Abstract

Fungal infections are a major public health problem resulting from the lack of public policies addressing these diseases, toxic and/or expensive therapeutic tools, scarce diagnostic tests, and unavailable vaccines. In this Perspective, we discuss the need for novel antifungal alternatives, highlighting new initiatives based on drug repurposing and the development of novel antifungals.
Keywords: Antifungals drugs, Antifungal pipeline, Fungal infections, Emerging fungal species, Neglected diseases
The Need for New Antifungals
Nearly 6 million different species of fungi exist, and about 600 of them can cause human disease. Fungal diseases represent a health concern, because of high mortality rates and constant problems with antifungal resistance.1 There are no licensed vaccines to prevent fungal diseases.
Fungal diseases have an extreme impact on public health. More than one million humans die every year from fungal diseases, a dramatic scenario that is aggravated by scarce funding for research,2 lack of awareness of public health authorities3 reduced global access to antifungals,4 antifungal resistance,5 and insufficient alternatives for accurate diagnosis.6 The number of cases of fungal infections is still growing, notably in immunocompromised patients, but there are still a few options of treatment and the ones available are linked to high costs and toxicity.4,7−9 Many mycoses require hospitalization, and the most appropriate drugs for combating them have low availability in territories where fungal infections are more prevalent.3,9−11 This problem is aggravated by the growing emergence of species resistant to the available therapies.12−15
There are four main families of antifungals: polyenes, which bind to ergosterol and produce membrane pores; azoles, which inhibit sterol 14 demethylase and reduce the levels of ergosterol at the plasma membrane; echinocandins, which inhibit the synthesis of β-1,3-glucan in the wall, and pyrimidine analogues, which inhibit the synthesis of RNA and DNA.16−19 The most frequently used antifungals are all associated with therapeutic failures. Amphotericin B, for example, is highly toxic, and its less toxic pharmaceutic preparations are excessively expensive.7,9 Among the azoles, an increase in the antifungal resistance has been continuously reported in Candida spp., Cryptococcus spp. and Aspergillus spp.20−22 Emergence of resistance can be associated with the development of aneuploidy when azoles are used as monotherapy.23−26 Even the newest azoles, including isavuconazole, which is indicated mainly for the treatment of aspergillosis, candidiasis, and mucormycosis, were linked to the emergence of resistant isolates.27 The resistance mechanisms are likely similar to those that lead to fluconazole resistance.27,28 Echinocandins have also been associated with resistance due to amino acid changes in the FKS-subunits of glucan synthase.16 Monotherapy with pyrimidine analogues drug is also commonly associated with resistance.29−31 The need for more alternatives to fight fungal infections led to the search for novel drugs and cellular targets. Recently, turbinmicin was identified as a promising and safe antifungal targeting the vesicular trafficking pathway.32 Other examples include small molecules targeting protein splicing33 and hydrazycins inhibiting the synthesis of fungal sphingolipids.34
Antifungal resistance is now a topic that necessarily includes Candida auris, an emerging fungal pathogen that has gained great importance in recent years due to its intrinsic resistance to antifungals and great ability to spread in the environment, affecting especially immunocompromised patients in the hospital environment.35−37C. auris was first reported in 2009 at a Japanese hospital and since then, it was associated with disease in more than 30 countries from all continents.20,37−41 In the US, for example, the Centers for Disease Control and Prevention (CDC) reported a significant increase in C. auris infections, from 309 cases in 2013 to 1012 cases in 2018.42 The impact of the emergence of C. auris on public health is directly linked to the fact that C. auris can be resistant to azoles, echinocandins, and polyenes.39,43 In fact, multidrug-resistant (MDR) isolates have been frequently reported worldwide, including resistance to two or more classes of antifungals.13,44−46 Some of the C. auris isolates show an alarmingly low susceptibility to amphotericin B.12,13,20,35,41,42 In this scenario, drugs affecting C. auris growth have been continuously investigated, with considerable progress reported for molecules affecting the fungal mitochondria, plasma membrane, biofilms, and cell wall.47,48
Antifungal resistance is not only problematic in C. auris. Aspergillus species, in particular A. fumigatus, are mostly responsible for mold infections, affecting mainly immunocompromised patients.49Aspergillus spp. frequently cause fatal respiratory diseases.50,51 In this genus, novel resistant variants of previously susceptible pathogens have been reported.5 Mold infections caused by non-Aspergillus species have also increased significantly, including cases of life-threatening infections.51,52 Such diseases range from superficial to severe invasive infections including immunocompetent patients.52,53Fusarium, Scedosporium, Lomentospora, and mucormycete species are the main causative agents of these mold infections.41,50,54 Several of them are resistant to the currently available antifungals.50,54
The already dynamic picture connecting fungal diseases and public health has become even more complex since the beginning of 2020 with the pandemic scenario of coronavirus disease (COVID-19) caused by SARS-CoV-2. Fungal diseases were associated with COVID-19 mostly in individuals with weakened immune status in intensive care units (ICUs).55,56 Mucormycosis, a mold infection mostly caused by Rhizopus species, was frequently associated with COVID-19 with high morbidity and mortality.14,57 Diseases caused by Aspergillus, Lichtheimia, Mucor, Lomentospora and Candida auris also impacted COVID-19 patients negatively.55,56,58 This complex scenario illustrates the urgency for new antifungal therapies.
A Better Use for Existing Tools: The Optimized Application of the Currently Available Antifungals
A few years ago, Kneale and colleagues claimed that “national governments without access to antifungal drugs should address this health system deficiency urgently to improve clinical outcomes from serious fungal disease”, a conclusion based on the striking variability in the price of antifungals between countries.4 In fact, in developing countries the cost of treating mycoses patients with liposomal amphotericin B can exceed USD 100,000.9 Amphotericin B and flucytosine are not available in most of the countries that are heavily affected by fungal diseases.59 This scenario illustrates the need for rethinking the access to antifungal treatment and diagnosis. As previously proposed, rapid diagnostic tools and the rational use of already available antifungal agents would likely have a major impact in reducing deaths.60
The successful, rational use of antifungals in clinics demands a plan of access, as illustrated in the case of cryptococcal meningitis. In this disease, it was recently demonstrated that, compared to the treatment currently recommended by the World Health Organization (WHO), a regimen of a single dose of 10 mg/kg of liposomal amphotericin B followed by 14 days of treatment with 100 mg/kg/day of flucytosine and 1200 mg/day of fluconazole had a similar efficacy and fewer adverse events.61 This observation will likely benefit clinical practice. However, as well pointed out by Falci and Pasqualotto,62a major issue is whether this regimen can be made available and affordable in regions that are most heavily affected by cryptococcosis, including the sub-Saharan Africa, Latin America, and Southeast Asia. They properly remind readers that only four countries in sub-Saharan Africa have access to liposomal amphotericin B62 and it is unavailable in most public health systems in Latin America. The situation is similar, if not worse, for other fungal neglected diseases. Therefore, changes in clinical practice and the development of novel antifungals must be accompanied by a plan of effective implementation for populations that are affected by fungal diseases.
New Antifungal Alternatives
More effective, less toxic, and affordable antifungal drugs have the potential to save thousands of lives. The pace of knowledge generation of antifungals is slower than that of, for instance, antibacterial agents.63 However, in recent years, solid progress has been made in the field of antifungals, as follows below.
Drug Repurposing
Repurposing of drugs consists of finding new applications for already known and clinically approved drugs.64,65 Repurposing is cheaper and faster than the conventional process of drug development discovery.66−69 A classic–and the oldest–example of drug repurposing is aspirin, which was marketed in 1899 as an analgesic and first repositioned in the 1980s as an antiplatelet aggregation drug.70
In the case of pathogenic fungi, drug repurposing emerged as a promising approach to combat C. neoformans,47,71−74Candida spp. (including C. auris),47,75−82Aspergillus spp.,83Scedosporium and Lomentospora species,54 the chromoblastomycosis agents Fonsecaea pedrosoi, F. monophora, F. nubica, Cladophialophora carrionii, Phialophora verrucosa, Exophiala jeanselmei, E. dermatitidis, and Rhinocladiella similis,84 and the dimorphic fungi Sporothrix spp.85 These studies revealed a collection of molecules with potential to be applied in the treatment of the diseases caused by members of the above-mentioned genera. In fact, examples of antifungal candidates that were clinically tested are available. For instance, sertraline, a selective serotonin reuptake inhibitor antidepressant, showed in vivo and in vitro anticryptococcal activity.86,87 The antifungal mechanism of action of sertraline remains unknown. However, a few putative mechanisms have been suggested, including cell death mediated by the insertion of sertraline into the phospholipid membrane layers of intracellular organelles,88 inhibition of ergosterol synthesis through sertraline binding to lanosterol 14-demethylase (CYP51) protein,89 and alterations in the lipid metabolism.90 Sertraline was clinically tested as an adjunctive antifungal therapy in HIV-infected individuals with cryptococcal meningitis.91 Antifungal treatment using sertraline as an adjuvant resulted in faster cryptococcal cerebrospinal fluid clearance with decreased relapse.92 However, serious adverse events were associated with sertraline treatment, including persistent psychosis and aggressive behavioral changes, which led to treatment interruption.91 Although sertraline failed to achieve the expected therapeutic effects, novel derivatives of this drugs were recently designed by scaffold hopping, and they showed improved anticryptococcal activity both in vitro and in vivo.93 Similarly, a derivative of haloperidol caused fungal membrane damage and down-regulation of ERG11 and MDR1 genes when used in combination with fluconazole.94 These observations illustrate how drug repurposing and development of new drugs can be efficiently connected. There are several ongoing initiatives for repurposing drugs against cryptococcosis and other mycoses,47,68,73,76,95−98 which may result in new therapeutic alternatives in a near future.
Antifungals under Development
In the following paragraphs, we will illustrate the recent activity resulting in candidates for developing novel antifungals.
Inhibition of the 14-demethylase enzyme is a known mechanism of action of the classic azoles and the recently identified antifungals opelconazole (PC945) and oteseconazole (VT-1161). Opelconazole (developed by Pulmocide Ltd., London, UK) is an inhaled triazole presenting wide-spectrum activity against Candida (including C. auris), Aspergillus, Rhyzopus, and Cryptococcus spp.99 Opelconazole acts through the inhibition of lanosterol 14-demethylase (CYP51A1), impairing the conversion of lanosterol to ergosterol. Deficient ergosterol synthesis affects fungal membranes and prevents growth.99 Preclinical studies showed that nebulization with opelconazole resulted in an increased drug concentration in the lungs of rats.28,99,100 The triazole manifested no safety concerns in preclinical studies101 and showed persistent action on epithelial cells and hypha in vitro. In mice, intranasal treatment with opelconazole was more efficient against A. fumigatus than voriconazole or posaconazole.102 Opelconazole was successfully and safely used to treat a refractory Aspergillus bronchial infection in a lung transplant patient.103 A human clinical trial revealed that opelconazole was well tolerated in both healthy subjects and subjects with mild asthma, with no evidence of acute bronchospasm/irritancy.100 Phase 2 clinical trials with lung transplant patients are ongoing in the United States (ClinicalTrials.gov Identifier: NCT05037851), which places opelconazole as a promising candidate to be clinically used as an antifungal agent.
Another new antifungal targeting 14-demethylase is oteseconazole (VT-1161), developed by Mycovia Pharmaceuticals, USA. Oteseconazole is an orally administered azole that was recently approved by the U.S. Food and Drug Administration agency (FDA) for the treatment of recurrent vulvovaginal candidiasis.104 Oteseconazole also showed activity in vitro against several fungal species such as Cryptococcus spp., Mucorales, Coccidioides immitis, Trichophyton rubrum, and Rhizopus arrhizus.11
Rezafungin (CD101) is a second-generation echinocandin105 that is in Phase 3 of development (ClinicalTrials.gov Identifier: NCT02734862). Rezafungin manifests promising pharmacokinetic/pharmacodynamic properties and, as other echinocandins, acts as an inhibitor of β-1,3-d-glucan synthase, a cell-wall enzyme complex required to maintain the regular cell-wall structure.99,105 Rezafungin results from the addition of a choline moiety to the echinocandin structure resulting in a higher affinity for 1,3-β-d-glucan synthase.106 This modification led to a prolonged half-life with improvement of in vitro activity against the pathogens Candida spp. (including C. auris), Pneumocystis jirovecii, Trichophyton mentagrophytes, Trichophyton rubrum, Microsporum gypseum, and Aspergillus spp.28,106−108 This new echinocandin manifested optimized pharmacokinetic/pharmacodynamic profiles after single or weekly intravenous doses.28,108
Ibrexafungerp is a triterpenoid that also targets 1,3-β-d-glucan synthase. The drug was approved by the FDA in January 2021 for the treatment of vaginal infections with Candida. Ibrexafungerp is mainly recommended for the treatment of candidiasis among patients with refractory responses to echinocandin and/or azoles. Ibrexafungerp is orally bioavailable, making it the only oral glucan synthase inhibitor developed so far. Besides being active against Candida species, ibrexafungerp also controls the growth of a variety of Aspergillus species.28,109−111
Some of the new antifungals in the development pipeline exert their activities through novel mechanisms of action. Fosmanogepix (APX001) is a novel antifungal that is currently in Phase 2 trial (ClinicalTrials. gov Identifier: NCT04240886). It controls fungal growth through the inhibition of Gwt1, an enzyme responsible for anchoring mannoproteins through the glycosylphosphatidylinositol (GPI) biosynthesis pathway at the fungal cell surface.112−114 These anchored mannoproteins are essential for fungal adhesion to mucosal and epithelial surfaces within the host prior to colonization.99 An essential part of the GPI biosynthesis pathway is an acyl transfer reaction that is catalyzed by Gwt1. This enzyme is responsible for adding an acyl group to the inositol unit of glucosaminyl phosphatidylinositol (GlcN-PI), a key step in the biosynthesis of GPI anchors.114 The active compound of fosmanogepix, manogepix, has broad spectrum activity, including antifungal effects against Candida spp., Cryptococcus spp., Aspergillus spp., Fusarium spp., Scedosporium spp., and L. prolificans.114−119 Fosmanogepix can reach the central nervous system, which places this drug as potential candidate to fight cryptococcal meningitis.28,114,120,121 A recent study in mice demonstrated that fosmanogepix is synergistic with amphotericin B against invasive pulmonary aspergillosis, invasive mucormycosis, and invasive fusariosis.122
Olorofim (F901318) belongs to the orotomides class of antifungals. Olorofim interrupts pyrimidine synthesis by inhibiting the enzyme dihydroorotate dehydrogenase.11,123 Dihydroorotate dehydrogenase participates in the de novo pyrimidine biosynthesis pathway, which is required for DNA, RNA, protein, and cell wall syntheses.123 This drug is a potential anti-Aspergillus candidate, being active against azole resistant strains.28,113,124−126 Olorofim also presents activity at relatively low MICs against L. prolificans and species of Scedosporium,127Histoplasma, Blastomyces, and Coccidioides.28,128 The drug is also active against dermatophytes species such as Trichophyton spp., Epidermophyton spp., and Microsporum spp.129 Olorofim lacks activity against Candida, Cryptococcus, and Mucorales species. Currently, olorofim is in phase 2 trial for invasive fungal infections in patients without other alternatives, and in phase 3 trial for comparison with amphotericin B for invasive aspergillosis.28,113,125
ATI-2307 (T-2307) is an arylamidine that presents antifungal activity against Cryptococcus spp.,130,131Candida spp. including C. auris,131−135 and Aspergillus spp.131 Its mechanism of action requires the inhibition of the respiratory complexes III and IV in fungal mitochondria.136 This drug reaches the mitochondria through a polyamine transporter related to the uptake of spermine and spermidine. ATI-2307 then promotes disruption of the mitochondrial membrane potential, leading to dysfunction.136−138 ATI-2307 passed through phase 1 trial.139 Phase 2 study is about to begin.113
Perspectives
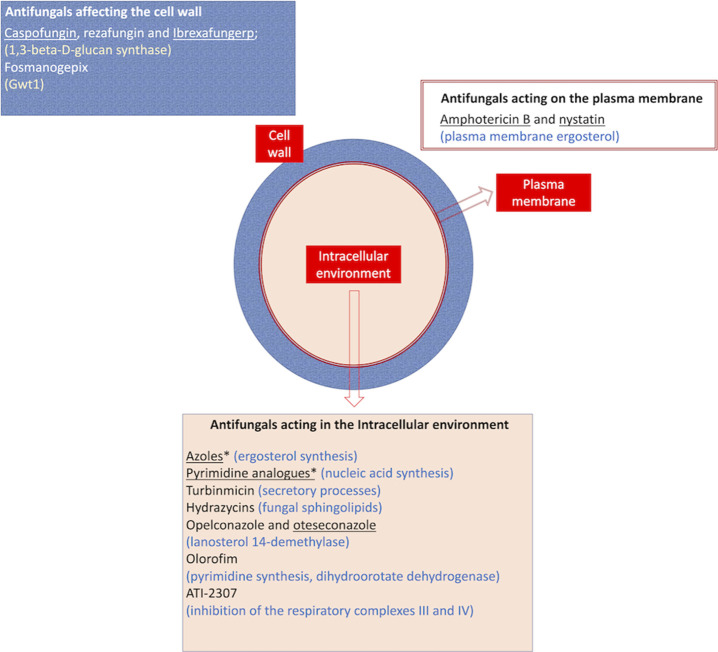
Currently available and under development antifungals are summarized in Figure 1.
Figure 1.
Summary of the current scenario of antifungal therapy. Drugs were classified according to their cellular sites of action, and their targets are described in parentheses. Antifungals that were approved for clinical use were underlined, while the other drugs are under different stages of development. Asterisks denote drug families that have been extensively reviewed before, so individual compounds were not listed. Please refer to Figure 2 for structural details of the listed antifungals.
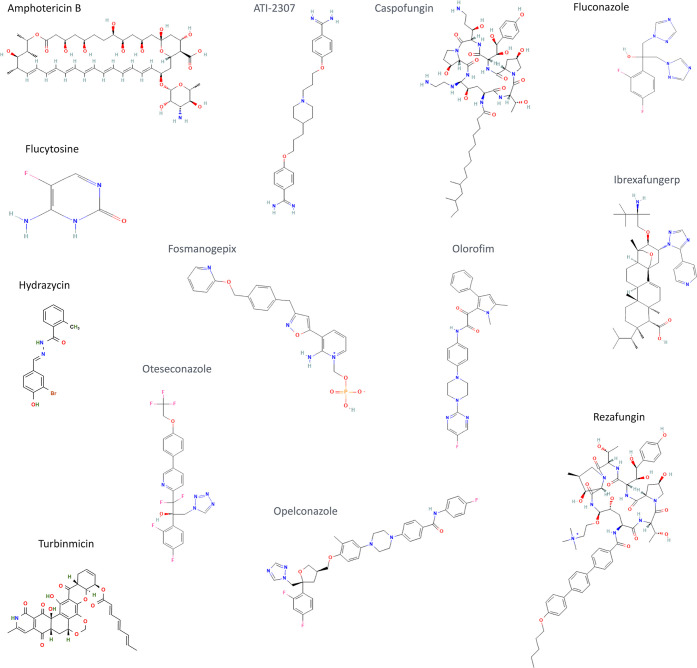
Figure 2.
Structural aspects of clinically approved or under development antifungals. Structures were obtained from Pubchem (https://pubchem.ncbi.nlm.nih.gov; no permission for reproduction required, according to the Pubchem citation guidelines; https://pubchemdocs.ncbi.nlm.nih.gov/citation-guidelines). Amphotericin B, fluconazole, and flucytosine illustrate some of the structural aspects of polyenes, azoles, and pyrimidine analogues, respectively.
The need for changes in the fight against fungal infections is unquestionable. Considering the urgency in improving treatment, prevention and diagnosing of fungal infections, we believe some multidisciplinary actions could attenuate the damage imposed by these diseases on public health. We list below actions that we believe would be beneficial for fighting fungal diseases:
-
(1)
Increase the awareness that fungal diseases are widely neglected, and that programs to fight them are necessary worldwide. In this sense, WHO developed the first global fungal priority pathogens list to define research and development priorities to align investments with public health needs.140 This is a commendable action that will likely stimulate funding agencies to launch programs to combat the most important fungal diseases. In addition, promoting the teaching of Medical Mycology in undergraduate or graduate programs of medical and biological sciences will likely expand the awareness of fungal diseases at all levels.
-
(2)
Improve funding to stimulate research and technological development in the field of fungal diseases. As already discussed in this manuscript, funding for fungal research is smaller than that available for other areas with similar impact on public health. As scientists in this field, we empirically see that we and other colleagues must compete for funding with scientists from all fields of infectious diseases. Mycology-focused programs aiming at developing tools to prevent, treat and combat fungal diseases will likely have a tremendous impact on the generation of knowledge and innovation in this area.
-
(3)
Make the rational use of already existing tools accessible globally. The understanding that fungal diseases mostly affect neglected populations is essential for the implementation of programs to combat lethal mycoses with governmental support. The clear correlation between low socioeconomic status and incidence of lethal mycoses is an alert to public health decision makers that making tools accessible is not less important than developing and/or using them rationally.
Author Contributions
CRediT: Haroldo Cesar de Oliveira conceptualization (equal), writing-original draft (lead); Barbara T. Bezerra conceptualization (supporting), validation (supporting), writing-original draft (supporting); Marcio L. Rodrigues conceptualization (lead), writing-review & editing (lead).
We received no specific funding for this article but received scholarships from the Inova Program of Fiocruz (H.C.O.), the Undergraduate Program (PIBIC) of Fiocruz, sponsored by the National Council for Scientific and Technological Development (CNPq) (B.T.B.), the Fulbright Commission (M.L.R.) and the Scientific Productivity Program of CNPq (M.L.R.).
The authors declare no competing financial interest.
Author Status
M.L.R. is currently on leave from the position of associate professor at the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil.
References
- Banerjee S.; Denning D.; Chakrabarti A. One Health Aspects & Priority Roadmap for Fungal Diseases : A Mini-Review. Indian Journal of Medical Research 2021, 153 (3), 311. 10.4103/ijmr.IJMR_768_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. L.; Albuquerque P. C. Searching for a Change: The Need for Increased Support for Public Health and Research on Fungal Diseases. PLoS Negl Trop Dis 2018, 12 (6), e0006479. 10.1371/journal.pntd.0006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M. L.; Nosanchuk J. D. Fungal Diseases as Neglected Pathogens: A Wake-up Call to Public Health Officials. PLoS Negl Trop Dis 2020, 14 (2), e0007964. 10.1371/journal.pntd.0007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneale M.; Bartholomew J. S.; Davies E.; Denning D. W. Global Access to Antifungal Therapy and Its Variable Cost. J. Antimicrob. Chemother. 2016, 71 (12), 3599–3606. 10.1093/jac/dkw325. [DOI] [PubMed] [Google Scholar]
- Fisher M. C.; Alastruey-Izquierdo A.; Berman J.; Bicanic T.; Bignell E. M.; Bowyer P.; Bromley M.; Brüggemann R.; Garber G.; Cornely O. A.; Gurr; Sarah J.; Harrison T. S.; Kuijper E.; Rhodes J.; Sheppard D. C.; Warris A.; White P. L.; Xu J.; Zwaan B.; Verweij P. E. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol 2022, 20 (9), 557–571. 10.1038/s41579-022-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickes B. L.; Wiederhold N. P. Molecular Diagnostics in Medical Mycology. Nat. Commun. 2018, 9 (1), 5135. 10.1038/s41467-018-07556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniado-Laborín R.; Cabrales-Vargas M. N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam Micol 2009, 26 (4), 223–227. 10.1016/j.riam.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tverdek F. P.; Kofteridis D.; Kontoyiannis D. P. Antifungal Agents and Liver Toxicity: A Complex Interaction. Expert Rev. Anti Infect Ther 2016, 14 (8), 765–776. 10.1080/14787210.2016.1199272. [DOI] [PubMed] [Google Scholar]
- Borba H. H. L.; Steimbach L. M.; Riveros B. S.; Tonin F. S.; Ferreira V. L.; Bagatim B. A. Q.; Balan G.; Pontarolo R.; Wiens A. Cost-Effectiveness of Amphotericin B Formulations in the Treatment of Systemic Fungal Infections. Mycoses 2018, 61 (10), 754–763. 10.1111/myc.12801. [DOI] [PubMed] [Google Scholar]
- Chen T.; Mwenge L.; Lakhi S.; Chanda D.; Mwaba P.; Molloy S. F.; Gheorghe A.; Griffiths U. K.; Heyderman R. S.; Kanyama C.; Kouanfack C.; Mfinanga S.; Chan A. K.; Temfack E.; Kivuyo S.; Hosseinipour M. C.; Lortholary O.; Loyse A.; Jaffar S.; Harrison T. S.; Niessen L. W.; Team A. T. Healthcare Costs and Life-Years Gained From Treatments Within the Advancing Cryptococcal Meningitis Treatment for Africa (ACTA) Trial on Cryptococcal Meningitis: A Comparison of Antifungal Induction Strategies in Sub-Saharan Africa. Clin Infect Dis 2019, 69 (4), 588–595. 10.1093/cid/ciy971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G.; Paterson D. L. How Urgent Is the Need for New Antifungals?. Expert Opin Pharmacother 2021, 22 (14), 1857–1870. 10.1080/14656566.2021.1935868. [DOI] [PubMed] [Google Scholar]
- Sarma S.; Upadhyay S. Current Perspective on Emergence, Diagnosis and Drug Resistance. Infect Drug Resist 2017, 10, 155–165. 10.2147/IDR.S116229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K.; Woodworth K.; Walters M.; Berkow E. L.; Jackson B.; Chiller T.; Vallabhaneni S. Candida Auris: The Recent Emergence of a Multidrug-Resistant Fungal Pathogen. Med. Mycol 2019, 57 (1), 1–12. 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- Hoenigl M.; Seidel D.; Carvalho A.; Rudramurthy S. M.; Arastehfar A.; Gangneux J.-P.; Nasir N.; Bonifaz A.; Araiza J.; Klimko N.; Serris A.; Lagrou K.; Meis J. F.; Cornely O. A.; Perfect J. R.; White P. L.; Chakrabarti A.; The Emergence of COVID-19 Associated Mucormycosis: A Review of Cases from 18 Countries. Lancet Microbe 2022, 3 (7), e543–e552. 10.1016/S2666-5247(21)00237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart S. R.; Etienne K. A.; Vallabhaneni S.; Farooqi J.; Chowdhary A.; Govender N. P.; Colombo A. L.; Calvo B.; Cuomo C. A.; Desjardins C. A.; Berkow E. L.; Castanheira M.; Magobo R. E.; Jabeen K.; Asghar R. J.; Meis J. F.; Jackson B.; Chiller T.; Litvintseva A. P. Simultaneous Emergence of Multidrug-Resistant Candida Auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis 2017, 64 (2), 134–140. 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L. A.; Gow N. A. R.; Munro C. A. Fungal Echinocandin Resistance. Fungal Genetics and Biology 2010, 47 (2), 117–126. 10.1016/j.fgb.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar P. Management of Invasive Fungal Infections: A Role for Polyenes. J. Antimicrob. Chemother. 2011, 66 (3), 457–465. 10.1093/jac/dkq479. [DOI] [PubMed] [Google Scholar]
- Loyse A.; Dromer F.; Day J.; Lortholary O.; Harrison T. S. Flucytosine and Cryptococcosis: Time to Urgently Address the Worldwide Accessibility of a 50-Year-Old Antifungal. J. Antimicrob. Chemother. 2013, 68 (11), 2435–2444. 10.1093/jac/dkt221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolus H.; Pierson S.; Lagrou K.; van Dijck P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. Journal of Fungi 2020, 6 (4), 321. 10.3390/jof6040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pristov K. E.; Ghannoum M. A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clinical Microbiology and Infection 2019, 25 (7), 792–798. 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- Vermeulen E.; Lagrou K.; Verweij P. E. Azole Resistance in Aspergillus Fumigatus: A Growing Public Health Concern. Curr. Opin Infect Dis 2013, 26 (6), 493–500. 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- Francois I.; Cammue B.; Borgers M.; Ausma J.; Dispersyn G.; Thevissen K. Azoles: Mode of Antifungal Action and Resistance Development. Effect of Miconazole on Endogenous Reactive Oxygen Species Production in Candida Albicans. Antiinfect Agents Med. Chem. 2006, 5 (1), 3–13. 10.2174/187152106774755554. [DOI] [Google Scholar]
- Hope W.; Stone N. R. H.; Johnson A.; McEntee L.; Farrington N.; Santoro-Castelazo A.; Liu X.; Lucaci A.; Hughes M.; Oliver J. D.; Giamberardino C.; Mfinanga S.; Harrison T. S.; Perfect J. R.; Bicanic T. Fluconazole Monotherapy Is a Suboptimal Option for Initial Treatment of Cryptococcal Meningitis Because of Emergence of Resistance. mBio 2019, 10 (6), e02575-19. 10.1128/mBio.02575-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arastehfar A.; Hilmioğlu-Polat S.; Daneshnia F.; Hafez A.; Salehi M.; Polat F.; Yaşar M.; Arslan N.; Hoşbul T.; Ünal N.; Metin D. Y.; Gürcan Ş.; Birinci A.; Koç A. N.; Pan W.; Ilkit M.; Perlin D. S.; Lass-Flörl C. Recent Increase in the Prevalence of Fluconazole-Non-Susceptible Candida Tropicalis Blood Isolates in Turkey: Clinical Implication of Azole-Non-Susceptible and Fluconazole Tolerant Phenotypes and Genotyping. Front Microbiol 2020, 11, 587278. 10.3389/fmicb.2020.587278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp C.; Bohner F.; Kocsis K.; Varga M.; Szekeres A.; Bodai L.; Willis J. R.; Gabaldón T.; Tóth R.; Nosanchuk J. D.; Vágvölgyi C.; Gácser A. Triazole Evolution of Candida Parapsilosis Results in Cross-Resistance to Other Antifungal Drugs, Influences Stress Responses, and Alters Virulence in an Antifungal Drug-Dependent Manner. mSphere 2020, 5 (5), e00821-20. 10.1128/mSphere.00821-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.-S.; Rodriguez-Manzano J.; Moser N.; Moniri A.; Malpartida-Cardenas K.; Miscourides N.; Sewell T.; Kochina T.; Brackin A.; Rhodes J.; Holmes A. H.; Fisher M. C.; Georgiou P. Rapid Detection of Azole-Resistant Aspergillus Fumigatus in Clinical and Environmental Isolates by Use of a Lab-on-a-Chip Diagnostic System. J. Clin Microbiol 2020, 58 (11), e00843-20. 10.1128/JCM.00843-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth M.; Ostrosky-Zeichner L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. Journal of Fungi 2020, 6 (4), 324. 10.3390/jof6040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan A.; Wolfe A.; Williamson J. C. Antifungal Resistance and the Role of New Therapeutic Agents. Curr. Infect Dis Rep 2022, 24, 105. 10.1007/s11908-022-00782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block E. R.; Jennings A. E.; Bennett J. E. 5-Fluorocytosine Resistance in Cryptococcus Neoformans. Antimicrob. Agents Chemother. 1973, 3 (6), 649–656. 10.1128/AAC.3.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. E.; Alexander B. D.; Perfect J.. Drug Resistance in Cryptococcus Neoformans. In Antimicrobial Drug Resistance; Humana Press: Totowa, NJ, 2009; pp 967–985. 10.1007/978-1-60327-595-8_20. [DOI] [Google Scholar]
- Billmyre R. B.; Applen Clancey S.; Li L. X.; Doering T. L.; Heitman J. 5-Fluorocytosine Resistance Is Associated with Hypermutation and Alterations in Capsule Biosynthesis in Cryptococcus. Nat. Commun. 2020, 11 (1), 127. 10.1038/s41467-019-13890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F.; Zhao M.; Braun D. R.; Ericksen S. S.; Piotrowski J. S.; Nelson J.; Peng J.; Ananiev G. E.; Chanana S.; Barns K.; Fossen J.; Sanchez H.; Chevrette M. G.; Guzei I. A.; Zhao C.; Guo L.; Tang W.; Currie C. R.; Rajski S. R.; Audhya A.; Andes D. R.; Bugni T. S. A Marine Microbiome Antifungal Targets Urgent-Threat Drug-Resistant Fungi. Science (1979) 2020, 370 (6519), 974–978. 10.1126/science.abd6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.; Tharappel A. M.; Xu J.; Lang Y.; Green C. M.; Zhang J.; Lin Q.; Chaturvedi S.; Zhou J.; Belfort M.; Li H. Small-Molecule Inhibitors for the Prp8 Intein as Antifungal Agents. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (2), e2008815118. 10.1073/pnas.2008815118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor V.; Rella A.; Farnoud A. M.; Singh A.; Munshi M.; Bryan A.; Naseem S.; Konopka J. B.; Ojima I.; Bullesbach E.; Ashbaugh A.; Linke M. J.; Cushion M.; Collins M.; Ananthula H. K.; Sallans L.; Desai P. B.; Wiederhold N. P.; Fothergill A. W.; Kirkpatrick W. R.; Patterson T.; Wong L. H.; Sinha S.; Giaever G.; Nislow C.; Flaherty P.; Pan X.; Cesar G. V.; de Melo Tavares P.; Frases S.; Miranda K.; Rodrigues M. L.; Luberto C.; Nimrichter L.; del Poeta M. Identification of a New Class of Antifungals Targeting the Synthesis of Fungal Sphingolipids. mBio 2015, 6 (3), e00647-15. 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo Ruiz G.; Lorenz A. What Do We Know about the Biology of the Emerging Fungal Pathogen of Humans Candida Auris?. Microbiol Res. 2021, 242, 126621. 10.1016/j.micres.2020.126621. [DOI] [PubMed] [Google Scholar]
- Spivak E. S.; Hanson K. E. Candida Auris: An Emerging Fungal Pathogen. J. Clin Microbiol 2018, 56 (2), e01588-17. 10.1128/JCM.01588-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoubeaux G.; Coste A. T.; Imbert C.; Hennequin C. Overview about Candida Auris: What’s up 12 Years after Its First Description?. Journal of Medical Mycology 2022, 32 (2), 101248. 10.1016/j.mycmed.2022.101248. [DOI] [PubMed] [Google Scholar]
- Černáková L.; Roudbary M.; Brás S.; Tafaj S.; Rodrigues C. F. Candida Auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22 (9), 4470. 10.3390/ijms22094470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.; Bing J.; Hu T.; Ennis C. L.; Nobile C. J.; Huang G. Candida Auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PLoS Pathog 2020, 16 (10), e1008921. 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K.; Makimura K.; Hasumi Y.; Nishiyama Y.; Uchida K.; Yamaguchi H. Candida Auris Sp. Nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009, 53 (1), 41–44. 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. Z. P.; Schwartz I. S. Emerging Fungal Infections: New Patients, New Patterns, and New Pathogens. Journal of Fungi 2019, 5 (3), 67. 10.3390/jof5030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyaolu A.; Okorie C.; Marinkovic A.; Abbasi A. F.; Prakash S.; Mangat J.; Hosein Z.; Haider N.; Chan J. Candida Auris : An Overview of the Emerging Drug-Resistant Fungal Infection. Infect Chemother 2022, 54 (2), 236. 10.3947/ic.2022.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordalewska M.; Lee A.; Park S.; Berrio I.; Chowdhary A.; Zhao Y.; Perlin D. S. Understanding Echinocandin Resistance in the Emerging Pathogen Candida Auris. Antimicrob. Agents Chemother. 2018, 62 (6), e00238-18. 10.1128/AAC.00238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo A. L.; Júnior J. N. A.; Guinea J. Emerging Multidrug-Resistant Candida Species. Curr. Opin Infect Dis 2017, 30 (6), 528–538. 10.1097/QCO.0000000000000411. [DOI] [PubMed] [Google Scholar]
- Sears D.; Schwartz B. S. Candida Auris: An Emerging Multidrug-Resistant Pathogen. Int. J. Infect Dis 2017, 63, 95–98. 10.1016/j.ijid.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Chowdhary A.; Voss A.; Meis J. F. Multidrug-Resistant Candida Auris: “new Kid on the Block” in Hospital-Associated Infections?. J. Hosp Infect 2016, 94 (3), 209–212. 10.1016/j.jhin.2016.08.004. [DOI] [PubMed] [Google Scholar]
- de Oliveira H. C.; Castelli R. F.; Reis F. C. G.; Samby K.; Nosanchuk J. D.; Alves L. R.; Rodrigues M. L. Screening of the Pandemic Response Box Reveals an Association between Antifungal Effects of MMV1593537 and the Cell Wall of Cryptococcus Neoformans, Cryptococcus Deuterogattii, and Candida Auris. Microbiol Spectr 2022, 10.1128/spectrum.00601-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J.; Liu N.; Huang Y.; Yang W.; Sheng C. Small Molecules for Combating Multidrug-Resistant Superbug Candida Auris Infections. Acta Pharm. Sin B 2022, 12, 4056. 10.1016/j.apsb.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J.-P. Aspergillus Fumigatus and Aspergillosis. Clin Microbiol Rev. 1999, 12 (2), 310–350. 10.1128/CMR.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Garcia A.; Pellon A.; Rementeria A.; Buldain I.; Barreto-Bergter E.; Rollin-Pinheiro R.; de Meirelles J. V.; Xisto M. I. D. S.; Ranque S.; Havlicek V.; Vandeputte P.; Govic Y.; le Bouchara J.-P.; Giraud S.; Chen S.; Rainer J.; Alastruey-Izquierdo A.; Martin-Gomez M. T.; López-Soria L. M.; Peman J.; Schwarz C.; Bernhardt A.; Tintelnot K.; Capilla J.; Martin-Vicente A.; Cano-Lira J.; Nagl M.; Lackner M.; Irinyi L.; Meyer W.; de Hoog S.; Hernando F. L. Scedosporium and Lomentospora: An Updated Overview of Underrated Opportunists. Med. Mycol 2018, 56 (suppl_1), S102–S125. 10.1093/mmy/myx113. [DOI] [PubMed] [Google Scholar]
- Thornton C. R. Detection of the ‘Big Five’ Mold Killers of Humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Advances in Applied Microbiology 2020, 110, 1–61. 10.1016/bs.aambs.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Douglas A. P.; Chen S. C.-A.; Slavin M. A. Emerging Infections Caused by Non- Aspergillus Filamentous Fungi. Clinical Microbiology and Infection 2016, 22 (8), 670–680. 10.1016/j.cmi.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Lamoth F.; Kontoyiannis D. P. Therapeutic Challenges of Non- Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob. Agents Chemother. 2019, 63 (11), e01244-19. 10.1128/AAC.01244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin-Pinheiro R.; Borba-Santos L. P.; da Silva Xisto M. I. D.; de Castro-Almeida Y.; Rochetti V. P.; Rozental S.; Barreto-Bergter E. Identification of Promising Antifungal Drugs against Scedosporium and Lomentospora Species after Screening of Pathogen Box Library. Journal of Fungi 2021, 7 (10), 803. 10.3390/jof7100803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K.-H.; Lee S.-H. COVID-19 and Fungal Diseases. Antibiotics 2022, 11 (6), 803. 10.3390/antibiotics11060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.-M.; Lai C.-C.; Yu W.-L. COVID-19 Associated Mucormycosis – An Emerging Threat. Journal of Microbiology, Immunology and Infection 2022, 55 (2), 183–190. 10.1016/j.jmii.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K.; Agolli A.; H. Patel M.; Garimella R.; Devi M.; Garcia E.; Amin H.; Domingue C.; del Castillo R. G.; Sanchez-Gonzalez M. High Mortality Co-Infections of COVID-19 Patients: Mucormycosis and Other Fungal Infections. Discoveries 2021, 9 (1), e126. 10.15190/d.2021.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestel C.; Anderson E.; Forsberg K.; Lyman M.; de Perio M. A.; Kuhar D.; Edwards K.; Rivera M.; Shugart A.; Walters M.; Dotson N. Q. Candida Auris Outbreak in a COVID-19 Specialty Care Unit — Florida, July–August 2020. MMWR Morb Mortal Wkly Rep 2021, 70 (2), 56–57. 10.15585/mmwr.mm7002e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAFFI . Global Action Fund For Fungal Infections. https://www.gaffi.org/. October 2, 2022.
- Denning D. W. Minimizing Fungal Disease Deaths Will Allow the UNAIDS Target of Reducing Annual AIDS Deaths below 500 000 by 2020 to Be Realized. Philosophical Transactions of the Royal Society B: Biological Sciences 2016, 371 (1709), 20150468. 10.1098/rstb.2015.0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis J. N.; Lawrence D. S.; Meya D. B.; Kagimu E.; Kasibante J.; Mpoza E.; Rutakingirwa M. K.; Ssebambulidde K.; Tugume L.; Rhein J.; Boulware D. R.; Mwandumba H. C.; Moyo M.; Mzinganjira H.; Kanyama C.; Hosseinipour M. C.; Chawinga C.; Meintjes G.; Schutz C.; Comins K.; Singh A.; Muzoora C.; Jjunju S.; Nuwagira E.; Mosepele M.; Leeme T.; Siamisang K.; Ndhlovu C. E.; Hlupeni A.; Mutata C.; van Widenfelt E.; Chen T.; Wang D.; Hope W.; Boyer-Chammard T.; Loyse A.; Molloy S. F.; Youssouf N.; Lortholary O.; Lalloo D. G.; Jaffar S.; Harrison T. S. Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis. New England Journal of Medicine 2022, 386 (12), 1109–1120. 10.1056/NEJMoa2111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falci D. R.; Pasqualotto A. C. Single-Dose Amphotericin B for Cryptococcal Meningitis. New England Journal of Medicine 2022, 387 (4), 380–381. 10.1056/NEJMc2206274. [DOI] [PubMed] [Google Scholar]
- de Oliveira H.; Castell R.; Alves L.; Nosanchuk J.; Salama E.; Seleem M.; Rodrigues M. Identification of 4 Compounds from the Pharmakon Library with Antifungal Activity against Candida Auris and Species of Cryptococcus. Med. Mycol 2022, 60, myac033. 10.1093/mmy/myac033. [DOI] [PubMed] [Google Scholar]
- Kaul G.; Shukla M.; Dasgupta A.; Chopra S. Update on Drug-Repurposing: Is It Useful for Tackling Antimicrobial Resistance?. Future Microbiol 2019, 14 (10), 829–831. 10.2217/fmb-2019-0122. [DOI] [PubMed] [Google Scholar]
- Nosengo N. Can You Teach Old Drugs New Tricks?. Nature 2016, 534 (7607), 314–316. 10.1038/534314a. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Cheng L. W.; Chan K. L.; Tam C. C.; Mahoney N.; Friedman M.; Shilman M. M.; Land K. M. Antifungal Drug Repurposing. Antibiotics (Basel) 2020, 9 (11), 812. 10.3390/antibiotics9110812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushpakom S.; Iorio F.; Eyers P. A.; Escott K. J.; Hopper S.; Wells A.; Doig A.; Guilliams T.; Latimer J.; McNamee C.; Norris A.; Sanseau P.; Cavalla D.; Pirmohamed M. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov 2019, 18 (1), 41–58. 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Truong M.; Monahan L. G. G.; Carter D. A. A.; Charles I. G. G. Repurposing Drugs to Fast-Track Therapeutic Agents for the Treatment of Cryptococcosis. PeerJ. 2018, 6, e4761. 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan S.; Grootendorst P.; Lexchin J.; Cunningham C.; Greyson D. The Cost of Drug Development: A Systematic Review. Health Policy (New York) 2011, 100 (1), 4–17. 10.1016/j.healthpol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Jourdan J.-P.; Bureau R.; Rochais C.; Dallemagne P. Drug Repositioning: A Brief Overview. J. Pharm. Pharmacol. 2020, 72 (9), 1145–1151. 10.1111/jphp.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S. A.; de Oliveira H. C.; Agreda-Mellon D.; Lucio J.; Mendes-Giannini M. J. S.; García-Cambero J. P.; Zaragoza O. Identification of Off-Patent Drugs That Show Synergism with Amphotericin B or That Present Antifungal Action against Cryptococcus Neoformans and Candida Spp. Antimicrob. Agents Chemother. 2020, 64 (4), e01921-19. 10.1128/AAC.01921-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts A.; DiDone L.; Koselny K.; Baxter B. K.; Chabrier-Rosello Y.; Wellington M.; Krysan D. J. A Repurposing Approach Identifies Off-Patent Drugs with Fungicidal Cryptococcal Activity, a Common Structural Chemotype, and Pharmacological Properties Relevant to the Treatment of Cryptococcosis. Eukaryot Cell 2013, 12 (2), 278–287. 10.1128/EC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira H. C.; Joffe L. S.; Simon K. S.; Castelli R. F.; Reis F. C. G.; Bryan A. M.; Borges B. S.; Medeiros L. C. S.; Bocca A. L.; del Poeta M.; Rodrigues M. L. Fenbendazole Controls In Vitro Growth, Virulence Potential, and Animal Infection in the Cryptococcus Model. Antimicrob. Agents Chemother. 2020, 64 (6), e00286-20. 10.1128/AAC.00286-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe L. S.; Schneider R.; Lopes W.; Azevedo R.; Staats C. C.; Kmetzsch L.; Schrank A.; del Poeta M.; Vainstein M. H.; Rodrigues M. L. The Anti-Helminthic Compound Mebendazole Has Multiple Antifungal Effects against Cryptococcus Neoformans. Front Microbiol 2017, 8, 535. 10.3389/fmicb.2017.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall G.; Chaturvedi A. K.; Wormley F. L.; Wiederhold N. P.; Patterson H. P.; Patterson T. F.; Lopez-Ribot J. L. Screening a Repurposing Library for Inhibitors of Multidrug-Resistant Candida Auris Identifies Ebselen as a Repositionable Candidate for Antifungal Drug Development. Antimicrob. Agents Chemother. 2018, 62 (10), e01084-18. 10.1128/AAC.01084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.-S.; Roma J. S.; Shen M.; Fernandes C. M.; Tsang P. S.; Forbes H. E.; Boshoff H.; Lazzarini C.; del Poeta M.; Zheng W.; Williamson P. R. Identification of Antifungal Compounds against Multidrug Resistant Candida Auris Utilizing a High Throughput Drug Repurposing Screen. Antimicrob. Agents Chemother. 2021, e01305-20. 10.1128/AAC.01305-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousfi H.; Cassagne C.; Ranque S.; Rolain J.-M.; Bittar F. Repurposing of Ribavirin as an Adjunct Therapy against Invasive Candida Strains in an In Vitro Study. Antimicrob. Agents Chemother. 2019, 63 (10), e00263-19. 10.1128/AAC.00263-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldesouky H. E.; Salama E. A.; Li X.; Hazbun T. R.; Mayhoub A. S.; Seleem M. N. Repurposing Approach Identifies Pitavastatin as a Potent Azole Chemosensitizing Agent Effective against Azole-Resistant Candida Species. Sci. Rep 2020, 10 (1), 7525. 10.1038/s41598-020-64571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.; Fatima Z.; Ahmad K.; Hameed S. Repurposing of Respiratory Drug Theophylline against Candida Albicans: Mechanistic Insights Unveil Alterations in Membrane Properties and Metabolic Fitness. J. Appl. Microbiol. 2020, 129 (4), 860–875. 10.1111/jam.14669. [DOI] [PubMed] [Google Scholar]
- Siles S. A.; Srinivasan A.; Pierce C. G.; Lopez-Ribot J. L.; Ramasubramanian A. K. High-Throughput Screening of a Collection of Known Pharmacologically Active Small Compounds for Identification of Candida Albicans Biofilm Inhibitors. Antimicrob. Agents Chemother. 2013, 57 (8), 3681–3687. 10.1128/AAC.00680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall G.; Herrera N.; Lopez-Ribot J. L. Repositionable Compounds with Antifungal Activity against Multidrug Resistant Candida Auris Identified in the Medicines for Malaria Venture’s Pathogen Box. J. Fungi (Basel) 2019, 5 (4), 92. 10.3390/jof5040092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira H. C.; Monteiro M. C.; Rossi S. A.; Pemán J.; Ruiz-Gaitán A.; Mendes-Giannini M. J. S.; Mellado E.; Zaragoza O. Identification of Off-Patent Compounds That Present Antifungal Activity Against the Emerging Fungal Pathogen Candida Auris. Front Cell Infect Microbiol 2019, 9, 83. 10.3389/fcimb.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Reis T. F.; Horta M. A. C.; Colabardini A. C.; Fernandes C. M.; Silva L. P.; Bastos R. W.; Fonseca M. V. de L.; Wang F.; Martins C.; Rodrigues M. L.; Silva Pereira C.; del Poeta M.; Wong K. H.; Goldman G. H. Screening of Chemical Libraries for New Antifungal Drugs against Aspergillus Fumigatus Reveals Sphingolipids Are Involved in the Mechanism of Action of Miltefosine. mBio 2021, 12 (4), e01458-21. 10.1128/mBio.01458-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R. A.; Joffe L. S.; Alves G. M.; Figueiredo-Carvalho M. H. G.; Brito-Santos F.; Amaral A. C. F.; Rodrigues M. L.; Almeida-Paes R. A Screening of the MMV Pathogen Box® Reveals New Potential Antifungal Drugs against the Etiologic Agents of Chromoblastomycosis. PLoS One 2020, 15 (5), e0229630. 10.1371/journal.pone.0229630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba-Santos L. P.; Vila T.; Rozental S. Identification of Two Potential Inhibitors of Sporothrix Brasiliensis and Sporothrix Schenckii in the Pathogen Box Collection. PLoS One 2020, 15 (10), e0240658. 10.1371/journal.pone.0240658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai B.; Wu C.; Wang L.; Sachs M. S.; Lin X. The Antidepressant Sertraline Provides a Promising Therapeutic Option for Neurotropic Cryptococcal Infections. Antimicrob. Agents Chemother. 2012, 56 (7), 3758–3766. 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treviño-Rangel R. de J.; Villanueva-Lozano H.; Hernández-Rodríguez P.; Martínez-Reséndez M. F.; García-Juárez J.; Rodríguez-Rocha H.; González G. M. Activity of Sertraline against Cryptococcus Neoformans : In Vitro and in Vivo Assays. Med. Mycol 2016, 54 (3), 280–286. 10.1093/mmy/myv109. [DOI] [PubMed] [Google Scholar]
- Chen J.; Korostyshevsky D.; Lee S.; Perlstein E. O. Accumulation of an Antidepressant in Vesiculogenic Membranes of Yeast Cells Triggers Autophagy. PLoS One 2012, 7 (4), e34024. 10.1371/journal.pone.0034024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowri M.; Jayashree B.; Jeyakanthan J.; Girija E. K. Sertraline as a Promising Antifungal Agent: Inhibition of Growth and Biofilm of Candida Auris with Special Focus on the Mechanism of Action in Vitro. J. Appl. Microbiol. 2020, 128 (2), 426–437. 10.1111/jam.14490. [DOI] [PubMed] [Google Scholar]
- Breuer M. R.; Dasgupta A.; Vasselli J. G.; Lin X.; Shaw B. D.; Sachs M. S. The Antidepressant Sertraline Induces the Formation of Supersized Lipid Droplets in the Human Pathogen Cryptococcus Neoformans. Journal of Fungi 2022, 8 (6), 642. 10.3390/jof8060642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware D. R.; Nalintya E.; Rajasingham R.; Kirumira P.; Naluyima R.; Turya F.; Namanda S.; Rutakingirwa M. K.; Skipper C. P.; Nikweri Y.; Hullsiek K. H.; Bangdiwala A. S.; Meya D. B. Adjunctive Sertraline for Asymptomatic Cryptococcal Antigenemia: A Randomized Clinical Trial. Med. Mycol 2020, 58 (8), 1037–1043. 10.1093/mmy/myaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhein J.; Morawski B. M.; Hullsiek K. H.; Nabeta H. W.; Kiggundu R.; Tugume L.; Musubire A.; Akampurira A.; Smith K. D.; Alhadab A.; Williams D. A.; Abassi M.; Bahr N. C.; Velamakanni S. S.; Fisher J.; Nielsen K.; Meya D. B.; Boulware D. R.; Efficacy of Adjunctive Sertraline for the Treatment of HIV-Associated Cryptococcal Meningitis: An Open-Label Dose-Ranging Study. Lancet Infect Dis 2016, 16 (7), 809–818. 10.1016/S1473-3099(16)00074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Yun Z.; Ji C.; Tu J.; Yang W.; Li J.; Liu N.; Sheng C. Discovery of Novel Sertraline Derivatives as Potent Anti- Cryptococcus Agents. J. Med. Chem. 2022, 65 (9), 6541–6554. 10.1021/acs.jmedchem.1c01845. [DOI] [PubMed] [Google Scholar]
- Ji C.; Liu N.; Tu J.; Li Z.; Han G.; Li J.; Sheng C. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect Dis 2020, 6 (5), 768–786. 10.1021/acsinfecdis.9b00197. [DOI] [PubMed] [Google Scholar]
- Wall G.; Lopez-Ribot J. L. Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity. Antimicrob. Agents Chemother. 2020, 64 (9), e00924-20. 10.1128/AAC.00924-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong M.; Monahan L. G.; Carter D. A.; Charles I. G. Repurposing Drugs to Fast-Track Therapeutic Agents for the Treatment of Cryptococcosis. PeerJ. 2018, 6, e4761. 10.7717/peerj.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P.; Patterson T. F.; Srinivasan A.; Chaturvedi A. K.; Fothergill A. W.; Wormley F. L.; Ramasubramanian A. K.; Lopez-Ribot J. L. Repurposing Auranofin as an Antifungal: In Vitro Activity against a Variety of Medically Important Fungi. Virulence 2017, 8 (2), 138–142. 10.1080/21505594.2016.1196301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon G. L. L.; McEntee L.; Johnson A.; Farrington N.; Whalley S.; Livermore J.; Natal C.; Washbourn G.; Bibby J.; Berry N.; Lestner J.; Truong M.; Owen A.; Lalloo D.; Charles I.; Hope W. Repurposing and Reformulation of the Antiparasitic Agent Flubendazole for Treatment of Cryptococcal Meningoencephalitis, a Neglected Fungal Disease. Antimicrob. Agents Chemother. 2018, 62 (4), e01909-17. 10.1128/AAC.01909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl M.; Sprute R.; Egger M.; Arastehfar A.; Cornely O. A.; Krause R.; Lass-Flörl C.; Prattes J.; Spec A.; Thompson G. R.; Wiederhold N.; Jenks J. D. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021, 81 (15), 1703–1729. 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass L.; Murray A.; Davis A.; Woodward K.; Albayaty M.; Ito K.; Strong P.; Ayrton J.; Brindley C.; Prosser J.; Murray J.; French E.; Haywood P.; Wallis C.; Rapeport G. Safety and Nonclinical and Clinical Pharmacokinetics of PC945, a Novel Inhaled Triazole Antifungal Agent. Pharmacol Res. Perspect 2021, 9 (1), e00690. 10.1002/prp2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.; Cass L.; Ito K.; Pagani N.; Armstrong-James D.; Dalal P.; Reed A.; Strong P. PC945, a Novel Inhaled Antifungal Agent, for the Treatment of Respiratory Fungal Infections. Journal of Fungi 2020, 6 (4), 373. 10.3390/jof6040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley T.; Alanio A.; Kelly S. L.; Sehra G.; Kizawa Y.; Warrilow A. G. S.; Parker J. E.; Kelly D. E.; Kimura G.; Anderson-Dring L.; Nakaoki T.; Sunose M.; Onions S.; Crepin D.; Lagasse F.; Crittall M.; Shannon J.; Cooke M.; Bretagne S.; King-Underwood J.; Murray J.; Ito K.; Strong P.; Rapeport G. In Vitro and In Vivo Antifungal Profile of a Novel and Long-Acting Inhaled Azole, PC945, on Aspergillus Fumigatus Infection. Antimicrob. Agents Chemother. 2017, 61 (5), e02280-16. 10.1128/AAC.02280-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani N.; Armstrong-James D.; Reed A. Successful Salvage Therapy for Fungal Bronchial Anastomotic Infection after – Lung Transplantation with an Inhaled Triazole Anti-Fungal PC945. Journal of Heart and Lung Transplantation 2020, 39 (12), 1505–1506. 10.1016/j.healun.2020.09.015. [DOI] [PubMed] [Google Scholar]
- Hoy S. M. Oteseconazole: First Approval. Drugs 2022, 82 (9), 1017–1023. 10.1007/s40265-022-01734-y. [DOI] [PubMed] [Google Scholar]
- Garcia-Effron G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. Journal of Fungi 2020, 6 (4), 262. 10.3390/jof6040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Effron G. Rezafungin—Mechanisms of Action, Susceptibility and Resistance: Similarities and Differences with the Other Echinocandins. Journal of Fungi 2020, 6 (4), 262. 10.3390/jof6040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S. Cell Wall-Modifying Antifungal Drugs. Current Topics in Microbiology and Immunology 2019, 425, 255–275. 10.1007/82_2019_188. [DOI] [PubMed] [Google Scholar]
- Szymański M.; Chmielewska S.; Czyżewska U.; Malinowska M.; Tylicki A. Echinocandins – Structure, Mechanism of Action and Use in Antifungal Therapy. J. Enzyme Inhib Med. Chem. 2022, 37 (1), 876–894. 10.1080/14756366.2022.2050224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraskevicius J.; Petraitis V.; Davainis L.; Zucenka A. The Feasibility of Ibrexafungerp for the Treatment of Fungal Infections in Patients with Hematological Malignancies. Journal of Fungi 2022, 8 (5), 440. 10.3390/jof8050440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D. Ibrexafungerp for the Treatment of Vulvovaginal Candidiasis. Drugs of Today 2022, 58 (4), 149. 10.1358/dot.2022.58.4.3381586. [DOI] [PubMed] [Google Scholar]
- Sobel R.; Nyirjesy P.; Ghannoum M.; Delchev D.; Azie N.; Angulo D.; Harriott I.; Borroto-Esoda K.; Sobel J. Efficacy and Safety of Oral Ibrexafungerp for the Treatment of Acute Vulvovaginal Candidiasis: A Global Phase 3, Randomised, Placebo-controlled Superiority Study (VANISH 306). BJOG 2022, 129 (3), 412–420. 10.1111/1471-0528.16972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K.; Horii T.; Miyazaki M.; Watanabe N.; Okubo M.; Sonoda J.; Nakamoto K.; Tanaka K.; Shirotori S.; Murai N.; Inoue S.; Matsukura M.; Abe S.; Yoshimatsu K.; Asada M. Efficacy of Oral E1210, a New Broad-Spectrum Antifungal with a Novel Mechanism of Action, in Murine Models of Candidiasis, Aspergillosis, and Fusariosis. Antimicrob. Agents Chemother. 2011, 55 (10), 4543–4551. 10.1128/AAC.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlin M. J.; Meyers M. J. Repurposing and Optimization of Drugs for Discovery of Novel Antifungals. Drug Discov Today 2022, 27 (7), 2008–2014. 10.1016/j.drudis.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J.; Ibrahim A. S. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. Journal of Fungi 2020, 6 (4), 239. 10.3390/jof6040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.; Wang N.; Carter C. L.; Zimmerman M.; Dartois V.; Shaw K. J.; Perlin D. S.; Zhao Y. Therapeutic Potential of Fosmanogepix (APX001) for Intra-Abdominal Candidiasis: From Lesion Penetration to Efficacy in a Mouse Model. Antimicrob. Agents Chemother. 2021, 65 (4), e02476-20. 10.1128/AAC.02476-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam T.; Alkhazraji S.; Alqarihi A.; Jeon H. H.; Gu Y.; Kapoor M.; Shaw K. J.; Ibrahim A. S. APX001 Is Effective in the Treatment of Murine Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2019, 63 (2), e01713-18. 10.1128/AAC.01713-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhazraji S.; Gebremariam T.; Alqarihi A.; Gu Y.; Mamouei Z.; Singh S.; Wiederhold N. P.; Shaw K. J.; Ibrahim A. S. Fosmanogepix (APX001) Is Effective in the Treatment of Immunocompromised Mice Infected with Invasive Pulmonary Scedosporiosis or Disseminated Fusariosis. Antimicrob. Agents Chemother. 2020, 64 (3), e01735-19. 10.1128/AAC.01735-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A.; Huband M. D.; Flamm R. K.; Bien P. A.; Castanheira M. In Vitro Activity of APX001A (Manogepix) and Comparator Agents against 1,706 Fungal Isolates Collected during an International Surveillance Program in 2017. Antimicrob. Agents Chemother. 2019, 63 (8), e00840-19. 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A.; Huband M. D.; Flamm R. K.; Bien P. A.; Castanheira M. Antimicrobial Activity of Manogepix, a First-in-Class Antifungal, and Comparator Agents Tested against Contemporary Invasive Fungal Isolates from an International Surveillance Programme (2018–2019). J. Glob Antimicrob Resist 2021, 26, 117–127. 10.1016/j.jgar.2021.04.012. [DOI] [PubMed] [Google Scholar]
- Hoenigl M.; Sprute R.; Arastehfar A.; Perfect J. R.; Lass-Flörl C.; Bellmann R.; Prattes J.; Thompson G. R.; Wiederhold N. P.; al Obaidi M. M.; Willinger B.; Arendrup M. C.; Koehler P.; Oliverio M.; Egger M.; Schwartz I. S.; Cornely O. A.; Pappas P. G.; Krause R. Invasive Candidiasis: Investigational Drugs in the Clinical Development Pipeline and Mechanisms of Action. Expert Opin Investig Drugs 2022, 31 (8), 795–812. 10.1080/13543784.2022.2086120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraitiene R.; Petraitis V.; Maung B. B. W.; Mansbach R. S.; Hodges M. R.; Finkelman M. A.; Shaw K. J.; Walsh T. J. Efficacy and Pharmacokinetics of Fosmanogepix (APX001) in the Treatment of Candida Endophthalmitis and Hematogenous Meningoencephalitis in Nonneutropenic Rabbits. Antimicrob. Agents Chemother. 2021, 65 (3), e01795-20. 10.1128/AAC.01795-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremariam T.; Gu Y.; Alkhazraji S.; Youssef E.; Shaw K. J.; Ibrahim A. S. The Combination Treatment of Fosmanogepix and Liposomal Amphotericin B Is Superior to Monotherapy in Treating Experimental Invasive Mold Infections. Antimicrob. Agents Chemother. 2022, 66 (7), e00380-22. 10.1128/aac.00380-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Pré S.; Beckmann N.; Almeida M. C.; Sibley G. E. M.; Law D.; Brand A. C.; Birch M.; Read N. D.; Oliver J. D. Effect of the Novel Antifungal Drug F901318 (Olorofim) on Growth and Viability of Aspergillus Fumigatus. Antimicrob. Agents Chemother. 2018, 62 (8), e00231-18. 10.1128/AAC.00231-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen C.; Lu H.; Jiang Y. Novel Promising Antifungal Target Proteins for Conquering Invasive Fungal Infections. Front Microbiol 2022, 13, 911322. 10.3389/fmicb.2022.911322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff L.; Dittmer S.; Furnica D.-T.; Buer J.; Steinmann E.; Rath P.-M.; Steinmann J. Inhibition of Azole-Resistant Aspergillus Fumigatus Biofilm at Various Formation Stages by Antifungal Drugs, Including Olorofim. J. Antimicrob. Chemother. 2022, 77 (6), 1645–1654. 10.1093/jac/dkac062. [DOI] [PubMed] [Google Scholar]
- Escribano P.; Gómez A.; Reigadas E.; Muñoz P.; Guinea J. In Vitro Activity of Olorofim against Aspergillus Fumigatus Sensu Lato Clinical Isolates: Activity Is Retained against Isolates Showing Resistance to Azoles and/or Amphotericin B. Clinical Microbiology and Infection 2022, 28, 1291.E7–1291.E10. 10.1016/j.cmi.2022.05.013. [DOI] [PubMed] [Google Scholar]
- Escribano P.; Gómez A.; Reigadas E.; Muñoz P.; Guinea J. EUCAST-Obtained Olorofim MICs against Aspergillus and Scedosporium Species and Lomentospora Prolificans Showed High Agreements between Visual Inspection and Spectrophotometric Readings. Antimicrob. Agents Chemother. 2022, 66, e00849-22. 10.1128/aac.00849-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P.; Najvar L. K.; Jaramillo R.; Olivo M.; Birch M.; Law D.; Rex J. H.; Catano G.; Patterson T. F. The Orotomide Olorofim Is Efficacious in an Experimental Model of Central Nervous System Coccidioidomycosis. Antimicrob. Agents Chemother. 2018, 62 (9), e00999-18. 10.1128/AAC.00999-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbzadeh Ardakani E.; Sharifirad A.; Pashootan N.; Nayebhashemi M.; Zahmatkesh M.; Enayati S.; Razzaghi-Abyaneh M.; Khalaj V. Olorofim Effectively Eradicates Dermatophytes In Vitro and In Vivo. Antimicrob. Agents Chemother. 2021, 65 (12), e01386-21. 10.1128/AAC.01386-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H.; Fukuda Y.; Mitsuyama J.; Tashiro M.; Tanaka A.; Takazono T.; Saijo T.; Yamamoto K.; Nakamura S.; Imamura Y.; Miyazaki T.; Kakeya H.; Yamamoto Y.; Yanagihara K.; Mukae H.; Kohno S.; Izumikawa K. In Vitro and in Vivo Antifungal Activities of T-2307, a Novel Arylamidine, against Cryptococcus Gattii: An Emerging Fungal Pathogen. J. Antimicrob. Chemother. 2017, 72 (6), 1709–1713. 10.1093/jac/dkx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama J.; Nomura N.; Hashimoto K.; Yamada E.; Nishikawa H.; Kaeriyama M.; Kimura A.; Todo Y.; Narita H. In Vitro and In Vivo Antifungal Activities of T-2307, a Novel Arylamidine. Antimicrob. Agents Chemother. 2008, 52 (4), 1318–1324. 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P.; Najvar L. K.; Jaramillo R.; Olivo M.; Patterson H.; Connell A.; Fukuda Y.; Mitsuyama J.; Catano G.; Patterson T. F. The Novel Arylamidine T-2307 Demonstrates In Vitro and In Vivo Activity against Candida Auris. Antimicrob. Agents Chemother. 2020, 64 (3), e02198-19. 10.1128/AAC.02198-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P.; Najvar L. K.; Fothergill A. W.; Bocanegra R.; Olivo M.; McCarthy D. I.; Fukuda Y.; Mitsuyama J.; Patterson T. F. The Novel Arylamidine T-2307 Demonstrates in Vitro and in Vivo Activity against Echinocandin-Resistant Candida Glabrata. J. Antimicrob. Chemother. 2016, 71 (3), 692–695. 10.1093/jac/dkv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P.; Najvar L. K.; Fothergill A. W.; Bocanegra R.; Olivo M.; McCarthy D. I.; Kirkpatrick W. R.; Fukuda Y.; Mitsuyama J.; Patterson T. F. The Novel Arylamidine T-2307 Maintains In Vitro and In Vivo Activity against Echinocandin-Resistant Candida Albicans. Antimicrob. Agents Chemother. 2015, 59 (2), 1341–1343. 10.1128/AAC.04228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H.; Yamada E.; Shibata T.; Uchihashi S.; Fan H.; Hayakawa H.; Nomura N.; Mitsuyama J. Uptake of T-2307, a Novel Arylamidine, in Candida Albicans. J. Antimicrob. Chemother. 2010, 65 (8), 1681–1687. 10.1093/jac/dkq177. [DOI] [PubMed] [Google Scholar]
- Yamashita K.; Miyazaki T.; Fukuda Y.; Mitsuyama J.; Saijo T.; Shimamura S.; Yamamoto K.; Imamura Y.; Izumikawa K.; Yanagihara K.; Kohno S.; Mukae H. The Novel Arylamidine T-2307 Selectively Disrupts Yeast Mitochondrial Function by Inhibiting Respiratory Chain Complexes. Antimicrob. Agents Chemother. 2019, 63 (8), e00374-19. 10.1128/AAC.00374-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T.; Takahashi T.; Yamada E.; Kimura A.; Nishikawa H.; Hayakawa H.; Nomura N.; Mitsuyama J. T-2307 Causes Collapse of Mitochondrial Membrane Potential in Yeast. Antimicrob. Agents Chemother. 2012, 56 (11), 5892–5897. 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold N. P. Review of T-2307, an Investigational Agent That Causes Collapse of Fungal Mitochondrial Membrane Potential. J. Fungi (Basel) 2021, 7 (2), 130. 10.3390/jof7020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appili Therapeutics . A novel, broad spectrum, clinical stage antifungal to address severe and difficult-to-treat invasive fungal infections. Appili Therapeutics. www.appilitherapeutics.com/ati-2307. October 2, 2022.
- WHO. WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization. https://www.who.int/publications/i/item/9789240060241. October 25, 2022.