Abstract
Significance:
Chronic wounds are associated with significant morbidity, marked loss of quality of life, and considerable economic burden. Evidence-based risk prediction to guide improved wound prevention and treatment is limited by the complexity in their etiology, clinical underreporting, and a lack of studies using large high-quality datasets.
Recent Advancements:
The objective of this review is to summarize key components and challenges in the development of personalized risk prediction tools for both prevention and management of chronic wounds, while highlighting several innovations in the development of better risk stratification.
Critical Issues:
Regression-based risk prediction approaches remain important for assessment of prognosis and risk stratification in chronic wound management. Advances in statistical computing have boosted the development of several promising machine learning (ML) and other semiautomated classification tools. These methods may be better placed to handle large number of wound healing risk factors from large datasets, potentially resulting in better risk prediction when combined with conventional methods and clinical experience and expertise.
Future Directions:
Where the number of predictors is large and heterogenous, the correlations between various risk factors complex, and very large data sets are available, ML may prove a powerful adjuvant for risk stratifying patients predisposed to chronic wounds. Conventional regression-based approaches remain important, particularly where the number of predictors is relatively small. Translating estimated risk derived from ML algorithms into practical prediction tools for use in clinical practice remains challenging.
Keywords: chronic wounds, risk prediction, risk stratification, wound management, personalized therapy

Vladica M. Veličković, MD, PhD
SCOPE
Chronic wounds are associated with a diverse range of etiologies and complex interactions of risk factors. There is an urgent need for reliable, validated risk prediction tools that can better target an individual patient's unique set of predisposing factors that influence both their risk of disease progression and response to treatment once chronic wounds develop. The aim of this review is to summarize recent advancements in computing technologies to support risk prediction in other diseases and describe both the challenges in transferring these tools into clinical support for chronic wounds (Table 1).
Table 1.
Scope: design, outcomes, and methods
| Scope Component | |
|---|---|
| Study design | Systematic reviews Meta-analyses Randomized Controlled Trials Cohort studies (prospective or retrospective) Case–control studies |
| End-points and outcomes | Wound healing In-hospital amputation Hospital-acquired pressure ulcers Wound care management decision making |
| Risk prediction methods | Logistic regression Cox regression Classification trees Gradient-boosted decision tree models Multiclass classification models Machine learning classification Bayesian classification Random forest methods Fuzzy clustering Linear discriminant classification |
TRANSLATIONAL RELEVANCE
Recent advancements in computing power and statistical risk prediction in cardiovascular and endocrine disease may be transferrable to complex, high-burden diseases such as chronic wounds. The growing availability of large clinical databases, disease registries, and administrative datasets provides a fertile base for the study and development of risk prediction tools for the targeted triaging and management of chronic wounds.
CLINICAL RELEVANCE
Improved risk prediction is essential for the early identification of patients at highest risk for progressing to chronic wounds. This may enable early intervention in clinical practice to both prevent disease progression and support targeted, personalized management of disease once progressed, accounting for an individual patient's unique set of risk factors.
INTRODUCTION
Complex wounds that fail to progress through expected healing phases in a timely manner are classified in the group of hard-to-heal wounds or chronic wounds.1 Chronic wounds are associated with significant morbidity, marked loss of quality of life and utility, and considerable economic and societal burden, both within the United States and globally.2 Recent US estimates across all wound types put Medicare spending alone at USD 28–97 billion annually.3 Data from other countries further support the considerable costs of chronic wounds. Canadian expenditure on diabetic foot ulcers alone is estimated at $509 million annually,4 while chronic wound expenditure in the United Kingdom is estimated to be € 4.5–5.3 billion.5–8
A 2017 systematic review of 36 international cost-of-illness studies covering payer, hospital, patient, and societal perspectives estimated mean 1-year costs to the public payer ranging from $11,000 (USD) per chronic venous leg ulcer up to $44,200 for every diabetic foot ulcer.4 Current projections suggest that these costs will continue to increase into the future.
Despite chronic wounds representing a major public health challenge, current cost estimates likely underestimate the true burden of disease due to underreporting,1,9 and a lack of quality prevalence studies, particularly at the global level.1 Critically, the timely identification, intervention, and personalized management of complex chronic wounds are further limited by a lack of reliable, validated risk prediction and prognostic tools. This is, in part, secondary to the complexity and range of comorbidities associated with chronic, often treatment-refractory wounds.7 These include such diverse chronic conditions as diabetes, chronic renal disease, venous insufficiency, peripheral vascular disease, and hypertension, in addition to pressure injuries secondary to lack of mobility and/or poor nutrition.
This complexity in etiology, variability in the underlying pathophysiology, and the attendant risk factors that each condition imparts are reflected in the large number of different guidelines currently available for the treatment and management of chronic wounds. The objective of this review is to summarize the key components and challenges in the development of personalized risk prediction tools for the prevention and management of chronic wounds, and highlight several promising innovations in the development of better risk stratification and prognosis tools, including machine learning (ML) and other broader artificial intelligence (AI)-based applications.
RISK PREDICTION AND CHRONIC WOUNDS
Personalized medicine and wound healing
As knowledge and treatments for the management of chronic wounds evolve, treatment guidelines are increasingly emphasizing a more targeted approach that combines personalized medicine with evidence-based risk prediction for better patient-level and health care usage cost outcomes. Specifically, better tailoring of treatment to individual patients means a broader consideration of the factors that drive disease, including patient-related factors (pathology, comorbidity, allergy, medications, psychosocial, and pain); wound-related factors (duration/senescence and size, area and depth, wound bed condition, ischemia, inflammation/infection, anatomical site, and treatment response); health care professional skills and knowledge; and resource treatment-related factors (health care system, availability, suitability, effectiveness, and cost/reimbursement).10 The development and application of predictive tools for risk stratification may aid both targeted prevention of chronic wounds or improved, personalized treatment and management once wounds develop.11
Barriers to targeted chronic wounds management
Current standard of care for the management of chronic wounds covers multiple stages, including debridement, surgical drainage (where indicated), wound bed preparation, dressings and antimicrobial management of infection, and wound bioburden.9 Many conventional local therapies, growth factors, and dressing and biomaterial technologies for the management and treatment of chronic wounds remain generic. These interventions are commonly used in wound management irrespective of etiology or underlying risk factors that may impair wound healing. Moreover, the large number of available treatments for which evidence of effectiveness across the various phases of chronic wound disease is limited.
This lack of validated, primary evidence is more pronounced in the biotechnological sphere, where promising technologies such as functional biomaterials and dressings are often insufficiently tested and trialled, making it difficult to establish causative associations between wound management technology and improved clinical and/or cost outcomes.8,12,13 These limitations extend to the economic evaluation of treatments for chronic wounds, both pharmaceutical and technological. A recent systematic review of model-based economic evaluations of venous leg ulcer treatments found that the reporting quality was generally low, particularly with regard to the reporting of evidence supporting the structure of each model used to translate the efficacy favoring a chronic wound intervention reported in the clinical trial setting into an economic cost-benefit or cost-utility.14
The heterogenous nature of chronic wounds makes personalized ulcer management challenging. A key component of personalized treatment of chronic wounds is better risk prediction. Being able to reliably predict which patients are likely to experience impaired ulcer healing would be a critical step in the tailoring of wound therapies to the individual patient and being able to respond in a timely manner when chronic wounds prove refractory to initial treatment. Predictive diagnostics have been identified as a critical component in the targeted prevention of various pathologies that characteristically drive poor wound healing.11 Risk prediction may also need to appreciate systematic differences in health care delivery across different settings.15
Of the various etiologies or underlying disease that may present as chronic wounds, diabetes is one area that has seen recent advancements in personalized diabetes care. The development of risk prediction models that combine clinical and phenotypic data with biomarkers and genetic data to estimate individualized risk of diabetic complications have be used to risk-stratify and monitor patients to prevent or delay the development of such complications.16 However, while analytical tools for the development of robust risk prediction for the prevention or targeted management of chronic wounds are evolving rapidly, their application in real-world clinical practice remains limited.
Building risk prediction models for chronic wounds
In its simplest form, risk prediction models are mathematical equations that combine various patient-level risk factor data to estimate the probability of a future adverse event or poor clinical outcome, whether that be the initial development of a chronic wound or its subsequent response to therapy.17 As such, a critical component of any risk prediction tool is the availability of high-quality risk factor data. Risk factors can be divided into potentially modifiable risk factors (e.g., smoking, alcohol, obesity, malnutrition, diabetes cardiovascular disease) and nonmodifiable factors (e.g., age, genetic predisposition). Accessing a reasonably complete suite of risk factors required for reliable risk prediction in chronic wound can be challenging, given the large number of demographics, lifestyle, comorbidity, and other clinical factors that predispose to chronic wound disease or moderate their response to treatment.18–20
Despite so-called “big data” being recognized as generally lacking in wound healing outcome analysis,21 high-quality real-world data such as disease registries are proving an increasingly valuable source of chronic wound risk factor data. These include high-coverage, national quality registries such as the Swedish National Quality Registry for Ulcer Treatment (RiksSår),22–24 and the Danish National Patient Register.25
Such large, national disease registries are typically characterized by excellent coverage and data quality and, unlike clinical trials, cover a broad spectrum of patient type and risk factor profiles that better characterize real-world clinical practice. Powering risk prediction tools on data collected from real-world clinical settings further improves the generalizability and utility of such tools to every-day clinical practice. The capacity to further link these registries to administrative data and electronic health records also provides opportunities to use novel methods such as ML and data mining to better identify relevant combinations of risk factors in the building of targeted, personalized risk prediction.26,27
Definitions and terminology
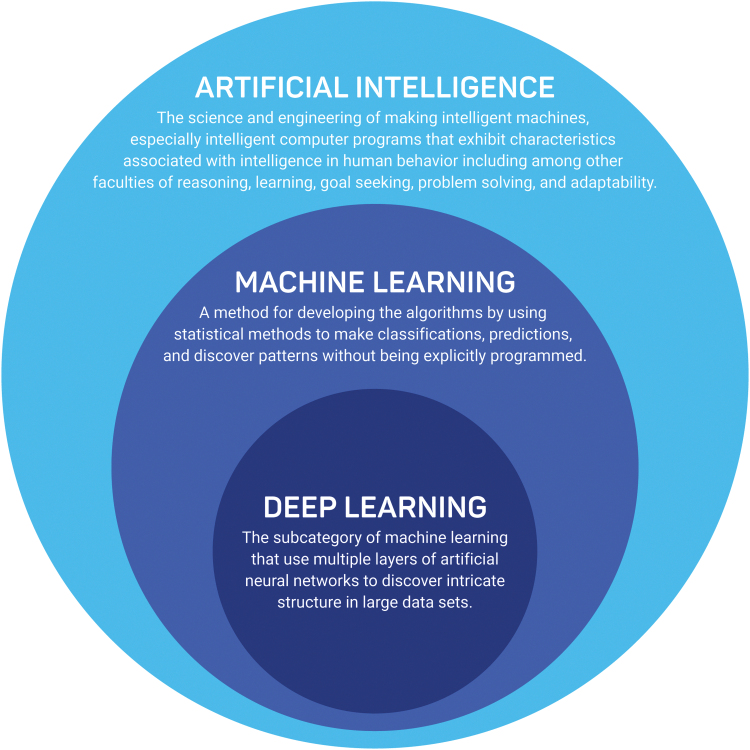
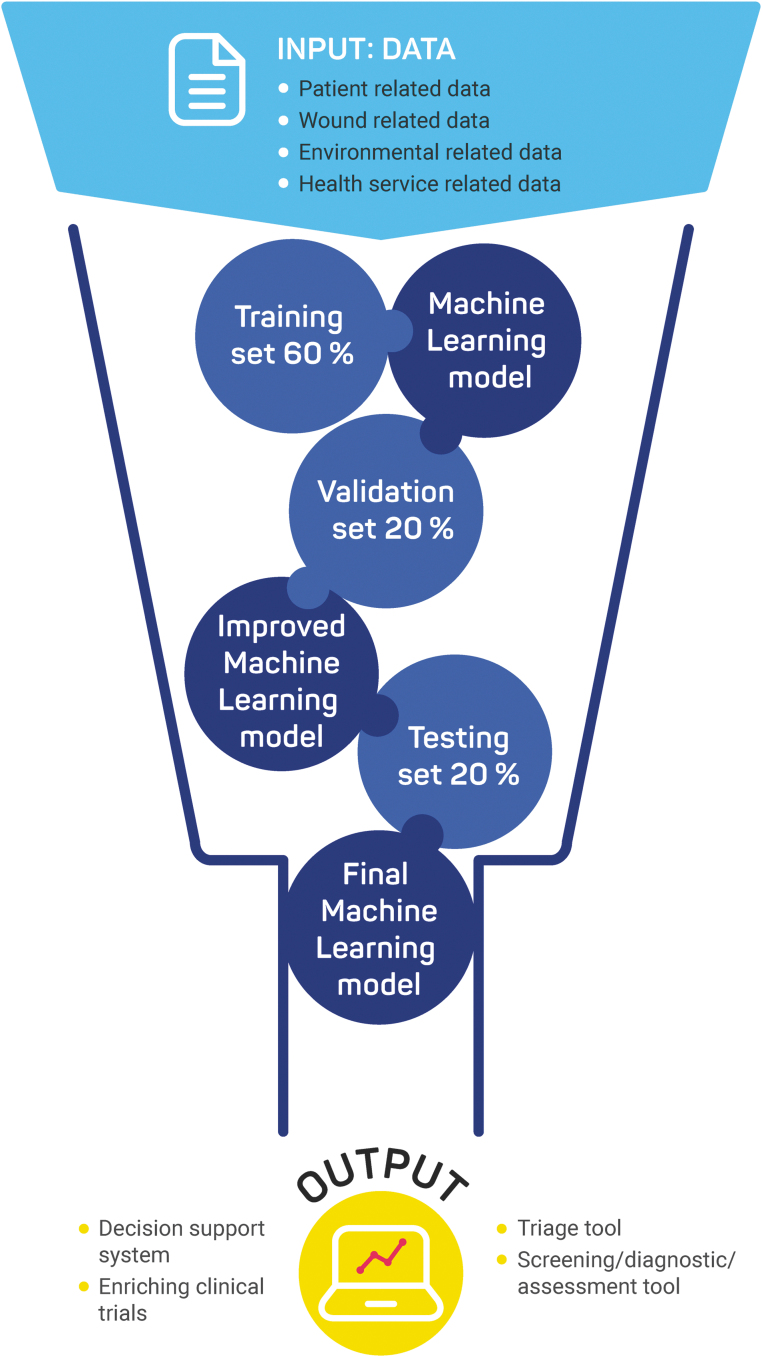
AI, ML, and deep learning tend to be used interchangeably and therefore, it is essential to define those concepts separately. AI can be defined as “the science and engineering of making intelligent machines, especially intelligent computer programs that exhibit characteristics associated with intelligence in human behavior including among other faculties of reasoning, learning, goal seeking, problem solving, and adaptability.”28 ML and deep learning are the subsets of AI, and deep learning is a subset of ML (Fig. 1). To paraphrase Arthur Samuel, who coined the term, ML is a method for developing the models by using mathematical methods to make classifications and predictions, and discover patterns without being explicitly programmed.29 Deep learning is the subcategory of ML that uses multiple layers of artificial neural networks to discover intricate structure in large data sets.30 Figure 2 describes the typical stages involved in developing and validating an ML model.
Figure 1.
Difference between Artificial Intelligence, Machine Learning, and Deep Learning.
Figure 2.
Typical Machine Learning model development steps.
Apart from the above-depicted definitions, considerable confusion exists around the individualized or personalized medicine approach. In the medical literature, several terms can be found (personalized medicine, precision medicine, stratified medicine, P4, and patient- and person-centered medicine), which are not synonyms, although frequently used interchangeably. From a very ambitious definition of “personalized medicine,” which often is described as “genomics-based knowledge that promises the ability to approach each patient as the biological individual he or she is,”31 the challenge of predicting individual outcomes has led to a gradual reinterpretation of the term “personalized medicine,” being replaced by “stratified medicine” in United Kingdom32,33 and by “precision medicine” in the United States.34
The PROGRESS consortium defined stratified medicine as the targeting of treatments (pharmacological or nonpharmacological) according to the biological or clinical characteristics shared by subgroups of patients.35 They highlight the distinction between purely prognostic factors (that affect outcome irrespective of treatment) and those predictive of treatment response.36 However, recently introduced “patient-centric medicine” has a much broader scope defined as “individual's specific health needs and desired health outcomes” as “the driving force behind all health care decisions and quality measurements” in which “patients are partners with their health care providers, and providers treat patients not only from a clinical perspective, but also from an emotional, mental, spiritual, social, and financial perspective.”37
The risk prediction models typically include clinical, genetic, and care-related factors and environmental, demographic, and other factors of importance for patient-related outcomes. Although judging based on the spectrum of factor, category, and outcome measures used in the risk prediction models, they can be described in certain situations as developments under the scope of patient-centric medicine. However, still, the majority of risk prediction models has a humbler approach and can be instead described under the scope of stratified/precision medicine. Even today, we lack a consistent analytical approach that can account for multiple patient attributes combined to inform treatment decisions adequately at the individual level.
Access to high-quality and representative data is just the first of several key phases in the development of clinically meaningful risk prediction models. Ideally, models balance statistical performance with clinical usefulness, achieving a satisfactory level of discrimination, calibration, and face validity.23 Current best practice guidelines highlight the importance of appropriate variable selection strategies, assessment of model performance, and external validation as three of the key steps in the development of good risk prediction.24 Variable selection, the identification of which combination of strong risk or prognostic factors best predicts the clinical outcome of interest, is a key determinant of both model performance and its ultimate utility in clinical or value-based payment settings. Although conventional selection methods generalized from linear regression analyses such as forward selection and backward elimination remain popular,25 such approaches have many disadvantages, such as instability of the selection and suboptimal model performance.
Moreover, these standard selection methods may be less practical for real-world risk prediction in chronic wound, where the number of potential candidate risk factors is typically large and the a priori evidence-based supporting of one variable over another is often limited. In the case of chronic wounds, the use of shrinkage of estimated regression coefficients, penalized likelihood, resampling, and stability testing may offer better alternatives to standard automated step-wise variable selection, particularly when combined with expert clinical opinion.26
Model stability (the robustness of the model to small changes in the training dataset), calibration (agreement between the estimated and observed event risks), and discrimination (the ability of the model to correctly identify which chronic wound patients progress to an adverse clinical outcome and which do not) are key elements in assessing performance. These can be assessed at internal validation of a risk prediction model, that is, without access to new, independent data, for example, using bootstrapping techniques. External validation is central to establishing the model's generalizability to different or new subgroups of chronic wound patients.14,27–29 External validation typically takes the form of assessing the performance of a risk prediction model in a separate dataset and can reveal key mismatches between a model's discrimination and calibration, which may reflect genuine differences in the cohort used to derive of the original prediction model and real-world settings.
One well-publicized example involved the Framingham Risk Score (FRS), commonly used in risk prediction for cardiovascular endpoints,33 whereas an FRS-based risk prediction model of coronary artery disease (CAD) returned similar levels of discrimination in Asian and non-Asian cohorts; only on subsequent external validation was it apparent that the same model markedly overestimated the absolute risk of CAD, by 276% in men and 102% in women.34 Similarly, an American College of Cardiology/American Heart Association (ACC/AHA) risk model overestimated CAD risk by 75% to 150% in validation cohorts.34 These examples highlight the common problem of poor calibration of absolute risk predictions.32
The emergence of large, high-quality national wound registries provides further opportunities to optimize the performance of chronic wound risk prediction by dividing these large databases into meaningful training and testing datasets, including by region or health care provider.33 Similarly, electronic medical records (EMRs) are proving an increasing valuable resource for both developing and validating risk prediction in chronic wounds. A 2020 study of 620,356 chronic wounds (various etiologies) from the EMRs of 261,398 patients in the United States was able to predict wound healing with an area under the curve of 0.72,34 although this was less than the accuracy observed in other studies. Whatever the data source or stage of model development, it is essential that the future of risk prediction in chronic wounds follows best practice recommendations both in terms of model development17,35 and in reporting.36
Innovative methods for improving risk prediction in chronic wounds
What is broadly acknowledged is that risk prediction in chronic wounds is challenging, given the high level of heterogeneity in patient, disease, monitoring, treatment, and health care system factors that correlate with the various clinical outcomes. Conventional risk prediction models based on linear combinations of risk factors remain an important cornerstone of any attempt to quantify a patient's individual risk status.37 Decision tree models are also well established in risk stratification and can be useful for both clinical outcome prediction and ranking the relative importance of competing risk factors to that prediction, may result in adequate risk prediction relative to conventional linear regression.
Risk prediction studies in chronic wound research employing conventional or algorithmic classification schemes generally tend to focus on three main outcomes: (1) wound healing; (2) in-hospital adverse events; and (3) wound management decision making (for example, specialist referral or optimal treatment recommendation). Characteristics of a selection of risk prediction studies for each of these outcomes are summarized in Tables 2–4, respectively.
Table 2.
Characteristics of wound risk prediction studies with wound healing outcome
| Study | Observation Period | End-Point | Sample Size | Model Predictors | Model Type and Performance |
|---|---|---|---|---|---|
| Cho et al (2019) | January 2014–September 2018 | Wound healing by end week 12 | 620,356 wounds from 261,398 patients | Demographic (including age, sex, and smoking status) Patient level clinical (including wound number and comorbidities) Wound factors (area, location, and etiology) |
Logistic model AUC: 0.712 Classification tree model: 0.717 |
| Berezo et al (2021) | January 2012–July 2021 | Wound not healing by end week 4, 8 and 12 from treatment start | 1,220,576 wounds | 187 covariates, including patient demographics, comorbidities, and wound factors. | Machine learning gradient-boosted decision tree models AUC: 4 weeks: 0.854 8 weeks: 0.855 12 weeks: 0.853 |
| Chakraborty (2019) | Not reported | Wound healing | 153 images of wounds | Visual wound features | Fuzzy c-means clustering for wound image segmentation vs standard computational learning schemes including decision tree, naive Bayesian and random forest. Accuracy: Fuzzy clustering = 93.8% Decision Tree = 84.3% Linear Discriminant = 85.7% Naive Bayesian = 78.7% |
| Fife and Horn (2020) | July 2003–July 2011 | Venous leg ulcer healing | 26,713 venous leg ulcers (split 90% development model & 10% validation sample) | Various demographic, clinical, and wound factors | Test-validate logistic regression confirmed wound size, age (days), number of concurrent wounds, evidence of infection/bioburden, being non-ambulatory and hospitalization for any reason significantly predicted healing. |
| Ubbink et al (2015) | November 2007–April 2012 | Time to complete wound healing | 1660 wounds | Various demographic, clinical, and wound factors identified from the literature and national expert panel | Cox and linear regression analysis identified five independent predictors: wound location, infection, size, duration, and patient age. |
Table 3.
Characteristics of wound risk prediction studies—in-hospital outcomes
| Study | Observation Period | Endpoint | Sample Size | Model Predictors | Model Type and Performance |
|---|---|---|---|---|---|
| Xie et al (2021) | 2009–2020 | In-hospital amputation in patients with diabetic foot ulcer | 618 patients | Demographic features, medical and medication history, clinical and laboratory data, Wagner Ulcer Classification, Wound, Ischemia, foot Infection (WIfI) Classification | Light Gradient Boosting Machine multiclass classification model AUC: Minor amputation: 0.85 Major amputation: 0.86 Nonamputation: 0.90 |
| Cramer et al (2019) | 2001–2012 | Hospital-acquired pressure ulcers in intensive care units | 50,581 admissions | Demographic parameters, diagnosis codes, laboratory values, and vitals in the first 24 h of admission. | Braden score vs machine learning weighted linear regression. Braden score: Precision = 0.09 Recall = 0.50 ML weighted: Precision = 0.09 Recall = 0.71 |
Table 4.
Characteristics of wound risk prediction studies–wound management decision-making outcomes
| Study | Observation Period | Endpoint | Sample Size | Model Predictors | Model Type and Performance |
|---|---|---|---|---|---|
| Mombini et al (2021) | Not reported | Wound care decisions | Not reported | Amount and presence of unhealthy tissue, wound visual features | Machine learning-based Shapley logistic regression: F1 score = 0.938 |
A U.S study of 620,356 chronic wounds (multiple etiologies) from 261,398 patients reported that a classification tree-based prediction model (AUC = 0.72) was broadly equivalent to conventional regression modeling (AUC = 0.71) in predicting wound healing within 12 weeks.38 This study highlights the importance of power and the availability of large datasets for driving reliable classification-based risk prediction. ML may not always outperform traditional approaches, and indeed may return relatively poor classification or prognostication, particularly where the training datasets are small.23,36,37 The very large number of risk factors at play, frequent interactions between competing risk factors, and nonlinear relationships between risk factors and, for example, delayed healing time, motivate the application of more innovative and sophisticated statistical solutions.39
Promising new approaches include ML methods. ML employs automated statistical algorithms to identify and then learn to recognize patterns in data, for example, combinations of risk factors that optimally predict development of a chronic wound. Three basic classes of ML methods are commonly used in risk prediction: supervised, unsupervised, and reinforcement learning.40 Supervised learning algorithms (where the outcome is known, and the aim is to learn what covariate patterns best predict the outcomes) have been suggested to be associated with superior accuracy in identifying treatment responders in other diseases.19,41
These techniques can be applied to large, real-world datasets such as patient registries, administrative datasets, and electronic health records to efficiently risk stratify patients based on their underlying risk factor profile. A recent 2021 study of 1,220,576 wounds from 425,163 patients, sourced from electronic health records in the United States, reported that ML-derived risk prediction models accurately predicted wounds at risk of not healing (AUC 0.86).39 The sheer volume of risk factors studied (187 demographic, comorbidity, and wound characteristics) coupled to the very large sample size of over 1 million wounds would have been logistically and computationally challenging. In another study, ML models based on Light Gradient Boosting Machine (LightGBM) algorithms could accurately classify the risk of major inpatient amputation (AUC 0.86) in a large, real-world cohort of patients with diabetic foot ulcers.42
ML models may be additionally useful as screening tools.43 Furthermore, an ML model trained on the electronic health records of 50,851 admissions to tertiary intensive care units outperformed the Braden score in predicting patients who subsequently developed chronic pressure ulcers.43 ML can also be used in decision support. Mombini et al applied ML to the analysis of a large volume of patient and image data of visual wound features to accurately predict treatment and referral decisions.44 More broadly, studies combining telemedicine with automated computational learning approaches for monitoring wound development and predicting patients in need of intervention or a change in treatment are currently underway.45
Another promising class of risk prediction methods is the use of multistate models to allow for dynamic risk prediction.46,47 A limitation of conventional approaches to risk prediction in chronic wounds is a patient's risk for, say delayed wound healing, is often assessed at a single point-in-time only (e.g., at baseline). However, a chronic wound patient's real-world risk profile is likely to vary and fluctuate over time, depending upon how their underlying disease is being managed or otherwise. Multistate models allow a patient to move between varying risk states, and can result in better estimation of the true level risk associated with a particular factor.
A critical aspect when making the leap from prognosis to a treatment recommendation is the causality. Risk prediction should thus form only one part of a decision support system to guide choices in the management of chronic wounds. The volume and heterogeneity of potential confounders of treatment response in chronic wound management make it difficult to isolate potentially causal pathways amenable to treatment, particularly when the risk prediction models are trained and tested in real-world datasets. This has, in part, formed an important driver of the increased popularity of ML in this field.46
Although the application of deep-learning models for building confounder-invariant risk prediction in chronic wounds has thus far been limited, the method has recently shown promise in the use of MRIs in the diagnosis of HIV,47 and the prediction of lung adenocarcinoma through CT.48 The combination of such methods for data-driven causal hypothesis and future application of causal inference methods may bring personalized, targeted chronic wound management one step closer.49–51
Nevertheless, such novel ML methods should be taken with caution and should not be universally applied to any research question, despite demonstrated superiority in specific areas. A 2020 review of 453 articles published between 2015 and 2019 on ML predictive models for the diagnosis of chronic diseases noted the large variety of ML methods employed and the lack of standard methods for determining the optimal approach.43 This may, in part, reflect the recency and novelty of many of these ML approaches.
CLINICAL USEFULNESS
Routine clinical practice requires simple, interpretable models of risk that use predictors that are easy to measure and not overly time-consuming.17 Although ML methods are attractive, they are very data dependent. Those models can lack the interpretability of predictor models grounded in subject matter knowledge. Furthermore, the clinical utility of algorithmic predictions needs to consider the benefit-harm consequences for patients of false positive and false negatives. For example, a higher rate of false positives may be acceptable in real-world clinical practice if it avoids undue harm.32,51 The trade-off between these choices should therefore be balanced with practical clinical needs. ML may play an important role for identifying complex combinations and/or interactions between various risk factors if large data sets are available.
However, the ultimate tool for use in real-world clinical practice needs to be pragmatic, intuitive, and actionable. Some performance measures have been proposed recently to quantify the ability of a prediction model to improve decision making.25,52,53 These measures consider the differential clinical consequences of false-positive versus true-positive classifications in a summary measure for clinical usefulness. ML-based risk prediction is not in itself sufficient to establish causal relationships between risk factors and wound outcomes and the general lack of standards or best practice in the development and application of the reviewed ML-based risk prediction has likely contributed to this lack of transparency and the relatively poor uptake of such technology in the clinical setting.
In terms of future work assessing the clinical usefulness of ML in wound management, this study group is currently undertaking a formal study applying ML methods to real-world data from the Swedish RiksSår quality registry for patients with difficult-to-heal wounds, to identify clusters of predictive factors that best predict wound class (healable vs maintenance vs nonhealable) in clinical practice.
CONCLUSION
Conventional regression-based approach to risk prediction will remain important, particularly where the number of predictors is relatively small, and assumptions underlying the chosen model form such as linearity are carefully assessed. Furthermore, translating estimated risk derived from ML algorithms into practical, standalone prediction tools for use in everyday clinical practice (such as personalized risk calculators or nomograms) can be challenging. Where the number of predictors is large and heterogenous (as is characteristic of chronic wounds), the relationships and correlations between various risk factors are complex, and very large data sets are available, ML may prove a powerful adjuvant for risk-stratifying patients predisposed to or living with chronic wounds.
TAKE-HOME MESSAGES
Chronic wounds are associated with considerable morbidity, loss of quality of life. and significant economic burden globally.
Reliable risk prediction is urgently needed for earlier identification and better management of disease.
Recent advancements in computing power and statistical methods based on ML and analysis of big data show promise for the development of personalized risk stratification in the real-world management of chronic wounds.
Abbreviations and Acronyms
- ACC
American College of Cardiology
- AHA
American Heart Association
- AI
artificial intelligence
- AUC
area under the curve
- CAD
coronary artery disease
- CT
computed tomography
- EMR
electronic medical records
- FRS
Framingham risk score
- GBM
gradient boosting machine
- HIV
human immunodeficiency virus
- ML
machine learning
- MRI
magnetic resonance imaging
- P4
predictive, preventive, personalized, participatory
- RiksSår
Swedish National Quality Registry for Ulcer Treatment
ACKNOWLEDGMENTS AND FUNDING SOURCES
Writing of this article is supported by unrestricted research grant from HARTMANN GROUP Advanced Predictive Analytic (APA) Program. This study is partially supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Award Number 1R01124789-01A1 and partially supported by National Science Foundation (NSF) Center to Stream Health care in Place (#C2SHiP) CNS Award Number 2052578.
AUTHORs' CONTRIBUTION
V.V.: conceptualization, methodology, writing—review and editing, supervision, and project administration
T.S.: conceptualization, methodology, investigation, writing—original draft, and writing—review and editing
M.C.: writing—review and editing
S.P.: writing—review and editing
D.G.A.: writing—review and editing
E.S.: writing—review and editing
AUTHOR DISCLOSURES AND GHOSTWRITING
V.V. is an employee of HARTMANN GROUP.
T.S. received compensation from serving on advisory boards for Biogen; speaker fees from Biogen and Novartis.
M.C. received speaker fees from Arjo.
S.P. declares no competing interests for this publication
D.G.A. This study is partially supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Award Number 1R01124789-01A1 and partially supported by National Science Foundation (NSF) Center to Stream Health care in Place (#C2SHiP) CNS Award Number 2052578
E.S. declares no competing interests
The content of this article was expressly written by the authors listed.
ABOUT THE AUTHORS
Vladica Veličković, MD, PhD candidate in Health Technology Assessment, is Global Senior Health Economist, lead of Health Economics and Outcome Research department, Advanced Predictive Analytic (APA) Program in HARTMANN GROUP, and Associate Researcher at Institute of Public Health, Medical Decision Making and HTA, UMIT, Austria.
Tim Spelman, PhD, MD, is Head of Statistics at MSBase, senior scientist at the Department of Clinical Neuroscience at the Karolinska Institute and lead statistician at the Department of Health Services Research at the Peter MacCallum Cancer Centre.
Michael Clark, PhD, is wound biologist and professor at School of Health Science, Birmingham, and Welsh Wound Innovation Centre. Professor Clark was a member of the coordinating guideline development group for the 2009 and 2014 International Pressure Ulcer Prevention and Treatment guidelines. He is an active member of the International Compression Club, which has produced several consensus documents in peer-reviewed publications on limb compression.
Sebastian Probst, DClinPrac, MNS, BNS, RN, is Full Professor of Tissue Viability and Wound Care, Geneva School of Health Sciences, University of Applied Sciences and Arts Western Switzerland, Adjunct Professor Faculty of Medicine Nursing and Health Sciences, Monash University, Melbourne, Australia. Prof. Probst is president of the European Wound Management Association, and a Council Member of Wound DACH
David G. Armstrong, PhD, is Professor of Surgery with Tenure at the University of Southern California and Visiting Professor of Medicine at University of Manchester College of Medicine. Dr. Armstrong has produced more than 600 peer-reviewed research articles in dozens of scholarly medical journals, as well as over 100 books or book chapters.
Ewout Steyerberg, PhD, is Professor of Clinical Biostatistics and Medical Decision Making at Leiden University Medical Center and a Professor of Medical Decision Making at Erasmus MC. Prof. Steyerberg is mainly known for his seminal work on prediction modeling. His textbook “Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating” has become both a practical guide and reference work for anyone involved in prediction research in medicine. He has published over 1,000 peer-reviewed articles, many in collaboration with clinical researchers, both in methodological and medical journals. His h-index exceeds 150.
REFERENCES
- 1. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann Epidemiol 2019;29:8–15; doi: 10.1016/j.annepidem.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–1602; doi: 10.1016/s0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21(1):27–32. doi: 10.1016/j.jval.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 4. Chan B, Cadarette S, Wodchis W, et al. Cost-of-illness studies in chronic ulcers: A systematic review. J Wound Care 2017;26(Supp 4):S4–S14; doi: 10.12968/jowc.2017.26.sup4.s4 [DOI] [PubMed] [Google Scholar]
- 5. Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: Costs and outcomes. Int Wound J 2018;15(1):29–37; doi: 10.1111/iwj.12814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sen CK. Human Wound and Its Burden: Updated 2020 compendium of estimates. Adv Wound Care (New Rochelle) 2021;10(5):281–292; doi: 10.1089/wound.2021.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calne S. (ed). Hard-to-Heal Wounds: A Holistic Approach. MEDICAL EDUCATION PARTNERSHIP: London, United Kingdom; 2008. [Google Scholar]
- 8. Avishai E, Yeghiazaryan K, Golubnitschaja O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J 2017;8(1):23–33; doi: 10.1007/s13167-017-0081-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. FrykbergRobert G. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4(9):560–582; doi: 10.1089/wound.2015.0635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Norman G, Westby MJ, Rithalia AD, et al. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev 2018;6(6):CD012583; doi: 10.1002/14651858.cd012583.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Layer A, McManus E, Levell NJ. A systematic review of model-based economic evaluations of treatments for venous leg ulcers. PharmacoEconomics - Open 2020;4(2):211–222; doi: 10.1007/s41669-019-0148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: A systematic review. JAMA 2011;306(15):1688–1698; doi: 10.1001/jama.2011.1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nijpels G, Beulens JW, van der Heijden AA, et al. Innovations in personalised diabetes care and risk management. Eur J Prev Cardiol 2019;26(2_Suppl):125–132; doi: 10.1177/2047487319880043 [DOI] [PubMed] [Google Scholar]
- 14. Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis research strategy (PROGRESS) 3: Prognostic model research. PLoS Med 2013;10(2):e1001381; doi: 10.1371/journal.pmed.1001381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dasari N, Jiang A, Skochdopole A, et al. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin Plast Surg 2021;35(03):153–158; doi: 10.1055/s-0041-1731460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ennis WJ, Hoffman RA, Gurtner GC, et al. Wound healing outcomes: Using big data and a modified intent-to-treat method as a metric for reporting healing rates. Wound Repair Regen 2017;25(4):665–672; doi: 10.1111/wrr.12575 [DOI] [PubMed] [Google Scholar]
- 17. Neziraj M, Hellman P, Kumlien C, et al. Prevalence of risk for pressure ulcers, malnutrition, poor oral health and falls – a register study among older persons receiving municipal health care in southern Sweden. BMC Geriatr 2021;21(1):265; doi: 10.1186/s12877-021-02205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madsen UR, Hyldig N, Juel K. Outcomes in patients with chronic leg wounds in Denmark: A nationwide register-based cohort study. Int Wound J 2022;19(1):156–168; doi: 10.1111/iwj.13607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fife CE, Horn SD. The wound healing index for predicting venous leg ulcer outcome. Adv Wound Care 2020;9(2):68–77; doi: 10.1089/wound.2019.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Serena TE, Fife CE, Eckert KA, et al. A new approach to clinical research: Integrating clinical care, quality reporting, and research using a wound care network-based learning healthcare system. Wound Repair Regen 2017;25(3):354–365; doi: 10.1111/wrr.12538 [DOI] [PubMed] [Google Scholar]
- 21. Jordan MI, Mitchell TM. Machine learning: Trends, perspectives, and prospects. Science 2015;349(6245):255–260; doi: 10.1126/science.aaa8415 [DOI] [PubMed] [Google Scholar]
- 22. Beam AL, Kohane IS. Big data and machine learning in health care. JAMA 2018;319(13):1317–1318; doi: 10.1001/jama.2017.18391 [DOI] [PubMed] [Google Scholar]
- 23. Christodoulou E, Ma J, Collins GS, et al. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol 2019;110:12–22; doi: 10.1016/j.jclinepi.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 24. Royston P, Moons KG, Altman DG, et al. Prognosis and prognostic research: Developing a prognostic model. BMJ 2009;338:b604; doi: 10.1136/bmj.b604 [DOI] [PubMed] [Google Scholar]
- 25. Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 2006;26(6):565–574; doi: 10.1177/0272989x06295361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heinze G, Wallisch C, Dunkler D. Variable selection–a review and recommendations for the practicing statistician. Biom J 2018;60(3):431–449; doi: 10.1002/bimj.201700067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hingorani AD, van der Windt DA, Riley RD, et al. Prognosis research strategy (PROGRESS) 4: Stratified medicine research. BMJ 2013;346:e5793; doi: 10.1136/bmj.e5793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD Statement. BMC Med 2015;13(1):1; doi: 10.1136/bmj.g7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramspek CL, Jager KJ, Dekker FW, et al. External validation of prognostic models: What, why, how, when and where? Clin Kidney J 2021;14(1):49–58; doi: 10.1093/ckj/sfaa188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson PWF, D'Agostino RB, Levy D, et al. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837–1847; doi: 10.1161/01.cir.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 31. Levy WC, Anand IS. Heart Failure Risk Prediction Models: What Have We Learned? JACC Heart Fail 2014;2(5):437–439; doi: 10.1016/j.jchf.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 32. Van Calster B, McLernon DJ, Van Smeden M, et al. Calibration: The Achilles heel of predictive analytics. BMC Med 2019;17(1):230; doi: 10.1186/s12916-019-1466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riley RD, Ensor J, Snell KIE, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: Opportunities and challenges. BMJ 2016;353:i3140; doi: 10.1136/bmj.i3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cho SK, Mattke S, Gordon H, et al. Development of a model to predict healing of chronic wounds within 12 weeks. Advan Wound Care 2019;9(9):516–524; doi: 10.1089/wound.2019.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ubbink DT, Lindeboom R, Eskes AM, et al. Predicting complex acute wound healing in patients from a wound expertise centre registry: A prognostic study. Int Wound J 2015;12(5):531–536; doi: 10.1111/iwj.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Austin PC, Harrell Jr FE, Steyerberg EW. Predictive performance of machine and statistical learning methods: Impact of data-generating processes on external validity in the “large N, small p” setting. Stat Methods Med Res 2021;30(6):1465–1483; doi: 10.1177/09622802211002867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Austin PC, Tu JV, Lee DS. Logistic regression had superior performance compared with regression trees for predicting in-hospital mortality in patients hospitalized with heart failure. J Clin Epidemiol 2010;63(10):1145–1155; doi: 10.1016/j.jclinepi.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 38. Berezo ML, Budman J, Deutscher D, et al. Predicting chronic wound healing time using machine learning to support real-time clinical decisions. Adv Wound Care (New Rochelle) 2021;11(6): doi: 10.1089/wound.2021.0073 [DOI] [Google Scholar]
- 39. Ayodele TO. New Advances in Machine Learning Available. London, UK: InTech, 2010. [Google Scholar]
- 40. Weng SF, Reps J, Kai J, et al. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One 2017;12(4):e0174944; doi: 10.1371/journal.pone.0174944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie P, Li Y, Deng B, et al. An explainable machine learning model for predicting in-hospital amputation rate of patients with diabetic foot ulcer. Int Wound J 2021;19(4):910–918; doi: 10.1111/iwj.13691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cramer EM, Seneviratne MG, Sharifi H, et al. Predicting the Incidence of Pressure Ulcers in the Intensive Care Unit Using Machine Learning. EGEMS (Wash DC) 2019;7(1):49; doi: 10.5334/egems.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Du C, Li Y, Xie P, et al. The amputation and mortality of inpatients with diabetic foot ulceration in the COVID-19 pandemic and postpandemic era: A machine learning study. Int Wound J 2021; 10.1111/iwj.13723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mombini H, Bengisu T, Diane S, et al. An Explainable Machine Learning Model for Chronic Wound Management Decisions. AMCIS 2021 Proceedings. 18. 2021. [Google Scholar]
- 45. Chakraborty C. Computational approach for chronic wound tissue characterization. Inform Med Unlocked 2019;17:100162; doi: 10.1016/j.imu.2019.100162 [DOI] [Google Scholar]
- 46. Upshaw JN, Konstam MA, Klaveren DV, et al. Multistate model to predict heart failure hospitalizations and all-cause mortality in outpatients with heart failure with reduced ejection fraction: Model derivation and external validation. Circ Heart Fail 2016;9(8):e003146; doi: 10.1161/circheartfailure.116.003146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith IL, Nixon JE, Sharples L. Power and sample size for multistate model analysis of longitudinal discrete outcomes in disease prevention trials. Stat Med 2021;40(8):1960–1971; doi: 10.1002/sim.8882 [DOI] [PubMed] [Google Scholar]
- 48. Holzinger A, Langs G, Denk H, et al. Causability and explainability of artificial intelligence in medicine. WIREs Data Mining and Knowledge Discovery 2019;9(4):e1312; doi: 10.1002/widm.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao Q, Adeli E, Pohl KM. Training confounder-free deep learning models for medical applications. Nat Commun 2020;11(1):6010; doi: 10.1038/s41467-020-19784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang H, Wu Z, Xing EP. Removing confounding factors associated weights in deep neural networks improves the prediction accuracy for healthcare applications. Pac Symp Biocomput 2019;24:54–65; doi: 10.1142/9789813279827_0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trikalinos TA, Siebert U, Lau J. Decision-analytic modeling to evaluate benefits and harms of medical tests: Uses and limitations. Med Decis Making 2009;29(5):E22–E29; doi: 10.1177/0272989X09345022 [DOI] [PubMed] [Google Scholar]
- 52. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: A framework for some traditional and novel measures. Epidemiol 2010;21(1):128–138; doi: 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016;352:i6; doi: 10.1136/bmj.i6 [DOI] [PMC free article] [PubMed] [Google Scholar]