Abstract
Nanoparticles based on biodegradable polymers have been shown to be excellent herbicide carriers, improving weed control and protecting the active ingredient in the crop fields. Metribuzin is often found in natural waters, which raises environmental concerns. Nanoencapsulation of this herbicide could be an alternative to reduce its losses to the environment and improve gains in its efficiency. However, there is a paucity of information about the behavior of nanoformulations of herbicides in environmental matrices. In this study, the stability of nanoencapsulated metribuzin in polymeric nanoparticles (nanoMTZ) was verified over time, as well as its dissipation in different soils, followed by the effects on soil enzymatic activity. The physiological parameters and control effects of nanoMTZ on Ipomoea grandifolia plants were investigated. No differences were verified in the half-life of nanoencapsulated metribuzin compared to a commercial formulation of the herbicide. Moreover, no suppressive effects on soil enzymatic activities were observed. The retention of nanoMTZ in the tested soils was lower compared to its commercial analogue. However, the mobility of nanoencapsulated metribuzin was not greatly increased, reflecting a low risk of groundwater contamination. Weed control was effective even at the lowest dose of nanoMTZ (48 g a.i. ha–1), which was consistent with the higher efficiency of nanoMTZ compared to the conventional herbicide in inhibiting PSII activity and decreasing pigment levels. Overall, we verified that nanoMTZ presented a low environmental risk, with increased weed control.
Keywords: nanopesticide, sorption−desorption, leaching, biodegradation, chemical control, soil enzyme activity
1. Introduction
Herbicides are the pesticides most commonly detected in the environment due to the large volumes applied in cropping fields.1 Several studies have reported the presence of preemergence herbicides in groundwater and surface waters,2−6 such as metribuzin (4-amino-6-tert-butyl-4,5-dihydro-3-methylthio-1,2,4-triazin-5-one), which has been pointed out as a potentially leachable compound, even though its persistence in the environment is low.5,7 Metribuzin is one of the organic microcontaminants most commonly found at high concentrations in Brazil8 and was detected in 20 out of 113 freshwater samples (3–8 ng L–1).9 Moreover, metribuzin is found at concentrations above the drinking water limits in Europe (0.1 mg L–1),10,11 the United States,12 and Norway.13
Metribuzin is an acid herbicide (pKa = 1) of the triazinone group, with high water solubility (SW = 1200 mg L–1), moderate lipophilicity (log KOW = 1.7) and persistence (DT50 = 7–19 days), and high mobility in soils.7,14 It inhibits photosystem II (PSII) and is indicated for the control of broadleaf weeds and some grasses before and after emergence, in soybean, tomato, potato, and sugarcane cropping fields.7 Metribuzin has been applied in lower doses (from 240 g a.i. ha–1) to higher doses (up to 1920 g a.i. ha–1) in different crops, such as potato, soybean, and wheat.15−18 For soybean, metribuzin is applied at doses up to 480 g a.i. ha–1 in the United States and Brazil, to avoid the potential phytotoxicity induced by higher-dose applications.19,20
Moderate availability and the persistence of metribuzin in soils are related to low levels of organic matter (OM) and clay, as well as high pH.21,22 The effects on the growth and survival of earthworms (Octodrilus complanatus) are reported for agricultural soils,23 in addition to suppressive effects on soil microorganisms.24 In water, metribuzin has been described as highly toxic to macrophytes and algae in mesocosm experiments under laboratory conditions.25 For microalgae (Chlamydomonas reinhardtii), metribuzin caused an inhibitory effect on photosynthetic activity due to the generation of reactive oxygen species.26 Furthermore, metribuzin presents moderate to high toxicity to mammals, birds, and fish.7 In humans, it can induce negative effects in the reproductive system.7,27
Global concern about the risks of pesticides (such as metribuzin) to humans and other nontarget organisms has encouraged research into the development of polymeric nanocarriers for pesticides, which could improve environment protection.28−32 These nanocarrier systems are promising, as they demonstrate biodegradability, low toxicity, lower environmental impacts, and increased efficiency against target organisms compared to their conventional analogues.33−36 In addition, nanoencapsulation modifies the fate of pesticides once released into the environment (such as mobility, sorption, and degradation) so that controlled changes in formulation properties are the key to achieving better targeting and reducing losses of active ingredients into the environment.34
Recently, Diyanat and Saeidian37 verified the good efficiency of poly(epsilon-caprolactone) (PCL) nanoparticles loaded with metribuzin for the control of purslane (Portulaca oleracea) and their safety for preemergence applications in soybean crops. Nanoformulations demonstrated better performance on weed control compared to their commercial formulations, even at doses lower than those recommended for conventional formulations.38−40 Results indicating that PCL nanocarrier systems are potentially safer for nontarget organisms have also been reported.41 Nonetheless, gaps in the behavior and fate of these nanoformulations in the environment indicate the need for further investigation.
The potential applications of nanopesticides are widely reported in the literature.33−36 Knowledge on the fate and behavior of nanobased pesticides in different environmental matrices is essential for safer use of nanotechnology in agriculture. Regulatory issues are identified as a major concern for understanding the environmental life cycle of these new products.42 Therefore, the applicability of methods to assess the fate of pesticides in the environment needs to be tested and validated for nanopesticides, considering that nanoparticles may demonstrate different behavior and characteristics from conventional active ingredients.43 Moreover, when analyzing the behavior of nanopesticides in solutions and the soil matrix, it is important to consider information on the characteristics of the nanoparticles, such as those obtained with light scattering and particle tracking methods.43 In addition, it is essential to compare the characteristics of nano- and conventional formulations of pesticides, which could help to improve the design of the nanoparticles.44 Once released into the environment, effects of nanoparticles and active ingredients on soil quality must be evaluated in the context of risk assessment toward sustainability. Some soil enzymes have been shown to be sensitive to the presence of nanoparticles, acting as environmental indicators for soil quality.45−47
Our group is dedicated to understanding the possible mechanisms of action of nanoherbicides encapsulated into PCL nanoparticles, such as atrazine.48,49 The uptake and distribution mode of nanoparticles in plant tissues can be affected by morphological and physiological traits of the plant, in addition to the characteristics of the nanoparticles. More information on the mode of action of a nanoformulation is essential for complete understanding of the behavior of nanopesticides in the environment and of their effectiveness for weed control.50
Thus, this research aimed to (i) evaluate the behavior of metribuzin encapsulated into PCL nanoparticles (nanoMTZ) in three types of soil and (ii) compare the environmental fate and effectiveness on weed control of nanoMTZ and a commercial formulation of metribuzin (MTZ). For soil studies, radiometric techniques (14C-metribuzin) were used to mark and track the fate of nanoMTZ. Soil enzyme activities were evaluated after the application of nanoMTZ and a commercial formulation of metribuzin in soils. The efficiency of preemergence application on Ipomoea grandifolia plants was also assessed. This study aims to better understand how nanoparticles can be developed to decrease environmental impacts and increase the effectiveness of herbicide formulations, contributing to the development of more sustainable agriculture.
2. Results and Discussion
2.1. Characterization of Nanoformulations Containing Metribuzin
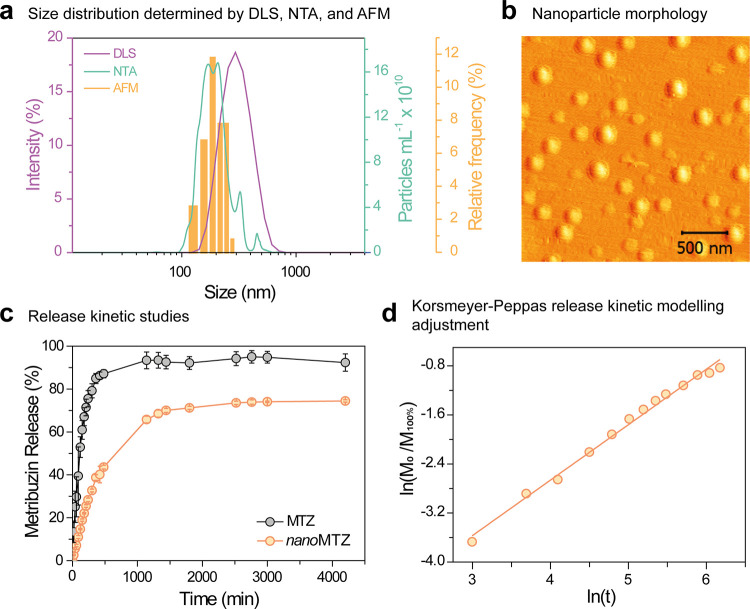
The suspensions of empty nanoparticles [without active ingredient (a.i.)] and those containing metribuzin (nanoMTZ) remained stable during the 120 days of monitoring (Figure S1a). The mean hydrodynamic size of both the nanoparticles, measured by the DLS technique, was 289 ± 3 nm. Identical values of the polydispersity index (PDI) were also found (0.09 ± 0.02), indicating monodispersed nanoparticles in the formulation (Figure S1b). Considering surface charges, the herbicide encapsulation did not seem to interfere with surface chemistry, as ζ-potential values of −31.6 ± 0.3 and −32.3 ± 0.4 mV were obtained for nanoMTZ and, empty nanoparticles, respectively (Figure S1c). With the variation in pH (2–11) in an aqueous medium, the nanoparticles showed changes in surface charges, expressed by the ζ-potential (Figure S1d), but remained stable in terms of size and PDI, indicating good chemical stability (Figure S1e). The size distributions determined by nanoparticle tracking analysis (NTA) and atomic force microscopy (AFM) for nanoMTZ were 223 ± 2 and 195 ± 35 nm (n = 39), respectively (Figure 1). These values are slightly lower than those obtained by dynamic light scattering analysis (DLS), however, they are inherent to the techniques used, with evaluations in a more dilute medium (NTA) and dry nanoparticles (AFM). Regarding morphology, the micrograph obtained by AFM shows the spherical shape of the PCL nanoparticles loaded with metribuzin. These results are consistent with the literature, for the encapsulation of atrazine in PCL nanoparticles.28,37,49 The nanoMTZ formulation showed high encapsulation efficiency of the technical and radiolabeled herbicide (74.8 ± 0.5 and 82.5 ± 0.1%, respectively) (Table S1), confirming the stability of this nanoformulation. Diyanat and Saeidian,37 following the stability of PCL nanoparticles as carriers of metribuzin, also verified characteristics similar to those shown in the current study. Compared with nanoformulations of atrazine, a more hydrophobic herbicide than metribuzin, the systems were similar with respect to characteristics as a function of time, but the encapsulation efficiencies for atrazine and 14C-atrazine were approximately 15 and 10% higher than nanoformulations with metribuzin and 14C-metribuzin, respectively.28,49
Figure 1.
Size distribution and morphology of nanoMTZ nanoparticles and in vitro release profile of metribuzin. (a) Size distribution by DLS, NTA, and AFM techniques; (b) AFM micrograph of nanoMTZ nanoparticles; (c) in vitro release profile of encapsulated and unencapsulated metribuzin in PCL nanocapsules; and (d) adjustment of nanoMTZ release kinetics to the Korsmeyer–Peppas mathematical model.
In vitro release kinetics for unencapsulated metribuzin showed that herbicide migration to the acceptor medium is rapid, with a percentage release >94% in 24 h (Figure 1c). Nanoencapsulation altered the release profile of metribuzin, reducing its release rate to the acceptor medium, and causing the release percentage to reach values of around 70% within 24 h. A more expressive variation occurred between the systems in the initial 8 h when the percentage of release for nanoencapsulated metribuzin was 40–45% lower than that of unencapsulated metribuzin. Release profile data for nanoMTZ were fitted to the Korsmeyer–Peppas mathematical model (Figure 1d). The correlation coefficient (R2 = 0.9916) indicated that the model was suitable for analyzing the cumulative release rate of metribuzin below 60%, with the constant K value being 1.87 × 10–3 min–1. The value determined for the release exponent (n) was 0.9045, indicating that the release of metribuzin from the nanoMTZ, through the cellulose membrane involved an anomalous Type II transport mechanism (0.85 < n). Volova et al.18 also reported a similar release mechanism for metribuzin encapsulated in poly(3-hydroxybutyrate) microparticles. In this mechanism, the release rate is controlled by the swelling and relaxation of the polymer matrix, which occurs soon after the imbibition of water in the system.28,51,52
2.2. Biodegradation Assay
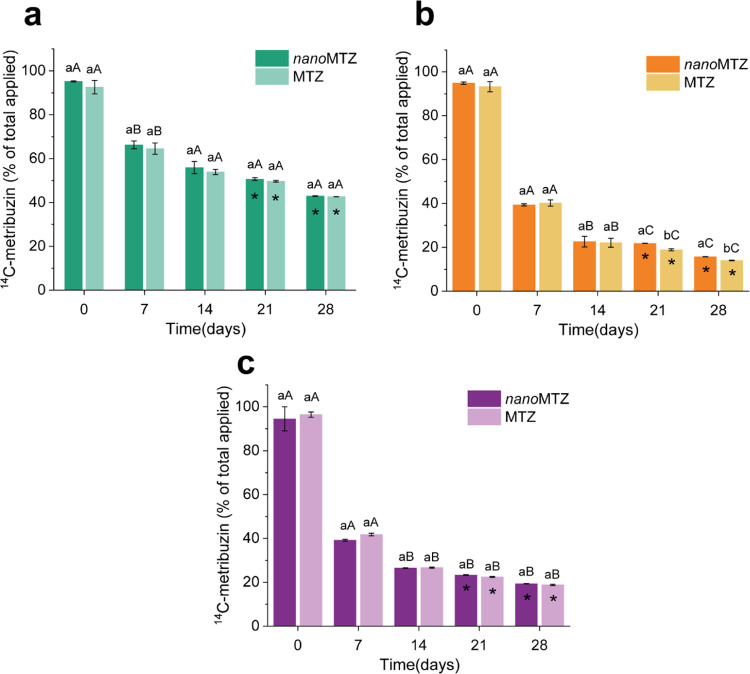
The bioavailability of 14C-metribuzin (as nanoMTZ and commercial MTZ) in soils decreased over the 28 days of incubation (Figure 2). After 21 days of incubation, there was a significant interaction between soil and formulation types, which affected the availability of the herbicide in the soil. Up to 14 days of incubation, the soil factor was responsible for the greatest differences in herbicide availability. For clay soil, 42.92 ± 0.15% (nanoMTZ) and 42.67 ± 0.05% (MTZ) of the herbicide was bioavailable at the end of the incubation period (Figure 2a). Smaller amounts of 14C-metribuzin were found in SL-1 (15.70 ± 0.09% nanoMTZ and 13.99 ± 0.13% MTZ) (Figure 2b) and SL-2 (19. 32 ± 0.11% nanoMTZ and 18.82 ± 0.28% MTZ) (Figure 2c), at the end of the same period. After 21 days of incubation in SL-1, nanoMTZ was more available compared to the commercial metribuzin (p < 0.05). At 28 days of incubation, the same pattern was observed for SL-2. Acidic herbicides, such as metribuzin (pKa = 1), show greater availability (lower retention) in soils with pH > pKa (Clay soil, Table S2), where they remain in the anionic form.7,53,54NanoMTZ showed similar bioavailability compared to the commercial formulation. These results were evidenced mainly for the clay soil, with higher sorption sites (clay content, OM, and cation exchange capacity—CEC) (Table S2). The negative charges of PCL nanoparticles could promote lower retention due to electrostatic repulsion caused by soil charges.55 In the present study, a ζ-potential of ∼−30 mV was found for nanoMTZ. Furthermore, this retention may be influenced by the greater affinity of the herbicide molecule with the PCL nanoparticles than with the soil components.56
Figure 2.
Availability of nanoMTZ and MTZ formulations in soils (clay (a) SL-1 (b), and SL-2 (c)) in soils over 28 days. Bars indicate the mean ± standard error of the mean of extracted residue (bioavailable metribuzin in soil). Different lowercase letters indicate significant differences between formulations within the same soil, while different uppercase letters indicate significant differences between soils for the same formulation, by Tukeyʼs test (p < 0.05), for each incubation time. Asterisks in the columns indicate interaction of soil and formulation factors for determining soil herbicide availability, for each incubation time (p < 0.05).
From the portions of herbicide extracted from the soil over the 28 days of incubation, the data were fitted to first-order kinetic models and the active half-lives were then calculated for each soil and formulation type (Table 1). The DT50 values were similar among the soils and the formulations (11.36 to 17.55 days), indicating that nanoencapsulation did not interfere with the metribuzin persistence in the environment. Kah et al.57 found that PCL nanoparticles also did not affect the degradation kinetics of the herbicide atrazine. The same authors indicated that the herbicide release could have occurred faster than the degradation kinetics by the carrier and/or that the herbicide associated with the nanoparticle could undergo biotic or abiotic degradation in the soil.
Table 1. Biodegradation Parameters of the Nanoformulation and Commercial Formulation of 14C-Metribuzin, 28 after Incubation, in Different Soils.
| formulation |
|||
|---|---|---|---|
| parameters | soil | nanoMTZ | MTZ |
| C0 ± sea (%) | clay | 89.14 ± 8.94 | 91.14 ± 6.56 |
| SL-1 | 84.10 ± 14.76 | 90.63 ± 12.89 | |
| SL-2 | 84.20 ± 14.93 | 84.29 ± 15.75 | |
| k ± se (day–1) | clay | 0.0417 ± 0.0095 | 0.0406 ± 0.0067 |
| SL-1 | 0.0395 ± 0.0161 | 0.0610 ± 0.0173 | |
| SL-2 | 0.0483 ± 0.0182 | 0.0485 ± 0.0193 | |
| R2 | clay | 0.88 | 0.93 |
| SL-1 | 0.67 | 0.83 | |
| SL-2 | 0.71 | 0.68 | |
| p-value | clay | <0.1 | <0.1 |
| SL-1 | <0.1 | <0.1 | |
| SL-2 | <0.1 | <0.1 | |
| DT50 (days) | clay | 16.62 | 17.07 |
| SL-1 | 14.35 | 14.29 | |
| SL-2 | 17.55 | 11.36 | |
Standard error of mean (n = 2).
Persistence and degradation are dependent on factors such as temperature, humidity, and soil type. The persistence data found in this study are close to the values found in the literature for metribuzin (19 to 138 days), although in most studies degradation occurred more quickly in the soils (especially in the first month following the pesticide application).15,58−60 Nanoformulations are reported to increase the half-life of pesticides in the environment, indicating greater availability over time, as well as to protect pesticides from degradation.34,61 However, in the present paper, no effects of nanoparticles on the persistence of metribuzin in soils were verified, even with a slower and modified release of the herbicide by the PCL nanoparticles. Kah et al.62 also found similarities between the half-life of a nanoencapsulated insecticide and the commercial formulation in contrasting soils. The degradation of active ingredients seems to be little affected by nanoencapsulation, although photodegradation might be reduced, prolonging the protection of active ingredients by nanoformulations.34
The formation of bounded residue (portion of herbicide or its metabolites strongly retained in the soil) increased during the degradation of metribuzin in all soils, for both formulations (54–70% in relation to the total applied) (Table S3), possibly due to higher energy chemical interactions, such as the formation of covalent bonds, and physical trapping of more polar molecules, such as metribuzin.63 In addition, some of these strongly retained compounds may be products of herbicide transformation in the environment, considering the low herbicide retention in the soil.22,64,65 However, any formed residues did not affect the degradation and mineralization rates of metribuzin, regardless of the soils and formulations tested.
More than 40% of the 14C fractions of the a.i. extracted from the soil after up to 7 days of incubation were derived from parental 14C-metribuzin. The other fractions were divided into up to five metabolites identified by thin-layer chromatography (TLC), with increasing percentages of metabolites with time (Figure S2). The nature of the metabolites was not identified in this study, due to the absence of proper 14C-standards. The European Food Safety Authority (EFSA) risk analysis report66 for metribuzin indicated diketometribuzin and desamino-diketometribuzin as relevant products of metribuzin transformation. These products are also considered as moderately and little persistent in the environment and of low risk to soil organisms. Mutua et al.60 verified the presence of these same metabolites in soils exposed to metribuzin over 63 days of incubation, with an accumulation close to 30% for each metabolite, mainly in soils treated with organic waste. There was no interference of encapsulation of metribuzin in the transformation of 14C-metribuzin in the studied soils, so that the formation of degradation products from the a.i. was similar for all soils, indicating the release and availability of the active ingredient for further degradation.
The formation and accumulation of 14CO2 (mineralized herbicide) during the herbicide incubation period for nanoMTZ was 6.98 ± 0.48% in clay soil, differing from the commercial formulation that showed 11.83 ± 0.72% of 14CO2 (p < 0.05) (Table S3). For SL-1, metribuzin mineralization was more pronounced compared to other soils (p < 0.05), with ∼19% mineralized herbicide at the end of 28 days of incubation (Table S3). In this same period, the SL-2 soil presented 14CO2 accumulation of 14.86 ± 0.06% and 13.54 ± 0.20% for nanoMTZ and MTZ, respectively (Table S3). It is important to emphasize that in soils with lower clay contents, the nanoMTZ presented similar mineralization to the soils treated with MTZ, indicating that the soil microbiota participated in the degradation of the active ingredient in a similar way, regardless of the nanoform. The mineralization rates in all studied soils were <20% of total metribuzin applied, over 28 days of incubation, depending on the soil type as well as the applied formulation (Figure 2). Guimarães et al.15 also found slow mineralization of metribuzin over 70 days of incubation (3.20% of the total 14C-metribuzin applied) in different soils, where a large part of the herbicide transformation was due to the formation of metabolites in the soil.
2.3. Soil Enzyme Activity Assays
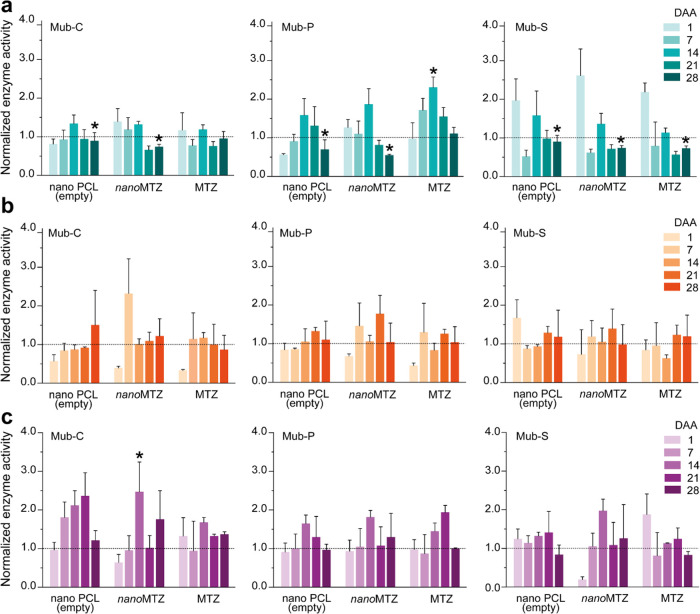
Soil enzyme activities showed some significant differences between control (samples without addition of nanoparticles and herbicides) and treated samples (p < 0.01) (Figure 3). For clay soil (Figure 3a), activities of Mub-C, Mub-P, and Mub-S were significantly different for nanoMTZ and PCL at 28 days after application (DAA). Soils exposed to MTZ showed significantly different activities of Mub-P and Mub-S significantly different to those of the control samples at 14 and 28 DAA, respectively. For SL-1 soils (Figure 3b), no significant differences between the control soils and those exposed to the different treatments were verified at p < 0.01. For SL-2 soils (Figure 3c), significant differences between control samples and those exposed to nanoMTZ were verified at 14 DAA for Mub-C.
Figure 3.
Enzymatic activity of the clay (a), SL-1 (b), and SL-2 (c) soils, normalized in relation to the control (dashed line, i.e., samples without the addition of nanoparticles and pesticides). Error bars indicate the standard deviation (biological replicates n = 3, technical replicates n = 6). For enzymatic activities, significant differences from the control (Tukeyʼs test, p < 0.01) are indicated by an asterisk (*). DAA = days after application of the treatments.
Metribuzin is rapidly biodegraded in soils, however, it can precipitate negative effects on soil microorganisms, including changes in microbial composition.67 In a previous study, MTZ did not affect the number of proteolytic microorganisms in loamy soil but significantly reduced the population after 7 days of exposure to a high concentration of the a.i. (1200 mg kg–1).68 In the present article, when using the MTZ concentration recommended by the manufacturer, no systemic or extensive effects of the a.i. (pure or encapsulated) were verified for the soil enzymatic activities. The absence of effects of PCL nanoparticles on bioavailability, mineralization, mobility, and persistence of the a.i. in the three investigated soil types (Figure 2, Tables 1 and 2) may account for the reduced evidence of a clear pattern between the effects of MTZ and nanoMTZ on the tested soil enzymatic activities. Kah et al.62 also found no major differences between dehydrogenase activities in soils exposed to pure bifenthrin or bifenthrin encapsulated into polymeric nanoparticles.
Table 2. Sorption and Desorption Parameters of Nanoformulation and Commercial Formulation of 14C-Metribuzin in Different Soilsa.
|
nanoMTZ |
MTZ |
|||||
|---|---|---|---|---|---|---|
| parameters | clay | SL-1 | SL-2 | clay | SL-1 | SL-2 |
| Sorption | ||||||
| Kd (mL g–1) | 0.90 ± 0.02 bB | 1.04 ± 0.01 bA | 0.52 ± 0.02 bC | 1.24 ± 0.03 aB | 1.37 ± 0.04 aA | 0.65 ± 0.04 aC |
| KOC (mL g–1) | 32.81 ± 0.59 | 199.29 ± 1.81 | 52.71 ± 1.70 | 45.29 ± 0.97 | 264.21 ± 7.32 | 65.71 ± 4.29 |
| Kf (mL g–1) | 0.67 ± 0.01 | 0.83 ± 0.03 | 0.44 ± 0.03 | 1.25 ± 0.02 | 1.30 ± 0.01 | 0.62 ± 0.01 |
| 1/nsorption | 0.88 ± 0.01 | 0.90 ± 0.02 | 0.92 ± 0.04 | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.96 ± 0.01 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| % sorptionb | 47.24 ± 0.45 | 50.88 ± 0.22 | 34.26 ± 0.73 | 55.43 ± 0.53 | 57.84 ± 0.53 | 39.37 ± 1.56 |
| Desorption | ||||||
| Kd (mL g–1) | 2.28 ± 0.06 bB | 2.52 ± 0.00 bA | 1.50 ± 0.08 bC | 1.24 ± 0.02 aB | 3.23 ± 0.05 aA | 1.96 ± 0.07 aC |
| KOC (mL g–1) | 83.44 ± 2.09 | 484.60 ± 0.82 | 157.01 ± 7.65 | 107.72 ± 0.71 | 620.41 ± 10.12 | 198.23 ± 7.11 |
| Kf (mL g–1) | 1.62 ± 0.03 | 1.96 ± 0.22 | 1.13 ± 0.07 | 2.74 ± 0.23 | 2.49 ± 0.23 | 1.72 ± 0.14 |
| 1/ndesorption | 0.90 ± 0.01 | 0.93 ± 0.03 | 0.93 ± 0.03 | 0.98 ± 0.02 | 0.92 ± 0.03 | 0.95 ± 0.02 |
| H | 0.98 | 0.97 | 0.99 | 1.00 | 1.05 | 1.01 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| % desorptionc | 38.74 ± 0.57 | 36.44 ± 0.04 | 47.28 ± 1.10 | 32.96 ± 0.11 | 30.96 ± 0.11 | 41.54 ± 0.98 |
Values correspond to the mean ± standard error of the mean (n = 2). Lowercase letters indicate the difference in the formulations within each soil, and uppercase letters indicate the difference in each soil within each formulation, by Tukeyʼs test (p < 0.05). Kd = sorption–desorption coefficients, KOC = organic carbon normalized sorption–desorption coefficients, Kf = soil–water distribution coefficients (Freundlich coefficients), and 1/n = slope of the Freundlich curve.
Percentage of total assets applied to the soil.
Percentage in relation to the total asset remaining in the soil after sorption.
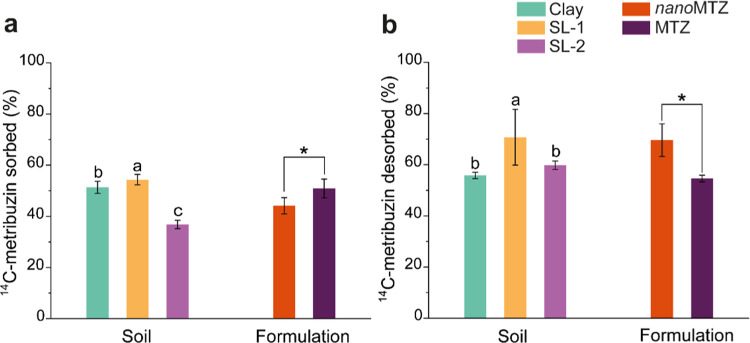
Although all metribuzin concentrations in soils reached <10% of the total mass initially added after 28 days (Table S4) and the metribuzin degradation by the soil microorganisms did not appear to be greatly affected by the formulation type. Soils exposed to nanoMTZ showed a higher number of significant differences in soil enzymes (p < 0.01) compared to those exposed to MTZ. The slightly lower retention of the a.i. when encapsulated into the polymeric nanoparticles compared to the commercial formulation of MTZ (Figure 4a) might be responsible for these apparently slightly more pronounced effects of the nanoherbicide on soil enzymes since the a.i. may be more bioavailable to the microbiota.
Figure 4.
Percentages of 14C-metribuzin sorbed (a) and desorbed (b) from the nanoMTZ and MTZ formulations applied to soils (clay, SL-1, and SL-2). Bars represent the mean ± standard error of the mean (n = 2) of each factor (soil and formulation) separately. Different lowercase letters indicate significant differences among the soils, while asterisks represent significant differences among the formulation types by Tukeyʼs test (p < 0.05). The interaction of factors was not significant according to the variance test (F > 0.05).
It is also noteworthy that the effects of the treatments on the soil enzymatic activities generally occurred after 14 DAA, being stimulant at 14 DAA (for Mub-P of clay soil exposed to MTZ, and Mub-C for SL-1 exposed to nanoMTZ), after which the enzyme activities are suppressed at lower levels compared to those of the control soils, especially in the case of exposure to nanoMTZ at 28 DAA, indicating possible risks to the soil bacteria. In the current paper, the initial increase in enzymatic activity may be related to the availability of organic substrates for soil microorganisms,69 such as products from the degradation of PCL nanoparticles and MTZ, as well as to the slow release of the a.i. from the nanoparticles (Figure 1), acting thus as a source of organic substrates for the soil microbiota over time. In addition to the supplemental energy source and the addition of a pollutant that might cause harm to the microorganisms, stimulatory or suppressive effects on soil enzymes can also be considered as changes in the soil microbial community, or an indication of microbial adaptation to the products.70,71
For better comprehension of nanoparticle effects in the soil microbiota, validation of environmental indicators for nanopesticides is necessary, which can aid safe use of these new nanoproducts.47,72 In this article, Mub-C, Mub-P, and Mub-S indicated significant effects at 28 days following the application of the nanopesticide in the clay soil, acting thus as potential environmental indicators for the presence of a nanopesticide in agricultural soils. Further research is needed to improve the comprehensive risk assessment of these novel products. Experiments could involve field tests to reproduce the effects of nanoformulations on soil enzymes under more realistic conditions. Multiple applications of the nanoherbicide over consecutive production cycles and genomic analysis of the microbial community composition could also be evaluated, to ensure environmental safety and protection of human health.47
2.4. Retention and Mobility Tests of the Nanoformulations in the Soils
The sorption kinetics of nanoMTZ and MTZ formulations in soils showed similar behavior (herbicide sorption > 70%), over 48 h of incubation (Figure S3). The formulations were sorbed immediately after reaching the soils, attaining sorption stability 24 h after their application (>88% of the total sorbed during the kinetic test).
The sorption isotherms for nanoMTZ and MTZ, represented by Kf and 1/n values, presented linear behavior for the different soils tested, according to the classification by Giles et al.73 (1/n or slope of curves close to 1) (Table 2 and Figure S4). The Kd values indicated lower sorption for nanoMTZ (0.52–0.90 mL g–1) compared to MTZ (0.65–1.37 mL g–1) for all soils (p < 0.05). The same behavior was found for the soil with lower clay content for nanoMTZ and MTZ (SL-2; 0.65 ± 0.04 mL g–1). In soils with higher clay content (Clay and SL-1), sorption was significantly higher (p < 0.05). However, in all of these scenarios, Kd and Kf values indicated low retention of 14C-metribuzin, whether or not nanoencapsulated (Table 2). The Freundlich coefficient (Kf) and isotherms are very important to describe the behavior of pesticide molecules in the soil since the concentrations of pesticides can be different in the environment. Kd is also important because it represents the behavior of this pesticide in a specific scenario (such as soil type, crop system, and environment), considering a recommended dose. In both coefficients, the increase in values represents the increase in herbicide sorption in the soil. Rigi et al.22 reported that metribuzin sorption (Kf) in different soils increased with the herbicide concentration, indicating nonsaturation of sorption sites. The Kf values found in the literature for metribuzin are consistent with those found in the present study, indicating low retention of the herbicide in the soils.22,74−76 Even though metribuzin retention was low, it may be associated with clay fractions and soil OM (considering that iron and aluminum oxide fractions can be more present in tropical soils).22,77−80 The low sorption of the acid herbicide metribuzin can also be explained by the fact that it occurs in its anionic form when pH > pKa,53,54,81 as in the studied soils. For the nanoMTZ, which showed the lowest sorption by soils, a repulsion between the nanoparticles with a negative surface and the negatively charged soil particles could have occurred. These results were also observed by Rashidipour et al.82 for Pectin/Chitosan/Tripolyphosphate nanoparticles as carriers of the herbicide paraquat, which had a negative charge (−24.3 mV) conferred by anionic pectin and tripolyphosphate.
Higher KOC values were found for SL-1 (199.29 ± 1.81 for nanoMTZ and 264.21 ± 7.32 for MTZ) (Table 2). Therefore, even though SL-1 had the lowest organic carbon (OC) content among the tested soils, OC had greater effects on metribuzin retention compared to the other mineral soil components. Furthermore, the KOC data also suggest that the mobility of metribuzin when associated or not with nanoparticles was similar. Mobility classifications of the pesticide in soil adopted by regulatory agencies around the world indicated that metribuzin is a very mobile herbicide.66,83−85 Considering the FAO (2000) classification, nanoMTZ and MTZ are categorized as moderately mobile to mobile in soils (log KOC 1.51–2.29 and 1.66–2.42, respectively, calculated based on data presented in Table 2). The risk analysis presented by EFSA66 in the European Union indicated MTZ as a highly mobile pesticide in soils, based on the KOC values (Table 2), which may also be applicable to nanoMTZ. In Brazil, according to the classification given by the Brazilian Institute for the Environment and Renewable Natural Resources (IBAMA),85 metribuzin is considered to be of low adsorption, based on Kf data (0–5 mL g–1), for both the commercial MTZ and the nanoMTZ (Table 2).
Desorption Kd values were between 0.52–1.04 mL g–1 for soils treated with nanoMTZ and 0.65–1.37 mL g–1 for soils treated with MTZ (Table 2), differing significantly from each other (p < 0.05). Desorption was more pronounced in SL-1 for both formulations (desorption KOC of 484.60 ± 0.82 for nanoMTZ and 264.620.41 ± 10.12 for MTZ), possibly due to fewer sites for sorption in this soil, allowing weaker and more reversible connections between both formulations and the soil particles (hysteresis values, H, close to 1). The SL-1 soil presented the lowest CEC among the tested soils, and its sites were also largely saturated (Table S2). The desorption behavior was also linear (1/n close to 1) as in sorption, showing that the resuspension of the a.i. in the soils, either as nanoMTZ or commercial MTZ, occurs as a function of their concentrations in the medium (Figure S4). Herbicides with low sorption have greater desorption capacity.86 Considering that acidic herbicides have weak interactions with the soil,81 resuspension of metribuzin in the soil solution is expected, as observed in this study.
The percentage of a.i. sorbed into the soil did not show interaction effects between the soil and formulations, and only the isolated effect of these variables was verified (Figure 4). More details of sorption–desorption percentages are available in the Supporting Material (Table S4). SL-1 showed the highest retention capacity of metribuzin (54.36 ± 2.03%), followed by clay soil (51.33 ± 2.38%) (Figure 4a). Only 36.81 ± 1.63% of the herbicide was retained in the sandy soil (SL-2). Part of the product retained in the soil returned to the solution, suggesting bioavailability to the soil microbiota and to plants, and favoring dissipation in the environment. More than 70% of the total a.i. initially retained in the soil returned to the soil solution of the SL-1, while for the other soils, only between 55 and 59% of the metribuzin was available in the soil solutions (Figure 4b). In total, 6.7% less retention of the nanoformulation was verified in soils compared to the commercial formulation of metribuzin (Figure 4a). Due to its lower affinity with the soils, 69.63 ± 6.39% of the total nanoMTZ initially retained returned to the soil solution, compared to 54.61 ± 1.29% for MTZ (Figure 4b). In addition to surface charges, the increased solubility of metribuzin given by encapsulation into polymeric nanoparticles can also contribute to the greater availability of nanoMTZ in the soil solution, which might reflect the greater number of statistically significant differences found for soils exposed to nanoMTZ in relation to the control samples compared to the soils exposed to PCL and MTZ (p < 0.01, Figure 3; p < 0.05, Table S4). The increased solubility of metribuzin when encapsulated into PCL particles might occur because the surface of these polymeric nanocapsules is stabilized in water by the surfactant polysorbate 80, despite the oil core and hydrophobic characteristics of the polymer.49 The surfactant plays an important role in the hydrophilic-lipophilic balance of nanoformulations, giving the nanoparticles an amphiphilic character.87,88 Thus, the interaction of polysorbate 80 with nanoparticles keeps its polar head facing the aqueous medium, which improves interactions with the aqueous medium and, consequently, its solubility.
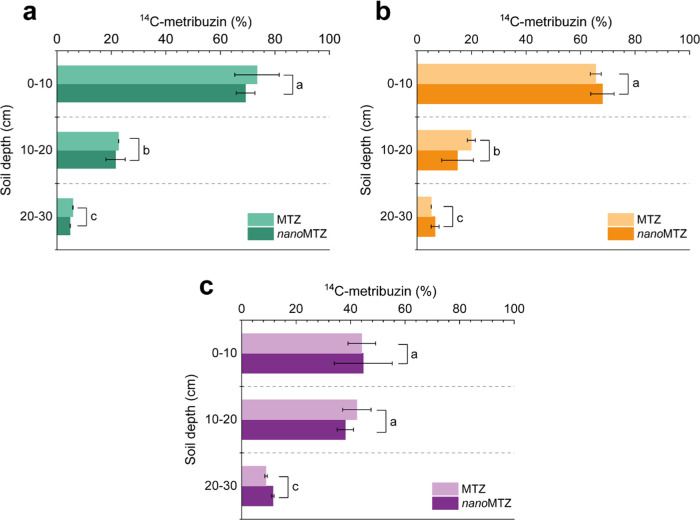
There was no interaction effect between the variables depth and type of formulation on the mobility of the a.i. in each soil, when testing each soil separately (Figure 5). The formulations were similarly retained in the first 10 cm of the soil profile (60.84 ± 1.66%). Values >87% of the total herbicide were concentrated in the first 20 cm, which suggests a greater presence in the seed bank, conferring greater weed control. Only 7.17 ± 3.74% of the herbicide was distributed in the deeper portions of the soil. However, the 14C-metribuzin collected in the leachate did not exceed 0.60 ± 0.01% for the nanoMTZ, and 0.63 ± 0.02% for the MTZ, after 48 h of simulated rain. These results indicate low mobility of the formulations in the soil profile (beyond 30 cm in depth), even in extreme conditions of rainfall and low soil retention. For both formulations, Diyanat and Saeidian37 found herbicide retention at a depth of 5–10 cm of the soil profile, with a reduction in the presence of nanoMTZ at a depth of 10–15 cm (0.06%) compared to commercial metribuzin (8.03%). Fernández-Pérez et al.89 found a reduction in metribuzin contents in the leachate solution using a lignin-based formulation of metribuzin. Maqueda et al.90 also pointed out a reduction in the concentration of metribuzin in the soil profile using a clay-gel matrix formulation.
Figure 5.
Mobility of nanoMTZ (a) and MTZ (b) formulations in the soil profile (clay, SL-1, and SL-2). Bars represent the mean ± standard error of the mean (n = 2). Different lowercase letters indicate significant differences between the depths for each soil separately, according to Tukeyʼs test (p < 0.05). Symbols can cover up error bars.
For polar compounds, such as metribuzin, sorption mechanisms can occur through ion exchange, charge transfer, ligand exchange, and cationic or salt bridges in aqueous medium.78 The PCL-based metribuzin nanopesticide also has polar characteristics, mainly due to the surfactants responsible for the stability of the nanoparticles in water. For PCL-based atrazine nanopesticide, Pereira et al.29 reported that the nanocapsules increased the mobility of the a.i. in soil due to its reduced adsorption in the soil compared to the commercial formulation. However, the same authors found that this increase in the availability of the a.i. in the soil was beneficial, as there was an increase in the effectiveness of control of the target organism due to the nanoencapsulation of the pesticide. Similar results were found for metribuzin in this article, indicating that the polarity characteristics of PCL nanoparticles promote higher herbicide solubility and availability in soil without increasing the risks of groundwater contamination.
2.5. Weed Control and Inhibition of Physiological Parameters
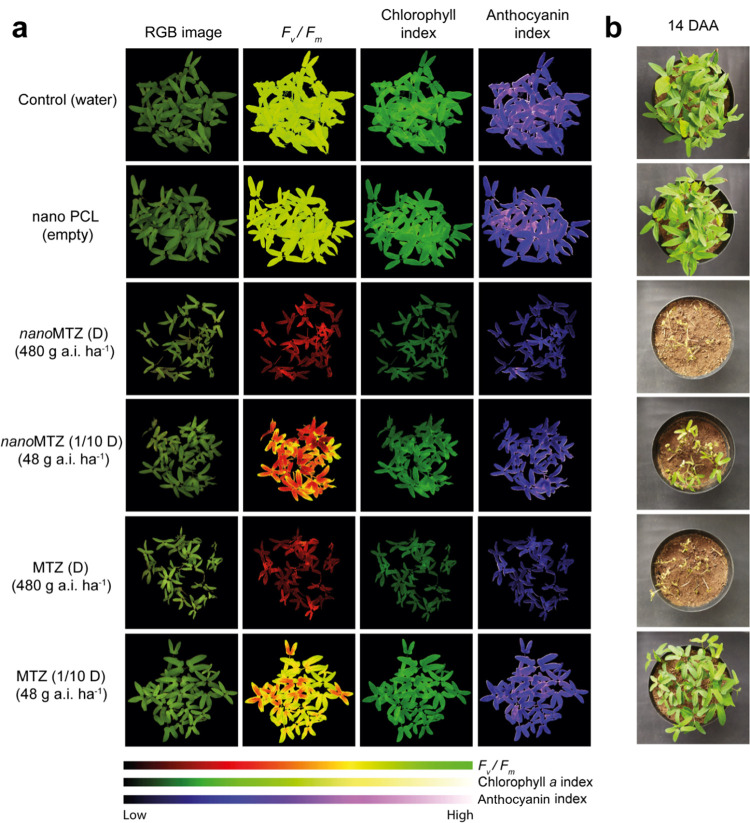
At 14 DAA, effective control of I. grandifolia could be observed in nanoMTZ and MTZ for the highest dose (480 g a.i. ha–1), and nanoMTZ for the lowest dose (48 g a.i. ha–1) (Figure 6b). The effects of the nanoMTZ formulation (480 g a.i. ha–1) in reducing the number of live plants were detected from 7 DAA onward, compared to the other treatments (Figure S5a). The initial similarity in the number of live plants among the treatments, at 3 DAA (Figure S5a), was consistent with plant germination, which occurred between 3 and 7 DAA. Furthermore, the effects of metribuzin, a PSII inhibitor, were already expected from 7 DAA onward (Figure 6a). At 14 DAA, the nanoMTZ and a commercial MTZ induced a reduction of 94% in the number of plants compared to the control when applied at the highest dosage, followed by application of the nanoMTZ diluted 10 times (52%). PCL nanoparticles and 10-fold diluted commercial MTZ showed similar responses to the control samples for all evaluated days, indicating no effects of these treatments on the number of plants. Plants treated with nanoMTZ formulations (regardless of the dose) and commercial MTZ (480 g a.i. ha–1) induced similar reductions in shoot and root dry masses compared to the control samples (around 67 and 65% for shoot and root dry masses, respectively) (Figure S5b). In contrast, the decreases in root and shoot dry masses induced by MTZ at 48 g a.i. ha–1 were much less intense (23.9 and 22.7% relative to the control). These results indicate that the encapsulation potentiated the activity of the herbicide metribuzin in relation to commercial MTZ, allowing maintenance of its activity even when applied at a reduced dosage (48 g a.i. ha–1). These results were corroborated by the analysis of the physiological parameters of the plants (Figure S5b). The nanoMTZ and MTZ (at 480 g a.i. ha–1) led to the same steep inhibitions of PSII activity and chlorophyll a and anthocyanin indices (by 77.9, 46.3, and 47.3% in relation to the control). However, when the 10-fold lower dosage was used, a difference between nanoMTZ and MTZ was observed. While nanoMTZ at 48 g a.i. ha–1 inhibited the PSII activity by 36.7%, the inhibition induced by MTZ at this dosage was 10.9%. Regarding the chlorophyll a and anthocyanin indices, both were negatively affected by nanoMTZ at 48 g a.i. ha–1, but not by MTZ at this dosage. The PCL nanoparticles without the a.i. did not induce a reduction in shoot and root mass of plants at 14 DAA compared to the control samples or affect the physiological parameters of the plants (Figure S5b).
Figure 6.
Physiological parameters (a) at 7 days after treatment application (DAA), and control at 14 DAA (b), of I. grandifolia plants with the application of nanoMTZ and MTZ. The multispectral images of the I. grandifolia plants at 7 DAA are displayed on the left (b), representing the values plotted in the graphs presented in the Supporting Material (Figure S5).
The effectiveness of PCL nanoparticles as a carrier system for atrazine, aiming at weed control has been evidenced by previous studies from our group.29,38−40,91 Even when applying 10-fold lower doses of the nanoformulation, the pre- and postemergence herbicidal activity of atrazine against susceptible weeds was not compromised.38,39,91 In the present research, the MTZ incorporation into PCL nanoparticles was also shown to potentiate the preemergent herbicidal activity against I. grandifolia plants. The nanoMTZ induced an earlier decrease in the number of live plants compared to commercial MTZ. Moreover, when both formulations were applied at a 10-fold lower concentration, nanoMTZ showed a higher decrease in dry mass accumulation and inhibition of physiological parameters than commercial MTZ. The QB-binding site on the D1 protein of PSII is the primary target of MTZ in plants, which acts by interrupting the electron transport from QA to QB.92,93 This action, in addition to decreasing CO2 assimilation, leads to the generation of reactive oxygen species, which cause oxidative damage to different cellular components, including chlorophylls and anthocyanins. Both the compromised carbohydrate synthesis and the induction of oxidative stress are related to the decrease in biomass accumulation and to the plant mortality.94
Previous studies have reported a higher uptake rate of organic compounds by plant roots due to the negative charge of nanocarriers.95,96 Positively charged nanoparticles tend to adhere more to the soil particles and the root tissue surface (both negatively charged), while negative particles, such as nanoMTZ (∼−30 mV), may have lower soil retention (Table 2) and better penetration through symplastic and apoplastic pathways into the roots, being transported by vascular tissues to the shoot.50,97−100 Therefore, the greater the herbicide uptake, the more a.i. can reach the site of action and promote weed control.49 Diyanat and Saeidian,37 using a PCL nanoparticle as a carrier of metribuzin (size of 263 nm, PDI of 0.32, and ζ-potential of −27 mV), obtained reduced germination of P. oleracea plants with the preemergence application of nanoMTZ (600 g a.i. ha–1) in relation to the plants exposed to a commercial formulation and to untreated samples. However, when the control efficiency was measured (shoot and root dry masses), differences were not observed between treatments. The authors indicated that the dose used may have been sufficient to quickly and completely prevent plant growth, without affecting the mass after 14 DAA. In the present study, using other doses (480 and 48 g a.i. ha–1), lower than those used by Diyanat and Saeidian,37 differences were observed in control and physiological parameters. It is noteworthy that even at a higher recommended dose (600 g a.i. ha–1), the authors found that the genotoxicity in onion cells was reduced with the nanoencapsulation of metribuzin. Furthermore, they did not observe side effects in the crop (soybean).
Weed control using lower doses of herbicides than the conventional formulations is one of the main objectives of developing nanobased formulations. For Wu et al.,101 studies such as the one presented in this article are needed to better understand the interaction between a nanoherbicide, a target species, and the environment, so that the development of effective and environmentally safe nanopesticides is possible. In our study, nanoMTZ showed similar environmental behavior to MTZ and low risks to soil enzymes. The dose of nanoMTZ diluted to 1/10 was effective in controlling I. grandifolia and points to the possibility of reducing the dose of nanoMTZ. Nanoformulation improves the targeting of the a.i. to the site of action, maintaining the productʼs efficacy even with a reduction in the applied dose, reducing therefore the amount of a.i. needed for crop protection.91 The use of lower doses of herbicides is desirable as it reduces the long-term effects of residues of these herbicides in agricultural areas and the toxicity to the environment, maintaining the sustainability of agricultural production.102,103 In this sense, nanoMTZ demonstrated the potential to be used in the production system at reduced doses, characterizing an efficient and sustainable tool for weed control. Studies with further scenarios, using other crop species and weeds, are necessary before introducing these nanoformulations into crop systems.
3. Conclusions
Nanoencapsulation had no effects on the environmental fate of metribuzin, which was similarly available for degradation, retention, and transport processes between the nanoformulation and the commercial formulation in the different soils tested. The bioavailability of the a.i. for degradation in the soil was not altered, indicating that soil microorganisms possibly use the polymer nanoparticles and the herbicide as a source of energy, enabling processes of biodegradation that were corroborated by the stimulating effects on the soil enzymatic activities. Significant effects of PCL, nanoMTZ, and MTZ for soil enzymes were reported 14 days after their application in the soils, especially for the clay soil, indicating microbial adaptation to the products. The retention of all formulations occurred linearly, so that the sites in the soils were not saturated, regardless of the soil. The mobility of the nanoformulation occurs to a great extent up to 20 cm from the soil surface, which is considered as the seed bank and area of the main action of herbicides applied in preemergence. Despite the distribution in the soil profile, the nanoformulation did not present risks for groundwater contamination. The greater bioavailability of the nanoMTZ in soil was confirmed by the greater control of weed plants, obtained even at the dose 10 times lower than the recommended dose, indicating efficiency in the delivery of the herbicide to the plants by PCL nanoparticles. Considering this dose reduction and increased control effectiveness, smaller amounts of a.i. will be available for dissipation in the environment.
4. Materials and Methods
4.1. Materials
Polycaprolactone (Mn ∼ 80,000 Da), sorbitane monostearate (Mw: 430.63 g mol–1), and polysorbate 80 (Mn ∼ 1310 Da) were purchased from Sigma-Aldrich (Sigma-Aldrich, Chem. Co.) and caprylic/capric triglyceride (Myritol 318) from Basf Co. Ltd, Brazil. Technical-grade metribuzin (95%) and the conventional formulation (Sencor 500 CG), 14C-metribuzin (95%) with a specific activity of 2.3 Bq mg–1 (American Radiolabeled Chemicals, Inc., St. Louis, MO), and scintillation solution (Ultima Gold, PerkinElmer, Waltham, MA) were also used. Acetone ACS (>99.5%) and high-performance liquid chromatography (HPLC)-grade methanol were purchased from Dinâmica Co. Ltd, Brazil.
The substrates (4-methylumbelliferone-phosphate (Mub-P), 4-methylumbelliferone-glucopyranoside (Mub-C), and 4-methylumbelliferone-sulfate (Mub-S)) and fluorophore (4-methylumbelliferone, Mub) for the enzyme assays were purchased from Sigma-Aldrich. Other reagents (analytical grade or better) were purchased from local suppliers.
4.2. Nanoformulation Preparation
The method used to prepare the nanoformulation was proposed by Grillo et al.28 for the preparation of PCL nanoparticles as a carrier for the herbicide atrazine and adapted for metribuzin. The preparation consisted of an organic phase, composed of 100 mg of PCL dissolved in 30 mL of acetone, under magnetic stirring with heating (55 °C) until the polymer was completely dissolved. Next, 200 mg of Myritol, 40 mg of sorbitane monostearate, and 10 mg of technical-grade metribuzin were added to this organic phase. Subsequently, 30 mL of ultrapure water and 60 mg of polysorbate 80 were homogenized under magnetic stirring for the aqueous phase. The organic solution was then poured into the aqueous solution and kept under stirring for 20 min at room temperature. The resulting suspension was concentrated on a rotary evaporator to a volume of 10 mL (1 mg of metribuzin mL–1). For the behavior studies, the formulation was also prepared as described above, however, in the preparation step of the organic phase, 14C-metribuzin was added, together with metribuzin technical (2 mg mL–1), to reach the final concentration of 0.4 mg mL–1.
4.3. Characterization and Stability of the Nanoformulation
The stability of the nanoformulation was monitored 0, 15, 30, 60, 90, and 120 days after preparation. Average size, polydispersity index (PDI), ζ-potential, and nanoparticle concentration measurements were performed by photon correlation spectroscopy (dynamic light scattering—DLS), microelectrophoresis, and nanoparticle tracking analysis (NTA). For DLS and microelectrophoresis measurements, taken in triplicate, the samples were diluted in ultrapure water (1:1000, v/v) and analyzed in ZetaSizer Nano ZS90 equipment (Malvern Instruments, United Kingdom), with a fixed angle detector at 90°, at 25 °C. For analysis by tracking nanoparticles, samples were diluted in ultrapure water (1:30,000, v/v) and measurements were taken by collecting 60 s videos, from five injections at 25 °C. NanoSight LM 10 cell (532 nm) equipment was used, with a CMOS camera and NanoSight software (version 3.2).
The chemical stability of the nanoparticles was measured as a function of pH variation. The pH of the dilution water was adjusted with solutions of HCl and NaOH (0.05 M), and the nanoformulation of PCL, containing and not containing the herbicide metribuzin, was diluted in these solutions in a proportion of 1:1000. The pH was measured before and after the dilution of the nanoformulations, and measurements were made for size, polydispersity index, and ζ-potential by DLS, as well as in the characterization step.
The morphology of nanoparticles containing metribuzin was evaluated using a micrograph obtained by atomic force microscopy (AFM). The nanoparticle suspension was diluted in ultrapure water (1:30,000, v/v), 10 μL was deposited on a silicon surface, dried in a desiccator, and observed in Nanosurf Easy Scan 2 Basic AFM—Pattern BT02217 equipment (Nanosurf, Switzerland). The analysis was carried out in noncontact mode, using a TapAl-G cantilever (BudgetSensors, Bulgaria) with a scan rate of 90 Hz. The image was processed using Gwyddion software.
4.4. Encapsulation Efficiency
The encapsulation efficiency for metribuzin was evaluated by high-performance liquid chromatography (HPLC), using a UV–vis detector operating at 290 nm, equipped with a Luna 5 μm C18(2) column 100 Å 250 mm × 4.6 mm, maintained at 40 °C. The mobile phase consisted of a methanol:water (80:20, v/v) solution, with a flow rate of 0.8 mL min–1. The detection limit presented was 5.7 ng mL–1 and the quantification limit was 19.1 ng mL–1. The samples were submitted to the ultrafiltration/centrifugation method, using Microcon 10 kDa regenerated cellulose ultrafiltration units (Millipore). The fraction not retained on the filter was used for quantification in HPLC, and the encapsulation efficiency was determined considering the amount initially added to the filter.
For the nanoformulation prepared for the behavior studies, using 14C-metribuzin, the fraction not retained on the filter was added to 10 mL of scintillating solution and analyzed in liquid scintillation counting (LSC) (Tri-Carb 2910 TR LSA, LSA PerkinElmer, Waltham, MA) for the quantification of radioactivity (% unencapsulated).
4.5. In Vitro Release Assays
The experiments to evaluate the release of metribuzin were carried out in triplicate, under light agitation and at room temperature (25 ± 2 °C), in a system composed of a donor and an acceptor medium, separated by a cellulose membrane (Visking, with size 1000 Da size-exclusion pore). The acceptor medium consisted of 100 mL of an aqueous solution of polysorbate 80 (0.25%, v/v) and the donor medium per 0.5 ml of sample (0.4 mg mL–1 of a.i.). The cross-sectional area was 3.46 cm2, in which the pores ensured that only the nonassociated herbicide passed into the acceptor medium until the systems reached equilibrium. Samples were collected from the acceptor compartment as a function of time and metribuzin quantified by HPLC. The evaluation of the mechanism involved in the release of metribuzin was performed by fitting the measurements to the Korsmeyer–Peppas semiempirical kinetic model (eq 1).104,105
| 1 |
where Mt corresponds to the absolute cumulative amount of metribuzin released in time t, M∞ is the total amount of herbicide released in infinite time, K is a constant that considers the structural and geometric aspects of the system, and n is a release exponent, indicative of the mechanism of herbicide release, which is semiempirically valid for the Fickian and non-Fickian diffusion process.52,106 The analysis was performed using the linearized equation of the model, applied to the measures that precede the region, where Mt/M∞ < 0.6.
4.6. Preparation of Commercial Metribuzin Formulation
To study the behavior of the formulations, 14C-metribuzin was used together with the commercial herbicide (70% purity), considering the recommended dose of 480 g a.i. ha–1 for the soybean crop. The same dose recommendation was considered for the studies of biodegradation, soil enzymatic activity, and sorption–desorption, in the preparation of the working solution of nanoMTZ and MTZ solutions, with and without radiolabel, calculated in relation to the mass of soil per experimental unit. In the leaching study, the area of the glass column (19.52 cm2) was considered for the calculation of the doses of the formulations.
4.7. Collection and Preparation of Soil Samples
Samples of three soils (Clay, Sandy loam-1 (SL-1) and Sandy loam-2 (SL-2)) were collected from the 0–20 cm layer, with total removal of the vegetation cover layer, in agricultural areas of experimental studies at the “Luiz de Queiroz” School of Agriculture (ESALQ/USP), Piracicaba, São Paulo, Brazil. The collected samples were sieved (2 mm) and stored at 4 ± 2 °C until the studies were carried out. The physicochemical properties of these soils are shown in Table S1 in the Supporting Material.
4.8. Biodegradation and Mineralization of Formulations
The biodegradation test was carried out according to the guidelines described in OECD guidelines 307.107 In the flask preparation and incubation stage, 50 g of each sieved soil (2 mm) (corrected for soil moisture) was added to biometric flasks as per Bartha and Pramer.108 The humidity of each soil was adjusted to 75% of field capacity, and the flasks were incubated in a dark room at 20 ± 2 °C, for 7 days, until the application of the formulations. At this stage, the bottles were weighed so that the humidity could be adjusted throughout the study, when necessary.
After the initial incubation period, 200 μL of each working solution containing the metribuzin formulations were applied to each vial and homogenized. The flasks were stored in a dark room (20 ± 2 °C) for a period of 28 days after application (DAA), during which time the mineralization and degradation of the formulations applied to the soils were evaluated.
For mineralization, traps to capture evolved 14CO2, containing 10 mL of NaOH (0.01 M), added to the handle of the biometric flask, were collected in duplicate, 10 mL of scintillating solution were added, and the radioactivity was measured in LSC. Traps were replaced every week. For degradation, the herbicide was extracted from the soil through two sequential extractions of methanol (60 and 40 mL) and one of chloroform (40 mL). In each extraction, using Teflon flasks, the suspensions were shaken (200 rpm for 1 h), centrifuged (800g for 15 min), and the supernatant collected.15 The radioactivity of the total volume extracted was measured by LSC, constituting the bioavailable fraction of the herbicide in the soil. For the unavailable fraction, the soil remaining from the extractions was dried at room temperature, ground, and homogenized for burning (3 aliquots of 0.2 g) in a biological oxidizer (OX500, R.J. Harvey Instrument Corporation, Tappan, NY). The 14CO2 evolved after the combustion of the samples, fixed in 10 mL of scintillating solution, was quantified in LSC. Data on the total 14C-metribuzin extracted were fitted to a first-order kinetic model (eq 2)
| 2 |
where C is the percentage of herbicide at time t (%), C0 is the percentage of herbicide at time zero (%), k is the degradation rate constant (days), and t is the incubation time (days). The herbicide degradation half-life (DT50), defined as the time required for 50% of the herbicide to be degraded in the soil, was calculated by DT50 = ln 2/k, based on the radioactivity of the herbicide extracted from the soil.
Thin-layer chromatography (TLC) analysis was performed according to the US Environmental Protection Agency method 507109,110 for the quantification of parental 14C-metribuzin and metabolites formed during the days of incubation. The samples extracted from the soil in the degradation stage were concentrated in a rotary evaporator up to a volume of 10 mL; 100 μL aliquots of these concentrated solutions were applied to TLC plates (60F254, EMD Millipore), together with the 14C-metribuzin standard, and eluted with 100 mL of acetonitrile:chloroform (1:1, v/v), in the saturated chamber.15 The eluted plates were dried at room temperature for 30 min and taken to phosphorescent film sensitization for 24 h. After this period, autoradiography images were generated on the radio scanner (Packard, Cyclone, PerkinElmer). The images obtained indicated the percentage of parental metribuzin and metabolites available in the soil contained in each sample submitted to extraction. The identification of the positions of the metabolites on the TLC plates and comparisons was possible due to the sensitization of the phosphorescent plates by the 14C present in the molecules formed from the parental 14C-metribuzin, used as a standard in the analysis.
4.9. Soil Enzymatic Activity
For the soil enzymatic activity assays, 100 g of soil was used, in 250 mL flasks. As in the study of biodegradation (14C), the moisture in the soils was adjusted and maintained at 75% of the field capacity. In each vial, nanoMTZ and MTZ formulations were applied at a dose of 480 g a.i. ha–1. The soils were incubated, also for 28 days, and the analysis of the enzymatic activity in the soils was carried out at 24 h, and 7, 14, 21, and 28 DAA.
Acid phosphatase, β-1,4-glucosidase, and arylsulfatase (linked to Mub substrate: Mub-P, Mub-C, and Mub-S) were chosen as hydrolases indicative of enzymatic activity in the soil. These enzymes play important roles in biogeochemical cycles, are resistant to a wide range of physical factors in soil environments, and demonstrate important markers of the effects of nanoparticles in soil.45,70
The soil enzyme activity assays were based on a sensitive fluorometric technique.45 At each evaluation time, 1 g of soil was collected from the flasks, dried at room temperature, and sieved (2 mm). Enzyme extraction was performed in 0.5 g of soil, with 25 mL of a 50 mM sodium acetate buffer solution (pH 5.2, equal to the soil samples) for 30 min under constant agitation, followed by centrifugation (5 min at 1700g). A 30 mM stock solution of Mub was prepared in dimethyl sulfoxide and stored in the dark at 4 °C. Working solutions (200 μM) of each substrate (Mub-P, Mub-C, Mub-S) were prepared from the sodium acetate buffer solutions. In a 96-well black polystyrene microplate (Corning), 50 μL of each substrate and 150 μL of soil extract were added to each well separately. For each sample and each substrate, six technical replicas were prepared. Blank samples consisting only of soil extract and substrate solutions were also prepared and analyzed. Replicates of all treatments were randomly shifted into multiwell microplates. Details on the soil enzymes assays are presented at the Supporting Material. At the same time as the soil sampling for analysis of enzymatic activities, a 1 g aliquot of each soil was sampled to quantify the residual content of metribuzin using 2 mL of acetonitrile under stirring for 1 h, followed by filtration (0.45 μm) and quantification by HPLC.
4.10. Sorption–Desorption of Metribuzin Formulations
The sorption–desorption test was performed based on the OECD guidelines 106.111 The study was carried out in three sequential tests, one to define the soil:solution ratio (step I), another to determine the time needed to obtain the equilibrium and sorption kinetics (step II), and, finally, the sorption–desorption as a function of a.i. concentrations in the soil (step III). In steps I and II (Table S4 and Figure S1), the ratio of 1:1 (m/v) and the equilibrium time of 24 h were defined. In step III, the experimental units consisted of 50 mL Teflon tubes, containing 10 g of soil and 10 mL of a CaCl2 solution (0.01 M) containing 14C-metribuzin in nanoMTZ and commercial MTZ formulations.
The tubes were incubated 12 h before herbicide application with 90% of the final volume (at all stages) of CaCl2 (0.01 M) and shaken at 180 rpm on a shaking table. The remaining 10% of the volume was prepared and applied with nanoMTZ and commercial MTZ formulations. After application, the tubes were shaken for another 24 h and subsequently centrifuged at 800 g for 15 min;15 1 mL aliquots were removed from the supernatant in duplicate, added to 10 mL of scintillator solution, and analyzed in LSC for quantification of radioactivity (5 min). The portion sorbed into the soil was determined by the difference between the total radioactivity of the 14C-metribuzin applied and the radioactivity obtained in the solution.
In step III, the isotherms were built with five doses of active ingredient (120, 240, 480, 960, and 1920 g a.i. ha–1), following the same protocol described above. After sorption, the supernatant was discarded and 10 mL of a new herbicide-free CaCl2 solution (0.01 M) was added to the soil. The tubes were shaken for another 24 h and centrifuged. The supernatant was collected again (1 mL), and the quantification of radioactivity was performed. The total desorbed was calculated as a function of the asset remaining in the soil after sorption.
The sorption–desorption coefficients, Kd (mL g–1), were obtained considering the a.i. concentrations in the soil (Cs, μg g–1) and in the solution (Ce, μg mL–1), through eq 3. The normalized sorption–desorption coefficients for organic carbon, KOC (mL g–1), were determined by eq 4. The soil–water distribution coefficients (Freundlich coefficient), Kf (mL g–1), were calculated by eq 5. Furthermore, the reversibility of sorption, hysteresis (H), was calculated based on the values of 1/n, according to eq 6.
| 3 |
| 4 |
| 5 |
| 6 |
4.11. Leaching of Metribuzin Formulations in the Soil Profile
Column leaching testing was performed based on the OECD guidelines 312.112 The experimental units consisted of glass columns (50 cm) filled with soil up to 30 cm deep. As a filter and containment of the soil, glass wool and washed sand were used at the lower conical end (HCl 0.01 M, in sufficient quantity for total cleaning of the sample, with subsequent washing in deionized water, raising the pH to 7 and drying in an oven at 100 °C). Before applying the 14C-metribuzin formulations, the columns were capillary saturated with a 0.01 M CaCl2 solution and drained for 15 min. Applications of nanoMTZ and MTZ formulations were made at the top of the columns (878 μL of the working solution), and subsequently, the rainfall simulation system was started (200 mm in 48 h). Glass wool disks and inverted funnels were used at the top of the column so that the hoses of the peristaltic pump (model ISM942, Ismatec, Wertheim, Germany) would not touch the soil containing the radioisotope and so that preferential water paths would not form, with the rain smeared on the glass wool.
During the 48 h of the test, samples of 10 mL of the leachate solution were collected in triplicate, after 50, 100, 125, 150, and 200 mm of rain. The samples were added to 10 mL of scintillating solution, and the radioactivity was quantified in LSC. After 48 h of simulated rain, the columns were sectioned into 10 cm portions, which were placed on aluminum trays to dry at room temperature. After drying, the samples were ground, homogenized, and, in triplicate (0.2 g aliquots of soil), burned in a biological oxidizer (OX500, R.J. Harvey Instrument Corporation, Tappan, NY). The percentages of active leached beyond 30 cm in depth and distributed along the soil profile were calculated in relation to the total 14C-metribuzin initially applied to the soil.
4.12. Control Efficacy and Aspects of Photosynthetic Activity and Vigor of I. grandifolia
Pots containing 1 L of soil were prepared, with the same SL-1 used in the behavior tests (Table S2). For each experimental unit, 25 seeds of viola cord, I. grandifolia Lam. (synonymous with Stictocardia tiliifolia (Desr.) Hallier f.), were sown and, after 24 h, preemergence treatments were applied (4 repetitions): water (control), nanoparticles without the herbicide, the metribuzin nanoformulation at doses of 480 and 48 g a.i. ha–1, and the commercial formulation of metribuzin applied in the same doses. For the application of treatments, the area of the pots (0.019 m2) and a 3 mL volume of the formulations, standardized for all treatments, were considered. At 3, 7, 11, and 14 days after application (DAA), the number of live plants was counted, and, at 14 DAA, the shoot and root dry mass of the experimental units was measured.
SeedReporter equipment (PhenoVation B.V., Wageningen, Netherlands) was used to evaluate the physiological performance of I. grandifolia plants at 7 DAA of the herbicides, following the procedures described by Oliveira et al.113 The data were calculated using SeedReporter software, version 5.5.1. To obtain the chlorophyll a and anthocyanin indices, the leaves were illuminated with a broad-band white light (3000 K; 450 to 780 nm), with a previously adjusted intensity to avoid overload. In a few seconds, reflectance images were acquired using optical filters at 540, 640, 710, and 770 nm to generate the multispectral images (2448 × 2448 pixels corresponding to 3.69 μm pixel–1). The chlorophyll a and anthocyanin indices were calculated according to the formulas presented by Oliveira et al.113 To evaluate the photosynthetic activity of the leaves, the fast chlorophyll fluorescence method based on the Kautsky induction curve was used. Plants were dark-adapted for 30 min prior to image acquisition. Thirty-six high-intensity amber LED sources (peak at 620 nm) were used to excite the chlorophyll under a saturating light intensity (6320 μmol m–2 s–1) that closed all of the reaction centers. Fluorescence signals were transmitted to a CCD chip through an interference filter (730 nm) that blocked the LED sources. F0 (basal fluorescence) was measured in the dark-adapted state, when the reaction centers were open. After application of the saturating light flash, Fm (maximum fluorescence) was measured, and the variable fluorescence (Fv) was calculated as the difference between Fm and F0. The Fv/Fm ratio (which is equivalent to the maximum quantum yield of PSII) was then obtained.
4.13. Statistical Analysis
Data on extracted and bound residues, enzymatic activity (for MUB independently associated with a specific substrate), the concentration of herbicide extracted in the study of enzymatic activity, Kd of sorption and desorption, % sorption and desorption, and % leaching were submitted to an analysis of variance (ANOVA) of two factors. Data referring to the enzymatic activity (for MUB independently associated with a specific substrate), the number of live plants, dry mass of shoots and roots, and physiological parameters of the plants were subjected to a one-way analysis of variance. When necessary, the data were transformed to meet the analysis assumptions (homoscedasticity and homogeneity of variances). Later, when significant differences were detected (p < 0.05 for the F test), the means were compared by the Tukeyʼs test (p < 0.05). For the enzyme activities, significance levels were at 0.01 for the F and Tukeyʼs tests. The figures were plotted using Origin 2020 (Version 9.7.0.185, OriginLab Corporation, Northampton, MA) to represent the degradation and sorption–desorption models and for the other biodegradation and leaching data.
Acknowledgments
The authors are grateful to the Fundação de Amparo à Pesquisa de São Paulo (FAPESP, grants #2017/21004-5; #2019/04758-1; #2018/23608-8; #2020/12644-3; #2017/15220-7), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Capes (PrInt/Capes #88887.468162/2019-00), Associação dos Produtores de Soja e Milho do Estado de Mato Grosso (APROSOJA—Projeto Agrocientista), and Instituto Mato-Grossense do Agronegócio (IAGRO—MT).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnanoscienceau.1c00055.
Encapsulation efficiency; physical–chemical properties of soils; bounded residues and 14CO2 accumulated in 28 days after the pesticide application; concentration of metribuzin extracted from enzymatic activity assay; percentage of sorption 14C-metribuzin in the first step of sorption studies; characterization and stability of the nanoMTZ formulation over 120 days after encapsulation; parental 14C-metribuzin and metabolites formed in the biodegradation study; sorption kinetics; sorption and desorption isotherms; and number of live plants and physiological parameters measured in the plant assay (PDF)
Author Contributions
V.T., L.B.C., and J.A.G. contributed to investigation, methodology, and writing–original draft. R.F.P., G.V.M.-G., and H.C.O. involved in methodology and writing–original draft. V.L.T. contributed to investigation, methodology, supervision. V.T., L.B.C., H.C.O., and L.F.F. contributed to conceptualization, writing–review and editing, and supervision. All authors revised and gave approval for the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- dos Santos E. A.; Correia N. M.; Botelho R. G. Herbicides Residues in Water Bodies - A Review. Rev. Bras. Herbic. 2013, 12, 188–201. 10.7824/rbh.v12i2.245. [DOI] [Google Scholar]
- Dores E. F. G. C.; Navickiene S.; Cunha M. L. F.; Carbo L.; Ribeiro M. L.; De-Lamonica-Freire E. M. Multiresidue Determination of Herbicides in Environmental Waters from Primavera Do Leste Region (Middle West of Brazil) by SPE-GC-NPD. J. Braz. Chem. Soc. 2006, 17, 866–873. 10.1590/S0103-50532006000500008. [DOI] [Google Scholar]
- Cerdeira A. L.; Desouza M. D.; Queiroz S. C. N.; Ferracini V. L.; Bolonhezi D.; Gomes M. A. F.; Rosa M. A.; Balderrama O.; Rampazzo P.; Queiroz R. H. C.; Neto C. F.; Matallo M. B. Leaching and Half-Life of the Herbicide Tebuthiuron on a Recharge Area of Guarany Aquifer in Sugarcane Fields in Brazil. J. Environ. Sci. Health, Part B 2007, 42, 635–639. 10.1080/03601230701465593. [DOI] [PubMed] [Google Scholar]
- De Armas E. D.; Monteiro R. T. R.; Antunes P. M.; Dos Santos M. A. P. F.; De Camargo P. B.; Abakerli R. B. Spatial-Temporal Diagnostic of Herbicide Occurrence in Surface Waters and Sediments of Corumbataí River and Main Affluents. Quim. Nova 2007, 30, 1119–1127. 10.1590/s0100-40422007000500013. [DOI] [Google Scholar]
- Britto F. B.; Do Vasco A. N.; Pereira A. P. S.; Méllo A. V.; Nogueira L. C. Herbicidas No Alto Rio Poxim, Sergipe e Os Riscos de Contaminação Dos Recursos Hídricos. Rev. Ciênc. Agron. 2012, 43, 390–398. 10.1590/S1806-66902012000200024. [DOI] [Google Scholar]
- Moreira J. C.; Peres F.; Simões A. C.; Pignati W. A.; Dores E. d. C.; Vieira S. N.; Strüssmann C.; Mott T. Contaminação de Águas Superficiais e de Chuva Por Agrotóxicos Em Uma Região Do Estado Do Mato Grosso. Ciênc. Saúde Coletiva 2012, 17, 1557–1568. 10.1590/S1413-81232012000600019. [DOI] [PubMed] [Google Scholar]
- Lewis K. A.; Tzilivakis J.; Warner D. J.; Green A. An International Database for Pesticide Risk Assessments and Management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. 10.1080/10807039.2015.1133242. [DOI] [Google Scholar]
- Montagner C. C.; Vidal C.; Acayaba R. D. Contaminantes Emergentes Em Matrizes Aquáticas Do Brasil: Cenário Atual e Aspectos Analíticos, Ecotoxicológicos e Regulatórios. Quim. Nova 2017, 40, 1094–1110. 10.21577/0100-4042.20170091. [DOI] [Google Scholar]
- Albuquerque A. F.; Ribeiro J. S.; Kummrow F.; Nogueira A. J. A.; Montagner C. C.; Umbuzeiro G. A. Pesticides in Brazilian Freshwaters: A Critical Review. Environ. Sci. Process. Impacts 2016, 18, 779–787. 10.1039/c6em00268d. [DOI] [PubMed] [Google Scholar]
- Jacobsen C. S.; van der Keur P.; Iversen B. V.; Rosenberg P.; Barlebo H. C.; Torp S.; Vosgerau H.; Juhler R. K.; Ernstsen V.; Rasmussen J.; Brinch U. C.; Jacobsen O. H. Variation of MCPA, Metribuzine, Methyltriazine-Amine and Glyphosate Degradation, Sorption, Mineralization and Leaching in Different Soil Horizons. Environ. Pollut. 2008, 156, 794–802. 10.1016/j.envpol.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Pot V.; Benoit P.; Le Menn M.; Eklo O. M.; Sveistrup T.; Kværner J. Metribuzin Transport in Undisturbed Soil Cores under Controlled Water Potential Conditions: Experiments and Modelling to Evaluate the Risk of Leaching in a Sandy Loam Soil Profile. Pest Manage. Sci. 2011, 67, 397–407. 10.1002/ps.2077. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency - USEPA. Health Advisory Summary: Metribuzine; 1992.
- Haarstad K.; Ludvigsen G. H. Ten Years of Pesticide Monitoring in Norwegian Ground Water. Groundwater Monit. Rem. 2007, 27, 75–89. 10.1111/j.1745-6592.2007.00153.x. [DOI] [Google Scholar]
- PubChem. https://pubchem.ncbi.nlm.nih.gov/source/hsdb/68441/24.
- Guimarães A. C. D.; Mendes K. F.; dos Reis F. C.; Campion T. F.; Christoffoleti P. J.; Tornisielo V. L. Role of Soil Physicochemical Properties in Quantifying the Fate of Diuron, Hexazinone, and Metribuzin. Environ. Sci. Pollut. Res. 2018, 25, 12419–12433. 10.1007/s11356-018-1469-5. [DOI] [PubMed] [Google Scholar]
- Mendes K. F.; Sousa R. N.; Takeshita V.; Alonso F. G.; Régo A. P. J.; Tornisielo V. L. Cow bone char as a sorbent to increase sorption and decrease mobility of hexazinone, metribuzin and quinclorac in soil. Geoderma 2019, 343, 40–49. 10.1016/j.geoderma.2019.02.009. [DOI] [Google Scholar]
- Arsenijevic N.; De Avellar M.; Butts L.; Arneson N. J.; Werle R. Influence of Sulfentrazone and Metribuzin Applied Preemergence on Soybean Development and Yield. Weed Technol. 2021, 35, 210–215. 10.1017/wet.2020.99. [DOI] [Google Scholar]
- Volova T.; Shumilova A.; Zhila N.; Sukovatyi A.; Shishatskaya E.; Thomas S. Efficacy of Slow-Release Formulations of Metribuzin and Tribenuron Methyl Herbicides for Controlling Weeds of Various Species in Wheat and Barley Stands. ACS Omega 2020, 5, 25135–25147. 10.1021/acsomega.0c02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. US EPA, Pesticide Product Label, Metribuzin 4L,04/15/2020 https://www3.epa.gov/pesticides/chem_search/ppls/042750-00361-20200415.pdf (accessed Nov 30, 2021).
- accessed Nov 30
- Fuscaldo F.; Bedmar F.; Monterubbianesi G. Persistence of Atrazine, Metribuzin and Simazine Herbicides in Two Soils. Pesqui. Agropecu. Bras. 1999, 34, 2037–2044. 10.1590/S0100-204X1999001100009. [DOI] [Google Scholar]
- Rigi M. R.; Farahbakhsh M.; Rezaei K. Adsorption and Desorption Behavior of Herbicide Metribuzin in Different Soils of Iran. J. Agric. Sci. Technol. 2015, 17, 777–787. [Google Scholar]
- Travlos I. S.; Gkotsi T.; Roussis I.; Kontopoulou C. K.; Kakabouki I.; Bilalis D. J. Effects of the Herbicides Benfluralin, Metribuzin and Propyzamide on the Survival and Weight of Earthworms (Octodrilus Complanatus). Plant, Soil Environ. 2017, 63, 117–124. 10.17221/811/2016-PSE. [DOI] [Google Scholar]
- Santric L.; Radivojevic L.; Saric-Krsmanovic M.; Gajic-Umiljendic J.; Djurovic-Pejcev R. Microbial Activity in Different Soils in Response to Metribuzin Treatment. Pestic. i fitomedicina 2019, 34, 123–130. 10.2298/pif1902123s. [DOI] [Google Scholar]
- Fairchild J. F.; Sappington L. C. Fate and Effects of the Triazinone Herbicide Metribuzin in Experimental Pond Mesocosms. Arch. Environ. Contam. Toxicol. 2002, 43, 198–202. 10.1007/s00244-002-1208-1. [DOI] [PubMed] [Google Scholar]
- Almeida A. C.; Gomes T.; Langford K.; Thomas K. V.; Tollefsen K. E. Oxidative Stress Potential of the Herbicides Bifenox and Metribuzin in the Microalgae Chlamydomonas Reinhardtii. Aquat. Toxicol. 2019, 210, 117–128. 10.1016/j.aquatox.2019.02.021. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency - USEPA. Contaminant Candidate List Regulatory Determination Support Document for Naphthalene, 2003, p 6.
- Grillo R.; dos Santos N. Z. P.; Maruyama C. R.; Rosa A. H.; de Lima R.; Fraceto L. F. Poly(E{open}-Caprolactone)Nanocapsules as Carrier Systems for Herbicides: Physico-Chemical Characterization and Genotoxicity Evaluation. J. Hazard. Mater. 2012, 231–232, 1–9. 10.1016/j.jhazmat.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Pereira A. E. S.; Grillo R.; Mello N. F. S.; Rosa A. H.; Fraceto L. F. Application of Poly(Epsilon-Caprolactone) Nanoparticles Containing Atrazine Herbicide as an Alternative Technique to Control Weeds and Reduce Damage to the Environment. J. Hazard. Mater. 2014, 268, 207–215. 10.1016/j.jhazmat.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Campos E. V. R.; De Oliveira J. L.; Da Silva C. M. G.; Pascoli M.; Pasquoto T.; Lima R.; Abhilash P. C.; Fernandes Fraceto L. Polymeric and Solid Lipid Nanoparticles for Sustained Release of Carbendazim and Tebuconazole in Agricultural Applications. Sci. Rep. 2015, 5, 13809 10.1038/srep13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y.; Wu Y.; Zhao C.; Xu Y.; Lu J.; Xiang S.; Zong F.; Wu X. Polymeric Nanoparticles as a Metolachlor Carrier: Water-Based Formulation for Hydrophobic Pesticides and Absorption by Plants. J. Agric. Food Chem. 2017, 65, 7371–7378. 10.1021/acs.jafc.7b02197. [DOI] [PubMed] [Google Scholar]
- de Oliveira J. L.; Campos E. V. R.; Pereira A. E. S.; Pasquoto T.; Lima R.; Grillo R.; de Andrade D. J.; dos Santos F. A.; Fraceto L. F. Zein Nanoparticles as Eco-Friendly Carrier Systems for Botanical Repellents Aiming Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 1330–1340. 10.1021/acs.jafc.7b05552. [DOI] [PubMed] [Google Scholar]
- Pascoli M.; Lopes-Oliveira P. J.; Fraceto L. F.; Seabra A. B.; Oliveira H. C. State of the Art of Polymeric Nanoparticles as Carrier Systems with Agricultural Applications: A Minireview. Energy, Ecol. Environ. 2018, 3, 137–148. 10.1007/s40974-018-0090-2. [DOI] [Google Scholar]
- Kah M.; Walch H.; Hofmann T. Environmental Fate of Nanopesticides: Durability, Sorption and Photodegradation of Nanoformulated Clothianidin. Environ. Sci.: Nano 2018, 5, 882–889. 10.1039/c8en00038g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Guo Z.; Ullah S.; Melagraki G.; Afantitis A.; Lynch I. Nanotechnology and Artificial Intelligence to Enable Sustainable and Precision Agriculture. Nat. Plants 2021, 7, 864–876. 10.1038/s41477-021-00946-6. [DOI] [PubMed] [Google Scholar]
- Nisha Raj S.; Anooj E. S.; Rajendran K.; Vallinayagam S. A Comprehensive Review on Regulatory Invention of Nano Pesticides in Agricultural Nano Formulation and Food System. J. Mol. Struct. 2021, 1239, 130517 10.1016/j.molstruc.2021.130517. [DOI] [Google Scholar]
- Diyanat M.; Saeidian H. The Metribuzin Herbicide in Polycaprolactone Nanocapsules Shows Less Plant Chromosome Aberration than Non-Encapsulated Metribuzin. Environ. Chem. Lett. 2019, 17, 1881–1888. 10.1007/s10311-019-00912-x. [DOI] [Google Scholar]
- Preisler A. C.; Pereira A. E. S.; Campos E. V. R.; Dalazen G.; Fraceto L. F.; Oliveira H. C. Atrazine Nanoencapsulation Improves Pre-Emergence Herbicidal Activity against Bidens Pilosa without Enhancing Long-Term Residual Effect on Glycine Max. Pest Manage. Sci. 2020, 76, 141–149. 10.1002/ps.5482. [DOI] [PubMed] [Google Scholar]
- Sousa G. F. M.; Gomes D. G.; Campos E. V. R.; Oliveira J. L.; Fraceto L. F.; Stolf-Moreira R.; Oliveira H. C. Post-Emergence Herbicidal Activity of Nanoatrazine against Susceptible Weeds. Front. Environ. Sci. 2018, 6, 12. 10.3389/fenvs.2018.00012. [DOI] [Google Scholar]
- de Sousa B. T.; Pereira A. d. E. S.; Fraceto L. F.; de Oliveira H. C.; Dalazen G. Effectiveness of Nanoatrazine in Post-Emergent Control of the Tolerant Weed Digitaria Insularis. J. Plant Prot. Res. 2020, 60, 185–192. 10.24425/jppr.2020.133311. [DOI] [Google Scholar]
- Assalin M. R.; Dos Santos L. D. L.; Souza D. R. C.; Rosa M. A.; Duarte R. C. R. M.; Castanha R. F.; Donaire P. P. R.; Durán N. Ecotoxicity Evaluation: Preparation of Poly-ϵ-Caprolactone and Chitosan Nanoparticles as Carriers of Thiamethoxam Pesticide. J. Phys.: Conf. Ser. 2019, 1323, 012017. 10.1088/1742-6596/1323/1/012017. [DOI] [Google Scholar]
- Kah M.; Johnston L. J.; Kookana R. S.; Bruce W.; Haase A.; Ritz V.; Dinglasan J.; Doak S.; Garelick H.; Gubala V. Comprehensive Framework for Human Health Risk Assessment of Nanopesticides. Nat. Nanotechnol. 2021, 16, 955–964. 10.1038/s41565-021-00964-7. [DOI] [PubMed] [Google Scholar]
- Kookana R. S.; Boxall A. B. A.; Reeves P. T.; Ashauer R.; Beulke S.; Chaudhry Q.; Cornelis G.; Fernandes T. F.; Gan J.; Kah M.; Lynch I.; Ranville J.; Sinclair C.; Spurgeon D.; Tiede K.; Van Den Brink P. J. Nanopesticides: Guiding Principles for Regulatory Evaluation of Environmental Risks. J. Agric. Food Chem. 2014, 62, 4227–4240. 10.1021/jf500232f. [DOI] [PubMed] [Google Scholar]
- Kah M.; Kookana R. S.; Gogos A.; Bucheli T. D. A Critical Evaluation of Nanopesticides and Nanofertilizers against Their Conventional Analogues. Nat. Nanotechnol. 2018, 13, 677–684. 10.1038/s41565-018-0131-1. [DOI] [PubMed] [Google Scholar]
- Peyrot C.; Wilkinson K. J.; Desrosiers M.; Sauvé S. Effects of Silver Nanoparticles on Soil Enzyme Activities with and without Added Organic Matter. Environ. Toxicol. Chem. 2014, 33, 115–125. 10.1002/etc.2398. [DOI] [PubMed] [Google Scholar]
- Asadishad B.; Chahal S.; Akbari A.; Cianciarelli V.; Azodi M.; Ghoshal S.; Tufenkji N. Amendment of Agricultural Soil with Metal Nanoparticles: Effects on Soil Enzyme Activity and Microbial Community Composition. Environ. Sci. Technol. 2018, 52, 1908–1918. 10.1021/acs.est.7b05389. [DOI] [PubMed] [Google Scholar]
- Galhardi J. A.; Fraceto L. F.; Wilkinson K. J.; Ghoshal S. Soil Enzyme Activities as an Integral Part of the Environmental Risk Assessment of Nanopesticides. J. Agric. Food Chem. 2020, 68, 8514–8516. 10.1021/acs.jafc.0c04344. [DOI] [PubMed] [Google Scholar]
- Bombo A. B.; Pereira A. E. S.; Lusa M. G.; De Medeiros Oliveira E.; De Oliveira J. L.; Campos E. V. R.; De Jesus M. B.; Oliveira H. C.; Fraceto L. F.; Mayer J. L. S. A Mechanistic View of Interactions of a Nanoherbicide with Target Organism. J. Agric. Food Chem. 2019, 67, 4453–4462. 10.1021/acs.jafc.9b00806. [DOI] [PubMed] [Google Scholar]
- Takeshita V.; de Sousa B. T.; Preisler A. C.; Carvalho L. B.; Pereira A. d. E. S.; Tornisielo V. L.; Dalazen G.; Oliveira H. C.; Fraceto L. F. Foliar Absorption and Field Herbicidal Studies of Atrazine-Loaded Polymeric Nanoparticles. J. Hazard. Mater. 2021, 418, 126350 10.1016/j.jhazmat.2021.126350. [DOI] [PubMed] [Google Scholar]
- Su Y.; Ashworth V.; Kim C.; Adeleye A. S.; Rolshausen P.; Roper C.; White J.; Jassby D. Delivery, Uptake, Fate, and Transport of Engineered Nanoparticles in Plants: A Critical Review and Data Analysis. Environ. Sci. Nano 2019, 6, 2311–2331. 10.1039/c9en00461k. [DOI] [Google Scholar]
- Korsmeyer R. W.; Gurny R.; Doelker E.; Buri P.; Peppas N. A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- Singh A.; Dhiman N.; Kar A. K.; Singh D.; Purohit M. P.; Ghosh D.; Patnaik S. Advances in Controlled Release Pesticide Formulations: Prospects to Safer Integrated Pest Management and Sustainable Agriculture. J. Hazard. Mater. 2020, 385, 121525 10.1016/j.jhazmat.2019.121525. [DOI] [PubMed] [Google Scholar]
- Spadotto C. A.; Hornsby A. G. Soil Sorption of Acidic Pesticides. J. Environ. Qual. 2003, 32, 949. 10.2134/jeq2003.9490. [DOI] [PubMed] [Google Scholar]
- Spadotto C. A.; Locke M. A.; Bingner R. L.; Mingoti R. Estimating Sorption of Monovalent Acidic Herbicides at Different PH Levels Using a Single Sorption Coefficient. Pest Manage. Sci. 2020, 76, 2693–2698. 10.1002/ps.5815. [DOI] [PubMed] [Google Scholar]
- Zhao J.; Tang J.; Dang T. Influence of Extracellular Polymeric Substances on the Heteroaggregation between CeO2 Nanoparticles and Soil Mineral Particles. Sci. Total Environ. 2022, 806, 150358 10.1016/j.scitotenv.2021.150358. [DOI] [PubMed] [Google Scholar]
- Silva M. d. S.; Cocenza D. S.; Grillo R.; Melo N. F. S. d.; Tonello P. S.; de Oliveira L. C.; Cassimiro D. L.; Rosa A. H.; Fraceto L. F. Paraquat-Loaded Alginate/Chitosan Nanoparticles: Preparation, Characterization and Soil Sorption Studies. J. Hazard. Mater. 2011, 190, 366–374. 10.1016/j.jhazmat.2011.03.057. [DOI] [PubMed] [Google Scholar]
- Kah M.; Machinski P.; Koerner P.; Tiede K.; Grillo R.; Fraceto L. F.; Hofmann T. Analysing the Fate of Nanopesticides in Soil and the Applicability of Regulatory Protocols Using a Polymer-Based Nanoformulation of Atrazine. Environ. Sci. Pollut. Res. 2014, 21, 11699–11707. 10.1007/s11356-014-2523-6. [DOI] [PubMed] [Google Scholar]
- Lechón Y.; García-Valcárcel A. I.; Matienzo T.; Sánchez-Brunete C.; Tadeo J. L. Laboratory and Field Studies on Metribuzin Persistence in Soil and Its Prediction by Simulation Models. Toxicol. Environ. Chem. 1997, 63, 47–61. 10.1080/02772249709358516. [DOI] [Google Scholar]
- Perceval J.; Benoit P.; Stenrød M.; Eklo O. M.; Charmay M. P.; Barriuso E. Availability and Biodegradation of Metribuzin in Alluvial Soils as Affected by Temperature and Soil Properties. Weed Res. 2007, 47, 517–526. 10.1111/j.1365-3180.2007.00589.x. [DOI] [Google Scholar]
- Mutua G. K.; Ngigi A. N.; Getenga Z. M. Degradation Characteristics of Metribuzin in Soils within the Nzoia River Drainage Basin, Kenya. Toxicol. Environ. Chem. 2016, 98, 800–813. 10.1080/02772248.2015.1128938. [DOI] [Google Scholar]
- Fojtová D.; Vašíčková J.; Grillo R.; Bílková Z.; Šimek Z.; Neuwirthová N.; Kah M.; Hofman J. Nanoformulations Can Significantly Affect Pesticide Degradation and Uptake by Earthworms and Plants. Environ. Chem. 2019, 16, 470–481. 10.1071/EN19057. [DOI] [Google Scholar]
- Kah M.; Weniger A. K.; Hofmann T. Impacts of (Nano)Formulations on the Fate of an Insecticide in Soil and Consequences for Environmental Exposure Assessment. Environ. Sci. Technol. 2016, 50, 10960–10967. 10.1021/acs.est.6b02477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen W. C.; Harper S. S.. The Retention Process: Mechanisms. In Pesticides in the Soil Environment: Processes, Impacts, and Modeling; Cheng H. H., Ed.; Soil Science Society of America: Madson, 1990; pp 51–77. 10.2136/sssabookser2.c3. [DOI] [Google Scholar]
- Khoury R.; Geahchan A.; Coste C. M.; Cooper J. F.; Bobe A. Retention and Degradation of Metribuzin in Sandy Loam and Clay Soils of Lebanon. Weed Res. 2003, 43, 252–259. 10.1046/j.1365-3180.2003.00341.x. [DOI] [Google Scholar]
- Saritha J. D.; Ramprakash T.; Rao P. C.; Madhavi M. Persistence of Metribuzin in Tomato Growing Soils and Tomato Fruits. Nat. Environ. Pollut. Technol. 2017, 16, 505–508. [Google Scholar]
- Conclusion Regarding the Peer Review of the Pesticide Risk Assessment of the Active Substance Metribuzin. EFSA J. 2008, 6, 145r 10.2903/j.efsa.2008.145r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi R. M.; Heiskanen I.; Ahtiainen J. H.; Rahkonen A.; Mäntykoski K.; Welling L.; Laitinen P.; Ruuttunen P. Microbial Toxicity and Impacts on Soil Enzyme Activities of Pesticides Used in Potato Cultivation. Appl. Soil Ecol. 2009, 41, 293–304. 10.1016/j.apsoil.2008.12.002. [DOI] [Google Scholar]
- Santric L.; Radivojevic L.; Saric-Krsmanovic M.; Gajic-Umiljendic J.; Djurovic-Pejcev R. The Effect of Metribuzin on the Density of Proteolytic Microorganisms and Proteolytic Activity in Different Types of Soil. Pestic. i fitomedicina 2018, 33, 233–240. 10.2298/pif1804233s. [DOI] [Google Scholar]
- Nottingham A. T.; Turner B. L.; Chamberlain P. M.; Stott A. W.; Tanner E. V. J. Priming and Microbial Nutrient Limitation in Lowland Tropical Forest Soils of Contrasting Fertility. Biogeochemistry 2012, 111, 219–237. 10.1007/s10533-011-9637-4. [DOI] [Google Scholar]
- Li B.; Chen Y.; Liang W.-z.; Mu L.; Bridges W. C.; Jacobson A. R.; Darnault C. J. G. Influence of Cerium Oxide Nanoparticles on the Soil Enzyme Activities in a Soil-Grass Microcosm System. Geoderma 2017, 299, 54–62. 10.1016/j.geoderma.2017.03.027. [DOI] [Google Scholar]
- Montes de Oca-Vásquez G.; Solano-Campos F.; Vega-Baudrit J. R.; López-Mondéjar R.; Odriozola I.; Vera A.; Moreno J. L.; Bastida F. Environmentally Relevant Concentrations of Silver Nanoparticles Diminish Soil Microbial Biomass but Do Not Alter Enzyme Activities or Microbial Diversity. J. Hazard. Mater. 2020, 391, 122224 10.1016/j.jhazmat.2020.122224. [DOI] [PubMed] [Google Scholar]
- Fraceto L. F.; Grillo R.; de Medeiros G. A.; Scognamiglio V.; Rea G.; Bartolucci C. Nanotechnology in Agriculture: Which Innovation Potential Does It Have?. Front. Environ. Sci. 2016, 4, 20. 10.3389/fenvs.2016.00020. [DOI] [Google Scholar]
- Giles C. H.; MacEwan T. H.; Nakhwa S. N.; Smith D. Studies in Adsorption. Part XI.* A System of Classification of Solution Adsorption Isotherms, and Its Use in Diagnosis of Adsorption Mechanisms and in Measurement of Specific Surface Areas of Solids. J. Chem. Soc. 1960, 3973. 10.1039/JR9600003973. [DOI] [Google Scholar]
- Johnson R. M.; Pepperman A. B. Mobility of Atrazine from Alginate Controlled Release Formulations. J. Environ. Sci. Health, Part B 1995, 30, 27–47. 10.1080/03601239509372926. [DOI] [Google Scholar]
- Lagat S. C.; Lalah J. O.; Kowenje C. O.; Getenga Z. M.. Metribuzin Mobility in Soil Column As Affected By Environmental and Physico-Chemical Parameters in Mumias Sugarcane Zone, Kenya 2011, 6 (3), 27–33..
- Saritha J. D.; Prakash T. R.; Madhavi M.; Rao P. C. Leaching Potential of Metribuzin in Agricultural Soils Leaching Potential of Metribuzin in Agricultural Soils. Int. J. Chem. Stud. 2017, 5, 1459–1462. [Google Scholar]
- Harper S. S. Sorption of Metribuzin in Surface and Subsurface Soils of the Mississippi Delta Region. Weed Sci. 1988, 36, 84–89. 10.1017/s004317450007449x. [DOI] [Google Scholar]
- Kah M.; Brown C. D. Adsorption of Ionisable Pesticides in Soils. Rev. Environ. Contam. Toxicol. 2006, 188, 149–217. 10.1007/978-0-387-32964-2_5. [DOI] [PubMed] [Google Scholar]
- Majumdar K.; Singh N. Effect of Soil Amendments on Sorption and Mobility of Metribuzin in Soils. Chemosphere 2007, 66, 630–637. 10.1016/j.chemosphere.2006.07.095. [DOI] [PubMed] [Google Scholar]
- Maqueda C.; Villaverde J.; Sopeña F.; Undabeytia T.; Morillo E. Effects of Soil Characteristics on Metribuzin Dissipation Using Clay-Gel-Based Formulations. J. Agric. Food Chem. 2009, 57, 3273–3278. 10.1021/jf803819q. [DOI] [PubMed] [Google Scholar]
- Pintado S.; Montoya M. R.; Rodríguez Mellado J. M. Imidazolinone Herbicides in Strongly Acidic Media: Speciation and Electroreduction. C. R. Chim. 2011, 14, 957–962. 10.1016/j.crci.2011.01.004. [DOI] [Google Scholar]
- Rashidipour M.; Maleki A.; Kordi S.; Birjandi M.; Pajouhi N.; Mohammadi E.; Heydari R.; Rezaee R.; Rasoulian B.; Davari B. Pectin/Chitosan/Tripolyphosphate Nanoparticles: Efficient Carriers for Reducing Soil Sorption, Cytotoxicity, and Mutagenicity of Paraquat and Enhancing Its Herbicide Activity. J. Agric. Food Chem. 2019, 67, 5736–5745. 10.1021/acs.jafc.9b01106. [DOI] [PubMed] [Google Scholar]
- FAO. APPENDIX 2 Parameters of pesticides that influence processes in the soil The parameters used to characterize the properties of pesticides are: https://www.fao.org/3/X2570E/X2570E06.htm (accessed Oct 25, 2021).
- USEPA.Guidance for Reporting on the Environmental Fate and Transport of the Stressors of Concern in Problem Formulations. http://www.epa.gov/pesticides/science/efed/policy_guidance/team_authors/endangered_species_reregistration_workgroup/esa_reporting_fate.pdf (accessed Oct 25, 2021).
- IBAMA. Avaliação ambiental para registro de agrotóxicos, seuscomponentes e afins de uso agrícola. http://www.ibama.gov.br/agrotoxicos/avaliacao-ambiental/avaliacao-ambiental-para-registro-de-agrotoxicos-seus-componentes-e-afins-de-uso-agricola (accessed Oct 25, 2021).
- Mamy L.; Barriuso E. Desorption and Time-Dependent Sorption of Herbicides in Soils. Eur. J. Soil Sci. 2007, 58, 174–187. 10.1111/j.1365-2389.2006.00822.x. [DOI] [Google Scholar]
- Pohlmann A. R.; Fonseca F. N.; Paese K.; Detoni C. B.; Coradini K.; Beck R. C.; Guterres S. S. Poly(ε-Caprolactone) Microcapsules and Nanocapsules in Drug Delivery. Expert Opin. Drug Delivery 2013, 10, 623–638. 10.1517/17425247.2013.769956. [DOI] [PubMed] [Google Scholar]
- Joshi A. S.; Gahane A.; Thakur A. K. Deciphering the Mechanism and Structural Features of Polysorbate 80 during Adsorption on PLGA Nanoparticles by Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy. RSC Adv. 2016, 6, 108545–108557. 10.1039/c6ra07699h. [DOI] [Google Scholar]
- Fernández-Pérez M.; Villafranca-Sánchez M.; Flores-Céspedes F.; Pérez-García S.; Daza-Fernández I. Prevention of Chloridazon and Metribuzin Pollution Using Lignin-Based Formulations. Environ. Pollut. 2010, 158, 1412–1419. 10.1016/j.envpol.2009.12.040. [DOI] [PubMed] [Google Scholar]
- Maqueda C.; Villaverde J.; Sopeña F.; Undabeytia T.; Morillo E. Novel System for Reducing Leaching of the Herbicide Metribuzin Using Clay-Gel-Based Formulations. J. Agric. Food Chem. 2008, 56, 11941–11946. 10.1021/jf802364t. [DOI] [PubMed] [Google Scholar]
- Oliveira H. C.; Stolf-Moreira R.; Martinez C. B. R.; Grillo R.; De Jesus M. B.; Fraceto L. F. Nanoencapsulation Enhances the Post-Emergence Herbicidal Activity of Atrazine against Mustard Plants. PLoS One 2015, 10, e0132971 10.1371/journal.pone.0132971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vítek P.; Veselá B.; Klem K. Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging. Sensors 2020, 20, 1015. 10.3390/s20041015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglino B.; Grinzato A.; Pagliano C. Binding Properties of Photosynthetic Herbicides with the Qb Site of the D1 Protein in Plant Photosystem Ii: A Combined Functional and Molecular Docking Study. Plants 2021, 10, 1501. 10.3390/plants10081501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwani S. I.; Arif I. A.; Khan H. A.. Modes of Action of Different Classes of Herbicides. In Herbicides, Physiology of Action, and Safety, Kelton J.; Sarunite L., Eds.; IntechOpen: London, 2014; Vol. 1, pp 165–185. [Google Scholar]
- Zhu Z. J.; Wang H.; Yan B.; Zheng H.; Jiang Y.; Miranda O. R.; Rotello V. M.; Xing B.; Vachet R. W. Effect of Surface Charge on the Uptake and Distribution of Gold Nanoparticles in Four Plant Species. Environ. Sci. Technol. 2012, 46, 12391–12398. 10.1021/es301977w. [DOI] [PubMed] [Google Scholar]
- Li J.; Tappero R. V.; Acerbo A. S.; Yan H.; Chu Y.; Lowry G. V.; Unrine J. M. Effect of CeO2 Nanomaterial Surface Functional Groups on Tissue and Subcellular Distribution of Ce in Tomato (Solanum Lycopersicum). Environ. Sci. Nano 2019, 6, 273–285. 10.1039/c8en01287c. [DOI] [Google Scholar]
- Ma X.; Quah B. Effects of Surface Charge on the Fate and Phytotoxicity of Gold Nanoparticles to Phaseolus Vulgaris. J. Food Chem. Nanotechnol. 2016, 2, 57–65. 10.17756/jfcn.2016-011. [DOI] [Google Scholar]
- Ristroph K. D.; Astete C. E.; Bodoki E.; Sabliov C. M. Zein Nanoparticles Uptake by Hydroponically Grown Soybean Plants. Environ. Sci. Technol. 2017, 51, 14065–14071. 10.1021/acs.est.7b03923. [DOI] [PubMed] [Google Scholar]
- Spielman-Sun E.; Lombi E.; Donner E.; Howard D.; Unrine J. M.; Lowry G. V. Impact of Surface Charge on Cerium Oxide Nanoparticle Uptake and Translocation by Wheat (Triticum Aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. 10.1021/acs.est.7b00813. [DOI] [PubMed] [Google Scholar]
- Prasad A.; Astete C. E.; Bodoki A. E.; Windham M.; Bodoki E.; Sabliov C. M. Zein Nanoparticles Uptake and Translocation in Hydroponically Grown Sugar Cane Plants. J. Agric. Food Chem. 2018, 66, 6544–6551. 10.1021/acs.jafc.7b02487. [DOI] [PubMed] [Google Scholar]
- Wu J.; Zhai Y.; Monikh F. A.; Arenas-Lago D.; Grillo R.; Vijver M. G.; Peijnenburg W. J. G. M. The Differences between the Effects of a Nanoformulation and a Conventional Form of Atrazine to Lettuce: Physiological Responses, Defense Mechanisms, and Nutrient Displacement. J. Agric. Food Chem. 2021, 69, 12527–12540. 10.1021/acs.jafc.1c01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Choudhary A.; Kaur H.; Mehta S.; Husen A. Smart Nanomaterial and Nanocomposite with Advanced Agrochemical Activities. Nanoscale Res. Lett. 2021, 16, 156 10.1186/s11671-021-03612-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari G.; Kalita S.; Hussain Z.; Deka N. C.; Meena B. R. Nanoencapsulation to Control Herbicide Residues and Resistances: A Review. Asian J. Microbiol., Biotechnol. Environ. Sci. 2021, 23, 140–148. [Google Scholar]
- Peppas N. A.; Korsmeyer R. W. Dynamically Swelling Hydrogels in Controlled Release Applications. Hydrogels Med. Pharm. 1986, 1987, 109–136. [Google Scholar]
- Siepmann J.; Peppas N. A. Modeling of Drug Release from Delivery Systems Based on Hydroxypropyl Methylcellulose (HPMC). Adv. Drug Delivery Rev. 2012, 64, 163–174. 10.1016/j.addr.2012.09.028. [DOI] [PubMed] [Google Scholar]
- Hayashi T.; Kanbe H.; Okada M.; Suzuki M.; Ikeda Y.; Onuki Y.; Kaneko T.; Sonobe T. Formulation Study and Drug Release Mechanism of a New Theophylline Sustained-Release Preparation. Int. J. Pharm. 2005, 304, 91–101. 10.1016/j.ijpharm.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Cooperation and Development. Test No. 307: Aerobic and Anaerobic Transformation in Soil. 2002, (No. April), , 1–17.
- Bartha R.; Pramer D. Features of a Flask and Method for Measuring the Persistence and Biological Effects of Pesticides in Soil. Soil Sci. 1965, 100, 68–70. 10.1097/00010694-196507000-00011. [DOI] [Google Scholar]
- USEPA. Fate, Transport and Transformation Test Guidelines OPPTS 835.3300 Soil Biodegradation. 1998, (No. January), , p 10.
- Sherma J. Recent Advances in Thin-Layer Chromatography of Pesticides. J. AOAC Int. 1999, 82, 48–53. 10.1093/jaoac/82.1.48. [DOI] [PubMed] [Google Scholar]
- OECD Guidelines for the Testing of Chemicals: Adsorption - Desorption Using a Batch Equilibrium Method. 2000, pp 1–45.
- OECD Guidelines for the Testing of Chemicals: Leaching in Soil Columns. 2004, (No. April), , pp 1–15.
- Oliveira N. M.; de Medeiros A. D.; Nogueira M. d. L.; Arthur V.; Mastrangelo T. d. A.; Barboza da Silva C. Hormetic Effects of Low-Dose Gamma Rays in Soybean Seeds and Seedlings: A Detection Technique Using Optical Sensors. Comput. Electron. Agric. 2021, 187, 106251. 10.1016/j.compag.2021.106251. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.