Abstract
Informed consent and shared decision making (SDM) are crucial portions of preoperative patient management. Informed consent is a standard for surgery from both a legal and ethical standpoint, involving disclosure of potential risks of a procedure and ensuring patient understanding of these risks. SDM is a process in which a clinician and patients decide between two or more treatment plans, taking into account the patient's goals and values. SDM is a particularly important aspect of patient-centered care when two or more treatment options exist or in situations where an indicated treatment may not align with the patient's long-term goals. This article details aspects of and issues surrounding informed consent and SDM.
Keywords: informed consent, shared decision making, patient-centered care, palliative care
Informed Consent
The clinical practice of “informed consent” arose in the second half of the 20th century, reflecting a shift from the prior paternalistic view of medicine to one of patient autonomy and self-determination. 1 Initially rooted in clinical research, informed consent is now considered a standard for surgical treatment from both a legal and an ethical standpoint. 2 Legally, informed consent was initially outlined in the case Canterbury v. Spence in 1972, in which the United States Court of Appeals, District of Columbia Circuit listed several included steps in an informed consent conversation. They defined that physicians must disclose (1) the condition being treated, (2) the nature of the proposed treatment, (3) the anticipated results, (4) recognized alternative forms of treatment, and (5) recognized risks, benefits, and complications of the proposed treatment. 3 4
The process of informed consent can be broken down into three stages: physician disclosure, patient understanding, and patient decision making. 5 The first stage, physician disclosure, involves the provision of information by the physician to the patient and their families. This includes providing information on the benefits, risk, possible complications, and alternatives to a surgical procedure. These should also include potential quality-of-life complications such as need for a skilled nursing facility, change in independence, and impact on day-to-day activities.
In the second stage, patient understanding, the surgeon must assess how well the patient understands the information that is provided to them. This may require repetition or rephrasing. Even with ideal communication, it is frequent that patients do not recall the entirety of the conversation. For instance, in a study conducted on adult rectal cancer patients treated with either an abdominoperineal resection or low anterior resection, 47% of patients could not recall a preoperative discussion of risk to sexual function, and 57% could not recall a discussion of the risk to urinary function and half of the patients could not recall having a discussion regarding postoperative bowel function. 6 Only 20% could recall specific aspects regarding their probability of survival. However, over 70% recalled a discussion on quality-of-life topics such as body image, functional outcomes, and the appearance of stomas and scars. For most, the discussion was centered on returning to activity, work, and regular day-to-day life. 6 These results demonstrate the dichotomy between the outcomes that patients value versus those the surgeons consider and demonstrate the proportion of information patients retain from consent discussions.
Finally, in the decision-making phase, the patient discusses the information relayed to them with their family, friends, and providers prior to making an informed decision. The final decision that the patient makes must coincide with their values and goals. The consent form must contain clear descriptions of the planned procedure, lists the risks of the procedure, and documentation that the patient had the chance to ask questions. 5 Lastly, it is authorized by the physician's signature and signature of the patient or surrogate decision-maker.
To many surgeons, the informed consent process becomes a routine part of their daily practice. The provision of information including the risks, benefits, and alternatives to a procedure is repeated to each patient. However, individual patients are unique in their understanding and comprehension of the information provided. The consent process is one of many opportunities to employ shared decision making (SDM) in the perioperative setting.
Shared Decision Making
SDM is a process in which patients and clinicians formulate a clinical plan together, using clinical evidence, the clinician's experience, and patient's informed preferences. 7 In general, the provider presents facts and details about a clinical plan or procedure, the provider and patient (and their family members) discuss all potential options, and then a plan is made together based on the patient's long-term goals and preferences. 8 SDM can be incorporated into the informed consent process, or may be an entirely separate area of conversation and decision making. SDM is particularly relevant when multiple acceptable treatment options are available, and is especially needed in surgical practice, where decisions must be made about treatments that are irreversible or have temporary or permanent harmful consequences.
SDM has been associated with improved patient satisfaction, decrease in patient anxiety, and decrease in “decisional regret.” 9 By involving patients throughout the decision-making process, patients feel a greater degree of “buy-in” and are more likely to be satisfied with their decision to pursue a particular treatment. Additionally, when engaging in SDM, patients often choose less expensive, more conservative treatment options, particularly at the end of life. This potential for reducing health care costs has led to the inclusion of SDM as a metric and requirement in various health care policies, including the Affordable Care Act. 7
The definition of SDM varies, but typically includes three key elements, as described by Légaré and Witteman. 10 First, both the provider and patient must realize that there is a health care decision to be made. This may be a situation in which two or more treatments are clinical options (such as the decision to proceed with surgery vs an alternate option that was not initially considered, such as the option of not pursuing surgery in a frail elderly patient). Second, both parties should understand the best available evidence on the topic. This does require the clinician to (1) be aware of the existing evidence, including evidence regarding quality-of-life outcomes, and (2) be able to convey this information to the patient. Decision aids and other patient-directed resources are helpful in this aim. Third, a decision should be made that uses these data and considers the values and preferences of the patient. This decision can be made with varying amounts of input from the provider versus the patient, and has been proposed as a continuum, with the patient and the clinical situation dictating the degree of input from each party in the final decision. 11 It is important that the provider respect the opinions and values of the patient, with the self-awareness that their personal opinions may differ from their patients'.
Importantly, SDM should not be interpreted to imply patient-only decision making and should involve an equal degree of participation from both the patient and surgeon. It has been shown that surgeons more commonly employ SDM in situations in which they feel operative intervention is exceedingly risky, in hopes that patients will select nonoperative care. 12 However, in these situations it is particularly necessary for surgeons to impart their recommendation, even if it is nonoperative management, as the patient often wants to hear this opinion directly. For instance, in a study of patients diagnosed with a life-threatening illness, including a new diagnosis of cancer, >50% of patients wanted their physician to share responsibility in the decision making. 13 It is also acceptable in SDM for the clinician to make the final decision, if desired by the patient, if that decision is based on the patient's stated goals and wishes.
Tools for Shared Decision Making
Decision Aids
The quality of the communication between the patient and provider is essential with SDM. Poor-quality communication limits the patient's knowledge of prognosis and treatment options, management of symptoms, and use of treatments consistent with their preferences. 14 Decision aids are designed to promote informed decisions about treatment options and can come in a variety of forms including print, video, or other media. Decision aids used in clinical decision making can increase knowledge, increase the accuracy of risk perceptions, and increase congruency between informed values and care choices. 15 Compared with usual care strategies, individuals who use decision aids have an improved perception of involvement in decision making. 14 These tools should be easily understood by both the patient and provider, should be written at a low reading level, and should contain data and graphics that can be easily understood by all patients. 16
Decision aids have shown promise in the colorectal surgery patient population. For example, a rectal cancer patient decision aid was created and implemented at a tertiary care hospital in Ottawa. 17 This aid provided information on outcomes and lifestyle changes after a low anterior resection versus an abdominoperineal resection for mid to low rectal cancer. It was found to improve patient knowledge by 37.5% and reduced decisional conflict by 24%. Remarkably, 7.1% of patients preferred a permanent stoma compared with zero patients at baseline after exposure to the decision aid, and almost all patients stated they would recommend the decision aid to others.
Best Case/Worst Case Framework
An additional well-described tool for patient communication and SDM is the best case/worst case framework, by Schwarze and coworkers from the University of Wisconsin. 18 19 20 In this tool, physicians draw two parallel lines on paper, one for each treatment option. The best case, worst case, and likely case scenarios for each option are then located on each line, with focus on qualitative descriptions of each of these scenarios, rather than numbers or percentages. The goal is to visually represent the two treatment options in a narrative way that is accessible to patients, to allow them to make informed decisions when engaging in SDM 21 ( Fig. 1 ). This allows patients to visualize a set of possible outcomes and the path to achieve each outcome. This framework has been associated with improved SDM in a variety of clinical situations, including in conversations with elderly oncology patients and acutely ill general surgery patients. 19
Fig. 1.
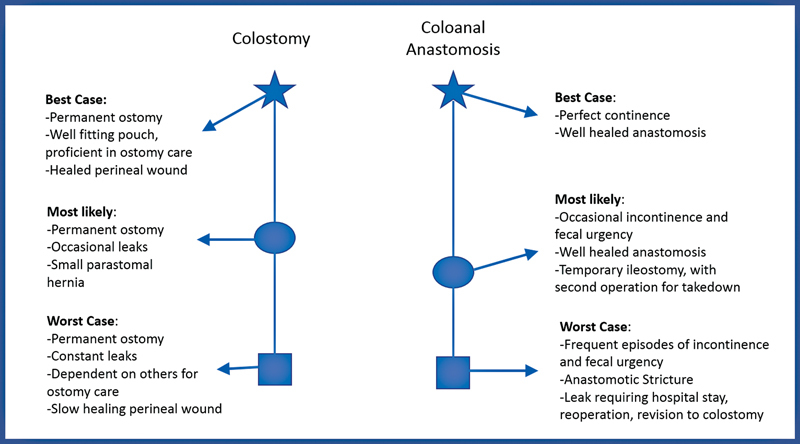
Example of the best case/worst case scenario decision tool focusing on a common discussion in colorectal surgery—coloanal anastomosis versus permanent colostomy for distal rectal cancer. (Adapted from framework described by Schwarze and coworkers. 18 19 20 )
Shared Decision Making and Surgical Palliative Care
SDM may incorporate palliative care, particularly in discussions with frail and elderly patients. Surgical palliative care has more recently been defined as treatment of suffering and the promotion of quality of life for seriously ill patients and their families undergoing surgical care . 22 In the only existing surgical palliative care textbook, Mosenthal and Dunn describe surgical palliative care based on several core principles. 23 They state that surgical palliative care should be considered throughout the continuum of surgical illness, may be delivered in parallel to disease-modifying treatment, and should consider treatments based on their ability to meet the patients' goals rather than their disease-modifying ability. 23 The authors also state that “shared decision making around surgery is the foundation of quality surgical palliative care,” highlighting the interaction between SDM and palliative care. 23
Surgeons are often hesitant to incorporate palliative care in their preoperative discussions. However, the provision of palliative care to surgical patients has been associated with increased quality of life, improved pain and symptom management, higher satisfaction with care, and reduced health care costs. 24 Patients receiving palliative care consultations either within 30 days before or 90 days after a high-risk surgery had better ratings of overall end-of-life care, communication, and support, as reported by families of patients who died within 90 days of high-risk surgery. 25 This multidisciplinary and collaborative approach to surgical care has even resulted in significantly reduced postoperative morbidity. Yet, surgeons generally report limited knowledge and comfort to introducing palliative care to their patients. 26 As a result, only 4 to 38% of surgical patients receive palliative care at some point prior to their death. 27 In general, surgeons report a broad exposure to end-of-life care and understand the goals of palliation but find difficulty in communicating realistic estimates of risk and benefit to patients and their families who are struggling with decision making for high-risk surgery. 26 Incorporation of palliative care teams in the preoperative setting may greatly help with SDM in these instances. There are two ongoing clinical trials regarding the role of presurgical palliative care team involvement in the care of surgical oncology patients, the SCOPE trial and the PERIOP-PC trial. 28 29 These trials and additional studies are needed to further determine the optimal way to incorporate palliative care into the presurgical setting and provide ways to identify and improve care of surgical patients who may benefit from palliative care services.
Shared Decision Making Surrounding a Stoma
Many patient discussions in colorectal surgery involve the topic of creation or reversal of an ostomy. The decision between abdominoperineal resection versus low anterior resection, timing and staging of total proctocolectomy for ulcerative colitis, discussions of the risks and benefits of diversion, and consideration for difficult ostomy reversal all revolve around patients' opinions regarding a stoma. In these situations, it is important that the provider share available information regarding quality of life with a stoma in the setting of the patient's clinical condition and respect the viewpoints and values of the patient. There is a need for well-written decision aids (such as those described by Wu et al and referenced above 17 ), as well as a need for continued research regarding ostomy quality of life in various groups. Communication regarding stomas also presents an opportunity for novel means of sharing information with patients. For example, young patients with inflammatory bowel disease stated a desire to have “stoma buddies,” peers with an existing stoma, from whom they could learn and interact with. 30 Living with a stoma, whether permanent or temporary, is life changing and patients seek clear and timely discussion with their physicians regarding life with a stoma in addition to a thorough review of all treatment options. The provision of additional pre- and postoperative education and support such as incorporating the services of a wound and ostomy nursing team, local support groups, and home health services may be needed to help patients adjust to life with a stoma.
Shared Decision Making in Urgent/Emergent Surgery
SDM is important in both the elective surgical setting and the setting of an emergent life-threatening illness. In urgent and emergent colorectal surgery cases, such as a large bowel obstruction in a patient with severe malnutrition, colonic volvulus in an elderly and infirm patient, or a diverting stoma in a patient with a decubitus ulcer and poor functional status, patients often present in a frail, decompensated state, and SDM is especially important. Often, this interaction can be quite complex as the patient may not be able to participate in these discussions, due to either altered mental status, intubation, or obtunded state from critical illness. In these scenarios, the surgeon must rely on the patient proxy's or patient advocate to aid in the decision making. This type of SDM, particularly by proxy, is especially delicate as the decision to proceed or not with surgery may result in irreversible morbidity and even mortality. Additionally, the surrogate may not appreciate the gravity of the situation, adding an additional barrier toward providing adequate care in these emergent, potentially end-of-life scenarios. 31
Prior to emergent surgery in severely ill patients, it is often important to draw attention to a patient's previously stated wishes and desires, to ensure surgery is in line with these goals. The previously mentioned “best case/worst case” decision aid has been studied and found to be helpful in these patients as well, with particular focus on using descriptive language, painting stories rather than statistics, and allowing time to discuss with patients and their families even in the emergent setting. 18 There is also a need for specific risk anticipation tools in these patients, as these patients frequently do not match existing data on elective surgery patients. New data collection tools, such as the Emergency General Surgery (EGS)-Targeted Module of the ACS National Surgical Quality Improvement Program (ACS NSQIP) registry may provide more information for these patients in the future. Finally, engagement of the palliative care teams can be helpful for facilitating SDM with these patients and their families.
Cultural Considerations in Shared Decision Making
Numerous factors can influence a patient's experience with SDM, including their race/ethnicity, religion, family support, and gender. Notably, a study evaluating the impact of race/ethnicity on patient satisfaction with treatment decisions in colorectal cancer found that patients identifying as Black race or other race demonstrated the lowest rates of decisional satisfaction. 32 Patients of minority race have also been found to have lower rates of trust in the health care system and higher rates of perceived discrimination compared with those of majority race. 32 For these reasons, particular attention should be paid to SDM for these patients, with a focus on developing trust, understanding their concerns, and creating a supportive environment for SDM. It may be that additional time is needed to adequately engage in SDM and develop trust with these patients. It is also important that pictorial decision aids and patient photos reflect patients with a variety of race, ethnicity, and gender, to adequately represent the patient audience. Additionally, patients may have specific concerns related to surgery and their religion, such as particular considerations regarding ostomy care or fasting. It is important to create an environment in which these concerns can be discussed and improve education of the surgical team to address these concerns if able.
Conclusion
Both SDM and informed consent are important aspects of preoperative patient-centered care. The two can be synergistic, as informed consent can be an opportunity for SDM, and SDM can lead into more formal informed consent discussions. As preoperative discussions often involve potentially life-altering decisions, it is crucial that surgeons become facile in SDM to ensure optimum care for patients and their families.
Footnotes
Conflict of Interest None declared.
References
- 1.Jefford M, Moore R. Improvement of informed consent and the quality of consent documents. Lancet Oncol. 2008;9(05):485–493. doi: 10.1016/S1470-2045(08)70128-1. [DOI] [PubMed] [Google Scholar]
- 2.Bernat J L, Peterson L M. Patient-centered informed consent in surgical practice. Arch Surg. 2006;141(01):86–92. doi: 10.1001/archsurg.141.1.86. [DOI] [PubMed] [Google Scholar]
- 3.Murray B. Informed consent: what must a physician disclose to a patient? Virtual Mentor. 2012;14(07):563–566. doi: 10.1001/virtualmentor.2012.14.7.hlaw1-1207. [DOI] [PubMed] [Google Scholar]
- 4.Canterbury v Spence. 464 F.2d. 772, 782 D.C. Cir. 1972
- 5.Childers R, Lipsett P A, Pawlik T M. Informed consent and the surgeon. J Am Coll Surg. 2009;208(04):627–634. doi: 10.1016/j.jamcollsurg.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Scheer A S, O'Connor A M, Chan B P. The myth of informed consent in rectal cancer surgery: what do patients retain? Dis Colon Rectum. 2012;55(09):970–975. doi: 10.1097/DCR.0b013e31825f2479. [DOI] [PubMed] [Google Scholar]
- 7.Oshima Lee E, Emanuel E J. Shared decision making to improve care and reduce costs. N Engl J Med. 2013;368(01):6–8. doi: 10.1056/NEJMp1209500. [DOI] [PubMed] [Google Scholar]
- 8.Frosch D L, Kaplan R M. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17(04):285–294. doi: 10.1016/s0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 9.Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, Poenaru D. Shared decision making in surgery: a meta-analysis of existing literature. Patient. 2020;13(06):667–681. doi: 10.1007/s40271-020-00443-6. [DOI] [PubMed] [Google Scholar]
- 10.Légaré F, Witteman H O. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood) 2013;32(02):276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 11.Kon A A. The shared decision-making continuum. JAMA. 2010;304(08):903–904. doi: 10.1001/jama.2010.1208. [DOI] [PubMed] [Google Scholar]
- 12.Baggett N D, Schulz K, Buffington A. Surgeon use of shared decision-making for older adults considering major surgery: a secondary analysis of a randomized clinical trial. JAMA Surg. 2022;157(05):406–413. doi: 10.1001/jamasurg.2022.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degner L F, Sloan J A. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45(09):941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 14.Austin C A, Mohottige D, Sudore R L, Smith A K, Hanson L C. Tools to promote shared decision making in serious illness: a systematic review. JAMA Intern Med. 2015;175(07):1213–1221. doi: 10.1001/jamainternmed.2015.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stacey D, Légaré F, Lewis K. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4(04):CD001431. doi: 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu R C, Boushey R P, Scheer A S. Evaluation of the rectal cancer patient decision aid: a before and after study. Dis Colon Rectum. 2016;59(03):165–172. doi: 10.1097/DCR.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 17.Wu R, Boushey R, Potter B, Stacey D. The evaluation of a rectal cancer decision aid and the factors influencing its implementation in clinical practice. BMC Surg. 2014;14:16. doi: 10.1186/1471-2482-14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor L J, Nabozny M J, Steffens N M. A framework to improve surgeon communication in high-stakes surgical decisions: best case/worst case. JAMA Surg. 2017;152(06):531–538. doi: 10.1001/jamasurg.2016.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong M L, Nicosia F M, Smith A K. “You have to be sure that the patient has the full picture”: adaptation of the best case/worst case communication tool for geriatric oncology. J Geriatr Oncol. 2022;13(05):606–613. doi: 10.1016/j.jgo.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruser J M, Taylor L J, Campbell T C. “Best case/worst case”: training surgeons to use a novel communication tool for high-risk acute surgical problems. J Pain Symptom Manage. 2017;53(04):711–7.19E7. doi: 10.1016/j.jpainsymman.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kruser J M, Nabozny M J, Steffens N M. “Best case/worst case”: qualitative evaluation of a novel communication tool for difficult in-the-moment surgical decisions. J Am Geriatr Soc. 2015;63(09):1805–1811. doi: 10.1111/jgs.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosenthal A C, Murphy P A.Interdisciplinary model for palliative care in the trauma and surgical intensive care unit: Robert Wood Johnson Foundation Demonstration Project for Improving Palliative Care in the Intensive Care Unit Crit Care Med 200634(11, Suppl):S399–S403. [DOI] [PubMed] [Google Scholar]
- 23.Mosenthal A C, Dunn G P. New York, NY: Oxford University Press; 2019. Surgical Palliative Care. [Google Scholar]
- 24.Lilley E J, Khan K T, Johnston F M. Palliative care interventions for surgical patients: a systematic review. JAMA Surg. 2016;151(02):172–183. doi: 10.1001/jamasurg.2015.3625. [DOI] [PubMed] [Google Scholar]
- 25.Yefimova M, Aslakson R A, Yang L. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155(02):138–146. doi: 10.1001/jamasurg.2019.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwanabol P A, Kanters A E, Reichstein A C. Characterizing the role of U.S. surgeons in the provision of palliative care: a systematic review and mixed-methods meta-synthesis. J Pain Symptom Manage. 2018;55(04):1196–1.215E8. doi: 10.1016/j.jpainsymman.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Heller D R, Jean R A, Chiu A S. Regional differences in palliative care utilization among geriatric colorectal cancer patients needing emergent surgery. J Gastrointest Surg. 2019;23(01):153–162. doi: 10.1007/s11605-018-3929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shinall M C, Jr, Hoskins A, Hawkins A T. A randomized trial of a specialist palliative care intervention for patients undergoing surgery for cancer: rationale and design of the Surgery for Cancer with Option of Palliative Care Expert (SCOPE) trial. Trials. 2019;20(01):713. doi: 10.1186/s13063-019-3754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslakson R A, Chandrashekaran S V, Rickerson E.A multicenter, randomized controlled trial of perioperative palliative care surrounding cancer surgery for patients and their family members (PERIOP-PC) J Palliat Med 201922(S1):44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.members of the stoma PPI team . Dibley L, Czuber-Dochan W, Wade T. Patient decision-making about emergency and planned stoma surgery for IBD: a qualitative exploration of patient and clinician perspectives. Inflamm Bowel Dis. 2018;24(02):235–246. doi: 10.1093/ibd/izx043. [DOI] [PubMed] [Google Scholar]
- 31.Pieracci F M, Ullery B W, Eachempati S R. Prospective analysis of life-sustaining therapy discussions in the surgical intensive care unit: a housestaff perspective. J Am Coll Surg. 2008;207(04):468–476. doi: 10.1016/j.jamcollsurg.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Hawley S T, Morris A M. Cultural challenges to engaging patients in shared decision making. Patient Educ Couns. 2017;100(01):18–24. doi: 10.1016/j.pec.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


