Abstract
Background
Previous studies have suggested the applicability of three classifications of subsolid nodules (SSNs). However, few studies have unraveled the natural history of the three types of SSNs.
Methods
A retrospective study from two medical centers between November 2007 and November 2017 was conducted to explore the long‐term follow‐up results of three different types of SSNs, which were divided into pure ground‐glass nodules (pGGNs), heterogeneous ground‐glass nodules (hGGNs), and real part‐solid nodules (rPSNs).
Results
A total of 306 consecutive patients, including 361 SSNs with long‐term follow‐up, were reviewed. The median growth times of pGGNs, hGGNs, and rPSNs were 7.7, 6.0, and 2.0 years, respectively. For pGGNs, the median period of development into rPSNs was 4.6 years, while that of hGGNs was 1.8 years, and the time from pGGNs to hGGNs was 3.1 years (p < 0.05). In SSNs with an initial lung window consolidation tumor ratio (LW‐CTR) >0.5 and mediastinum window (MW)‐CTR >0.2, all cases with growth were identified within 5 years. Meanwhile, in SSNs whose LW‐CTR and MW‐CTR were 0, it took over 5 years to detect nodular growth. Pathologically, 90.6% of initial SSNs with LW‐CTR >0 were invasive carcinomas (invasive adenocarcinoma and micro‐invasive adenocarcinoma). Among patients with rPSNs in the initial state, 100.0% of the final pathological results were invasive carcinoma. Cox regression showed that age (p = 0.038), initial maximal diameter (p < 0.001), and LW‐CTR (p = 0.002) were independent risk factors for SSN growth.
Conclusions
pGGNs, hGGNs, and rPSNs have significantly different natural histories. Age, initial nodule diameter, and LW‐CTR are important risk factors for SSN growth.
Keywords: heterogenous ground‐glass nodules (hGGNs), natural course, pulmonary adenocarcinoma, subsolid nodules (SSNs)
Pure ground‐glass nodule pGGNs, heterogeneous ground‐glass nodules (hGGNs), and real part‐solid nodules (rPSNs) have significantly different natural histories. hGGNs are an intermediate form,pGGN‐hGGN‐rPSN has a step‐by‐step evolutionary relationship. It is necessary to distinguish hGGNs from the preceding PSNs and to manage them separately.
INTRODUCTION
The prevalence of subsolid nodules (SSNs) in patients who undergo chest computed tomography (CT) ranges from 1.8% to 2.6%. 1 , 2 Previous research has shown that persistent SSNs have a sluggish development trend and a 29–34% risk of malignancy. 3 , 4 Prospective and retrospective studies on the natural course of SSNs demonstrated that nodule growth and the progression of tumors could be found with long‐term follow‐up, 5 , 6 therefore 5 years of follow‐up are required for persistently stable nodules according to the Fleischner Society 2017 guidelines 7 and the 2022 National Comprehensive Cancer Network (NCCN) Guidelines for Non‐Small Cell Lung Cancer. 8 However, several recent studies have found that SSNs stable for 5 years required increased follow‐up, and follow‐up beyond 5 years may detect more lung cancers, 9 , 10 , 11 , 12 but guidelines on follow‐up strategies of SSNs have not been updated based on these results.
SSNs can be divided into pure ground‐glass nodules (pGGNs) and part‐solid nodules (PSNs) based on the presence of solid components on the lung window (LW) according to Fleischner Society 2017 guidelines. 7 , 13 Many guidelines recommend distinct therapeutic modalities for pGGNs and PSNs due to their different biological characteristics and prognoses. However, it is always controversial in comprehending and quantifying solid components in SSNs. 14 Solid components in some PSNs (real PSNs [rPSNs]) can be seen in both the LW and mediastinum window (MW), whereas others are only observed in the LW (heterogeneous ground‐glass nodules [hGGNs]). hGGNs could develop into rPSNs in 2.1 years according to reports from past studies. 15 The clinicopathologic behaviors of rPSNs appear to be worse than those of hGGNs. 16
However, previous studies on the natural process of SSNs mainly compared pGGNs and PSNs separated by the LW, 1 , 17 , 18 , 19 and did not provide enough information on the natural history with regard to the solid component on different window settings. This study aims to investigate the natural history and clinicopathological aspects of pGGNs, hGGNs, and rPSNs with long‐term follow‐up.
MATERIALS AND METHODS
Study design and participants
We designed a two‐centre, retrospective cohort study of adults aged 18 years who underwent CT scan for any reason, regardless of their smoking history, between November 2007 and November 2017 at Peking University People's Hospital and AMHT Group Aerospace 731 Hospital. The inclusion criteria for SSNs were as follows. First, the persistence of the SSNs for at least 6 months was confirmed after the initial CT examination. Second, the included SSNs had a long axial diameter of 3 cm or less. Third, each SSN had been evaluated using CT images with a section thickness of 1.5 mm or less. Finally, SSNs with long‐term follow‐ups of at least 5 years or a follow‐up within 5 years but growing.
The smoking status of the participants was taken from the hospital information system. Never‐smokers were defined as adults who had never smoked or had smoked <100 cigarettes in their lifetime. Participants with a previous history of lung cancer at the time of baseline screening and those with unknown history on smoking status were excluded. Individuals with data on smoking status but not the amount of smoking were included. This study was approved by the Institutional Review Board (IRB) of Peking University People's Hospital (No.2022PHB031‐001) and AMHT Group Aerospace 731 Hospital (No. 2022‐0601‐01). The IRB waived the need for written informed consent from the participants.
Patients characteristics and classification of SSNs
Patients characteristics including age, gender, smoking history, cancer history, family history of cancer, number of concurrent SSNs, and pathologic diagnoses of patients who underwent resection were collected. In this study, the SSNs were classified into three groups on the basis of their texture (Figure 1). Nodules that showed a homogeneous hazy area of increased opacity when viewed using the LW were defined as pGGNs. hGGNs were defined as SSNs only with a solid component on the LW. rPSNs were characterized as SSNs with a solid component on both the LW and the MW. 20
FIGURE 1.
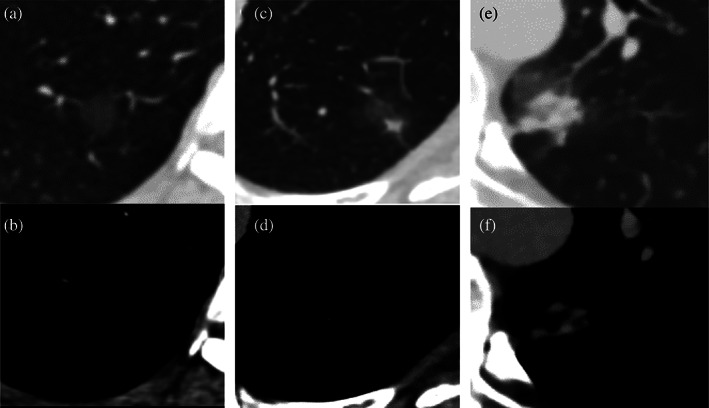
Categories of subsolid nodules. (a, b) Pure ground‐glass nodules: no solid component on both lung and mediastinum windows. (c, d) Heterogeneous ground‐glass nodules: solid component only visible on the lung window. (e, f) Real part‐ solid nodules: a solid component can be seen in both the lung and mediastinum windows.
Radiologic assessment
Unenhanced chest CT scans were performed using 128‐slice multidetector CT (Somatom Force; Siemens Healthcare) or 256‐slide multi‐detector CT (Revolution CT; GE Healthcare) at a peak tube voltage of 100–120 kV and a reference tube current of 100–720 mA. Images were reconstructed with sections of ≤1.25 mm thickness. The LWs were set at a window width of 1500 Hounsfield units (HU) and a window level −500 HU. MWs for the measurement of solid components were set at a window width of 350 HU and a window level of 40 HU. All images were interpreted by board‐certified experienced chest radiologists initially. One radiologist (with 10 years’ experience) and one radiologist‐trained thoracic surgeon (with 6 years’ experience) reviewed the CT images of patients independently and labeled any increase in the size of an SSN, change from pGGN to hGGN, change from pGGN to rPSN, change from hGGN to rPSN, or increase in the size of a solid component. If there was any disagreement between the two readers, the results would be adjudicated by a third radiologist and three physicians unanimously confirmed the final results. None of the three readers was aware of the clinical, histopathological or growth data.
SSN size (the longest diameter of the nodule) was measured using the LW. The solid component size was measured in both LW and MW settings. The LW consolidation tumor ratio (LW‐CTR) was defined as the ratio of the solid component size measured at the LW (LW‐SCS) to SSN size. The MW‐CTR was defined as the ratio of the solid component size measured at the MW (MW‐SCS) to SSN size.
SSN growth was defined as (1) an interval increase ≥2 mm in size, (2) an interval increase ≥2 mm in the solid component of a PSN (including hGGNs and rPSNs), (3) the appearance of a new solid component of any size in pGGNs. 21 Resolution of an SSN was defined as the disappearance or reduction of ≥2 mm in total size or solid component from initial detection. 22 If the SSN did not meet the definition of growth and resolution, we judged it as stable or without growth.
Follow‐up
Growth interval was the time from the initial to subsequent CT scans during which the same GGN was observed to satisfy the definition of growth. The total observation time was the interval between the initial and final CT scans of the same SSN or the interval between the initial CT scan and the last intervention received. The clinicians determined the interval of the subsequent chest CT scan. All patients accepted scheduled follow‐ups by telephone or outpatient clinic visits. The outcome of the nodules was documented, and the deadline for follow‐up was May 2022.
Pathological examination
Decisions regarding invasive procedures for pathologic evaluations were made by the attending thoracic surgeon. Postoperative pathological types were classified into benign and lung adenocarcinoma (LUAD), including invasive adenocarcinoma (IAC), micro‐invasive adenocarcinoma (MIA), and its precancerous lesions, including adenocarcinoma in situ (AIS) and atypical adenomatous hyperplasia (AAH), documenting and classifying the pathologic reports on surgical specimens and pathologic staging in accordance with the 8th edition staging system of the American Joint Committee on Cancer. 23 One board‐certified pathologist made all diagnoses and reviewed them with another experienced pathologist. The final result was unanimously decided by two pathologists.
Statistical analysis
The independent samples t‐test or Mann–Whitney U test was used to compare continuous variables, and categorical variables were compared by Pearson's chi‐square test or Fisher's exact test. Univariate analyses for factors affecting SSN growth were analyzed using Kaplan–Meier analyses. Differences between curves were determined using the log‐rank test. Multivariate Cox proportional hazards regression analysis with backward stepwise selection was performed to identify independent predictors of interval growth and outcome. Predictors (p < 0.05) in the univariate analysis were included in a multivariable analysis. All statistical analyses were performed using SPSS 26.0 for Windows (SPSS Inc.) and R software, version 3.5.1 (http://www.rproject.org/). p values <0.05 were considered statistically significant.
RESULTS
Number of included patients and SSNs
The flowchart of the study is shown in Figure 2. During the study period, 28 791 participants with reported pulmonary ground‐glass opacities underwent chest CT scan at the two different medical centers. A total of 20 646 people were excluded for three reasons: lack of SSN features or diagnosis of inflammation on radiography (acute respiratory distress syndrome, interstitial lung disease, pulmonary infection, edema, or hemorrhage), transient presence of nodules (present for less than 6 months), absence of thin‐slice CT, and initial maximum diameter exceeding 30 mm. For the remaining 8145 patients, a total of 7839 patients were excluded for the following reasons: follow‐up of less than 5 years for target stable SSNs, detailed clinical data could not be obtained, and decrease in maximal diameter of 2 mm or more. A total of 306 patients with 361 nodules were included according to the inclusion criteria finally. They were divided into the growth group and the stable group according to whether they met the growth criteria.
FIGURE 2.
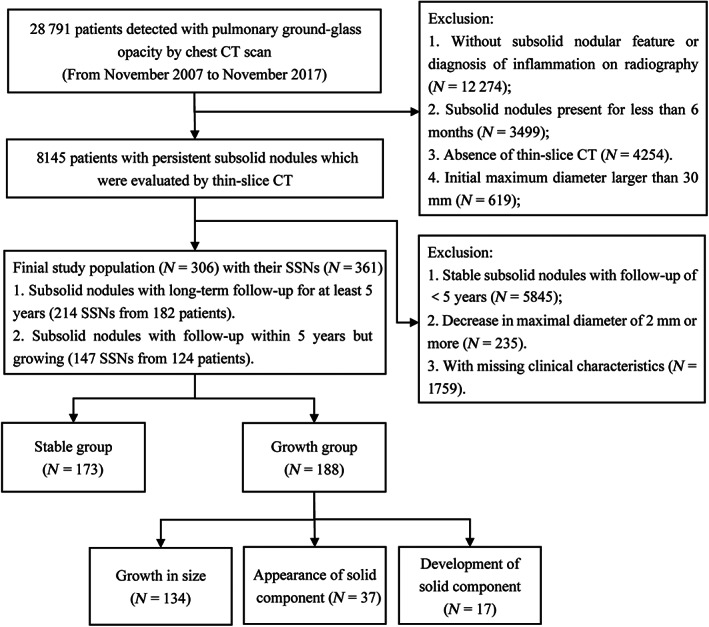
Flow diagram for the patient selection in this study.
Baseline clinical characteristics of the patients and radiologic features of included SSNs
Of the 306 patients, 163 (53.3%) patients were categorized into the SSN growth group and 143 (46.7%) patients were categorized into the SSN stable group. The clinical characteristics of patients are listed in Table 1. The median age was 67 (interquartile range [IQR] 59–77) years old and 91 (29.7%) subjects were male. In addition, 139 (45.4%) subjects had multiple SSNs.
TABLE 1.
Clinical characteristics of patients with SSN growth and SSN stable under long‐term follow‐up
| Characteristic | Total patients (n = 306) | Patients with SSN growth (n = 163) | Patients with SSN stable (n = 143) | p value |
|---|---|---|---|---|
| Age | 67.0 (59.0–77.0) | 71.0 (63.0–80.0) | 64.0 (56.0–71.0) | <0.001 |
| Sex | 0.059 | |||
| Female | 215 (70.3%) | 107 (65.6%) | 108 (75.5%) | |
| Male | 91 (29.7%) | 56 (34.4%) | 35 (24.5%) | |
| Smoking history | 0.289 | |||
| Current smoker | 19 (6.2%) | 13 (8.0%) | 6 (4.2%) | |
| Ex‐smoker | 28 (9.2%) | 15 (9.2%) | 13 (9.1%) | |
| Nonsmoker | 259 (84.6%) | 135 (82.8%) | 124 (86.7%) | |
| Pack‐years | 20.0 (10.3–30.0) | 20.0 (12.5–30.0) | 15.0 (5.0–30.0) | 0.073 |
| Previous history of lung disease | 0.208 | |||
| Emphysema | 3 (1.0%) | 2 (1.2%) | 1 (0.7%) | |
| Bronchiectasis | 5 (1.6%) | 5 (3.1%) | 0 (0.0%) | |
| Chronic bronchitis | 8 (2.6%) | 5 (3.1%) | 3 (2.1%) | |
| Tuberculosis | 12 (3.9%) | 6 (3.7%) | 6 (4.2%) | |
| Previous history of cancer | ||||
| Lung cancer | 69 (22.5%) | 36 (22.1%) | 33 (23.1%) | 0.836 |
| Other cancer | 81 (26.5%) | 38 (23.3%) | 43 (30.1%) | 0.181 |
| Family history of cancer | 33 (10.8%) | 17 (10.4%) | 16 (11.2%) | 0.977 |
| Number of concurrent SSNs per patient | 0.562 | |||
| 1 | 261(85.3%) | 142 (87.1%) | 119 (83.2%) | |
| 2 | 35 (11.4%) | 17 (10.4%) | 18 (12.6%) | |
| 3 | 10 (3.3%) | 4 (2.5%) | 6 (4.2%) | |
Note: Values are expressed as a number (%) or the median (interquartile range).
Abbreviation: SSN, subsolid nodule.
Among 361 SSN lesions, 233 (64.5%) were pGGNs, 93 (25.8%) were hGGNs, and 35 (9.7%) were rPSNs. At the initial CT scan, the initial median nodule size was 7.0 (IQR 5.6–9.0) mm, the median initial diameter of the solid component in the LW was 2.3 (IQR 1.8–3.8) mm, and the median initial diameter of the solid component in the MW was 2.8 (1.4–3.5) mm. The median follow‐up period was 70.2 (IQR 59.8–87.5) months (Table 2). Significant differences were found in nodular type, initial diameter, LW solid component size, CTR in the LW and the MW, vacuole sign, vascular sign, and pleural retraction between the growth and stable groups.
TABLE 2.
Radiologic features of SSNs with growth and stable under long‐term follow‐up
| Feature | Total SSNs (n = 361) | SSNs with growth (n = 188) | SSNs stable (n = 173) | p value |
|---|---|---|---|---|
| Initial nodule type | <0.001 | |||
| pGGN | 233 (64.5%) | 106 (56.3%) | 127 (73.4%) | |
| hGGN | 93 (25.8%) | 52 (27.7%) | 41 (23.7%) | |
| rPSN | 35 (9.7%) | 30 (16.0%) | 5 (2.9%) | |
| Lesion location | 0.148 | |||
| RUL | 122 (33.8%) | 70 (37.2%) | 52 (30.1%) | |
| RML | 20 (5.5%) | 8 (4.3%) | 12 (6.9%) | |
| RLL | 67 (18.62%) | 34 (18.1%) | 33 (19.1%) | |
| LUL | 109 (30.2%) | 60 (31.9%) | 49 (28.3%) | |
| LLL | 43 (11.9%) | 16 (8.5%) | 27 (15.6%) | |
| Initial diameter (mm) | 7.0 (5.6–9.0) | 8.0 (6.0–12.0) | 6.4 (5.4–7.6) | <0.001 |
| Initial diameter classification (mm) | <0.001 | |||
| 2.0–3.9 | 10 (2.8%) | 9 (4.8%) | 1 (0.6%) | |
| 4.0–5.9 | 92 (25.5%) | 29 (15.4%) | 63 (36.4%) | |
| 6.0–7.9 | 127 (35.2%) | 48 (25.5%) | 79 (45.7%) | |
| 8.0–9.9 | 58 (16.0%) | 34 (18.1%) | 24 (13.9%) | |
| ≥10.0 | 74 (20.5%) | 68 (36.2%) | 6 (3.5%) | |
| Initial diameter of solid component in LW (mm) | 2.3 (1.8–3.8) | 3.0 (2.0–4.5) | 2.0 (1.5–2.3) | <0.001 |
| Initial diameter of solid component in MW (mm) | 2.8 (1.4–3.5) | 2.4 (1.2–3.4) | 1.8 (1.7–3.6) | 0.766 |
| Initial mean CT value (HU) | −705.8 ± 85.8 | −703.5 ± 86.5 | −708.3 ± 85.2 | 0.601 |
| CTR classification in LW | 0.003 | |||
| 0 | 233 (64.5%) | 106 (56.4%) | 127 (73.4%) | |
| 0.01–0.25 | 53 (14.7%) | 32 (17.0%) | 21 (12.1%) | |
| 0.26–0.50 | 62 (17.2%) | 39 (20.7%) | 23 (13.3%) | |
| >0.5 | 13 (3.6%) | 11 (5.9%) | 2 (1.2%) | |
| CTR classification in MW | <0.001 | |||
| 0 | 326 (90.3%) | 158 (84.0%) | 168 (97.1%) | |
| 0.01–0.10 | 7 (1.9%) | 7 (3.7%) | 0 (0.0%) | |
| 0.11–0.20 | 15 (4.2%) | 12 (6.4%) | 3 (1.7%) | |
| 0.21–0.30 | 6 (1.7%) | 6 (3.2%) | 0 (0.0%) | |
| 0.31–0.40 | 2 (0.6%) | 2 (1.1%) | 0 (0.0%) | |
| 0.41–0.50 | 4 (1.1%) | 2 (1.1%) | 2 (1.2%) | |
| >0.5 | 1 (0.3%) | 1 (0.5%) | 0 (0.0%) | |
| Radiologic findings | ||||
| Lobulated sign | 31 (8.6%) | 20 (10.6%) | 11 (6.4%) | 0.147 |
| Vacuole sign | 54 (15.0%) | 40 (21.3%) | 14 (8.1%) | <0.001 |
| Vascular sign | 159 (44.0%) | 98 (52.1%) | 61 (35.3%) | 0.001 |
| Pleural adhesion | 87 (24.1%) | 38 (20.2%) | 49 (28.3%) | 0.072 |
| Pleural retraction | 2 (1.2%) | 13 (6.9%) | 15 (4.2%) | 0.006 |
| Follow‐up duration (months) | 70.2 (59.8–87.5) | 62.0 (46.3–86.8) | 73.8 (68.1–88.4) | <0.001 |
| Time to nodule enlargement (months) | NA | 35.0 (21.3–55.0) | NA | NA |
| Total number of CT scans per patient | 7.0 (4.0–9.0) | 5.0 (4.0–8.0) | 7.0 (6.0–10.0) | <0.001 |
Note: Values are expressed as a number (%) or the mean ± standard deviation or median (IQR). Initial diameter of the solid component was measured when hGGNs and rPSNs were detected on the initial chest CT.
Abbreviations: CT, computed tomography; CTR, maximum diameter of consolidation relative to the maximum tumor diameter; hGGN, heterogeneity ground‐glass nodule; IQR, interquartile range; LLL, left lower lobe; LUL, left upper lobe; LW, lung window; MW, mediastinum window; pGGN, pure ground‐glass nodule; RLL, right lower lobe; RML, right middle lobe; rPSN, real part‐solid nodule; RUL, right upper lobe; SSN, subsolid nodule.
Finally, 54 SSNs underwent surgical resections, of which 37 (68.5%) were wedge resections, eight (14.8%) were segmental resections, and nine (16.7%) were lobectomies. According to the pathology reports, there were 32 (59.3%) IACs, 18 (33.3%) MIAs, one (1.9%) AIS, two (3.7%) AAHs, and one (1.9%) focal fibrosis. Lymph node dissection or sampling was performed according to the surgeon's decision during the surgery, and no lymph node metastasis was detected (Table 3).
TABLE 3.
Pathological features and surgical methods for SSNs with growth and stable under long‐term follow‐up
| Feature/surgical method | Total SSNs (n = 54) | SSNs with growth (n = 51) | SSNs stable (n = 3) | p value |
|---|---|---|---|---|
| Pathologic diagnosis of resected cases | 0.108 | |||
| Focal fibrosis | 1 (1.9%) | 1 (2.0%) | 0 (0.0%) | |
| AAH | 2 (3.7%) | 1 (2.0%) | 1 (33.3%) | |
| AIS | 1 (1.9%) | 1 (2.0%) | 0 (0.0%) | |
| MIA | 18 (33.3%) | 17 (33.3%) | 1 (33.3%) | |
| IAC | 32 (59.3%) | 31 (60.8%) | 1 (33.3%) | |
| Surgical type | 0.732 | |||
| Wedge resection | 37 (68.5%) | 35 (68.6%) | 2 (66.7%) | |
| Segmentectomy | 8 (14.8%) | 7 (13.7%) | 1 (33.3%) | |
| Lobectomy | 9 (16.7%) | 9 (17.6%) | 0 (0.0%) |
Note: Values are expressed as a number (%).
Abbreviations: AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; IAC, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; SSN, subsolid nodule.
The natural course of different SSNs
During the follow‐up of 233 pGGNs on initial CT, 76 (32.6%) nodules were found to have increased in total size by more than 2 mm, 19 (8.2%) exhibited a solid component appearance in the LW and 11 (4.7%) in the MW, and 127 (54.5%) remained stable. Thirty‐seven (39.8%) among the 93 hGGNs demonstrated an increase in total diameter of more than 2 mm, eight (8.6%) showed a rise of solid component size of more than 2 mm in the LW, seven (7.5%) showed the appearance of a solid component in the MW, and 41 (44.1%) remained stable. Twenty‐one (60.0%) of the 35 initial rPSNs showed growth in the total diameter of more than 2 mm, nine (25.7%) showed a rise in SCS of more than 2 mm, and five (14.3%) remained stable (Figure 3). Thirty‐five of these nodules showed growth after more than 5 years of stability.
FIGURE 3.
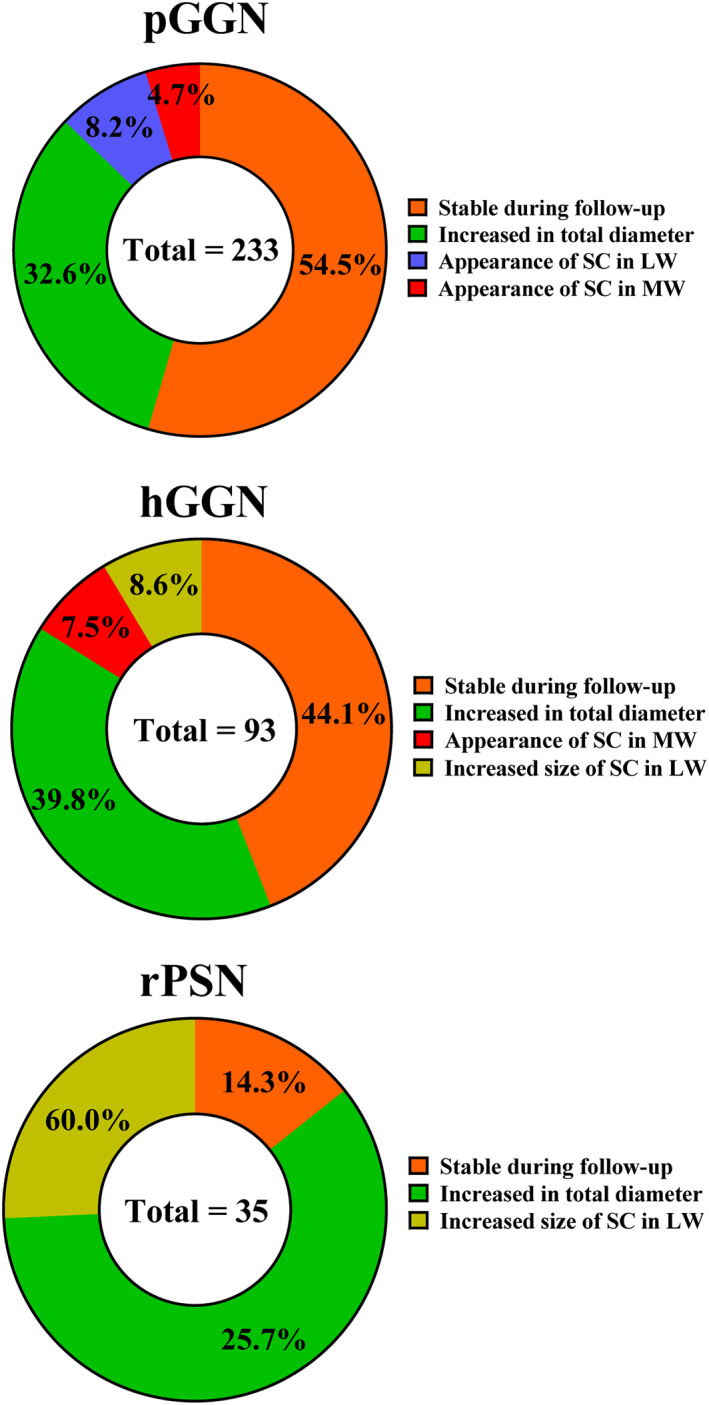
The percentages of all growth patterns in pure ground‐glass nodules (pGGNs), heterogeneous ground‐glass nodules (hGGNs), and real part‐solid nodules (rPSNs). LW, lung window; MW, mediastinum window; SC, solid component
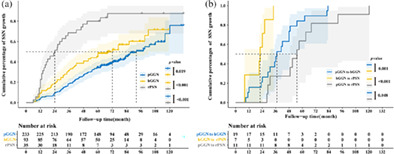
The 1‐, 2‐, 3‐, 4‐, and 5‐year cumulative percentages of SSN growth were 5.8%, 15.0%, 26.4%, 34.4%, and 43.2%, respectively, for the whole group. The natural course of different SSNs is shown in Figure 4. The cumulative growth percentage of SSNs differed significantly between the pGGN and hGGN + rPSN (i.e., PSN in the conventional classification) groups (p < 0.001) (Figure 4a). The median time to growth was 7.7 years for pGGNs, and the median time was 6.0 years for hGGN growth. In contrast, the rPSN group had a median growth time of 2.0 years. There were significant differences in growth periods among the three groups (p < 0.05), as shown in Figure 4b.
FIGURE 4.
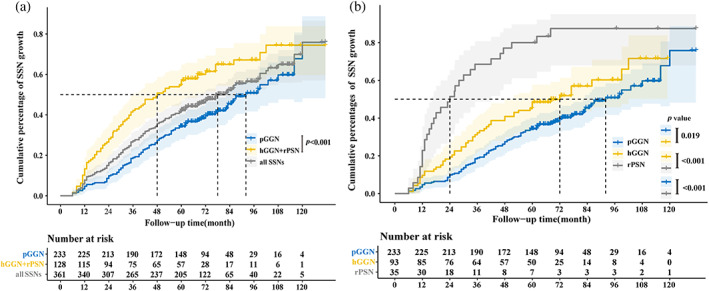
The Kaplan–Meier plot for time to subsolid nodules (SSNs) growth. Curves with different colors represent the cumulative growth percentage of pure ground‐glass nodules (pGGNs), heterogeneous ground‐glass nodules (hGGNs), and real part‐ solid nodules (rPSNs), respectively. (a) All enrolled SSNs (the pGGN group vs. the hGGN + rPSN group). There was a significant difference in the cumulative percentage of SSN growth between the pGGN group and the hGGN + rPSN group (p < 0.001). (b) All enrolled SSNs (the pGGN group vs. the hGGN group vs. the rPSN group). There was a significant difference in the cumulative percentage of SSN growth among the pGGN, hGGN, and PSN groups.
In the subgroup analysis of growth mode, Figure 5 depicts the time when the SSN grew by 2 mm or more on the maximum diameter, the time when solid components appear in the MW, and the solid component size increased by more than 2 mm based on the consistency of SSNs. When the SSN growth was evaluated by the maximum diameter increasing by more than 2 mm, the growth of hGGNs and pGGNs was relatively slower than that of rPSNs (p < 0.001). However, the growth curves of hGGNs and pGGNs were comparable (p = 0.176; Figure 5a). When solid component appearance in the MW was taken as the growth indicator, the cumulative growth percentages of pGGNs and hGGNs were significantly different (p < 0.001; Figure 5b). The cumulative percentage growths of hGGNs and rPSNs did not differ significantly when the growth index was defined as an increase in solid content of more than 2 mm (p = 0.465; Figure 5c).
FIGURE 5.
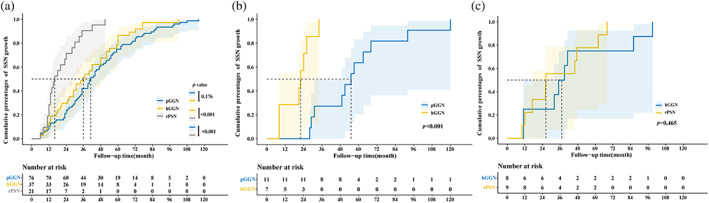
The Kaplan–Meier plot for time to subsolid nodule (SSN) growth based on different outcomes. (a) Time until the SSN grows by 2 mm or more according to the category of the SSN. Each pair of growth curves except for that for pure ground‐glass nodules (pGGNs) and heterogeneous ground‐glass nodules (hGGNs) showed a statistically significant difference. (b) Time until the appearance of a solid component in the mediastinum window (MW) according to the category of the SSN. The two groups were statistically significant on the curve during the follow‐up. (c) Time until the solid component size increased by more than 2 mm according to the category of the SSN. The two groups were not statistically significant on the curve during the follow‐up. rPSN, real part‐solid nodules
Time for and probability of pGGNs and hGGNs progressing to part‐solid nodules
During the follow‐up, 19 (8.2%) of 233 pGGNs on initial CT transformed into hGGNs and 11 (4.7%) pGGNs converted into rPSNs. Seven (7.5%) of the 93 hGGNs initially examined by CT developed into rPSNs.
The median time for 19 pGGNs to develop into hGGNs was 3.1 (IQR 2.0–4.1) years. For seven hGGNs, the median time to develop into rPSNs was 1.8 (IQR 0.7–2.2) years. The median time for a pGGN to develop into an rPSN was 4.6 (IQR 2.6–5.7) years. Statistically significant differences (p < 0.05) existed among the time of pGGN and hGGN progression to further conditions (Figure S1).
Analysis of risk factors related to SSN growth
Using multivariate Cox proportional hazards regression analysis to predict the growth of SSNs, three factors were found to be independent risk factors predicting tumor growth: age (hazard ratio [HR] = 1.013, 95% confidence interval [CI] 1.001–1.026), initial maximal diameter (HR = 1.097, 95% CI 1.067–1.129), and CTR in LW (HR = 3.721, 95% CI 1.618–8.554) (Table 4).
TABLE 4.
Univariable and multivariable analyses of characteristics to predict SSN growth
| Variable | Univariate analysis a | Multivariate analysis b | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.031 | 1.018–1.043 | <0.001 | 1.013 | 1.001–1.026 | 0.038 |
| Sex | 1.510 | 1.119–2.039 | 0.007 | 1.317 | 0.964–1.799 | 0.084 |
| Type of SSN | 1.813 | 1.481–2.219 | <0.001 | 1.256 | 0.792–1.991 | 0.333 |
| Smoking status (current or former vs. never) | 1.247 | 0.852–1.825 | 0.255 | |||
| History of lung cancer (yes vs. no) | 1.071 | 0.767–1.496 | 0.687 | |||
| History of other cancer (yes vs. no) | 0.809 | 0.579–1.129 | 0.213 | |||
| History of chronic lung disease (yes vs. no) | 1.301 | 0.804–2.106 | 0.284 | |||
| Family history of cancer (yes vs. no) | 1.063 | 0.668–1.693 | 0.795 | |||
| Solitary pulmonary nodule (yes vs. no) | 0.980 | 0.735–1.306 | 0.889 | |||
| Location (right vs. left) | 1.076 | 0.804–1.440 | 0.623 | |||
| Vascular sign (yes vs. no) | 1.701 | 1.277–2.267 | <0.001 | 1.207 | 0.935–1.723 | 0.126 |
| Lobulated sign (yes vs. no) | 1.499 | 0.941–2.388 | 0.088 | |||
| Vacuole sign (yes vs. no) | 2.218 | 1.560–3.152 | <0.001 | 1.209 | 0.807–1.814 | 0.358 |
| Pleural retraction (yes vs. no) | 3.465 | 1.957–6.136 | <0.001 | 0.825 | 0.408–1.668 | 0.593 |
| Initial maximal diameter (mm) | 1.127 | 1.101–1.153 | <0.001 | 1.097 | 1.067–1.129 | <0.001 |
| Initial mean CT value (HU) | 1.001 | 0.999–1.002 | 0.413 | |||
| CTR in LW | 8.692 | 4.005–18.632 | <0.001 | 3.721 | 1.618–8.554 | 0.002 |
Abbreviations: CI, confidence interval; CT, computed tomography; CTR, maximum diameter of consolidation relative to the maximum tumor diameter; HR, hazard ratio; LW, lung window; SSN, subsolid nodule.
Kaplan–Meier analyses with the log‐rank test.
Multivariate Cox proportional hazards regression analysis with backward stepwise selection.
Relationship between CTR and interval growth and pathological type
The relationship between the CTR on the initial CT, the interval growth time, and the type of pathology is shown in Figure 6. In the LW‐CTR 0.01–0.25 group, the proportions of cumulative growth in 2, 3, and 5 years were 11.3%, 22.6%, and 45.3%, respectively. In the LW‐CTR 0.26–0.50 group, the 2‐, 3‐, and 5‐year cumulative growth ratios were 40.3%, 54.8%, and 61.3%, respectively. The 2‐, 3‐, and 5‐year cumulative growth rates for the CTR >50% group were 38.5%, 61.5%, and 84.6%, respectively. In the growth group, SSN growth in the LW within 5 years was detected in all patients with LW‐CTR >50%. In contrast, 11.2% (26 of 233) of LW‐CTR = 0 patients required more than 5 years to show any growth (Figure 6a). In the LW‐CTR subgroup analysis, patients with CTR ≤25% and 50% had significantly slower SSN growth compared to those with CTR >25% and 50% (p > 0.05) (Figure 6b,c).
FIGURE 6.
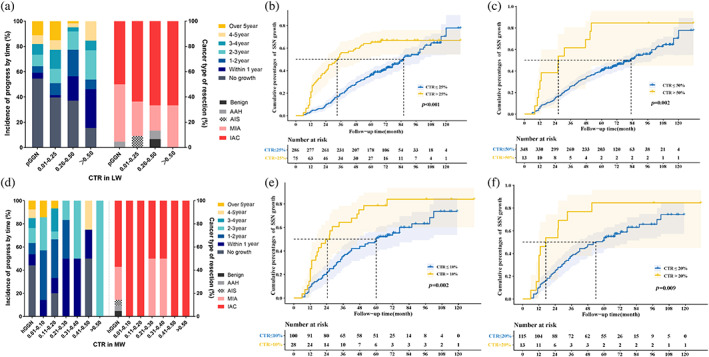
The associations between the consolidation tumor ratio (CTR) at the initial CT examination and the time interval until detection of subsolid nodule growth and the histologic subtype. (a) Incidence of progress by time and cancer type of resection in different lung window‐CTR (LW‐CTR) groups. (b) The LW‐CTR >25% group shows a significantly higher cumulative growth percentage than the ≤25% group (p < 0.001). (c) The LW‐CTR >50% group shows a significantly higher cumulative growth percentages than the ≤50% group (p = 0.002). (d) Incidence of progress by time and cancer type of resection in different mediastinum window‐CTR (MW‐CTR) groups. (e) The MW‐CTR >10% group shows a significantly higher cumulative growth percentage than the ≤10% group (p = 0.002). (f) The MW‐CTR >20% group exhibits a considerably higher cumulative growth percentage than the ≤20% group (p = 0.009).
The proportions of growth within 2, 3, and 5 years for MW‐CTR 0.01–0.10 nodules were 57.1%, 85.7%, and 85.7%, respectively. The proportions of growth within 2, 3, and 5 year for CTR 0.11–0.20 nodules were 46.7%, 53.3%, and 73.3%, respectively. Nodules with CTR >20% had 2‐, 3‐, and 5‐year growth ratios of 53.8%, 76.9%, and 84.6%, respectively. In the SSN growth group, all patients except those with MW‐CTR ≤20% displayed detectable nodule growth within 5 years. A total of 7.5% (seven of 93 cases) of hGGN patients required more than 5 years of follow‐up to detect nodule growth (Figure 6d). In the MW‐CTR subgroup analysis, patients with CTR ≤10% and 20% had slower SSN growth (p < 0.05) (Figure 6e,f).
When measuring initial CTR in the LW, the proportions of final pathologically confirmed IAC and MIA were 95.5% in the CTR 0 group, 90.9% in the CTR 0.01–0.25 group, 86.7% in the CTR 0.26–0.50 group, and 100.0% in the CTR >0.50 group. The proportions of MIA + IAC were confirmed as 85.7% in the hGGN group and 100.0% in the rPSN group.
DISCUSSION
Persistent SSNs exhibit slow growth 22 and a favorable prognosis. 24 Currently, the major guidelines divide SSNs into pGGNs and PSNs based on the absence or presence of a solid component in the LW and manage them based on their respective diameters. 25 , 26 , 27 , 28 Despite significant advances in radiology, the interpretation of solid components of the SSN, including the window and measurement methods that should be used to measure the solid component, remains contentious. An estimated 36.4% of pulmonary nodules are classified differently, according to research findings. Disagreements primarily center on whether the SSN has a solid component in the LW and whether the solid component is greater than 5 mm. 14 It is well known that the following management of SSNs is primarily decided by the presence and size of the solid component in SSNs. Such a significant percentage of classification differences will lead to distinct decision‐making.
Kakinuma et al. 15 introduced the tomographic characteristics in the MW of the SSNs into the classification and improved the SSN classification procedure until 2016. The results of their study showed that the growth pattern differed between hGGNs and rPSNs (both sorted in PSNs), necessitating additional research to determine whether this intermediate nodule category is appropriate. Some studies have begun to investigate the applicability of the three classifications in clinical interpretation and their correlation with the degree of pathological infiltration, 29 as well as the differences in pathological types and prognosis between rPSNs and hGGNs. 30 However, few studies have focused on the long‐term follow‐up outcomes for pGGNs, hGGNs, and rPSNs, which would allow for more accurate management decisions.
Using the presence of solid components in the MW as the standard for interpretation, it was found that pGGNs and hGGNs differ significantly. The median time for a pGGN to develop into an rPSN was 4.6 years, the average time for an hGGN to develop into an rPSN was 1.8 years, and the average time for a pGGN to develop into an hGGN was 3.1 years. Moreover, 8.2% of pGGN patients progressed to an hGGN and 7.5% of hGGN patients progressed to an rPSN, which was higher than the 5.2% of pGGN patients who progressed to an rPSN, confirming the higher risk of progression for hGGNs. These differences suggest that hGGN is an intermediate form and that perhaps pGGN‐hGGN‐rPSN has a step‐by‐step evolutionary relationship. Regarding the 2 mm diameter increase in SSNs, there were significant differences between pGGNs, hGGNs, and rPSNs, but no difference was seen between pGGNs and hGGNs, which may suggest that hGGNs are more similar to pGGNs in regard to natural behavior. The hGGN is an intermediate form in genomics, and its genome structure is closer to that of pGGNs, which explains why the natural course of hGGNs is more similar to that of pGGNs from a genetic standpoint. 31 Given the differences in natural biological behavior and genetic level between the three types of SSNs, it is necessary to separate hGGNs from previous PSN types and manage them in a manner similar to pGGNs.
According to the 2017 Fleshiner Society guidelines, the growth of the solid component is mainly judged through the LW, and the progression from hGGN to rPSN without solid size enlargement in LW is not considered as a growth indicator. However, hGGNs and rPSNs have different biological behaviors and natural histories based on both previous reports and our studies. The growth of the solid component in SSNs should not be determined solely by the increase in the LW, and an additional definition is needed.
Age, initial nodule diameter, and LW‐CTR were found to be significant risk factors for SSN growth, which is consistent with previously reported findings. 1 , 11 , 17 , 24 , 32 Concurrently, we further investigated the relationship between LW‐CTR/MW‐CTR and nodule growth. Previous results showed that with initial LW‐CTR >0, all tumors grew within 3 years, whereas 16% of SSNs with initial LW‐CTR = 0 grew beyond 3 years. Seventy percent of initial CTR >25% and 4% of CTR 0% had invasive carcinoma. 24 In our long follow‐up data, among those growth SSNs with MW‐CTR >0 (rPSNs), only 76.6% (23/30) grew within 3 years, while 93.3% (28/30) grew within 5 years. Similarly, based on the initial CTR, 95.5% (21/22) of patients with pGGNs had a final outcome of MIA + IAC, whereas 90.6% (29/32) of patients with LW‐CTR >0 were MIA + IAC. A total of 85.7% (18/21) of the final pathologies among patients with hGGNs at the initial state were MIA and IAC, while 100% (11/11) of rPSNs were invasive carcinoma (MIA and IAC). Consequently, it is evident from the LW‐CTR and MW‐CTR that different types of SSNs must be managed differently based on the solid components of the LWs and MWs. CTR also has a high reference value for nodule management.
Nevertheless, since the natural history of SSNs with long‐term follow‐up beyond 5 years is currently unknown, the appropriate duration of follow‐up needs to be determined. According to the most recent research, the total follow‐up period for SSNs has been extended to 5 years, 7 and long‐term follow‐up studies spanning up to 10 years have revealed that some SSNs continue to grow after 5 years of stability. 9 , 12 Our results validate this consensus. In terms of SSN growth, the three types of SSN exhibited significantly different progression times, with a median progression time of 7.7 years for pGGNs, 6.0 years for hGGNs, and 2.0 years for rPSNs. According to current management guidelines, the recommended 5‐year follow‐up may be somewhat arbitrary. 8 Given the comprehensive stepwise natural course of SSN growth in this study, due to its infrequent and consistently sluggish biological behavior, CT follow‐up for patients with pGGNs should be at least 7–9 years. Patients with hGGNs must be followed for 5–7 years, while those with rPSNs must be followed for 3–5 years to define any growth. In light of these findings, surveillance CT scans may be performed less frequently, or follow‐up examinations may be discontinued in some patients if no nodular growth is observed, as previously described during follow‐up. 33
Our research has several limitations. First, this was a retrospective study with data from only two institutions, which may introduce bias to the selection process. Second, this study only included Asian populations, which may have distinct demographic differences from white populations. Third, not all growing nodules have their malignant nature confirmed by pathology. Consequently, the criteria for surgical intervention following the development of nodules warrant additional research. Fourth, although two‐dimensional scale measurement is frequently used clinically to judge nodule growth, as well as in our study, it is unable to accurately evaluate the size change of SSNs and their volume doubling time, which warrant additional research. Finally, only clinical and conventional CT parameters were used in this study. In the future it should be possible to investigate the natural course of SSNs by radiomic or delta (longitudinal analysis).
In conclusion, this retrospective study confirmed that three SSN types classified by LWs and MWs have distinct natural histories, that hGGNs are an intermediate form, and that the evolutionary relationship between pGGN, hGGN, and PSN may be hierarchical. PSNs should therefore be further subdivided into hGGNs and rPSNs, which will not only benefit the precise management of PSNs but will also provoke discussion involving the conditional interpretation of the solid components of SSNs. Age, initial nodule diameter, and LW‐CTR are independent risk factors for SSN growth.
This study may provide new insights into the long‐term natural course of SSN growth at different intervals and contribute to the development of follow‐up guidelines for lung SSNs within lung cancer screening programs and management strategies.
AUTHOR CONTRIBUTIONS
Zhedong Zhang: Conceptualization, data collection, formal analysis, methodology, investigation, writing – original draft, writing – review and editing. Li Xinzhou: Conceptualization, methodology, investigation, writing – review and editing, supervision. Xianjun Min: Resources, methodology, investigation. Hao Li: Conceptualization, methodology, investigation. Qingyi Qi: Resources, methodology, radiological data collection, investigation. Chao Sun: Resources, radiological data collection, investigation. Kunkun Sun: Resources, pathological data collection, investigation. Fan Yang: Conceptualization, funding acquisition, supervision, project administration. Xiao Li: Conceptualization, writing – review and editing, funding acquisition, supervision, project administration.
FUNDING INFORMATION
This work was supported by the Beijing Municipal Natural Science Foundation (grant number 7212122), the National Natural Science Foundation of China (grant number 92059203), and the Science Foundation of AMHT (grant number 2021YK04).
CONFLICT OF INTEREST STATEMENT
The authors declare no commercial or financial relationships that could be construed as a potential conflict of interest existed during the conduct of the research.
Supporting information
Supporting Information Figure S1. Category of subsolid nodules status conversion. (a) A new solid component (yellow arrow) appeared in the lung window of a pure ground‐glass nodule (pGGN). During the regular follow‐up period of 35 months, the pGGN developed into a heterogeneous ground‐glass nodule (hGGN). (b) A new solid component (yellow arrow) appeared in the mediastinum window (MW) of the pGGN. During the follow‐up period of 62 months, the pGGN developed into a real part‐ solid nodule (rPSN). (c) A new solid component (yellow arrow) appeared in the MW of the hGGN. During the follow‐up period of 33 months, the hGGN developed into an rPSN. (d) Differences in the time of status conversion are statistically significant.
Zhang Z, Zhou L, Min X, Li H, Qi Q, Sun C, et al. Long‐term follow‐up of persistent pulmonary subsolid nodules: Natural course of pure, heterogeneous, and real part‐solid ground‐glass nodules. Thorac Cancer. 2023;14(12):1059–1070. 10.1111/1759-7714.14845
Zhedong Zhang and Lixin Zhou contributed equally to this work and share first authorship.
Contributor Information
Fan Yang, Email: yangfan@pku.edu.cn.
Xiao Li, Email: dr.lixiao@163.com.
REFERENCES
- 1. Kim YW, Kwon BS, Lim SY, Lee YJ, Park JS, Cho Y, et al. Lung cancer probability and clinical outcomes of baseline and new subsolid nodules detected on low‐dose CT screening. Thorax. 2021;76(10):980–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Resp J. 2013;42(6):1659–67. [DOI] [PubMed] [Google Scholar]
- 3. Kakinuma R, Muramatsu Y, Kusumoto M, Tsuchida T, Tsuta K, Maeshima AM, et al. Solitary pure ground‐glass nodules 5 mm or smaller: frequency of growth. Radiology. 2015;276(3):873–82. [DOI] [PubMed] [Google Scholar]
- 4. Henschke CI, Yip R, Smith JP, Wolf AS, Flores RM, Liang M, et al. CT screening for lung cancer: part‐solid nodules in baseline and annual repeat rounds. Am J Roentgenol. 2016;207(6):1176–84. [DOI] [PubMed] [Google Scholar]
- 5. Lee JH, Park CM, Lee SM, Kim H, McAdams HP, Goo JM. Persistent pulmonary subsolid nodules with solid portions of 5 mm or smaller: their natural course and predictors of interval growth. Eur Radiol. 2016;26(6):1529–37. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi Y, Fukui T, Ito S, Usami N, Hatooka S, Yatabe Y, et al. How long should small lung lesions of ground‐glass opacity be followed? J Thorac Oncol. 2013;8(3):309–14. [DOI] [PubMed] [Google Scholar]
- 7. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung A, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017;284(1):228–43. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network . National Comprehensive Cancer Network Clinical Practice Guidelines in Non‐Small Cell Lung Cancer. Version 1 2022. Accessed 25 Dec 2021.https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450.
- 9. Lee HW, Jin K, Lee J, Kim DK, Chung HS, Heo EY, et al. Long‐term follow‐up of ground‐glass nodules after 5 years of stability. J Thorac Oncol. 2019;14(8):1370–7. [DOI] [PubMed] [Google Scholar]
- 10. Herskovitz E, Solomides C, Barta J, Evans N, Kane G. Detection of lung carcinoma arising from ground glass opacities (GGO) after 5 years ‐ a retrospective review. Respir Med. 2022;196:106803. [DOI] [PubMed] [Google Scholar]
- 11. Tang E, Chen C, Wu CC, Wu M, Yang T, Liang H, et al. Natural history of persistent pulmonary subsolid nodules: long‐term observation of different interval growth. Heart Lung Circ. 2019;28(11):1747–54. [DOI] [PubMed] [Google Scholar]
- 12. Lee JH, Lim WH, Hong JH, Nam JG, Hwang EJ, Kim H, et al. Growth and clinical impact of 6‐mm or larger subsolid nodules after 5 years of stability at chest CT. Radiology. 2020;295(2):448–55. [DOI] [PubMed] [Google Scholar]
- 13. Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part‐solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11(8):1204–23. [DOI] [PubMed] [Google Scholar]
- 14. van Riel SJ, Sánchez CI, Bankier AA, Naidich DP, Verschakelen J, Scholten ET, et al. Observer variability for classification of pulmonary nodules on low‐dose CT images and its effect on nodule management. Radiology. 2015;277(3):863–71. [DOI] [PubMed] [Google Scholar]
- 15. Kakinuma R, Noguchi M, Ashizawa K, Kuriyama K, Maeshima AM, Koizumi N, et al. Natural history of pulmonary subsolid nodules: a prospective multicenter study. J Thorac Oncol. 2016;11(7):1012–28. [DOI] [PubMed] [Google Scholar]
- 16. Lai J, Li Q, Fu F, Zhang Y, Li Y, Liu Q, et al. Subsolid lung adenocarcinomas: radiological, clinical and pathological features and outcomes. Semin Thorac Cardiovasc Surg. 2022;34(2):702–10. [DOI] [PubMed] [Google Scholar]
- 17. Gao C, Yan J, Luo Y, Wu L, Pang P, Xiang P, et al. The growth trend predictions in pulmonary ground glass nodules based on radiomic CT features. Front Oncol. 2020;10:580809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo X, Jia X, Zhang D, Feng H, Dou Y, Shi G. Indeterminate pulmonary subsolid nodules in patients with no history of cancer: growing prediction. Diagn Interv Radiol. 2022;28(3):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kobayashi Y, Sakao Y, Deshpande GA, Fukui T, Mizuno T, Kuroda H, et al. The association between baseline clinical–radiological characteristics and growth of pulmonary nodules with ground‐glass opacity. Lung Cancer. 2014;83(1):61–6. [DOI] [PubMed] [Google Scholar]
- 20. Revel MP, Mannes I, Benzakoun J, Guinet C, Léger T, Grenier P, et al. Subsolid lung nodule classification: a CT criterion for improving interobserver agreement. Radiology. 2018;286(1):316–25. [DOI] [PubMed] [Google Scholar]
- 21. Matsuguma H, Mori K, Nakahara R, Suzuki H, Kasai T, Kamiyama Y, et al. Characteristics of subsolid pulmonary nodules showing growth during follow‐up with CT scanning. Chest. 2013;143(2):436–43. [DOI] [PubMed] [Google Scholar]
- 22. Hiramatsu M, Inagaki T, Inagaki T, Matsui Y, Satoh Y, Okumura S, et al. Pulmonary ground‐glass opacity (GGO) lesions‐large size and a history of lung cancer are risk factors for growth. J Thorac Oncol. 2008;3(11):1245–50. [DOI] [PubMed] [Google Scholar]
- 23. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. [DOI] [PubMed] [Google Scholar]
- 24. Sawada S, Yamashita N, Sugimoto R, Ueno T, Yamashita M. Long‐term outcomes of patients with ground‐glass opacities detected using CT scanning. Chest. 2017;151(2):308–15. [DOI] [PubMed] [Google Scholar]
- 25. Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–e120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bai C, Choi CM, Chu CM, Anantham D, Chung‐Man HJ, Khan AZ, et al. Evaluation of pulmonary nodules: clinical practice consensus guidelines for Asia. Chest. 2016;150(4):877–93. [DOI] [PubMed] [Google Scholar]
- 27. American College of Radiology . Lung‐RADS Version 1.1. 2019. https://www.acr.org/‐/media/ACR/Files/RADS/Lung‐RADS/LungRADSAssessmentCategoriesv1‐1.pdf?la=en Accessed June 21, 2019.
- 28. Jeong HLJKYKMCCYY. Korean Society of Thoracic Radiology Guideline for lung cancer screening with low‐dose CT. J Korean Soc Radiol. 2012;67(issue):349–65. 10.3348/jksr.2012.67.5.349 [DOI] [Google Scholar]
- 29. Wu F, Chen P, Wu CC, Kuo P, Tsao S, Chien C, et al. Semiquantative visual assessment of sub‐solid pulmonary nodules ≦3 cm in differentiation of lung adenocarcinoma spectrum. Sci Rep. 2017;7(1):15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin J, Xi J, Liang J, Zhan C, Jiang W, Lin Z, et al. Solid components in the mediastinal window of computed tomography define a distinct subtype of subsolid nodules in clinical stage I lung cancers. Clin Lung Cancer. 2021;22(4):324–31. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Sun Z, Xiao R, Qi Q, Li X, Huang H, et al. Stepwise evolutionary genomics of early‐stage lung adenocarcinoma manifesting as pure, heterogeneous and part‐solid ground‐glass nodules. Br J Cancer. 2022;127(4):747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qi L, Wu B, Tang W, Zhou L, Huang Y, Zhao S, et al. Long‐term follow‐up of persistent pulmonary pure ground‐glass nodules with deep learning–assisted nodule segmentation. Eur Radiol. 2020;30(2):744–55. [DOI] [PubMed] [Google Scholar]
- 33. Wu L, Gao C, Kong N, Lou X, Xu M. The long‐term course of subsolid nodules and predictors of interval growth on chest CT: a systematic review and meta‐analysis. Eur Radiol. 2022;33(3):2075–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1. Category of subsolid nodules status conversion. (a) A new solid component (yellow arrow) appeared in the lung window of a pure ground‐glass nodule (pGGN). During the regular follow‐up period of 35 months, the pGGN developed into a heterogeneous ground‐glass nodule (hGGN). (b) A new solid component (yellow arrow) appeared in the mediastinum window (MW) of the pGGN. During the follow‐up period of 62 months, the pGGN developed into a real part‐ solid nodule (rPSN). (c) A new solid component (yellow arrow) appeared in the MW of the hGGN. During the follow‐up period of 33 months, the hGGN developed into an rPSN. (d) Differences in the time of status conversion are statistically significant.


