Abstract
Frailty assessment and prehabilitation can be incrementally implemented in a multidisciplinary, multiphase pathway to improve patient care. To start, modifications can be made to a surgeon's practice with existing resources while adapting standard pathways for frail patients. Frailty screening can identify patients in need of additional assessment and optimization. Personalized utilization of frailty data for optimization through prehabilitation can improve postoperative outcomes and identify patients who would benefit from adapted care. Additional utilization of the multidisciplinary team can lead to improved outcomes and a strong business case to add additional members of the team.
Keywords: frailty, prehabilitation, geriatric assessment, functional recovery, optimization
Challenges in providing appropriate colorectal surgery care to patients with frailty are often driven by the unique balance of a patient's diagnosis, preoperative assessment, decision for and type of surgery, and options for perioperative care. 1 Patients are often excluded from standard or advanced care due to chronological age or treated without accounting for their individual vulnerabilities and goals when these challenges are not met with multidisciplinary care. 2 This puts older adults with frailty at risk of poor surgical outcomes as age is a nonmodifiable risk factor. 3 4 Therefore, surgeons should base care decisions on multidimensional health status and frailty rather than chronological age with the overall goal of treatment of colorectal disease while maintaining functional independence and quality of life (QoL). 5 6 It should be noted that frailty-driven decisions should be made in younger patients, as well. Young, frail patients, such as those with inflammatory bowel disease, are vulnerable to the same biases as older adults in treatment planning. 7
Frailty Screening as Part of a Multiphase Pathway
When defining frailty, it can be broadly thought of as an accumulation of deficits resulting in an inability to tolerate stress. 8 Fried's phenotypic definition (3 of 5 traits: slow walking speed, impaired grip strength, self-reported declining activity level, unintended weight loss, and exhaustion) is the basis for objective evaluation of frailty is useful to define domains for screening. 9 10 Accurate assessment of frailty in older adults facilitates opportunities to identify and address vulnerabilities, which can lead to improved, patient-centered outcomes.
The literature is ever expanding in frailty assessment tools predicting poor surgical outcomes. 11 12 13 14 15 16 17 18 However, these indices can become increasingly overwhelming to surgeons when not paired with actionable interventions for guidance in care and improvement in outcomes. Ultimately, frailty assessment is most meaningful in an individual patient when it is paired with an opportunity for directed intervention, which requires a multidisciplinary team. 1 8 19 The “frailty fatigue” that sets in when surgeons are seeing report after report of the effects of frailty on patient outcomes with few resources to intervene can limit the surgeon's subsequent engagement with multidisciplinary evaluation and optimization.
Implementation of Multidisciplinary, Multiphase Pathway
To help surgeons implement a multidisciplinary, multiphase pathway in their practices, a multidisciplinary team of surgeons and geriatric oncologist proposed incremental implementation of patient-centered inverventions 1 ( Fig. 1 ). To ensure the team culture evolved as interventions were enacted, implementation of frailty screening was not recommended until stage 3 in the pathway. To start with, surgeons should ensure they are appropriately selecting patients for minimally invasive surgery. Historically there were concerns about the use of minimally invasive surgery in older adults. However, three meta-analyses, including data from randomized controlled trials, comparing laparoscopic and open colorectal surgery in older adults demonstrate lower complication rates in the laparoscopic group. 20 21 22 Additionally, a retrospective cohort study comparing minimally invasive colorectal surgery between older (≥70 years) and younger adults reported a greater reduction in deaths among older adults (absolute difference 7.0 vs. 2.1%; adjusted odds ratio [aOR] 3.01; 95% confidence interval [CI]: 1.31–7.33 vs. aOR 0.31; 95% CI: 0.05–1.38). 23 To date, there are no randomized controlled trials evaluating the use of minimally invasive surgery in frail patients, but since the older adult cohorts include frail and nonfrail patients, we are left to extrapolate that minimally invasive surgery also benefits frail patients.
Fig. 1.
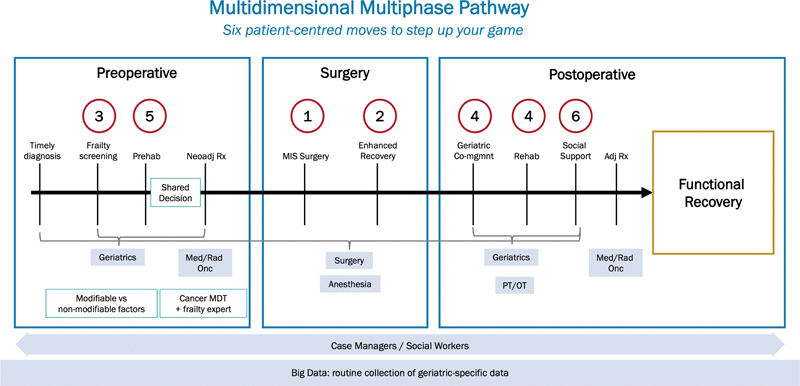
Multidimensional multiphase pathway. Stepwise implementation of six patient-centered interventions to improve care of older adults undergoing colorectal surgery. (Reproduced with permission of Montroni et al. 1 ).
In stage 2, the group recommended implementation of an adapted enhanced recovery protocol (ERP) for older, frail adults. Clinicians prescribing medications in the perioperative period should consider patients' unique medical history and current medications. Medications that meet Beer's criteria as potentially inappropriate medications for older adults should be used with caution or avoided altogether in older adults. 24 For example, care should be taken when prescribing nonsteroidal anti-inflammatory drugs (NSAIDs) to patients with renal insufficiency. Gabapentin, which can cause dizziness, drowsiness, and confusion in older adults, especially when taken with opioids, should be avoided. 25 Older adults benefit from ERPs, especially when protocols are adapted to patients' individual comorbidities and risk profiles. 26 27 28 29 30 Specifically, independence is preserved at a greater rate in older adults in ERPs as shown in a randomized controlled trial of 150 older adults (>70 years; home discharge 87 vs. 67%; p = 0.005). 31
Frailty Screening in Colorectal Surgery Patients
To institute frailty screening, surgeons should collaborate with frailty experts in their institution. Ideal targets for frailty screening tools include those that assess mobility, cognitive function, comorbidities, and nutritional status. While the comprehensive geriatric assessment (CGA) 32 is considered the benchmark for frailty assessment as it is both an assessment and focused optimization intervention, 33 this is time-consuming and requires a team with geriatrics expertise.
Frailty screening, in some cases, can be as effective as the CGA in predicting postoperative complications. 13 34 35 In a prospective, multicenter study of 263 patients ≥70 years undergoing surgery for solid tumors, Huisman et al reported that a brief “Timed Up and Go” (TUG) test (i.e., the time a patient requires to get out of a chair, walk 3 m and return to the chair), predicted major postoperative complications (odds ratio [OR] = 3.43; 95% CI: 1.13–10.36) and was associated with a prolonged length of stay (OR = 4.21; 95% CI: 1.10–24.73). 36 Jones et al, in a prospective cohort study, evaluated 81 patients >65 years undergoing elective colorectal surgery and showed that more than one fall in the 6 months prior to the operation was associated with a higher rate of postoperative complications (59 vs. 25%; p = 0.04) and postoperative institutionalization (52 vs. 6%; p < 0.001). Patients with three or more falls in the preceding 6 months had a 005 chance of postoperative complications. 37 Similarly, the 11-point Modified Frailty Index (m-FI) and the Risk Analysis Index (RAI), a 14-question survey measuring frailty among surgical patients, have also been shown to predict prolonged length of stay, need for intensive care unit (ICU) admission, discharge to nursing home, and short- and long-term mortality after various surgical procedures including colectomy. 38 39 40 41 42 43 44
Cognitive function can be assessed by the Mini-Cog (a 3-item recall test and a clock drawing task) as shown by Culley et al, in a study of 211 orthopaedic surgery patients. Twenty-four percent of the patients were identified with preoperative cognitive impairment (Mini-Cog score ≤ 2), which was associated with increased postoperative incidence of delirium (21 vs. 7%; OR = 4.52; 95%CI: 1.30–15.68) in this prospective study. 45 Cognitive impairment, again measured by the Mini-Cog, was also observed in 21% of 1,003 patients older than 70 years before undergoing major elective oncologic surgery in the prospective, multicenter Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery (GOSAFE) study. 46 A compelling reason to evaluate for cognitive impairment preoperatively is to predict and prepare patients and caregivers for the likelihood of postoperative delirium as preoperative cognitive impairment is one of the strongest predictors of postoperative delirium. 47 Also, importantly, delirium can be prevented in up to 50% of patients by utilizing a delirium prevention bundle. 48
Frailty can also be assessed in the emergency setting, as shown by Zattoni et al, using the Flemish version of the Triage Risk Screening Tool (fTRST) to aid in decision-making for frail, older patients. The fTRST, a simple method to estimate postoperative risk, evaluates five weighted metrics including experiencing cognitive decline (2 points), living alone or having no help available at home (1 point), having reduced mobility or falls in the past 6 months (1 point), being hospitalized in the past 3 months (1 point), and requiring polypharmacy (≥5 different medications; 1 point). This prospective study evaluated 110 frail, older patients undergoing emergency abdominal surgery for a variety of indications and demonstrated that an fTRST ≥ 2 was predictive of increased morbidity, mortality, and length of stay. 49 Ideally, frailty screening will result in referral to a multidisciplinary team for patients with identified vulnerability. However, as surgeons start using frailty tools to screen their patients, they will gain objective information about their own outcomes in their patient population, which will benefit both surgeons and patients. Table 1 summarizes some of the available frailty screening tools utilized in colorectal surgery patients.
Table 1. Selected frailty screening tools evaluated in colorectal surgery patients.
| Tool | Acronym | Range of scores | Cut-off indicating frailty | Population tested |
|---|---|---|---|---|
| Geriatric-8 | G8 | 0–17 | ≤14 | CRC 12 |
| Timed Up & Go Test | TUG | n/a | ≥20 s | CRC 36 |
| 4-m gait speed | gait speed | 0–2 m/s | <0.8–1.0 m/s | CRC 14 |
| 6-minute walk test | 6MWT | n/a | <20 m | CRS a 15 |
| Question about falls last 6 mo | falls | n/a | ≥2 | CRC 37 |
| Risk Analysis Index | RAI | 0–81 | ≥30 | Noncardiac including CRS 16 |
| Modified Frailty Index (11-item) | mFI | 0–11 | >3 | CRC 77 |
| Modified Frailty Index (5-item) | mFI | 0–5 | ≥2 | CRS 17 |
| Multidimensional Prognostic Index | MPI | 0–1.0 | >0.33 | CRC 18 |
| Flemish version of the Triage Risk Screening Tool | fTRST | 0–6 | ≥2 | Emergency surgery including CRS 49 |
Abbreviations: CRC, colorectal cancer; CRS, colorectal surgery.
also used as measure of recovery of function.
Source: Adapted from Saur et al. 8
Geriatric Co-management as an Extension of Frailty Screening
If a geriatric specialist is part of the multidisciplinary team, geriatric co-management can be undertaken starting in the preoperative period and carried postoperatively. To supplement the work of geriatricians, practices can utilize other specialists to complete portions of the geriatric assessment and provide geriatric-related optimization such as adult/geriatric nurse practitioners, social workers, nurse navigators, pharmacists, dieticians, rehabilitative medicine physicians, physical and occupational therapists, psychologists, and psychiatrists. 50 Shahrokni et al retrospectively studied the effects of geriatricians co-managing a cohort of 1,020 patients in the perioperative period who underwent cancer surgery for a variety of cancer types who required at least a 1-day hospital stay and compared this group to 872 similar patients who were treated with standard surgical service management (i.e., were not seen by a geriatrician). This cohort study found the adjusted probability of death within 90 days in the geriatric co-managed group was less than half the rate in the standard management group (4.3 vs. 8.9%; 95% CI: 2.3–6.9; p < 0 0.001). While the two groups had similar complication rates, the geriatric co-management group had greater utilization of supportive care services (e.g., physical therapy, speech and swallow rehabilitation, nutrition services), which may have contributed to the decreased mortality rate in this group. In addition, although not specifically studied, the geriatricians may have identified and addressed risk factors for geriatric-specific complications (e.g., risk of delirium, falls, etc.). 51 Similarly, in a study of 310 patients ≥70 years undergoing elective colorectal surgery, 107 were assigned to usual care and 203 to multidisciplinary, CGA-based care, based on their preoperative comorbidities and independence level. Although the patients in the multidisciplinary/CGA care group had more overall complications (75.9 vs. 56.1%; p < 0.001), as expected based on their comorbidities, they also had a lower incidence of geriatric-specific complications (delirium 11.3 vs. 29.2%; p < 0.001; geriatric syndromes 10.3 vs. 26.2%; p < 0.001). 52
Screening for Social Frailty
Social frailty is an incompletely explored concept in surgical patients. Frailty tools such as the fTRST include a component of social frailty, living alone, which has been shown to be associated with increased postoperative vulnerability. Older and frail adults rely on social relationships to effectively navigate complex care pathways, such as those used to treat many colorectal diseases. 53 While this topic has been widely discussed, large, prospective studies evaluating the effects of social frailty have not been undertaken.
Hawkins et al, in an observational study, evaluated 63 patients undergoing lower-extremity amputation and showed that increased social integration (i.e., the number of contacts and interactions in a patient's social network) was associated with improved postoperative function and QoL. 54 Another prospective study of 972 consecutive patients undergoing colorectal cancer resection showed that increased social support and decreased psychological distress improved health-related QoL at 1 year after surgery. 55 A systematic review of 19 randomized trials by Gardner et al showed that providing practical social support was effective in enabling home-based health behavior change in frail, older adults. 56 In this study, subjects with social support were more likely to have been instructed regarding positive behavioral changes and environmental changes. The utilization of social services and an individualized care path resulted in improvement in social functioning and general health. Screening for and improving social frailty is an important function of the multidisciplinary team in a fully functioning multimodality, multiphase care pathway as seen in Fig. 1 .
Prehabilitation and Multidisciplinary Optimization
Prehabilitation refers to a multidisciplinary, multifaceted, intervention to prevent or minimize surgery-related functional decline and improve perioperative outcomes. 57 Multimodal prehabilitation can include exercise training, nutritional therapy, and anxiety reduction strategies along with optimization of comorbidities and smoking/alcohol cessation. Prehabilitation programs vary based on recommended duration, location, and specific multidisciplinary components of the intervention. 58 59 60 61 62 A systematic review and meta-analysis of 26 studies with heterogeneous methodologies compared the impact of prehabilitation versus no prehabilitation on outcomes after major abdominal surgery and demonstrated that patients receiving prehabilitation had significantly lower rates of overall (OR = 0.61; 95%CI: 0.43–0.86), pulmonary (OR = 0.41; 95%CI: 0.25–0.67), and cardiac complications (OR = 0.46; 95%CI: 0.22–0.98). 63 However, this study did not report patients' ages or whether patients were frail and the definition of prehabilitation was not standardized across the different studies.
Ideal prehabilitation should be tailored to the results of the geriatric assessment and while components can be prescribed generally by surgeons (e.g., increase activity, increase protein or caloric intake, etc.), more formal recommendations are typically made by the multidisciplinary team based on the geriatric assessment. Therefore, prehabilitation is best considered an important component part of a multiphase, multidisciplinary pathway for vulnerable patients rather than a stand-alone intervention. 1 19
While the duration of prehabilitation should be individualized to patients' needs and circumstances, intervention may range from as short as 5 days to as long as 6 weeks. Many of the studies evaluating prehabilitation utilized prolonged programs and most models suggest a duration of 4 to 6 weeks for prehabilitation, when possible. 64 Exercise programs, the mainstay of prehabilitation, may be concentrated at home, an outpatient facility, or an inpatient unit and may include walking, functional activities, balance exercises, and resistance and strength training. The implementation of home-based wearable technology was evaluated in a prospective, observational study; patients wore a tracking device for 30 days prior to surgery and patients taking >5,000 steps/d were classified as active. Of the 99 study patients, 40.4% ( n = 40) were active and experienced fewer overall complications (27.5 vs. 55.9%; p = 0.005) and serious complications (5 vs. 20.3%; p = 0.03). Furthermore, increased preoperative activity was associated with a decreased risk of any postoperative complication (OR = 0.38; 95%CI: 0.15–0.90) on multivariable analysis. 65 As this was a nonrandomized trial, it should be noted that preoperative activity level was likely associated with the degree of frailty and, therefore, decreased activity may be a surrogate marker impaired functional status greater likelihood of complications. However, wearable technologies have the potential to increase access to objective data on preoperative function and optimization. Additionally, wearable devices can be used throughout the perioperative period. Kane et al evaluated postoperative activity, as measured via a wearable device, and 30-day readmission rates. On the day prior to discharge, patients who were readmitted were significantly less active compared with those who were not (median [interquartile range (IQR)], 424 [267–875] vs. 1378 [714–2340]; p < 0.001). In addition, the percent of patients who had an activity return to baseline was significantly lower among readmitted patients (median: 15.1 vs. 31.8%; p = 0.004). 66
Weight loss and poor nutritional status are predictors of worse outcomes in older adults. 67 Nutritional optimization, another pillar of prehabilitation, can potentially decrease ileus incidence and severity, improve appetite, promote normoglycemia, attenuate the perioperative inflammatory response, and provide sufficient protein intake to maintain lean body mass. 8 68 In addition, nutrition supplementation can synergize with exercise activity as shown when liver and muscle glycogen stores are increased and completion of exercise facilitated by ingesting 140 g of carbohydrates 3 hours before exercising. 69 Dieticians are often part of the multidisciplinary team performing the geriatric assessment and make recommendations regarding nutrition and patient-specific protein and calorie intake, when possible.
Prehabilitation programs also typically include a psychosocial domain involving patient education, lifestyle modifications, and management of anxiety and depression. 58 59 60 The incidence of depression increases with age, and depression is associated with worse pos-operative outcomes, longer postoperative recovery times, increased health care utilization, and higher rates of postoperative delirium. 70 Kristjansson et al showed that depression, as assessed by the Geriatric Depression Scale (GDS), was an independent predictor of postoperative complications in a prospective study of 182 patients older than 70 years undergoing surgery for colorectal cancer (OR: 3.68; 95%CI: 0.96–14.08). 71 Symptoms of anxiety, depression, pain and fatigue, and QoL are improved with utilization of relaxation techniques (e.g., deep breathing, progressive muscle relaxation, meditation), guided imagery, and problem-solving, and coping strategies preoperatively. 72
Carli et al performed a randomized superiority trial evaluating the effectiveness of preoperative prehabilitation versus postoperative rehabilitation in 110 frail patients (Fried frailty index ≥ 2) undergoing surgery for colorectal cancer. 73 In this study, patients (mean age: 78 years) were provided with exercise, nutrition, and psychological interventions. Seventy-nine percent of the patients underwent a minimally invasive surgical approach. This study reported no differences between the groups in terms of the 30-day Comprehensive Complications Index, 30-day overall and severe complications, length of hospital stay, hospital readmissions, recovery of walking capacity, and patient-reported outcome measures. While this study did not support prehabilitation over rehabilitation, this may be explained by both the intensity of the prehabilitation and rehabilitation programs and involvement of the established care pathway including minimally invasive surgery, enhanced recovery pathways, and intensive multimodal optimization. A secondary analysis of the dataset from this randomized trial evaluated only frail patients (Fried Frailty criteria > 2; n = 55) and found that patients who did not achieve a minimum walking distance of 400 m in 6 minutes had a higher 30-day complication rate than those who did (61 vs. 21%; p = 0.009). 74 This implies that prehabilitation can also be utilized as a frailty metric and that patients who are not able to be optimized prior to surgery may benefit from adapted care in the context of shared decision-making and with a focus on patient goals. Montroni et al evaluated the QoL after major cancer surgery in a cohort of 942 patients and showed that postoperative complications (Clavien-Dindo (CD) I-II complications: OR = 1.67; 95% CI = 1.17–2.38; p = 0.004; Clavien-Dindo (CD) III-IV complications: OR = 2.20; 95% CI = 1.34–3.61; p = 0.002) correlated with worsening QoL. 75 This finding reiterates the importance of optimization, when possible, and shared decision-making to ensure treatment is aligned with the patient's goals after objective assessment of risks. 1
Implementation of prehabilitation has been variable given the diversity of surgical practices in the United States. Like the other elements in the pathway proposed in Fig. 1 , prehabilitation can be implemented incrementally starting with existing resources. An example is proposed in Fig. 2 . The ideal, fully realized, prehabilitation program was proposed by Carli et al where the prehabilitation clinic, with a perioperative physician, exercise specialist, nutritionist, anxiety-coping specialist, and smoking cessation specialist, is embedded in the preoperative clinic. A patient is screened for frailty and individualized prehabilitation is prescribed based on the data. Medical comorbidity evaluation and optimization is occurring simultaneously. When a patient is deemed functionally and medically optimized, they are “cleared” for surgery. 76
Fig. 2.
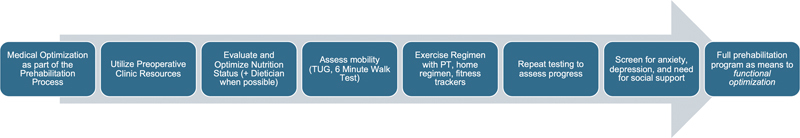
Incremental adoption of a prehabilitation pathway. A prehabilitation pathway can be started with available resources and incrementally expanded to a full program with the end point of functional optimization. PT, physical therapy; TUG, Timed-up-and-go.
Implementation of the PeRioperative Evaluation and Planning Pathway at Pennsylvania Hospital
The PeRioperative Evaluation and Planning (PREP) Program at Pennsylvania Hospital is based on the modified pathway presented in Fig. 3 . Over a 5-year period, a geriatric surgery was started using existing resources in the cancer center including physical therapy, nutrition, and palliative care specialists to implement frailty screening and personalized prehabilitation. Using the framework presented in a previous publication, 19 a pilot of the PREP program was started by hiring a full-time geriatric nurse practitioner for the surgical clinic in collaboration with the established geriatrics team. A business case was presented to hospital administration based on billing and cost-savings secondary to decreased length of stay and decreased readmission, and a full-time geriatrics nurse practitioner and geriatrician were funded by the hospital. Frailty assessment, postoperative, and functional outcomes as well as associated financial implications are tracked prospectively and will be reported in subsequent publications.
Fig. 3.
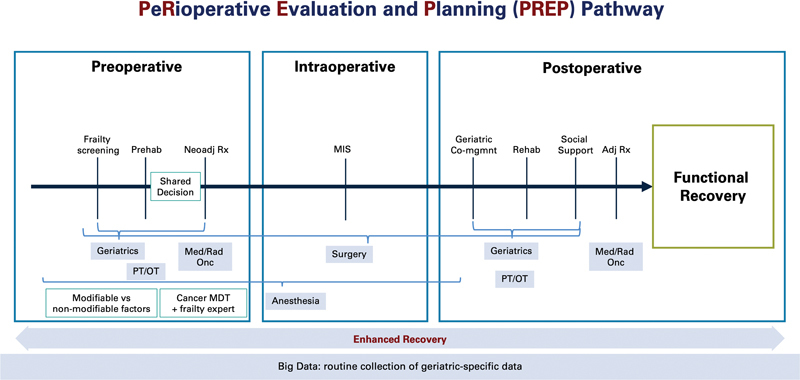
The PeRioperative Evaluation and Planning (PREP) pathway. The multiphase, multidisciplinary pathway for evaluation and optimization of patients in the perioperative period. (Reproduced with permission of Montroni et al. 1 ).
Conclusion
In conclusion, frailty screening and prehabilitation can be used and implemented as part of a PREP program for optimal management of frail patients undergoing colorectal surgery. Specific, geriatric-oriented, interventions can be undertaken with the help of a multidisciplinary team. Incremental adoption of patient-centered interventions can improve clinical outcomes, align care with patient goals, and help make a business case to expand the program.
Footnotes
Conflict of Interest None declared.
References
- 1.Montroni I, Saur N M, Shahrokni A, Suwanabol P A, Chesney T R. Surgical considerations for older adults with cancer: a multidimensional, multiphase pathway to improve care. J Clin Oncol. 2021;39(19):2090–2101. doi: 10.1200/JCO.21.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DuMontier C, Loh K P, Bain P A. Defining undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol. 2020;38(22):2558–2569. doi: 10.1200/JCO.19.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahrokni A, Alexander K. The age of talking about age alone is over. Ann Surg Oncol. 2019;26(01):12–14. doi: 10.1245/s10434-018-6983-7. [DOI] [PubMed] [Google Scholar]
- 4.Korc-Grodzicki B, Downey R J, Shahrokni A, Kingham T P, Patel S G, Audisio R A. Surgical considerations in older adults with cancer. J Clin Oncol. 2014;32(24):2647–2653. doi: 10.1200/JCO.2014.55.0962. [DOI] [PubMed] [Google Scholar]
- 5.Saur N M, Montroni I. Geriatric comanagement: a secret ingredient of the elusive recipe. JAMA Netw Open. 2020;3(08):e209460. doi: 10.1001/jamanetworkopen.2020.9460. [DOI] [PubMed] [Google Scholar]
- 6.Soto-Perez-de-Celis E, Li D, Yuan Y, Lau Y M, Hurria A. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(06):e305–e316. doi: 10.1016/S1470-2045(18)30348-6. [DOI] [PubMed] [Google Scholar]
- 7.Faye A S, Wen T, Soroush A. Increasing prevalence of frailty and its association with readmission and mortality among hospitalized patients with IBD. Dig Dis Sci. 2021;66(12):4178–4190. doi: 10.1007/s10620-020-06746-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons . Saur N M, Davis B R, Montroni I. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the perioperative evaluation and management of frailty among older adults undergoing colorectal surgery. Dis Colon Rectum. 2022;65(04):473–488. doi: 10.1097/DCR.0000000000002410. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(01):17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Robinson T N, Walston J D, Brummel N E. Frailty for surgeons: Review of a National Institute on Aging Conference on Frailty for Specialists. J Am Coll Surg. 2015;221(06):1083–1092. doi: 10.1016/j.jamcollsurg.2015.08.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berian J R, Wolf J H, Kunitake H. Much to do about frailty. Dis Colon Rectum. 2022;65(04):457–460. doi: 10.1097/DCR.0000000000002381. [DOI] [PubMed] [Google Scholar]
- 12.van Walree I C, Scheepers E, van Huis-Tanja L. A systematic review on the association of the G8 with geriatric assessment, prognosis and course of treatment in older patients with cancer. J Geriatr Oncol. 2019;10(06):847–858. doi: 10.1016/j.jgo.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Huisman M G, Audisio R A, Ugolini G. Screening for predictors of adverse outcome in onco-geriatric surgical patients: a multicenter prospective cohort study. Eur J Surg Oncol. 2015;41(07):844–851. doi: 10.1016/j.ejso.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Bessems S AM, Konsten J LM, Vogelaar J FJ. Frailty screening by Geriatric-8 and 4-meter gait speed test is feasible and predicts postoperative complications in elderly colorectal cancer patients. J Geriatr Oncol. 2021;12(04):592–598. doi: 10.1016/j.jgo.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Moriello C, Mayo N E, Feldman L, Carli F. Validating the six-minute walk test as a measure of recovery after elective colon resection surgery. Arch Phys Med Rehabil. 2008;89(06):1083–1089. doi: 10.1016/j.apmr.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 16.George E L, Hall D E, Youk A. Association between patient frailty and postoperative mortality across multiple noncardiac surgical specialties. JAMA Surg. 2021;156(01):e205152. doi: 10.1001/jamasurg.2020.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Khamis A, Warner C, Park J. Modified frailty index predicts early outcomes after colorectal surgery: an ACS-NSQIP study. Colorectal Dis. 2019;21(10):1192–1205. doi: 10.1111/codi.14725. [DOI] [PubMed] [Google Scholar]
- 18.Pata G, Bianchetti L, Rota M. Multidimensional Prognostic Index (MPI) score has the major impact on outcome prediction in elderly surgical patients with colorectal cancer: The FRAGIS study. J Surg Oncol. 2021;123(02):667–675. doi: 10.1002/jso.26314. [DOI] [PubMed] [Google Scholar]
- 19.Saur N M, Montroni I, Shahrokni A. Care of the geriatric colorectal surgical patient and framework for creating a geriatric program: a compendium from the 2019 American Society of Colon and Rectal Surgeons Annual Meeting. Dis Colon Rectum. 2020;63(11):1489–1495. doi: 10.1097/DCR.0000000000001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seishima R, Okabayashi K, Hasegawa H. Is laparoscopic colorectal surgery beneficial for elderly patients? A systematic review and meta-analysis. J Gastrointest Surg. 2015;19(04):756–765. doi: 10.1007/s11605-015-2748-9. [DOI] [PubMed] [Google Scholar]
- 21.Antoniou S A, Antoniou G A, Koch O O, Pointner R, Granderath F A. Laparoscopic colorectal surgery confers lower mortality in the elderly: a systematic review and meta-analysis of 66,483 patients. Surg Endosc. 2015;29(02):322–333. doi: 10.1007/s00464-014-3672-x. [DOI] [PubMed] [Google Scholar]
- 22.Vallribera Valls F, Landi F, Espín Basany E. Laparoscopy-assisted versus open colectomy for treatment of colon cancer in the elderly: morbidity and mortality outcomes in 545 patients. Surg Endosc. 2014;28(12):3373–3378. doi: 10.1007/s00464-014-3597-4. [DOI] [PubMed] [Google Scholar]
- 23.Chesney T R, Quereshy H A, Draginov A, Chadi S A, Quereshy F A. Benefits of minimally-invasive surgery for sigmoid and rectal cancer in older adults compared with younger adults: do older adults have the most to gain? J Geriatr Oncol. 2020;11(05):860–865. doi: 10.1016/j.jgo.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 24.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . Panel A GSBCUE. American Geriatrics Society 2019 Updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(04):674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 25.Fleet J L, Dixon S N, Kuwornu P J. Gabapentin dose and the 30-day risk of altered mental status in older adults: a retrospective population-based study. PLoS One. 2018;13(03):e0193134. doi: 10.1371/journal.pone.0193134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tejedor P, Pastor C, Gonzalez-Ayora S, Ortega-Lopez M, Guadalajara H, Garcia-Olmo D. Short-term outcomes and benefits of ERAS program in elderly patients undergoing colorectal surgery: a case-matched study compared to conventional care. Int J Colorectal Dis. 2018;33(09):1251–1258. doi: 10.1007/s00384-018-3057-z. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Ayora S, Pastor C, Guadalajara H. Enhanced recovery care after colorectal surgery in elderly patients. Compliance and outcomes of a multicenter study from the Spanish working group on ERAS. Int J Colorectal Dis. 2016;31(09):1625–1631. doi: 10.1007/s00384-016-2621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Suo J, Jiang J, Wang C, Zhao Y Q, Cao X. Effectiveness of fast-track rehabilitation vs conventional care in laparoscopic colorectal resection for elderly patients: a randomized trial. Colorectal Dis. 2012;14(08):1009–1013. doi: 10.1111/j.1463-1318.2011.02855.x. [DOI] [PubMed] [Google Scholar]
- 29.Forsmo H M, Erichsen C, Rasdal A, Körner H, Pfeffer F. Enhanced Recovery After Colorectal Surgery (ERAS) in elderly patients is feasible and achieves similar results as in younger patients. Gerontol Geriatr Med. 2017;3:2.333721417706299E15. doi: 10.1177/2333721417706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia Y, Jin G, Guo S. Fast-track surgery decreases the incidence of postoperative delirium and other complications in elderly patients with colorectal carcinoma. Langenbecks Arch Surg. 2014;399(01):77–84. doi: 10.1007/s00423-013-1151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostermann S, Morel P, Chalé J J. Randomized controlled trial of enhanced recovery program dedicated to elderly patients after colorectal surgery. Dis Colon Rectum. 2019;62(09):1105–1116. doi: 10.1097/DCR.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 32.National Institutes of Health Consensus Development Conference Statement: geriatric assessment methods for clinical decision-making. J Am Geriatr Soc. 1988;36(04):342–347. doi: 10.1111/j.1532-5415.1988.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 33.Puts M TE, Alibhai S MH. Fighting back against the dilution of the comprehensive geriatric assessment. J Geriatr Oncol. 2018;9(01):3–5. doi: 10.1016/j.jgo.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 34.PACE participants . Audisio R A, Pope D, Ramesh H S. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol. 2008;65(02):156–163. doi: 10.1016/j.critrevonc.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Katlic M R, Coleman J, Khan K, Wozniak S E, Abraham J H. Sinai abbreviated geriatric evaluation: development and validation of a practical test. Ann Surg. 2019;269(01):177–183. doi: 10.1097/SLA.0000000000002597. [DOI] [PubMed] [Google Scholar]
- 36.Huisman M G, van Leeuwen B L, Ugolini G. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One. 2014;9(01):e86863. doi: 10.1371/journal.pone.0086863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones T S, Dunn C L, Wu D S, Cleveland J C, Jr, Kile D, Robinson T N. Relationship between asking an older adult about falls and surgical outcomes. JAMA Surg. 2013;148(12):1132–1138. doi: 10.1001/jamasurg.2013.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall D E, Arya S, Schmid K K. Development and initial validation of the Risk Analysis Index for measuring frailty in surgical populations. JAMA Surg. 2017;152(02):175–182. doi: 10.1001/jamasurg.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall D E, Arya S, Schmid K K. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152(03):233–240. doi: 10.1001/jamasurg.2016.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dirks R C, Edwards B L, Tong E. Sarcopenia in emergency abdominal surgery. J Surg Res. 2017;207:13–21. doi: 10.1016/j.jss.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 41.van der Windt D J, Bou-Samra P, Dadashzadeh E R, Chen X, Varley P R, Tsung A. Preoperative Risk Analysis Index for frailty predicts short-term outcomes after hepatopancreatobiliary surgery. HPB (Oxford) 2018;20(12):1181–1188. doi: 10.1016/j.hpb.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Keller D S, Bankwitz B, Nobel T, Delaney C P. Using frailty to predict who will fail early discharge after laparoscopic colorectal surgery with an established recovery pathway. Dis Colon Rectum. 2014;57(03):337–342. doi: 10.1097/01.dcr.0000442661.76345.f5. [DOI] [PubMed] [Google Scholar]
- 43.Panayi A C, Orkaby A R, Sakthivel D. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. 2019;218(02):393–400. doi: 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahl T S, Graham L A, Hawn M T. Association of the modified frailty index with 30-day surgical readmission. JAMA Surg. 2017;152(08):749–757. doi: 10.1001/jamasurg.2017.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Culley D J, Flaherty D, Fahey M C. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127(05):765–774. doi: 10.1097/ALN.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SIOG surgical task force/ESSO GOSAFE study group . Montroni I, Rostoft S, Spinelli A. GOSAFE: Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery—early analysis on 977 patients. J Geriatr Oncol. 2020;11(02):244–255. doi: 10.1016/j.jgo.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Watt J, Tricco A C, Talbot-Hamon C. Identifying older adults at risk of delirium following elective surgery: a systematic review and meta-analysis. J Gen Intern Med. 2018;33(04):500–509. doi: 10.1007/s11606-017-4204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddiqi N, Harrison J K, Clegg A. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev. 2016;3:CD005563. doi: 10.1002/14651858.CD005563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zattoni D, Montroni I, Saur N M. A simple screening tool to predict outcomes in older adults undergoing emergency general surgery. J Am Geriatr Soc. 2019;67(02):309–316. doi: 10.1111/jgs.15627. [DOI] [PubMed] [Google Scholar]
- 50.Williams G RWK, Weaver K E, Lesser G J. Capacity to provide geriatric specialty care for older adults in community oncology practices. Oncologist. 2020;25(12):1032–1038. doi: 10.1634/theoncologist.2020-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shahrokni A, Tin A L, Sarraf S. Association of geriatric comanagement and 90-day postoperative mortality among patients aged 75 years and older with cancer. JAMA Netw Open. 2020;3(08):e209265. doi: 10.1001/jamanetworkopen.2020.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarazona-Santabalbina F J, Llabata-Broseta J, Belenguer-Varea Á, Álvarez-Martínez D, Cuesta-Peredo D, Avellana-Zaragoza J A. A daily multidisciplinary assessment of older adults undergoing elective colorectal cancer surgery is associated with reduced delirium and geriatric syndromes. J Geriatr Oncol. 2019;10(02):298–303. doi: 10.1016/j.jgo.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Dent E, Morley J E, Cruz-Jentoft A J. Physical frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J Nutr Health Aging. 2019;23(09):771–787. doi: 10.1007/s12603-019-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hawkins A T, Pallangyo A J, Herman A M. The effect of social integration on outcomes after major lower extremity amputation. J Vasc Surg. 2016;63(01):154–162. doi: 10.1016/j.jvs.2015.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez-Saenz de Tejada M, Bilbao A, Baré M. Association of social support, functional status, and psychological variables with changes in health-related quality of life outcomes in patients with colorectal cancer. Psychooncology. 2016;25(08):891–897. doi: 10.1002/pon.4022. [DOI] [PubMed] [Google Scholar]
- 56.Gardner B, Jovicic A, Belk C. Specifying the content of home-based health behaviour change interventions for older people with frailty or at risk of frailty: an exploratory systematic review. BMJ Open. 2017;7(02):e014127. doi: 10.1136/bmjopen-2016-014127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Minnella E M, Carli F. Prehabilitation and functional recovery for colorectal cancer patients. Eur J Surg Oncol. 2018;44(07):919–926. doi: 10.1016/j.ejso.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 58.Waite I, Deshpande R, Baghai M, Massey T, Wendler O, Greenwood S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg. 2017;12(01):91. doi: 10.1186/s13019-017-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chia C LKMS, Mantoo S K, Tan K Y. “Start to finish trans-institutional transdisciplinary care”: a novel approach improves colorectal surgical results in frail elderly patients. Colorectal Dis. 2016;18(01):O43–O50. doi: 10.1111/codi.13166. [DOI] [PubMed] [Google Scholar]
- 60.Mazzola M, Bertoglio C, Boniardi M. Frailty in major oncologic surgery of upper gastrointestinal tract: How to improve postoperative outcomes. Eur J Surg Oncol. 2017;43(08):1566–1571. doi: 10.1016/j.ejso.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 61.Hoogeboom T J, Dronkers J J, van den Ende C H, Oosting E, van Meeteren N L. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil. 2010;24(10):901–910. doi: 10.1177/0269215510371427. [DOI] [PubMed] [Google Scholar]
- 62.Oosting E, Jans M P, Dronkers J J. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2012;93(04):610–616. doi: 10.1016/j.apmr.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Kamarajah S K, Bundred J, Weblin J, Tan B HL. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery. 2020;167(03):540–549. doi: 10.1016/j.surg.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 64.Carli F, Bessissow A, Awasthi R, Liberman S. Prehabilitation: finally utilizing frailty screening data. Eur J Surg Oncol. 2020;46(03):321–325. doi: 10.1016/j.ejso.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Hedrick T L, Hassinger T E, Myers E. Wearable technology in the perioperative period: predicting risk of postoperative complications in patients undergoing elective colorectal surgery. Dis Colon Rectum. 2020;63(04):538–544. doi: 10.1097/DCR.0000000000001580. [DOI] [PubMed] [Google Scholar]
- 66.Kane W J, Hassinger T E, Myers E L. Wearable technology and the association of perioperative activity level with 30-day readmission among patients undergoing major colorectal surgery. Surg Endosc. 2022;36(02):1584–1592. doi: 10.1007/s00464-021-08449-3. [DOI] [PubMed] [Google Scholar]
- 67.Hamaker M E, Oosterlaan F, van Huis L H, Thielen N, Vondeling A, van den Bos F. Nutritional status and interventions for patients with cancer: a systematic review. J Geriatr Oncol. 2020;20:S1879–S4068. doi: 10.1016/j.jgo.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 68.Kamel H K.Sarcopenia and aging Nutr Rev 200361(5, Pt 1):157–167. [DOI] [PubMed] [Google Scholar]
- 69.Hargreaves M.Pre-exercise nutritional strategies: effects on metabolism and performance Can J Appl Physiol 200126(Suppl):S64–S70. [DOI] [PubMed] [Google Scholar]
- 70.Leung J M, Sands L P, Mullen E A, Wang Y, Vaurio L. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci. 2005;60(12):1563–1568. doi: 10.1093/gerona/60.12.1563. [DOI] [PubMed] [Google Scholar]
- 71.Kristjansson J M, Sr, Nesbakken A, Skovlund E, Bakka A, Johannessen H, Wyller T B. Which elements of a comprehensive geriatric assessment (CGA) predict post-operative complications and early mortality after colorectal cancer surgery? J Geriatr Oncol. 2010;1(02):57–65. [Google Scholar]
- 72.Parker P A, Pettaway C A, Babaian R J. The effects of a presurgical stress management intervention for men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2009;27(19):3169–3176. doi: 10.1200/JCO.2007.16.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carli F, Bousquet-Dion G, Awasthi R. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155(03):233–242. doi: 10.1001/jamasurg.2019.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillis C, Fenton T R, Gramlich L. Older frail prehabilitated patients who cannot attain a 400 m 6-min walking distance before colorectal surgery suffer more postoperative complications. Eur J Surg Oncol. 2021;47(04):874–881. doi: 10.1016/j.ejso.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 75.SIOG Surgical Task Force/ESSO GOSAFE Study Group . Montroni I, Ugolini G, Saur N M. Quality of life in older adults after major cancer surgery: the GOSAFE international study. J Natl Cancer Inst. 2022;114(07):969–978. doi: 10.1093/jnci/djac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carli F, Baldini G, Feldman L S. Redesigning the preoperative clinic: from risk stratification to risk modification. JAMA Surg. 2021;156(02):191–192. doi: 10.1001/jamasurg.2020.5550. [DOI] [PubMed] [Google Scholar]
- 77.Tatar C, Benlice C, Delaney C P. Modified frailty index predicts high-risk patients for readmission after colorectal surgery for cancer. Am J Surg. 2020;220(01):187–190. doi: 10.1016/j.amjsurg.2019.11.016. [DOI] [PubMed] [Google Scholar]


