Abstract
Intervertebral disc degeneration (IDD) is a major contributor to back, neck, and radicular pain. It is related to changes in tissue structure and function, including the breakdown of the extracellular matrix (ECM), aging, apoptosis of the nucleus pulposus, and biomechanical tissue impairment. Recently, an increasing number of studies have demonstrated that inflammatory mediators play a crucial role in IDD, and they are being explored as potential treatment targets for IDD and associated disorders. For example, interleukins (IL), tumour necrosis factor-α (TNF-α), chemokines, and inflammasomes have all been linked to the pathophysiology of IDD. These inflammatory mediators are found in high concentrations in intervertebral disc (IVD) tissues and cells and are associated with the severity of LBP and IDD. It is feasible to reduce the production of these proinflammatory mediators and develop a novel therapy for IDD, which will be a hotspot of future research. In this review, the effects of inflammatory mediators in IDD were described.
1. Introduction
Intervertebral disc degeneration (IDD) is a disease of the discs that link adjacent vertebrae, with structural damage leading to a degeneration of the discs and surrounding areas. The intervertebral disc (IVD) is a fibrocartilage tissue that joins the adjacent vertebral bodies in the spine. The nucleus pulposus (NP) is the central component of the IVD and is rich in elastic colloidal compounds, including proteoglycans and type II collagen [1]. IDD can be diagnosed and graded by conventional T2-weighted magnetic resonance images, in which the colour and homogeneity of the disc, distinction of nucleus and annulus, disc signal intensity, and disc height are the basis for grading [2]. IDD is associated with disc herniation, spondylosis, lumbar spinal stenosis, sagittal imbalance of the spinal-pelvic complex, and neurological symptoms, such as low back pain (LBP), limb numbness, and decreased muscle strength [3–5]. The most common symptom of IDD is LBP, which impacts the quality of life of middle-aged and elderly individuals while increasing the economic burden on families and society [6, 7]. Although current evidence-based medicine has identified IDD as the result of a variety of genetic, traumatic, inflammatory, lifestyle, aging, and nutritional variables, the pathogenic processes implicated in the development of IDD remain unclear [8–14]. Currently, treatment options include noninvasive therapy such as medications, multiple physical modalities, and multidisciplinary biopsychosocial rehabilitation; interventional treatments, such as intradiscal radiofrequency and epidural injections; regeneration by injecting solutions of papain and methylene blue into the disc; and surgical approaches, such as intervertebral fusion or artificial disc replacement. Despite advances in pain relief therapies, they provide only temporary relief and are associated with complications [15].
IDD progresses due to cellular and biochemical changes in the IVD microenvironment, resulting in progressive functional and structural damage. The main pathological features of IDD include the production of proinflammatory mediators, progressive loss of ECM, increased cellular senescence and apoptosis, and phenotypic changes in healthy NP cells [13, 14, 16, 17]. Many molecular biology studies have demonstrated increased expression of inflammatory mediators such as IL-1β, TNF-α, IL-6, IL-8, and IL-20 in degenerative IVD [18–23]. Increased plasma inflammatory mediator concentrations are related to the degree of IDD and the severity of LBP [24]. Advances in inflammatory mediator mechanisms will significantly promote the translation of molecular research into clinical practice, offering new paths for developing IDD medication. This review is aimed at discussing the research on the potential function of inflammatory mediators in IDD.
2. Upstream and Downstream Regulatory Networks
Disc degeneration was derived from several initializing factors, such as genetics, mechanical stress, aging, trauma, and environmental factors [25–29]. These initializing factors lead to morphological changes in the disc tissue and surrounding structures, including a series of changes such as rupture of the annulus fibrosus (AF), NP herniation, and calcification of the cartilage endplates (CE). Since the intervertebral disc is a nearly wholly enclosed avascular tissue with few sources of nutrition, accumulation of degraded organelles and waste materials that are difficult to metabolize occurs, and a closed acidic environment gradually develops, leading to an imbalance in the internal and external environment, which propagates inflammatory signals and causes a massive release of inflammatory mediators [1], including IL-1α, IL-1β, IL-2, IL-6, IL-8, IL-9, IL-10, IL-17, TNF-α, chemokines, the NLRP3 inflammasome, and nitric oxide. These inflammatory mediators can activate signalling pathways, such as the NF-κB, PI3-K/Akt/mTOR, TGF-β, JAK-STAT, Wnt/β-catenin, and MAPK pathways, resulting in a range of pathological responses within the IVD, including an enhanced inflammatory response, promote ECM degradation, accelerate cellular senescence, increased intracellular ROS, promotion of apoptosis or pyroptosis, regulation of NP cell proliferation, and increased angiogenesis and neoinnervation. Ultimately, this process exacerbates the development of IDD. A schematic diagram of this pathological process is shown in Figure 1.
Figure 1.
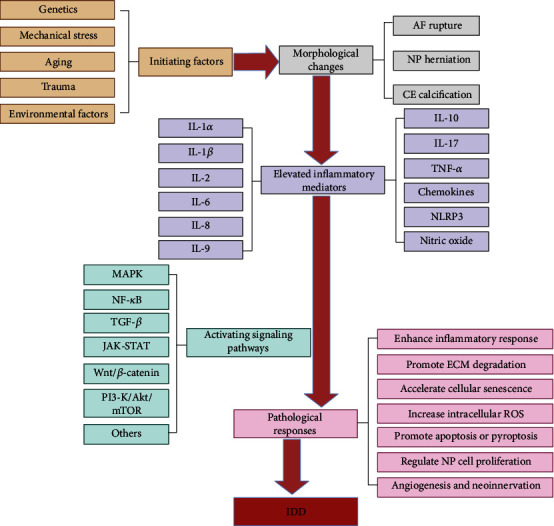
Diagram of IDD upstream and downstream regulation networks.
3. Sources of Inflammatory Mediators
Inflammatory mediators can be secreted by endogenous intervertebral disc cells and exogenous immune cells [30]. The normal aging process associated with genetic susceptibility leads to degeneration of the IVD, causing alterations in the ECM, such as a reduced number of functional cells, reduced proteoglycan content, malnutrition, dehydration, matrix breakdown, and calcification. Modifications in the ECM affect the typical response of the IVD to mechanical loading. The IVD becomes prone to microfissures and consequent ingrowth of nerve tissue and blood vessels. Fragments and microcrystals of the ECM may internally cause an inflammatory response, stimulating endogenous IVD cells to produce proinflammatory mediators such as IL-1β, IL-6, and IL-8, further promoting a chain reaction of tissue degeneration. In addition, NP is recognized by the immune system as nonself when exposed to tissues, such as through microfissures or protrusions, thereby recruiting inflammatory cells such as macrophages, endothelial cells, B cells, and T cells. These inflammatory cells can secrete inflammatory mediators. A brief overview of the various cells expressing different cytokines is presented in Figure 2.
Figure 2.
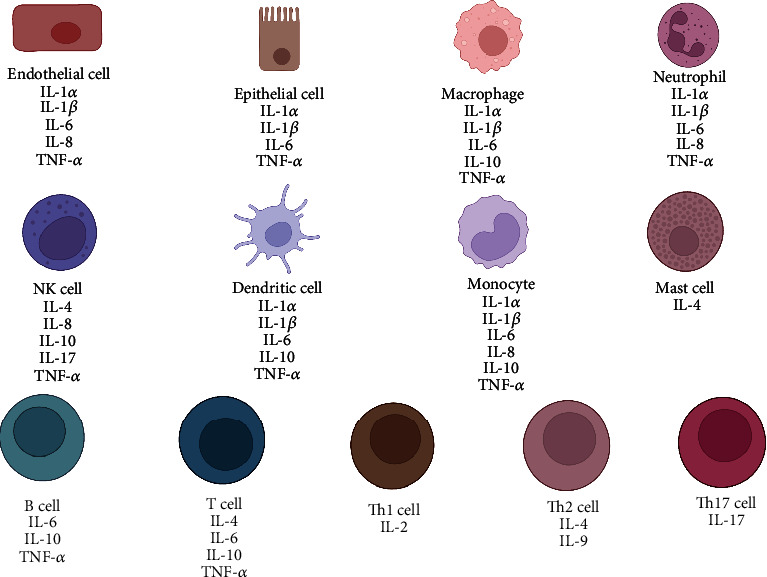
Schematic illustration of different cells expressing various cytokines.
4. Inflammatory Mediators
Table 1 shows the inflammatory mediators associated with IDD.
Table 1.
Inflammatory mediators associated with IDD progression.
| Name | Size (amino acids) | Chromosomal location | Gene | Origins | Receptor | Function in IDD | Signaling pathways |
|---|---|---|---|---|---|---|---|
| IL-1α | 271 | 2q14 | IL1A (IL1F1) | Neutrophil; macrophage; monocyte; endothelial cell; epithelial cell; dendritic cell | IL1R1; IL1R2 | Inhibit ECM synthesis; enhance inflammatory response; enhance bradykinin sensitivity | TGF-β; MAPK; NF-κB; Wnt/β-catenin; PI3K/Akt/mTOR |
| IL-1β | 269 | 2q14 | IL1B (IL1F2) | Neutrophil; macrophage; monocyte; endothelial cell; epithelial cell; dendritic cell | IL1R1; IL1R2 | Enhance inflammatory response; promote ECM degradation; accelerate cellular senescence; promote apoptosis and pyroptosis; regulate NP cell proliferation; increase intracellular ROS; increase angiogenesis and neoinnervation | TGF-β; MAPK; NF-κB; Wnt/β-catenin; PI3K/Akt/mTOR |
| IL-2 | 153 | 4q26-q27 | IL2 | Th1 cell | IL2RA; IL2RB; IL2RG | Regulate NP cell proliferation; promote apoptosis; promote ECM degradation | PI3K/Akt/mTOR; MAPK; JAK-STAT |
| IL-4 | 153 | 5q31.1 | IL4 | Th2 cell; NK cell; T cell; mast cell | IL4R | Inhibit inflammatory processes | JAK-STAT |
| IL-6 | 212 | 7p21 | IL6 (IFNB2) | Neutrophil; macrophage; T cell; B cell; monocyte; endothelial cell; epithelial cell; dendritic cell | IL6R | Enhance inflammatory response; promote apoptosis of neurons in the dorsal root ganglion; promote chondrocyte ferrogenesis | JAK-STAT; MAPK; PI3K/Akt/mTOR |
| IL-8 | 99 | 4q13-q21 | CXCL8 (IL8) | Neutrophil; NK cell; endothelial cell; monocyte | CXCR1; CXCR2 | Enhance inflammatory response; regulate angiogenesis | MAPK; JAK-STAT; NF-κB; PI3K/Akt/mTOR |
| IL-9 | 144 | 5q31.1 | IL9 | Th2 cell | IL9R | Enhance inflammatory response | MAPK; JAK-STAT |
| IL-10 | 178 | 1q31-q32 | IL10 | Macrophage; T cell; B cell; NK cell; monocyte; dendritic cell | IL10R1; IL10R2 | Enhance inflammatory response | MAPK; JAK-STAT; NF-κB |
| IL-17A | 155 | 6p12 | IL17A (CTLA8, IL17) | Th17 cell; NK cell | IL17RA; IL17RC | Enhance inflammatory response; promote ECM degradation; block autophagy in degenerating NP cells | MAPK; NF-κB; C/EBPβ/δ |
| TNF-α | 233 | 6p21.33 | TNF (TNFA, TNFSF2) | Endothelial cell; epithelial cell; macrophage; neutrophil; NK cell; dendritic; monocyte; B cell; T cell | TNFR1; TNFR2 | Enhance inflammatory response;1 promote ECM degradation;1 accelerate cellular senescence;1 promote apoptosis;1 regulate NP cell proliferation;1 increase angiogenesis and neoinnervation | MAPK; NF-κB; Notch; UPR/XBP1 |
| Chemokines | - | - | - | Endothelial cell; epithelial cell; B cell; T cell; NK cell | CR; CCR; CXCR; CX3CR | Enhance inflammatory response; promote ECM degradation; promote apoptosis; Increase angiogenesis and neoinnervation | NF-κB; PI3K/Akt/mTOR |
| NLRP3 | 1036 | 1q44 | NLRP3 (C1orf7, CIAS1, NALP3, PYPAF1) | Neutrophil; macrophage | - | Enhance inflammatory response; promote apoptosis | NF-κB |
4.1. Interleukin (IL)
4.1.1. IL-1α
IL-1α is a critical inflammatory mediator primarily released by monocytes, macrophages, dendritic cells, and endothelial cells [31]. IL-1α and IL-1β act in the same way, and their receptors share the same ligand binding and signal transduction pathways [32]. However, unlike IL-1β, IL-1α activity is not dependent on the inflammasome caspase-1 pathway [33]. Several studies have found that IL-1α levels in degenerative lumbar disc tissue are elevated compared with those in normal lumbar disc tissue and that IL-1α levels are positively associated with the severity of IDD [31, 34]. Previous meta-analyses revealed that the IL-1α (+889C/T) polymorphism was related to the increased incidence of IDD in Caucasian and Chinese Han populations [35, 36]. IL-1α has been found to accelerate IDD development by increasing extracellular matrix-degrading enzyme production and inhibiting extracellular matrix synthesis [37, 38]. IL-1α may also play a role in cartilage endplate degeneration by regulating MMP-3 and TIMP-3[39]. Furthermore, IL-1α could contribute to LBP by inducing IVDs to produce prostaglandin E2 and other inflammatory chemicals [40]. The sensitivity of bradykinin can be enhanced by IL-1α, which directly irritates nerve roots and hence contributes to IDD-induced neuralgia [41]. The synthesis and signal transduction pathways of IL-1α and IL-1β are shown in Figure 3. In conclusion, IL-1α is of paramount importance in the development of IDD.
Figure 3.
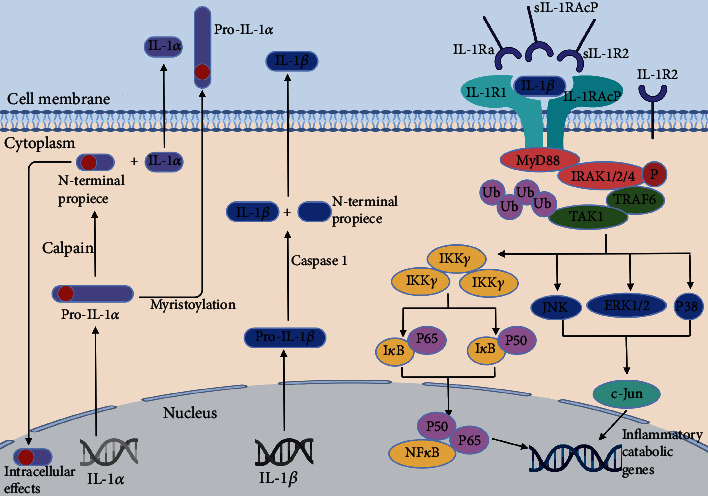
IL-1α and IL-1β synthesis and signal transduction pathways.
Two distinct genes encode IL-1α and IL-1β. Both proteins are produced as propeptide precursors (pro-IL-1α and pro-IL-1β). Pro-IL-1α is a physiologically active molecule with intracellular and extracellular effects. Pro-IL-1α has a nuclear localization sequence at its N-terminus and exists in high quantities in the nucleus. Pro-IL-1α is also produced as a membrane-bound cytokine after myristoylation, where it is most likely engaged in cell–cell interactions. Less frequently, the precursor form can be cleaved by a calpain-like protease to generate secreted IL-1α and an N-terminal peptide. Pro-IL-1α and the N-terminal peptide can be physiologically active after nuclear translocation. Caspase 1 cleaves pro-IL-1β into IL-1β, which may be released as a soluble, functional protein. Pro-IL1α, IL-1α, and IL-1β can all bind to IL1R1, allowing the recruitment of the IL1RAcP coreceptor. A series of events downstream of the IL-1R complex activate essential signalling proteins, such as mitogen-activated kinases (JNK, p38, and ERK1/2) and transcription factors, such as NF-κB (p65 and p50 subunits) and c-Jun (an AP-1 subunit), which regulate the expression of several inflammatory and catabolic genes. Signalling through the IL-1R complex can be modulated by the inhibitory effects of IL-1R2, sIL-1R2, sIL-1RAcP, and IL-1Ra.
4.1.2. IL-1β
IL-1β is a crucial inflammatory mediator with a wide range of actions and activities on various cells that can lead to various inflammatory processes. Systemically, IL-1β signalling generates an acute phase response, hypotension, vasodilation, and fever; locally, IL-1β signalling leads to an increase in adhesion molecules, which increases lymphocyte recruitment and amplifies the inflammatory response [42]. IL-1β expression has been demonstrated to be significantly increased in degenerative IVDs and is related to symptoms of LBP [43–46].
As shown in Figure 4, IL-1β may influence the development of IDD through several mechanisms. First, IL-1β can enhance the inflammatory response of the IVD by increasing the production of inflammatory mediators, such as IL-6, IL-8, IL-17, prostaglandin E2, chemokines, and the NLRP3 inflammasome [47–50]. Second, IL-1β regulates ADAMTS and MMP production in the IVD, resulting in ECM degradation [38, 51–53]. Third, the output of senescence-associated-galactosidase (SA-β-Gal) can be enhanced by IL-1β, indicating that this inflammatory mediator may accelerate IDD development by hastening cellular senescence [54–57]. Fourth, IL-1β can promote apoptosis and pyroptosis in NP cells by regulating the NF-κB and MAPK pathways, which hastens the development of IDD [50, 53, 58, 59]. Fifth, it was demonstrated that IL-1β regulated NP cell proliferation leading to the development of IDD [56, 60]. Additionally, IL-1β increases intracellular reactive oxygen species (ROS), and excessive ROS accumulation can lead to oxidative stress and the progression of IDD [61–63]. Finally, IL-1β might increase angiogenesis and neoinnervation inside IVDs by increasing the synthesis of vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and BDNF [64, 65]. In conclusion, IL-1β plays a significant role in IDD and may be a promising therapeutic target.
Figure 4.
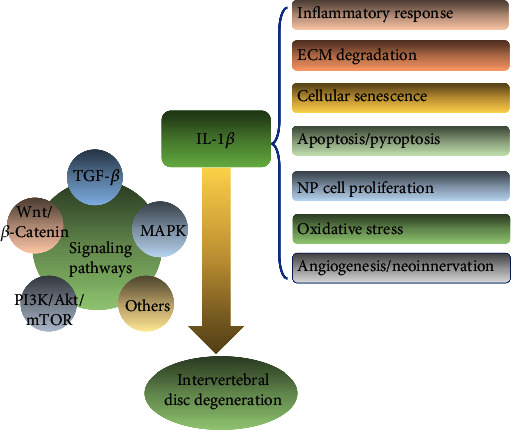
IL-1β is involved in multiple pathological processes of intervertebral disc degeneration.
4.1.3. IL-2
IL-2, found on 4q27, is mainly generated by mature T cells and acts as a growth factor for T and B cells, playing a role in their growth. IL-2 is increased in individuals with lumbar disc herniation and influences human NPC proliferation, apoptosis, and ECM degradation through the MAPK pathway [66]. Furthermore, IL-2 gene variations have been revealed as susceptibility factors for IDD, indicating that IL-2 may play a role in the development of IDD [67]. In conclusion, IL-2 has a function in IDD, but the exact mechanism is still unclear.
4.1.4. IL-4
IL-4 is a cytokine produced by T cells that regulates the activity of various immune cells. IL-4 is primarily generated by immune cells, but its receptors are found in various cell types and promote cell proliferation and differentiation, tissue regeneration, and neurological function. It was discovered that IL-4 expression was significantly higher in IDD patients than in healthy controls [68–70]. Interestingly, unlike IL-1, IL-4 exhibits direct anti-inflammatory actions by binding to the IL-4RA receptor on 16p12.1 and blocking the induction pathway of IL-1 and TNF-α [71–75]. In conclusion, IL-4 performs an anti-inflammatory function in IDD and can be used to treat this disorder.
4.1.5. IL-6
IL-6 is an important cytokine that can be secreted by T cells, macrophages, and NP cells. According to research, patients with disc degeneration have higher serum IL-6 levels than healthy controls [76, 77]. It has also been demonstrated that increased serum IL-6 levels are associated with disc degeneration-related LBP [78, 79]. Furthermore, IL-6 levels are linked to the degree of disc degeneration and pain intensity [80–83]. There are multiple potential mechanisms for IL-6 involvement in IDD. IL-6 accelerates the course of IDD by increasing the catabolic effects of IL-1β and TNF-α on NP cells through the JAK/STAT signalling pathway [84]. Moreover, IL-6 promotes apoptosis of neurons in the dorsal root ganglion, resulting in sensory impairment [85]. Furthermore, IL-6 promotes the degeneration of NP cells by blocking miR-10a-5p and hence the IL-6R signalling pathway, which in turn encourages chondrocyte ferrogenesis [86]. In conclusion, IL-6 plays an essential role in IDD and may be a target for future therapy.
4.1.6. IL-8
IL-8 is a chemokine with a distinct CXC amino acid sequence [87]. IL-8 expression is considerably higher in the disc tissue of IDD patients, indicating that it may have a role in the disease [88–90]. IL-8 can activate microglia in the spinal cord, promote the upregulation of neuroinflammatory markers such as IL-1β and TNF-α, and exacerbate the inflammatory response, aggravating the development of IDD [91]. IL-8 can also regulate angiogenesis by enhancing extracellular matrix survival, proliferation, and MMP-2 production through the MAPK signalling pathway, thereby affecting IDD progression [87, 92, 93].
4.1.7. IL-9
IL-9 is a polymorphic cytokine that regulates the Th2 inflammatory response [94]. IL-9 was shown to upregulate TNF-α and PGE2 production in NP cells, and its blood levels were positively associated with the degree of disc degeneration in IDD patients [95]. Therefore, IL-9 may play a role in the autoimmune inflammatory process in IDD, but the exact mechanism is not yet clear.
4.1.8. IL-10
Interleukin-10 (IL-10) is an important immune system regulator that regulates inflammation and tissue hemostasis [96]. IL-10 SNPs have been linked to IDD, suggesting that genetic alterations in IL-10 may lead to intervertebral disc imbalances and degeneration [97]. The expression of IL-10 is considerably higher in IDD patients, indicating the close relationship between this inflammatory cytokine and the disorder [70, 77]. Furthermore, in IDD animal models, IL-10 expression levels in several spinal components (bone, discs, and ligaments) were dramatically upregulated [98]. According to previous studies, IL-10 may hasten IDD development by intensifying the inflammatory response [99, 100]. To summarize, IL-10 plays a role in the degenerative process of IDD and can potentially be a new therapeutic target.
4.1.9. IL-17A
IL-17 is a cytokine primarily generated by the T helper 17 subsets of CD4+ T cells and plays a vital role in various inflammatory disorders [101, 102]. It has six members in its family, from IL-17A to IL-17F [103]. IL-17A, one of the most important members of the IL family, has been related to a range of degenerative illnesses [104, 105]. It has been demonstrated that IL-17A is more abundant in degenerative disc tissue than in normal tissue [96, 106, 107]. There are various probable theories for the mechanism of action. In NP cells, IL-17A can increase the production of inflammatory markers, such as IL-6, COX-2, MMPs, IFN-γ, and TNF-α [106, 108–110]. IL-17A has been found to regulate the development of IDD by modulating the ECM metabolism balance linked with ADAMTS-7 expression [107, 111, 112]. In addition, IL-17A may accelerate the development of IDD by blocking autophagy in human degenerative NP cells through stimulation of the PI3K/Akt/Bcl-2 signalling pathway [113, 114]. To summarize, the involvement of IL-17 in IDD is significant, and it may be an essential target for IDD treatment.
4.2. TNF-α
Tumour necrosis factor-alpha (TNF-α), located at 6p21.33, is mainly synthesized as a transmembrane protein and is turned into an active molecule following processing by specific enzymes, including TNF-α converting enzymes [115]. TNF-α is a proinflammatory cytokine linked to some pathological illnesses, including infections, autoimmune diseases, cancer, atherosclerosis, Alzheimer's disease, and inflammatory bowel disease [116–121]. TNF-α also regulates various developmental and immunological processes, including inflammation, differentiation, lipid metabolism, and apoptosis [122–124]. TNF-α has been linked to almost every component of the human immune system [125].
Studies have shown that TNF-α expression is upregulated in degenerative disc tissue more than in normal tissue [126–129]. TNF-α levels were also found to be positively associated with the severity of IDD [129–131]. In the absence of substantial deterioration, transgenic mice overexpressing human TNF-α exhibited early onset spontaneous disc herniation [132]. In a porcine model, lumbar discs treated with exogenous TNF-α displayed degenerative alterations, including annular fissures, loss of NP matrix, vascularization, and expression of IL-1β in the outer annulus, indicating that TNF-α is a driver of disc degeneration [133].
As shown in Figure 5, TNF-α binds to two receptors: TNF receptor type 1 (TNFR1) and TNF receptor type 2 (TNFR2). TNF-α may be implicated in IDD in many ways. TNF-α has been demonstrated in multiple studies to trigger IVDs by releasing many proinflammatory cytokines, including IL-1, IL-6, IL-8, IL-17, NO, and PGE2, and chemokines, which further exacerbate the inflammatory response of discs [134–137]. TNF-α also increases the synthesis of substance P, NGF, and VEGF, all of which can cause pain by sensitizing the nervous system and driving neurovascular development toward IVD [138, 139]. Furthermore, TNF-α stimulates ECM breakdown mostly via the NF-κB/MAPK signalling pathway [140–144]. TNF-α also interacts with its receptor and affects the JNK/ERK-MAPK and NF-κB signalling pathways in NPCs during IDD, upregulating proapoptotic proteins and downregulating antiapoptotic proteins, resulting in apoptosis [145–149]. Furthermore, TNF-α has been shown to cause premature senescence in NPCs [150, 151]. Additionally, TNF-α can affect the proliferation of NP cells via the JNK, NF-κB, Notch, UPR/XBP1, and p38 MAPK signalling pathways [152–156].
Figure 5.
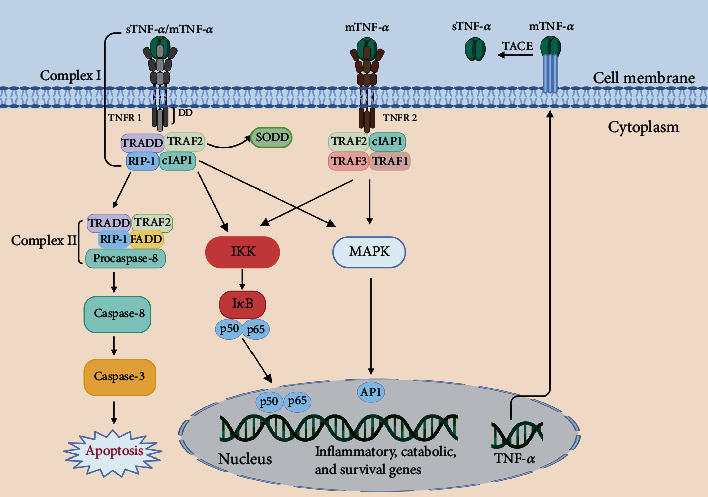
TNF-α signalling pathway.
TNF-α is generally found as a stable homotrimer known as mTNF-α. TACE, a metalloproteinase, can convert mTNF-α to sTNF-α. TNF-α works via two distinct receptors, TNFR1 and TNFR2. sTNF-α or mTNF-α may bind to transmembrane TNFR1, resulting in a conformational shift and release of the inhibitory SODD protein. Bound TNFR1 recruits several factors, including TRADD, RIP1, TRAF2, and cIAP 1 and 2, to form complex I, which signals via the NF-κB or MAPK pathway, and activate p65 or AP1. Complex I signalling causes inflammation (through chemokines and cytokines) and activates stromal catabolic genes (MMPs and ADAMTSs), as well as survival-promoting genes (cIAP1 and 2, cFLIP, TRAF1, and TRAF2). In addition, mTNF-α may also activate TNFR2, resulting in a similar complex and downstream signaling cascade. In specific circumstances, TNFR1 bound to sTNF-α may be internalized into complex II, causing procaspase 8 to be converted into caspase 8 and then caspase 3 to be activated, eventually leading to apoptosis.
4.3. Chemokines
Chemokines are significant second-order cytokines produced in response to stimuli and play an essential role in acute and chronic inflammation [134]. Based on the primary cysteine residues involved in disulphide bonding, chemokines have been categorized as C, CC, CXC, and CX3C [157]. According to a bioinformatics study, numerous chemokine genes may have a role in the development of IDD caused by inflammatory reactions [158]. CCL2, CCL5, CXCL6, CXCL12, CXCL20, C-X-C receptor 4 (CXCR4), and stromal cell-derived factor 1 (SDF1) expression is considerably elevated in IDD tissues [159–163]. Serum CCL3, CXCL12, and SDF1 levels have also been demonstrated to be positively associated with the degree of IDD [137, 162, 164, 165]. Chemokines may have a role in IDD through a variety of pathways. Zhang et al. [166] discovered that the CCL20/CCR6 pathway attracts IL-17-producing cells to degenerate IVDs and that IL-17 is implicated in the autoimmune process of IDD in a rat model. Furthermore, CXCL12 promotes ECM disintegration and enhances MMP production in human disc endplate chondrocytes [167]. SDF1/CXCR4 was discovered to be higher in degenerating intervertebral discs, and it promotes apoptosis of NPCs via the NF-B pathway, leading to IDD [168]. Furthermore, the SDF1/CXCR4 axis, via the PI3K/AKT pathway, can regulate VEC survival, migration, tube formation, and angiogenesis in human degenerative discs [169–171].
4.4. The NLRP3 Inflammasome
The NLRP3 inflammasome is a multiprotein complex in the cytoplasm that consists of a receptor, adaptor, and effector [172]. NLRP3 expression in IDD was observed to be considerably higher than that in normal disc tissue [173, 174]. There is further evidence from MRI and histology that NLRP3 is linked to the progression of IDD [175]. It has been demonstrated that overactivation of the NLRP3 inflammasome results in the overproduction of downstream IL-1, which is vital in the development of IDD [173]. Activation of the NLRP3 inflammasome can also cause apoptosis in NP cells [176, 177]. In addition, Propionibacterium acnes can activate the NLRP3 inflammasome via the TXNIP-NLRP3 pathway, causing pyroptosis of NP cells and IDD [178]. In summary, the NLRP3 inflammasome plays a crucial role in IDD, and more research is needed to discover its mechanism of action.
4.5. Nitric Oxide
NP cells can create nitric oxide (NO), and it was shown that NO production is enhanced in IDD and that its synthesis relies on nitric oxide synthase (NOS) [131]. TNF-α, IL-1β, lipopolysaccharide, and interferon-γ were discovered to promote NO production [89, 179]. Nitric oxide has proinflammatory effects, and its role as a vasodilator promotes vascular leakage, inhibits proteoglycans, and induces neuropathic pain, all of which contribute to IDD [180]. In addition, NO is regarded as a member of the ROS superfamily due to its similar effects to those of ROS, and ROS hasten intervertebral disc degeneration. The specific mechanism is shown in Figure 6.
Figure 6.
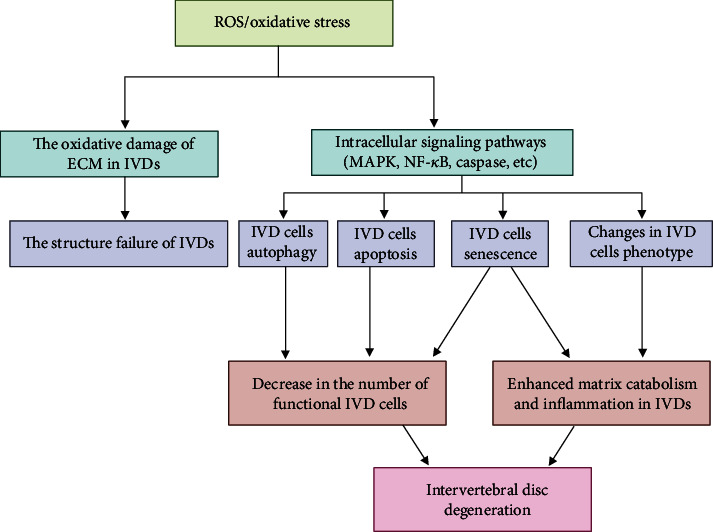
The role of ROS/oxidative stress in the development of IDD.
ROS alter the ECM of IVDs through oxidative modification, eventually impairing the structure of IVDs. ROS activate multiple signaling pathways, such as the MAPK and NF-κB pathways, thereby regulating autophagy, apoptosis, senescence, and the phenotype of IVD cells, thus reinforcing matrix degradation and inflammation and enhancing the decrease in the number of functional IVD cells. Ultimately, ROS/oxidative stress promotes the progression of IDD.
5. Therapeutic Prospects for IDD by Targeting Inflammation
The inflammatory response that mediates the degenerative cascade in IVDs is being targeted as a potential therapeutic or prognostic strategy. Currently, the main goals of therapies are to manage degenerated IVDs and relieve symptoms. The conventional approaches include systemic medicine and surgical decompression/discectomy. However, these methods are not aimed at the pathogenesis of IDD. In this section, we focused on reviewing and providing more information on novel anti-inflammation therapies for IDD, including intradiscal injections, gene therapies, MSC-based therapies, and exosome-based therapies.
5.1. Intradiscal Injections
Injecting medications into the IVD is one of the most straightforward ways to regulate inflammation in IVDs. TNF-α inhibitors are examples of medications administered in this way [181]. TNF inhibitors, such as infliximab and Atsttrin, have been shown to decrease the inflammatory response [182, 183]. Infliximab is an antibody against TNF-α. Injecting infliximab into the IVD of rats alleviated discomfort compared with the control groups [184]. Atsttrin is an inflammatory-related growth factor consisting of three pieces of progranulin. In a mouse model, this protein inhibited TNF-initiated inflammatory signaling by binding directly to TNF-α receptors [185]. Additionally, Atsttrin suppressed TNF-induced inflammatory cytokine production, including production of MMP-13, COX-2, iNOS, and IL-17, causing concomitant catabolic alterations in cartilage, disc height, and NP cells in ex vivo cultured rat discs [183].
The IL-1 inhibitor, IL-1 receptor antagonist (IL-1Ra), binds to the IL-1 receptor (IL-1R) and blocks the transmission of inflammatory signals [141]. IL-1Ra may have a therapeutic role in IDD, according to previous studies [38, 186, 187]. Injection of IL-1Ra into both degenerative and nondegenerative human IVD tissues reduced the production of matrix breakdown proteases, such as type II collagenase, gelatinase, and caseinase [38]. Another study revealed the therapeutic efficacy of IL-1Ra by applying polylactic-co-glycolic acid (PLGA) microspheres as a delivery vehicle. In NP cell cultures, IL-1Ra-PLGA microspheres attenuated the degradative effects of IL-1β on NP cells by suppressing NO production while restoring the levels of iNOS, IL-6, ADAMTS-4, and MMP-13 [186].
COX-2, which controls PGE2 production in inflammatory circumstances, is also a target for suppressing inflammation in IVDs [188]. In a rat model of disc herniation, epidural injections of COX-2 inhibitors resulted in satisfactory pain relief [189]. Additionally, the inhibitor of IkB kinase-b (IKKb), which is involved in NF-kB activation, is a novel candidate for treating inflammation in IVD. Intradiscal injection of IKKb downregulated the expression of TNF-α, IL-1β, and IL-6 in degenerative discs and neuropeptides in dorsal root ganglion neurons [190]. Despite promising results, injection of such molecules in IVDs may be ineffective owing to their short half-life and the complicated microenvironment of degenerative IVDs [30]. Furthermore, the potential risk of IDD caused by puncturing should be noted.
The injection of phytochemicals derived from medicinal plants has been researched in recent years because of its cost-effectiveness and biological functions. According to previous in vivo and in vitro studies, various phytochemicals, including resveratrol [191], mangiferin [192], epigallocatechin-3-gallate [177], chlorogenic acid [193], celastrol [194], isofraxidin [195], higenamine [196], sesamin [197, 198], honokiol [176], naringin [199, 200], baicalein [201], berberine [53], wogonin [52], and luteoloside [202]. Most of these phytochemicals inhibit the IL-1β-induced or TNF-α-induced inflammatory response and extracellular matrix degradation in NP cells. Although satisfactory therapeutic effects of phytochemicals in IDD have been reported, the metabolic processes, organ distribution, and toxicity of different doses still need to be investigated.
5.2. Gene Therapies
With the ability of locally modifying the expression of a certain gene and production of the corresponding protein, gene therapy offers longer sustained effects in IDD [203]. A study published in 1997 proposed genetic modifications as a positional treatment for IDD [204]. In this study, a retrovirus vector was developed to transduce IL-1Ra into bovine chondrocyte cells. Injection of cells overexpressing IL-1Ra significantly downregulated MMP3 for 14 days in degenerative IVD tissue, reducing IL-1-mediated matrix degradation and halting the deterioration of IDD. In a rabbit model, NP cells transfected with TGF-β1 demonstrated increased proteoglycan production [205]. Consistent with this finding, TGF-β1-transfected senescent NP cells of humans also enhanced the synthesis of proteoglycan and collagen [206, 207].
The safety of gene therapy may restrict its application in clinical settings. For the treatment of chronic IDD, high dosage exposure and long-term usage may induce oncogenesis, which is a critical concern [208]. Improvement in the reliability of viral vector designs and expression control of transgenes might allow the safe use of gene therapy.
5.3. MSC-Based Therapies
In recent years, many cell-based treatments to regenerate IVDs have been developed [209, 210]. Among the candidates, MSCs have the best potential for IVD regeneration, which is attributed to their autologous transplantation ability [211]. MSCs boosted collagen type II expression and slowed the apoptosis process of NP cells [212]. Additionally, IVD tissue survived for 6 months in rabbits with the concomitance of MSCs [213]. However, the number of transplanted MSCs is important [214]. In addition to their multidifferentiation capability, the immunomodulatory role of MSCs has been revealed [215, 216]. MSCs participate in inflammation by releasing cytokines, which directly interact with degenerative NP cells [217]. In vitro studies showed that MSCs cocultured with rat NP cells inhibited the expression of proinflammatory cytokines, including IL-3, IL-6, IL-11, IL-15, and TNF-α [218]. In a clinical trial, LBP was significantly alleviated by three months of MSC injection, and the authors concluded that MSCs stimulated the regeneration of IVD and had immunomodulatory characteristics [219]. In another 2-year follow-up study, after the injection of umbilical cord-derived MSCs into IVDs, LBP and lumbar function were improved and maintained during the duration of follow-up [220]. Although benefits and promising outcomes of MSC-based therapies have been observed, the mechanisms have still not been clearly elucidated by animal experiments, and most of the clinical studies were case reports with limited sample sizes.
5.4. Exosome-Based Therapies
Exosomes and exosomal miRNAs have been the focus of IDD therapy in recent years. The potential mechanisms reported in previous studies could be categorized as angiogenesis of the ECM, senescence, metabolic homeostasis, proliferation, apoptosis, and oxidative stress [221]. Additionally, exosomes and exosomal miRNAs also play an important role in the regulation of inflammation in IVDs [222]. By downregulating LRG1, BMSC-derived exosomal miR-129-5p attenuated the activation of the p38 MAPK pathway to inhibit macrophage polarization from the M1 to M2 phenotype, which resulted in the release of anti-inflammatory mediators and prevented apoptosis of NP cells as well as degradation of ECM [223]. NLRP3, a member of the inflammasome, is a crucial component of innate immunity and participates in several proinflammatory processes [224]. NLRP3 can be extremely upregulated in the development of IDD [225]. By blocking the NLRP3/caspase-1 pathway, MSC-derived exosomal miR-410 reversed the expression of IL-1β and IL-18, reducing LPS-induced pyroptosis in NP cells [226]. Similarly, human umbilical cord mesenchymal stem cell- (hucMSC-) derived miR-26a-5p affected mRNA methyltransferase (METTL14) and m6A methylation in NP cells, which downregulated the expression of NLRP3, leading to the inhibition of pyroptosis and the release of proinflammatory cytokines [227]. As a novel therapy, more studies focused on the role of exosomes in IDD treatment are expected.
6. Conclusion
IDD is a prevalent musculoskeletal illness that produces LBP and negatively impacts quality of life. Recent research has revealed that various inflammatory mediators, such as IL-1β, TNF-α, IL-6, IL-17, chemokines, and the NLRP3 inflammasome, play an essential role in IDD. Most research has found that inflammatory mediators have a role in the development of IDD primarily through the control of the inflammatory response, IVD cell proliferation, senescence, apoptosis, pyroptosis, autophagy, ECM degradation, and oxidative stress. Targeting these inflammatory mediators may lead to future optimum IDD treatment. Clinical investigations have recently revealed that inhibiting IL-1β and TNF-α is a promising future therapy for IDD. More research into IDD-related inflammatory mediators is needed to help us understand the molecular pathophysiology of IDD and provide novel ideas for future IDD therapy based on inflammatory mediators.
Contributor Information
Yong Hai, Email: yong.hai@ccmu.edu.cn.
Yunzhong Cheng, Email: chengyunzhong@163.com.
Data Availability
The data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Zhangfu Li and Honghao Yang collected the documents and finished the manuscript. Yong Hai and Yunzhong Cheng supervised and revised the manuscript. All authors have read and approved the content of the manuscript. Zhangfu Li and Honghao Yang are co-first authors of this article, contributing equally to the design and drafting of the manuscript.
References
- 1.Roberts S., Evans H., Trivedi J., Menage J. Histology and pathology of the human intervertebral disc. Journal of Bone and Joint Surgery . 2006;88(Supplement 2):10–14. doi: 10.2106/JBJS.F.00019. [DOI] [PubMed] [Google Scholar]
- 2.Pfirrmann C. W., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine . 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 3.Barrey C., Jund J., Noseda O., Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. European Spine Journal . 2007;16(9):1459–1467. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopal D., Ho A. L., Shah A., Chi J. H. Advances in Experimental Medicine and Biology . Springer; 2012. Molecular basis of intervertebral disc degeneration; pp. 114–133. [DOI] [PubMed] [Google Scholar]
- 5.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. European Spine Journal . 2008;17(Supplement 4):452–458. doi: 10.1007/s00586-008-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergroesen P. P., Kingma I., Emanuel K. S., et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis and Cartilage . 2015;23(7):1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Liao Z., Wu X., Song Y., et al. Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. Journal of Cellular and Molecular Medicine . 2019;23(8):5737–5750. doi: 10.1111/jcmm.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teraguchi M., Yoshimura N., Hashizume H., et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama Spine Study. Osteoarthritis and Cartilage . 2017;25(7):1122–1131. doi: 10.1016/j.joca.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Dario A. B., Ferreira M. L., Refshauge K. M., Lima T. S., Ordoñana J. R., Ferreira P. H. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: a systematic review of twin studies. The Spine Journal . 2015;15(5):1106–1117. doi: 10.1016/j.spinee.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Sivan S. S., Wachtel E., Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochimica et Biophysica Acta . 2014;1840(10):3181–3189. doi: 10.1016/j.bbagen.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Hangai M., Kaneoka K., Kuno S., et al. Factors associated with lumbar intervertebral disc degeneration in the elderly. The Spine Journal . 2008;8(5):732–740. doi: 10.1016/j.spinee.2007.07.392. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y. X. J. Postmenopausal Chinese women show accelerated lumbar disc degeneration compared with Chinese men. Journal of Orthopaedic Translation . 2015;3(4):205–211. doi: 10.1016/j.jot.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams M. A., Roughley P. J. What is intervertebral disc degeneration, and what causes it? Spine . 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 14.Wang S. Z., Rui Y. F., Tan Q., Wang C. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Research & Therapy . 2013;15(5):p. 220. doi: 10.1186/ar4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L., Manchikanti L., Kaye A. D., Abd-Elsayed A. Treatment of discogenic low back pain: current treatment strategies and future options-a literature review. Current Pain and Headache Reports . 2019;23(11):p. 86. doi: 10.1007/s11916-019-0821-x. [DOI] [PubMed] [Google Scholar]
- 16.Roughley P. J. Biology of intervertebral disc aging and Degeneration. Spine . 2004;29(23):2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 17.Priyadarshani P., Li Y., Yao L. Advances in biological therapy for nucleus pulposus regeneration. Osteoarthritis and Cartilage . 2016;24(2):206–212. doi: 10.1016/j.joca.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki S., Fujita N., Fujii T., et al. Potential involvement of the IL-6/JAK/STAT3 pathway in the pathogenesis of intervertebral disc degeneration. Spine . 2017;42(14):E817–e824. doi: 10.1097/BRS.0000000000001982. [DOI] [PubMed] [Google Scholar]
- 19.Risbud M. V., Shapiro I. M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nature Reviews Rheumatology . 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke J. G., Watson R. W. G., McCormack D., Dowling F. E., Walsh M. G., Fitzpatrick J. M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. Journal of Bone and Joint Surgery. British Volume . 2002;84-B(2):196–201. doi: 10.1302/0301-620X.84B2.0840196. [DOI] [PubMed] [Google Scholar]
- 21.Doita M., Kanatani T., Harada T., Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine . 1996;21(2):235–241. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 22.Huang K. Y., Lin R. M., Chen W. Y., Lee C. L., Yan J. J., Chang M. S. IL-20 may contribute to the pathogenesis of human intervertebral disc herniation. Spine . 2008;33(19):2034–2040. doi: 10.1097/BRS.0b013e31817eb872. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H., Suguro T., Okazima Y., Motegi M., Okada Y., Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine . 1996;21(2):218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 24.Lai A., Moon A., Purmessur D., et al. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. The Spine Journal . 2016;16(3):420–431. doi: 10.1016/j.spinee.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasto L. A., Ngo K., Leme A. S., et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. The Spine Journal . 2014;14(3):416–423. doi: 10.1016/j.spinee.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vo N., Seo H. Y., Robinson A., et al. Accelerated aging of intervertebral discs in a mouse model of progeria. Journal of Orthopaedic Research . 2010;28(12):1600–1607. doi: 10.1002/jor.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon C. H., Jacobs L., Kim J. H., et al. Part 2: quantitative proton T2 and sodium magnetic resonance imaging to assess intervertebral disc degeneration in a rabbit model. Spine . 2012;37(18):E1113–E1119. doi: 10.1097/BRS.0b013e3182583447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samartzis D., Karppinen J., Chan D., Luk K. D., Cheung K. M. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis and Rheumatism . 2012;64(5):1488–1496. doi: 10.1002/art.33462. [DOI] [PubMed] [Google Scholar]
- 29.Livshits G., Popham M., Malkin I., et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Annals of the Rheumatic Diseases . 2011;70(10):1740–1745. doi: 10.1136/ard.2010.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinos M., Almeida C. R., Caldeira J., Cunha C., Gonçalves R. M., Barbosa M. A. Inflammation in intervertebral disc degeneration and regeneration. Journal of the Royal Society Interface . 2015;12(104, article 20141191) doi: 10.1098/rsif.2014.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P. B., Tang W. J., Wang K., Zou K., Che B. Expressions of IL-1alpha and MMP-9 in degenerated lumbar disc tissues and their clinical significance. European Review for Medical and Pharmacological Sciences . 2017;21(18):4007–4013. [PubMed] [Google Scholar]
- 32.Wesche H., Korherr C., Kracht M., Falk W., Resch K., Martin M. U. The interleukin-1 receptor accessory protein (IL-1RAcP) is essential for IL-1-induced activation of interleukin-1 receptor-associated kinase (IRAK) and stress-activated protein kinases (SAP kinases) The Journal of Biological Chemistry . 1997;272(12):7727–7731. doi: 10.1074/jbc.272.12.7727. [DOI] [PubMed] [Google Scholar]
- 33.Kwak A., Lee Y., Kim H., Kim S. Intracellular interleukin (IL)-1 family cytokine processing enzyme. Archives of Pharmacal Research . 2016;39(11):1556–1564. doi: 10.1007/s12272-016-0855-0. [DOI] [PubMed] [Google Scholar]
- 34.Cai F., Zhu L., Wang F., et al. The paracrine effect of degenerated disc cells on healthy human nucleus pulposus cells is mediated by MAPK and NF-κB pathways and can be reduced by TGF-β1. DNA and Cell Biology . 2017;36(2):143–158. doi: 10.1089/dna.2016.3230. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z., Qu Z., Fu C., et al. Interleukin 1 polymorphisms contribute to intervertebral disc degeneration risk: a meta-analysis. PLoS One . 2016;11(6, article e0156412) doi: 10.1371/journal.pone.0156412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Ma H., Bi D., Qiu B. Association of interleukin 1 gene polymorphism with intervertebral disc degeneration risk in the Chinese Han population. Bioscience Reports . 2018;38(4) doi: 10.1042/BSR20171627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solovieva S., Kouhia S., Leino-Arjas P., et al. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology . 2004;15(5):626–633. doi: 10.1097/01.ede.0000135179.04563.35. [DOI] [PubMed] [Google Scholar]
- 38.Phillips K. L. E., Jordan-Mahy N., Nicklin M. J., Le Maitre C. L. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Annals of the Rheumatic Diseases . 2013;72(11):1860–1867. doi: 10.1136/annrheumdis-2012-202266. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J. F., Wang G. L., Zhou Z. J., Fang X. Q., Chen S., Fan S. W. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and interleukins in vertebral cartilage endplate. Orthopaedic Surgery . 2018;10(4):306–311. doi: 10.1111/os.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rannou F., Corvol M. T., Hudry C., et al. Sensitivity of anulus fibrosus cells to interleukin 1β: comparison with articular chondrocytes. Spine . 2000;25(1):17–23. doi: 10.1097/00007632-200001010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Olmarker K., Nutu M., Storkson R. Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine . 2003;28(15):1635–1641. doi: 10.1097/01.BRS.0000083162.35476.FF. [DOI] [PubMed] [Google Scholar]
- 42.Navone S. E., Marfia G., Giannoni A., et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histology and Histopathology . 2017;32(6):523–542. doi: 10.14670/HH-11-846. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Che M., Xin J., Zheng Z., Li J., Zhang S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomedicine & Pharmacotherapy . 2020;131, article 110660 doi: 10.1016/j.biopha.2020.110660. [DOI] [PubMed] [Google Scholar]
- 44.Lyu F. J., Cui H., Pan H., et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Research . 2021;9(1):p. 7. doi: 10.1038/s41413-020-00125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altun I. Cytokine profile in degenerated painful intervertebral disc: variability with respect to duration of symptoms and type of disease. The Spine Journal . 2016;16(7):857–861. doi: 10.1016/j.spinee.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder M., Viezens L., Schaefer C., et al. Chemokine profile of disc degeneration with acute or chronic pain. Journal of Neurosurgery: Spine . 2013;18(5):496–503. doi: 10.3171/2013.1.SPINE12483. [DOI] [PubMed] [Google Scholar]
- 47.Jia J., Nie L., Liu Y. Butyrate alleviates inflammatory response and NF-kappaB activation in human degenerated intervertebral disc tissues. International Immunopharmacology . 2020;78, article 106004 doi: 10.1016/j.intimp.2019.106004. [DOI] [PubMed] [Google Scholar]
- 48.Chen F., Jiang G., Liu H., et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1β/NF-κB-NLRP3 inflammasome positive feedback loop. Bone Research . 2020;8:p. 10. doi: 10.1038/s41413-020-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brand F. J., Forouzandeh M., Kaur H., Travascio F., de Rivero Vaccari J. P. Acidification changes affect the inflammasome in human nucleus pulposus cells. Journal of Inflammation . 2016;13(1):p. 29. doi: 10.1186/s12950-016-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Z., Tang P., Dong W., et al. SIRT1 alleviates IL-1β induced nucleus pulposus cells pyroptosis via mitophagy in intervertebral disc degeneration. International Immunopharmacology . 2022;107, article 108671 doi: 10.1016/j.intimp.2022.108671. [DOI] [PubMed] [Google Scholar]
- 51.Zhan S., Wang K., Song Y., et al. Long non-coding RNA HOTAIR modulates intervertebral disc degenerative changes via Wnt/β-catenin pathway. Arthritis Research & Therapy . 2019;21(1):p. 201. doi: 10.1186/s13075-019-1986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang W., Zhou X., Wang J., et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. International Immunopharmacology . 2018;65:539–549. doi: 10.1016/j.intimp.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 53.Lu L., Hu J., Wu Q., et al. Berberine prevents human nucleus pulposus cells from IL1beta induced extracellular matrix degradation and apoptosis by inhibiting the NF-κB pathway. International Journal of Molecular Medicine . 2019;43(4):1679–1686. doi: 10.3892/ijmm.2019.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao C. Q., Wang L. M., Jiang L. S., Dai L. Y. The cell biology of intervertebral disc aging and degeneration. Ageing Research Reviews . 2007;6(3):247–261. doi: 10.1016/j.arr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Yang M., Peng Y., Liu W., Zhou M., Meng Q., Yuan C. Sirtuin 2 expression suppresses oxidative stress and senescence of nucleus pulposus cells through inhibition of the p53/p21 pathway. Biochemical and Biophysical Research Communications . 2019;513(3):616–622. doi: 10.1016/j.bbrc.2019.03.200. [DOI] [PubMed] [Google Scholar]
- 56.Li X., Lin F., Wu Y., et al. Resveratrol attenuates inflammation environment-induced nucleus pulposus cell senescence in vitro. Bioscience Reports . 2019;39(5) doi: 10.1042/BSR20190126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Chen Z. B., Yu Y. B., Wa Q. B., Zhou J. W., He M., Cen Y. The role of quinazoline in ameliorating intervertebral disc degeneration by inhibiting oxidative stress and anti-inflammation via NF-κB/MAPKs signaling pathway. European Review for Medical and Pharmacological Sciences . 2020;24(4):2077–2086. doi: 10.26355/eurrev_202002_20387. [DOI] [PubMed] [Google Scholar]
- 58.Wang K., Chen T., Ying X., et al. Ligustilide alleviated IL-1β induced apoptosis and extracellular matrix degradation of nucleus pulposus cells and attenuates intervertebral disc degeneration in vivo. International Immunopharmacology . 2019;69:398–407. doi: 10.1016/j.intimp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 59.Zhang K., Ding W., Sun W., et al. Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis . 2016;21(1):13–24. doi: 10.1007/s10495-015-1180-7. [DOI] [PubMed] [Google Scholar]
- 60.Wang S. L., Yu Y. L., Tang C. L., Lv F. Z. Effects of TGF-β1 and IL-1β on expression of ADAMTS enzymes and TIMP-3 in human intervertebral disc degeneration. Experimental and Therapeutic Medicine . 2013;6(6):1522–1526. doi: 10.3892/etm.2013.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nasto L. A., Robinson A. R., Ngo K., et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. Journal of Orthopaedic Research . 2013;31(7):1150–1157. doi: 10.1002/jor.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou G., Lu H., Chen M., Yao H., Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Archives of Gerontology and Geriatrics . 2014;59(3):665–669. doi: 10.1016/j.archger.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Liu Q., Jin L., Shen F. H., Balian G., Li X. J. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells-a potential novel treatment for intervertebral disc degeneration. The Spine Journal . 2013;13(11):1571–1580. doi: 10.1016/j.spinee.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu Y. H., Lin R. M., Chiu Y. S., Liu W. L., Huang K. Y. Effects of IL-1β, IL-20, and BMP-2 on intervertebral disc inflammation under hypoxia. Journal of Clinical Medicine . 2020;9(1) doi: 10.3390/jcm9010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwon W. K., Moon H. J., Kwon T. H., Park Y. K., Kim J. H. The role of hypoxia in angiogenesis and extracellular matrix regulation of intervertebral disc cells during inflammatory reactions. Neurosurgery . 2017;81(5):867–875. doi: 10.1093/neuros/nyx149. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z., Wang G., Zhu X., Geng D., Yang H. Interleukin-2 is upregulated in patients with a prolapsed lumbar intervertebral disc and modulates cell proliferation, apoptosis and extracellular matrix metabolism of human nucleus pulposus cells. Experimental and Therapeutic Medicine . 2015;10(6):2437–2443. doi: 10.3892/etm.2015.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanaei S., Abdollahzade S., Sadr M., et al. Association of interleukin 2, interleukin 12, and interferon-γ with intervertebral disc degeneration in Iranian population. BMC Medical Genetics . 2020;21(1):p. 143. doi: 10.1186/s12881-020-01081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hanaei S., Abdollahzade S., Sadr M., et al. The role of interleukin 4 and IL-4RA in intervertebral disc degeneration: investigation of single nucleotide polymorphisms in genes and a systematic review & meta-analysis of IL-4 expression level. British Journal of Neurosurgery . 2020;34(1):66–71. doi: 10.1080/02688697.2019.1698010. [DOI] [PubMed] [Google Scholar]
- 69.Wang K., Bao J. P., Yang S., et al. A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. European Spine Journal . 2016;25(5):1428–1434. doi: 10.1007/s00586-015-4349-4. [DOI] [PubMed] [Google Scholar]
- 70.Akyol S., Eraslan B. S., Etyemez H., Tanriverdi T., Hanci M. Catabolic cytokine expressions in patients with degenerative disc disease. Turkish Neurosurgery . 2010;20(4):492–499. doi: 10.5137/1019-5149.JTN.3394-10.1. [DOI] [PubMed] [Google Scholar]
- 71.Te Velde A. A., Klomp J. P., Yard B. A., De Vries J. E., Figdor C. G. Modulation of phenotypic and functional properties of human peripheral blood monocytes by IL-4. Journal of Immunology . 1988;140(5):1548–1554. [PubMed] [Google Scholar]
- 72.Schuerwegh A. J., Dombrecht E. J., Stevens W. J., Van Offel J. F., Bridts C. H., De Clerck L. S. Influence of pro-inflammatory (IL-1α, IL-6, TNF-α, IFN-γ) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis and Cartilage . 2003;11(9):681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 73.Chowdhury T. T., Bader D. L., Lee D. A. Anti-inflammatory effects of IL-4 and dynamic compression in IL-1β stimulated chondrocytes. Biochemical and Biophysical Research Communications . 2006;339(1):241–247. doi: 10.1016/j.bbrc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 74.Rachakonda P. S., Rai M. F., Manning K., Schmidt M. F. Expression of canine interleukin-4 in canine chondrocytes inhibits inflammatory cascade through STAT6. Cytokine . 2008;44(1):179–184. doi: 10.1016/j.cyto.2008.07.470. [DOI] [PubMed] [Google Scholar]
- 75.Assirelli E., Pulsatelli L., Dolzani P., et al. Human osteoarthritic cartilage shows reduced in vivo expression of IL-4, a chondroprotective cytokine that differentially modulates IL-1β-stimulated production of chemokines and matrix-degrading enzymes in vitro. PLoS One . 2014;9(5, article e96925) doi: 10.1371/journal.pone.0096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weber K. T., Alipui D. O., Sison C. P., et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Research & Therapy . 2016;18:p. 3. doi: 10.1186/s13075-015-0887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiaogang M., Quanshan H., Liping Z., Kaken H. The expression of cytokine and its significance for the intervertebral disks of Kazakhs. Journal of Clinical Laboratory Analysis . 2017;31(5) doi: 10.1002/jcla.22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedersen L. M., Schistad E., Jacobsen L. M., Røe C., Gjerstad J. Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: a 12-month prospective study. Brain, Behavior, and Immunity . 2015;46:132–136. doi: 10.1016/j.bbi.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 79.Deng X., Zhao F., Kang B., Zhang X. Elevated interleukin-6 expression levels are associated with intervertebral disc degeneration. Experimental and Therapeutic Medicine . 2016;11(4):1425–1432. doi: 10.3892/etm.2016.3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schistad E. I., Espeland A., Pedersen L. M., Sandvik L., Gjerstad J., Røe C. Association between baseline IL-6 and 1-year recovery in lumbar radicular pain. European Journal of Pain . 2014;18(10):1394–1401. doi: 10.1002/j.1532-2149.2014.502.x. [DOI] [PubMed] [Google Scholar]
- 81.Guo Y., Li C., Shen B., et al. Is there any relationship between plasma IL-6 and TNF-α levels and lumbar disc degeneration? A retrospective single-center study. Disease Markers . 2022;2022:8. doi: 10.1155/2022/6842130.6842130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guan Y., Wang S., Wang J., et al. Gene polymorphisms and expression levels of interleukin-6 and interleukin-10 in lumbar disc disease: a meta-analysis and immunohistochemical study. Journal of Orthopaedic Surgery and Research . 2020;15(1):p. 54. doi: 10.1186/s13018-020-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hiyama A., Suyama K., Sakai D., Tanaka M., Watanabe M. Correlational analysis of chemokine and inflammatory cytokine expression in the intervertebral disc and blood in patients with lumbar disc disease. Journal of Orthopaedic Research . 2022;40(5):1213–1222. doi: 10.1002/jor.25136. [DOI] [PubMed] [Google Scholar]
- 84.Studer R. K., Vo N., Sowa G., Ondeck C., Kang J. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-α. Spine . 2011;36(8):593–599. doi: 10.1097/BRS.0b013e3181da38d5. [DOI] [PubMed] [Google Scholar]
- 85.Murata Y., Rydevik B., Nannmark U., et al. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-alpha in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine . 2011;36(12):926–932. doi: 10.1097/BRS.0b013e3181e7f4a9. [DOI] [PubMed] [Google Scholar]
- 86.Bin S., Xin L., Lin Z., Jinhua Z., Rui G., Xiang Z. Targeting miR-10a-5p/IL-6R axis for reducing IL-6-induced cartilage cell ferroptosis. Experimental and Molecular Pathology . 2021;118, article 104570 doi: 10.1016/j.yexmp.2020.104570. [DOI] [PubMed] [Google Scholar]
- 87.Li A., Varney M. L., Valasek J., Godfrey M., Dave B. J., Singh R. K. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis . 2005;8(1):63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 88.Kang J. D., Georgescu H. I., McIntyre-Larkin L., Stefanovic-Racic M., Donaldson W. F., III, Evans C. H. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine . 1996;21(3):271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 89.Shamji M. F., Setton L. A., Jarvis W., et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis and Rheumatism . 2010;62(7):1974–1982. doi: 10.1002/art.27444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., Chee A., Shi P., et al. Intervertebral disc cells produce interleukins found in patients with back pain. American Journal of Physical Medicine & Rehabilitation . 2016;95(6):407–415. doi: 10.1097/PHM.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Navone S. E., Peroglio M., Guarnaccia L., et al. Mechanical loading of intervertebral disc modulates microglia proliferation, activation, and chemotaxis. Osteoarthritis and Cartilage . 2018;26(7):978–987. doi: 10.1016/j.joca.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 92.Moon H. J., Yurube T., Lozito T. P., et al. Effects of secreted factors in culture medium of annulus fibrosus cells on microvascular endothelial cells: elucidating the possible pathomechanisms of matrix degradation and nerve in-growth in disc degeneration. Osteoarthritis and Cartilage . 2014;22(2):344–354. doi: 10.1016/j.joca.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li A., Dubey S., Varney M. L., Dave B. J., Singh R. K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. Journal of Immunology . 2003;170(6):3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 94.Hauber H. P., Bergeron C., Hamid Q. IL-9 in allergic inflammation. International Archives of Allergy and Immunology . 2004;134(1):79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y., Zhao Y., Li J., et al. Interleukin-9 promotes TNF-α and PGE2 release in human degenerated intervertebral disc tissues. Spine . 2016;41(21):1631–1640. doi: 10.1097/BRS.0000000000001621. [DOI] [PubMed] [Google Scholar]
- 96.Banimostafavi E. S., Fakhar M., Abediankenari S., et al. Determining serum levels of IL-10 and IL-17 in patients with low back pain caused by lumbar disc degeneration. Infectious Disorders Drug Targets . 2021;21(5) doi: 10.2174/1871526520666200820093924. [DOI] [PubMed] [Google Scholar]
- 97.Hanaei S., Abdollahzade S., Sadr M., Mirbolouk M. H., Khoshnevisan A., Rezaei N. Association of IL10 and TGFB single nucleotide polymorphisms with intervertebral disc degeneration in Iranian population: a case control study. BMC Medical Genetics . 2018;19(1):p. 59. doi: 10.1186/s12881-018-0572-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Holm S., Mackiewicz Z., Holm A. K., et al. Pro-inflammatory, pleiotropic, and anti-inflammatory TNF-α, IL-6, and IL-10 in experimental porcine intervertebral disk degeneration. Veterinary Pathology . 2009;46(6):1292–1300. doi: 10.1354/vp.07-VP-0179-K-FL. [DOI] [PubMed] [Google Scholar]
- 99.Chen J., Mei Z., Huang B., et al. IL-6/YAP1/β-catenin signaling is involved in intervertebral disc degeneration. Journal of Cellular Physiology . 2019;234(5):5964–5971. doi: 10.1002/jcp.27065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim H., Hong J. Y., Lee J., Jeon W. J., Ha I. H. IL-1β promotes disc degeneration and inflammation through direct injection of intervertebral disc in a rat lumbar disc herniation model. The Spine Journal . 2021;21(6):1031–1041. doi: 10.1016/j.spinee.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 101.Park H., Li Z., Yang X. O., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology . 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim J. S., Jordan M. S. Diversity of IL-17-producing T lymphocytes. Cellular and Molecular Life Sciences . 2013;70(13):2271–2290. doi: 10.1007/s00018-012-1163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu S., Song X., Chrunyk B. A., Shanker S., Hoth L. R. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nature Communications . 2013;4:p. 1888. doi: 10.1038/ncomms2880. [DOI] [PubMed] [Google Scholar]
- 104.Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual Review of Immunology . 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 105.Moseley T. A., Haudenschild D. R., Rose L., Reddi A. H. Interleukin-17 family and IL-17 receptors. Cytokine & Growth Factor Reviews . 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 106.Gabr M. A., Jing L., Helbling A. R., et al. Interleukin‐17 synergizes with IFNγ or TNFα to promote inflammatory mediator release and intercellular adhesion molecule‐1 (ICAM‐1) expression in human intervertebral disc cells. Journal of Orthopaedic Research . 2011;29(1):1–7. doi: 10.1002/jor.21206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian P., Li Z. J., Fu X., Ma X. L. Role of interleukin-17 in chondrocytes of herniated intervertebral lumbar discs. Experimental and Therapeutic Medicine . 2015;10(1):81–87. doi: 10.3892/etm.2015.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yao Z., Fanslow W. C., Seldin M. F., et al. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity . 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 109.Li J. K., Nie L., Zhao Y. P., et al. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. Journal of Translational Medicine . 2016;14:p. 77. doi: 10.1186/s12967-016-0833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suyama K., Sakai D., Hirayama N., et al. Effects of interleukin-17A in nucleus pulposus cells and its small-molecule inhibitors for intervertebral disc disease. Journal of Cellular and Molecular Medicine . 2018;22(11):5539–5551. doi: 10.1111/jcmm.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ouyang Z. H., Wang W. J., Yan Y. G., Wang B., Lv G. H. The PI3K/Akt pathway: a critical player in intervertebral disc degeneration. Oncotarget . 2017;8(34):57870–57881. doi: 10.18632/oncotarget.18628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S. S., Zhang W., Zhang Y. Q., et al. IL-17A enhances ADAMTS-7 expression through regulation of TNF-α in human nucleus pulposus cells. Journal of Molecular Histology . 2015;46(6):475–483. doi: 10.1007/s10735-015-9640-5. [DOI] [PubMed] [Google Scholar]
- 113.Hu B., Wang J., Wu X., Chen Y., Yuan W., Chen H. Interleukin-17 upregulates vascular endothelial growth factor by activating the JAK/STAT pathway in nucleus pulposus cells. Joint, Bone, Spine . 2017;84(3):327–334. doi: 10.1016/j.jbspin.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 114.He W. S., Zou M. X., Yan Y. G., et al. Interleukin-17A promotes human disc degeneration by inhibiting autophagy through the activation of the phosphatidylinositol 3-kinase/Akt/Bcl2 signaling pathway. World Neurosurgery . 2020;143:e215–e223. doi: 10.1016/j.wneu.2020.07.117. [DOI] [PubMed] [Google Scholar]
- 115.Bachmeier B. E., Nerlich A. G., Weiler C., Paesold G., Jochum M., Boos N. Analysis of tissue distribution of TNF-α, TNF-α-receptors, and the activating TNF-α-converting enzyme suggests activation of the TNF-α system in the aging intervertebral disc. Annals of the New York Academy of Sciences . 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- 116.Locksley R. M., Killeen N., Lenardo M. J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell . 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 117.Aggarwal B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nature Reviews Immunology . 2003;3(9):745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 118.Dichamp I., Bourgeois A., Dirand C., Herbein G., Wendling D. Increased nuclear factor-kappaB activation in peripheral blood monocytes of patients with rheumatoid arthritis is mediated primarily by tumor necrosis factor-alpha. The Journal of Rheumatology . 2007;34(10):1976–1983. [PubMed] [Google Scholar]
- 119.Ragab S. M., Safan M. A., Obeid O. M., Sherief A. S. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and tumor necrosis factor-alpha (TNF-α) and their relation to premature atherosclerosis in β-thalassemia children. Hematology . 2015;20(4):228–238. doi: 10.1179/1607845414Y.0000000180. [DOI] [PubMed] [Google Scholar]
- 120.Swardfager W., Lanctôt K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biological Psychiatry . 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 121.Brynskov J., Foegh P., Pedersen G., et al. Tumour necrosis factor α converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut . 2002;51(1):37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wallach D. The TNF family: only the surface has been scratched. Seminars in Immunology . 2014;26(3):181–182. doi: 10.1016/j.smim.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Herbein G., Mahlknecht U., Batliwalla F., et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature . 1998;395(6698):189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 124.Aggarwal B. B., Gupta S. C., Kim J. H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood . 2012;119(3):651–665. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wallach D. The TNF cytokine family: one track in a road paved by many. Cytokine . 2013;63(3):225–229. doi: 10.1016/j.cyto.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 126.Le Maitre C. L., Hoyland J. A., Freemont A. J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Research & Therapy . 2007;9(4):p. R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohtori S., Inoue G., Eguchi Y., et al. Tumor necrosis factor-α-immunoreactive cells in nucleus pulposus in adolescent patients with lumbar disc herniation. Spine . 2013;38(6):459–462. doi: 10.1097/BRS.0b013e3182739cb4. [DOI] [PubMed] [Google Scholar]
- 128.Andrade P., Visser-Vandewalle V., Philippens M., et al. Tumor necrosis factor-α levels correlate with postoperative pain severity in lumbar disc hernia patients: opposite clinical effects between tumor necrosis factor receptor 1 and 2. Pain . 2011;152(11):2645–2652. doi: 10.1016/j.pain.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 129.Liang H., Yang X., Liu C., Sun Z., Wang X. Effect of NF-kB signaling pathway on the expression of MIF, TNF-α, IL-6 in the regulation of intervertebral disc degeneration. Journal of Musculoskeletal & Neuronal Interactions . 2018;18(4):551–556. [PMC free article] [PubMed] [Google Scholar]
- 130.Weiler C., Nerlich A. G., Bachmeier B. E., Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine . 2005;30(1):44–53. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 131.Park J. Y., Kuh S. U., Park H. S., Kim K. S. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. Journal of Spinal Disorders & Techniques . 2011;24(6):352–357. doi: 10.1097/BSD.0b013e3181fee4df. [DOI] [PubMed] [Google Scholar]
- 132.Gorth D. J., Shapiro I. M., Risbud M. V. Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death & Disease . 2018;10(1):p. 7. doi: 10.1038/s41419-018-1246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kang R., Li H., Rickers K., Ringgaard S., Xie L., Bünger C. Intervertebral disc degenerative changes after intradiscal injection of TNF-α in a porcine model. European Spine Journal . 2015;24(9):2010–2016. doi: 10.1007/s00586-015-3926-x. [DOI] [PubMed] [Google Scholar]
- 134.Gruber H. E., Hoelscher G. L., Ingram J. A., Bethea S., Cox M., Hanley E. N., Jr. Proinflammatory cytokines modulate the chemokine CCL2 (MCP-1) in human annulus cells in vitro: CCL2 expression and production. Experimental and Molecular Pathology . 2015;98(1):102–105. doi: 10.1016/j.yexmp.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 135.Moon H. J., Kim J. H., Lee H. S., et al. Annulus fibrosus cells interact with neuron-like cells to modulate production of growth factors and cytokines in symptomatic disc degeneration. Spine . 2012;37(1):2–9. doi: 10.1097/BRS.0b013e31820cd2d8. [DOI] [PubMed] [Google Scholar]
- 136.Kepler C. K., Markova D. Z., Hilibrand A. S., et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine . 2013;38(21):E1291–E1299. doi: 10.1097/BRS.0b013e3182a42bc2. [DOI] [PubMed] [Google Scholar]
- 137.Wang J., Tian Y., Phillips K. L. E., et al. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis and Rheumatism . 2013;65(3):832–842. doi: 10.1002/art.37819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abe Y., Akeda K., An H. S., et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine . 2007;32(6):635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 139.Ohba T., Haro H., Ando T., et al. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. Journal of Orthopaedic Research . 2009;27(2):229–235. doi: 10.1002/jor.20727. [DOI] [PubMed] [Google Scholar]
- 140.Yang S., Li L., Zhu L., et al. Aucubin inhibits IL‐1β‐ or TNF‐α-induced extracellular matrix degradation in nucleus pulposus cell through blocking the miR-140-5p/CREB1 axis. Journal of Cellular Physiology . 2019;234(8):13639–13648. doi: 10.1002/jcp.28044. [DOI] [PubMed] [Google Scholar]
- 141.Hoyland J. A., Le Maitre C., Freemont A. J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology . 2008;47(6):809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 142.Wang C., Yu X., Yan Y., et al. Tumor necrosis factor-α: a key contributor to intervertebral disc degeneration. Acta Biochimica et Biophysica Sinica Shanghai . 2017;49(1):1–13. doi: 10.1093/abbs/gmw112. [DOI] [PubMed] [Google Scholar]
- 143.Kim J. S., Ellman M. B., Yan D., et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. Journal of Cellular Physiology . 2013;228(9):1884–1896. doi: 10.1002/jcp.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang J., Markova D., Anderson D. G., Zheng Z., Shapiro I. M., Risbud M. V. TNF-α and IL-1β Promote a Disintegrin-like and Metalloprotease with Thrombospondin Type I Motif-5-mediated Aggrecan Degradation through Syndecan-4 in Intervertebral Disc. The Journal of Biological Chemistry . 2011;286(46):39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang J., Wang X., Liu H., et al. TNF‐α enhances apoptosis by promoting chop expression in nucleus pulposus cells: role of the MAPK and NF‐κB pathways. Journal of Orthopaedic Research . 2019;37(3):697–705. doi: 10.1002/jor.24204. [DOI] [PubMed] [Google Scholar]
- 146.Yu W., Fu J., Liu Y., Wu Y., Jiang D. Osteogenic protein-1 inhibits nucleus pulposus cell apoptosis through regulating the NF-κB/ROS pathway in an inflammation environment. Bioscience Reports . 2018;38(6) doi: 10.1042/BSR20181530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147.Gruber H. E., Hoelscher G. L., Bethea S., Hanley E. N., Jr. Mitochondrial membrane potential and nuclear and gene expression changes during human disc cell apoptosis: in vitro and in vivo annulus findings. Spine . 2015;40(12):876–882. doi: 10.1097/BRS.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 148.Guo Z., Gao W. S., Wang Y. F., Gao F., Wang W., Ding W. Y. MiR-502 Suppresses TNF-α-Induced Nucleus Pulposus Cell Apoptosis by Targeting TARF2. BioMed Research International . 2021;2021:11. doi: 10.1155/2021/5558369.5558369 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Zhu H., Sun B., Shen Q. TNF-α induces apoptosis of human nucleus pulposus cells via activating the TRIM14/NF-κB signalling pathway. Artif Cells Nanomed Biotechnol . 2019;47(1):3004–3012. doi: 10.1080/21691401.2019.1643733. [DOI] [PubMed] [Google Scholar]
- 150.Purmessur D., Walter B. A., Roughley P. J., Laudier D. M., Hecht A. C., Iatridis J. A role for TNFα in intervertebral disc degeneration: A non-recoverable catabolic shift. Biochemical and Biophysical Research Communications . 2013;433(1):151–156. doi: 10.1016/j.bbrc.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie J., Li B., Zhang P., Wang L., Lu H., Song X. Osteogenic protein-1 attenuates the inflammatory cytokine-induced NP cell senescence through regulating the ROS/NF-κB pathway. Biomedicine & Pharmacotherapy . 2018;99:431–437. doi: 10.1016/j.biopha.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 152.Lin X., Lin Q. MiRNA-495-3p attenuates TNF-α induced apoptosis and inflammation in human nucleus pulposus cells by targeting IL5RA. Inflammation . 2020;43(5):1797–1805. doi: 10.1007/s10753-020-01254-5. [DOI] [PubMed] [Google Scholar]
- 153.Cheng S., Li X., Jia Z., et al. The inflammatory cytokine TNF‐α regulates the biological behavior of rat nucleus pulposus mesenchymal stem cells through the NF‐κB signaling pathway in vitro. Journal of Cellular Biochemistry . 2019;120(8):13664–13679. doi: 10.1002/jcb.28640. [DOI] [PubMed] [Google Scholar]
- 154.Wang H., Tian Y., Wang J., et al. Inflammatory Cytokines Induce NOTCH Signaling in Nucleus Pulposus Cells: The Journal of Biological Chemistry . 2013;288(23):16761–16774. doi: 10.1074/jbc.M112.446633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen L., Xie Z. Y., Liu L., et al. Nuclear factor-kappa B-dependent X-box binding protein 1 signalling promotes the proliferation of nucleus pulposus cells under tumour necrosis factor alpha stimulation. Cell Proliferation . 2019;52(2, article e12542) doi: 10.1111/cpr.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang X. H., Hong X., Zhu L., et al. Tumor necrosis factor alpha promotes the proliferation of human nucleus pulposus cells via nuclear factor-κB, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. Experimental Biology and Medicine . 2015;240(4):411–417. doi: 10.1177/1535370214554533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beider K., Abraham M., Peled A. Chemokines and chemokine receptors in stem cell circulation. Frontiers in Bioscience . 2008;13:6820–6833. doi: 10.2741/3190. [DOI] [PubMed] [Google Scholar]
- 158.Fan N., Yuan S., Hai Y., et al. Identifying the potential role of IL-1β in the molecular mechanisms of disc degeneration using gene expression profiling and bioinformatics analysis. Journal of Orthopaedic Surgery . 2022;30(1) doi: 10.1177/23094990211068203. [DOI] [PubMed] [Google Scholar]
- 159.Pattappa G., Peroglio M., Sakai D., et al. CCL5/RANTES is a key chemoattractant released by degenerative intervertebral discs in organ culture. European Cells & Materials . 2014;27 doi: 10.22203/ecm.v027a10. [DOI] [PubMed] [Google Scholar]
- 160.Grad S., Bow C., Karppinen J., et al. Systemic blood plasma CCL5 and CXCL6: potential biomarkers for human lumbar disc degeneration. European Cells & Materials . 2016;31:1–10. doi: 10.22203/eCM.v031a01. [DOI] [PubMed] [Google Scholar]
- 161.Zhong H., Zhou Z., Guo L., et al. The miR-623/CXCL12 axis inhibits LPS-induced nucleus pulposus cell apoptosis and senescence. Mechanisms of Ageing and Development . 2021;194, article 111417 doi: 10.1016/j.mad.2020.111417. [DOI] [PubMed] [Google Scholar]
- 162.Zhang H., Zhang L., Chen L., Li W., Li F., Chen Q. Stromal cell-derived factor-1 and its receptor CXCR4 are upregulated expression in degenerated intervertebral discs. International Journal of Medical Sciences . 2014;11(3):240–245. doi: 10.7150/ijms.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Willems N., Tellegen A. R., Bergknut N., et al. Inflammatory profiles in canine intervertebral disc degeneration. BMC Veterinary Research . 2016;12:p. 10. doi: 10.1186/s12917-016-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Er Z. J., Yin C. F., Wang W. J., Chen X. J. Serum CXCL12/SDF-1 level is positively related with lumbar intervertebral disc degeneration and clinical severity. Innate Immunity . 2020;26(5):341–350. doi: 10.1177/1753425919895086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang Y. L., Li B., Zhou Z. H. A cross-sectional study: serum CCL3/MIP-1α levels may reflect lumbar intervertebral disk degeneration in Han Chinese people. Journal of Pain Research . 2018;11:497–503. doi: 10.2147/JPR.S152349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Y., Liu L., Wang S., et al. Production of CCL20 on nucleus pulposus cells recruits IL-17-producing cells to degenerated IVD tissues in rat models. Journal of Molecular Histology . 2016;47(1):81–89. doi: 10.1007/s10735-015-9651-2. [DOI] [PubMed] [Google Scholar]
- 167.Zhang H., Zhu T., Zhang L., Wu Q. Stromal cell‑derived factor‑1 induces matrix metalloproteinase expression in human endplate chondrocytes, cartilage endplate degradation in explant culture, and the amelioration of nucleus pulposus degeneration in vivo. International Journal of Molecular Medicine . 2018;41(2):969–976. doi: 10.3892/ijmm.2017.3278. [DOI] [PubMed] [Google Scholar]
- 168.Liu Z., Ma C., Shen J., Wang D., Hao J., Hu Z. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Molecular Medicine Reports . 2016;14(1):783–789. doi: 10.3892/mmr.2016.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang H., Wang P., Zhang X., Zhao W., Ren H., Hu Z. SDF1/CXCR4 axis facilitates the angiogenesis via activating the PI3K/AKT pathway in degenerated discs. Molecular Medicine Reports . 2020;22(5):4163–4172. doi: 10.3892/mmr.2020.11498. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Zhang H., Wang P., Zhang X., Zhao W., Ren H., Hu Z. SDF1/CXCR7 signaling axis participates in angiogenesis in degenerated discs via the PI3K/AKT pathway. DNA and Cell Biology . 2019;38(5):457–467. doi: 10.1089/dna.2018.4531. [DOI] [PubMed] [Google Scholar]
- 171.Zhang H., He B. SDF1/CXCR4 axis plays a role in angiogenesis during the degeneration of intervertebral discs. Molecular Medicine Reports . 2019;20(2):1203–1211. doi: 10.3892/mmr.2019.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gross O., Thomas C. J., Guarda G., Tschopp J. The inflammasome: an integrated view. Immunological Reviews . 2011;243(1):136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 173.Chen Z. H., Jin S. H., Wang M. Y., et al. Enhanced NLRP3, caspase-1, and IL- 1β levels in degenerate human intervertebral disc and their association with the grades of disc degeneration. The Anatomical Record . 2015;298(4):720–726. doi: 10.1002/ar.23059. [DOI] [PubMed] [Google Scholar]
- 174.Tang P., Zhu R., Ji W. P., et al. The NLRP3/Caspase-1/Interleukin-1β axis is active in human lumbar cartilaginous endplate degeneration. Clinical Orthopaedics and Related Research . 2016;474(8):1818–1826. doi: 10.1007/s11999-016-4866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang W., Li G., Luo R., et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis- dependent nucleus pulposus cell pyroptosis. Experimental & Molecular Medicine . 2022;54(2):129–142. doi: 10.1038/s12276-022-00729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tang P., Gu J. M., Xie Z. A., et al. Honokiol alleviates the degeneration of intervertebral disc via suppressing the activation of TXNIP-NLRP3 inflammasome signal pathway. Free Radical Biology & Medicine . 2018;120:368–379. doi: 10.1016/j.freeradbiomed.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 177.Tian Y., Bao Z., Ji Y., Mei X., Yang H. Epigallocatechin-3-Gallate protects H2O2-Induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 Activation. Drug Design, Development and Therapy . 2020;14:2113–2122. doi: 10.2147/DDDT.S251623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.He D., Zhou M., Bai Z., Wen Y., Shen J., Hu Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochemical and Biophysical Research Communications . 2020;526(3):772–779. doi: 10.1016/j.bbrc.2020.03.161. [DOI] [PubMed] [Google Scholar]
- 179.Hashizume H., Kawakami M., Nishi H., Tamaki T. Histochemical demonstration of nitric oxide in herniated lumbar discs. A clinical and animal model study. Spine . 1997;22(10):1080–1084. doi: 10.1097/00007632-199705150-00005. [DOI] [PubMed] [Google Scholar]
- 180.De Geer C. M. Cytokine involvement in biological inflammation related to degenerative disorders of the intervertebral disk: a narrative review. Journal of Chiropractic Medicine . 2018;17(1):54–62. doi: 10.1016/j.jcm.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goupille P., Mulleman D., Paintaud G., Watier H., Valat J. P. Can sciatica induced by disc herniation be treated with tumor necrosis factor alpha blockade? Arthritis and Rheumatism . 2007;56(12):3887–3895. doi: 10.1002/art.23051. [DOI] [PubMed] [Google Scholar]
- 182.Likhitpanichkul M., Torre O. M., Gruen J., Walter B. A., Hecht A. C., Iatridis J. C. Do mechanical strain and TNF-α interact to amplify pro-inflammatory cytokine production in human annulus fibrosus cells? Journal of Biomechanics . 2016;49(7):1214–1220. doi: 10.1016/j.jbiomech.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ding H., Wei J., Zhao Y., Liu Y., Liu L., Cheng L. Progranulin derived engineered protein Atsttrin suppresses TNF-α-mediated inflammation in intervertebral disc degenerative disease. Oncotarget . 2017;8(65):109692–109702. doi: 10.18632/oncotarget.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Evashwick-Rogler T. W., Lai A., Watanabe H., et al. Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine . 2018;1(2, article e1014) doi: 10.1002/jsp2.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tang W., Lu Y., Tian Q. Y., et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science . 2011;332(6028):478–484. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gorth D. J., Mauck R. L., Chiaro J. A., et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1beta-mediated degradation of nucleus pulposus in vitro. Arthritis Research & Therapy . 2012;14(4):p. R179. doi: 10.1186/ar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Swamy G., Salo P., Duncan N., Jirik F., Matyas J. IL-1Ra deficiency accelerates intervertebral disc degeneration in C57BL6J mice. JOR Spine . 2022;5(2, article e1201) doi: 10.1002/jsp2.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bjarnason I. The impact of recent data on our understanding of the roles of COX-1 and COX-2 in gastrointestinal pathophysiology. Clinical Drug Investigation . 2007;27(Supplement 1):7–13. doi: 10.2165/00044011-200727001-00003. [DOI] [PubMed] [Google Scholar]
- 189.Kawakami M., Matsumoto T., Hashizume H., Kuribayashi K., Tamaki T. Epidural injection of cyclooxygenase-2 inhibitor attenuates pain-related behavior following application of nucleus pulposus to the nerve root in the rat. Journal of Orthopaedic Research . 2002;20(2):376–381. doi: 10.1016/S0736-0266(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 190.Kobori S., Miyagi M., Orita S., et al. Inhibiting IκB Kinase-β downregulates inflammatory cytokines in injured discs and neuropeptides in dorsal root ganglia innervating injured discs in rats. Spine (Phila Pa 1976) . 2014;39(15):1171–1177. doi: 10.1097/BRS.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 191.Wuertz K., Quero L., Sekiguchi M., et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine (Phila Pa 1976) . 2011;36(21):E1373–E1384. doi: 10.1097/BRS.0b013e318221e655. [DOI] [PubMed] [Google Scholar]
- 192.Yu H., Hou G., Cao J., Yin Y., Zhao Y., Cheng L. Mangiferin Alleviates Mitochondrial ROS in Nucleus Pulposus Cells and Protects against Intervertebral Disc Degeneration via Suppression of NF- κ B Signaling Pathway. Oxidative Medicine and Cellular Longevity . 2021;2021:27. doi: 10.1155/2021/6632786.6632786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ge Q., Ying J., Shi Z., et al. Chlorogenic Acid retards cartilaginous endplate degeneration and ameliorates intervertebral disc degeneration via suppressing NF- κB signaling. Life Sciences . 2021;274, article 119324 doi: 10.1016/j.lfs.2021.119324. [DOI] [PubMed] [Google Scholar]
- 194.Chen J., Xuan J., Gu Y. T., et al. Celastrol reduces IL-1β induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomedicine & Pharmacotherapy . 2017;91:208–219. doi: 10.1016/j.biopha.2017.04.093. [DOI] [PubMed] [Google Scholar]
- 195.Su X., Liu B., Gong F., et al. Isofraxidin attenuates IL‐1β‐induced inflammatory response in human nucleus pulposus cells. Journal of Cellular Biochemistry . 2019;120(8):13302–13309. doi: 10.1002/jcb.28604. [DOI] [PubMed] [Google Scholar]
- 196.Bai X., Ding W., Yang S., Guo X. Higenamine inhibits IL-1β-induced inflammation in human nucleus pulposus cells. Bioscience Reports . 2019;39(6) doi: 10.1042/BSR20190857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li K., Li Y., Xu B., Mao L., Zhao J. Sesamin inhibits lipopolysaccharide-induced inflammation and extracellular matrix catabolism in rat intervertebral disc. Connective Tissue Research . 2016;57(5):347–359. doi: 10.1080/03008207.2016.1182998. [DOI] [PubMed] [Google Scholar]
- 198.Li K., Lv C. Intradiscal injection of sesamin protects from lesion-induced degeneration. Connective Tissue Research . 2020;61(6):594–603. doi: 10.1080/03008207.2019.1651847. [DOI] [PubMed] [Google Scholar]
- 199.Gao G., Chang F., Zhang T., et al. Naringin protects against interleukin 1β (IL-1β)-Induced human nucleus pulposus cells degeneration via downregulation nuclear factor kappa B (NF-κB) pathway and p53 expression. Medical Science Monitor . 2019;25:9963–9972. doi: 10.12659/MSM.918597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen R., Gao S., Guan H., et al. Naringin protects human nucleus pulposus cells against TNF- α -induced inflammation, oxidative stress, and loss of cellular homeostasis by enhancing autophagic flux via AMPK/SIRT1 activation. Oxidative Medicine and Cellular Longevity . 2022;2022:17. doi: 10.1155/2022/7655142.7655142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Jin H., Wang Q., Wu J., et al. Baicalein inhibits the IL-1β-Induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration in vivo. Inflammation . 2019;42(3):1032–1044. doi: 10.1007/s10753-019-00965-8. [DOI] [PubMed] [Google Scholar]
- 202.Lin J., Chen J., Zhang Z., et al. Luteoloside inhibits IL-1β-Induced apoptosis and catabolism in nucleus pulposus cells and ameliorates intervertebral disk degeneration. Frontiers in Pharmacology . 2019;10:p. 868. doi: 10.3389/fphar.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen S., Luo M., Kou H., Shang G., Ji Y., Liu H. A review of gene therapy delivery systems for intervertebral disc degeneration. Current Pharmaceutical Biotechnology . 2020;21(3):194–205. doi: 10.2174/1389201020666191024171618. [DOI] [PubMed] [Google Scholar]
- 204.Wehling P., Schulitz K. P., Robbins P. D., Evans C. H., Reinecke J. A. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine (Phila Pa 1976) . 1997;22(10):1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 205.Nishida K., Kang J. D., Gilbertson L. G., et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976) . 1999;24(23):2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 206.Lee Y. J., Kong M. H., Song K. Y., Lee K. H., Heo S. H. The relation between Sox9, TGF-beta1, and proteoglycan in human intervertebral disc cells. Journal of Korean Neurosurgical Association . 2008;43(3):149–154. doi: 10.3340/jkns.2008.43.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tan Y., Hu Y., Tan J. Extracellular matrix synthesis and ultrastructural changes of degenerative disc cells transfected by Ad/CMV-hTGF-beta 1. Chinese Medical Journal . 2003;116(9):1399–1403. [PubMed] [Google Scholar]
- 208.Takeoka Y., Yurube T., Nishida K. Gene therapy approach for intervertebral disc degeneration: an update. Neurospine . 2020;17(1):3–14. doi: 10.14245/ns.2040042.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hajiesmailpoor A., Mohamadi O., Emami P., Farzanegan G., Ghorbani M. Overview of stem cell therapy in intervertebral disc disease: clinical perspectives. Current Stem Cell Research & Therapy . 2022 doi: 10.2174/1574888x17666220628123912. [DOI] [PubMed] [Google Scholar]
- 210.Croft A. S., Illien-Jünger S., Grad S., Guerrero J., Wangler S., Gantenbein B. The application of mesenchymal stromal cells and their homing capabilities to regenerate the intervertebral disc. International Journal of Molecular Sciences . 2021;22(7):p. 3519. doi: 10.3390/ijms22073519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Steffen F., Smolders L. A., Roentgen A. M., Bertolo A., Stoyanov J. Bone marrow-derived mesenchymal stem cells as autologous therapy in dogs with naturally occurring intervertebral disc disease: feasibility, safety, and preliminary results. Tissue Engineering. Part C, Methods . 2017;23(11):643–651. doi: 10.1089/ten.tec.2017.0033. [DOI] [PubMed] [Google Scholar]
- 212.Qi L., Wang R., Shi Q., Yuan M., Jin M., Li D. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. Journal of Bone and Mineral Metabolism . 2019;37(3):455–466. doi: 10.1007/s00774-018-0953-9. [DOI] [PubMed] [Google Scholar]
- 213.Miyamoto T., Muneta T., Tabuchi T., et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Research & Therapy . 2010;12(6):p. R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Richardson S. M., Kalamegam G., Pushparaj P. N., et al. Mesenchymal stem cells in regenerative medicine: focus on articular cartilage and intervertebral disc regeneration. Methods . 2016;99:69–80. doi: 10.1016/j.ymeth.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 215.Ferreira J. R., Teixeira G. Q., Neto E., et al. IL-1β-pre-conditioned mesenchymal stem/stromal cells’ secretome modulates the inflammatory response and aggrecan deposition in intervertebral disc. European Cells & Materials . 2021;41:431–543. doi: 10.22203/eCM.v041a28. [DOI] [PubMed] [Google Scholar]
- 216.Heo J. S., Choi Y., Kim H.-S., Kim H. O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. International Journal of Molecular Medicine . 2016;37(1):115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Urits I., Capuco A., Sharma M., et al. Stem cell therapies for treatment of discogenic low back pain: a comprehensive review. Current Pain and Headache Reports . 2019;23(9):p. 65. doi: 10.1007/s11916-019-0804-y. [DOI] [PubMed] [Google Scholar]
- 218.Li X., Wu A., Han C., et al. Bone marrow-derived mesenchymal stem cells in three-dimensional co-culture attenuate degeneration of nucleus pulposus cells. Aging (Albany NY) . 2019;11(20):9167–9187. doi: 10.18632/aging.102390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Orozco L., Soler R., Morera C., Alberca M., Sánchez A., García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation . 2011;92(7):822–828. doi: 10.1097/TP.0b013e3182298a15. [DOI] [PubMed] [Google Scholar]
- 220.Pang X., Yang H., Peng B. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Physician . 2014;17(4):E525–E530. doi: 10.36076/ppj.2014/17/E525. [DOI] [PubMed] [Google Scholar]
- 221.Li Z., Wu Y., Tan G., Xu Z., Xue H. Exosomes and exosomal miRNAs: a new therapy for intervertebral disc degeneration. Frontiers in Pharmacology . 2022;13, article 992476 doi: 10.3389/fphar.2022.992476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bhujel B., Shin H. E., Choi D. J., Han I. Mesenchymal stem cell-derived exosomes and intervertebral disc regeneration: review. International Journal of Molecular Sciences . 2022;23(13):p. 7306. doi: 10.3390/ijms23137306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cui S., Zhang L. MicroRNA-129-5p shuttled by mesenchymal stem cell-derived extracellular vesicles alleviates intervertebral disc degeneration via blockade of LRG1-mediated p38 MAPK activation. Journal of Tissue Engineering . 2021;12:14. doi: 10.1177/20417314211021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shao B. Z., Xu Z.-Q., Han B.-Z., Su D.-F., Liu C. NLRP3 inflammasome and its inhibitors: a review. Frontiers in Pharmacology . 2015;6:p. 262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chao-Yang G., Peng C., Hai-Hong Z. Roles of NLRP3 inflammasome in intervertebral disc degeneration. Osteoarthritis and Cartilage . 2021;29(6):793–801. doi: 10.1016/j.joca.2021.02.204. [DOI] [PubMed] [Google Scholar]
- 226.Zhang J., Zhang J., Zhang Y., et al. Mesenchymal stem cells-derived exosomes ameliorate intervertebral disc degeneration through inhibiting pyroptosis. Journal of Cellular and Molecular Medicine . 2020;24(20):11742–11754. doi: 10.1111/jcmm.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yuan X., Li T., Shi L., Miao J., Guo Y., Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Molecular Medicine . 2021;27(1):p. 91. doi: 10.1186/s10020-021-00355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding authors upon request.


