Abstract
Microbial communities are shaped by positive and negative interactions ranging from competition to mutualism. In the context of the mammalian gut and its microbial inhabitants, the integrated output of the community has important impacts on host health. Cross-feeding, the sharing of metabolites between different microbes, has emergent roles in establishing communities of gut commensals that are stable, resistant to invasion, and resilient to external perturbation. In this review, we first explore the ecological and evolutionary implications of cross-feeding as a cooperative interaction. We then survey mechanisms of cross-feeding across trophic levels, from primary fermenters to H2 consumers that scavenge the final metabolic outputs of the trophic network. We extend this analysis to also include amino acid, vitamin and cofactor cross-feeding. Throughout, we highlight evidence for the impact of these interactions on each species’ fitness as well as host health. Understanding cross-feeding illuminates an important aspect of microbe-microbe and host-microbe interactions that establishes and shapes our gut communities.
Introduction
Microorganisms in nature do not typically exist in monoculture under constant conditions, but instead form complex communities that withstand fluctuating environments. The interactions between these microbes produce synergistic responses that often cannot be predicted by studying individual species on their own; competitive and antagonistic interactions restrict the growth of some members, while the combined metabolic activity of the community buffers against perturbation during nutrient stress. Many of these ecological dynamics are mediated by diffusible metabolites which can act as nutrient sources, inhibitory compounds, or signalling molecules.
The human body is an important habitat for microbial colonization, hosting ~100 trillion microbial symbionts1. Of the body sites that are colonized, microorganisms inhabiting the intestinal tract, or gut microbiome, are by far the most abundant and diverse, and include bacteria alongside lesser studied fungi, viruses, and other microbes. The gut microbiome is home to more than 1000 different bacterial species at the population level and at least 150 species in an individual2. These microbes have far-ranging impacts on health, determined by their encoded metabolic capacity and interactions with the host.
Cross-feeding is the exchange of metabolites as energy and nutrients among different species or strains of microorganisms3. In the context of the gut microbiome, metabolite sharing can also occur between host and microbe, though we will focus on microbe-microbe interactions in this review. Microbial cross-feeding is not unique to the gut microbiome and occurs in many, if not all, natural communities. Both anecdotal evidence and genome-scale analysis highlight the breadth of cross-feeding across microbial communities. For example, mapping interspecies metabolic exchanges predicted from genome sequences across 800 host-associated and free-living microbial communities identified co-occurring subcommunities of species with a significantly higher-than-chance potential for cross-feeding4. The observed high frequency of cross-feeding is consistent with division of labour in microbial communities, a common theme among all biological systems. Furthermore, ill-defined metabolic requirements that are provided through cross-feeding in a community setting may explain our difficulty culturing various microbes axenically5.
In addition to its pervasive presence, understanding cross-feeding in the context of the gut is important to understand the microbiome’s impact on host health. Cross-feeding is an important mechanism shaping community composition, response to perturbation, the community’s integrated metabolome, and together their emergent interactions that affect the host. In this review, we will describe the ecological forces and consequences of cross-feeding interactions, then explore cross-feeding mechanisms of different nutrients in the gut microbiome and their impact on host health.
Fitness impacts of cross-feeding
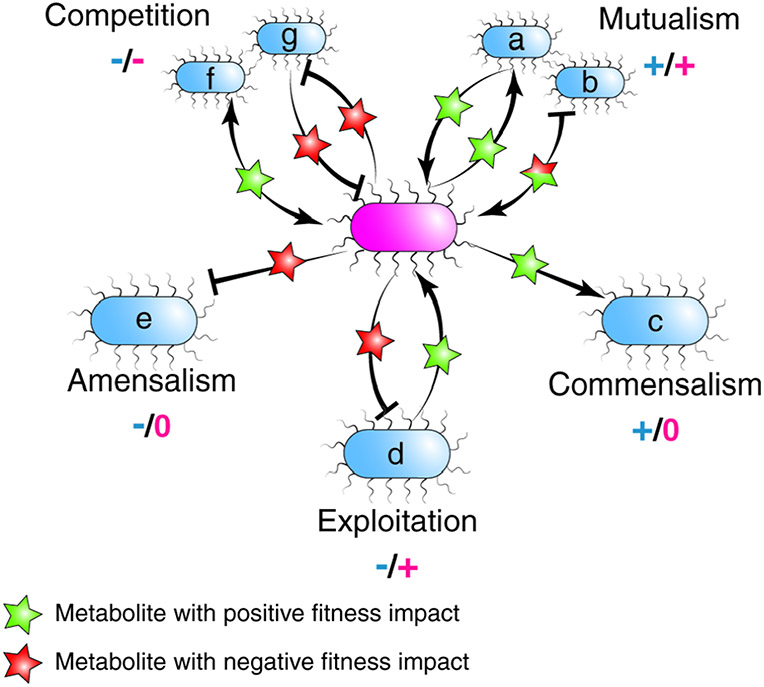
To understand the significance of cross-feeding in the gut microbiome, we first consider the role it plays in shaping community composition, stability, and resilience. Members of microbial communities, including those in the gut microbiome, interact with and impact the growth of other microbes in ways that can be classified in terms of the fitness of members involved (Figure 1)6. On the negative side, competition (−/−) lowers the fitness of both involved, while amensalism (−/0) harms one species while the other is unaffected. On the positive side, exploitation/parasitism/predation (+/−) benefits one member to the detriment of the other, commensalism (+/0) benefits one member and is neutral to the other, and mutualism (+/+) benefits both members. These interactions can occur both intra- and interspecifically, though cross-feeding is usually considered to be an interspecific interaction.
Figure 1. Landscape of microbe-microbe interactions.
Microbe-microbe interactions can result in positive (+) or negative (−) impacts on fitness of participating species. These interactions are often mediated by diffusible metabolites, shown here as stars. Cross-feeding can result in mutualism (a-b), commensalism (c) or exploitation (d). These interactions are often through the production of a metabolite by one species that benefits or harms another species (a, c, d, e, f, g), but can also occur when a harmful metabolite to one species is consumed by and benefits another species (b). Amensalism (e) and competition (f-g) can also occur through metabolite exchange, but do not represent cross-feeding interactions.
Cross-feeding in the gut microbiome can be exploitative, commensalistic or mutualistic (Figure 1a-d). Exploitation can occur when a consumer benefits from a substrate produced by a partner organism while also changing the environment in a way that harms this producer, such as by secreting a toxic waste product (Figure 1d). Alternatively, commensalistic cross-feeding results when one microbe feeds on metabolites produced by another microbe with no impact to the latter (Figure 1c).
Cross-feeding interactions that are mutualistic occur when two species each feed on a metabolite produced by the other (Figure 1a), or when one microbe feeds on a metabolite from another and also modifies the environment in a way that benefits the producer (Figure 1b). These cooperative interactions are termed syntrophy, classically defined as a form of “obligately mutualistic metabolism” where together the partners exploit substrates that neither could metabolize alone under a given set of conditions7. The quintessential example of syntrophy is interspecies H2 transfer (discussed in detail below). Other examples of syntrophy include reciprocal feeding on metabolites produced by partner microbes. For example, in the Drosophila melanogaster gut, Acetobacter pomorum feeds on lactate produced by Lactobacillus plantarum and in turn produces essential amino acids required for L. plantarum growth (discussed further in the amino acid cross-feeding section)8.
The community stability paradox
Understanding the extent of mutualistic cross-feeding interactions in the gut microbiome is fundamental to explaining some of its core characteristics: extraordinary diversity of species and remarkable stability in maintaining the same taxa over the course of years to decades9. In ecological theory, true syntrophic interactions lead to interacting species that “boom and bust” together, resulting in unstable, low diversity communities susceptible to invasion and characteristic of dysbiosis. Furthermore, cooperative interactions involving public goods, defined as compounds or functions released into the extracellular environment for a collective benefit10, are susceptible to promoting cheaters that utilize these compounds but do not contribute to their production and threaten to collapse cooperative behaviour. For comparison, competitive interactions can stabilize microbial communities by reducing the strength of mutualism and blocking any one species from outcompeting the rest of the community11. Different studies come to different conclusions about the abundance of cooperative cross-feeding in the gut microbiome; while some report that these communities are dominated by cooperative interactions3,12,13, others report that competition is the primary microbial interaction14,15. These discrepancies highlight two key questions: what is the true frequency of cooperative cross-feeding in the gut microbiome, and how do communities remain stable in the presence of cross-feeding interactions?
The answer to these questions likely lies with ecological forces in the gut that reductionist experiments and computational models fail to measure or predict. For example, measuring the frequency of cross-feeding interactions using pairwise measurements between species fails to account for higher order interactions with other species, computational models for gene content or metabolic flux often fail to consider gene regulation, and in vitro models typically neglect host factors. Furthermore, interactions between microbes can be communalistic or mutualistic depending on the context. For example, the same pair of cross-feeding strains can exhibit a range of interactions depending on nutrient availability, from competition in high nutrient conditions to obligate mutualism in low nutrient conditions16. In general, nutrient addition releases one or both species in a syntrophic interaction from dependence on the other, shifting mutualistic interactions to either commensalistic or parasitic17. Such parasitic interactions can result in uncontrolled growth of the faster-growing species and exclusion of the slow-grower17.
By considering these complexities, multiple hypotheses for how cooperation stably exists in the gut microbiome consider the contextual details of cross-feeding interactions11. These explanations converge on two mechanisms that prevent cooperative interactions from forming run-away positive feedback loops: (1) blocking the overgrowth of cooperative species and (2) weakening the strength of cooperative interactions so that coupling between species is dampened. Mechanisms that block overgrowth of cooperative species include the presence of competitive interactions and host-mediated limitations on microbial growth such as immune regulation. Alternatively, the strength of cooperative cross-feeding can be dampened by spatial structure, which increases physical distance between interacting partners and increases the likelihood that sharing occurs between clonemates rather than through cross-feeding. Functional redundancy can also replace a single strong cooperative interaction with several weak ones11. Future studies that begin to account for these unseen factors will help to provide better understanding of the ecology of our microbiome and the role that cross-feeding plays in driving broader community dynamics.
Why and how does cross-feeding occur?
Why and how do microbes release public goods? As to the “why” of it, we must first address whether cross-feeding is an evolved trait or a serendipitous occurrence. In some cases, there is evidence that bacteria are adapted to rely on mutualistic cross-feeding interaction. For example, syntrophic bacteria involved in interspecies H2 transfer often have highly reduced genomes, lacking key components for anaerobic respiration but specialized towards their given role in the interaction (see below for discussion on H2 transfer)18. In another example, a recent paper suggests that certain gut bacteria secrete enzymes for polysaccharide digestion for the benefit of a cross-feeding species, which in turn provides a different benefit to the former organism19. As described below, the release of public goods can also be serendipitous as a result of cell lysis, overflow metabolism, or other processes.
As to the “how” of public good secretion, there are a handful of proposed mechanisms. High molecular weight polysaccharides and proteins are usually degraded outside of the cell and release sugars available for others. Intracellular metabolites may be released through cell lysis, for example through the action of host or microbial toxins, starvation or lytic phages. Indeed, lytic phage have been shown to play a role in nutrient release in both natural and laboratory settings20,21.
Apart from cell lysis, intracellular metabolites can also be released from live cells. Sometimes this may occur by free diffusion across lipid membranes or ‘accidental’ leakage through transporters. In some cases, overflow metabolism may contribute to the availability of intracellular metabolites to neighbouring bacteria. This phenomenon is based on metabolic models that optimize growth rate, rather than efficiency, under a given nutrient environment22. During overflow metabolism, growth rate under high resource supply is faster using fermentation (thereby secreting acetate) than aerobic respiration22. Overflow metabolism has been largely studied in E. coli but is also observed in the gut symbiont Bacteroides thetaiotaomicron, which secretes acetate, formate, succinate and propionate as major metabolic by-products23. These metabolites are all easily derived from fermentative glycolysis by-products and their accumulation is predicted from metabolic models that allow overflow metabolism.
Another hypothesis for metabolite leakage is that under starvation, cells secrete non-degradable metabolites, such as some amino acids, in order to prevent their toxic buildup24. Alternatively, in a phenomenon called “noise-averaging cooperation”, noisy regulation of metabolic enzymes at the single cell level causes a subset of cells to excrete metabolites that are able to be used by their genetically identical kin25. This sharing of metabolites allows related bacteria to “average out” their communal metabolism and together improve collective growth. However, in a diverse community these metabolites are available to other species and can result in interspecies cross-feeding25. Finally, metabolic flux analysis suggests that secretion of many metabolic by-products is ‘costless’ to the producer under certain conditions, depending on which nutrients are limiting and the carbon-nitrogen ratio in the cell26. In this setting, there would be no selective pressure to prevent release of these metabolites from the cell.
An outline of cross-feeding in the gut microbiome
Metabolites that undergo cross-feeding in the microbiome can broadly be classified into two groups: those used directly in central metabolism (sugars and electron donors/acceptors), and those that are essential nutrients and require biosynthesis or uptake (amino acids, cofactors, vitamins).
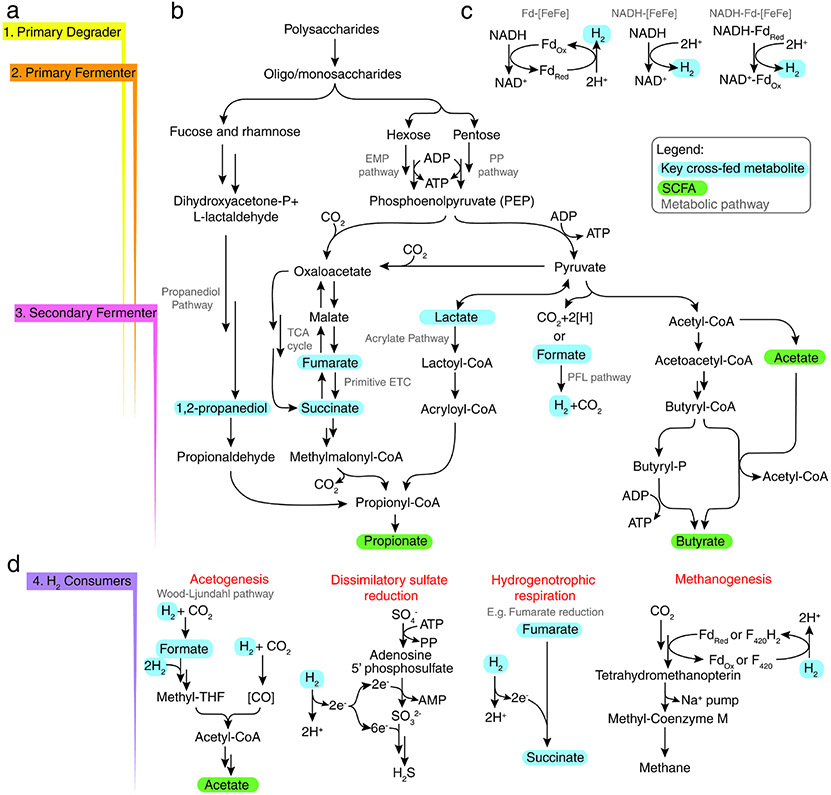
In terms of central metabolism, cross-feeding establishes trophic levels (Figure 2a), where primary degraders (first trophic level) with specialized machinery hydrolyze complex polysaccharides, releasing oligo- and monosaccharides accessible to other species. Primary fermenters (second trophic level) liberate these sugars themselves or acquire them from other microbes, and funnel these sugars through glycolysis, producing phosphoenolpyruvate (PEP) that is used for substrate level phosphorylation and generating organic acids (e.g. formate, acetate, succinate) or alcohols (e.g. 1,2-propanediol) (Figure 2b). Next, secondary fermenters (third trophic level) can make use of these by-products in various fermentative or respiratory pathways of their own, generating short chain fatty acids (SCFA: acetate, butyrate, propionate). Finally, molecular hydrogen (H2) produced by primary/secondary fermenters (Figure 2c) serves as an electron donor for sulfate reducing bacteria (SRB), methanogens and acetogens (fourth trophic level; Figure 2d).
Figure 2. Central metabolism pathways involved in cross-feeding.
An overview of central metabolism pathways involved in cross-feeding interactions. (a) Flags along the left show how the pathways in (b) roughly divide into four trophic levels through cross-feeding. Key cross-fed intermediates released by primary degraders/fermenters, highlighted in blue, can be released and utilized by secondary fermenters ultimately for the production of SCFA, highlighted in green. Throughout, H2 is produced in one of two ways: first, the Pyruvate-Formate Lyase (PFL) pathway that splits formate into H2 and CO2, and second through the oxidation of NADH. (c) NADH oxidation can be coupled to ferredoxin reduction and reoxidation by ferredoxin (Fd)-dependent hydrogenase (Fd-[FeFe]), directly by an NADH-dependent hydrogenase (NADH-[FeFe]), or directly by a bifurcating NADH-Fdred-dependent hydrogenase (NADH-Fdred-[FeFe]). (d) H2 is consumed by various types of metabolism, indicated in red. Pathway stoichiometry is not represented in this figure.
Given the complexity of intestinal biogeography, does the gut microbiome truly function in trophic levels? In support of the notion, some trophic roles are mutually exclusive; for example, no known microbes are both primary degraders and methanogens27. Furthermore, while many bacteria encode the potential to fill multiple trophic roles (e.g., many acetogens also encode extracellular polysaccharide hydrolases), this potential is not realized as bacteria shift to specific trophic roles based on the environment and community context28. Correlational analysis of human microbiome data also identifies trophic networks29, as does in situ modelling30. While such modelling does not account for all contributing parameters, a framework dictated by trophic levels allows for coarse-grained understanding of community function that is too complex to achieve using fine-grained approaches focused on single species30.
First trophic level: Primary degraders sharing sugar in the gut
While there are examples of primary degraders across diverse bacterial phyla in the gut, members of the order Bacteroidales are central to the carbon food web31. These bacteria exist at densities ranging 109-1010 CFU/g feces and are comprised of three dominant genera: Bacteroides, Parabacteroides and Prevotella. The primary niche of Bacteroidales in the gut is the degradation of plant polysaccharides that escape digestion by human enzymes in the small intestine. Genes required for growth on a particular polysaccharide are encoded in gene clusters termed polysaccharide utilization loci (PUL), which may include surface proteins that bind polysaccharides (canonically SusD), surface-associated enzymes that cleave them (glycoside hydrolases/polysaccharide lyases, GH/PL, canonically SusG), transporters for import (canonically SusC), and periplasmic GH for degradation into monosaccharides32. Since different PULs are specific to different substrates, each species’ capabilities depend on the PULs it encodes. The presence of surface exposed GH, usually encoding N-terminal signal peptidase II sequences for lipoprotein attachment, allows a species to produce public goods33. These surface exposed GH/PL can also be secreted in outer membrane vesicles based on their lipoprotein export sequence33,34. In this way, outer membrane vesicles can themselves act as public goods.
Due to their role as primary degraders, Bacteroides spp. are often considered keystone species in a community with disproportionately large impacts that ripple throughout the ecosystem35. Taking it a step further, PULs themselves are important drivers of community composition. For example, various PUL mutants in Bacteroides uniformis support significantly lower growth of cross-feeding firmicute butyrate producers, and thus lower butyrate production36.
It could be tempting to draw a simple model where PUL presence predicts the availability of cross-fed substrates and thus the abundance of secondary fermenters that rely on these substrates. However, empirical data demonstrates that whether cross-feeding occurs depends on the glycan and interacting species involved. There are two factors that contribute to this specificity. The first is the ability of recipient species to import and metabolize the mono- or oligosaccharides derived from the targeted polysaccharide, which requires the necessary machinery to utilize the released sugars. The second factor is how the primary degrader metabolizes the polysaccharide. Different species encoding the same PULs release different types, amounts and proportions of mono/oligosaccharides. For example, while Bacteroides caccae, B. ovatus, B. uniformis and B. thetaiotaomicron all metabolize inulin, different amounts of oligosaccharides versus fructose are released33. Primary degraders can also operate through either selfish or unselfish mechanisms. While unselfish mechanisms produce public goods, selfish metabolism utilizes minimal extracellular hydrolysis to prevent loss to other microbes. Yeast α-mannan degradation by B. thetaiotaomicron is one example of the latter, where surface exposed endo-α-mannosidases mediate infrequent cleavage of the mannan backbone, producing relatively large oligosaccharides that are imported for periplasmic endo- and exo-α-1,6-mannanases37. Similarly, B. ovatus adopts a selfish strategy for hemicellulose degradation, but ‘unselfish’ strategies for degradation of other xylooligosaccharides38. Usually, extracellular polysaccharide digestion that provides public goods to other species is thought to be commensalistic or exploitative, i.e. it does not benefit the primary degrader. However, in one specific example a mutualistic interaction has been proposed: extracellular digestion of inulin by GH secreted by Bacteroides ovatus is dispensable for its own usage of the fiber yet allows Bacteroides vulgatus to feed on the liberated oligo/monosaccharides19. B. vulgatus in turn enhances the growth of B. ovatus through an unknown mechanism. The authors propose that this reciprocal benefit provides selection to maintain these extracellular hydrolases in B. ovatus.
There is a plethora of other substrates and species involved in cross-feeding polysaccharides in the gut microbiome. One notable interaction contributing to disease is cross-feeding of host-derived sialic acid between Bacteroides spp. and enteric pathogens including E. coli, Salmonella enterica serovar Typhimurium (S. Typhimurium) and Clostridium difficile39,40. Bacteroides spp. sialidase activity, which liberates monomers from glycoproteins and oligomers, promotes pathogen expansion in both colitis and post-antibiotic infection models39,40. Other cross-fed substrates include arabinogalactan41, inulin42, pectin43, xantham gum44, and xylitol45. Further characterization of the genes and enzymes responsible for metabolizing these complex substrates and the species that utilize the liberated substrates will allow for greater understanding of specificity during polysaccharide cross-feeding.
Second and third trophic levels: Cross-feeding fermentation by-products
After liberation by primary degraders, energy from sugars is harvested through the combined action of members of the second and third trophic levels. Collectively, sugars are funnelled into one of three upper pathways (Entner-Doudoroff; Embden-Meyerhof-Parnas [glycolysis]; Pentose Phosphate) to produce PEP which is directed through various lower fermentative pathways and ultimately generates short chain fatty acids (SCFA: butyrate, propionate, acetate) and gases (CO2, H2). Key intermediates are generated from PEP (Figure 2b)45,46. First, PEP can be used to produce fumarate, which acts as a terminal electron acceptor during anaerobic respiration, producing succinate which is subsequently decarboxylated to propionate52. Second, PEP can be converted to pyruvate through substrate level phosphorylation, and subsequently fermented to propionate via lactate (acrylate pathway), propionate via succinate, or butyrate via acetyl-CoA51. Third, acetate is a key intermediate produced via acetyl-CoA hydrolysis or by acetogens using the Wood-Ljungdahl pathway, and used in an alternate pathway for butyrate production using butyryl-CoA:acetate CoA-transferase (acetyl-CoA pathway)47. Finally, deoxy-sugar fermentation (i.e. fucose and rhamnose) produces propionate through the propanediol utilization pathway and the key intermediate 1,2-propanediol45.
Individual cells do not usually ferment sugar all the way to SCFAs, but instead secrete intermediates as a result of overflow metabolism. Which intermediates are released depends on factors that optimize energy harvest and growth rate, including CO2/H2 partial pressures and redox balance (NAD+/NADH) in the cell46. These intermediates are taken up by other cells and fermentation is completed, producing SCFA. The net outcome of this cross-feeding is that the three major SCFA (acetate, propionate, butyrate) accumulate to high concentrations in the gut, peaking in the proximal colon, while other fermentation intermediates (e.g. fumarate, lactate, succinate, 1,2-propanediol) do not48. Acetate is produced by many bacteria while propionate and butyrate production is less widespread; for example, Lachnospiraceae (e.g. Anaerostipes, Roseburia, Eubacterium), and Faecalibacterium prausnitzii are prominent butyrate producers while Bacteroides spp., Negativicutes and some Clostridium are major propionate producers45,47.
For many species, cross-feeding fermentative intermediates is an integral part of their lifestyle in the gut. Acetate cross-feeding is particularly important, since it is consistently the most abundant SCFA in the gut across populations48, and substrate for production of butyrate, a physiologically important SCFA in human health49. Underscoring its importance, acetate is also required for growth of most butyrogenic bacteria50,51. Lactate is used to a lesser degree, supporting ~20% of all butyrate production in the gut, in a pathway that first requires oxidation to pyruvate (Figure 2b)52,53. Growing cross-feeding species together often results in higher butyrate levels, growth rate and/or biomass of each species compared to these species cultured alone. For example, Bifidobacterium spp. ferment fructooligosaccharides to produce acetate and lactate, which is used by butyrate producing firmicutes including F. prausnitzii, Roseburia, and Eubacterium spp.47,54. Fructooligosaccharide supplementation therefore increases the abundance of both Bifidobacterium and butyrate-producing firmicutes55,56. Acetate and formate produced by Bifidobacterium spp. are also thought to be important for butyrate producing Clostridia in the infant gut during the transition from breast milk to solid food57.
The trophic behaviour of a given species depends on the presence of their cross-feeding partner(s). For example, colonization of a gnotobiotic mouse with B. thetaiotaomicron and Eubacterium rectale together versus alone results in both species shifting towards a more specialized trophic role; B. thetaiotaomicron upregulates of a variety of PULs for glycan degradation, while E. rectale downregulates GHs and upregulates enzymes for acetate metabolism28. The fitness cost of these pathways is also affected. For instance, genes required for 1,2-propanediol utilization in Lactobacillus reuteri confer a fitness burden in the mouse gut that is reversed in the presence of Bifidobacterium breve, a 1,2-propanediol producer58.
Fourth trophic level: Interspecies H2 transfer
Molecular hydrogen (H2) is an important electron sink in many pathways for anaerobic growth. For these bacteria, proton reduction is coupled to ferredoxin, formate, NADH and FADH2 oxidation, all of which result in H2 production (Figure 2b-c)59. However, the low redox potential of H+/H2 means that these reactions are only favourable in low H2 partial pressure and its buildup can slow or inhibit growth, usually by inhibiting NADH oxidation (Figure 2c). Removal of H2 by partner bacteria or archaea, a process called interspecies H2 transfer, is critical for growth of these bacteria and a classic example of syntrophy. These interactions are often obligate59, but facultative relationships also exist if alternative intracellular metabolites can be reduced instead of protons. Nonetheless, use of protons allows higher ATP yield and so is favourable59,60.
Several types of metabolism consume H2 as part of a strategy to generate ATP (Figure 2d). These include hydrogenotrophic methanogenesis (4H2 + CO2 → CH4 + 2H2O), acetogenesis (4H2 + CO2 → CH3COOH + 2H2O; Wood-Ljungdahl pathway), and various forms of hydrogenotrophic respiration46. In general, hydrogenotrophic respiration utilizes H2 as an electron donor for the electron transport chain that ultimately reduces terminal electron acceptors (e.g. fumarate, nitrate, sulfate)61. A specific type of hydrogenotrophic respiration is dissimilatory sulfate reduction (4H2 + SO42− + H+ → HS− + 4H2O), which involves a specific strategy for activating sulfate with ATP and ultimately reducing it to H2S62. All of these forms of H2 consumption ultimately generate a H+ or Na+ gradient that drives ATP synthase. Hydrogenotrophic respiration cleaves H2 in the periplasm into protons, which directly contribute to the proton motive force, and electrons, which enter the electron transport chain61. Additional proton motive force can be generated in some species when oxidation of electron carriers (e.g. ferredoxin) is coupled to translocating H+ or Na+ across the membrane by ferredoxin:NAD oxidoreductase (Rnf) or ferredoxin:H+ oxidoreductase (Ech)62,63. This strategy is used to generate ATP during dissimilatory sulfate reduction and acetogenesis. Alternatively, during hydrogenotrophic methanogenesis, energy conservation occurs during a methyl transfer reaction by the membrane-bound methyltransterase Mtr that translocates Na+ across the membrane64.
These metabolic strategies for H2 consumption are utilized by diverse and distinct taxa of microbes in the gut. Sulfate reducing bacteria (SRB) predominantly belong to the genus Desulfovibrio, while all known methanogens are Archaea and belong to diverse orders including Methanobacteriales and Methanomassiliicoccales, with Methanobrevibacter smithii prominent in the human gut65. Acetogenesis and other forms of hydrogenotrophic respiration are found in diverse phyla, though acetogens are primarily firmicutes60. Importantly, hydrogenotrophic respiration is essential for the virulence of several enteric pathogens including Helicobacter pylori and S. Typhimurium66.
In contrast to SRB and hydrogenotrophic methanogens whose growth is limited by H2 availability, acetogens utilizing the Wood-Ljungdahl pathway generally have more metabolic flexibility. In addition to H2, these microbes can use alternate electron donors for CO2 reduction including carbon monoxide or organic carbon sources such as sugars or alcohols. Furthermore, many acetogens lack formate dehydrogenase, the enzyme for the first step in the Wood-Ljungdahl pathway (reduction of CO2 to formate using H2)67. Therefore, these bacteria benefit from cross-feeding on formate produced by other species in the gut68. On the other hand, SRB growth is limited by availability of sulfate, which it obtains in part through cross-feeding mediated by Bacteroides-encoded sulfatases that liberate sulfate from host glycans (e.g. chondroitin sulfate)69.
Based solely on energetic favourability, SRB can outcompete methanogens for H2 (as long as sulfate is not limiting), and methanogens can outcompete acetogens70. Despite this apparent metabolic stratification, acetogens are more prevalent and abundant than SRB or methanogens60. The success of acetogens may be explained by their ability to use alternative electron donors to H2 and other encoded strategies for energy harvest such as polysaccharide degradation. Indeed, in communities that contain methanogens and SRB, acetogens provide only a minor contribution to H2 consumption71.
Several examples in vitro and in gnotobiotic mice demonstrate that pairing sugar fermenting gut microbes with H2 consuming species increases cell density and can impact metabolic output of the interacting species69. For example, the relative abundances of the methanogenic Archaea Methanobacteriaceae and H2 producing Christensenellaceae72 are positively associated in different human populations across several studies. In vitro, M. smithii produces more methane when paired with Christensenella minuta compared to other H2 producers, possibly owing to the high H2 production of C. minuta and physical aggregation with M. smithii. In turn, C. minuta shifts its metabolism from butyrate to acetate production with potential impacts on host health72.
Amino acid cross-feeding
Amino acids are an important currency in microbial communities. They act as an energy source by entering central metabolism, as a nitrogen source for transamination reactions, and as an essential nutrient for amino acid auxotrophs. In contrast to the carbon food web, which centers around Bacteroidales due to their glycan degrading capacity, the nitrogen food web is driven by diverse species including many firmicutes such as Clostridium, Fusobacterium, Actinomyces, Propionibacterium, and Peptostreptococci31,73. Indeed, many Clostridia degrade amino acids through oxidative, reductive or coupled pathways, including the Stickland reaction (Figure 3a)46. Together, these species drive nitrogen cross-feeding in the gut, which can be exchanged in two forms: ammonium (NH4+) and amino acids.
Figure 3. Nitrogen metabolism in the gut.
(a) Stickland metabolism couples reduction of one amino acid (left) with oxidation of a second amino acid (right) via electron carriers indicated by [H]. Deamination of the amino acids results in production of carboxylic acid intermediates and ammonium. Stoichiometric equivalents are indicated by n. (b) Deamination of urea by ureases also produces ammonia. (c) Nitrogen and fiber metabolism vary along the length of the colon. Whereas ample dietary fiber in the proximal colon leads to high concentrations of SCFA, amino acid fermentation is more common in the distal colon. Ammonium and SCFA concentrations shown are estimated from human samples74.
Ammonium is abundant and microbially produced in the colon, where concentrations increase distally (Figure 3c)74. It acts as an important nutrient for Bacteroides spp. growth, since some Bacteroides spp. cannot grow on amino acids as the sole nitrogen source75. In vivo, B. thetaiotaomicron shows preferential use of NH4+ for arginine and lysine biosynthesis by upregulating alternative enzymes depending on ammonium availability75. Bacteroides spp. cross-feed NH4+ produced by diverse bacteria during amino acid fermentation (Figure 3a)76, or from urea, which is excreted in abundance into the intestine (Figure 3b). In this case, cross-feeding occurs when urease expressing species, including members of all dominant phyla, liberate NH4+ for utilization by urease non-producers77.
The second way that nitrogen is exchanged during cross-feeding is in the form of amino acids. More energy can be conserved by using amino acids anabolically, thereby relieving the necessity for amino acid biosynthesis, compared to catabolic pathways that funnel them into central metabolism. Empirical evidence that amino acid biosynthesis is energetically costly leads to the hypothesis that auxotrophs should be common78. The loss of amino acid biosynthetic genes is consistent with the Black Queen Hypothesis, which suggests that gene loss provides a selective advantage if the essential function/nutrient is provided in the environment, for example through the release of public goods79.
Amino acid auxotrophy is widespread: metabolic pathway analysis suggests that 24%80 to 98%81 of bacteria lack the ability to synthesize at least one amino acid. A more recent analysis of >12,000 communities included in the Earth Microbiome Project, including free-living and host-associated natural communities, identified amino acid auxotrophs in virtually all communities82. Some caution should be taken when interpreting these data because cryptic amino acid biosynthetic pathways can lead to erroneous over-prediction of auxotrophy83,84. Nonetheless, applying the same pipeline to host-associated (e.g. gut microbiome) and free-living species highlights the increased frequency of auxotrophy in host-associated microbes (in Earth Microbiome Project data, 45% vs 25% of species, respectively)82. This observation is consistent with increased nutrient availability in host-associated environments.
Despite the increased frequency of predicted auxotrophy in host-associated environments, notable examples of cross-feeding between amino acid auxotrophs are documented in free-living settings including food fermentation85-88. Synthetic communities of amino acid auxotrophs are also used to probe the principles of cross-feeding and mutualism in community ecology. For example, syntrophic growth of 14 auxotrophic E. coli mutants demonstrates that sharing energetically expensive amino acids (e.g. methionine, lysine, isoleucine, arginine and aromatics) promotes stronger interactions than cross-feeding inexpensive amino acids89. These interactions are dependent on nutrient availability; for example, cross-feeding amino acids between S. cerevisiae and lactic acid bacteria is dependent on nitrogen availability to allow S. cerevisiae to engage in ‘costless’ overflow metabolism of amino acids that subsequently become available to auxotrophic Lactobacilli87. Cross-feeding experiments using synthetic consortia can also probe how and when cooperation can evolve90-92. Addressing these basic science questions has important implications to understanding resilience to nutrient change and resistance to invasion12.
In contrast to limited amino acid availability in free-living systems, bacteria in the mammalian gastrointestinal tract may have ample access to protein and amino acids. Thus, is amino acid cross-feeding between auxotrophic microbes relevant in the gut? While there is certainly in vitro and in silico evidence that gut microbes can cross-feed amino acids93,94, whether this is an important amino acid source in vivo is unclear. In the small intestine, dietary protein is abundant and studies using labelled proteins or nitrogen sources indicate that microbes subsist on this source95,96. In the distal colon, fermentable glycans become scarce and amino acids are more frequently utilized for fermentation (Figure 3c). Here, fermentable fiber is depleted, as are SCFA that inhibit proteolytic degradation of proteins97, and so amino acid fermentation becomes favourable. It is feasible that the fermentation of amino acids restricts their use by auxotrophs and encourages mutualistic cross-feeding.
There is little direct in vivo evidence for amino acid cross-feeding in the gut. One example in a simplified system, the Drosophila gut, demonstrates that L. plantarum produces lactate through central metabolism, which is used by A. pomorum to generate amino acids that are essential for L. plantarum growth8. While these essential amino acids are usually present in the diet, the authors propose that this form of cross-feeding protects the microbial community from perturbation should the host diet lack these nutrients8. Interestingly, the mutualistic interaction also decreases the fly’s preference for a protein rich diet through a mechanism mediated by lactate and A. pomorum. In the human gut, a similar exchange of lactate and amino acids has been demonstrated in vitro between Lactobacillus rhamnosus and Bacteroides caccae13. Finally, in gnotobiotic mice carrying a four membered consortium of engineered amino acid auxotrophs, evidence of cross-feeding was stronger on a low-protein diet than high-protein diet98. This is consistent with the hypothesis that dietary protein can rescue auxotrophic requirements in the gut microbiome.
Taken together, these studies establish the potential for amino acid cross-feeding in the human gut microbiome. Although the relative contribution of these interactions to microbial growth in the human gut requires further study, it is notable that bacterial metabolites of amino acid metabolism have important impacts on host health including obesity99, neurologic disorders100, and drug metabolism101. Future studies that discriminate mechanisms of amino acid cross-feeding in vivo will be important to understanding and predicting the contribution of the microbiome to these host phenotypes.
Vitamin cross-feeding
Apart from cross-feeding major carbon and nitrogen sources, micronutrients including vitamins and cofactors are also cross-fed in the gut microbiome. Vitamins are well suited to inter-microbial cross-feeding in the distal gut: (i) they are essential for enzymatic function in vital cellular pathways, (ii) they are energetically expensive to synthesize, so consequently prototrophs regulate biosynthesis and auxotrophs are common, (iii) they are commonly imported from the environment using dedicated transporters, and (iv) in contrast to amino acids and central metabolism substrates, only low concentrations (nanomolar range) are required for growth. As evidence for the latter point, in a genome wide screen of E. coli auxotrophs, vitamin auxotrophs emerged as ideal cross-feeding collaborators able to grow rapidly in the presence of a prototrophic partner yet do not grow in their absence102. The authors attribute this quality to the low concentrations of vitamins required for rapid growth as compared to large amounts of amino acid or nucleotides that are required.
Bacteria synthesize/import and therefore potentially cross-feed multiple B vitamins (thiamine [B1], riboflavin [B2], niacin [B3], pantothenate [B5], pyridoxine [B6], biotin [B7], folate [B9], corrinoids [B12]), quinones (including menaquinone [K2], and ubiquinone [CoQ]) cellular antioxidants (glutathione and ergothioneine)103,104. While there is less evidence of cross-feeding antioxidants or quinones, there is extensive literature on cross-feeding B vitamins105,106
Vitamin B12 belongs to a group of closely related compounds known as corrinoids and is perhaps the most widely studied example of vitamin cross-feeding in microbial communities. Corrinoids meet all the characteristics for an ideal cross-fed metabolite: (i) they are important for the growth of many microbes, as ~75% of bacteria encode corrinoid dependent enzymes107; (ii) roughly half of bacteria are auxotrophs, as corrinoid biosynthesis is expensive, involving ~30 enzymatic steps108; (iii) import is common, as different forms of corrinoids and their biosynthetic intermediates are efficiently captured by dedicated scavenging/transport systems (BtuBFCD)109; and (iv) only nanomolar amounts are required for growth. B12 consumed in food is absorbed in the ileum with bioavailability of ~50% depending on the food, and saturated at ~2 μg/meal110. Therefore, depending on diet, corrinoids in the distal colon can be of both dietary and microbial origin. Dietary B12 that reaches the colon is most likely converted into other corrinoids by the microbiome111. However, under limiting dietary B12, which can occur in diets low in animal products, evidence supports that microbial corrinoid production and cross-feeding stabilizes the community. First, direct in vivo evidence demonstrates that B. thetaiotaomicron, a B12 auxotroph, relies on uptake from the environment, since transporter mutants are outcompeted by the wildtype strain in monocolonized mice but not in mice co-colonized with corrinoid prototrophs; lower-efficiency transporters may substitute when corrinoids are abundant112. Second, a B12 deficient diet has almost no effect on the composition of the gut microbiome, suggesting that prototrophs are able to supply corrinoids to auxotrophs113, though host storage of B12 in the liver and release through enterohepatic circulation may also contribute48. B12 can also be shared with other metabolites involved in central carbon metabolism, such as the exchange of corrinoids and 1,2-propanediol between Eubacterium hallii (corrinoid producer and 1,2-propanediol consumer) and A. mucuniphila (corrinoid consumer and 1,2-propanediol producer) at the mucosal border114.
Thiamine (B1) provides a second example of cross-feeding in the gut. Thiamine biosynthesis involves two distinct branches that produce stable intermediates (a 5-membered 4-methyl-5-(2-hydroxyethyl)thiazole ring [THZ] and a 6-membered 4-amino-5-hydroxymethyl-2-methylpyrimidine ring [HMP]) which are coupled together using the enzyme ThiE, then phosphorylated to the active cofactor, thiamine pyrophosphate (TPP). Alternatively, thiamine itself can be phosphorylated to TPP115. Many gut and marine bacteria are auxotrophic for the biosynthesis of both THZ and HMP or for the ability to combine THZ and HMP, while import pathways for these compounds are widely reported116. In a synthetic community, E. coli mutants in THZ/HMP biosynthesis or Thiamine-phosphate synthase (ThiE) can support each other by sharing these intermediates117. These observations suggest that thiamine and its intermediates can be readily shared among bacteria.
There is similar evidence of cross-feeding for other B vitamins. In silico predictions demonstrate widespread auxotrophy of B vitamins, where ~20% of a typical microbial population (by relative abundance) is auxotrophic for each vitamin, apart from B12 auxotrophy which reaches ~50% prevelance108. Modelling suggests that B vitamins drive modules of interacting gut microbes based on complementary prototroph/auxotroph networks118. Experimentally, varying dietary B vitamin (B1, B2, B3, B5, B6, B7, B9, and B12) in vivo and in vitro supports that prototrophs protect auxotrophs from nutrient deficit108,113. In vitro, the concentration of B vitamins (B2, B3, and B5) increases in spent media during growth of human complex communities and adding excess B-vitamins does not increase the abundance of auxotrophs, suggesting that prototrophs can supply auxotrophs under these conditions108. As an in vivo example, in the termite gut, the acetogen Treponema primitia requires folate (B9) for growth, which it receives from other gut microbes including Lactococcus lactis and Serratia grimesii119. T. primitia may in turn produce biotin, pyridoxal phosphate and coenzyme A to support growth of Treponema azotonutricium120.
Notably, similar to amino acid auxotrophy predictions, prediction of vitamin dependency based on genomic data is not always supported by in vitro evidence121. This may partially be due to the conditional essentiality of vitamins. For example, in many microbes vitamin B12 is essential for growth in minimal medium due to the corrinoid-dependent methionine biosynthesis enzyme MetH; in species encoding an alternative B12-independent enzyme MetE, B12 is not an essential vitamin under these conditions107. Similarly, biotin is essential for aspartate biosynthesis and supplementation of this amino acid can lower the biotin requirement of some species122. In silico modelling further predicts that vitamins B1, B5, B9, B12 and K are required or dispensable dependent on growth conditions118. In Bacteroides, B vitamin biosynthesis or transport was conditionally important for fitness depending on the conditions (in vivo versus in vitro), species, and community composition 112,123. Taken together, these observations suggest that genomic predictions and in vitro growth requirements only partially predict the role of B vitamin biosynthesis and cross-feeding in the gut microbiome.
Unlike central metabolism by-products, which may be released from producer cells with little or no fitness cost due to overflow metabolism or extracellular polysaccharide degradation, vitamins are energetically expensive biosynthetic end-products: in this context, does vitamin release represent an altruistic behaviour by the vitamin producers? Altruism, a form of cooperation where an individual incurs a fitness cost while providing a fitness benefit to others, can be explained when it helps the reproduction of genetically similar individuals more than distant relatives124 and restricts the success of cheaters that do not contribute to the common resource125. There is some evidence for altruism in the gut microbiome124, but in the context of B vitamins alternate explanations for their release are also feasible. For example, while the cobamide precursor cobinamide inhibits the growth of auxotrophic E. coli, conversion to B12 relieves this toxicity126. This suggests that B12 assembly may constitute a means of protection from toxic biosynthetic intermediates and the release of excess B12 is a by-product of this process126. Furthermore, only limited amounts of B12 are secreted, which puts limits on the growth of freeloaders126. Consistent with low levels of vitamin secretion, little evidence exists for the active export of vitamins, though bidirectional transporters or exporters are predicted in some prototrophic species116,118. Few dedicated exporters are characterized except for flavin export for the purpose of extracellular electron transport127.
Differing ability to release a vitamin to the environment might explain variation in the ability of prototrophs to support the growth of auxotrophs. For example, in the case of thiamine, both E. coli and B. thetaiotaomicron encode biosynthetic pathways as well as import pathways for thiamine or its intermediates. Whereas E. coli mutants can support each other by cross-feeding thiamine intermediates117, B. thetaiotaomicron mutants defective in biosynthesis are readily outcompeted by wildtype in media lacking exogenous thiamine128. Pairing various butyrate-producing firmicutes under B vitamin limitation similarly demonstrates that only some prototrophic strains support growth of auxotrophs121. Future work characterizing the mechanisms of vitamin release, whether it be ‘accidental’ (i.e. leakage or lysis) or through active efflux, will shed light on these observations. Moreover, most investigations of vitamin cross-feeding have used in vitro conditions and genetically engineered strains; it is possible that undiscovered mechanisms (e.g. transport or biosynthesis) are used in natural environments.
Iron, heme and siderophores
Minerals (Zn, Mn, Cu, Se, Fe, S, Co, Cu) are essential for both bacteria and the host for the function of metalloproteins involved in vital processes such as DNA replication, response to oxidative stress and ATP production. These cofactors are derived from the diet, and minerals that escape human absorption reach the colon where they are used by microbes. Bacteria capture minerals such as iron from the environment either directly (e.g., using FeoABC transport systems to transport soluble ferrous Fe2+) or through scavenging molecules (e.g., siderophores or heme/hemophores for insoluble ferric Fe3+)129. While cross-feeding of iron itself has not been documented in the gut, these scavenging molecules are readily exchanged between species. Further, some siderophores such as yersiniabactin function as metallophores that bind other metals such as Cu, Zn and Mn130, expanding the scope of mineral cross-feeding among members of the gut microbiome.
Siderophores are structurally diverse and require a cognate receptor for uptake, including an outer membrane TonB-dependent transporter and periplasmic binding protein/ATP-transporter to facilitate translocation into the cell131. In the context of the gut microbiome, competition for iron is particularly important as it contributes to virulence of enteric pathogens129. During infection, the host secretes iron binding proteins lactoferrin and calprotectin as a nutritional immunity strategy to starve pathogens of iron. Likewise, production of siderophores is not required for colonization of commensals (e.g. Bifidobacterium, commensal E. coli) in a homeostatic state132, but is important for competition between commensals and pathogens (e.g. Salmonella) during infection133,134.
Similar to vitamins, siderophores and heme are energetically expensive to produce and thus some non-siderophore-producing bacteria express receptors that allow piracy of siderophores produced by other species (xenosiderophores). Gut Bacteroides spp. encode neither heme nor siderophores and obtain iron through the uptake of heme precursors (e.g. protoporphyrin)135, transferrin136, and xenosiderophores in a species-specific manner131. During infection or colitis when microbial access to iron is limited, fitness of Bacteroides spp. becomes dependent on their ability to capture siderophores from Enterobacteriacae (i.e. salmochelin and enterobactin). Indeed, B. thetaiotaomicron mutants defective in the xusABC locus responsible for salmochelin/enterobactin uptake exhibit no fitness defect in a healthy gut but are outcompeted during Salmonella infection or colitis134.
What effect does siderophore piracy have on the fitness of the producing organism? Although a negative impact may be expected, experimental studies of Salmonella burden in mice colonized with wildtype or XusABC-deficient B. thetaiotaomicron strains reveal no impact on pathogen load134. Notably, these studies were performed in conventionally raised mice in which B. thetaiotaomicron only represented a small proportion of the microbiome; it is possible that the combined action of many siderophore-utilizing species would impact the fitness of the producing organism. Alternatively, mutualistic cross-feeding can increase the fitness of both parties during siderophore piracy. For example, during polymicrobial intra-abdominal infections, synergism between E. coli and B. fragilis leads to lesion and abscess formation. This synergism is at least partially attributed to E. coli hemoglobin protease (Hbp), which is secreted, degrades hemoglobin, binds heme, and delivers it for uptake137. Hbp can be intercepted by B. fragilis, which in turn improves E. coli growth through mechanisms that remain ill-defined137. Environmental Enteric Dysfunction, a form of undernutrition characterized by overgrowth of proteobacteria, chronic malabsorption, and susceptibility to infection, provides a second example. During Environmental Enteric Dysfunction, mutualistic cross-feeding occurs between E. coli and Bacteriodes spp., where Bacteroides spp. enhance carbohydrate access for E. coli through the liberation of sialic acid from mucin, and E. coli enhances iron acquisition of Bacteroides through siderophore secretion, hemoglobin degradation and heme biosynthesis138. This interaction is dependent on the combination of malnourished and inflammatory conditions, when Bacteroides spp. degrade host mucins and iron is limiting. Understanding the mechanisms and consequences of iron acquisition and cross-feeding during these dysbiotic states could aid in the development of therapeutic strategies that ameliorate microbiome-associated disease risks.
Outlook
Despite the seeming simplicity of microbial cross-feeding, outcomes of cross-feeding in microbial communities are clearly complex. As highlighted in the examples above, these interactions can result in commensalism, exploitation or mutualism, can be context dependent, influenced by the participating species and nutrient conditions, and can introduce a throng of eco-evolutionary conundrums involving community stability and altruism. Depending on context, cross-feeding interactions can be an integral function of a healthy microbiota (for example, through promoting growth of butyrate producers) and also contribute to disease (for example, by promoting pathogen expansion). These disparate outcomes may be inherent to metabolites released as public goods that can be accessed by other members of a microbial community.
Despite these challenges, cross-feeding interactions in the gut are worthy of continued study. Various strategies described here simplify cross-feeding, for example studying pairwise interactions or breaking complex communities into trophic levels. Integrating the findings of these studies create the foundation for developing comprehensive models that integrate cross-feeding into community dynamics predictions. Models incorporating proteomic, metabolomic and genomic data continue to increase in accuracy as they harness advancing technologies such as untargeted metabolomics, stable isotope tracking139, and more powerful ecological and computation models (e.g. Ordinary Differential Equation-based models140, Genome-scale Metabolic Modelling141). However, limitations to these approaches exist, including a lack of integration of host factors such as spatial structure and microbe-host interactions and our limited understanding of the metabolic function of diverse species present in the gut. Integration of these host factors and continued investigation of uncharacterized functions in diverse gut microbes remain important goals. Together, these efforts work towards the goal of understanding the composition and metabolic output of gut microbial communities, the impacts of these communities on their host, and how these outputs and impacts can be manipulated for therapeutic benefit.
Cross-feeding, the sharing of metabolites between different microbes, plays an important role in shaping the gut microbiome. This review describes ecological and evolutionary implications of cross-feeding and dissects specific mechanisms that enable carbon, nitrogen, vitamin and cofactor cross-feeding.
Acknowledgements
Support for this work was provided by the National Institute of Health grants R01 AT010014, R35 GM118159, and R01 DK133798 (to A.L.G.), and by the Damon Runyon Cancer Research Foundation (to E.J.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, and Gordon JI (2007). The human microbiome project. Nature 449, 804–810. 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Germerodt S, Bohl K, Lück A, Pande S, Schröter A, Kaleta C, Schuster S, and Kost C (2016). Pervasive selection for cooperative cross-feeding in bacterial communities. PLoS Comput. Biol 12. 10.1371/journal.pcbi.1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zelezniak A, Andrejev S, Ponomarova O, Mende DR, Bork P, and Patil KR (2015). Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl. Acad. Sci 112, 6449–6454. 10.1073/pnas.1421834112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zengler K, Toledo G, Rappé M, Elkins J, Mathur EJ, Short JM, and Keller M (2002). Cultivating the uncultured. Proc. Natl. Acad. Sci 99, 15681–15686. 10.1073/pnas.252630999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faust K, and Raes J (2012). Microbial interactions: From networks to models. Nat. Rev. Microbiol 10, 538–550. 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 7.Morris BEL, Henneberger R, Huber H, and Moissl-Eichinger C (2013). Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev 37, 384–406. 10.1111/1574-6976.12019. [DOI] [PubMed] [Google Scholar]
- 8.Henriques SF, Dhakan DB, Serra L, Francisco AP, Carvalho-Santos Z, Baltazar C, Elias AP, Anjos M, Zhang T, Maddocks ODK, et al. (2020). Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour. Nat. Commun 11, 4236. 10.1038/s41467-020-18049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dethlefsen L, and Relman DA (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci 108, 4554–4561. 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith P, and Schuster M (2019). Public goods and cheating in microbes. Curr. Biol 29, R442–R447. 10.1016/j.cub.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Coyte KZ, Schluter J, and Foster KR (2015). The ecology of the microbiome: Networks, competition, and stability. Science 350, 663–666. 10.1126/science.aad2602. [DOI] [PubMed] [Google Scholar]
- 12.Machado D, Maistrenko OM, Andrejev S, Kim Y, Bork P, Patil KR, and Patil KR (2021). Polarization of microbial communities between competitive and cooperative metabolism. Nat. Ecol. Evol 5, 195–203. 10.1038/s41559-020-01353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnúsdóttir S, Heinken A, Kutt L, Ravcheev DA, Bauer E, Noronha A, Greenhalgh K, Jäger C, Baginska J, Wilmes P, et al. (2017). Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat. Biotechnol 35, 81–89. 10.1038/nbt.3703. [DOI] [PubMed] [Google Scholar]
- 14.Venturelli OS, Carr AV, Fisher G, Hsu RH, Lau R, Bowen BP, Hromada S, Northen T, and Arkin AP (2018). Deciphering microbial interactions in synthetic human gut microbiome communities. Mol. Syst. Biol 14. 10.15252/msb.20178157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faust K, Bauchinger F, Laroche B, de Buyl S, Lahti L, Washburne AD, Gonze D, and Widder S (2018). Signatures of ecological processes in microbial community time series. Microbiome 6, 120. 10.1186/s40168-018-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoek TA, Axelrod K, Biancalani T, Yurtsev EA, Liu J, and Gore J (2016). Resource availability modulates the cooperative and competitive nature of a microbial cross-feeding mutualism. PLoS Biol. 14, e1002540. 10.1371/journal.pbio.1002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammarlund SP, Chacón JM, and Harcombe WR (2019). A shared limiting resource leads to competitive exclusion in a cross-feeding system. Environ. Microbiol 21, 759–771. 10.1111/1462-2920.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaka T, Kato S, Shimoyama T, Ishii S, Abe T, and Watanabe K (2008). The genome of Pelotomaculum thermopropionicum reveals niche-associated evolution in anaerobic microbiota. Genome Res. 18, 442–448. 10.1101/gr.7136508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S, Foster KR, and Comstock LE (2016). The evolution of cooperation within the gut microbiota. Nature 533, 255–259. 10.1038/nature17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daly RA, Roux S, Borton MA, Morgan DM, Johnston MD, Booker AE, Hoyt DW, Meulia T, Wolfe RA, Hanson AJ, et al. (2019). Viruses control dominant bacteria colonizing the terrestrial deep biosphere after hydraulic fracturing. Nat. Microbiol 4, 352–361. 10.1038/s41564-018-0312-6. [DOI] [PubMed] [Google Scholar]
- 21.Fazzino L, Anisman J, Chacón JM, Heineman RH, and Harcombe WR (2020). Lytic bacteriophage have diverse indirect effects in a synthetic cross-feeding community. ISME J. 14, 123–134. 10.1038/s41396-019-0511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basan M, Hui S, Okano H, Zhang Z, Shen Y, Williamson JR, and Hwa T (2015). Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528, 99–104. 10.1038/nature15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catlett JL, Catazaro J, Cashman M, Carr S, Powers R, Cohen MB, and Buan NR (2020). Metabolic feedback inhibition influences metabolite secretion by the human gut symbiont Bacteroides thetaiotaomicron. mSystems 5. 10.1128/msystems.00252-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zampieri M, Hörl M, Hotz F, Müller NF, and Sauer U (2019). Regulatory mechanisms underlying coordination of amino acid and glucose catabolism in Escherichia coli. Nat. Commun 10, 3354. 10.1038/s41467-019-11331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez JG, and Wingreen NS (2022). Noisy metabolism can promote microbial cross-feeding. Elife 11. 10.7554/eLife.70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco AR, Moel M, and Segrè D (2019). Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat. Commun 10, 103. 10.1038/s41467-018-07946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gralka M, Szabo R, Stocker R, and Cordero OX (2020). Trophic interactions and the drivers of microbial community assembly. Curr. Biol 30, R1176–R1188. 10.1016/j.cub.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, Shah N, Wang C, Magrini V, Wilson RK, et al. (2009). Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci 106, 5859–5864. 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Goffau MC, Jallow AT, Sanyang C, Prentice AM, Meagher N, Price DJ, Revill PA, Parkhill J, Pereira DIA, and Wagner J (2022). Gut microbiomes from Gambian infants reveal the development of a non-industrialized Prevotella-based trophic network. Nat. Microbiol 7, 132–144. 10.1038/s41564-021-01023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang T, Goyal A, Dubinkina V, and Maslov S (2019). Evidence for a multi-level trophic organization of the human gut microbiome. PLOS Comput. Biol 15, e1007524. 10.1371/journal.pcbi.1007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pérez Escriva P, Fuhrer T, and Sauer U (2022). Distinct N and C cross-feeding networks in a synthetic mouse gut consortium. mSystems 7. 10.1128/msystems.01484-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grondin JM, Tamura K, Déjean G, Abbott DW, and Brumer H (2017). Polysaccharide utilization loci: fueling microbial communities. J. Bacteriol 199. 10.1128/JB.00860-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakoff-Nahoum S, Coyne MJ, and Comstock LE (2014). An ecological network of polysaccharide utilization among human intestinal symbionts. Curr. Biol 24, 40–49. 10.1016/j.cub.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valguarnera E, Scott NE, Azimzadeh P, and Feldman MF (2018). Surface exposure and packing of lipoproteins into outer membrane vesicles are coupled processes in Bacteroides. mSphere 3. 10.1128/mSphere.00559-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Liao C, Wu L, Tang J, Chen J, Lei C, Zheng L, Zhang C, Liu YY, Xavier J, et al. (2022). Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 16, 2040–2055. 10.1038/s41396-022-01253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Qian Y, Zhou Z, Ertmer S, Vivas EI, Lan F, Hamilton JJ, Rey FE, Anantharaman K, and Venturelli OS (2022). Polysaccharide utilization loci in Bacteroides determine population fitness and community-level interactions. Cell Host Microbe 30, 200–215.e12. 10.1016/j.chom.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuskin F, Lowe EC, Temple MJ, Zhu Y, Cameron EA, Pudlo NA, Porter NT, Urs K, Thompson AJ, Cartmell A, et al. (2015). Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 517, 165–169. 10.1038/nature13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogowski A, Briggs JA, Mortimer JC, Tryfona T, Terrapon N, Lowe EC, Baslé A, Morland C, Day AM, Zheng H, et al. (2015). Glycan complexity dictates microbial resource allocation in the large intestine. Nat. Commun 6, 7481. 10.1038/ncomms8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YL, Chassard C, Hausmann M, Von Itzstein M, and Hennet T (2015). Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun 6. 10.1038/ncomms9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, et al. (2013). Microbiotaliberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502, 96–99. 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cartmell A, Muñoz-Muñoz J, Briggs JA, Ndeh DA, Lowe EC, Baslé A, Terrapon N, Stott K, Heunis T, Gray J, et al. (2018). A surface endogalactanase in Bacteroides thetaiotaomicron confers keystone status for arabinogalactan degradation. Nat. Microbiol 3, 1314–1326. 10.1038/s41564-018-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moens F, Weckx S, and De Vuyst L (2016). Bifidobacterial inulin-type fructan degradation capacity determines cross-feeding interactions between Bifidobacteria and Faecalibacterium prausnitzii. Int. J. Food Microbiol 231, 76–85. 10.1016/j.ijfoodmicro.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Luis AS, Briggs J, Zhang X, Farnell B, Ndeh D, Labourel A, Baslé A, Cartmell A, Terrapon N, Stott K, et al. (2018). Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol 3, 210–219. 10.1038/s41564-017-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrowski MP, La Rosa SL, Kunath BJ, Robertson A, Pereira G, Hagen LH, Varghese NJ, Qiu L, Yao T, Flint G, et al. (2022). Mechanistic insights into consumption of the food additive xanthan gum by the human gut microbiota. Nat. Microbiol 7, 556–569. 10.1038/s41564-022-01093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krautkramer KA, Fan J, and Bäckhed F (2021). Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol 19, 77–94. 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- 46.Fischbach MA, and Sonnenburg JL (2011). Eating for two: How metabolism establishes interspecies interactions in the gut. Cell Host Microbe 10, 336–347. 10.1016/j.chom.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louis P, and Flint HJ (2009). Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett 294, 1–8. 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 48.Green R, Allen LH, Bjørke-Monsen A-LL, Brito A, Guéant J-LL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh B-HH, et al. (2017). Vitamin B12 deficiency. Nat. Rev. Dis. Prim 3, 17040. 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 49.Canani RB (2011). Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol 17, 1519. 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan SH, Holtrop G, Lobley GE, Calder AG, Stewart CS, and Flint HJ (2004). Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr 91, 915–923. 10.1079/BJN20041150. [DOI] [PubMed] [Google Scholar]
- 51.Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, and Flint HJ (2000). Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol 66, 1654–1661. 10.1128/AEM.66.4.1654-1661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weghoff MC, Bertsch J, and Müller V (2015). A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol 17, 670–677. 10.1111/1462-2920.12493. [DOI] [PubMed] [Google Scholar]
- 53.Belenguer A, Holtrop G, Duncan SH, Anderson SE, Calder AG, Flint HJ, and Lobley GE (2011). Rates of production and utilization of lactate by microbial communities from the human colon. FEMS Microbiol. Ecol 77, 107–119. 10.1111/j.1574-6941.2011.01086.x. [DOI] [PubMed] [Google Scholar]
- 54.Rios-Covian D, Gueimonde M, Duncan SH, Flint HJ, and de los Reyes-Gavilan CG (2015). Enhanced butyrate formation by cross-feeding between Faecalibacterium prausnitzii and Bifidobacterium adolescentis. FEMS Microbiol. Lett 362, fnv176. 10.1093/femsle/fnv176. [DOI] [PubMed] [Google Scholar]
- 55.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, and Delzenne NM (2011). Prebiotic effects of wheat arabinoxylan related to the increase in Bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6, e20944. 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, and Flint HJ (2006). Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol 72, 3593–3599. 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsukuda N, Yahagi K, Hara T, Watanabe Y, Matsumoto H, Mori H, Higashi K, Tsuji H, Matsumoto S, Kurokawa K, et al. (2021). Key bacterial taxa and metabolic pathways affecting gut short-chain fatty acid profiles in early life. ISME J. 15, 2574–2590. 10.1038/s41396-021-00937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng CC, Duar RM, Lin X, Perez-Munoz ME, Tollenaar S, Oh J-H, van Pijkeren J-P, Li F, van Sinderen D, Gänzle MG, et al. (2020). Ecological importance of cross-feeding of the intermediate metabolite 1,2-propanediol between bacterial gut symbionts. Appl. Environ. Microbiol 86. 10.1128/AEM.00190-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stams AJM, and Plugge CM (2009). Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol 7, 568–577. 10.1038/nrmicro2166. [DOI] [PubMed] [Google Scholar]
- 60.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, and Gordon JI (2010). Dissecting the in vivo metabolic potential of two human gut acetogens. J. Biol. Chem 285, 22082–22090. 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benoit SL, Maier RJ, Sawers RG, and Greening C (2020). Molecular hydrogen metabolism: a widespread trait of pathogenic bacteria and protists. Microbiol. Mol. Biol. Rev 84. 10.1128/MMBR.00092-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Price MN, Ray J, Wetmore KM, Kuehl JV, Bauer S, Deutschbauer AM, and Arkin AP (2014). The genetic basis of energy conservation in the sulfate-reducing bacterium Desulfovibrio alaskensis G20. Front. Microbiol 5. 10.3389/fmicb.2014.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poehlein A, Schmidt S, Kaster A-K, Goenrich M, Vollmers J, Thürmer A, Bertsch J, Schuchmann K, Voigt B, Hecker M, et al. (2012). An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7, e33439. 10.1371/journal.pone.0033439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurth JM, Op den Camp HJM, and Welte CU (2020). Several ways one goal—methanogenesis from unconventional substrates. Appl. Microbiol. Biotechnol 104, 6839–6854. 10.1007/s00253-020-10724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borrel G, Brugère J-F, Gribaldo S, Schmitz RA, and Moissl-Eichinger C (2020). The host-associated archaeome. Nat. Rev. Microbiol 18, 622–636. 10.1038/s41579-020-0407-y. [DOI] [PubMed] [Google Scholar]
- 66.Maier L, Vyas R, Cordova CD, Lindsay H, Schmidt TSB, Brugiroux S, Periaswamy B, Bauer R, Sturm A, Schreiber F, et al. (2013). Microbiota-derived hydrogen fuels Salmonella Typhimurium invasion of the gut ecosystem. Cell Host Microbe 14, 641–651. 10.1016/J.CHOM.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Trischler R, Roth J, Sorbara MT, Schlegel X, and Müller V (2022). A functional Wood–Ljungdahl pathway devoid of a formate dehydrogenase in the gut acetogens Blautia wexlerae , Blautia luti and beyond. Environ. Microbiol 24, 3111–3123. 10.1111/1462-2920.16029. [DOI] [PubMed] [Google Scholar]
- 68.Laverde Gomez JA, Mukhopadhya I, Duncan SH, Louis P, Shaw S, Collie-Duguid E, Crost E, Juge N, and Flint HJ (2019). Formate cross-feeding and cooperative metabolic interactions revealed by transcriptomics in co-cultures of acetogenic and amylolytic human colonic bacteria. Environ. Microbiol 21, 259–271. 10.1111/1462-2920.14454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, and Gordon JI (2013). Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc. Natl. Acad. Sci 110, 13582–13587. 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith NW, Shorten PR, Altermann E, Roy NC, and McNabb WC (2020). Competition for hydrogen prevents coexistence of human gastrointestinal hydrogenotrophs in continuous culture. Front. Microbiol 11. 10.3389/fmicb.2020.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fonty G, Joblin K, Chavarot M, Roux R, Naylor G, and Michallon F (2007). Establishment and development of ruminal hydrogenotrophs in methanogen-free lambs. Appl. Environ. Microbiol 73, 6391–6403. 10.1128/AEM.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruaud A, Esquivel-Elizondo S, de la Cuesta-Zuluaga J, Waters JL, Angenent LT, Youngblut ND, and Ley RE (2020). Syntrophy via interspecies H2 transfer between Christensenella and Methanobrevibacter underlies their global cooccurrence in the human gut. MBio 11. 10.1128/mBio.03235-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith EA, and Macfarlane GT (1996). Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J. Appl. Bacteriol 81, 288–302. 10.1111/j.1365-2672.1996.tb04331.x. [DOI] [PubMed] [Google Scholar]
- 74.Macfarlane GT, Gibson GR, and Cummings JH (1992). Comparison of fermentation reactions in different regions of the human colon. J. Appl. Bacteriol 72, 57–64. 10.1111/j.1365-2672.1992.tb04882.x. [DOI] [PubMed] [Google Scholar]
- 75.Liu H, Shiver AL, Price MN, Carlson HK, Trotter VV, Chen Y, Escalante V, Ray J, Hern KE, Petzold CJ, et al. (2021). Functional genetics of human gut commensal Bacteroides thetaiotaomicron reveals metabolic requirements for growth across environments. Cell Rep. 34, 108789. 10.1016/j.celrep.2021.108789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vince AJ, and Burridge SM (1980). Ammonia production by intestinal bacteria: the effects of lactose, lactulose and glucose. J. Med. Microbiol 13, 177–191. 10.1099/00222615-13-2-177. [DOI] [PubMed] [Google Scholar]
- 77.Ni J, Shen T-CD, Chen EZ, Bittinger K, Bailey A, Roggiani M, Sirota-Madi A, Friedman ES, Chau L, Lin A, et al. (2017). A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci. Transl. Med 9. 10.1126/scitranslmed.aah6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heizer EM, Raiford DW, Raymer ML, Doom TE, Miller RV, and Krane DE (2006). Amino acid cost and codon-usage biases in 6 prokaryotic genomes: A whole-genome analysis. Mol. Biol. Evol 23, 1670–1680. 10.1093/molbev/msl029. [DOI] [PubMed] [Google Scholar]
- 79.Morris JJ, Lenski RE, and Zinser ER (2012). The black queen hypothesis: Evolution of dependencies through adaptive gene loss. MBio 3. 10.1128/mBio.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D’Souza G, Waschina S, Pande S, Bohl K, Kaleta C, and Kost C (2014). Less is more: Selective advantages can explain the prevalent loss of biosynthetic genes in bacteria. Evolution (N. Y) 68, 2559–2570. 10.1111/evo.12468. [DOI] [PubMed] [Google Scholar]
- 81.Mee MT, and Wang HH (2012). Engineering ecosystems and synthetic ecologies. Mol. Biosyst 8, 2470. 10.1039/c2mb25133g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu JSL, Correia-Melo C, Zorrilla F, Herrera-Dominguez L, Wu MY, Hartl J, Campbell K, Blasche S, Kreidl M, Egger A-S, et al. (2022). Microbial communities form rich extracellular metabolomes that foster metabolic interactions and promote drug tolerance. Nat. Microbiol 7, 542–555. 10.1038/s41564-022-01072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Price MN, Zane GM, Kuehl JV, Melnyk RA, Wall JD, Deutschbauer AM, and Arkin AP (2018). Filling gaps in bacterial amino acid biosynthesis pathways with high-throughput genetics. PLOS Genet. 14, e1007147. 10.1371/journal.pgen.1007147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tramontano M, Andrejev S, Pruteanu M, Klünemann M, Kuhn M, Galardini M, Jouhten P, Zelezniak A, Zeller G, Bork P, et al. (2018). Nutritional preferences of human gut bacteria reveal their metabolic idiosyncrasies. Nat. Microbiol 3, 514–522. 10.1038/s41564-018-0123-9. [DOI] [PubMed] [Google Scholar]
- 85.Lin L, Du R, Wang Y, Wu Q, and Xu Y (2022). Regulation of auxotrophic lactobacilli growth by amino acid cross-feeding interaction. Int. J. Food Microbiol 377, 109769. 10.1016/j.ijfoodmicro.2022.109769. [DOI] [PubMed] [Google Scholar]
- 86.Kapetanakis GC, Gournas C, Prévost M, Georis I, and André B (2021). Overlapping roles of yeast transporters Aqr1, Qdr2, and Qdr3 in amino acid excretion and cross-feeding of lactic acid bacteria. Front. Microbiol 12. 10.3389/fmicb.2021.752742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ponomarova O, Gabrielli N, Sévin DC, Mülleder M, Zirngibl K, Bulyha K, Andrejev S, Kafkia E, Typas A, Sauer U, et al. (2017). Yeast creates a niche for symbiotic lactic acid cacteria through nitrogen overflow. Cell Syst. 5, 345–357.e6. 10.1016/j.cels.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prado MR, Blandón LM, Vandenberghe LPS, Rodrigues C, Castro GR, Thomaz-Soccol V, and Soccol CR (2015). Milk kefir: composition, microbial cultures, biological activities, and related products. Front. Microbiol 6. 10.3389/fmicb.2015.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mee MT, Collins JJ, Church GM, and Wang HH (2014). Syntrophic exchange in synthetic microbial communities. Proc. Natl. Acad. Sci 111. 10.1073/pnas.1405641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pande S, Merker H, Bohl K, Reichelt M, Schuster S, de Figueiredo LF, Kaleta C, and Kost C (2014). Fitness and stability of obligate cross-feeding interactions that emerge upon gene loss in bacteria. ISME J. 8, 953–962. 10.1038/ismej.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harcombe W (2010). Novel cooperation experimentally evolved between species. Evolution (N. Y) 64, 2166–2172. 10.1111/j.1558-5646.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 92.Fritts RK, Bird JT, Behringer MG, Lipzen A, Martin J, Lynch M, and McKinlay JB (2020). Enhanced nutrient uptake is sufficient to drive emergent cross-feeding between bacteria in a synthetic community. ISME J. 14, 2816–2828. 10.1038/s41396-020-00737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goyal A, Wang T, Dubinkina V, and Maslov S (2021). Ecology-guided prediction of cross-feeding interactions in the human gut microbiome. Nat. Commun 12, 1335. 10.1038/s41467-021-21586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Medlock GL, Carey MA, McDuffie DG, Mundy MB, Giallourou N, Swann JR, Kolling GL, and Papin JA (2018). Inferring metabolic mechanisms of interaction within a defined gut microbiota. Cell Syst. 7, 245–257.e7. 10.1016/j.cels.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davila A-M, Blachier F, Gotteland M, Andriamihaja M, Benetti P-H, Sanz Y, and Tomé D (2013). Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res 68, 95–107. 10.1016/j.phrs.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Stoll B, Henry J, Reeds PJ, Yu H, Jahoor F, and Burrin DG (1998). Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr 128, 606–614. 10.1093/jn/128.3.606. [DOI] [PubMed] [Google Scholar]
- 97.Smith E., and Macfarlane G. (1998). Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol 25, 355–368. 10.1111/j.1574-6941.1998.tb00487.x. [DOI] [Google Scholar]
- 98.Ziesack M, Gibson T, Oliver JKW, Shumaker AM, Hsu BB, Riglar DT, Giessen TW, DiBenedetto NV, Bry L, Way JC, et al. (2019). Engineered interspecies amino acid cross-feeding increases population evenness in a synthetic bacterial consortium. mSystems 4. 10.1128/mSystems.00352-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perna S, Alalwan TA, Alaali Z, Alnashaba T, Gasparri C, Infantino V, Hammad L, Riva A, Petrangolini G, Allegrini P, et al. (2019). The role of glutamine in the complex interaction between gut microbiota and health: a narrative review. Int. J. Mol. Sci 20, 5232. 10.3390/ijms20205232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Z, Ruan J, Li D, Wang M, Han Z, Qiu W, and Wu G (2021). The role of intestinal bacteria and gut–brain axis in hepatic encephalopathy. Front. Cell. Infect. Microbiol 10. 10.3389/fcimb.2020.595759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, and Turnbaugh PJ (2013). Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 341, 295–298. 10.1126/science.1235872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Noto Guillen M, Rosener B, Sayin S, and Mitchell A (2021). Assembling stable syntrophic Escherichia coli communities by comprehensively identifying beneficiaries of secreted goods. Cell Syst. 12, 1064–1078.e7. 10.1016/j.cels.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheah IK, and Halliwell B (2021). Ergothioneine, recent developments. Redox Biol. 42, 101868. 10.1016/j.redox.2021.101868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dumitrescu DG, Gordon EM, Kovalyova Y, Seminara AB, Duncan-Lowey B, Forster ER, Zhou W, Booth CJ, Shen A, Kranzusch PJ, et al. (2022). A microbial transporter of the dietary antioxidant ergothioneine. Cell 0. 10.1016/j.cell.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Putnam EE, and Goodman AL (2020). B vitamin acquisition by gut commensal bacteria. PLOS Pathog. 16, e1008208. 10.1371/journal.ppat.1008208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uebanso T, Shimohata T, Mawatari K, and Takahashi A (2020). Functional roles of B-vitamins in the gut and gut microbiome. Mol. Nutr. Food Res 64, 2000426. 10.1002/mnfr.202000426. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Rodionov DA, Gelfand MS, and Gladyshev VN (2009). Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 10, 78. 10.1186/1471-2164-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma V, Rodionov DA, Leyn SA, Tran D, Iablokov SN, Ding H, Peterson DA, Osterman AL, and Peterson SN (2019). B-vitamin sharing promotes stability of gut microbial communities. Front. Microbiol 10. 10.3389/fmicb.2019.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Degnan PH, Barry NA, Mok KC, Taga ME, and Goodman AL (2014). Human gut microbes use multiple transporters to distinguish vitamin B12 analogs and compete in the gut. Cell Host Microbe 15, 47–57. 10.1016/j.chom.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watanabe F (2007). Vitamin B12 sources and bioavailability. Exp. Biol. Med 232, 1266–1274. 10.3181/0703-MR-67. [DOI] [PubMed] [Google Scholar]
- 111.Allen RH, and Stabler SP (2008). Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am. J. Clin. Nutr 87, 1324–1335. 10.1093/ajcn/87.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, Knight R, and Gordon JI (2009). Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6, 279–289. 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lurz E, Horne RG, Määttänen P, Wu RY, Botts SR, Li B, Rossi L, Johnson-Henry KC, Pierro A, Surette MG, et al. (2020). Vitamin B12 deficiency alters the gut microbiota in a murine model of colitis. Front. Nutr 7. 10.3389/fnut.2020.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belzer C, Chia LW, Aalvink S, Chamlagain B, Piironen V, Knol J, and de Vos WM (2017). Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. MBio 8. 10.1128/mBio.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jurgenson CT, Begley TP, and Ealick SE (2009). The structural and biochemical foundations of thiamin biosynthesis. Annu. Rev. Biochem 78, 569–603. 10.1146/annurev.biochem.78.072407.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romine MF, Rodionov DA, Maezato Y, Osterman AL, and Nelson WC (2017). Underlying mechanisms for syntrophic metabolism of essential enzyme cofactors in microbial communities. ISME J. 11, 1434–1446. 10.1038/ismej.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sathe RRM, Paerl RW, and Hazra AB (2022). Exchange of vitamin B1 and its biosynthesis intermediates shapes the composition of synthetic microbial cocultures and reveals complexities of nutrient sharing. J. Bacteriol 204. 10.1128/jb.00503-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molina Ortiz JP, Read MN, McClure DD, Holmes A, Dehghani F, and Shanahan ER (2022). High throughput genome scale modeling predicts microbial vitamin requirements contribute to gut microbiome community structure. Gut Microbes 14. 10.1080/19490976.2022.2118831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Graber JR, and Breznak JA (2005). Folate cross-feeding supports symbiotic homoacetogenic spirochetes. Appl. Environ. Microbiol 71, 1883–1889. 10.1128/AEM.71.4.1883-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenthal AZ, Matson EG, Eldar A, and Leadbetter JR (2011). RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture. ISME J. 5, 1133–1142. 10.1038/ismej.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soto-Martin EC, Warnke I, Farquharson FM, Christodoulou M, Horgan G, Derrien M, Faurie JM, Flint HJ, Duncan SH, and Louis P (2020). Vitamin biosynthesis by human gut butyrate-producing bacteria and cross-feeding in synthetic microbial communities. MBio 11, 1–18. 10.1128/mBio.00886-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Broquist HP, and Snell EE (1951). Biotin and bacterial growth. J. Biol. Chem 188, 431–444. 10.1016/S0021-9258(18)56187-1. [DOI] [PubMed] [Google Scholar]
- 123.Wu M, McNulty NP, Rodionov DA, Khoroshkin MS, Griffin NW, Cheng J, Latreille P, Kerstetter RA, Terrapon N, Henrissat B, et al. (2015). Genetic determinants of in vivo fitness and diet responsiveness in multiple human gut Bacteroides. Science 350. 10.1126/science.aac5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simonet C, and McNally L (2021). Kin selection explains the evolution of cooperation in the gut microbiota. Proc. Natl. Acad. Sci 118, e2016046118. 10.1073/PNAS.2016046118/SUPPL_FILE/PNAS.2016046118.SD01.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Travisano M, and Velicer GJ (2004). Strategies of microbial cheater control. Trends Microbiol. 12, 72–78. 10.1016/j.tim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 126.Gude S, Pherribo GJ, and Taga ME (2022). A salvaging strategy enables stable metabolite provisioning among free-living bacteria. mSystems 7. 10.1128/msystems.00288-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khan MT, Duncan SH, Stams AJM, van Dijl JM, Flint HJ, and Harmsen HJM (2012). The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic–anoxic interphases. ISME J. 6, 1578–1585. 10.1038/ismej.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Costliow ZA, and Degnan PH (2017). Thiamine acquisition strategies impact metabolism and competition in the gut microbe Bacteroides thetaiotaomicron. mSystems 2. 10.1128/mSystems.00116-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Celis AI, and Relman DA (2020). Competitors versus collaborators: micronutrient processing by pathogenic and commensal human-associated gut bacteria. Mol. Cell 78, 570–576. 10.1016/j.molcel.2020.03.032. [DOI] [PubMed] [Google Scholar]
- 130.Behnsen J, Zhi H, Aron AT, Subramanian V, Santus W, Lee MH, Gerner RR, Petras D, Liu JZ, Green KD, et al. (2021). Siderophore-mediated zinc acquisition enhances enterobacterial colonization of the inflamed gut. Nat. Commun 2021 121 12, 1–15. 10.1038/s41467-021-27297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rocha ER, and Krykunivsky AS (2017). Anaerobic utilization of Fe(III)-xenosiderophores among Bacteroides species and the distinct assimilation of Fe(III)-ferrichrome by Bacteroides fragilis within the genus. Microbiologyopen 6, e00479. 10.1002/mbo3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lanigan N, Bottacini F, Casey PG, Motherway MOC, and van Sinderen D (2017). Genome-wide search for genes required for bifidobacterial growth under iron-limitation. Front. Microbiol 8. 10.3389/fmicb.2017.00964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christiaen SEA, O’Connell Motherway M, Bottacini F, Lanigan N, Casey PG, Huys G, Nelis HJ, van Sinderen D, and Coenye T (2014). Autoinducer-2 Plays a Crucial Role in Gut Colonization and Probiotic Functionality of Bifidobacterium breve UCC2003. PLoS One 9, e98111. 10.1371/journal.pone.0098111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu W, Winter MG, Spiga L, Hughes ER, Chanin R, Mulgaonkar A, Pennington J, Maas M, Behrendt CL, Kim J, et al. (2020). Xenosiderophore utilization promotes Bacteroides thetaiotaomicron resilience during colitis. Cell Host Microbe 27, 376–388.e8. 10.1016/j.chom.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halpern D, and Gruss A (2015). A sensitive bacterial-growth-based test reveals how intestinal Bacteroides meet their porphyrin requirement. BMC Microbiol. 15, 282. 10.1186/s12866-015-0616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Manfredi P, Lauber F, Renzi F, Hack K, Hess E, and Cornelis GR (2015). New Iron Acquisition System in Bacteroidetes. Infect. Immun 83, 300–310. 10.1128/IAI.02042-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Otto BR, Van Dooren SJM, Dozois CM, Luirink J, and Oudega B (2002). Escherichia coli hemoglobin protease autotransporter contributes to synergistic abscess formation and heme-dependent growth of Bacteroides fragilis. Infect. Immun 70, 5–10. 10.1128/IAI.70.1.5-10.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huus KE, Hoang TT, Creus-Cuadros A, Cirstea M, Vogt SL, Knuff-Janzen K, Sansonetti PJ, Vonaesch P, and Finlay BB (2021). Cross-feeding between intestinal pathobionts promotes their overgrowth during undernutrition. Nat. Commun 12. 10.1038/s41467-021-27191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schofield WB, Zimmermann-Kogadeeva M, Zimmermann M, Barry NA, and Goodman AL (2018). The stringent response determines the ability of a commensal bacterium to survive starvation and to persist in the gut. Cell Host Microbe 24, 120. 10.1016/J.CHOM.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clark RL, Connors BM, Stevenson DM, Hromada SE, Hamilton JJ, Amador-Noguez D, and Venturelli OS (2021). Design of synthetic human gut microbiome assembly and butyrate production. Nat. Commun 12. 10.1038/S41467-021-22938-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garza DR, Van Verk MC, Huynen MA, and Dutilh BE (2018). Towards predicting the environmental metabolome from metagenomics with a mechanistic model. Nat. Microbiol 2018 34 3, 456–460. 10.1038/s41564-018-0124-8. [DOI] [PubMed] [Google Scholar]