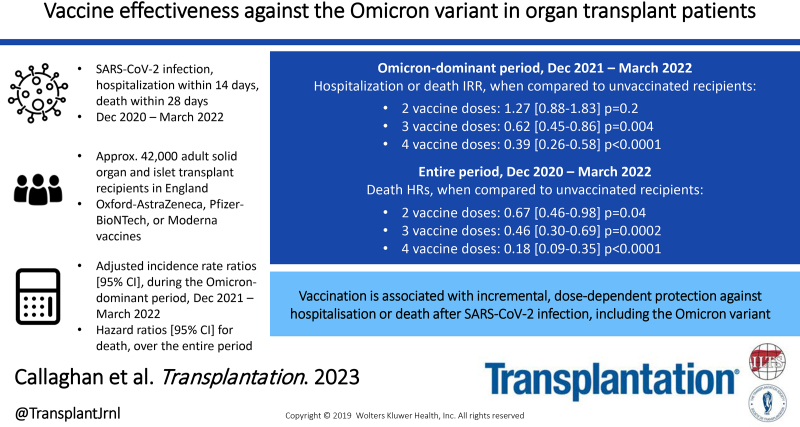
Background.
The effectiveness of vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.1.1.529 Omicron variant in immunosuppressed solid organ and islet transplant (SOT) recipients is unclear.
Methods.
National registries in England were linked to identify SARS-CoV-2 positive tests, noninjury hospitalization within 14 d, and deaths within 28 d between December 7, 2020, and March 31, 2022 in adult SOT recipients. Incidence rate ratios (IRRs) for infection, and hospitalization or death, were adjusted for recipient demographics and calendar month for the Omicron-dominant period (December 20, 2021, to March 31, 2022). Mortality risk following SARS-CoV-2 infection was adjusted for recipient demographics and dominant variant using a Cox proportional-hazards model for the entire time period.
Results.
During the Omicron-dominant period, infection IRRs (95% confidence intervals) were higher in those receiving 2, 3, and 4 vaccine doses than in unvaccinated patients (1.25 [1.08-1.45], 1.46 [1.28-1.67], and 1.79 [1.54-2.06], respectively). However, hospitalization or death IRRs during this period were lower in those receiving 3 or 4 vaccine doses than in unvaccinated patients (0.62 [0.45-0.86] and 0.39 [0.26-0.58], respectively). Risk-adjusted analyses for deaths after SARS-CoV-2 infection between December 7, 2020, and March 31, 2022, found hazard ratios (95% confidence intervals) of 0.67 (0.46-0.98), 0.46 (0.30-0.69), and 0.18 (0.09-0.35) for those with 2, 3, and 4 vaccine doses, respectively, when compared with the unvaccinated group.
Conclusions.
In immunosuppressed SOT recipients, vaccination is associated with incremental, dose-dependent protection against hospitalization or death after SARS-CoV-2 infection, including against the Omicron variant.
INTRODUCTION
Vaccination has been shown to reduce the risk of severe outcomes after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the general population.1-3 The clinical trials to establish vaccine efficacy, which subsequently enabled emergency-use authorization, were performed when the wild-type or Alpha variant were the dominant SARS-CoV-2 strains in circulation. In November 2021, the B.1.1.529 variant, first observed in cases from Botswana and South Africa, was designated as the Omicron variant and identified as a variant of concern by the World Health Organization.4 The variant is characterized by numerous mutations, with a significant number located within the receptor binding domain; it was therefore predicted to be less susceptible to neutralizing antibodies elicited by previous SARS-CoV-2 infection or vaccination.5 Subsequent publications have reported on reduced vaccine effectiveness (VE) and breakthrough infection with this variant in the general population following 2 or more vaccine doses.6-9
Solid organ and islet transplant (SOT) recipients were not included in vaccine efficacy trials despite having a significantly higher mortality risk following SARS-CoV-2 infection than the general population.10 Because of the requirement for continual systemic immunosuppression to prevent allograft rejection, vaccine responses are generally impaired in SOT recipients,11 and suboptimal responses to SARS-CoV-2 vaccination have been similarly reported in this population during the Alpha- and Delta-variant-dominant periods.12,13 Thus, the emergence of the Omicron variant with predicted relative resistance to established SARS-CoV-2 vaccines is a particular concern for the vulnerable SOT patient population, a concern heightened by the coincidental reduction in most countries in mandated nonpharmacological interventions (NPIs) such as mask wearing and social distancing. However, although small-scale serological studies in transplant recipients have confirmed limited postvaccination neutralizing antibody responses to the Omicron variant,14 how this relates to protection from hospitalization or death is not clear, and large-scale national analyses of SOT recipients during the Omicron wave have not been reported.
Here, we report on a risk-adjusted national registry study aiming to determine real-world VE in SOT recipients during the Omicron-dominant period in England. Three key outcomes were analyzed: incidence of testing positive for SARS-CoV-2, incidence of noninjury hospitalization within 14 d of testing positive for SARS-CoV-2, and risk of death within 28 d following a positive test for SARS-CoV-2.
MATERIALS AND METHODS
Study Cohort and Outcome Definitions
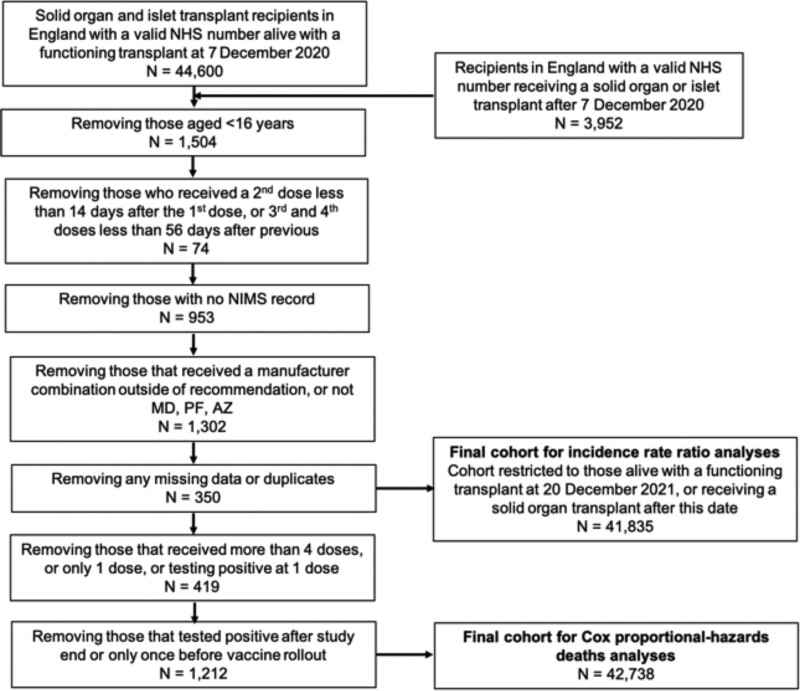
The at-risk cohort we examined were patients aged 16 y or more, residing in England, that were recipients of an organ or organs from a deceased or live donor with a functioning graft on December 7, 2020, and those transplanted between that date and March 31, 2022 (Figure 1).
FIGURE 1.
Study inclusion and exclusion criteria and patient flow. AZ, ChAdOx1-S vaccine; MD, mRNA-1273 vaccine; NHS, National Health Service; NIMS, National Immunisation Management Service; PF, BNT162b2 vaccine.
We only included those patients who received 2 homologous doses of Pfizer-BioNTech BNT162b2 mRNA vaccine (BNT162b2), Oxford University-AstraZeneca ChAdOx1-S vaccine (ChAdOx1-S), or Moderna mRNA-1273 vaccine (mRNA-1273) as their first 2 doses (or “spine”). Recipients of any other vaccine type as a first or second dose or those who received a different vaccine type as a second dose (ie, a heterologous schedule) were excluded because of small numbers and the inability to attribute any protective effect to a particular vaccine spine.
SOT recipients who were unmatched in the National Immunisation Management Service register were excluded, as were those without a valid National Health Service (NHS) number, a unique identifier for all patients in England for care provided by the NHS. Patients who had >1 episode of SARS-CoV-2 positive infection during the study period had outcomes analyzed for their first infection only. Individuals with a SARS-CoV-2 positive infection before December 7, 2020, were excluded from the Cox proportional-hazards analysis. Patients who received >4 vaccine doses were censored at the time of their fifth dose.
Outcomes of interest were the date of testing positive for SARS-CoV-2, noninjury hospitalization via emergency care within 14 d of a positive test, and patient death within 28 d of a positive test. Cohorts were followed until March 31, 2022, for SARS-CoV-2 infections and until April 28, 2022, for hospitalization or death (to allow for 28 d exposure). This study period covered an era when there was unrestricted access to testing for SARS-CoV-2 in the United Kingdom.
Vaccination status was defined as “unvaccinated” if the recipient had not received a vaccine dose during the study period or was ≤14 d after a first vaccine dose; “1 dose” if >14 d after the first dose and ≤14 d after the second dose; “2 doses” if >14 d after the second dose and ≤14 d after a third dose; “3 doses” if >14 d after a third dose and ≤14 d after a fourth dose; and “4 doses” if >14 d after a fourth dose.
Vaccination Program
The vaccination program commenced in December 2020, with most SOT recipients completing their first 2 doses by July 2021. They were eligible to receive a third dose from September 2021 and a fourth dose from January 2022.15 The vaccination program in the United Kingdom required the third does or subsequent doses to be either BNT162b2 or mRNA-1273 vaccine type in general. Other than prioritization for early receipt, SOT recipients were not assigned any particular vaccine. National policy mandated a 10- to 12-wk gap between first and second vaccine doses, irrespective of vaccine type, and a minimum of 3 mo between the second and third doses and the third and fourth doses.16 Vaccine rollout for the general population in the United Kingdom, including SOT recipients, has been described previously.12,17,18
The UK Health Security Agency designated Omicron as the dominant SARS-CoV-2 variant in the United Kingdom from December 2021,18 with Alpha- and Delta-dominant periods described as before.12 SARS-CoV-2 variant information was not available for each individual patient testing positive.
Study Design, Data Sources, Statistical Analyses, and Ethical Approval
This was a national retrospective cohort study enabled by linkage of 5 national registries in England, as described previously.12,19 Unlike previous analyses from this group, positive tests included both lateral flow (antigen-based) tests reported to the UK Government and laboratory-confirmed tests for SARS-CoV-2 RNA. Data on the number of positive SARS-CoV-2 tests in the general population of England were obtained from the UK Government (https://coronavirus.data.gov.uk). Data on patient symptoms were not available.
In line with publications related to this pandemic, noninjury hospitalization within 14 d or death within 28 d of SARS-CoV-2 positive infection was assumed to be because of, or related to, COVID-19. Causes of death were not definitively known.
Demographic characteristics (type of organ received, time since transplant, sex, age, ethnicity, and NHS region) for SOT recipients were summarized and stratified by vaccination status. Differences in characteristics between groups of SOT recipients were tested univariately using the chi-square test. Three risk periods (<90 d, 90 d–1 y, and >1 y posttransplant) were arbitrarily selected a priori in an attempt to account for the changing burden of immunosuppression after transplantation.
In order to provide estimates of VE within the Omicron-dominant period, data from December 20, 2021, to March 31, 2022, were analyzed. To minimize temporal bias and to take into account variations in community prevalence of SARS-CoV-2 infections during that period, infection incidence rate was defined as the number of events divided by the person-time at risk, stratified by the 6 demographic characteristics above and calendar month and, in some analyses, vaccine type. A Poisson regression model was used to derive infection incidence rate ratios (IRRs) with 95% confidence intervals adjusted for the variables above, in which IRR is the risk-adjusted incidence rate in vaccinated recipients divided by the incidence rate for unvaccinated recipients. Hospitalization IRRs and hospitalization or death IRRs were adjusted for the same variables as infection IRRs.
To further investigate findings regarding risk of death, an additional analysis was conducted using Cox proportional-hazards modeling. The analysis period covered December 7, 2020, to March 31, 2022, rather than the Omicron-dominant period alone, with deaths observed to April 28, 2022. A Cox proportional-hazards model was used to estimate the hazard ratio of risk of death after SARS-CoV-2 positive infection, adjusting for type of organ received, time since transplant, sex, age, ethnicity, NHS region, vaccination status, and vaccine type. Unadjusted Kaplan-Meier estimates of patient survival from the day of SARS-CoV-2 positive infection were stratified by vaccination status and vaccine type and were compared using the log-rank test.
Analyses were undertaken using SAS, version 9.4 (SAS Institute Inc, Cary, NC), and STATA, version 14.2.
Ethical approval was as described previously.12
RESULTS
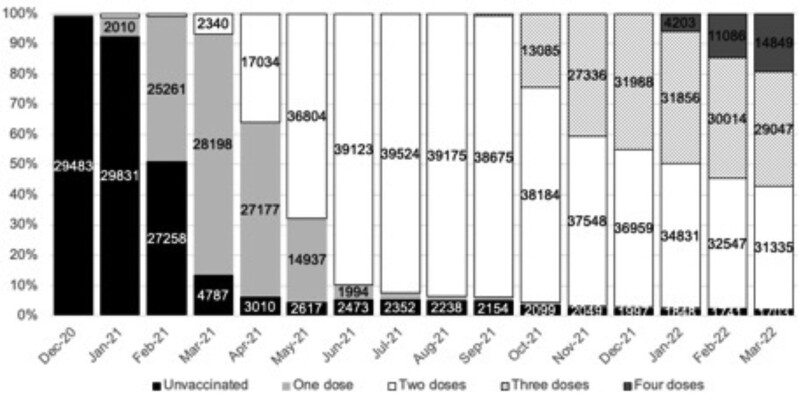
By March 31, 2022, 2646 (6.2%) SOT recipients meeting study inclusion criteria were unvaccinated, 3822 (8.9%) had received 2 doses, 18 725 (43.8%) had received 3 doses, and 17 545 (41.1%) had received 4 doses (Table 1). Vaccination in SOT recipients began in December 2020, with the majority of patients having received 2 doses by June 2021; rollout of third doses began in September 2021 and fourth doses in January 2022 (Figure 2). The median (interquartile range) interval between first and second vaccine doses was 77 d (70–79 d), with intervals between the second and third doses, and third and fourth doses, of 191 d (182–205 d) and 105 d (95–123 d), respectively. There were no differences in intervals between vaccine types (data not shown). The overwhelming majority of SOT recipients received 1 or more vaccines after their transplant (Figure 1). The median (interquartile range) time between transplantation and first vaccine dose effective date was 6.5 y (2.6–12.5 y). Of the 3952 patients who were transplanted after the start of the study date, 3194 were vaccinated with 1 or more doses before their transplant.
TABLE 1.
Demographic characteristics of unvaccinated and vaccinated solid organ and islet transplant recipients on March 31, 2022
| Variable | Vaccination status | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | Two doses | Three doses | Four doses | ||||||
| n | % | n | % | n | % | n | % | ||
| Total | 2646 | 6.2 | 3822 | 8.9 | 18 725 | 43.8 | 17 545 | 41.1 | |
| Transplant type | |||||||||
| Kidneya | 1827 | 6 | 2748 | 9 | 13 234 | 43.5 | 12 583 | 41.4 | <0.0001 |
| SPKb | 97 | 6 | 118 | 7.3 | 741 | 45.5 | 671 | 41.2 | |
| Liver | 532 | 7.1 | 620 | 8.3 | 3353 | 45 | 2950 | 39.6 | |
| Heart | 115 | 6.2 | 172 | 9.3 | 827 | 44.6 | 741 | 39.9 | |
| Lungc | 57 | 5 | 132 | 11.5 | 497 | 43.2 | 464 | 40.3 | |
| Intestinal and multiorgand | 18 | 6.9 | 32 | 12.4 | 73 | 28.2 | 136 | 52.5 | |
| Ethnicity | |||||||||
| White | 1629 | 5.1 | 2341 | 7.3 | 13 470 | 41.8 | 14 761 | 45.8 | <0.0001 |
| Asian | 359 | 7.4 | 807 | 16.7 | 2585 | 53.5 | 1080 | 22.4 | |
| Black | 438 | 18 | 425 | 17.5 | 1221 | 50.2 | 346 | 14.2 | |
| Other | 120 | 10.1 | 119 | 10 | 615 | 51.6 | 338 | 28.4 | |
| Unknown | 100 | 4.8 | 130 | 6.2 | 834 | 40 | 1020 | 48.9 | |
| Age (y) | |||||||||
| 16–49 | 1200 | 8.4 | 1777 | 12.4 | 6935 | 48.4 | 4428 | 30.9 | <0.0001 |
| 50+ | 1446 | 5.1 | 2045 | 7.2 | 11 790 | 41.5 | 13 117 | 46.2 | |
| Sex | |||||||||
| Male | 1661 | 6.4 | 2327 | 8.9 | 11 577 | 44.5 | 10 469 | 40.2 | <0.0001 |
| Female | 985 | 5.9 | 1495 | 8.9 | 7148 | 42.8 | 7076 | 42.4 | |
| Time from transplant | |||||||||
| <90 d | 111 | 12 | 68 | 7.3 | 619 | 66.8 | 129 | 13.9 | <0.0001 |
| 90 d to 1 y | 61 | 3 | 270 | 13.3 | 980 | 48.2 | 723 | 35.5 | |
| >1 y | 2474 | 6.2 | 3484 | 8.8 | 17 126 | 43.1 | 16 693 | 42 | |
| NHS region | |||||||||
| East of England | 267 | 5.1 | 369 | 7.1 | 2162 | 41.6 | 2403 | 46.2 | <0.0001 |
| London | 776 | 10.3 | 904 | 12 | 3822 | 50.6 | 2049 | 27.1 | |
| Midlands | 429 | 5.6 | 752 | 9.7 | 3337 | 43.2 | 3199 | 41.5 | |
| North East and Yorkshire | 357 | 5.3 | 550 | 8.2 | 2721 | 40.6 | 3067 | 45.8 | |
| North West | 308 | 6.2 | 525 | 10.5 | 2379 | 47.7 | 1773 | 35.6 | |
| South East | 308 | 4.8 | 461 | 7.2 | 2690 | 41.9 | 2965 | 46.2 | |
| South West | 201 | 4.8 | 261 | 6.3 | 1614 | 38.8 | 2089 | 50.2 | |
aIncludes single kidney, en bloc kidney, and double kidney transplants.
bSimultaneous pancreas-kidney transplants, including pancreas only, islet only, and simultaneous islet-kidney transplants.
cIncludes single lung, double lung, partial lung, and heart-lung transplants.
dIncludes if any other organ was transplanted with the intestine, as well as liver-kidney, heart-lung-liver, liver-pancreas, heart-kidney, heart-liver, and lung-liver transplants.
NHS, National Health Service; SPK, simultaneous pancreas-kidney transplant.
FIGURE 2.
Percentage of unvaccinated and vaccinated solid organ and islet transplant recipients through the study period. Numbers >1000 are shown on the graph. Recipients can be counted more than once in each month as they receive a vaccine. Numbers in each month do not add up to the summary data by the end of the study date because of patient deaths, incident transplants, and censoring.
Demographic characteristics of unvaccinated SOT recipients and those who had received 2, 3, or 4 vaccine doses by March 31, 2022, are shown in Table 1. Recipients who received 4 vaccine doses were more likely to be older, White, and female and to live outside London than those who did not. Of the 17 545 patients who received 4 vaccine doses, 9637 (54.9%) had been given 2 ChAdOx1-S doses followed by 2 mRNA vaccine doses (either BNT162b2 or mRNA-1273), whereas 7908 (45.1%) received 4 doses of mRNA vaccines (Table S1, SDC, http://links.lww.com/TP/C694).
SARS-CoV-2 Infections
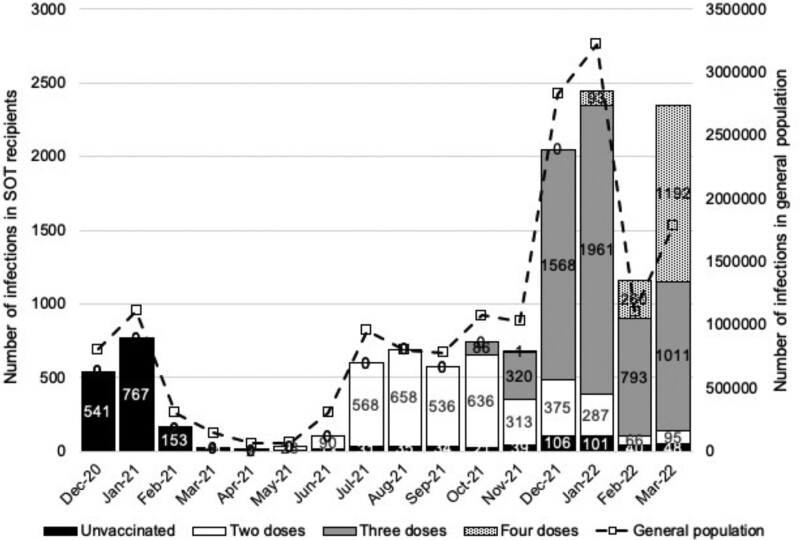
Overall, there were 12 454 SOT recipients with at least 1 SARS-CoV-2 infection between December 7, 2020, and March 31, 2022. Cases peaked in December 2020 to January 2021, July 2021 to September 2021, and December 2021 to January 2022, corresponding to Alpha, Delta, and Omicron variant surges in the general UK population (Figure 3). The first peak occurred before vaccine rollout, whereas for later peaks, the majority of those testing positive had been vaccinated. Demographic characteristics of SOT recipients with SARS-CoV-2 infections at the time of the last positive test are shown in Table 2, stratified by vaccination status. Data by vaccine type are shown in Table S2 (SDC, http://links.lww.com/TP/C694). The median (interquartile range) interval from latest vaccine-effective date before the last known positive SARS-CoV-2 infection result was 77 d (45–125 d) (Figure S1, SDC, http://links.lww.com/TP/C694). Of the 12 454 recipients who tested positive for SARS-CoV-2 at least once, just 608 (4.9%) had received 1 or more vaccines pretransplant.
FIGURE 3.
Number of SARS-CoV-2 positive infections per month in SOT recipients, by vaccination status compared with the general population in England, December 7, 2020, to March 31, 2022. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ and islet transplant.
TABLE 2.
Demographic characteristics of solid organ and islet transplant recipients with SARS-CoV-2 infection or death within 28 d of the latest positive test, by vaccination status, December 7, 2020, to March 31, 2022
| Variable | Unvaccinated | Two vaccine doses | Three vaccine doses | Four vaccine doses | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | Cases | Deaths | Cases | Deaths | ||||||
| n | n | % | n | n | % | n | n | % | n | n | % | ||
| Total | 1764 | 210 | 11.9 | 3465 | 265 | 7.6 | 5702 | 153 | 2.7 | 1523 | 14 | 0.9 | |
| Transplant type | |||||||||||||
| Kidneya | 1333 | 155 | 11.6 | 2533 | 191 | 7.5 | 4170 | 112 | 2.7 | 1133 | 10 | 0.9 | 0.90 |
| SPKb | 58 | 8 | 13.8 | 145 | 6 | 4.1 | 239 | 4 | 1.7 | 55 | 0 | 0 | |
| Liver | 279 | 27 | 9.7 | 493 | 29 | 5.9 | 809 | 19 | 2.3 | 199 | 2 | 1 | |
| Heart | 57 | 8 | 14 | 180 | 17 | 9.4 | 271 | 10 | 3.7 | 74 | 1 | 1.4 | |
| Lungc | 34 | 12 | 35.3 | 92 | 22 | 23.9 | 181 | 8 | 4.4 | 50 | 1 | 2 | |
| Intestinal and multiorgand | 3 | 0 | 0 | 22 | 0 | 0 | 32 | 0 | 0 | 12 | 0 | 0 | |
| Ethnicity | |||||||||||||
| White | 1029 | 122 | 11.9 | 2514 | 193 | 7.7 | 4456 | 111 | 2.5 | 1330 | 14 | 1.1 | 0.002 |
| Asian | 357 | 45 | 12.6 | 518 | 41 | 7.9 | 564 | 18 | 3.2 | 84 | 0 | 0 | |
| Black | 224 | 28 | 12.5 | 227 | 19 | 8.4 | 269 | 8 | 3 | 16 | 0 | 0 | |
| Other | 79 | 6 | 7.6 | 82 | 2 | 2.4 | 156 | 6 | 3.8 | 25 | 0 | 0 | |
| Unknown | 75 | 9 | 12 | 124 | 10 | 8.1 | 257 | 10 | 3.9 | 68 | 0 | 0 | |
| Age group (y) | |||||||||||||
| 16–49 | 745 | 27 | 3.6 | 1570 | 37 | 2.4 | 2329 | 8 | 0.3 | 530 | 2 | 0.4 | 0.05 |
| 50+ | 1019 | 183 | 18 | 1895 | 228 | 12 | 3373 | 145 | 4.3 | 993 | 12 | 1.2 | |
| Sex | |||||||||||||
| Male | 1064 | 135 | 12.7 | 2074 | 179 | 8.6 | 3399 | 109 | 3.2 | 871 | 9 | 1 | 0.6 |
| Female | 700 | 75 | 10.7 | 1391 | 86 | 6.2 | 2303 | 44 | 1.9 | 652 | 5 | 0.8 | |
| Time from transplant | |||||||||||||
| <90 d | 47 | 7 | 14.9 | 61 | 1 | 1.6 | 77 | 0 | 0 | 10 | 0 | 0 | 0.05 |
| 90 d to 1 y | 103 | 9 | 8.7 | 197 | 9 | 4.6 | 280 | 4 | 1.4 | 75 | 0 | 0 | |
| >1 y | 1614 | 194 | 12 | 3207 | 255 | 8 | 5345 | 149 | 2.8 | 1438 | 14 | 1 | |
| NHS region | |||||||||||||
| East of England | 177 | 33 | 18.6 | 353 | 24 | 6.8 | 712 | 12 | 1.7 | 225 | 1 | 0.4 | 0.002 |
| London | 537 | 47 | 8.8 | 563 | 35 | 6.2 | 1013 | 20 | 2 | 194 | 0 | 0 | |
| Midlands | 333 | 47 | 14.1 | 689 | 65 | 9.4 | 1035 | 38 | 3.7 | 257 | 3 | 1.2 | |
| North East and Yorkshire | 169 | 18 | 10.7 | 619 | 54 | 8.7 | 855 | 22 | 2.6 | 239 | 0 | 0 | |
| North West | 207 | 22 | 10.6 | 503 | 35 | 7 | 692 | 23 | 3.3 | 155 | 3 | 1.9 | |
| South East | 246 | 26 | 10.6 | 451 | 29 | 6.4 | 841 | 20 | 2.4 | 268 | 3 | 1.1 | |
| South West | 95 | 17 | 17.9 | 287 | 23 | 8 | 554 | 18 | 3.2 | 185 | 4 | 2.2 | |
aIncludes single kidney, en bloc kidney, and double kidney transplants.
bSimultaneous pancreas-kidney transplants, including pancreas only, islet only, and simultaneous islet-kidney transplants.
cIncludes single lung, double lung, partial lung, and heart-lung transplants.
dIncludes if any other organ was transplanted with the intestine, as well as liver-kidney, heart-lung-liver, liver-pancreas, heart-kidney, heart-liver, and lung-liver transplants.
NHS, National Health Service; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SPK, simultanoues pancreas-kidney transplant.
VE in the Omicron-dominant Period
This cohort comprised 41 835 SOT recipients, of whom 1997 remained unvaccinated. Between December 20, 2021, and March 31, 2022, the incidence rate of SARS-CoV-2 infections was 146.0 per 100 000 person-days for unvaccinated recipients, 168.1 per 100 000 person-days for those who received 2 vaccine doses, 183.6 per 100 000 person-days for those who received 3 doses, and 238.6 per 100 000 for those recipients who received 4 doses (Table 3). Compared with the unvaccinated SOT cohort, the risk-adjusted infection IRR was 1.25 (1.08–1.45), 1.46 (1.28–1.67), and 1.79 (1.54–2.06) for those receiving 2, 3, and 4 doses, respectively, indicating that vaccination did not reduce the risk of testing positive for SARS-CoV-2. When vaccine type was analyzed, no regimen showed a protective effect from SARS-CoV-2 infection. Risk-adjusted IRRs by vaccine status, demographic variables, and month are shown in Table S3 (SDC, http://links.lww.com/TP/C694).
TABLE 3.
SARS-CoV-2 infection incidence rates and risk-adjusted incidence rate ratios in solid organ and islet transplant recipients in the Omicron-dominant period, December 20, 2021, to March 31, 2022
| Vaccination status | Unvaccinated | Two doses | Three doses | Four doses | Risk-adjusted incidence rate ratio (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Incidence rate per 100 000 person-days | Cases | Incidence rate per 100 000 person-days | Cases | Incidence rate per 100 000 person-days | Cases | Incidence rate per 100 000 person-days | |||
| Total | 252 | 146.0 | 697 | 168.1 | 1.25 (1.08-1.45) | 0.002 | ||||
| 4857 | 183.6 | 1.46 (1.28-1.67) | <0.001 | |||||||
| 1522 | 238.6 | 1.79 (1.54-2.06) | <0.001 | |||||||
| By vaccine type | ||||||||||
| AZ spine only | 432 | 171.0 | 1.29 (1.11-1.52) | 0.001 | ||||||
| mRNA spine only | 265 | 163.6 | 1.20 (1.01-1.43) | 0.04 | ||||||
| AZ spine + mRNA booster | 2740 | 180.8 | 1.45 (1.27-1.66) | <0.001 | ||||||
| mRNA spine + mRNA booster | 2117 | 187.3 | 1.49 (1.30-1.70) | <0.001 | ||||||
| AZ spine + 2 mRNA boosters | 779 | 230.3 | 1.73 (1.49-2.01) | <0.001 | ||||||
| mRNA spine + 2 mRNA boosters | 743 | 248.1 | 1.84 (1.58-2.13) | <0.001 | ||||||
AZ, ChAdOx1-S vaccine; CI, confidence interval; mRNA, BNT162b2 or mRNA-1273 vaccines; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Severe event (hospitalization or death) data were also analyzed using IRR methods. During the Omicron-dominant period, there were 515 noninjury hospitalizations within 14 d of a positive SARS-CoV-2 test (Table 4). Vaccination was associated with a reduction in the risk of hospitalization and hospitalization or death following a positive test but only in those who had received 3 or more vaccine doses. The greatest protection from a severe event after SARS-CoV-2 infection occurred in those who had received 4 vaccines, particularly for those who received an mRNA vaccine spine and 2 mRNA boosters (Table 4).
TABLE 4.
Vaccine effectiveness against hospitalization and against hospitalization or death, by vaccine number and type, during the Omicron-dominant period December 20, 2021, to March 31, 2022
| Vaccination status | Person-years | Hospitalizations within 14 d of SARS-CoV-2 positive infection | Deaths within 28 d of SARS-CoV-2 positive infection | Hospitalizations within 14 d or deaths within 28 d of SARS-CoV-2 positive infection | Risk-adjusted hospitalization incidence rate ratio (hospitalization within 14 d of SARS-CoV-2 positive infection) | P | Risk-adjusted hospitalization or death incidence rate ratio (hospitalization within 14 d or death within 28 d of SARS-CoV-2 positive infection) | P |
|---|---|---|---|---|---|---|---|---|
| Unvaccinated | 497 | 38 | 10 | 43 | – | – | ||
| Two doses | 870.5 | 82 | 13 | 92 | 1.27 (0.86-1.88) | 0.2 | 1.27 (0.88-1.83) | 0.2 |
| Three doses | 6793 | 341 | 106 | 389 | 0.66 (0.46-0.93) | 0.02 | 0.62 (0.45-0.86) | 0.004 |
| Four doses | 1983 | 54 | 14 | 63 | 0.43 (0.27-0.66) | <0.0001 | 0.39 (0.26-0.58) | <0.0001 |
| By vaccine type | ||||||||
| Unvaccinated | 497 | 38 | 10 | 43 | – | – | ||
| AZ spine only | 520.5 | 52 | 8 | 57 | 1.36 (0.89-2.07) | 0.2 | 1.32 (0.89-1.97) | 0.2 |
| mRNA spine only | 350 | 30 | 5 | 35 | 1.15 (0.71-1.86) | 0.6 | 1.19 (0.76-1.87) | 0.4 |
| AZ spine + mRNA booster | 3944.4 | 194 | 65 | 220 | 0.66 (0.46-0.94) | 0.02 | 0.62 (0.44-0.87) | 0.01 |
| mRNA spine + mRNA booster | 2848.6 | 147 | 41 | 169 | 0.65 (0.45-0.94) | 0.02 | 0.62 (0.44-0.88) | 0.01 |
| AZ spine + 2 mRNA boosters | 1055.8 | 32 | 8 | 36 | 0.48 (0.29-0.79) | 0.004 | 0.42 (0.27-0.67) | <0.0001 |
| mRNA spine + 2 mRNA boosters | 927.1 | 22 | 6 | 27 | 0.36 (0.21-0.63) | <0.0001 | 0.35 (0.21-0.57) | <0.0001 |
“–” represents ‘Not relevant’ in this context.
AZ, ChAdOx1-S vaccine; mRNA, BNT162b2 or mRNA-1273 vaccines; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Deaths Within 28 d of a Last Reported Positive SARS-CoV-2 Test
Insufficient mortality events were observed to enable a meaningful Cox proportional-hazards analysis of death data during the Omicron-dominant period. Therefore, to expand on the IRR severe events analysis, a broader time period was used. Between December 7, 2020, and March 31, 2022, 12 454 SOT recipients had at least 1 SARS-CoV-2 infection, and 642 (5.2%) died within 28 d. Demographic characteristics of recipients dying after SARS-CoV-2 infection are shown in Table 2. Overall, of those SOT recipients who were unvaccinated at the time of positive test, 11.9% (210/1764) died within 28 d of a SARS-CoV-2 infection, compared with 7.6% of those who had received 2 doses (265/3465), 2.7% receiving 3 doses (153/5702), and 0.9% of those receiving 4 doses (14/1523).
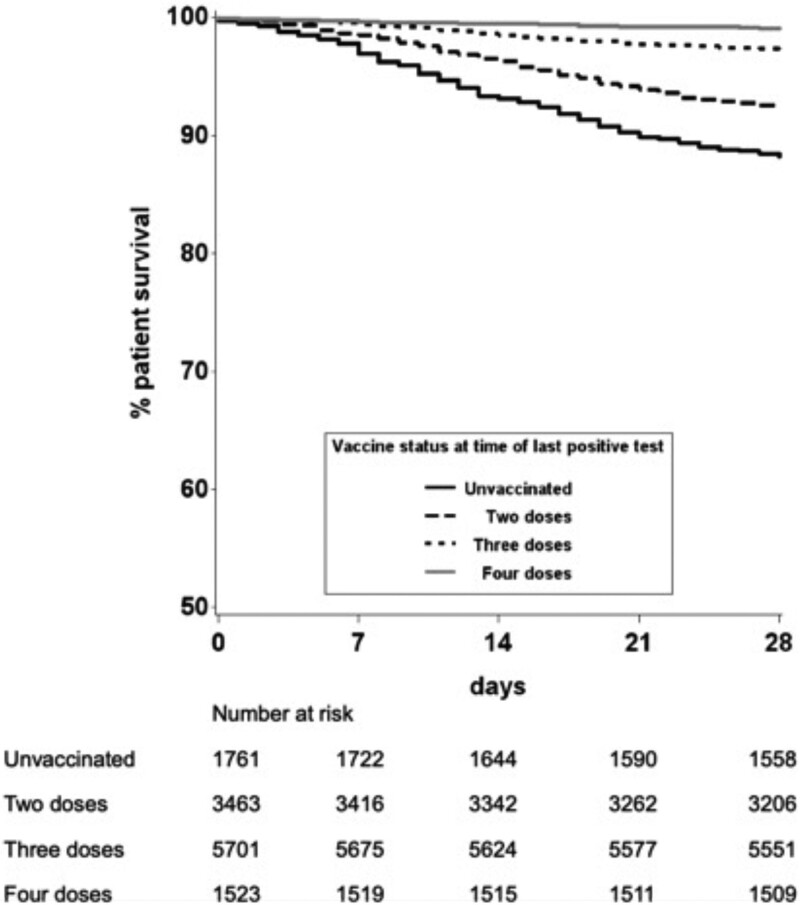
Patient survival from the day of last reported SARS-CoV-2 positive result was plotted using an unadjusted Kaplan-Meier analysis, stratified by vaccination status (Figure 4). SOT recipients who had received 2 doses, 3 doses, or 4 doses had a higher survival at 28 d than those who were unvaccinated (92.4%, 97.3%, and 99.1%, respectively, versus 88.2%, respectively; P < 0.0001).
FIGURE 4.
Unadjusted patient survival within 28 d of infection with SARS-CoV-2 from date of testing positive, by vaccination status, December 7, 2020, to March 31, 2022. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
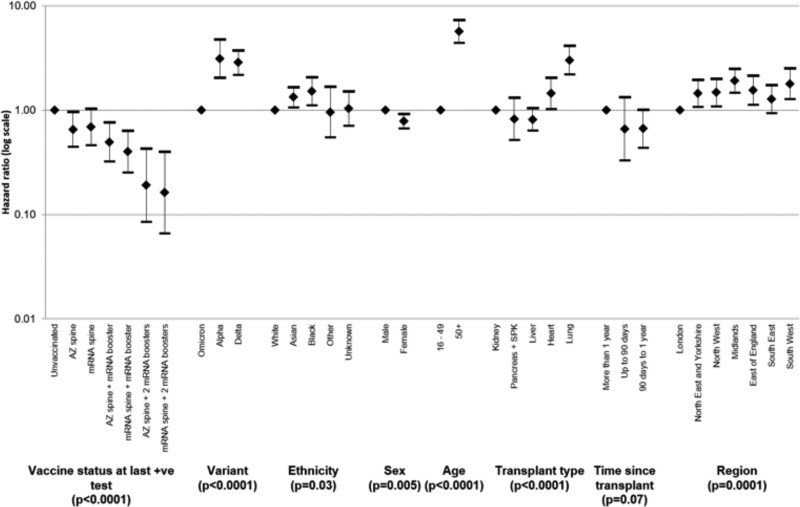
After risk-adjustment, a statistically significant increased chance of death within 28 d of a SARS-CoV-2 infection was found in those who were aged ≥50 y, from Black or Asian ethnic groups, male, and a recipient of a heart or lung transplant; those living outside of London (except for the South East); and those testing positive during the Alpha- or Delta-dominant periods (Figure 5 and Table S4, SDC, http://links.lww.com/TP/C694). The risk-adjusted hazard ratio (95% confidence interval) for death within 28 d of SARS-CoV-2 infection was 0.67 (0.46-0.98) for those with 2 vaccine doses, 0.46 (0.30-0.69) for those with 3 vaccine doses, and 0.18 (0.09-0.35) for those who had received 4 vaccine doses, in comparison to the unvaccinated group. The risk-adjusted hazard ratio for death within 28 d of SARS-CoV-2 infection during the Omicron-dominant period was significantly lower than that during the Alpha- and Delta-dominant periods (3.12 [2.04-4.78] and 2.86 [2.18-3.76], respectively).
FIGURE 5.
Hazard ratios (95% confidence intervals) of risk of death within 28 d of SARS-CoV-2 infection in solid organ and islet transplant recipients, by vaccination status, variant dominant period, and demographic characteristics, December 7, 2020, to March 31, 2022. AZ, ChAdOx1-S vaccine; mRNA, either BNT162b2 or mRNA-1273 vaccine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
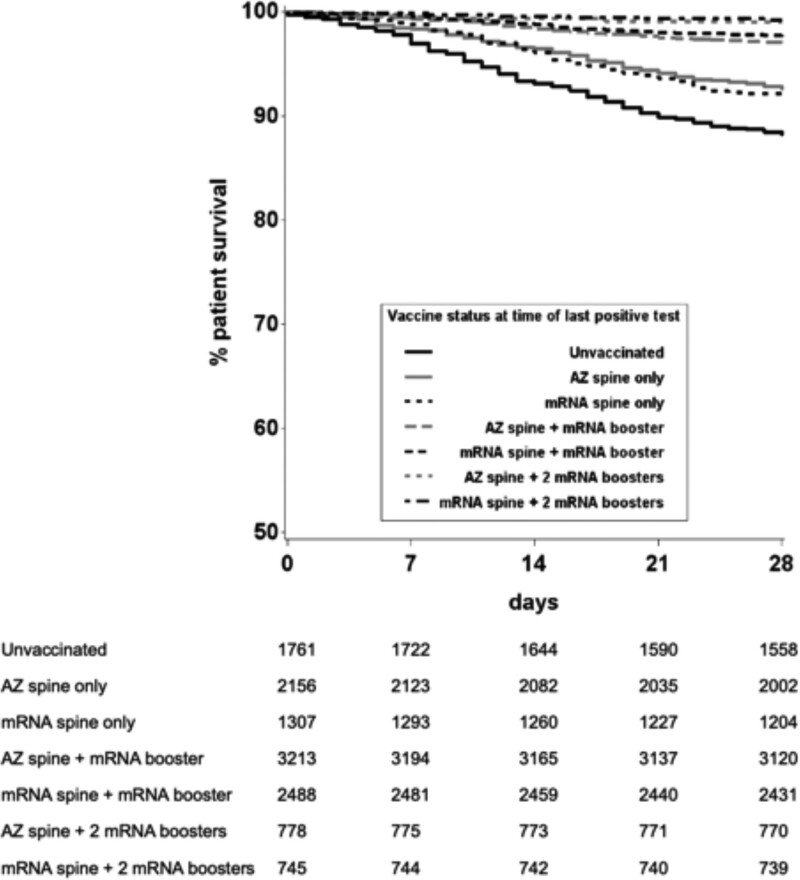
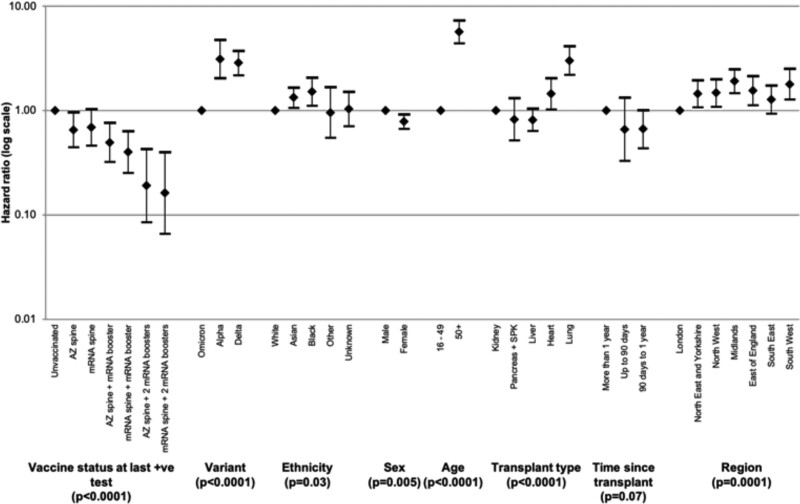
Differences in effectiveness between vaccine types were investigated with unadjusted and risk-adjusted analyses. Kaplan-Meier survival curves suggested that rates of death within 28 d following SARS-CoV-2 infection were lowest after vaccination with an mRNA spine followed by 2 mRNA boosters (Figure 6). Inclusion of vaccine type as a variable in the Cox proportional-hazards model allowed investigation of protective effect of different vaccine types (Figure 7 and Table S5, SDC, http://links.lww.com/TP/C694). Those receiving an mRNA spine followed by 2 mRNA boosters had the largest reduction in risk of death after SARS-CoV-2 infection (84%) when compared with unvaccinated recipients.
FIGURE 6.
Unadjusted patient survival from date of testing positive for SARS-CoV-2, by vaccination status and vaccine type, December 7, 2020, to March 31, 2022. AZ, ChAdOx1-S vaccine; mRNA, either BNT162b2 or mRNA-1273 vaccine; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
FIGURE 7.
Hazard ratios with 95% confidence intervals of risk of death within 28 d of testing positive for SARS-CoV-2 in solid organ and islet transplant recipients, by vaccination status, vaccine type, and demographic characteristics, December 7, 2020, to March 31, 2022. AZ, ChAdOx1-S vaccine; mRNA, BNT162b2 or mRNA-1273 vaccines; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
This national registry linkage analysis shows that the majority of SOT recipients in England received 3 or 4 doses of SARS-CoV-2 vaccines. There was an incremental benefit against hospitalization or death during the Omicron-dominant period in England for recipients of 3 or 4 doses compared with recipients of 2 doses or fewer. However, there was no vaccine-associated protection against the risk of testing positive for SARS-CoV-2 infection. As reported previously,12 demographic factors including age, ethnicity, and organ transplant type were associated with adverse outcomes.
The high number of mutations in the receptor binding domain of the Omicron variant raised concerns of reduced VE.20 VE following third or fourth doses including effectiveness against the Omicron variant in the general population have been recently reported.6,7,21-24 Grewal et al21 showed that a fourth vaccine dose was associated with a strong protection against severe outcomes due to the Omicron variant in vaccinated care home residents compared with unvaccinated residents. Similar to the findings in our study, Grewal et al21 also showed greater VE with each additional dose for all outcomes. Bar-on et al22 demonstrated that recipients of a fourth dose of BNTb162b had lower rates of confirmed SARS-CoV-2 infection and severe COVID-19 compared with 3-dose recipients. Andrews et al6 showed that, in the UK general population, immunization with 2 doses of ChAdOx1-S or BNT162b2 vaccine provided limited protection against symptomatic disease caused by the Omicron variant. A BNT162b2 or an mRNA-1273 booster after either the ChAdOx1-S or BNT162b2 primary course substantially increased protection. Although the above and other studies described outcomes in the general population following third and fourth doses of vaccine, large-scale studies describing outcomes in immunocompromised patients, including SOT recipients, are lacking. A number of studies report on immunogenicity, including both humoral and cell-mediated responses, in transplant recipients of 2 or more vaccine doses, but these reports do not include clinical outcome data. These studies have been reviewed by Napuri et al25 and Lee et al.26
Our study is one of the first to report outcomes during the Omicron era in SOT patients on a national scale.27 Pinto-Alvarez et al27 recently reported VE in a Colombian cohort of almost 7000 solid organ transplant recipients, showing that 3 vaccine doses appeared to provide almost 95% protection against death due to COVID-19 between March 2021 and May 2022. We were able to examine outcomes in a larger population, enabling us to specifically study VE during the Omicron-dominant period between December 2021 and March 2022. Our study suggests that 3 or 4 vaccine doses reduced severe events (hospitalization or death) after SARS-CoV-2 infection by between 40% and 60% during the Omicron era. Linkage of the 5 national registries that host data on immunization, infection, organ transplantation, hospitalizations, and survival allowed near real-time complete identification of new SARS-CoV-2 infections and severe events in this patient cohort. Inclusion and comprehensive follow-up of the entire at-risk population in England provided a more accurate effect estimate when comparing vaccinated versus unvaccinated SOT patients in the real world and is therefore likely to be translatable to similar patient populations in other countries.
Although the study findings of incremental protection with successive vaccine doses against severe outcomes following SARS-CoV 2 infections will be heartening, the finding of vaccination being associated with a higher risk of infection may seem counterintuitive. The absence of “sterilizing immunity” following vaccination in this patient population is not surprising because this is the same as the general population, with the benefit of vaccination predominantly being a reduced risk of hospitalization and death. Furthermore, it is possible that vaccinated SOT patients, with the confidence derived from vaccination, relaxed their NPIs more than unvaccinated patients and therefore were exposed to a higher risk of infection. The observational cohort study design does not allow us to accurately control for the confounding from relative uptake and adherence to NPIs and the associated risks of acquiring infection.
Because of the registry-based retrospective methodology, it is not possible to account for asymptomatic infections that were not confirmed with either a lateral flow or polymerase chain reaction test. Similar to published vaccine efficacy trials, it is not possible to disaggregate the protective influence of NPIs from any vaccine-derived protection. Approved antiviral treatments, including remdesivir, molnupiravir, and sotrovimab, for at-risk populations, such as SOT recipients, were available for use in the United Kingdom during the study period. It was not possible to identify patients who had received such antiviral treatments, and although it is unlikely any of the cohorts described in our study were systematically biased for or against receiving antiviral therapy, the absence of this information is a weakness.28 Furthermore, we were not able to analyze VE by immunosuppressant regimen because of incomplete data. Finally, it is not possible to rule out residual confounding due to factors that we were unable to control for or were unknown to the study team.
The SARS-CoV-2 pandemic has had significant on organ transplant services and patients across the globe.29 Furthermore, COVID-19 outcomes in SOT recipients are significantly worse than the general population.10,30 Our finding that there is incremental protection in vulnerable SOT recipients with each additional vaccine dose (at least up to the fourth vaccine dose), and that this extends to the Omicron variant, is heartening for patients and clinicians alike. Certainly, the mortality rate of <1% in those SOT recipient testing SARS-CoV-2 positive who had 4 vaccine doses is a very different perspective than the >10% mortality rates reported in this population at the onset of the pandemic.10 This is especially relevant because mandated NPI and universal access to polymerase chain reaction testing of symptomatic citizens have been stood down in most countries and SOT recipients can no longer rely on such measures for protection.
ACKNOWLEDGMENTS
Members of the Organ and Tissue Donation and Transplantation Clinical Team other than the authors already named are Dr Richard Baker, FRCP; Mr Marius Berman, FRCS; Ms Lisa Burnapp, MA; Mr Andrew Butler, FRCS; Mr John Casey, FRCS; Dr Akila Chandrasekar, FRCPath; Mr Ian Currie, FRCS; Ms Diana Garcia Saez, MD; Dr Gareth Jones, FRCP; Dr Dan Harvey, FFICM; Dr Ulrike Paulus, FRCPath; Dr Tracey Rees, FRCPath; Mr Sanjay Sinha, FRCS; Mr Rajamiyer Venkateswaran, FRCS; and Mr Alun Williams, FRCS. These colleagues are also affiliated with NHS Blood and Transplant. The authors would also like to acknowledge and thank colleagues in UK Health Security Agency and NHS Digital Tracing Service for sharing data, as well as Jamie Lopez Bernal, James Thomas, Tariq Malik, Anne Marie O’Connell, Julia Stowe, and Freya Kirsebom (all UK Health Security Agency), and Richard Little (NHS Blood and Transplant), for enabling database linkages.
Supplementary Material
Footnotes
C.J.C. and R.M.K.C. are joint first authors.
A list of members of the Organ and Tissue Donation and Transplantation Clinical Team is included under Acknowledgments.
The authors declare no funding or conflicts of interest.
C.J.C. and R.R. participated in research design and data analysis and cowrote the article. R.M.K.C., L.Mu., and H.W. participated in research design, co-led the data analysis, and participated in the writing of the article. G.P., D.G., L.Ma., D.T., S.W., J.P., I.U.-L., and D.M. participated in research design and the writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Supplemental Visual Abstract; http://links.lww.com/TP/C695.
REFERENCES
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. 2021. Available at https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern#:~:text=known%20confirmed%20B.-,1.1.,as%20compared%20to%20other%20VOCs.2022). Accessed December 15, 2022
- 5.European Centre for Disease Prevention and Control. Threat assessment brief: implications of the further emergence and spread of the SARS-CoV-2 B.1.1.529 variant of concern (Omicron) for the EU/EEA - first update. 2021. Available at https://www.ecdc.europa.eu/en/publications-data/covid-19-threat-assessment-spread-omicron-first-update. Accessed December 15, 2022
- 6.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med. 2022;387:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheikh A, Kerr S, Woolhouse M, et al. Severity of Omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022;22:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravanan R, Callaghan CJ, Mumford L, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20:3008–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckerle I, Rosenberger KD, Zwahlen M, et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor KL, Kim SJ, Smith G, et al. Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: a population-based cohort study from Canada. Am J Transplant. 2022;22:2228–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaba AH, Johnston TS, Aytenfisu TY, et al. A fourth dose of COVID-19 vaccine does not induce neutralization of the Omicron variant among solid organ transplant recipients with suboptimal vaccine response. Transplantation. 2022;106:1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Government UK. Joint Committee on Vaccination and Immunisation (JCVI) statement on COVID-19 vaccinations in 2022: 21 February 2022. 2022. Available at https://www.gov.uk/government/publications/joint-committee-on-vaccination-and-immunisation-statement-on-covid-19-vaccinations-in-2022/joint-committee-on-vaccination-and-immunisation-jcvi-statement-on-covid-19-vaccinations-in-2022-21-february-2022. Accessed December 15, 2022
- 16.NHS England. Updated JCVI guidance for vaccinating immunosuppressed individuals with a third primary dose. 2021. Available at https://www.england.nhs.uk/coronavirus/documents/updated-jcvi-guidance-for-vaccinating-immunosuppressed-individuals-with-a-third-primary-dose/. Accessed December 15, 2022
- 17.Ravanan R, Mumford L, Ushiro-Lumb I, et al. Two doses of SARS-CoV-2 vaccines reduce risk of death due to COVID-19 in solid organ transplant recipients: preliminary outcomes from a UK registry linkage analysis. Transplantation. 2021;105:e263–e264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Government UK. Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2020. Available at https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020. Accessed December 15, 2022
- 19.Williams SV, Whitaker HJ, Mumford L, et al. Effectiveness of COVID-19 vaccines against hospitalization and death with the SARS-CoV-2 Delta variant in solid organ and islet transplant recipients. Transplantation. 2022;106:e310–e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, Iketani S, Guo Y, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. [DOI] [PubMed] [Google Scholar]
- 21.Grewal R, Kitchen SA, Nguyen L, et al. Effectiveness of a fourth dose of Covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022;378:e071502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022;386:1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazit S, Saciuk Y, Perez G, et al. Short term, relative effectiveness of four doses versus three doses of BNT162b2 vaccine in people aged 60 years and older in Israel: retrospective, test negative, case-control study. BMJ. 2022;377:e071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stowe J, Andrews N, Kirsebom F, et al. Effectiveness of COVID-19 vaccines against Omicron and Delta hospitalisation, a test negative case-control study. Nat Commun. 2022;13:5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napuri NI, Curcio D, Swerdlow DL, et al. Immune response to COVID-19 and mRNA vaccination in immunocompromised individuals: a narrative review. Infect Dis Ther. 2022;11:1391–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee A, Wong SY, Chai LYA, et al. Efficacy of Covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto-Alvarez M, Fernandez-Nino JA, Arregoces-Castillo L, et al. Real-world evidence of COVID-19 vaccines effectiveness in solid-organ transplant recipient population in Colombia: a study nested in the Esperanza Cohort. Transplantation. 2023;107:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng B, Green ACA, Tazare J, et al. Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe Covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ. 2022;379:e071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nimmo A, Gardiner D, Ushiro-Lumb I, et al. The global impact of COVID-19 on solid organ transplantation: two years into a pandemic. Transplantation. 2022;106:1312–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.