Background.
Limited data and guidelines exist for using nirmatrelvir/ritonavir in solid organ transplant recipients stabilized on tacrolimus for the treatment of mild-to-moderate coronavirus disease. Concern exists regarding the impact of utilizing a 5-d course of nirmatrelvir/ritonavir with calcineurin inhibitors because of significant drug–drug interactions between ritonavir, a potent cytochrome P450 3A inhibitor, and other cytochrome P450 3A substrates, such as tacrolimus.
Methods.
We report the successful use of nirmatrelvir/ritonavir in 12 outpatient lung transplant recipients with confirmed severe acute respiratory syndrome coronavirus 2 infection stabilized on tacrolimus immunosuppression. All patients stopped tacrolimus and started nirmatrelvir/ritonavir 10 to 14 h after the last dose of tacrolimus. Tacrolimus was withheld and then reinitiated at a modified dose 48 h following the completion of nirmatrelvir/ritonavir therapy. Tacrolimus trough levels were checked during nirmatrelvir/ritonavir therapy and tacrolimus reinitiation.
Results.
Ten (10/12) patients were able to resume their original tacrolimus dose within 4 d of completing nirmatrelvir/ritonavir therapy and maintain therapeutic levels of tacrolimus. No patients experienced tacrolimus toxicity or acute rejection during the 30-d postcompletion of nirmatrelvir/ritonavir therapy.
Conclusions.
In this cohort of lung transplant recipients on tacrolimus, we demonstrated that nirmatrelvir/ritonavir can be safely used with close monitoring of tacrolimus levels and appropriate dose adjustments of tacrolimus. Further confirmatory studies are needed to determine the appropriate use of therapeutic drug monitoring and tacrolimus dose following completion of nirmatrelvir/ritonavir in the solid organ transplant population.
INTRODUCTION
Nirmatrelvir/ritonavir is an oral therapy authorized for use under an emergency use authorization (EUA) for the treatment of mild-to-moderate coronavirus disease for individuals at high risk for progression to severe coronavirus disease 2019 (COVID-19).1,2 The use of nirmatrelvir/ritonavir in the solid organ transplant (SOT) population is of interest as a viable treatment option because SOT recipients are at a high risk of morbidity and mortality from COVID-19.3 Although alternative therapies currently exist for the treatment of COVID-19, nirmatrelvir/ritonavir is an attractive option because of its oral route, efficacy, and availability in the outpatient setting.2 The antiviral activity of nirmatrelvir/ritonavir comprises 2 mechanisms.2 Nirmatrelvir acts as a protease inhibitor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and interferes with viral replication. Ritonavir, a potent cytochrome P450 (CYP) 3A inhibitor, acts as a pharmacokinetic booster for nirmatrelvir to allow for prolonged drug exposure in the body.4 In SOT, this drug interaction is of particular concern for patients stabilized on tacrolimus, cyclosporine, everolimus, or sirolimus because these medications are metabolized by CYP3A and have a narrow therapeutic index. The drug interaction from ritonavir inhibits the metabolism of tacrolimus, thus necessitating a dose reduction of tacrolimus to prevent toxicity.5,6 Reports of the intensity of the interaction between ritonavir and tacrolimus, however, vary from increasing tacrolimus levels 8-fold to requiring a 99% dose reduction in tacrolimus after starting lopinavir/ritonavir, resulting in a tacrolimus dose of 0.5 mg every 7 d.5,6 Therefore, the impact of a short 5-d course of ritonavir on tacrolimus levels remains uncertain, and concern for toxicity exists.7 One case report of 2 SOT recipients indicated that coadministration of tacrolimus and nirmatrelvir/ritonavir resulted in tacrolimus toxicity requiring hospitalization.8 Recently published guidelines suggest holding tacrolimus while receiving nirmatrelvir/ritonavir.9-14 However, the recommendation for timing and the dose of tacrolimus reinitiation varies from dosing by level to restarting the pretreatment dose at the conclusion of the treatment course.9,11-14 A challenge with utilizing dosing by level is the difficulty in obtaining laboratory blood measurements in patients with an active SARS-CoV-2 infection due to potential spread of disease, limited laboratory availability for infected patients, and the need for patients to quarantine. Therefore, a clear protocol that is not overly reliant on daily trough levels or timely return of trough levels would be preferable. We present 12 postlung transplant recipients diagnosed with SARS-CoV-2 and stabilized on tacrolimus immunosuppression who received a full course of nirmatrelvir/ritonavir.
CASE REPORT
Twelve outpatient bilateral lung transplant recipients with confirmed SARS-CoV-2 were started on nirmatrelvir/ritonavir within 5 d of onset of symptoms, which is in line with the guidelines put forth in the EUA. The University of California, San Francisco, institutional review board approved this study under protocol 13-10738. All study participants provided written informed consent. All patients had received either 2 doses of a messenger RNA or 1 dose of an adenovirus vaccination for SARS-CoV-2, and 83% (10/12) of the patients had received 1 or more additional/booster doses by the time of their diagnosis (Table 1). Baseline data for each of these patients including lung transplant indication, body mass index, current immunosuppression, and significant interacting medications were also evaluated. All patients stopped immediate-release tacrolimus and started nirmatrelvir/ritonavir 10 to 14 h after their last dose of tacrolimus. Patients were also instructed to hold all other daily medications with significant drug–drug interactions as identified in the EUA during 5 d of nirmatrelvir/ritonavir therapy.4 Of note, none of the patients were receiving a daily azole antifungal medication at the time of the SARS-CoV-2 diagnosis.
TABLE 1.
COVID-19 lung transplant cases
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/age, y | F/68 | F/35 | M/50 | F/32 | M/61 | M/71 | F/31 | M/50 | M/51 | M/64 | F/56 | M/44 |
| LTx indication | HP | ILD | ILD | CF | IPF | COPD | PVOD | HP | ILD | IPF | ILD | CF |
| Time since LTx, y | 1 | 2 | 1 | 3 | 12 | 2 | 12 | 0.25 | 5 | 3 | 13 | 4 |
| Duration of illness before starting NIR/R, d | 1 | 3 | 2 | 4 | 5 | 3 | 0 | 0 | 1 | 0 | 3 | 0 |
| Vaccination, no. dosesa | 3 | 2 | 4 | 1 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Latest BMI | 26.6 | 39.4 | 28.1 | 20.5 | 27 | 30.4 | 23.4 | 28.4 | 22.8 | 31 | 29.6 | 24.9 |
| eGFR | 70 | 82 | 100 | 52 | 66 | 75 | 99 | 90 | 90 | 54 | 63 | 67 |
| Baseline IS | PRED, TAC | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MPA | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF | PRED, TAC, MMF |
| Medications held during treatment | Rosuvastatin | Fluconazole (weekly), rosuvastatin | Tamsulosin | Fluticasone, tramadol | Atorvastatin | Pravastatin, diltiazem (if SBP <110) | None | Rosuvastatin | Atorvastatin | Rosuvastatin | None | Elexacaftor/tezacaftor/ivacaftor, loperamide, fluconazole (weekly) |
| FEV1 at time of diagnosis, L | 2.21 | 1.97 | 2.68 | 2.24 | 1.37 | 2.37 | 2.41 | 2.71 | 3.05 | 4.04 | 1.03 | 3.22 |
| Highest LOC | Home | Home | Home | Home | Home | Home | Home | Home | Home | Home | Home | Home |
| COVID-19 specific therapy | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R | NIR/R |
| Outcomes | Home | Admit | Home | Home | Home | Home | Home | Home | Home | Home | Home | Home |
All vaccines were messenger RNA (mRNA) COVID-19 vaccines with the exception of patient 4, who received a viral vector COVID-19 vaccine.
BMI, body mass index; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 s; HP, hypersensitivity pneumonitis; IPF, idiopathic pulmonary fibrosis; ILD, interstitial lung disease; IS, immunosuppression; LTx, lung transplant; LOC, level of care; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NIR/R, nirmatrelvir/ritonavir; PRED, prednisone; PVOD, pulmonary veno-occlusive disease; SBP, systolic blood pressure; TAC, tacrolimus
Tacrolimus levels were collected in the morning and each patient obtained a tacrolimus level between 3 and 5 d following initiation of nirmatrelvir/ritonavir therapy. Upon completion of therapy and resumption of tacrolimus, levels collected were verified 12- or 24-h troughs dependent on the frequency of tacrolimus that was resumed. Tacrolimus levels were compared with predetermined tacrolimus trough goals that had been derived from our center’s lung transplant protocol accounting for patient specific factors (Table 2). Six (50%) of the patients were within their specific trough goal during days 3 to 5 of nirmatrelvir/ritonavir therapy (Table 2). Four additional patients had a level that was below the tacrolimus trough goal. For 3 of these patients, the level was deemed an acceptable immunosuppression level given the patient’s active infection and therefore did not require additional interventions. One patient (patient 9) had a level 37% below the lower limit of the target range, which warranted an additional supplemental dosage of 0.5 mg of tacrolimus during nirmatrelvir/ritonavir therapy.
Table 2.
Tacrolimus troughs during and after nirmatrelvir/ritonavir therapy
| Patient 1 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | *Day 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 1.5/2 | 1.5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0 | 0.5/0 | 1/1 | 1/1 | 2/2 | 2/2 |
| Tac trough(goal 8–10) | 14.5a | 6.2 | 5.5 | 4.1 | 4.8 | 11.2 | ||||||||
| Patient 2 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | *Day 15 |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 2/2 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/ 0.5 | 0.5/ 0.5 | 2/2 | 2/2 | 2/2 | 2/2 |
| Tac trough(goal 8–10) | 10.9 | 9.7 | 7.8 | 8.7 | 8.2 | |||||||||
| Patient 3 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 2/2 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0.5 | 0.5/0.5 | 2/2 | 2/2 | 2/2 | |
| Tac trough(goal 8–10) | 7.7 | 11.0 | 8.0 | |||||||||||
| Patient 4 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 3.5/3.5 | 3.5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 | 3.5/3.5 | 3.5/3.5 | 3.5/3.5 | |
| Tac trough(goal 5–6) | 8.2 | 6.5 | ||||||||||||
| Patient 5 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | *Day 25 |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 0.5/1 | 0.5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0 | 0.5/0 | 0.5/1 | 0.5/1 | 0.5/1 | 1/1 |
| Tac trough(goal 6–8) | 5.2 | 6.7 | 4.3 | 8.6 | ||||||||||
| Patient 6 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5(am) | ||||||||
| Tac dose | 3/3.5 | 3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 | 3/3.5 | 3/3.5 | 3/3.5 | |
| Tac trough(goal 8–10) | 8.9 | 6.4 | 10.3 | |||||||||||
| Patient 7 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5(am) | ||||||||
| Tac dose | 1.5/2 | 1.5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0.5 | 0.5/0.5 | 1.5/2 | 1.5/2 | 1.5/2 | |
| Tac trough(goal 6–8) | 5.0 | 5.3 | 7.9 | |||||||||||
| Patient 8 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | *Day 14 |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D/3/4 | D4/5 | D5(am) | ||||||||
| Tac dose | 1/1.5 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0 | 0.5/0 | 1/0 | 0/1 | 1/1 | 1/1 |
| Tac trough(goal 10–14) | 9.7 | 16.8 | 16.5 | 7.8 | ||||||||||
| Patient 9 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 0.5/1 | 0.5/0 | 0/0 | 0/0 | 0/0 | 0.5/0 | 0/0 | 0/0 | 0.5/0 | 0.5/0 | 0.5/1 | 0.5/1 | 0.5/1 | |
| Tac trough(goal 7–8) | 4.5b | 4.4 | 8.2 | |||||||||||
| Patient 10 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 1/1 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0.5/0 | 0.5/0 | 1/1 | 1/1 | 1/1 | |
| Tac trough(goal 6–8) | 5.8 | 7.2 | 12.5 | |||||||||||
| Patient 11 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | *Day 16 |
| NIR/r | D1 (pm) | D1/2 | D2/3 | D3/4 | D4/5 | D5 (am) | ||||||||
| Tac dose | 1/1 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| Tac trough(goal 6–8) | 5.8 | 6.5 | 10.6 | 4.8 | ||||||||||
| Patient 12 | Baseline | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | |
| NIR/r | D1 | D2 | D3 | D4 | D5 | |||||||||
| Tac dose | 1.5/1.5 | 0.0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0.5 | 0.5/0.5 | 0.5/1.5 | 1.5/1.5 | 1.5/1.5 | 1.5/1.5 | |
| Tac trough(goal 6–8) | 6.4 | 8.0 | 5.9 |
All patients received 3 tablets (300 mg of nirmatrelvir, 100 mg of ritonavir) twice daily starting day 1 and completed a 5-d course. Tacrolimus dose is displayed as milligrams taken for the morning dose and the evening dose. Tacrolimus trough and goal levels (µg/L).
aTacrolimus dose was adjusted 7 d before day 0 in response to elevated tacrolimus trough; a repeated trough had not yet been drawn before NIR/r initiation.
bTacrolimus dose and trough goals were increased 10 d before day 0; a repeated trough had not yet been drawn prior to NIR/r initiation.
am, morning; D, dose; NIR/r, nirmatrelvir/ritonavir; pm, evening; Tac, tacrolimus.
Ten of the patients resumed a modified dose of tacrolimus at approximately 25% (rounded to the nearest 0.5 mg) of their baseline tacrolimus dose for 2 d after the completion of nirmatrelvir/ritonavir therapy. Patient 1 was started on 14% of the baseline tacrolimus dose (rather than 25%) and had a delayed time (12 d) until the full tacrolimus dose was restarted. Patient 11 deviated from our protocol by bypassing the stepwise dose escalation and instead restarted the full baseline tacrolimus dose at 48 h following the completion of nirmatrelvir/ritonavir therapy. The patient’s subsequent tacrolimus trough level on day 11 of 10.6 µg/L was still the above goal; however, by the next trough check on day 16, the trough had fallen without further dose modifications. Despite following the modified tacrolimus reinitiation protocol, patient 8 had a noticeable increase in the tacrolimus trough from a baseline, which persisted until day 14 when the patient was able to resume their baseline tacrolimus dose.
One patient was hospitalized and subsequently recovered for worsening SARS-CoV-2 infection following nirmatrelvir/ritonavir therapy; however, no patients were treated for acute rejection or experienced side effects associated with toxic tacrolimus troughs within 30 d of initiating nirmatrelvir/ritonavir.
DISCUSSION
Of the 12 lung transplant recipients who received nirmatrelvir/ritonavir, 10 (83%) of the patients were able to resume their pretreatment (baseline) tacrolimus dose by day 10 following the initiation of nirmatrelvir/ritonavir therapy without clinically relevant compromise of their tacrolimus trough. Patients 1 and 8 did not fully resume the baseline tacrolimus dose until days 12 and 16, respectively. We suspect that the subtherapeutic tacrolimus trough for patient 1 was confounded by a recent dose reduction before starting nirmatrelvir/ritonavir, which led to difficulty with therapeutic levels. Patient 1 had a tacrolimus dose decrease a week before initiating nirmatrelvir/ritonavir in response to a supratherapeutic tacrolimus level, and the repeat level had not yet been checked before starting nirmatrelvir/ritonavir therapy. Patient 1 was also the only patient who started nirmatrelvir/ritonavir 14 h after their last tacrolimus dose (rather than 12 h). The tacrolimus dose for patient 1 was increased with more caution because it was the first patient that we had use nirmatrelvir/ritonavir and the timing of enzymatic clearance in vivo was not established in the literature. With little published data available, we initiated tacrolimus slowly at 14% of patient’s previous dose on day 8 and slowly titrated up based on repeat levels. In retrospect, restarting only 14% of the baseline tacrolimus dose was too low because it might have resulted in a subtherapeutic level on day 10. By day 12, the patient required slightly higher doses than the baseline tacrolimus dose despite the patient taking 50% of the baseline tacrolimus dose for 2 d before the laboratory draw, which encouraged us to consider starting the baseline tacrolimus dose earlier following the completion of nirmatrelvir/ritonavir in subsequent patients.
Patient 8 had a prolonged supratherapeutic tacrolimus level during the nirmatrelvir/ritonavir therapy, which persisted past day 10. Subsequently, the full resumption of the baseline tacrolimus dose was delayed until day 16. It is unclear why this patient had prolonged enzyme inhibitory activity because the patient’s liver enzymes remained stable throughout, and 88% to 98% of the hepatic and intestinal CYP3A inhibition is proposed to have disappeared by 5 d after stopping ritonavir in other models (combined with lopinavir).10 This was the only patient that took nirmatrelvir/ritonavir 10 h after the last tacrolimus dose, yet it is still unclear why the tacrolimus level during nirmatrelvir/ritonavir therapy was high and subsequently remained elevated. Finally, patient 11 deviated from the protocol by starting the full baseline dose of tacrolimus 48 h after the completion of nirmatrelvir/ritonavir that resulted in an elevated tacrolimus level. Given this supratherapeutic level, it is reasonable to believe that a dose reduction when restarting tacrolimus, as recommended in our protocol, may have achieved a therapeutic concentration.
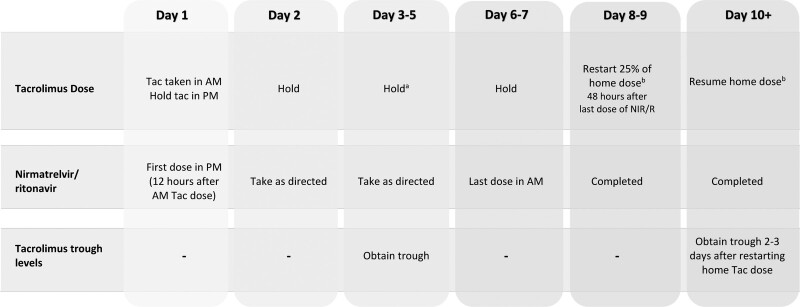
From these cases, we created a protocol for holding and reinitiating tacrolimus in a stepwise manner upon completion of a course of nirmatrelvir/ritonavir following a confirmatory diagnosis of SARS-CoV-2 (Figure 1). The purpose of the tacrolimus trough control during nirmatrelvir/ritonavir therapy is to verify tacrolimus exposure and ensure that a supplemental dose is not needed. The use of a “supplemental” dose for tacrolimus (ie, 0.5 mg once a week) has been proposed in other treatment protocols of a ritonavir-boosted protease inhibitor regimen.7 At this time, we are also recommending a second trough at 5 to 7 d after completing nirmatrelvir/ritonavir therapy to ensure that the tacrolimus trough level has remained stable. Following this second level, tacrolimus troughs are further monitored as deemed necessary by our outpatient providers.
FIGURE 1.
Protocol for monitoring lung transplant recipients with confirmed COVID-19 requiring nirmatrelvir/ritonavir. aSupplemental dose of tacrolimus if tacrolimus trough level is subtherapeutic. bAdjust tacrolimus based upon previous troughs if clinically indicated. am, morning; COVID-19, coronavirus disease 2019; NIR/R, nirmatrelvir/ritonavir; pm, evening; Tac, tacrolimus.
Our results are consistent with other reports suggesting that tacrolimus should be held when nirmatrelvir/ritonavir therapy is started, that tacrolimus troughs should be checked 3 to 5 d after starting nirmatrelvir/ritonavir and that tacrolimus can be reintroduced at partial or full dose 3 to 5 d after completing nirmatrelvir/ritonavir, ideally guided by therapeutic drug monitoring.9,12,13 However, in our experience, resuming 25% of the tacrolimus dose for 2 d rather than restarting the pretreatment dose 48 h after the completion of nirmatrelvir/ritonavir was appropriate in our patients. This algorithm was designed based on the half-life of nirmatrelvir/ritonavir and the time needed for enzyme upregulation of CYP3A to avoid toxic tacrolimus drug concentrations that could contribute to side effects and potential worsening of the SARS-CoV-2 active infection. Fortunately, none of our patients exhibited signs of rejection during the follow-up period, and only 1 patient was subsequently hospitalized for worsening of SARS-CoV-2 infection following the course of nirmatrelvir/ritonavir. It is worth noting, however, that all 12 patients had an immunosuppression regimen that contained prednisone. Given that coadministration of nirmatrelvir/ritonavir with prednisone has a potential to increase the area under the curve (AUC) of prednisone, although of undetermined clinical significance, this interaction may have augmented the patient’s overall immunosuppression and reduced the likelihood of the patients developing an episode of rejection.4
Our patient experiences presented in this short report have some limitations. This case series was limited to lung transplant recipients at a single academic medical center. As all patients were taking standard tacrolimus capsules, we do not have experience with extended-release tacrolimus formulations or utilizing tacrolimus in combination with mammalian target of rapamycin inhibitors (ie, sirolimus or everolimus). Drug–drug interactions were controlled for by stopping daily medications that had been known to have a significant interaction with ritonavir. Additionally, our center titrates and manages therapeutic drug monitoring of tacrolimus concentration based on tacrolimus trough rather than AUC measurements. It is possible that, despite maintaining an appropriate tacrolimus trough level, the AUC measurement may have been reduced while tacrolimus doses were held during nirmatrelvir/ritonavir therapy. Although AUC measurement may be a better predictor of acute rejection in SOT recipients than monitoring of tacrolimus trough concentrations, none of the patients in our study had shown to have acute rejection 30 d after starting nirmatrelvir/ritonavir therapy.15
Our proposed algorithm of tacrolimus titrations based on therapeutic drug monitoring is a practical guideline for transplant clinicians to utilize in a timely manner without requiring additional monitoring during the patient’s SARS-CoV-2 infection. In patients with accompanying immunologic risk factors, including recent transplant, history of rejection, or being on a medication regimen without prednisone, resuming a higher percentage of the baseline tacrolimus dose at 48 h in response to subtherapeutic tacrolimus troughs may be more appropriate. For this high-risk group, measuring an additional tacrolimus trough level around day 6 may be critical to accurately assess the tacrolimus elimination slope if concern for rejection exists.14
Because of laboratory infection control restrictions for patients actively infected with SARS-CoV-2, monitoring tacrolimus troughs during this period was challenging. Therapeutic drug monitoring of tacrolimus following the nirmatrelvir/ritonavir treatment course, though, remains important because the clearance of CYP3A enzymatic activity of nirmatrelvir/ritonavir may vary between patients as was evidenced by a small portion of our patients.11 Furthermore, given the risk of renal toxicity, neurotoxicity, and worsening SARS-CoV-2 infection from elevated tacrolimus levels or conversely risk of rejection from subtherapeutic levels, there should be caution with reinitiation of tacrolimus.
The short treatment course of nirmatrelvir/ritonavir presents a unique situation in which adjustments to immunosuppressive drugs are critical to monitor when initiating therapy and soon after therapy is complete. These cases illustrate the importance of coordination and communication of dosage changes for medications, taking into account the onset and offset of the pharmacokinetic drug interactions in this already complex patient population. As providers gain more experience with managing these drug interactions in this critical population, more patients will be able to utilize this valuable therapy.
Footnotes
The authors declare no funding or conflicts of interest.
K.W.D., B.Y., and R.F. participated in research design, writing of the paper, and data analysis. J.L., L.S., R.J., R.J.S., D.Q., J.P.S., K.B., and S.H. participated in writing of the paper and in data analysis. B.C. participated in writing of the paper.
REFERENCES
- 1.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. Accessed January 18, 2022.
- 2.EPIC-HR: study of oral PF-07321332/ritonavir compared with placebo in nonhospitalized high risk adults with COVID-19. ClinicalTrials.gov identifier: NCT04960202. Available at https://clinicaltrials.gov/ct2/show/NCT04960202. 2022. Accessed January 18, 2022.
- 3.Hasan I, Rashid T, Chirila RM, et al. Hepatorenal syndrome: pathophysiology and evidence-based management update. Rom J Intern Med. 2021;59:227–261. [DOI] [PubMed] [Google Scholar]
- 4.Fact sheet for healthcare providers: emergency use authorization for Paxlovid. Pfizer labs. Available at https://www.fda.gov/media/155050/download. 2021. Accessed January 17, 2022. [Google Scholar]
- 5.Schonder KS, Shullo MA, Okusanya O. Tacrolimus and lopinavir/ritonavir interaction in liver transplantation. Ann Pharmacother. 2003;37:1793–1796. [DOI] [PubMed] [Google Scholar]
- 6.Teicher E, Vincent I, Bonhomme-Faivre L, et al. Effect of highly active antiretroviral therapy on tacrolimus pharmacokinetics in hepatitis C virus and HIV co-infected liver transplant recipients in the ANRS HC-08 study. Clin Pharmacokinet. 2007;46:941–952. [DOI] [PubMed] [Google Scholar]
- 7.Kumar D, Humar A, Ison MG, et al. AST statement on oral antiviral therapy for COVID-19 for organ transplant recipients. Available at https://www.myast.org/sites/default/files/AST%20Statement%20on%20Oral%20Antiviral%20Therapy%20for%20COVID%20Jan%204%20%282%29.pdf. Accessed April 3, 2022.
- 8.Rose DT, Gandhi SM, Bedard RA, et al. Supratherapeutic tacrolimus concentrations with nirmatrelvir/ritonavir in solid organ transplant recipients requiring hospitalization: a case series using rifampin for reversal. Open Forum Infect Dis. 2022;9:ofac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange NW, Salerno DM, Jennings DL, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug-drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22:1925–1926. [DOI] [PubMed] [Google Scholar]
- 10.Stader F, Khoo S, Stoeckle M, et al. Stopping lopinavir/ritonavir in COVID-19 patients: duration of the drug interacting effect. J Antimicrob Chemother. 2020;75:3084–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbane S, H, irsch JS, Nair V. Special considerations for paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79:480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang AX, Koff A, Hao D, et al. Effect of nirmatrelvir/ritonavir on calcineurin inhibitor levels: early experience in four SARS-CoV-2 infected kidney transplant recipients. Am J Transplant. 2022;22:2117–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salerno DM, Jennings DL, Lange NW, et al. Early clinical experience with nirmatrelvir/ritonavir for the treatment of COVID-19 in solid organ transplant recipients. Am J Transplant. 2022;00:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemaitre F. Yes we can (use nirmatrelvir/ritonavir even in high immunological risk patients treated with immunosuppressive drugs)! Clin Pharmacokinet. 2022;61:1071–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet M, van Gelder T, Åsberg A, et al. Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 2019;41:261–307. [DOI] [PubMed] [Google Scholar]