Summary
The modern management of rectal cancers continues to evolve. With the release of data from new landmark randomized controlled trials (RAPIDO, PRODIGE-23), total neoadjuvant therapy (TNT) has moved to the forefront of locally advanced rectal cancer treatment and is considered a standard option in selected patients. Total neoadjuvant therapy promises enhanced systemic disease control, better treatment adherence and less time with an ostomy. However, TNT as currently described encompasses a number of different potential treatment options that differ significantly in terms of their radiation dosage, chemotherapy regimen and order of treatments administered. Being familiar with TNT regimens will be important for rectal cancer surgeons to appropriately advocate for their patients and optimize their outcomes. This article serves as a primer for the general surgeon and offers a pragmatic overview of the indications, realistic expected benefits and potential downsides of each TNT regimen.
For 15 years, the standard of care for locally advanced (clinical T3–4 or N-positive) rectal cancer in North America has consisted of neoadjuvant therapy (radiation or chemoradiotherapy) followed by total mesorectal excision, with systemic chemotherapy usually thereafter.1 The aim of preoperative therapy is to downstage or downsize tumours; it improves locoregional control, but distant metastases remain a concern. To address systemic relapse, several recent trials have been conducted in which systemic chemotherapy is applied earlier in the rectal cancer treatment sequence.
The term “total neoadjuvant therapy” (TNT) was developed and applied to a number of new multimodal treatment strategies for locally advanced rectal cancer that administer both radiation and systemic therapy before surgical resection. These strategies can vary substantially in their radiation dosages and fractionations, chemotherapy options and sequencing of modalities, but what they have in common is the administration of both radiation and full systemic chemotherapy (as opposed to lower radio-sensitizing chemotherapy doses) in the neoadjuvant setting, before surgery. Promising results released from recent landmark prospective randomized controlled trials (RCTs) show that TNT may improve disease-free survival (DFS), pathologic complete response (pCR) rates and chemotherapy completion rates.1,2 However, definitive curative-intent treatment still depends on complete surgical resection as standard treatment. Therefore, it is paramount that surgeons possess a detailed understanding of these new approaches in order to recognize when to advocate for TNT, when it is not needed and when it may be inappropriate. This article serves as an overview of the latest trials in TNT for rectal cancer treatment, to help guide surgeons in these discussions.
Evidence for TNT
Table 1 lists RCTs that used TNT for rectal cancers, and their results. The Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation (RAPIDO) trial showed TNT had lower disease-related treatment failure (hazard ratio [HR] 0.75, 95% confidence interval [CI] 0.60–0.95; p = 0.019) and distant metastatic disease (HR 0.69, 95% CI 0.54–0.90; p = 0.0048) at 3 years, compared with conventional neoadjuvant treatment. More patients treated with TNT also had a pCR (28.4 %; TNT v. 14.3%; standard, p < 0.0001), and 85% of patients treated with TNT completed their chemotherapy, compared with 67% of patients in the standard treatment arm.2
Table 1.
Landmark total neoadjuvant therapy randomized controlled trials
| Study (year) | Rectal cancers included | Treatment arms (no. of cycles) | N | Survival outcomes (%) | pCR (%) | Completed chemotherapy (%) | Received surgery (%) | R0 resection (%) | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| PRODIGE-23 (2020)1 | cT3 or cT4 | Standard: 5FU-CRT→Surgery | 230 | DFS: 69* OS: 88 |
12.1* | 75 | 89 | 94 | 46.5 |
| Induction: FOLFIRINOX(6)→ 5FU-CRT→Surgery | 231 | DFS: 76* OS: 91 |
27.8* | 81 | 94 | 95 | |||
| RAPIDO (2020)2 | cTa-b, or EMVI+, CN2, MRF+, or LPLN+ | Standard: 5FU-CRT→Surgery→ ±FOLFOX(12)/CAPOX(8) | 450 | DRTF: 30* OS: 89 |
14.3* | 66† | 89 | 90 | 54 |
| Consolidation: SCRT→FOLFOX(9)/ CAPOX(6)→Surgery Standard: 5FU/ OX-CRT→Surgery | 462 | DRTF: 24* OS: 89 |
28.4* | 85 | 92 | 90 | |||
| POLISH II (2019)3 | Primary or locally recurrent cT4, or a palpable fixed cT3 | Standard: 5FU/OX-CRT→Surgery | 254 | DFS: 43 OS: 49 |
12 | NR | 92 | 71 | 84 |
| Consolidation: SCRT→FOLFOX(3)→Surgery | 261 | DFS: 41 OS: 49 |
16 | 99 | 93 | 77 | |||
| STELLAR (2022)4 | Primary cT3–4 or N+ in mid to distal rectum | Standard: CAPE-CRT→Surgery→CAPOX(6) | 293 | DFS: 62 OS: 75* |
12.3* | 58 | 78.5 | 87.8 | 35 |
| Consolidation: SCRT→CAPOX(4) →Surgery→CAPOX(2) | 298 | DFS: 65 OS: 87* |
21.8* | 59 (98 neoadjuvant only) | 78.9 | 91.5 | |||
| CAO/ARO/ AIO-12 (2019)6 | cT3 if < 6 cm from AV; > cT3b if 6–12 cm from AV; cT4, or LPLN+ | Consolidation: 5FU/OX-RT→FOLFOX(3)→Surgery | 156 | NR | 25 | 85 | 91 | 90 | NR |
| Induction: FOLFOX(3)→ 5FU/OX-CRT→Surgery | 150 | NR | 17 | 92 | 95 | 92 | |||
| OPRA (2020)7 | AJCC stage III–IV | Consolidation: 5FU-CRT→ FOLFOX/CAPOX(4 mo)→Surgery | 155 | DFS: 77 DMFS: 83 |
NR | NR | 42*‡ | NR | 25 |
| Induction: FOLFOX/ CAPOX(4 mo)→5FU-CRT→Surgery | 152 | DFS: 78 DMFS: 81 |
NR | NR | 57*‡ | NR |
5FU = fluorouracil; AJCC = American Joint Committee on Cancer; AV = anal verge; CAO/ARO/AIO-12 = Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy trial; CAPOX = capecitabine combined with oxaliplatin; CAPE = capecitabine; CRT = chemoradiotherapy; DFS = disease-free survival; DMFS = distant metastasis-free survival; DRTF = disease-related treatment failure; EMVI+ = extramural vascular invasion; FOLFIRINOX = leucovorin calcium (folinic acid), fluorouracil, irinotecan hydrochloride and oxaliplatin; FOLFOX = leucovorin calcium (folinic acid), fluorouracil and oxaliplatin; LPLN+ = lateral pelvic lymph node involvement; MRF+ = mesorectal fascia involvement; NR = not reported; OPRA = Organ Preservation of Rectal Adenocarcinoma study; OS = overall survival; OX = oxaliplatin; pCR = pathologic complete response; PRODIGE-23 = Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer; RAPIDO = Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation trial; SCRT = short-course radiotherapy; STELLAR = Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer.
Indicates statistically significant outcome (p < 0.05).
Percentage of patients offered adjuvant chemotherapy who completed the course.
Inverse of organ preservation rate reported.
Similarly, the Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer (UNI-CANCER-PRODIGE-23) trial showed superior 3-year DFS compared with standard neoadjuvant chemoradiation (HR 0.69, 95% CI 0.49–0.097; p = 0.034).1 Three-year metastasis-free survival was also better in the TNT arm (HR 0.69, 95% CI 0.54–0.90; p = 0.0048), and there were increased rates of pCR (27.8%; TNT v. 12.1%; standard, p < 0.001). Of patients treated with TNT, 81% completed their chemotherapy, compared with 75% in the standard arm.1 In both studies, most patients had surgery in each group, showing that neoadjuvant chemotherapy adverse effects did not subsequently preclude an operation.1,2 The results from these 2 large, high-quality trials showed that offering TNT with both radiation and full chemotherapy before resection led to significantly better treatment adherence rates and improved outcomes. These studies did not necessarily offer additional chemotherapy; rather, they changed when it was given, compared with standard treatment. Although these studies were not powered to demonstrate an overall survival (OS) benefit, these data are compelling and support adoption of TNT in clinical practice.
However, many questions arise from these studies. Both trials showed benefits over standard neoadjuvant therapy, but the order of chemotherapy, the chemotherapy drugs and the radiation fractionation and dosages all differed. RAPIDO used short-course radiation (5 × 5 Gy over 8 days) followed by FOLFOX (a combination chemotherapy regimen that includes leucovorin calcium [folinic acid], fluorouracil and oxaliplatin; 9 cycles) or CAPOX (capecitabine combined with oxaliplatin; 6 cycles) chemotherapy. Adjuvant chemotherapy was optional. PRODIGE-23 started with FOLFIRINOX chemotherapy (leucovorin calcium [folinic acid], fluorouracil, irinotecan hydrochloride and oxaliplatin; 6 cycles), followed by long-course radiation (50 Gy over 5 weeks). RAPIDO used a novel primary outcome, “disease-related treatment failure” (defined as first locoregional recurrence, distant metastasis, new primary tumour or death due to treatment), whereas PRODIGE-23 used a more standard DFS.1,2 The RAPIDO trial involved only “high-risk” patients (at least 1 of the following: T4, extramural venous invasion, N2, mesorectal fascia or lateral pelvic node involvement) compared with patients in PRODIGE-23 (T3 or T4). Therefore, direct comparison between these studies is challenging. Furthermore, long-term data from these trials are not yet available. The only large, high-quality randomized controlled TNT study with long-term follow-up is the Polish II trial, which showed no DFS or OS benefit compared with standard neoadjuvant therapy at 7 years. However, the Polish II study differed from the RAPIDO and PRODIGE-23 trials in that they used only 3 cycles of FOLFOX chemotherapy in the neoadjuvant setting.3 A fourth randomized trial comparing TNT with standard chemoradiotherapy is the Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR) trial, in which short-course radiation was followed by neoadjuvant CAPOX (4 cycles), surgery, then adjuvant CAPOX (2 cycles). In the TNT arm, 98% of patients completed their neoadjuvant CAPOX, with only 59% progressing through surgery on to adjuvant chemotherapy. Unlike RAPIDO and Polish II, this trial also had mandatory adjuvant chemotherapy in the comparison group, which only 58% of patients completed.4 The STELLAR trial also showed a 3-year OS benefit of TNT compared with standard treatment (86.5% v. 75.1%). However, early Polish II data had similar results, which disappeared in long-term follow-up.3 A recent meta-analysis of TNT phase 2 and 3 RCTs showed higher rates of pCR compared with standard neoadjuvant therapy.5 Given the heterogeneity of these data, long-term follow-up studies are needed to confirm whether TNT is beneficial in terms of OS and in which patient subgroup.
Sequence of TNT
Sequence of TNT regimens can be classified as induction (chemotherapy first) or consolidation (radiation first). However, it is unclear which TNT sequence is superior. Fortunately, the literature does contain some clues on how to select between treatment regimens. Chemotherapy timing in relation to preoperative radiation in TNT was examined specifically in the Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy (CAO/ARO/AIO-12) phase 2 RCT.6 All patients received the same chemotherapy (FOLFOX) and long-course chemoradiotherapy (50.4 Gy in 28 fractions) before surgery. Half of the patients received chemotherapy first and the other half received radiation first. Consolidation resulted in better adherence with radiation (97% v. 91%) but worse adherence with chemotherapy compared with induction (85% v. 92%). However, pCR was better (25%) in the consolidation group compared with induction (17%). Notably, however, survival and recurrence data are not yet available.
The Organ Preservation of Rectal Adenocarcinoma (OPRA) trial also examined chemotherapy timing in relation to preoperative radiation in TNT using long-course chemoradiotherapy and FOLFOX or CAPOX.7 Currently, the trial results are available only in abstract form, but preliminary data demonstrate equivalent adherence to chemotherapy (82% induction v. 81% consolidation), DFS (78% v. 77%, respectively; p = 0.90) and metastasis-free survival (81% v. 83%; p = 0.86). The objective of this trial was to determine whether patients with a complete or near-complete clinical response to TNT could be observed with nonoperative management instead of receiving surgery. Organ preservation rates were substantial in both arms, but were significantly higher in the consolidation arm compared with induction (58% v. 43%, respectively; p = 0.01).
Treatment selection
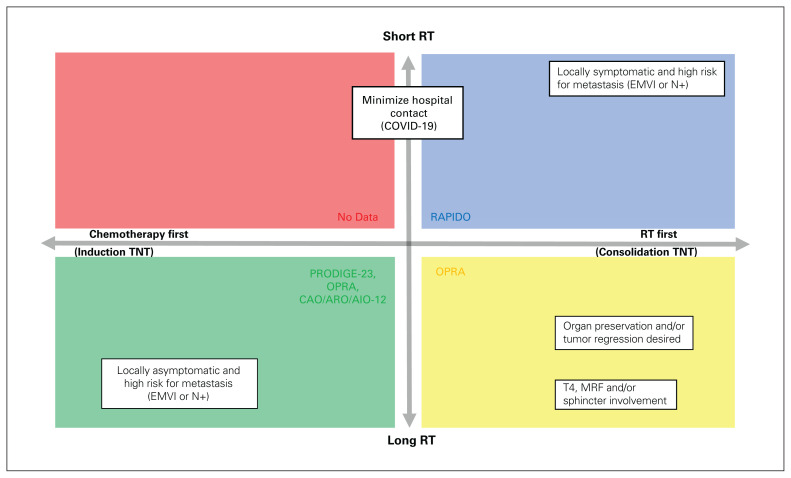
Given the evidence presented in these trials to this point, we can use the data presented to help select a TNT regimen most appropriate for a particular patient’s situation (Figure 1). In situations where more time for tumour regression is desired, a consolidation TNT regimen may be preferrable. Earlier radiation may also be offered for symptom relief for patients with tenesmus or bleeding from their cancer. Consolidation TNT may also be helpful if the patient becomes a candidate for organ preservation (i.e., low anterior resection instead of abdominal perineal resection). Following this approach, data from a large, nonrandomized phase 2 clinical trial of stage II–III rectal adenocarcinomas suggest that more chemotherapy doses and a longer interval between neoadjuvant chemoradiotherapy and surgery lead to higher pCR and DFS rates.8,9 The OPRA study authors even attempted to take all patients with a complete clinical response after consolidation TNT into a “watch-and-wait” approach. Can J Surg/J can chir 2023;66(2) E199 However, nonoperative management and surveillance is not currently considered standard of care, nor a recommended practice, and should be taken with caution or only in the context of a prospective trial. Conversely, induction TNT might be selected when earlier systemic control is a priority, such as tumours at increased risk for micrometastasis (e.g., lymph node involvement or extramural venous invasion). In reality, patients often present with multiple competing priorities — hence the importance of multidisciplinary discussion and patient-centred decision-making.
Fig. 1.
Decision-making matrix for considering neoadjuvant therapy for locally advanced rectal cancer. Radiation first can lead to standard neoadjuvant therapy or total neoadjuvant therapy depending on patient response and risk factors. CAO/ARO/AIO-12 = Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy trial; EMVI = extramural vascular invasion; MRF = mesorectal fascia; OPRA = Organ Preservation of Rectal Adenocarcinoma study; PRODIGE-23 = Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer; RAPIDO = Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation trial; RT = radiation therapy.
The choice between short- and long-course radiotherapy presents an additional important decision in the context of TNT. Short-course radiation means fewer hospital visits, which patients living at a distance from radiation treatment sites may prefer. Short-course radiation may also be a better option when given before chemotherapy for patients at higher risk of systemic disease, such as those in the RAPIDO trial.2 Patients who are symptomatic and at high risk of metastasis may want to receive short-course radiation first to treat their symptoms, but then move on quickly to chemotherapy, to receive the benefits of early systemic control as soon as possible. However, although both long- and short-course radiotherapy (with delay) can lead to downstaging, response rates are higher and more predictable with long-course. We favour long-course chemoradiotherapy consistent with the OPRA trial regimen for patients with T4b tumours, threatened or involved mesorectal fascia, or those with low rectal tumours who want to maximize their chances of organ preservation.7 Conversely, short-course radiation was not used in the “induction” TNT trials, so evidence is lacking using that treatment sequence.1,6,7
Surgery in patients treated with TNT
The time interval from radiation completion to surgery in a TNT protocol is much longer than the traditional 6–12 weeks, which has the potential to affect the quality of the surgical procedure. This has not resulted in worse perioperative outcomes in the experimental settings of the few studies to date, but it is unclear how this will bear out in routine clinical practice. R0 resection rates between experimental and control arms in the PRODIGE-23 and RAPIDO studies were not statistically different (95% in the experimental arm in PRODIGE-23 and 94% in the standard treatment group; 90% in both groups in the RAPIDO trial). Other important process measures potentially indicative of surgery difficulty — such as complete (v. incomplete) total mesorectal excision specimens, as well as postoperative complications — were also similar between TNT and standard of care arms in both studies.1,2 The PRODIGE-23 trial even measured intraoperative blood transfusion requirements, which were also similar between treatment arms.1 Anecdotally, the experience of patients at our own institution after TNT is that the perirectal tissues are more edematous and friable than those after conventional neoadjuvant chemoradiation. However, we have not noticed a reduction in our complete total mesorectal excision rates.
Remaining questions
Despite these exciting new studies in curative-intent management of rectal cancer, some additional treatment questions remain. For example, not all patients with locally advanced cancer require chemotherapy in the adjuvant setting.1 Therefore, providing chemotherapy preoperatively after a TNT regimen may be overtreating some patients. Magnetic resonance imaging can also overstage and unnecessarily commit some patients to chemotherapy toxicities.10 Furthermore, for reasons still poorly understood, some rectal cancers respond poorly to neoadjuvant treatment, and by pursuing TNT, we are delaying surgery, which may be the only effective treatment option.11 Finally, treatment toxicity may cause complications that can delay definitive surgery in some patients, although preliminary data suggest this is a rare occurrence.1,2,6
Given the existing evidence, it is currently impossible to predict which subset of patients with locally advanced rectal cancer will most benefit from chemotherapy and radiation. Future study will need to determine more effective prediction models to perhaps offer chemotherapy regimens selectively. Candidate examples include MRI-directed management,11 circulating tumour DNA12 or rectal cancer organoids.13
Conclusion
Surgeons have a fundamental role in the treatment of patients with rectal cancer. Although neoadjuvant or adjuvant treatments certainly can improve outcomes, definitive curative-intent treatment still depends on complete surgical resection as standard therapy. Patients with clinically advanced disease should be presented in multidisciplinary tumour boards for multimodality care with medical oncology, radiation oncology, radiology and pathology input. It is paramount that surgeons be active participants in these discussions. To do so effectively, a detailed understanding of these new approaches is required. Surgeons need to understand the treatments, benefits and risks of the approaches, and how they may affect their surgical procedure and their patients’ overall outcomes. We argue that TNT should be considered for (although not necessarily offered to) all patients with a clinically staged locally advanced rectal cancer (Box 1).
Box 1.
Key practice points
Surgeons should present patients with locally advanced rectal cancers at multidisciplinary tumour boards for multimodality care.
Total neoadjuvant therapy (TNT) means systemic chemotherapy and radiation before surgery.
Total neoadjuvant therapy should be considered (although not necessarily always given) for all locally advanced rectal cancers.
Indications for TNT include clinical T4 disease or close or involved circumferential resection margin, N2, lateral pelvic node involvement or extramural vascular invasion.
Total neoadjuvant therapy is associated with higher rates of pathologic complete response, better downstaging and downsizing, and improved chemotherapy completion rates over non-TNT approaches.
The current, rapidly evolving evidence supports TNT as a standard option for curative treatment of many locally advanced rectal cancers. Total neoadjuvant therapy leads to higher chemotherapy completion rates, pCR rates, improved DFS and decreased treatment-related failures. It should be most strongly considered in patients at high risk of local failure in whom a good local response is desired (clinical T4 disease, threatened or involved circumferential resection margin and lateral pelvic node involvement, and in those at high risk of distant failure, including N2 disease or extramural venous invasion). As surgeons, we must be aware of the potential implications of new treatment options, so we can advocate for our patients and collaborate effectively with our medical and radiation oncology colleagues and deliver the best and most effective curative surgery.
Footnotes
Competing interests: None declared.
Contributors’ statement: Garrett Johnson and Eric Hyun designed the study and acquired the data, which all of the authors analyzed. Garrett Johnson wrote the manuscript, which all of the authors revised critically for important intellectual content. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
References
- 1.Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCERPRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–15. [DOI] [PubMed] [Google Scholar]
- 2.Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29–42. [DOI] [PubMed] [Google Scholar]
- 3.Ciseł B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol 2019;30:1298–303. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, Tang Y, Hu C, et al. Multicenter, randomized, phase III Trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol 2022;40:1681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Jiang T, Xiao L, et al. Total Neoadjuvant Therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Oncologist 2021;26:e1555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol 2019;37:3212–22. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol 2020;38:4008. [Google Scholar]
- 8.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marco MR, Zhou L, Patil S, et al. Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum 2018;61:1146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillem JG, Díaz-González JA, Minsky BD, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol 2008;26:368–73. [DOI] [PubMed] [Google Scholar]
- 11.Stijns RCH, de Graaf EJR, Punt CJA, et al. Long-term oncological and functional outcomes of chemoradiotherapy followed by organsparing transanal endoscopic microsurgery for distal rectal cancer: the CARTS study. JAMA Surg 2019;154:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDuff SGR, Hardiman KM, Ulintz PJ, et al. Circulating tumor DNA predicts pathologic and clinical outcomes following neoadjuvant chemoradiation and surgery for patients with locally advanced rectal cancer. JCO Precis Oncol 2021;5:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganesh K, Wu C, O’Rourke KP, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med 2019;25:1607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]