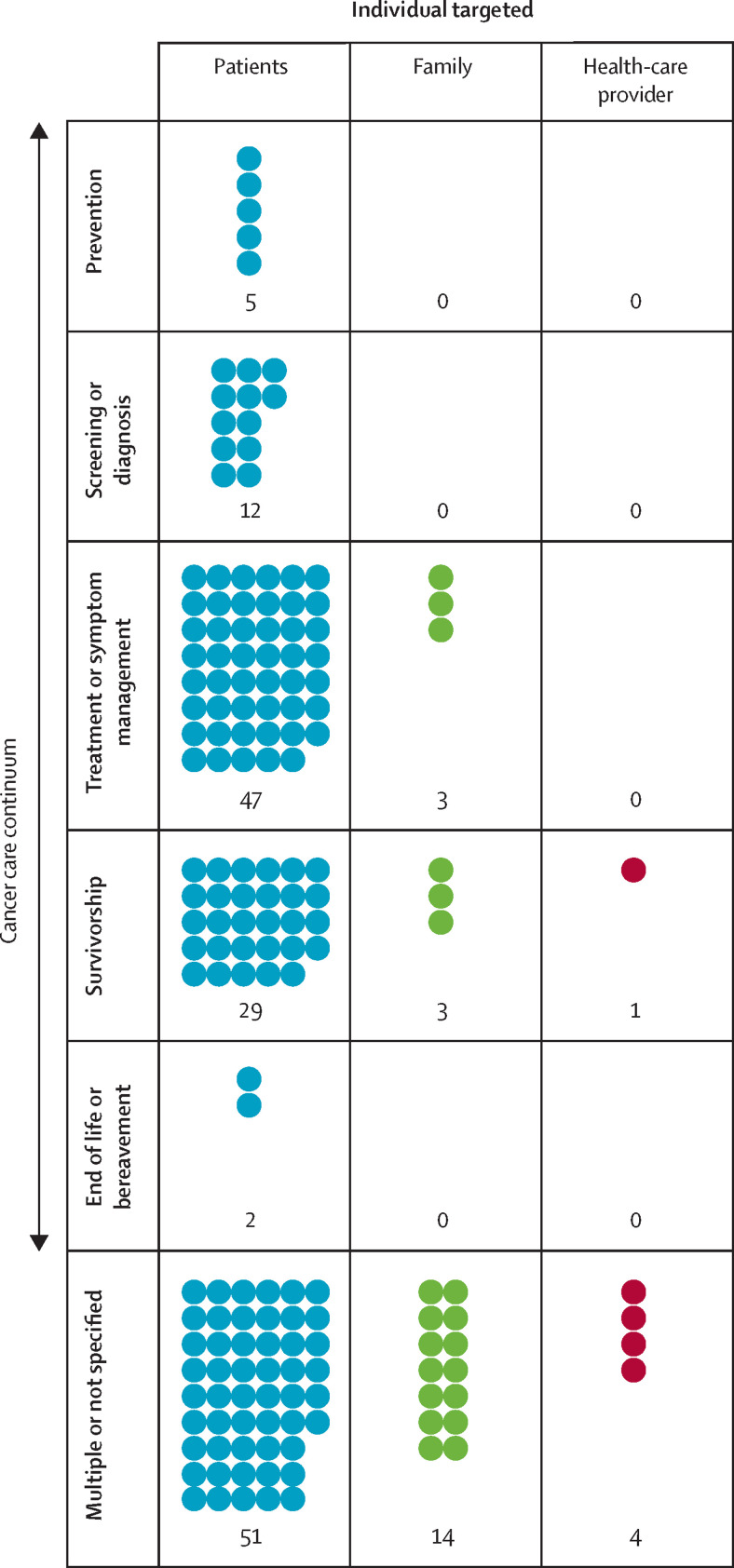
The frequencies of reviews targeting patients, family members, or health-care providers across the stages of the cancer care continuum are summarised in a visual evidence map in figure 4 . Readers can explore additional cross-tabulations of interest with the sortable worksheets of extracted data in appendix 3 on review details.
Figure 4.
Evidence map illustrating frequencies of reviews by individual targeted by intervention across the cancer care continuum
Interventions
Rather than focus on a single intervention delivery method, reviews tended to include studies of interventions across digital health and telehealth delivery methods. 87 (64·9%) included studies of interventions delivered entirely or in part by eHealth interventions, 78 (58·2%) of synchronous telehealth, and 78 (58·2%) of mobile applications. Reviews less commonly included studies of interventions that used email or secure asynchronous messaging platforms (n=39; 29·1%) or text or short messaging services (n=37; 27·6%). Other delivery components included use of wearable devices (n=12; 9·0%), social media (n=8; 6·0%), virtual reality (n=8; 6·0%), automated phone calls or interactive voice response calls (n=7; 5·2%), store-and-forward image transfer (n=6; 4·5%), electronic health records or patient portals (n=5; 3·7%), active or video games (n=4; 3·0%), and interactive robot-assisted therapy (3; 2·2%).
73 (54·5%) reviews did not restrict inclusion (or did not specify) on the basis of intervention type. Among reviews that addressed one or more specific types of interventions, 24 (17·9%) addressed health behaviour change, followed by 18 (13·4%) of psychosocial support or distress management, and 14 (10·4%) of disease detection or management. In addition, 124 reviews (92·5%) did not restrict (or did not specify) inclusion on the basis of the type of provider involvement in delivery. Only one review clearly specified inclusion of only fully automated programmes (ie, no human involvement), regarding the use of artificial intelligence (AI) for diagnosing skin cancer or melanoma from skin lesion pictures.22
Review design
29 reviews (21·6%) included a meta-analysis of all or a subset of their included studies. Among these 29 meta-analyses, digital health and telehealth interventions were compared with any comparator in 14 meta-analyses (48·3%) or to usual care in nine meta-analyses (31·0%). One meta-analysis compared genetic counselling delivered by telehealth versus in-person, determining that telehealth was not inferior to in-person counselling on the two evaluated outcomes of cancer-specific distress and knowledge. Across meta-analyses, the most examined outcome was quality of life: 11 meta-analyses reported positive effects of interventions relative to control and six reported null findings. Meta-analyses also frequently reported positive findings for interventions for outcomes including depression (nine positive effects, four null effects), anxiety (eight positive, three null), self-efficacy (five positive, no null), physical activity (four positive, one null), and cancer screenings (four positive, no null). The evidence was mixed for other outcomes like fatigue (five positive effects, five null effects), pain (three positive, four null), and distress (two positive, two null). Many reviews indicated that a meta-analysis was not done due to heterogeneity of methods and outcomes.13, 15, 18, 21, 23, 29, 40, 45, 46, 49, 60, 65, 69, 74, 75, 86, 88, 89, 90, 97, 100, 104, 110, 116, 118, 131, 134
113 reviews (84·3%) were termed systematic review; seven (5·2%) included the term scoping, and five (3·7%) included the term integrative. Methodological quality or risk of bias assessment is considered an essential reporting element for systematic reviews146 and 81 of the 113 systematic reviews (71·7%) reported such an assessment.
Implementation outcomes
83 (61·9%) of 134 reviews did not report implementation-related outcomes. This finding could either be because studies included in reviews did not report these outcomes or because reviews did not extract this information from studies. Among reviews that extracted implementation-related outcomes, 36 (26·9%) fell into the categories of acceptability, 32 (23·9%) of feasibility, and 29 (21·6%) of fidelity. None of the reviews extracted mid-stage to late-stage implementation outcomes like penetration or sustainability.
Discussion
To our knowledge, we conducted the first scoping review of literature reviews on the use of digital health and telehealth interventions across the cancer continuum for patients, caregivers, and health-care providers. The spread of digital health and telehealth in cancer care during the COVID-19 pandemic necessitated an overview of the state of this science, to identify gaps in what is known about remote cancer care delivery. Our scoping review revealed an extensive and recent body of review literature on digital health and telehealth interventions in cancer care. Overall, most reviews focused on interventions delivered to patients with cancer in the active treatment and survivorship phases using eHealth programs, synchronous calls by telephone or videoconferencing, and mobile applications. Common conclusions drawn by reviews were that digital health and telehealth interventions were feasible and effective, but the need for larger-scale and robust efficacy testing remains.
This scoping review identified several major gaps in the review literature in digital health and telehealth in cancer care. Specifically, there are opportunities to improve the knowledge base related to populations, interventions, review designs, and implementation outcomes. Systematic reviews addressing these gaps can help improve digital health and telehealth cancer care for all individuals by establishing the extent of the primary research and potential efficacy of interventions across the broad scope of remote cancer care.
Opportunities in populations
Most of the included reviews addressed digital health and telehealth interventions for individuals at risk for cancer (ie, cancer prevention), those receiving treatment (ie, patients), or those recovered from cancer (ie, survivors); however, several subpopulations were missing. For example, none of the reviews focused on older patients with cancer, which is a considerable gap given that more than half of individuals diagnosed with cancer are aged 65 years or older.147 Although often assumed that digital health or telehealth interventions are a poor fit for older adults, there is substantial promise for this type of care to support independent ageing.148 The adoption of digital technologies among older adults continues to rise,149 and digital health and telehealth interventions can help alleviate common health-care barriers faced by older adults, such as rurality150 and poverty.151 Moreover, one study of patients with uro-oncological cancer suggests similar interest in remote patient monitoring with digital technologies between older and younger patients, with older patients even reporting to be willing to engage with digital technologies as part of trials more frequently than younger patients.152
Three reviews addressed telehealth interventions for individuals facing advanced cancer, including those at the end of life.17, 23, 73 Characterising the use and benefits of digital health and telehealth interventions for these populations is important as many patients with terminal cancer and their families prefer palliative care delivered at home versus a health-care facility.153 Although bereavement care for families is traditionally a component of comprehensive palliative care and was included in several of the digital health and telehealth palliative care programs covered in reviews (eg, Dionne-Odom and colleagues and Pearce and colleagues),154, 155 none of the reviews specifically addressed family caregiver bereavement. This finding reflects the restricted focus on family cancer caregivers across reviews overall. Reviews have been conducted on digital health and telehealth bereavement care outside of oncology.156, 157 As caregivers’ health-related needs and grief reactions differ by disease context,158, 159 it is necessary to explore the availability, efficacy, and acceptability of digital health and telehealth interventions for cancer-related bereavement.
Opportunities in interventions
Digital health and telehealth interventions can range in their purpose to support “long-distance clinical health care, patient and professional health-related education, public health, and health administration”.4 Most identified reviews focused on long-distance cancer care, patient education, and public health (ie, cancer prevention interventions), with less focus on professional health-related education and administration. Synchronous telehealth services for long-distance cancer care were perhaps most commonly reviewed, in part due to a record of reimbursement before (and expanded upon during) the COVID-19 pandemic. In the example of US states and territories, telehealth services before the pandemic were reimbursed by government and private health insurance payers, although typically with requirements such as patients must have had previously received services in a licensed health-care facility. These requirements were largely dropped during the COVID-19 pandemic, and reimbursement policies continue to evolve. Monitoring professional reimbursement policy changes will be crucial to optimise the use and dissemination of telehealth services moving forward.
Five reviews did specifically address digital health and telehealth practices to support interdisciplinary collaboration and continuing education for oncology providers, with the end goal of improving cancer care for patients.25, 49, 82, 109, 127 However, no reviews addressed tools for oncology health-care provider wellbeing. Given the serious toll of the COVID-19 pandemic on health-care workers,160 there is an opportunity to establish viable digital health and telehealth interventions to mitigate occupational-health effects under the ongoing pandemic conditions.
Relatively few reviews included interventions with emerging technologies (eg, serious games and virtual reality) or integration with the internet of things (eg, wearable devices and smart speakers). One notable exception was a review by Albino de Queiroz and colleagues,55 which comprehensively described the utility and results of applying the internet of things to cancer care. Given the rapid evolution of the technical capacity of digital health, continually updating summaries of this literature will be crucial for the field to stay current and identify emerging opportunities to improve digital health interventions in cancer care.
There was also only one review focusing on digital health programs delivered without the input of any support personnel: a study of mobile applications that use AI to diagnose skin cancer or melanoma from skin lesion pictures, which concluded that evidence was insufficient (by the time of the review) to rely on AI to identify all diagnoses.22 No reviews specifically focused on entirely self-guided psychological, behavioural, or educational interventions delivered without the support of any personnel. Although there were reviews of interventions that could plausibly be fully automated,12, 13, 14, 18, 21, 36, 38, 52, 66 eligibility criteria did not require full automation, and incorporation of clinical support was unclear. Kiss and colleagues89 focused on self-guided interventions but included those with minimal facilitation. The public health effects of including professional guidance should be carefully weighed. Digital health interventions that include some guidance tend to result in greater improvements than stand-alone interventions.161, 162 Conversely, fully automated interventions provide greater scalability, accessibility, and cost-effectiveness.163 Rigorous reviews regarding this topic could help ascertain the benefits and costs of including professional guidance to better decide when more or less clinician support is warranted.
Opportunities in review designs
Approximately one-fifth of the reviews included a meta-analysis, and of those, most compared digital health and telehealth interventions with usual care or any kind of comparator. Only one meta-analysis compared telehealth with in-person care delivery. This unique review by Bracke and colleagues34 concluded that telehealth genetic counselling did not differ from in-person counselling regarding patients’ cancer-related distress or knowledge. Further reviews establishing how digital health and telehealth interventions intended for remote care delivery compare with in-person interventions on clinically salient outcomes might be important in ensuring that digital health and telehealth care becomes reimbursable,164 or remains reimbursable following expiration of COVID-19 public health emergency policies. Comparison with in-person care, however, is not pertinent for every research question. For example, payers might want to know whether it is equally beneficial to see a provider in-office versus by videoconference call, whereas consumers might be more interested in information to better choose between two or more digital health and telehealth programs. Importantly, none of the reviews attempted to compare different digital health and telehealth interventions to one another.
Another gap in the meta-analysis literature is that there were no meta-analyses identified that tested the effects of digital health or telehealth on real-world health-care utilisation data. Meta-analyses tended to focus on self-reported symptom questionnaires, which might reflect the primary literature tending to have self-reported endpoints. There were three meta-analyses that addressed objective clinical outcomes like range-of-motion and lymphoedema,14, 50, 52 with all three focusing on patients and survivors of breast cancer. Future meta-analyses regarding the effects of digital health and telehealth intervention on patient morbidity, mortality, and health-care utilisation factors (eg, office visits, use of emergency services, and hospitalisations) would provide crucial information to inform reimbursement and coverage decisions. Such reviews would also reveal the extent to which primary literature in digital health and telehealth has examined these outcomes, potentially revealing where gaps in the primary literature exist.
Regardless of the research question addressed by a systematic review, about 30% of the identified systematic reviews identified in our search did not conduct a methodological quality rating of the included studies, which is essential for interpreting findings. This outcome is consistent with an earlier meta-review of telehealth in cancer care5 that also identified a need to ensure consistent adherence to PRISMA guidelines, another opportunity for future improvement of this literature base.
Opportunities in implementation outcomes
About two in five reviews reported implementation-related outcomes. Of particular interest, because reimbursement and coverage of digital health and telehealth services remains in flux, only eight reviews directly addressed economic outcomes, with three specifically discussing these findings in a dedicated section.61, 68, 90 Across all eight reviews, the proportion of primary studies including health economic outcomes ranged from 0% (0/8)20 to 31% (6/19)90 of included studies, with outcomes including direct costs, cost minimisation, and cost-effectiveness (eg, on quality-adjusted life-years). As with mortality and health-care utilisation outcomes, more synthesised data on the various economic effects of digital health and telehealth interventions will be helpful to decision makers when considering coverage and implementation of such interventions.
Most of the implementation outcomes data were extracted from early-stage research studies, with few reviews focusing on the implementation of digital health and telehealth interventions into routine care. With a shift toward remotely delivered care precipitated by the COVID-19 pandemic, there might be opportunities to review studies focused on the use of digital health and telehealth practices within standard care, and eventual opportunities to describe mid-range to long-range implementation outcomes related to reach and sustainability of these practices. One of the earliest identified reviews, Sanchez and colleagues’ 2013 review, stands out in its application of implementation frameworks to their review of internet-based cancer prevention and control interventions.124 This review highlighted the need for more pragmatic trial designs to accelerate the implementation of effective digital health and telehealth interventions into routine care. Unfortunately, it seems that the field of cancer-related digital health and telehealth interventions is still struggling to implement this finding almost a decade later, given that reviews since that time frequently concluded that findings were preliminary and more robust, larger-scale studies were needed.
Limitations
Given that we conducted a scoping review of reviews, the identified gaps in the literature only establish that a review was not identified in a given area of remote cancer care, which might not generalise to the primary literature. Because we restricted the scope of our review to cancer, there could be reviews pertinent to identified gaps that are disease-agnostic. In addition, we did not extract review information pertinent to health equity, such as the racial or ethnic composition of samples or rurality. Several reviews indicated the generalisability of the included literature was restricted due to study samples comprising largely non-Hispanic White, highly educated, or breast cancer participants;44, 78, 111 however, there were reviews specifically focused on underserved populations and studies in low-income and middle-income countries.57, 109, 120, 131, 142
Conclusions
In this first-of-its-kind comprehensive scoping review of reviews on digital health and telehealth interventions across the cancer continuum, we summarised the existing review literature and identified several gaps. We identified an absence of review literature for older adults in cancer care, cancer-related bereavement, oncology provider wellness, and mid-range to long-range implementation outcomes. Additionally, there were few meta-analytic comparisons to traditional in-person care or between multiple digital health and telehealth programmes. Establishing the state of the science in these areas with high-quality reviews might help guide continued innovation in remote care delivery, particularly for older adults and bereaved families, and support the integration and sustainability of these interventions within standard oncology practice. This work will support continued growth in the reach and effects of digital health and telehealth interventions to reduce cancer risk and improve cancer care for all individuals.
Declaration of interests
BDG reports fees unrelated to this work from Sure Med Compliance and Elly Health, and was supported in part by a National Cancer Institute Cancer Center Support Grant supporting the Population Research, Interventions, and Measurements Core at Moffitt Cancer Center (P30 CA076292). CS was supported by a National Center for Complementary and Integrative Health T32 Fellowship (5T32AT003997-13). RJF and CAL were supported by the National Cancer Institute Cancer Center Support Grant (P30CA047904). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
This Review was undertaken in response to the Society of Behavioral Medicine Cancer Special Interest Group Technology and Remote Care Delivery Task Force 2022 Report.
Acknowledgments
Contributors
KMS led all aspects of the Review: conceptualisation, data curation (title and abstract screening, full-text screening, and data extraction), formal analysis, project administration, supervision, validation, and writing (original draft, review, and editing); KLT, CS, and BDG were involved in data curation (full-text screening and data extraction), validation, and writing (review and editing); RU and JVG were involved in data curation (title and abstract screening, full-text screening, and data extraction), validation, writing, and review; RJF was involved in conceptualisation, data curation (full text screening and data extraction), and writing (review and editing); CJ is the medical librarian and was responsible for devising (in collaboration with KMS) and executing the search strategy for this scoping review, and was involved in writing (original draft and writing, review, and editing); CAL was involved in conceptualisation, data curation (full text screening and data extraction), validation, writing (original draft), and writing (review and editing). All authors have had full access to the data and accept responsibility to submit for publication.
Supplementary Materials
References
- 1.Gupta A, Eisenhauer EA, Booth CM. The time toxicity of cancer treatment. J Clin Oncol. 2022;40:1611–1615. doi: 10.1200/JCO.21.02810. [DOI] [PubMed] [Google Scholar]
- 2.WHO Global strategy on digital health 2020–2025. 2021. https://www.who.int/docs/default-source/documents/gs4dhdaa2a9f352b0445bafbc79ca799dce4d.pdf [PubMed]
- 3.US Food and Drug Administration What is digital health? Sept 22, 2020. https://www.fda.gov/medical-devices/digital-health-center-excellence/what-digital-health
- 4.Office of the National Coordinator for Health Information Technology HealthIT.gov. Oct 17, 2019. https://www.healthit.gov/faq/what-telehealth-how-telehealth-different-telemedicine
- 5.Chan RJ, Crichton M, Crawford-Williams F, et al. The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: an overview of systematic reviews. Ann Oncol. 2021;32:1552–1570. doi: 10.1016/j.annonc.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services Medicare telemedicine health care provider fact sheet. Mar 17, 2020. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet
- 7.Shaffer K, Turner K, Siwik C, et al. Digital health and telehealth in cancer care: a protocol for a scoping review of reviews. July 18, 2022. https://inplasy.com/inplasy-2022-7-0089/ [DOI] [PMC free article] [PubMed]
- 8.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 9.NHS Centre for Reviews and Dissemination . 2nd edn. University of York; York: 2001. Undertaking systematic reviews of research on effectiveness: CRD's guidance for those carrying out or commissioning reviews. CRD report 4. [Google Scholar]
- 10.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38:65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acuna N, Vento I, Alzate-Duque L, Valera P. Harnessing digital videos to promote cancer prevention and education: a systematic review of the literature from 2013–2018. J Cancer Educ. 2020;35:635–642. doi: 10.1007/s13187-019-01624-0. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad M, Mohammad EB, Anshasi HA. Virtual reality technology for pain and anxiety management among patients with cancer: a systematic review. Pain Manag Nurs. 2020;21:601–607. doi: 10.1016/j.pmn.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Bu X, Ng PH, Xu W, et al. The effectiveness of virtual reality-based interventions in rehabilitation management of breast cancer survivors: systematic review and meta-analysis. JMIR Serious Games. 2022;10 doi: 10.2196/31395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agboola SO, Ju W, Elfiky A, Kvedar JC, Jethwani K. The effect of technology-based interventions on pain, depression, and quality of life in patients with cancer: a systematic review of randomized controlled trials. J Med Internet Res. 2015;17:e65. doi: 10.2196/jmir.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandeying N, Thongseiratch T. Online interventions to improve mental health of pediatric, adolescent, and young adult cancer survivors: a systematic review and meta-analysis. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.784615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari N, Wilson CM, Heneghan MB, Supiano K, Mooney K. How technology can improve communication and health outcomes in patients with advanced cancer: an integrative review. Support Care Cancer. 2022;30:6525–6543. doi: 10.1007/s00520-022-07037-y. [DOI] [PubMed] [Google Scholar]
- 18.Chirico A, Lucidi F, De Laurentiis M, Milanese C, Napoli A, Giordano A. Virtual reality in health system: beyond entertainment. A mini-review on the efficacy of VR during cancer treatment. J Cell Physiol. 2016;231:275–287. doi: 10.1002/jcp.25117. [DOI] [PubMed] [Google Scholar]
- 19.Forbes CC, Finlay A, McIntosh M, Siddiquee S, Short CE. A systematic review of the feasibility, acceptability, and efficacy of online supportive care interventions targeting men with a history of prostate cancer. J Cancer Surviv. 2019;13:75–96. doi: 10.1007/s11764-018-0729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curry J, Patterson M, Greenley S, Pearson M, Forbes CC. Feasibility, acceptability, and efficacy of online supportive care for individuals living with and beyond lung cancer: a systematic review. Support Care Cancer. 2021;29:6995–7011. doi: 10.1007/s00520-021-06274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow H, Hon J, Chua W, Chuan A. Effect of virtual reality therapy in reducing pain and anxiety for cancer-related medical procedures: a systematic narrative review. J Pain Symptom Manage. 2021;61:384–394. doi: 10.1016/j.jpainsymman.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Freeman K, Dinnes J, Chuchu N, et al. Algorithm based smartphone apps to assess risk of skin cancer in adults: systematic review of diagnostic accuracy studies. BMJ. 2020;368:m127. doi: 10.1136/bmj.m127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kane K, Kennedy F, Absolom KL, Harley C, Velikova G. Quality of life support in advanced cancer-web and technological interventions: systematic review and narrative synthesis. BMJ Support Palliat Care. 2021 doi: 10.1136/bmjspcare-2020-002820. [DOI] [PubMed] [Google Scholar]
- 24.Kaltenbaugh DJ, Klem ML, Hu L, Turi E, Haines AJ, Hagerty LJ. Using web-based interventions to support caregivers of patients with cancer: a systematic review. Oncol Nurs Forum. 2015;42:156–164. doi: 10.1188/15.ONF.156-164. [DOI] [PubMed] [Google Scholar]
- 25.Hong YA, Hossain MM, Chou WS. Digital interventions to facilitate patient-provider communication in cancer care: a systematic review. Psychooncology. 2020;29:591–603. doi: 10.1002/pon.5310. [DOI] [PubMed] [Google Scholar]
- 26.Hong HC, Min A, Kim YM. The effectiveness of digital self-management interventions on health outcomes among childhood cancer survivors: a systematic review and meta-analysis. J Adv Nurs. 2021;77:4387–4399. doi: 10.1111/jan.14925. [DOI] [PubMed] [Google Scholar]
- 27.Ayyoubzadeh SM, R Niakan Kalhori S, Shirkhoda M, Mohammadzadeh N, Esmaeili M. Supporting colorectal cancer survivors using eHealth: a systematic review and framework suggestion. Support Care Cancer. 2020;28:3543–3555. doi: 10.1007/s00520-020-05372-6. [DOI] [PubMed] [Google Scholar]
- 28.Badr H, Carmack CL, Diefenbach MA. Psychosocial interventions for patients and caregivers in the age of new communication technologies: opportunities and challenges in cancer care. J Health Commun. 2015;20:328–342. doi: 10.1080/10810730.2014.965369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bártolo A, Pacheco E, Rodrigues F, Pereira A, Monteiro S, Santos IM. Effectiveness of psycho-educational interventions with telecommunication technologies on emotional distress and quality of life of adult cancer patients: a systematic review. Disabil Rehabil. 2019;41:870–878. doi: 10.1080/09638288.2017.1411534. [DOI] [PubMed] [Google Scholar]
- 30.Bender JL, Yue RYK, To MJ, Deacken L, Jadad AR. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013;15:e287. doi: 10.2196/jmir.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhochhibhoya S, Dobbs PD, Maness SB. Interventions using mHealth strategies to improve screening rates of cervical cancer: a scoping review. Prev Med. 2021;143 doi: 10.1016/j.ypmed.2020.106387. [DOI] [PubMed] [Google Scholar]
- 32.Blackwood J, Rybicki K. Outcomes of telehealth-delivered physical activity programs in adult cancer survivors: a systematic review. Rehabil Oncol. 2021;39:128–136. [Google Scholar]
- 33.Bouma G, Admiraal JM, de Vries EGE, Schröder CP, Walenkamp AME, Reyners AKL. Internet-based support programs to alleviate psychosocial and physical symptoms in cancer patients: a literature analysis. Crit Rev Oncol Hematol. 2015;95:26–37. doi: 10.1016/j.critrevonc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Bracke X, Roberts J, McVeigh TP. A systematic review and meta-analysis of telephone vs in-person genetic counseling in BRCA1/BRCA2 genetic testing. J Genet Couns. 2021;30:563–573. doi: 10.1002/jgc4.1343. [DOI] [PubMed] [Google Scholar]
- 35.Bruce AF, Mallow JA, Theeke LA. The use of teledermoscopy in the accurate identification of cancerous skin lesions in the adult population: a systematic review. J Telemed Telecare. 2018;24:75–83. doi: 10.1177/1357633X16686770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y, Kang SJ. Computerized programs for cancer survivors with cognitive problems: a systematic review. J Cancer Surviv. 2019;13:911–920. doi: 10.1007/s11764-019-00807-4. [DOI] [PubMed] [Google Scholar]
- 37.Buneviciene I, Mekary RA, Smith TR, Onnela J-P, Bunevicius A. Can mHealth interventions improve quality of life of cancer patients? A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;157 doi: 10.1016/j.critrevonc.2020.103123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cazeau N. Mobile health interventions: examining medication adherence outcomes among patients with cancer. Clin J Oncol Nurs. 2021;25:431–438. doi: 10.1188/21.CJON.431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y-Y, Guan B-S, Li Z-K, Li X-Y. Effect of telehealth intervention on breast cancer patients' quality of life and psychological outcomes: a meta-analysis. J Telemed Telecare. 2018;24:157–167. doi: 10.1177/1357633X16686777. [DOI] [PubMed] [Google Scholar]
- 40.Cheng L, Liu F, Mao X, et al. The pediatric cancer survivors' user experiences with digital health interventions: a systematic review of qualitative data. Cancer Nurs. 2022;45:E68–E82. doi: 10.1097/NCC.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 41.Cheng L, Duan M, Mao X, Ge Y, Wang Y, Huang H. The effect of digital health technologies on managing symptoms across pediatric cancer continuum: a systematic review. Int J Nurs Sci. 2020;8:22–29. doi: 10.1016/j.ijnss.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho Y, Zhang H, Harris MR, Gong Y, Smith EL, Jiang Y. Acceptance and use of home-based electronic symptom self-reporting systems in patients with cancer: systematic review. J Med Internet Res. 2021;23 doi: 10.2196/24638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi J, Cho Y, Woo H. mHealth approaches in managing skin cancer: systematic review of evidence-based research using integrative mapping. JMIR Mhealth Uhealth. 2018;6:e164. doi: 10.2196/mhealth.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopp LM, Gastelum Z, Guerrero CH, Howe CL, Hingorani P, Hingle M. Lifestyle behavior interventions delivered using technology in childhood, adolescent, and young adult cancer survivors: a systematic review. Pediatr Blood Cancer. 2017;64:13–17. doi: 10.1002/pbc.26166. [DOI] [PubMed] [Google Scholar]
- 45.Lim NLY, Shorey S. Effectiveness of technology-based educational interventions on the empowerment related outcomes of children and young adults with cancer: a quantitative systematic review. J Adv Nurs. 2019;75:2072–2084. doi: 10.1111/jan.13974. [DOI] [PubMed] [Google Scholar]
- 46.Christopherson U, Wells SJ, Parker N, et al. Use of active video games with or without videoconferencing on health outcomes in adolescent and young adult cancer survivors: a systematic review. J Cancer Surviv. 2022;16:714–727. doi: 10.1007/s11764-021-01065-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coughlin S, Thind H, Liu B, Champagne N, Jacobs M, Massey RI. Mobile phone apps for preventing cancer through educational and behavioral interventions: state of the art and remaining challenges. JMIR Mhealth Uhealth. 2016;4:e69. doi: 10.2196/mhealth.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suh SR, Lee MK. Effects of nurse-led telephone-based supportive interventions for patients with cancer: a meta-analysis. Oncol Nurs Forum. 2017;44:E168–E184. doi: 10.1188/17.ONF.E168-E184. [DOI] [PubMed] [Google Scholar]
- 49.Mikles SP, Griffin AC, Chung AE. Health information technology to support cancer survivorship care planning: a systematic review. J Am Med Inform Assoc. 2021;28:2277–2286. doi: 10.1093/jamia/ocab134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo X, Chen Y, Chen J, et al. Effectiveness of mobile health-based self-management interventions in breast cancer patients: a meta-analysis. Support Care Cancer. 2022;30:2853–2876. doi: 10.1007/s00520-021-06568-0. [DOI] [PubMed] [Google Scholar]
- 51.Zheng C, Chen X, Weng L, et al. Benefits of mobile apps for cancer pain management: systematic review. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/17055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H, Xu H, Zhang ZX, Zhang Q. Efficacy of virtual reality-based interventions for patients with breast cancer symptom and rehabilitation management: a systematic review and meta-analysis. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox A, Lucas G, Marcu A, et al. Cancer survivors' experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19:e11. doi: 10.2196/jmir.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Darley A, Coughlan B, Furlong E. People with cancer and their family caregivers' personal experience of using supportive eHealth technology: a narrative review. Eur J Oncol Nurs. 2021;54 doi: 10.1016/j.ejon.2021.102030. [DOI] [PubMed] [Google Scholar]
- 55.Albino de Queiroz D, André da Costa C, Aparecida Isquierdo Fonseca de Queiroz E, Folchini da Silveira E, da Rosa Righi R. Internet of things in active cancer treatment: a systematic review. J Biomed Inform. 2021;118 doi: 10.1016/j.jbi.2021.103814. [DOI] [PubMed] [Google Scholar]
- 56.Wan SW, Chng YJD, Lim SH, Chong CS, Pikkarainen M, He H-G. A systematic review and meta-analysis on the effectiveness of web-based psychosocial interventions among patients with colorectal cancer. J Adv Nurs. 2022;78:1883–1896. doi: 10.1111/jan.15258. [DOI] [PubMed] [Google Scholar]
- 57.Thompson HS, Shelton RC, Mitchell J, Eaton T, Valera P, Katz A. Inclusion of underserved racial and ethnic groups in cancer intervention research using new media: a systematic literature review. J Natl Cancer Inst Monogr. 2013;2013:216–223. doi: 10.1093/jncimonographs/lgt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez-Rodriguez MM, Fernández-Millan A, Ruiz-Fernández MD, Dobarrio-Sanz I, Fernández-Medina IM. New technologies to improve pain, anxiety and depression in children and adolescents with cancer: a systematic review. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sezgin MG, Bektas H. The effect of web-based intervention programs on self-management and symptom management in patients with lymphoma: a systematic review of randomized controlled trials. Jpn J Nurs Sci. 2022;19 doi: 10.1111/jjns.12460. [DOI] [PubMed] [Google Scholar]
- 60.Delemere E, Maguire R. The role of Connected Health technologies in supporting families affected by paediatric cancer: a systematic review. Psychooncology. 2021;30:3–15. doi: 10.1002/pon.5542. [DOI] [PubMed] [Google Scholar]
- 61.Dickinson R, Hall S, Sinclair JE, Bond C, Murchie P. Using technology to deliver cancer follow-up: a systematic review. BMC Cancer. 2014;14:311. doi: 10.1186/1471-2407-14-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dorri S, Asadi F, Olfatbakhsh A, Kazemi A. A systematic review of electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer. 2020;27:25–46. doi: 10.1007/s12282-019-00982-3. [DOI] [PubMed] [Google Scholar]
- 63.Elepaño A, Fusingan AS, Yasay E, Sahagun JA. Mobile health interventions for improving colorectal cancer screening rates: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2021;22:3093–3099. doi: 10.31557/APJCP.2021.22.10.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Escriva Boulley G, Leroy T, Bernetière C, Paquienseguy F, Desfriches-Doria O, Préau M. Digital health interventions to help living with cancer: a systematic review of participants' engagement and psychosocial effects. Psychooncology. 2018;27:2677–2686. doi: 10.1002/pon.4867. [DOI] [PubMed] [Google Scholar]
- 65.Ester M, Eisele M, Wurz A, McDonough MH, McNeely M, Culos-Reed SN. Current evidence and directions for future research in eHealth physical activity interventions for adults affected by cancer: systematic review. JMIR Cancer. 2021;7 doi: 10.2196/28852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finch L, Janda M, Loescher LJ, Hacker E. Can skin cancer prevention be improved through mobile technology interventions? A systematic review. Prev Med. 2016;90:121–132. doi: 10.1016/j.ypmed.2016.06.037. [DOI] [PubMed] [Google Scholar]
- 67.Finnane A, Dallest K, Janda M, Soyer HP. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. 2017;153:319–327. doi: 10.1001/jamadermatol.2016.4361. [DOI] [PubMed] [Google Scholar]
- 68.Fournier DM. Comparing outcomes of genetic counseling options in breast and ovarian cancer: an integrative review. Oncol Nurs Forum. 2018;45:96–105. doi: 10.1188/18.ONF.96-105. [DOI] [PubMed] [Google Scholar]
- 69.Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients' symptoms: review of randomized trials. Support Care Cancer. 2018;26:337–351. doi: 10.1007/s00520-017-3882-6. [DOI] [PubMed] [Google Scholar]
- 70.Fung JYT, Lim H, Vongsirimas N, Klainin-Yobas P. Effectiveness of eHealth mindfulness-based interventions on cancer-related symptoms among cancer patients and survivors: a systematic review and meta-analysis. J Telemed Telecare. 2022 doi: 10.1177/1357633X221078490. 1357633X221078490. [DOI] [PubMed] [Google Scholar]
- 71.Furness K, Sarkies MN, Huggins CE, Croagh D, Haines TP. Impact of the method of delivering electronic health behavior change interventions in survivors of cancer on engagement, health behaviors, and health outcomes: systematic review and meta-analysis. J Med Internet Res. 2020;22 doi: 10.2196/16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gambalunga F, Iacorossi L, Notarnicola I, Serra V, Piredda M, De Marinis MG. Mobile health in adherence to oral anticancer drugs: a scoping review. Comput Inform Nurs. 2021;39:17–23. doi: 10.1097/CIN.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 73.Goodman W, Bagnall A-M, Ashley L, et al. The extent of engagement with telehealth approaches by patients with advanced cancer: systematic review. JMIR Cancer. 2022;8 doi: 10.2196/33355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haberlin C, O'Dwyer T, Mockler D, Moran J, O'Donnell DM, Broderick J. The use of eHealth to promote physical activity in cancer survivors: a systematic review. Support Care Cancer. 2018;26:3323–3336. doi: 10.1007/s00520-018-4305-z. [DOI] [PubMed] [Google Scholar]
- 75.Han CJ, Lee YJ, Demiris G. Interventions using social media for cancer prevention and management: a systematic review. Cancer Nurs. 2018;41:E19–E31. doi: 10.1097/NCC.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hernandez Silva E, Lawler S, Langbecker D. The effectiveness of mHealth for self-management in improving pain, psychological distress, fatigue, and sleep in cancer survivors: a systematic review. J Cancer Surviv. 2019;13:97–107. doi: 10.1007/s11764-018-0730-8. [DOI] [PubMed] [Google Scholar]
- 77.Heynsbergh N, Heckel L, Botti M, Livingston PM. Feasibility, useability and acceptability of technology-based interventions for informal cancer carers: a systematic review. BMC Cancer. 2018;18:244. doi: 10.1186/s12885-018-4160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hopstaken JS, Verweij L, van Laarhoven CJHM, Blijlevens NMA, Stommel MWJ, Hermens RPMG. Effect of digital care platforms on quality of care for oncological patients and barriers and facilitators for their implementation: systematic review. J Med Internet Res. 2021;23 doi: 10.2196/28869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houghton LC, Howland RE, McDonald JA. Mobilizing breast cancer prevention research through smartphone apps: a systematic review of the literature. Front Public Health. 2019;7:298. doi: 10.3389/fpubh.2019.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hwang N-K, Jung YJ, Park JS. Information and communications technology-based telehealth approach for occupational therapy interventions for cancer survivors: a systematic review. Healthcare. 2020;8:355. doi: 10.3390/healthcare8040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibeggazene S, Turner R, Rosario D, Bourke L. Remote interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:308. doi: 10.1186/s12885-021-07989-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janssen A, Brunner M, Keep M, et al. Interdisciplinary eHealth practice in cancer care: a review of the literature. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14111289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jongerius C, Russo S, Mazzocco K, Pravettoni G. Research-tested mobile apps for breast cancer care: systematic review. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamalumpundi V, Saeidzadeh S, Chi N-C, Nair R, Gilbertson-White S. The efficacy of web or mobile-based interventions to alleviate emotional symptoms in people with advanced cancer: a systematic review and meta-analysis. Support Care Cancer. 2022;30:3029–3042. doi: 10.1007/s00520-021-06496-z. [DOI] [PubMed] [Google Scholar]
- 85.Kang HS, Kim H-K, Park SM, Kim J-H. Online-based interventions for sexual health among individuals with cancer: a systematic review. BMC Health Serv Res. 2018;18:167. doi: 10.1186/s12913-018-2972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karim H, Choobineh H, Kheradbin N, Ravandi MH, Naserpor A, Safdari R. Mobile health applications for improving the sexual health outcomes among adults with chronic diseases: a systematic review. Digit Health. 2020;6 doi: 10.1177/2055207620906956. 2055207620906956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Keikha L, Maserat E, Mohammadzadeh Z. Telerehabilitation and monitoring physical activity in patient with breast cancer: systematic review. Iran J Nurs Midwifery Res. 2022;27:8–17. doi: 10.4103/ijnmr.ijnmr_472_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khoo S, Mohbin N, Ansari P, Al-Kitani M, Müller AM. mHealth interventions to address physical activity and sedentary behavior in cancer survivors: a systematic review. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18115798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiss N, Baguley BJ, Ball K, et al. Technology-supported self-guided nutrition and physical activity interventions for adults with cancer: systematic review. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kitamura C, Zurawel-Balaura L, Wong RKS. How effective is video consultation in clinical oncology? A systematic review. Curr Oncol. 2010;17:17–27. doi: 10.3747/co.v17i3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koç Z, Kaplan E, Tanrıverdi D. The effectiveness of telehealth programs on the mental health of women with breast cancer: a systematic review. J Telemed Telecare. 2022 doi: 10.1177/1357633X211069663. 1357633X211069663. [DOI] [PubMed] [Google Scholar]
- 92.Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer patients: a systematic review and meta-analysis. Telemed J E Health. 2018;24:397–405. doi: 10.1089/tmj.2017.0112. [DOI] [PubMed] [Google Scholar]
- 93.Larson JL, Rosen AB, Wilson FA. The effect of telehealth interventions on quality of life of cancer survivors: a systematic review and meta-analysis. Health Informatics J. 2020;26:1060–1078. doi: 10.1177/1460458219863604. [DOI] [PubMed] [Google Scholar]
- 94.Li J, Liu Y, Jiang J, Peng X, Hu X. Effect of telehealth interventions on quality of life in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud. 2021;122 doi: 10.1016/j.ijnurstu.2021.103970. [DOI] [PubMed] [Google Scholar]
- 95.Li J, Zhu C, Liu C, Su Y, Peng X, Hu X. Effectiveness of eHealth interventions for cancer-related pain, fatigue, and sleep disorders in cancer survivors: a systematic review and meta-analysis of randomized controlled trials. J Nurs Scholarsh. 2022;54:184–190. doi: 10.1111/jnu.12729. [DOI] [PubMed] [Google Scholar]
- 96.Li Y, Li J, Zhang Y, Ding Y, Hu X. The effectiveness of e-Health interventions on caregiver burden, depression, and quality of life in informal caregivers of patients with cancer: a systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud. 2022;127 doi: 10.1016/j.ijnurstu.2022.104179. [DOI] [PubMed] [Google Scholar]
- 97.Liptrott S, Bee P, Lovell K. Acceptability of telephone support as perceived by patients with cancer: a systematic review. Eur J Cancer Care. 2018;27 doi: 10.1111/ecc.12643. [DOI] [PubMed] [Google Scholar]
- 98.Magalhães B, Fernandes C, Martinez-Galiano JM, Santos C. Exploring the use of mobile applications by cancer patients undergoing chemotherapy: a scoping review. Int J Med Inform. 2020;144 doi: 10.1016/j.ijmedinf.2020.104293. [DOI] [PubMed] [Google Scholar]
- 99.Magalhães B, Fernandes C, Santos C, Martínez-Galiano JM. The use of mobile applications for managing care processes during chemotherapy treatments: a systematic review. Cancer Nurs. 2021;44:E339–E360. doi: 10.1097/NCC.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 100.Marthick M, McGregor D, Alison J, Cheema B, Dhillon H, Shaw T. Supportive care interventions for people with cancer assisted by digital technology: systematic review. J Med Internet Res. 2021;23 doi: 10.2196/24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marzorati C, Renzi C, Russell-Edu SW, Pravettoni G. Telemedicine use among caregivers of cancer patients: systematic review. J Med Internet Res. 2018;20:e223. doi: 10.2196/jmir.9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.da Mata LRF, da Silva AC, Pereira MG, de Carvalho EC. Telephone follow-up of patients after radical prostatectomy: a systematic review. Rev Lat Am Enfermagem. 2014;22:337–345. doi: 10.1590/0104-1169.3314.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matis J, Svetlak M, Slezackova A, Svoboda M, Šumec R. Mindfulness-based programs for patients with cancer via eHealth and mobile health: systematic review and synthesis of quantitative research. J Med Internet Res. 2020;22 doi: 10.2196/20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McAlpine H, Joubert L, Martin-Sanchez F, Merolli M, Drummond KJ. A systematic review of types and efficacy of online interventions for cancer patients. Patient Educ Couns. 2015;98:283–295. doi: 10.1016/j.pec.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 105.McCann L, McMillan KA, Pugh G. Digital interventions to support adolescents and young adults with cancer: systematic review. JMIR Cancer. 2019;5 doi: 10.2196/12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mehdizadeh H, Asadi F, Mehrvar A, Nazemi E, Emami H. Smartphone apps to help children and adolescents with cancer and their families: a scoping review. Acta Oncol. 2019;58:1003–1014. doi: 10.1080/0284186X.2019.1588474. [DOI] [PubMed] [Google Scholar]
- 107.Moradian S, Voelker N, Brown C, Liu G, Howell D. Effectiveness of internet-based interventions in managing chemotherapy-related symptoms in patients with cancer: a systematic literature review. Support Care Cancer. 2018;26:361–374. doi: 10.1007/s00520-017-3900-8. [DOI] [PubMed] [Google Scholar]
- 108.Moretto IG, Contim CLV. Santo FH do E. Telephone follow-up as a nursing intervention for patients receiving outpatient chemotherapy: integrative review. Rev Gaucha Enferm. 2019;40 doi: 10.1590/1983-1447.2019.20190039. (in Portuguese). [DOI] [PubMed] [Google Scholar]
- 109.Morris BB, Rossi B, Fuemmeler B. The role of digital health technology in rural cancer care delivery: a systematic review. J Rural Health. 2022;38:493–511. doi: 10.1111/jrh.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morrison KS, Paterson C, Toohey K. The feasibility of exercise interventions delivered via telehealth for people affected by cancer: a rapid review of the literature. Semin Oncol Nurs. 2020;36 doi: 10.1016/j.soncn.2020.151092. [DOI] [PubMed] [Google Scholar]
- 111.Okuyama S, Jones W, Ricklefs C, Tran ZV. Psychosocial telephone interventions for patients with cancer and survivors: a systematic review. Psychooncology. 2015;24:857–870. doi: 10.1002/pon.3704. [DOI] [PubMed] [Google Scholar]
- 112.Öter E, Özkan S, Çinar H. The effectiveness of using telemedicine to follow-up breast cancer during the COVID-19 pandemic: a scoping review. Turk J Oncol. 2022;37:93–99. [Google Scholar]
- 113.Ownsworth T, Chan RJ, Jones S, Robertson J, Pinkham MB. Use of telehealth platforms for delivering supportive care to adults with primary brain tumors and their family caregivers: a systematic review. Psychooncology. 2021;30:16–26. doi: 10.1002/pon.5549. [DOI] [PubMed] [Google Scholar]
- 114.Pang L, Liu Z, Lin S, et al. The effects of telemedicine on the quality of life of patients with lung cancer: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11 doi: 10.1177/2040622320961597. 2040622320961597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qan'ir Y, Song L. Systematic review of technology-based interventions to improve anxiety, depression, and health-related quality of life among patients with prostate cancer. Psychooncology. 2019;28:1601–1613. doi: 10.1002/pon.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramsey WA, Heidelberg RE, Gilbert AM, Heneghan MB, Badawy SM, Alberts NM. eHealth and mHealth interventions in pediatric cancer: a systematic review of interventions across the cancer continuum. Psychooncology. 2020;29:17–37. doi: 10.1002/pon.5280. [DOI] [PubMed] [Google Scholar]
- 117.Rat C, Hild S, Rault Sérandour J, et al. Use of smartphones for early detection of melanoma: systematic review. J Med Internet Res. 2018;20:e135. doi: 10.2196/jmir.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ream E, Hughes AE, Cox A, et al. Telephone interventions for symptom management in adults with cancer. Cochrane Database Syst Rev. 2020;6 doi: 10.1002/14651858.CD007568.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roberts AL, Fisher A, Smith L, Heinrich M, Potts HWW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2017;11:704–719. doi: 10.1007/s11764-017-0632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rossman AH, Reid HW, Pieters MM, et al. Digital health strategies for cervical cancer control in low- and middle-income countries: systematic review of current implementations and gaps in research. J Med Internet Res. 2021;23 doi: 10.2196/23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruco A, Dossa F, Tinmouth J, et al. Social media and mHealth technology for cancer screening: systematic review and meta-analysis. J Med Internet Res. 2021;23 doi: 10.2196/26759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saeidzadeh S, Kamalumpundi V, Chi N-C, Nair R, Gilbertson-White S. Web and mobile-based symptom management interventions for physical symptoms of people with advanced cancer: a systematic review and meta-analysis. Palliat Med. 2021;35:1020–1038. doi: 10.1177/02692163211006317. [DOI] [PubMed] [Google Scholar]
- 123.Salmani H, Ahmadi M, Shahrokhi N. The impact of mobile health on cancer screening: a systematic review. Cancer Inform. 2020;19 doi: 10.1177/1176935120954191. 1176935120954191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sanchez MA, Rabin BA, Gaglio B, et al. A systematic review of eHealth cancer prevention and control interventions: new technology, same methods and designs? Transl Behav Med. 2013;3:392–401. doi: 10.1007/s13142-013-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seiler A, Klaas V, Tröster G, Fagundes CP. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: a systematic review and meta-analysis. Psychooncology. 2017;26:1239–1253. doi: 10.1002/pon.4489. [DOI] [PubMed] [Google Scholar]
- 126.Singh S, Fletcher GG, Yao X, Sussman J. Virtual care in patients with cancer: a systematic review. Curr Oncol. 2021;28:3488–3506. doi: 10.3390/curroncol28050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soloe C, Burrus O, Subramanian S. The effectiveness of mHealth and eHealth tools in improving provider knowledge, confidence, and behaviors related to cancer detection, treatment, and survivorship care: a systematic review. J Cancer Educ. 2021;36:1134–1146. doi: 10.1007/s13187-021-01961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Son H, Hertsenberg L, La S, et al. Use of health information technologies for cancer survivorship care: a comprehensive review of the literature. J Inform Nurs. 2019;4:6–18. [Google Scholar]
- 129.Sotirova MB, McCaughan EM, Ramsey L, et al. Acceptability of online exercise-based interventions after breast cancer surgery: systematic review and narrative synthesis. J Cancer Surviv. 2021;15:281–310. doi: 10.1007/s11764-020-00931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suleman A, Vijenthira A, Berlin A, Prica A, Rodin D. The use of virtual care in patients with hematologic malignancies: a scoping review. Curr Oncol. 2022;29:892–900. doi: 10.3390/curroncol29020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tarver WL, Haggstrom DA. The use of cancer-specific patient-centered technologies among underserved populations in the United States: systematic review. J Med Internet Res. 2019;21 doi: 10.2196/10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Taylor S, Bellhouse S, Allsop M, Radford J, Yorke J. The role of e-Health in the delivery of care for patients with hematological cancers: a systematic literature review. Telemed J E Health. 2020;26:1093–1105. doi: 10.1089/tmj.2019.0231. [DOI] [PubMed] [Google Scholar]
- 133.Triberti S, Savioni L, Sebri V, Pravettoni G. eHealth for improving quality of life in breast cancer patients: a systematic review. Cancer Treat Rev. 2019;74:1–14. doi: 10.1016/j.ctrv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 134.Uy C, Lopez J, Trinh-Shevrin C, Kwon SC, Sherman SE, Liang PS. Text messaging interventions on cancer screening rates: a systematic review. J Med Internet Res. 2017;19:e296. doi: 10.2196/jmir.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ventura F, Ohlén J, Koinberg I. An integrative review of supportive e-health programs in cancer care. Eur J Oncol Nurs. 2013;17:498–507. doi: 10.1016/j.ejon.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 136.Viola A, Panigrahi G, Devine KA. Digital interventions for adolescent and young adult cancer survivors. Curr Opin Support Palliat Care. 2020;14:51–59. doi: 10.1097/SPC.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Lin Y, Chen J, Wang C, Hu R, Wu Y. Effects of internet-based psycho-educational interventions on mental health and quality of life among cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2020;28:2541–2552. doi: 10.1007/s00520-020-05383-3. [DOI] [PubMed] [Google Scholar]
- 138.Warrington L, Absolom K, Conner M, et al. Electronic systems for patients to report and manage side effects of cancer treatment: systematic review. J Med Internet Res. 2019;21 doi: 10.2196/10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wijeratne DT, Bowman M, Sharpe I, Srivastava S, Jalink M, Gyawali B. Text messaging in cancer-supportive care: a systematic review. Cancers. 2021;13 doi: 10.3390/cancers13143542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu A, Wang Y, Wu X. Effectiveness of e-health based self-management to improve cancer-related fatigue, self-efficacy and quality of life in cancer patients: systematic review and meta-analysis. J Adv Nurs. 2019;75:3434–3447. doi: 10.1111/jan.14197. [DOI] [PubMed] [Google Scholar]
- 141.Yajima C, Bowe C, Barber D, Dains J. Telehealth interventions for supportive management and early recognition of treatment-related symptoms in patients with hematologic malignancies. J Adv Pract Oncol. 2021;12:835–849. doi: 10.6004/jadpro.2021.12.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang D, Advani S, Waller J, et al. Mobile technologies and cervical cancer screening in low- and middle-income countries: a systematic review. JCO Glob Oncol. 2020;6:617–627. doi: 10.1200/JGO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang Q, Zhang L, Yin R, Fu T, Chen H, Shen B. Effectiveness of telephone-based interventions on health-related quality of life and prognostic outcomes in breast cancer patients and survivors-a meta-analysis. Eur J Cancer Care. 2018;27 doi: 10.1111/ecc.12632. [DOI] [PubMed] [Google Scholar]
- 144.Osborn J, Ajakaiye A, Cooksley T, Subbe CP. Do mHealth applications improve clinical outcomes of patients with cancer? A critical appraisal of the peer-reviewed literature. Support Care Cancer. 2020;28:1469–1479. doi: 10.1007/s00520-019-04945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cai T, Huang Y, Zhang Y, Lu Z, Huang Q, Yuan C. Mobile health applications for the care of patients with breast cancer: a scoping review. Int J Nurs Sci. 2021;8:470–476. doi: 10.1016/j.ijnss.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.American Cancer Society Cancer facts & figures 2022. 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- 148.Evangelista L, Steinhubl SR, Topol EJ. Digital health care for older adults. Lancet. 2019;393 doi: 10.1016/S0140-6736(19)30800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Faverio M. Share of those 65 and older who are tech users has grown in the past decade. Jan 13, 2022. https://www.pewresearch.org/fact-tank/2022/01/13/share-of-those-65-and-older-who-are-tech-users-has-grown-in-the-past-decade/
- 150.Smith AS, Trevelyan E. The older population in rural America: 2012–2016. September, 2019. https://www.census.gov/content/dam/Census/library/publications/2019/acs/acs-41.pdf
- 151.O'Brien E, Wu KB, Baer D. Older Americans in poverty: a snapshot. April, 2010. https://www.giaging.org/documents/2010-03-poverty.pdf
- 152.Rodler S, Buchner A, Stief CG, Heinemann V, Staehler M, Casuscelli J. Patients' perspective on digital technologies in advanced genitourinary cancers. Clin Genitourin Cancer. 2021;19:76–82.e6. doi: 10.1016/j.clgc.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 153.Cai J, Zhang L, Guerriere D, Fan H, Coyte PC. Where do cancer patients in receipt of home-based palliative care prefer to die and what are the determinants of a preference for a home death? Int J Environ Res Public Health. 2020;18:235. doi: 10.3390/ijerph18010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weaver MS, Shostrom VK, Neumann ML, Robinson JE, Hinds PS. Homestead together: pediatric palliative care telehealth support for rural children with cancer during home-based end-of-life care. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28921. [DOI] [PubMed] [Google Scholar]
- 155.Dionne-Odom JN, Azuero A, Lyons KD, et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33:1446–1452. doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pearce C, Wong G, Kuhn I, Barclay S. Supporting bereavement and complicated grief in primary care: a realist review. BJGP Open. 2021;5 doi: 10.3399/BJGPO.2021.0008. BJGPO.2021.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Laranjeira C, Moura D, Salci MA, et al. A scoping review of interventions for family bereavement care during the COVID-19 pandemic. Behav Sci. 2022;12:155. doi: 10.3390/bs12050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Secinti E, Lewson AB, Wu W, Kent EE, Mosher CE. Health-related quality of life: a comparative analysis of caregivers of people with dementia, cancer, COPD/emphysema, and diabetes and noncaregivers, 2015–2018 BRFSS. Ann Behav Med. 2021;55:1130–1143. doi: 10.1093/abm/kaab007. [DOI] [PubMed] [Google Scholar]
- 159.Houck JA. A comparison of grief reactions in cancer, HIV/AIDS, and suicide bereavement. J HIV AIDS Soc Serv. 2007;6:97–112. [Google Scholar]
- 160.Dillon EC, Stults CD, Deng S, et al. Women, younger clinicians', and caregivers' experiences of burnout and well-being during COVID-19 in a US healthcare system. J Gen Intern Med. 2022;37:145–153. doi: 10.1007/s11606-021-07134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Linardon J, Cuijpers P, Carlbring P, Messer M, Fuller-Tyszkiewicz M. The efficacy of app-supported smartphone interventions for mental health problems: a meta-analysis of randomized controlled trials. World Psychiatry. 2019;18:325–336. doi: 10.1002/wps.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13:127. doi: 10.1186/s12966-016-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Muñoz RF. Harnessing psychology and technology to contribute to making health care a universal human right. Cogn Behav Pract. 2022;29:4–14. [Google Scholar]
- 164.Aetna Prescription digital therapeutics. Jan 7, 2022. https://es.aetna.com/cpb/medical/data/900_999/0999.html
Uncited reference
- 165.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.