Abstract
Moraxella catarrhalis is one of the major otopathogens of otitis media (OM) in childhood. M. catarrhalis tends to form biofilm, which contributes to the chronicity and recurrence of infections, as well as resistance to antibiotic treatment. In this study, we aimed to investigate the effectiveness of antimicrobial blue light (aBL; 405 nm), an innovative nonpharmacological approach, for the inactivation of M. catarrhalis OM. M. catarrhalis either in planktonic suspensions or 24-h old biofilms were exposed to aBL at the irradiance of 60 mW cm−2. Under an aBL exposure of 216 J cm−2, a >4-log10 colony-forming units (CFU) reduction in planktonic suspensions and a >3-log10 CFU reduction in biofilms were observed. Both transmission electron microscopy and scanning electron microscopy revealed aBL-induced morphological damage in M. catarrhalis. Ultraperformance liquid chromatography results indicated that protoporphyrin IX and coproporphyrin were the two most abundant species of endogenous photosensitizing porphyrins. No statistically significant reduction in the viability of HaCaT cells was observed after an aBL exposure of up to 216 J cm−2. Collectively, our results suggest that aBL is potentially an effective and safe alternative therapy for OM caused byM. catarrhalis. Further in vivo studies are warranted before this optical approach can be moved to the clinics.
INTRODUCTION
Moraxella catarrhalis is a gram-negative mucosal bacterium that was previously regarded as a commensal of the oto-nasopharyngeal tract. More recently, it has been considered as an important pathogen of human disease (1,2). M. catarrhalis mainly affects the respiratory tract, particularly causing pediatric otitis media (OM) together with nontypeable Hemophilus influenzae (NTHi) and Streptococcus pneumoniae. OM is the most common bacterial infection in children and the main cause of antibiotic use within the pediatric population (3). However, due to the development of antibiotic resistance of these otopathogens (4), acute OM can still progress to chronic or recurrent infections, and complications with significant morbidity even occur (5). It has been reported that more than 90% of M. catarrhalis isolates are beta-lactamase positive (6). The extensive production of beta-lactamase enzymes makes M. catarrhalis resistant to antibiotic treatment, and no vaccine is available for this otopathogen, suggesting infections with M. catarrhalis may become more prevalent (6,7).
Moreover, M. catarrhalis tends to develop biofilms within the middle ear (8,9). Biofilms encase bacteria in an extracellular polymeric substance (EPS) and thus enhance resistance to the host immune response and antimicrobial killing (10). In previous clinical and in vivo animal model studies, M. catarrhalis has been found to form not only monomicrobial biofilms, but also polymicrobial biofilms with NTHi and S. pneumoniae (11–13), which contributes to nonresponsive OM and further resistance to antibiotics (14,15). For those cases, alternative physical therapies, such as myringotomy with insertion of a ventilating tube, are also used for OM. However, they are still considered risky, as these therapies may occasionally cause continuous otorrhea and other auditory impairments (5,16,17). Therefore, developing alternative approaches to treat OM is urgently needed.
Antimicrobial blue light (aBL) at 400–470 nm has been investigated as an innovative nonpharmacological approach for the treatment of localized infections (18,19). It poses promising antimicrobial activity without the involvement of exogenous photosensitizers (19,20). The exact antimicrobial mechanism of aBL remains unclear. A common hypothesis is that aBL can photo-excite endogenous photosensitizers (e.g., iron-free porphyrins) in bacterial cells and subsequently leads to the production of reactive oxidative species (ROS) (18–19,21). We previously reported that aBL is capable of inactivating a range of gram-positive and gram-negative bacteria (18,22,23), including multidrug-resistant strains (24,25).
However, aBL has yet to be investigated as a treatment for M. catarrhalis infections. The middle ear is readily accessible to a light source, and the translucent nature of the tympanic membrane facilitates light irradiation (16,26). As a local treatment approach, aBL avoids the systemic exposure to oral antibiotics and the subsequent side effects. In the present study, we investigated the efficacy and safety of aBL (405 nm) for the inactivation of M. catarrhalis and its potential as an alternative therapy for M. catarrhalis OM.
MATERIALS AND METHODS
Light source.
A single light-emitting diode with a peak emission at 405 nm and a full width at half-maximum (FWHM) of 25 nm (M405L2; Thorlabs, Newton, NJ) was used for irradiation. The irradiance was 60 mW cm−2, alevel that does not produce thermal effect according to our previous study (27). A power/energy meter (PM100D; Thorlabs) was used to measure light irradiance. The radiant exposure of light (J cm−2) was calculated as the product of irradiance (W cm−2) and irradiation duration (s).
Bacterial strains and culture conditions.
M. catarrhalis strain ATCC 25238 and five clinical isolates 108P13B1, 142P87B1, 46P73B1, 5P47B2 and 74P50B1 (kind gift from Dr. Timothy F. Murphy at the University at Buffalo) were studied. Bacteria were routinely cultured on chocolate agar plates in an incubator under the condition of 37°C, 5% CO2 or in brain heart infusion (BHI) broth overnight in an orbital incubator (34°C; 150 rpm).
Human epithelial cells and culture conditions.
The human keratinocyte cell line HaCaT (kindly donated by Dr. Bin Zheng at Massachusetts General Hospital) was used. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Grand Island, NY) added with 10% heat-inactivated fetal bovine serum, penicillin (100 Units mL−1) and streptomycin (100 μg mL−1) (Pen Strep; Life Technologies) and incubated at 37°C, 5% CO2 in a humidified incubator. Upon reaching about 70–80% confluence, the cells were washed with Dulbecco’s phosphate buffered saline (DPBS; Life Technologies) and digested for 5 min at 37°C with 0.25% trypsin/ethylenediamine tetraacetic acid (Life Technologies). The cell suspension was centrifuged, washed with DPBS and resuspended to a cell density of 106 cell mL−1 (measured by using a hemocytometer) in DPBS or DMEM without phenol red (Life Technologies) for next steps.
Inactivation of planktonic M. catarrhalis using aBL.
Moraxella catarrhalis were grown in BHI broth in the orbital incubator overnight. After the incubation, bacteria were centrifuged at 2500 g for 5 min, washed with PBS once and adjusted in PBS to an optical density at 600 nm (OD600nm) = 0.3 (approximately 108 CFU mL−1). Bacterial suspensions (3 mL) were then added into 35-mm petri dishes (Transwell®; Costar, New York, NY) prior to aBL exposure. During 405-nm aBL irradiation, bacteria suspensions were stirred using a 12-mm magnetic bar (20 rpm) to ensure uniform exposure of cells. Aliquots of 30 μL were taken at different time points of 0, 12, 24, 36, 48 and 60 min after the initiation of aBL, when aBL radiant exposures of 0, 43.2, 86.4, 129.6, 172.8 and 216 J cm−2 had been delivered (60 mW cm−2), respectively. The viability of bacteria (CFU counting) was measured using serial 10-fold dilution on chocolate agar plates (28). The experiment was conducted in three independent replicates.
Inactivation of M. catarrhalis biofilms using aBL.
Moraxella catarrhalis were grown in BHI broth overnight. The cell density of bacterial suspensions was adjusted to approximately 106 CFU mL−1 using BHI medium. Biofilms were formed in 96-well plates, and within each individual well, 100 μL of the bacterial suspension was added. After 24 h of incubation, aBL was delivered for varying time durations of 0, 15, 30, 45 and 60 min, when radiant exposures of 0, 54.0, 108.0, 162.0 and 216.0 J cm−2, respectively, were delivered. For each condition, three technical replicates (three wells) were used. After aBL exposures, each well containing biofilm was scraped with a 200-μL sterile pipette tip and the bacterial suspension was transferred to a 1.5-mL microcentrifuge tube. This procedure was repeated once with 100 μL fresh PBS. The total 200 μL suspension in each tube was then ultrasonicated (Bransonic M2800; Danbury, CT) for 5 min to disperse the bacterial cells. The samples were then serial diluted and plated on chocolate agar plates for CFU counting. The experiment was performed in triplicate.
Identification and quantification of endogenous photosensitizing porphyrins using ultraperformance liquid chromatography.
To perform ultraperformance liquid chromatography (UPLC; ACQUITY UPLC system; Waters, UT), bacteria were cultured overnight and then washed, centrifuged (at 13 500 g for 5 min), resuspended in 1.0 mL extraction solution (ethanol: dimethyl sulfoxide: acetic acid, 80:20:1 [vol/vol/vol]) and kept at −80°C for 24 h. The cells were then sonicated for 20 min to disrupt cells and centrifuged at 13 500 g for 6 min. The supernatant was collected as whole-cell lysates and then prepared to detect endogenous porphyrins as performed in a previous study (29). The porphyrins selected for quantification included: uroporphyrin, 7-carboxylporphyrin, 6-carboxylporphyrin, 5-carboxylporphyrin, coproporphyrin, protoporphyrin IX (PpIX) and Zinc protoporphyrin IX dihydrochloride (ZnPpIX). Using the bicinchoninic acid protein assay (Pierce® BCA Protein assay kit, Thermo Scientific, Rockford, IL) was used to measure the total protein content in each sample to calculate the porphyrin content per gram of protein.
Visualization of planktonic M. catarrhalis cells using transmission electron microscopy.
Transmission electron microscopy (TEM) was performed to examine the morphological and ultrastructural changes of bacteria induced by aBL treatment. In brief, M. catarrhalis were grown in BHI broth overnight and then exposed to 216 J cm−2 of aBL or left untreated. Then bacterial cells were fixed in 0.5 ml K2 buffer solution at 4°C for 2 h, which contains 1% paraformaldehyde and 1.25% glutaraldehyde. After centrifugation (13 500 g for 10 min) and removal of supernatant, another 1.5 ml K2 fixative was added without disturbing the cell pellet. The cell pellets were stored at 4°C overnight to be fixed and further processed as described previously (30) for TEM detection. The cell pellets were then postfixed, dehydrated and embedded. Ultrathin sections were finally observed by TEM (Philips CM-10; Eindhoven, the Netherlands). Multiple sections were analyzed microscopically, and images representing the most typical morphologies observed were presented.
Visualization of M. catarrhalis biofilms using scanning electron microscopy.
The ultrastructural changes of M. catarrhalis biofilms induced by aBL treatment were investigated by Scanning electron microscopy (SEM). Biofilms were formed on 10 × 10 mm coupons (ACLARCl® 33C Film; Electron Microscopy Sciences, Hatfield, PA) for 24 h in BHI broth in a 12-well microtiter plate. The 24-h biofilms were then exposed to 216 J cm−2 of aBL or left untreated. After being washed using PBS, the biofilms on the coupons were fixed in 0.1 m sodium cacodylate buffer containing 2.5% glutaraldehyde, 0.15% alcian blue and 0.15% safranin O at 4°C for 24 h. Then, the samples were further processed for microscopic scanning, which were described previously (22). The samples were finally examined using a high-resolution field emission SEM Hitachi S4800 (Beaverton, OR).
Examination of aBL cytotoxicity to human keratinocytes.
Human keratinocytes (HaCaT cells), one of the human epithelial cells, was used as the representative host cells in the present study (31). To test the cytotoxicity of aBL to normal human epithelial cells, suspensions of HaCaT cells (106 cells mL−1) were exposed to 405-nm aBL at the irradiance of 108 and 216 J cm−2 in 35-mm petri dishes, respectively. The viability of the cells was determined by flow cytometry after aBL exposure. Annexin V/Propidium Iodide (PI) Apoptosis Kit (Invitrogen; Thermo Fisher) was used to determine if cells were viable (32). The confluent cells after the incubation were collected and washed once in cold DPBS, and then resuspended in 1× annexin-binding buffer. Each 100 μL of the cell suspension was incubated with 5 μL of FITC annexin V and 1 μL of 100 μg mL−1 PI working solution at room temperature for 15 min. Immediately after the incubation, 400 μL of 1× annexin-binding buffer was added and the stained cells were analyzed using flow cytometry (Fortessa X-20; BD Biosciences, Franklin Lakes, NJ). The fluorescence emissions at 530 nm and 610 nm were measured.
Statistical analysis.
Data were presented as the mean ± standard error. All statistical analyses were performed on SPSS 19.0, and graphs were plotted by GraphPad Prism 7.0. The differences among groups were analyzed with a one-way ANOVA followed by Bonferroni’s multiple comparison test. P < 0.05 was considered statistically significant.
RESULTS
Susceptibilities of M. catarrhalis in planktonic suspensions to aBL inactivation
As shown in Fig. 1a, all CFU counts of M. catarrhalis strains in suspensions were reduced by aBL irradiation in suspensions. Over 3-log10 CFU reduction was observed in all the strains at the exposure of 172.8 J cm−2 aBL. When the exposure was increased to 216 J cm−2, over 4-log10 CFU reduction was found in the strains of ATCC 25238 and 74P50B1, while the strains of 46P73B, 5P74B2, 142P87B1 and 108P13B1 were completely eradicated (over 6-log10 CFU reduction) (Fig. 1a). In contrast, almost no viability change was observed in the suspensions of all the strains without being exposed to aBL during the equivalent period of time (data not shown). The TEM images showed that the cell membranes were disrupted and intracellular structures were distorted in M. catarrhalis cells after being exposed to 216 J cm−2 aBL (Fig. 1b).
Figure 1.
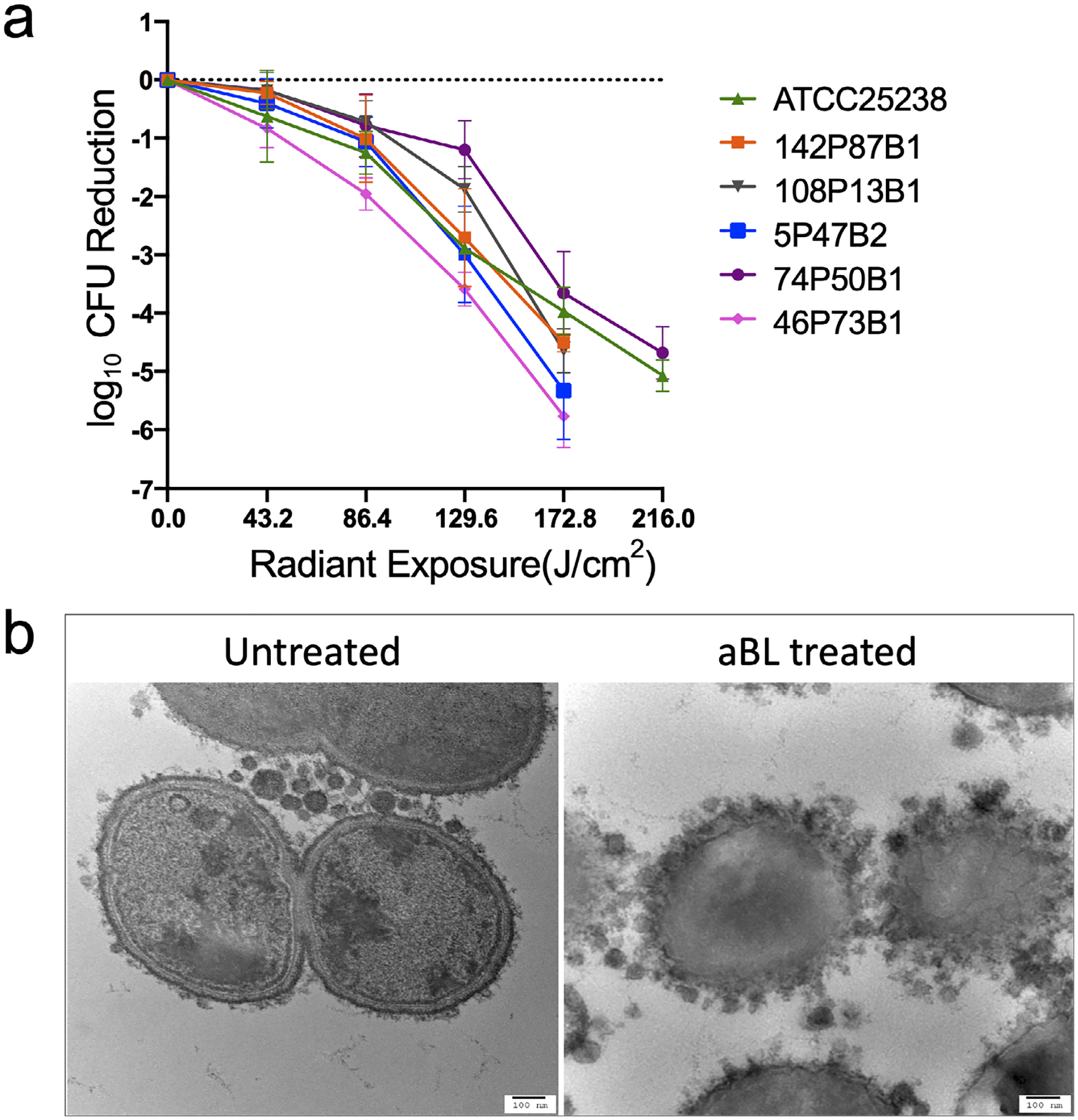
aBL inactivation of planktonic Moraxella catarrhalis. (a) Inactivation kinetics of M. catarrhalis (ATCC 25238, clinical isolates 108P13B1, 142P87B1, 46P73B1, 5P47B2, 74P50B1) in planktonic suspensions by 405-nm aBL. Values are the means of three independent experiments, and bars are SEM. (b) Transmission electron microscopy (TEM) images of untreated and 405-nm aBL-treated planktonic M. catarrhalis (ATCC 25238). Left: untreated M. catarrhalis cells; Right: M. catarrhalis cells treated with 216 J cm−2 aBL. Scale bar: 100 nm.
Susceptibilities of M. catarrhalis in biofilms to aBL inactivation
For the biofilm study, the most sensitive (strain 46P73B1) and the most tolerant strain (strain 74P50B1) to aBL inactivation of planktonic cells were selected, while ATCC 25238 was used as a reference strain. As shown in Fig. 2a, an approximately 4-log10 CFU reduction was achieved in the biofilms of both 46P73B1 and 74P50B1 at 216 J cm−2 exposure, while about 3-log10 CFU reduction was observed in ATCC 25238 biofilm under the same aBL exposure. In SEM images, untreated M. catarrhalis biofilms showed intact bacterial cells with EPS. After an aBL exposure of 216 J cm−2, cell wall damage in some bacteria cells and loss of EPS were observed (Fig. 2b).
Figure 2.

aBL inactivation of Moraxella catarrhalis in 24-h-old biofilms. (a) Inactivation kinetics of M. catarrhalis biofilms by 405-nm aBL. Values are the means of three independent experiments, and bars are SEM. (b) Scanning electron microscope images showing aBL-induced morphological changes in M. catarrhalis 46P73B1 biofilms after an aBL exposure of 216 J cm−2 (60 mW cm−2 and 60 min).
Species and quantification assay of endogenous photosensitizing porphyrins in M. catarrhalis
The presence of seven species of endogenous photosensitizing porphyrins, including PpIX, ZnPpIX, 7-carboxylporphyrin, 6-carboxylporphyrin, 5-carboxylporphyrin, uroporphyrin and coproporphyrin, were identified in M. catarrhalis cells. The UPLC quantitative analysis revealed that the most abundant species of endogenous porphyrins were PpIX and coproporphyrin (Table 1).
Table 1.
UPLC measurements of the concentrations of endogenous porphyrins in Moraxella catarrhalis. The table reports the concentrations of porphyrin/total protein weight (nmol g−1) followed by the constituent ratio (%) in brackets
| Moraxella catarrhalis strains | ||||||
|---|---|---|---|---|---|---|
| Porphyrin species | ATCC 25238 | 108P13B1 | 142P87B1 | 46P73B1 | 5P47B2 | 74P50B1 |
| Protoporphyrin IX | 2.48 (25.42) | 3.34 (21.39) | 0.63 (16.87) | 1.75 (15.31) | 1.06 (13.64) | 0.44 (13.16) |
| ZnPPIX | 6.45 (66.10) | 9.88 (63.18) | 1.88 (50.60) | 9.00 (78.57) | 5.04 (65.09) | 2.49 (75.00) |
| Uroporphyrin | 0.02 (0.16) | 0.03 (0.17) | 0.05 (1.27) | 0.06 (0.50) | 0.05 (0.65) | 0.04 (1.13) |
| 7-Carboxylporphyrin | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| 6-Carboxylporphyrin | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| 5-Carboxylporphyrin | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | 0.94 (12.09) | 0.00 (0.00) |
| Coproporphyrin | 0.81 (8.31) | 2.38 (15.25) | 1.16 (31.26) | 0.64 (5.63) | 0.66 (8.53) | 0.36 (10.72) |
| SUM | 9.75 (100.00) | 15.63 (100.00) | 3.72 (100.00) | 11.46 (100.00) | 7.74 (100.00) | 3.32 (100.00) |
Photo-cytotoxicity of aBL to normal human keratinocytes
We used FITC annexin V/PI assay to detect the viability, apoptosis or necrosis in HaCaT cells. Compared to the untreated cells, the viability of aBL-treated cells was reduced by approximately 5% (P = 0.144 ns) and 7% (P = 0.193 ns) at 108 and 216 J cm−2 aBL, respectively (Fig. 3).
Figure 3.
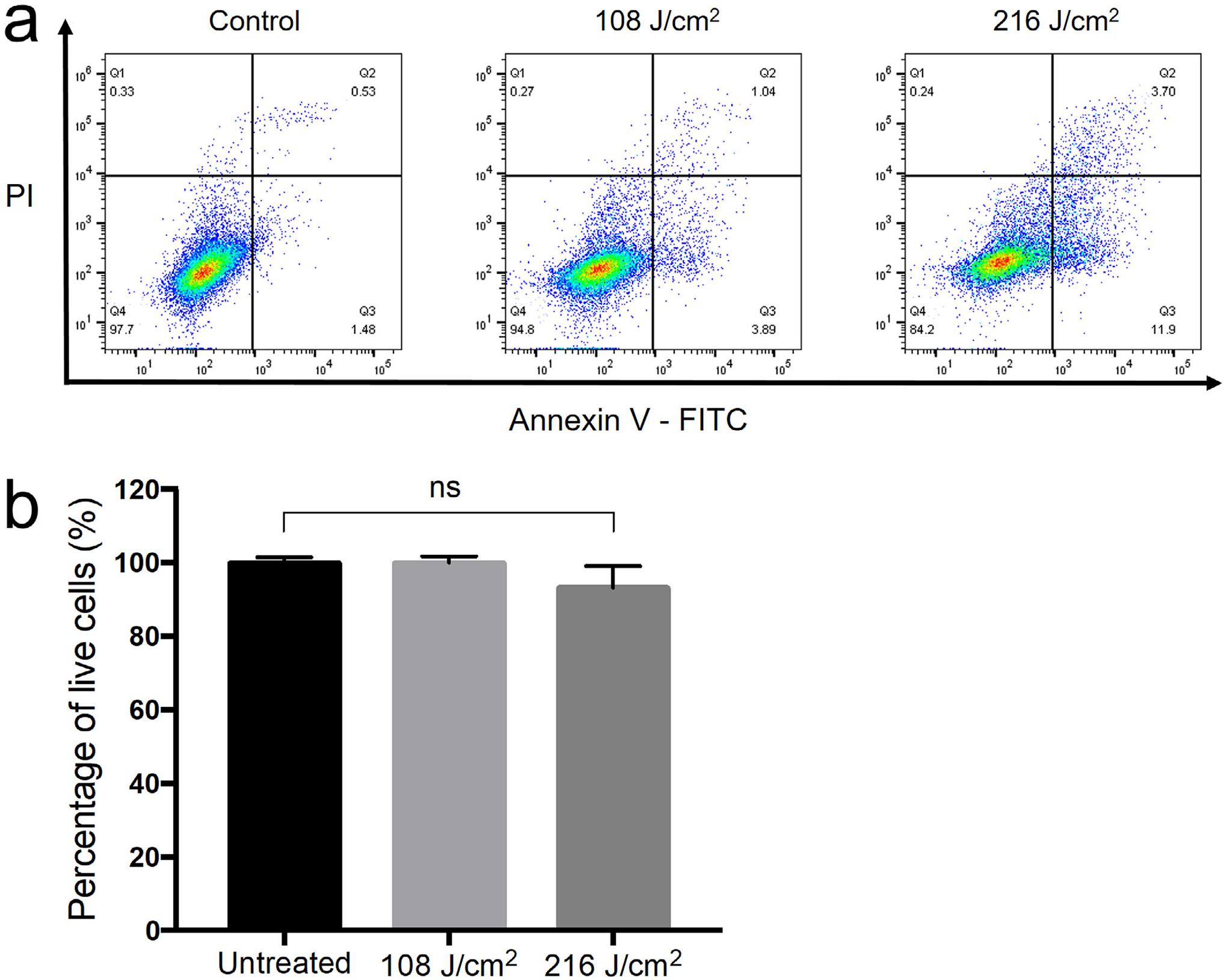
The effect of aBL on HaCaT cells. (a) Apoptosis and necrosis in HaCaT cells measured by flow cytometry with Annexin V-FITC/PI. Live cells: Annexin V negative and PI negative; apoptotic cells: Annexin V positive; necrotic cells: PI positive. (b) Percentage of live cells in untreated HaCaT cells and aBL-treated HaCaT cells at exposure of 108 J cm−2 and 216 J cm−2, respectively. Bars: standard errors. Ns, not significant.
DISCUSSION
Previous studies both in our laboratory and other research groups demonstrated that aBL effectively eradicated a panel of clinical pathogenic microbes, including Acinetobacter baumannii, Pseudomonas aeruginosa, Candida albicans, MRSA and Neisseria gonorrhea (22–23,25,29–30,33–40). In the present study, we showed that aBL was equally effective in inactivating M. catarrhalis, an otopathogen, both in suspensions and in biofilms. Biofilms play a significant role in persistent infections and enhance antibiotic resistance in OM. The successful eradication of M. catarrhalis biofilms by aBL suggests the potential of aBL as a novel treatment strategy against OM caused by M. catarrhalis, which is postulated to be biofilm-associated disease (5,41,42).
The aBL-mediated photo-destruction of various cellular components of bacteria, such as cellular capsules, proteins, lipids and genetic material, was observed in previous studies (18–19,43). Our results of the anti-bacterial effect of aBL against M. catarrhalis in vitro supported the findings of the previous studies. TEM images showed that the cell membranes were disrupted and intracellular structures were distorted in M. catarrhalis cells after the therapeutic exposures of aBL. SEM images revealed bacterial cell wall damage and loss of EPS in M. catarrhalis biofilms, indicating that the structure of biofilms was damaged by aBL.
It is commonly hypothesized that the mechanism of action of aBL is to photo-excite endogenous porphyrins in bacteria and, subsequently, generate cytotoxic ROS. According to the UPLC analysis of the present study, the most abundant endogenous porphyrin species in M. catarrhalis were PpIX and coproporphyrin. This is in agreement with previous studies, which reported that most gram-negative bacteria synthesize heme via PpIX pathway and/or coproporphyrin pathway (40,44,45). The presence of endogenous porphyrins in M. catarrhalis cells suggests that aBL elicits antimicrobial effects against M. catarrhalis via the photo-excitation porphyrins. It is well documented that porphyrins are highly efficient 1O2 generators (46,47), further supporting that aBL inactivation is mediated by 1O2 induced damage to bacterial cells(19,45). However, further work looking at the direct involvement of porphyrins in the photo-toxic effects of aBL against M. cattarhalis is warranted to fully corroborate our hypothesis.
We also evaluated whether aBL exhibited cytotoxic effect on the host cells by detecting apoptosis and necroptosis in human epithelial cells (HaCaT) after being exposed to aBL at the therapeutic exposures to inactivate M. catarrhalis. Our results indicated that there was no statistically significant loss of viability in HaCaT cells after aBL exposures, suggesting that aBL could selectively inactivate M. catarrhalis while preserved human host cells. In our previous study, the viability of HaCaT cells was evaluated by CCK-8 assays, with the results showing that the viability of HaCaT cells was reduced by 10% after an aBL exposure of up to 216 J cm−2 (22), which was consistent with the present result. Our other studies also demonstrated very minimal cytotoxic effect of aBL on the host cells and skin tissues at the therapeutic exposures of aBL to inactivate bacteria (22,30,40).
When using aBL in the clinics for the treatment of OM, one practical issue that needs to be addressed is the delivery of aBL to the infected sites. As we mentioned above, the middle ear is readily accessible to a light fiber. Photodynamic therapy using red light and exogenous photosensitizers has also been investigated to treat OM in an animal model (16), supporting the potential for aBL to be a viable option for the treatment of OM caused by M. catarrhalis. In our ongoing animal studies related to urogenital gonorrhea, a polymeric optical fiber has been developed for uniform diffusion of aBL within the vaginal canal, which further supports the potential translation of aBL for treatment of OM.
In conclusion, aBL is potentially a promising approach for the treatment of M. catarrhalis OM. Further studies will investigate the effectiveness of aBL against other otopathogens including NTHi and S. pneumoniae, and to evaluate the aBL effectiveness against OM in vivo.
Acknowledgements—
We would like to acknowledge Dr. Timothy F. Murphy at the University at Buffalo for providing us the clinical isolates of M. catarrhalis. We also would like to thank Neema Devi Kumar and William Fowle for their generous help in preparing TEM and SEM samples, Bin Zheng, Ph.D. for providing us the HaCaT cells and Tao Li for his support in performing the flow cytometry experiment.
FUNDING
This work was supported by the National Institutes of Health (R01AI123312 to T. D.). X.L. was supported by the National Natural Science Foundation of China (81903241 to X. L.).
Footnotes
This article is part of a Special Issue dedicated to Dr. Thomas Dougherty.
REFERENCES
- 1.Murphy TF and Parameswaran GI (2009) Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis 49, 124–131. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald M, Mulcahy R, Murphy S, Keane C, Coakley D and Scott T (1999) Transmission electron microscopy studies of Moraxella (Branhamella) catarrhalis. FEMS Immunol. Med. Microbiol 23, 57–66. [DOI] [PubMed] [Google Scholar]
- 3.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosen-feld RM, Sevilla XD, Schwartz RH, Thomas PA and Tunkel DE (2013) The diagnosis and management of acute otitis media. Pediatrics 131, e964–e999. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Cobos S, Moscoso M, Pumarola F, Arroyo M, Lara N, Perez-Vazquez M, Aracil B, Oteo J, Garcia E and Campos J (2014) Frequent carriage of resistance mechanisms to beta-lactams and biofilm formation in Haemophilus influenzae causing treatment failure and recurrent otitis media in young children. J. Antimicrob. Chemother 69, 2394–2399. [DOI] [PubMed] [Google Scholar]
- 5.Torretta S, Drago L, Marchisio P, Ibba T and Pignataro L (2019) Role of biofilms in children with chronic adenoiditis and middle ear disease. J. Clin. Med 8, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito R, Nonaka S, Fujinami Y, Matsuoka S, Nakajima S, Nishiyama H and Okamura N (2014) The frequency of BRO beta-lactamase and its relationship to antimicrobial susceptibility and serum resistance in Moraxella catarrhalis. J Infect Chemother 20, 6–8. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TF (2009) Vaccine development for Moraxella catarrhalis: rationale, approaches and challenges. Expert Rev. Vaccines 8, 655–658. [DOI] [PubMed] [Google Scholar]
- 8.Luke NR, Jurcisek JA, Bakaletz LO and Campagnari AA (2007) Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect. Immun 75, 5559–5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD and Kerschner JE (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Fuente-Nunez C, Reffuveille F, Fernandez L and Hancock RE (2013) Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol 16, 580–589. [DOI] [PubMed] [Google Scholar]
- 11.Perez AC and Murphy TF (2017) A Moraxella catarrhalis vaccine to protect against otitis media and exacerbations of COPD: An update on current progress and challenges. Hum Vaccin Immunother 13, 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakaletz LO (2012) Bacterial biofilms in the upper airway – evidence for role in pathology and implications for treatment of otitis media. Paediatr. Respir. Rev 13, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armbruster CE, Hong W, Pang B, Weimer KE, Juneau RA, Turner J and Swords WE (2010) Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. Mbio 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez AC, Pang B, King LB, Tan L, Murrah KA, Reimche JL, Wren JT, Richardson SH, Ghandi U and Swords WE (2014) Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathog. Dis 70, 280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermee Q, Cohen R, Hays C, Varon E, Bonacorsi S, Bechet S, Thollot F, Corrard F, Poyart C, Levy C and Raymond J (2019) Biofilm production by Haemophilus influenzae and Streptococcus pneumoniae isolated from the nasopharynx of children with acute otitis media. BMC Infect. Dis 19, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung JY, Kwon PS, Ahn JC, Ge R, Suh MW and Rhee CK (2009) In vitro and in vivo photodynamic therapy of otitis media in gerbils. Laryngoscope 119, 1781–1787. [DOI] [PubMed] [Google Scholar]
- 17.Steele DW, Adam GP, Di M, Halladay CH, Balk EM and Trikalinos TA (2017) Effectiveness of tympanostomy tubes for otitis media: a meta-analysis. Pediatrics 139, e20170125. [DOI] [PubMed] [Google Scholar]
- 18.Dai T, Gupta A, Murray CK, Vrahas MS, Tegos GP and Hamblin MR (2012) Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates 15, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Wang Y, Wang Y, Murray CK, Hamblin MR, Hooper DC and Dai T (2017) Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resistance Updates 33–35, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai T (2017) The antimicrobial effect of blue light: What are behind? Virulence 8, 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA and Tolkoff MJ (2005) Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob. Agents. Chemother 49, 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrer-Espada R, Liu X, Goh XS and Dai T (2019) Antimicrobial blue light inactivation of polymicrobial biofilms. Front. Microbiol 10, 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dai T, Gupta A, Huang YY, Sherwood ME, Murray CK, Vrahas MS, Kielian T and Hamblin MR (2013) Blue light eliminates community-acquired methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomed. Laser Surg 31, 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leanse LG, Harrington OD, Fang Y, Ahmed I, Goh XS and Dai T (2018) Evaluating the potential for resistance development to antimicrobial blue light (at 405 nm) in gram-negative bacteria: in vitro and in vivo studies. Front. Microbiol 9, 2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Harrington OD, Wang Y, Murray CK, Hamblin MR and Dai T (2017) In vivo investigation of antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii burn infections using bioluminescence imaging. J. Visual. Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luke-Marshall NR, Mang TS, Hansen LA and Campagnari AA (2014) Moraxella catarrhalis is susceptible to antimicrobial photodynamic therapy with Photofrin. Lasers Surg. Med 46, 712–717. [DOI] [PubMed] [Google Scholar]
- 27.Yoshino F and Yoshida A (2018) Effects of blue-light irradiation during dental treatment. Jpn. Dent. Sci. Rev 54, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jett BD, Hatter KL, Huycke MM and Gilmore MS (1997) Simplified agar plate method for quantifying viable bacteria. Biotechniques 23, 648–650. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Ferrer-Espada R, Baglo Y, Gu Y and Dai T (2019) Antimicrobial blue light inactivation of Neisseria gonorrhoeae: Roles of wavelength, endogenous photosensitizer, oxygen, and reactive oxygen species. Lasers Surg Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhu Y, Gupta A, Huang Y, Murray CK, Vrahas MS, Sherwood ME, Baer DG, Hamblin MR and Dai T (2014) Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis 209, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin B, Redmond SL, Rajkhowa R, Eikelboom RH, Atlas MD and Marano RJ (2013) Utilising silk fibroin membranes as scaffolds for the growth of tympanic membrane keratinocytes, and application to myringoplasty surgery. J Laryngol Otol 127(Suppl 1), S13–S20. [DOI] [PubMed] [Google Scholar]
- 32.Crowley LC, Marfell BJ, Scott AP and Waterhouse NJ (2016) Quantitation of apoptosis and necrosis by annexin v binding, propidium iodide uptake, and flow cytometry. Cold Spring Harbor Protocols 2016, pdb.prot087288. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H, Kochevar IE, Behlau I, Zhao J, Wang F, Wang Y, Sun X, Hamblin MR and Dai T (2017) Antimicrobial blue light therapy for infectious keratitis: ex vivo and in vivo studies. Invest. Ophthalmol. Vis. Sci 58, 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amin RM, Bhayana B, Hamblin MR and Dai T (2016) Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med 48, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai T, Gupta A, Huang YY, Yin R, Murray CK, Vrahas MS, Sherwood ME, Tegos GP and Hamblin MR (2013) Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother 57, 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai T and Hamblin MR (2017) Visible blue light is capable of inactivating Candida albicans and other fungal species. Photomed Laser Surg 35, 345–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhu Y, Chen J, Wang Y, Sherwood ME, Murray CK, Vrahas MS, Hooper DC, Hamblin MR and Dai T (2016) Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 7, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Chu Z, Ruan Z, Wang X, Dai T and Hu X (2018) Changes of intracellular porphyrin, reactive oxygen species, and fatty acids profiles during inactivation of methicillin-resistant Staphylococcus aureus by antimicrobial blue light. Front. Physiol 9, 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Wu X, Chen J, Amin R, Lu M, Bhayana B, Zhao J, Murray CK, Hamblin MR, Hooper DC and Dai T (2016) Antimicrobial blue light inactivation of gram-negative pathogens in biofilms. In vitro and in vivo studies. J. Infect. Dis 213, 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Ferrer-Espada R, Baglo Y, Goh XS, Held KD, Grad YH, Gu Y, Gelfand JA and Dai T (2019) Photoinactivation of Neisseria gonorrhoeae: a paradigm-changing approach for combating antibiotic-resistant gonococcal infection. J. Infect. Dis 220, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakaletz LO (2012) Bacterial biofilms in the upper airway - evidence for role in pathology and implications for treatment of otitis media. Paediatr. Respir. Rev 13, 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Post JC (2015) Direct evidence of bacterial biofilms in otitis media. 2001. Laryngoscope 125, 2003–2014. [DOI] [PubMed] [Google Scholar]
- 43.Fila G, Krychowiak M, Rychlowski M, Bielawski KP and Grinholc M (2018) Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: Quorum sensing signaling molecules, biofilm formation and pathogenicity. J. Biophotonics 11, e201800079. [DOI] [PubMed] [Google Scholar]
- 44.Choby JE and Skaar EP (2016) Heme synthesis and acquisition in bacterial pathogens. J. Mol. Biol 428, 3408–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cabral J and Ag R (2019) Blue light disinfection in hospital infection control: advantages, drawbacks, and pitfalls. Antibiotics 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanzilotto A, Kyropoulou M, Constable EC, Housecroft CE, Meier WP and Palivan CG (2018) Porphyrin-polymer nanocompartments: singlet oxygen generation and antimicrobial activity. J. Biol. Inorg. Chem 23, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsolekile N, Nelana S and Oluwafemi OS (2019) Porphyrin as diagnostic and therapeutic agent. Molecules 24, 2669. [DOI] [PMC free article] [PubMed] [Google Scholar]


