Abstract
Many enteric glia are located along nerve fibers in the gut mucosa where they form close associations with the epithelium lining the gastrointestinal tract. The gut epithelium is essential for absorbing nutrients, regulating fluid flux, forming a physical barrier to prevent the entry of pathogens and toxins into the host, and participating in immune responses. Disruptions to this epithelium are linked to numerous diseases, highlighting its central importance in maintaining health. Accumulating evidence indicates that glia regulate gut epithelial homeostasis. Observations from glial-epithelial co-cultures in vitro and mouse genetic models in vivo suggest that enteric glia influence several important features of the gut epithelium including barrier integrity, ion transport, and capacity for self-renewal. Here we review the evidence for enteric glial regulation of the intestinal epithelium, with a focus on these three features of its biology.
Background
The gastrointestinal (GI) tract, which consists of the stomach, small intestine, and large intestine (colon), is a vital organ responsible for digesting and absorbing nutrients from food, maintaining hydration, eliminating waste, and expelling pathogens while hosting a diverse community of commensal microbiota. Central to all these functions is the gut epithelium, which lines the entire lumen of the GI tract and is composed of a single layer of columnar epithelial cells. Reflecting the functional specialization of the different regions of the GI tract, this epithelium exhibits distinct architecture, cellular composition, and molecular features in each region. Three key properties of the epithelium are conserved in all regions: barrier function, regulation of fluid and ion transport, and the ability to self-renew. Disruption of any of these properties can have local and systemic impacts, leading to disease.
Enteric glia encircle neuronal soma within myenteric and submucosal ganglia but many are also found in the GI mucosa, where they intimately associate with epithelial cells (Figure 1). This close physical association has long raised the possibility that mucosal enteric glia directly regulate intestinal epithelial functions. Here, we review the data on glial regulation of the intestinal epithelium, with a focus on its barrier, transport, and self-renewal functions. We discuss the approaches that have been used to study glial-epithelial interactions and the somewhat contradictory findings regarding their significance in GI homeostasis.
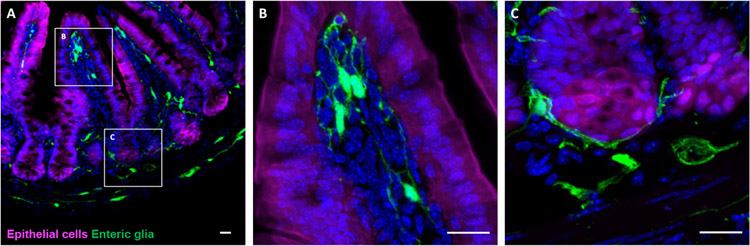
Figure 1. Enteric glia are found in close proximity to the intestinal epithelium.
(A) Immunohistochemical staining of a small intestinal cross-section from a Vil1CreRosa26tdTomato/+Plp1eGFP transgenic mouse in which enteric glia are labeled with green fluorescent protein (GFP) and intestinal epithelial cells are labeled with tdTomato. Mucosal glia closely associate with epithelial cells in villi (B) and in crypts (C). Scale bar = 20μm
1. Intestinal epithelial barrier integrity
The intestinal epithelium is a selectively permeable structure with regulated mechanisms for transcellular and paracellular movement of macromolecules in and out of the gut lumen. Apical junctional complexes including adherens junctions, tight junctions, and desmosomes, are formed by cell-cell adhesion molecules that interact with the actin cytoskeleton to seal gaps between neighboring epithelial cells. Disruption of these complexes can lead to entry of toxins, microorganisms, luminal antigens, or other noxious substances into the tissue, driving inflammation. Many diseases are associated with compromised integrity of this barrier ranging from inflammatory bowel disease (IBD) to autism spectrum disorder [1], Glia secrete factors that can influence epithelial barrier integrity.
1.1. Glial secreted factors
Enteric glial-derived factors decrease intestinal epithelial barrier permeability in vitro. For example, co-culture of the Caco-2 human colonic epithelial cell line with an immortalized rat enteric glial cell line or primary glia isolated from rat, mouse, or human myenteric plexus increases transepithelial resistance (TER) and reduces paracellular permeability [2]-[5]. Glial conditioned medium (CM) replicates these effects while co-culture with fibroblasts does not [2]-[4]. Caco-2 cells exhibit ~2-fold higher surface area and fewer gaps between cells when cultured with myenteric glia or glial CM [2]. These morphological changes are associated with increased levels and cell surface localization of tight junction proteins [2]-[4], [6]. Correspondingly, epithelial cells co-cultured with enteric glia upregulate genes involved in cell adhesion, including E-cadherin, laminins, and fibronectin [7].
Enteric glia also reduce intestinal epithelial permeability in the contexts of injury and infection. In an in vitro model of wound healing, for example, presence of enteric glia or glial CM promotes epithelial cell spreading [8], [9]. Similarly, Caco-2 cell monolayers exposed to the gut pathogen Shigella flexneri exhibit smaller and fewer foci of infection when co-cultured with myenteric glia or their CM, but not with fibroblasts [10]. In human colonic mucosal explants, glial CM blocks S. flexneri-induced epithelial desquamation [10]. Co-culture of intestinal epithelial cell lines with enteric glia can also mitigate the detrimental effects of pro-inflammatory stimuli including hypoxia, lipopolysaccharide, and Cytomix (cocktail of TNF-a, IFN-g, and IL-1b) on TER, paracellular permeability, and apical junctional complexes [4], [11], [12].
Several molecules have been proposed to mediate glial effects on the epithelial barrier [13]. The three factors most studied are reviewed below and in Table 1.
Table 1.
Putative glial-derived regulators of intestinal epithelial integrity
| Factor | Enteric glial cell (EGC) production |
In vitro evidence |
In vivo and ex vivo evidence (animal models) |
Human data | Other sources in the intestine |
|
|---|---|---|---|---|---|---|
| GNSO | ✓ Cell line [3] ✓ In situ in animal models [14] |
GSNO administration: ↑ TER and tight junction proteins [3] ↑ Inflammation-induced epithelial changes [10], [11], [15] Interference with GSNO activity: ↑ EGC-mediated increase in TER [3] ↑ EGC-mediated improvement in inflammation-induced epithelial changes [10], [11] |
GSNO administration: ↑ Permeability in explants [15] ↑ Infection-induced epithelial damage in explants [10] ↑ Infection-, injury- and inflammation-induced epithelial damage/permeability in vivo [3], [16]-[18] |
GSNO administration: ↑ Permeability in intestine ex vivo [3], [15] ↑ Infection-induced epithelial damage in intestine ex vivo [10] |
- Endothelium - Myofibroblasts - Stromal cells - Pericytes - Smooth muscle - Plasma cells - Others |
|
| GDNF | ✓ Primary EGCs and in situ in animal models [6], [19] ✓ EGCs in situ in human tissue [19]-[21] |
GDNF administration: ↑ IEC apoptosis [19] ↑ TER, wound healing [22] ↑ Permeability [22] ↑ Tight junction protein distribution [22], [23] ↑ Injury- and inflammation-induced epithelial impairments [12], [23] Interference with GDNF activity: ↑ EGC-mediated increase in TER [6], [12] ↑ EGC-mediated improvement in injury- and inflammation-induced epithelial impairments [6], [12] |
GDNF administration: ↑ Injury-induced epithelial damage and permeability in vivo [21], [23], [24] |
GDNF Administration: ↑ Inflammation-induced changes to tight junctions in enteric organoids [23] GDNF: ↑↑ Levels in inflamed intestine [19], [21], [23] |
- Smooth muscle - Epithelial cells (enterocytes and EECs) - Stromal cells - Others |
|
| Arachidonic acid metabolites | 15- HETE | ✓ Primary rat and human EGCs [9], [25] | 15-HETE administration: ↑ TER [25], [26] ↑ Permeability [25], [26] ↑ IEC spreading and tight junction protein distribution [25] |
Interference with 15-HETE signaling: ↑ Permeability in vivo [25] |
15-HETE: ↑↑ Levels in inflamed intestine [27], [28] ↑↑ Levels in EGC-CM from IBD patients [9], [25] |
- Neutrophils - Mast cells - Macrophages - Epithelial cells |
| 11β-PGF2α | ✓ Primary human EGCs [9] | 11β-PGF2α administration: ↑ Wound healing [9] |
11β-PGF2α: ↑ Levels and synthetic machinery EGCs from IBD patients [9] |
- Endothelium | ||
a). S-nitrosoglutathione (GSNO)
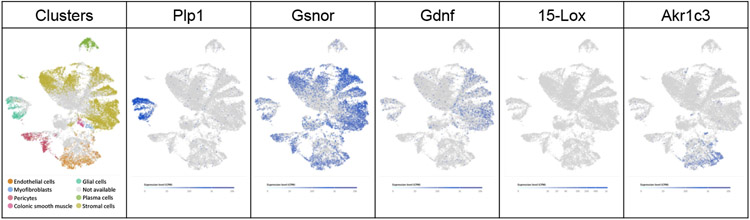
In elegant work, Savidge et al. used size-exclusion chromatography to identify GSNO as a critical “barrier-inducing factor” in glial CM that promotes TER of Caco-2 cell monolayers [3]. GSNO is a derivative of glutathione generated by the enzyme S-nitrosoglutathione reductase (GSNOR) that acts as a nitric oxide (NO) reservoir. In vitro, GSNO treatment is sufficient to promote epithelial barrier integrity at steady state and in the contexts of sterile and pathogen-induced inflammation [3], [10], [11], [15]. Pharmacological interference with GSNO or NO signaling abolishes the effects of enteric glia or their CM on intestinal epithelial cells [3], [4], [10], [11]. In vivo, administration of GSNO reduces mucosal inflammation in rodents with epithelial barrier disruption induced by genetic cell ablation, S. flexneri infection, endotoxemia, or sepsis, suggesting that this small molecule protects the epithelium in diverse contexts [3], [10], [17], [18]. GSNOR is widely expressed in the colon (Figure 2); thus, isolating the significance of glial GSNO production in vivo will be informative.
Figure 2. Enteric glia and other cells in the human colonic mesenchyme express machinery for barrier-modulating factors.
UMAP plots of gene expression (from adults with or without ulcerative colitis) [29]. Plp1 marks glia, Akr1c3 – Aldo-Keto Reductase Family 1 Member C3 (generates 11β-PGF2α).
b). Glial-derived neurotrophic factor (GDNF)
GDNF is a secreted protein initially identified as a glial-derived trophic factor for midbrain neurons. In the gut, the major source of GDNF is the mesenchyme but it is also produced by at least some enteric glia (Figure 2; [19]-[21 ]). Initial studies showed that GDNF does not affect TER [3]. Later work reported that GDNF selectively affects immature Caco-2 monolayers, causing a dose-dependent increase in TER [6], [22]. Glial CM exerts similar effects that are abolished by the depletion of GDNF from the CM or GDNF receptor blockade [6]. In vitro, GDNF administration accelerates wound closure by stimulating cell proliferation [22] and activates anti-apoptotic signaling pathways in colon cancer cell lines [19], exerting multiple effects on epithelial cell health. Correspondingly, GDNF supplementation reduces the permeability of epithelial cell barriers in myriad inflammatory contexts, including TNFα administration to organoids [6] and in vivo colitis models [23], [24]. Given that immortalized epithelial cells and enterocytes in situ produce GDNF themselves [22], the contributions of glial-derived GDNF to epithelial health in vivo will need to be unraveled.
c). Arachidonic acid (AA) metabolites
AA metabolites, such as 15-HETE and 11β-PGF2α, are fast-acting fatty acid signaling molecules that participate in inflammation. Enteric glia express the synthetic machinery for some AA metabolites including L-prostaglandin-D-synthase and 15-lipoxygenase (15-Lox) [25], [30]. 15-HETE and 11β-PGF2α are secreted by enteric glia and promote the integrity of the intestinal epithelial barrier in vitro. Treatment of epithelial monolayers with 15-HETE results in mildly decreased paracellular permeability, increased TER, more tight junction proteins, and enhanced cell spreading [25], [26]. 11β-PGF2α promotes wound healing of Caco-2 monolayers and blocking its signaling abolishes the beneficial effects of glial co-culture [9]. Enteric glia isolated from patients with IBD express lower levels of AA biosynthetic enzymes, secrete less 15-HETE and 11β-PGF2α, and are less effective at reducing permeability of epithelial monolayers than glia from healthy controls [9], [25].
1.2. In vivo evidence – are enteric glia necessary for epithelial barrier integrity?
Many of the observations above are from co-cultures of a single immortalized enteric glial cell line derived from the rat myenteric plexus [31] with one of two human epithelial cell lines, Caco-2 or IEC-6. Furthermore, none of the secreted factors identified is unique to enteric glia, at least at the transcriptional level (Figure 2). Therefore, understanding to what extent glia near the epithelium secrete these factors and how they influence barrier integrity in vivo is important. Several studies have probed these questions with conflicting findings (Table 2).
Table 2.
Mouse models of glial depletion and their epithelial phenotypes.
| Approach | Method | Timing | EGC loss | Barrier integrity |
Transport function |
Epithelial proliferation |
inflammation | Motility | References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical | 6-AN | Single IP dose | p1-p11 | X | MP EGCs present but abnormal | X | NR | NR | X | NR | [32] |
| Fluorocitrate | Daily IP x 7 days | Adult | X | MP EGCs present but abnormal | X | NR | NR | X | ✓ | [40] | |
| Genetic | hGFAP-HA; CL4-TCR | Constitutive | ✓ | ↑ # GFAP+ cells in MP and SMP | ✓ | NR | NR | ✓ | NR | [35] | |
| hGfapCre/ERT2; Rosa26iDTR | Single dose of TAM followed by two IP doses of DT (12-18h later) | p6-p7 | ✓ | ↑ GFAP IR | X | NR | NR | X | ✓ | [37] | |
| Pip1Cre/ERT2; Rosa26iDTR | ✓ | ↑ GFAP IR | X | NR | NR | X | ✓ | ||||
| Sox10Cre/ERT2; Rosa26iDTR | ✓ | ↑ GFAP IR | X | NR | NR | X | ✓ | ||||
| hGFAP-HSV-Tk | Continuous GCV (100m g/kg/day) | Adult | ✓ | ↑ GFAP IR in MP | ✓ | NR | NR | ✓ | NR | [33], [34] | |
| hGFAP-HSV-Tk transgenic | IP GCV (25mg/kg) twice a week | ✓ | ↑ GFAP IR and protein | X | NR | X | X | NR | [41] | ||
| hGFAP-HA | Adoptive transfer of activated HA-specific CD8+ T cells | ✓ | ↑ GFAP IR in MP | ✓ | ✓ | NR | X | ✓ | [36] | ||
| hGfapCre; Rosa26iDTR | Intracolonic DT injection | ✓ | ↑ GFAP IR and protein levels | NR | NR | NR | NR | NR | [41] | ||
| hGfapCre/ERT2; Rosa26DTA | Single IP dose (2mg) TAM and then continuous in chow | ✓ | ↑ GFAP IR in MP | X | NR | ✓ | X | NR | [38] | ||
| Plp1Cre/ERT2; Rosa26DTA | Single oral dose of TAM (8mg) | ✓ | ↑ #S100β+ cells in MP, muscuiaris externa and mucosa ↑ GFAP IR in MP |
X | NR | X | X | ✓ | [34] | ||
| Plp1Cre/ERT2; Rosa26iDTR | 4-OHT 1.5mg IP x 2 doses; Intracolonic injection of 20ng DT | NR | NR | NR | NR | NR | NR | NR | [41] | ||
| Plp1Cre/ERT2; Rosa26DTA | Single IP dose (2mg) TAM and then continuous in chow | ✓ | ↑ Plp1 mRNA and Plp1-Tomato+ cells | X | NR | X | X | NR | [38] | ||
| Plp1Cre/ERT2; hGfapCre/ERT2; Rosa26DTA | NR | NR | ✓ | NR | NR | ✓ | NR | ||||
✓ – Yes, phenotype observed; X – No, phenotype not observed; NR – not reported; IP – intraperitoneal; GCV – ganciclovir; TAM – Tamoxifen; IR – immunoreactivity; MP – myenteric plexus; SMP – submucosal plexus.
The earliest study investigated the effects of the CNS gliotoxin 6-aminonicotinamide (6-AN) in neonatal mice. The mice developed diarrhea without ultrastructural changes in myenteric neurons or the colonic epithelium [32]. Subsequent studies used gene promoters active in enteric glia, such as Gfap, Sox10, and Plp1, to genetically deplete cells and assess resulting deficits. In transgenic mice that express the herpes simplex virus thymidine kinase (HSV-TK) under the control of the human GFAP promoter, continuous administration of the substrate ganciclovir caused hemorrhagic inflammation selectively in the mid-to-distal small intestine [33]. This overt pathology was associated with ENS neurodegeneration and epithelial disruption [33], [34]. Defects in paracellular permeability were evident before overt inflammation [3], supporting a role for glia in barrier regulation. T cell-mediated autoimmune ablation of Gfap+ cells using different transgenic mice also caused severe intestinal pathology along with 100% mortality by postnatal day 8 [35]. When cell ablation was induced in these mice at later ages by T-cell adoptive transfer, however, the animals appeared well; yet they exhibited increased translocation of a 4.4kDa fluorophore from the gut lumen into circulation [36]. These observations supported a role for glia in epithelial barrier regulation but suggested that glial disruption was insufficient to provoke inflammation.
Recent studies have utilized selective expression of diphtheria toxin subunit A (DTA) or the DT receptor (DTR) to probe the roles of glia in epithelial regulation. Driving DTA or DT-mediated elimination of glia using the human GFAP, mouse Plp1, or mouse Sox10 promoters does not alter epithelial architecture, diminish myenteric neuronal number or cause intestinal inflammation in neonatal or adult mice [34], [37], [38]. In contrast, all these manipulations affect another key GI function, motility (Table 2). Targeting Plp1+ cells with DTA does not affect apical junctional complexes or the translocation of even small fluorophores (478Da) across the gut epithelium even though the vast majority of enteric glia are eliminated [34]. Similarly, chemogenetic disruption or stimulation of glial calcium signaling has no effect on permeability in ex vivo preparations [39]. Simultaneous targeting of Gfap+ and Plp1+ cells with DTA, in contrast, results in severe intestinal pathology associated with mortality within 9 days [38]. Epithelial permeability is increased within 48h of induction of DTA expression in this model, suggesting an early permeability deficit. A challenge with interpreting findings from these mice is that simultaneous targeting of both Gfap+ and Plp1+ cells would disrupt virtually all glia throughout the entire nervous system, presumably impacting many aspects of their health. Thus, on balance the cumulative data indicate that enteric glia secrete factors capable of influencing the intestinal epithelial barrier but are probably not essential for this role in vivo.
2. Ion transport
Closely related to the barrier property of the epithelium is its transport function, which allows direct absorption of micronutrients, macronutrients, and water. Transepithelial ion flux facilitated by enterocytes determines local and systemic fluid status, and is highly regulated by enteric neurons. Glia were initially considered dispensable for this because metabolic gliotoxins had no effect on transepithelial ion transport [40], [42] and genetic manipulation of glial Ca2+ signaling did not alter fecal water content [43]. Later studies, however, reported that diminishing or driving glial signaling can raise or lower the fecal water content, respectively [44], [45], indicating a role for glia. Myenteric glia secrete NO in response to cholinergic stimulation, which influences transepithelial ion transport [46]. In inflammatory colitis, this transport is disrupted and inhibition of glial function rescues this disruption [42]. These results suggest glial NO indirectly regulates electrogenic transport via its action on enteric neurons and, likely, epithelial and endothelial cells. Chemogenetic activation of Gfap+ cells causes trans-epithelial ion movement that is partially abolished by blocking fast synaptic transmission while inhibiting glial calcium signaling reduces neuron-evoked epithelial ion movement [39]. These observations are not due to defective epithelial permeability because manipulation of glial signaling does not alter TER or paracellular flux [39], [47]. In summary, glia regulate epithelial ion transport in collaboration with neuronal secretomotor circuits.
3. Regeneration and repair
The intestinal epithelium is highly proliferative with an average enterocyte turnover rate of ~4 days. This ability to self-renew depends on intestinal stem cells (ISCs) at crypt bases that give rise to all constituent epithelial cell types in a coupled process of migration and differentiation. Daughter cells migrate up the crypt-villus axis and are eventually extruded from the tips of the villi into the gut lumen. Niche factors like secreted Wnt ligands keep ISCs in an undifferentiated state.
Enteric nerve fibers and associated glia encircle intestinal epithelial crypts (Figure 1C), hinting that the ENS regulates the regenerative capacity of the epithelium. Observations in vitro suggest that glial factors mediate some effects. Enteric glia or their CM reduce the proliferation of immortalized and primary intestinal epithelial cells without affecting viability. Neutralization of TGF-β1 or loss of 15dPGJ2 synthesis reduces this anti-proliferative effect, suggesting that TGF-β1 and 15dPGJ2 are partially responsible [2], [30].
The effects of enteric glia on intestinal epithelial cell proliferation in vivo are less clear (Table 2). Chemical disruption of glial function with 6-AN or fluorocitrate does not cause crypt hyperplasia, but proliferation was not examined in detail [32], [40]. Genetic disruption of glia in GFAP-HSV-TK transgenic mice is associated with hyperproliferation of crypt cells, but only at ganciclovir doses that also disrupt non-glial cells and provoke inflammation [33], [34], [41]. In contrast, DTA-mediated elimination of the much broader Plp1+ population of enteric glia does not affect crypt size or cell proliferation [34]. A recent study compared the intestinal effects of driving DTA expression with the human GFAP and mouse Plp1 promoters and found that only the former reduced ISC number, stimulated crypt cell proliferation, and caused a defect in the epithelial regenerative response to radiation [38]. Taken together, studies to date suggest that a Plp1/Gfap+ population of cells, potentially but not necessarily glia (given the evidence for broader Gfap promoter activity [34], [41], [48]), is important for ISC regulation. Gfap+ cells express transcripts for several Wnts, offering a potential mechanism. Mice lacking Wntless, a protein necessary for Wnt secretion, in Gfap+ cells have normal epithelial architecture that becomes disrupted upon radiation exposure [38]. This observation suggests that secreted Wnts from Gfap+ cells are not essential for epithelial turnover in the healthy intestine but play a role in the regenerative response to injury.
Closing Remarks
The intestinal epithelium is a critical interface between an organism and its environment. Its barrier properties, transport functions, and ability to self-renew are fundamentally important for health. Enteric glia closely associate with enteric neurons, immune and epithelial cells, positioning them to influence epithelial functions directly and indirectly. Many studies have shown that enteric glia can secrete signals, ranging from trophic factors to small molecules, that can influence intestinal epithelial cells in vitro. In vivo, however, the essential roles of enteric glia are less clear. Studies to date demonstrate that glia modulate electrogenic ion transport but suggest that the majority of them are not necessary for maintaining barrier integrity or epithelial proliferation except in the context of specific injuries. These somewhat discrepant findings may be clues to the degree of functional redundancy in the gut and highlight the need for tools that enable manipulation of enteric glia with more precision. Moving forward, it will be exciting to uncover the contexts in which glial activity is most influential in vivo, identify the subpopulations that exert these effects, and ascertain the underlying molecular mechanisms.
Acknowledgements
This work was supported by the Richard and Susan Smith Family Foundation (Odyssey Award) and NIH R01DK130836 to M.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Leech B, Schloss J, and Steel A, “Association between increased intestinal permeability and disease: A systematic review,” Adv. Integr. Med, vol. 6, no. 1, pp. 23–34, Mar. 2019, doi: 10.1016/j.aimed.2018.08.003. [DOI] [Google Scholar]
- [2].Neunlist M et al. , “Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway,” Am. J. Physiol. Gastrointest. Liver Physiol, vol. 292, no. 1, pp. G231–241, Jan. 2007, doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- [3].Savidge TC et al. , “Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione,” Gastroenterology, vol. 132, no. 4, pp. 1344–1358, Apr. 2007, doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- [4].Xiao W-D, Chen W, Sun L-H, Wang W-S, Zhou S-W, and Yang H, “The protective effect of enteric glial cells on intestinal epithelial barrier function is enhanced by inhibiting inducible nitric oxide synthase activity under lipopolysaccharide stimulation,” Mol. Cell. Neurosci, vol. 46, no. 2, pp. 527–534, Feb. 2011, doi: 10.1016/j.mcn.2010.12.007. [DOI] [PubMed] [Google Scholar]
- [5].Soret R, Coquenlorge S, Cossais F, Meurette G, Rolli-Derkinderen M, and Neunlist M, “Characterization of human, mouse, and rat cultures of enteric glial cells and their effect on intestinal epithelial cells,” Neurogastroenterol. Motil, vol. 25, no. 11, pp. e755–e764, Nov. 2013, doi: 10.1111/nmo.12200. [DOI] [PubMed] [Google Scholar]
- [6].Meir M et al. , “Intestinal Epithelial Barrier Maturation by Enteric Glial Cells Is GDNF-Dependent,” Int. J. Mol. Sci, vol. 22, no. 4, p. 1887, Feb. 2021, doi: 10.3390/ijms22041887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Landeghem L et al. , “Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions,” BMC Genomics, vol. 10, p. 507, Nov. 2009, doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Van Landeghem L et al. , “Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF,” Am. J. Physiol. Gastrointest. Liver Physiol, vol. 300, no. 6, pp. G976–987, Jun. 2011, doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Coquenlorge S et al. , “The arachidonic acid metabolite 11β-ProstaglandinF2α controls intestinal epithelial healing: deficiency in patients with Crohn’s disease,” Sci. Rep, vol. 6, p. 25203, May 2016, doi: 10.1038/srep25203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Flamant M et al. , “Enteric glia protect against Shigella flexneri invasion in intestinal epithelial cells: a role for S-nitrosoglutathione,” Gut, vol. 60, no. 4, pp. 473–484, Apr. 2011, doi: 10.1136/gut.2010.229237. [DOI] [PubMed] [Google Scholar]
- [11].Cheadle GA, Costantini TW, Lopez N, Bansal V, Eliceiri BP, and Coimbra R, “Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown,” PloS One, vol. 8, no. 7, p. e69042, 2013, doi: 10.1371/journal.pone.0069042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xiao W et al. , “GDNF is involved in the barrier-inducing effect of enteric glial cells on intestinal epithelial cells under acute ischemia reperfusion stimulation,” Mol. Neurobiol, vol. 50, no. 2, pp. 274–289, Oct. 2014, doi: 10.1007/S12035-014-8730-9. [DOI] [PubMed] [Google Scholar]
- [13].de Mattos Coelho-Aguiar J et al. , “The Enteric Glial Network Acts in the Maintenance of Intestinal Homeostasis and in Intestinal Disorders,” in Glia in Health and Disease, Spohr T, Ed. IntechOpen, 2020. doi: 10.5772/intechopen.89170. [DOI] [Google Scholar]
- [14].Hou Y et al. , “Maintenance of Intestinal Homeostasis in Diarrhea-Predominant Irritable Bowel Syndrome by Electroacupuncture Through Submucosal Enteric Glial Cell-Derived S-Nitrosoglutathione,” Front. Physiol, vol. 13, p. 917579, Aug. 2022, doi: 10.3389/fphys.2022.917579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hagbom M et al. , “Neurotrophic Factors Protect the Intestinal Barrier from Rotavirus Insult in Mice,” mBio, vol. 11, no. 1, pp. e02834–19, Feb. 2020, doi: 10.1128/mBio.02834-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Costantini TW et al. , “Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells,” Am. J. Physiol.-Gastrointest. Liver Physiol, vol. 299, no. 6, pp. G1308–G1318, Dec. 2010, doi: 10.1152/ajpgi.00156.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Z, Zhang X, Zhou H, Liu W, and Li J, “Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats,” J. Trauma Acute Care Surg, vol. 80, no. 6, pp. 977–984, Jun. 2016, doi: 10.1097/TA.0000000000001008. [DOI] [PubMed] [Google Scholar]
- [18].Cheng B et al. , “Inhibition of platelet activation suppresses reactive enteric glia and mitigates intestinal barrier dysfunction during sepsis,” Mol. Med. Camb. Mass, vol. 28, no. 1, p. 127, Oct. 2022, doi: 10.1186/s10020-022-00556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Steinkamp M et al. , “Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells,” Gastroenterology, vol. 124, no. 7, pp. 1748–1757, Jun. 2003, doi: 10.1016/s0016-5085(03)00404-9. [DOI] [PubMed] [Google Scholar]
- [20].Bär KJ, Facer P, Williams NS, Tam PK, and Anand P, “Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung’s disease,” Gastroenterology, vol. 112, no. 4, pp. 1381–1385, Apr. 1997, doi: 10.1016/s0016-5085(97)70154-9. [DOI] [PubMed] [Google Scholar]
- [21].Chen H, Han T, Gao L, and Zhang D, “The Involvement of Glial Cell-Derived Neurotrophic Factor in Inflammatory Bowel Disease,” J. Interferon Cytokine Res, vol. 42, no. 1, pp. 1–7, Jan. 2022, doi: 10.1089/jir.2021.0116. [DOI] [PubMed] [Google Scholar]
- [22].Meir M et al. , “Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro,” Am. J. Physiol. Gastrointest. Liver Physiol, vol. 309, no. 8, pp. G613–624, Oct. 2015, doi: 10.1152/ajpgi.00357.2014. [DOI] [PubMed] [Google Scholar]
- [23].Meir M et al. , “Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease,” J. Clin. Invest, vol. 129, no. 7, pp. 2824–2840, Jun. 2019, doi: 10.1172/JCI120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang DK et al. , “Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis: GDNF regulates the intestinal barrier and inflammation,” J. Pathol, vol. 222, no. 2, pp. 213–222, Oct. 2010, doi: 10.1002/path.2749. [DOI] [PubMed] [Google Scholar]
- [25].Pochard C et al. , “Defects in 15-HETE Production and Control of Epithelial Permeability by Human Enteric Glial Cells From Patients With Crohn’s Disease,” Gastroenterology, vol. 150, no. 1, pp. 168–180, Jan. 2016, doi: 10.1053/j.gastro.2015.09.038. [DOI] [PubMed] [Google Scholar]
- [26].Ohata A, Usami M, and Miyoshi M, “Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation,” Nutrition, vol. 21, no. 7–8, pp. 838–847, Jul. 2005, doi: 10.1016/j.nut.2004.12.004. [DOI] [PubMed] [Google Scholar]
- [27].Krilis SA, Macpherson JL, de Carle DJ, Daggard GE, Talley NA, and Chesterman CN, “Small bowel mucosa from celiac patients generates 15-hydroxyeicosatetraenoic acid (15-HETE) after in vitro challenge with gluten,” J. Immunol. Baltim. Md 1950, vol. 137, no. 12, pp. 3768–3771, Dec. 1986. [PubMed] [Google Scholar]
- [28].Zijlstra FJ and Wilson JHP, “15-HETE is the main eicosanoid present in mucus of ulcerative proctocolitis,” Prostaglandins Leukot. Essent. Fatty Acids, vol. 43, no. 1, pp. 55–59, May 1991, doi: 10.1016/0952-3278(91)90133-P. [DOI] [PubMed] [Google Scholar]
- [29].Kinchen J et al. , “Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease,” Cell, vol. 175, no. 2, pp. 372–386.e17, Oct. 2018, doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bach-Ngohou K et al. , “Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2,” J. Physiol, vol. 588, no. Pt 14, pp. 2533–2544, Jul. 2010, doi: 10.1113/jphysiol.2010.188409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ruhl A, Trotter J, and Stremmel W, “Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat,” Neurogastroenterol. Motil, vol. 13, no. 1, pp. 95–106, Feb. 2001, doi: 10.1046/j.1365-2982.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- [32].Aikawa H and Suzuki K, “Enteric gliopathy in niacin-deficiency induced by CNS glio-toxin,” Brain Res., vol. 334, no. 2, pp. 354–356, May 1985, doi: 10.1016/0006-8993(85)90231-8. [DOI] [PubMed] [Google Scholar]
- [33].Bush TG et al. , “Fulminant Jejuno-Ileitis following Ablation of Enteric Glia in Adult Transgenic Mice,” Cell, vol. 93, no. 2, pp. 189–201, Apr. 1998, doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- [34].Rao M et al. , “Enteric Glia Regulate Gastrointestinal Motility but Are Not Required for Maintenance of the Epithelium in Mice,” Gastroenterology, vol. 153, no. 4, pp. 1068–1081.e7, Oct. 2017, doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Cornet A et al. , “Enterocolitis induced by autoimmune targeting of enteric glial cells: A possible mechanism in Crohn’s disease?,” Proc. Natl. Acad. Sci, vol. 98, no. 23, pp. 13306–13311, Nov. 2001, doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Aube A-C, “Changes in enteric neurone phenotype and intestinal functions in a transgenic mouse model of enteric glia disruption,” Gut, vol. 55, no. 5, pp. 630–637, May 2006, doi: 10.1136/gut.2005.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kovler ML et al. , “Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis,” Sci. Transl. Med, vol. 13, no. 612, p. eabg3459, Sep. 2021, doi: 10.1126/scitranslmed.abg3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baghdadi MB et al. , “Enteric glial cell heterogeneity regulates intestinal stem cell niches,” Cell Stem Cell, vol. 29, no. 1, pp. 86–100.e6, Jan. 2022, doi: 10.1016/j.stem.2021.10.004. [DOI] [PubMed] [Google Scholar]
- [39].Grubišić V and Gulbransen BD, “Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability,” J. Physiol, vol. 595, no. 11, pp. 3409–3424, Jun. 2017, doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nasser Y et al. , “Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function,” Am. J. Physiol. Gastrointest. Liver Physiol, vol. 291, no. 5, pp. G912–927, Nov. 2006, doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- [41].Yuan R et al. , “Enteric Glia Play a Critical Role in Promoting the Development of Colorectal Cancer,” Front. Oncol, vol. 10, p. 595892, 2020, doi: 10.3389/fonc.2020.595892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].MacEachern SJ et al. , “Inhibiting Inducible Nitric Oxide Synthase in Enteric Glia Restores Electrogenic Ion Transport in Mice With Colitis,” Gastroenterology, vol. 149, no. 2, pp. 445–455.e3, Aug. 2015, doi: 10.1053/j.gastro.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].McClain JL, Fried DE, and Gulbransen BD, “Agonist-Evoked Ca2+ Signaling in Enteric Glia Drives Neural Programs That Regulate Intestinal Motility in Mice,” Cell. Mol. Gastroenterol. Hepatol, vol. 1, no. 6, pp. 631–645, Nov. 2015, doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McClain JL et al. , “Ca2+ Responses in Enteric Glia Are Mediated by Connexin-43 Hemichannels and Modulate Colonic Transit in Mice,” Gastroenterology, vol. 146, no. 2, pp. 497–507.e1, Feb. 2014, doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L, and Gulbransen BD, “Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility,” Am. J. Physiol. Gastrointest. Liver Physiol, vol. 315, no. 4, pp. G473–G483, Oct. 2018, doi: 10.1152/ajpgi.00155.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].MacEachern SJ, Patel BA, McKay DM, and Sharkey KA, “Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus: Enteric glial regulation of ion transport,” J. Physiol, vol. 589, no. 13, pp. 3333–3348, Jul. 2011, doi: 10.1113/jphysiol.2011.207902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cavin J-B, Cuddihey H, MacNaughton WK, and Sharkey KA, “Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: the role of the enteric nervous system,” Am. J. Physiol.-Gastrointest. Liver Physiol, vol. 318, no. 2, pp. G254–G264, Feb. 2020, doi: 10.1152/ajpgi.00186.2019. [DOI] [PubMed] [Google Scholar]
- [48].Scholten D et al. , “Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice,” Gastroenterology, vol. 139, no. 3, pp. 987–998, Sep. 2010, doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]