Abstract
Background
Clostridioides difficile (formerly known as Clostridium difficile) is a bacterium that can cause potentially life‐threatening diarrheal illness in individuals with an unhealthy mixture of gut bacteria, known as dysbiosis, and can cause recurrent infections in nearly a third of infected individuals. The traditional treatment of recurrent C difficile infection (rCDI) includes antibiotics, which may further exacerbate dysbiosis. There is growing interest in correcting the underlying dysbiosis in rCDI using of fecal microbiota transplantation (FMT); and there is a need to establish the benefits and harms of FMT for the treatment of rCDI based on data from randomized controlled trials.
Objectives
To evaluate the benefits and harms of donor‐based fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile infection in immunocompetent people.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 31 March 2022.
Selection criteria
We considered randomized trials of adults or children with rCDI for inclusion. Eligible interventions must have met the definition of FMT, which is the administration of fecal material containing distal gut microbiota from a healthy donor to the gastrointestinal tract of a person with rCDI. The comparison group included participants who did not receive FMT and were given placebo, autologous FMT, no intervention, or antibiotics with activity against C difficile.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. proportion of participants with resolution of rCDI and 2. serious adverse events. Our secondary outcomes were 3. treatment failure, 4. all‐cause mortality, 5. withdrawal from study, 6. rate of new CDI infection after a successful FMT, 7. any adverse event, 8. quality of life, and 9. colectomy. We used the GRADE criteria to assess certainty of evidence for each outcome.
Main results
We included six studies with 320 participants. Two studies were conducted in Denmark, and one each in the Netherlands, Canada, Italy, and the US. Four were single‐center and two were multicenter studies. All studies included only adults. Five studies excluded people who were severely immunocompromised, with only one study including 10 participants who were receiving immunosuppressive therapy out of the 64 enrolled; these were similarly distributed between the FMT arm (4/24 or 17%) and comparison arms (6/40 or 15%). The route of administration was the upper gastrointestinal tract via a nasoduodenal tube in one study, two studies used enema only, two used colonoscopic only delivery, and one used either nasojejunal or colonoscopic delivery, depending on a clinical determination of whether the recipient could tolerate a colonoscopy. Five studies had at least one comparison group that received vancomycin. The risk of bias (RoB 2) assessments did not find an overall high risk of bias for any outcome.
All six studies assessed the efficacy and safety of FMT for the treatment of rCDI.
Pooled results from six studies showed that the use of FMT in immunocompetent participants with rCDI likely leads to a large increase in resolution of rCDI in the FMT group compared to control (risk ratio (RR) 1.92, 95% confidence interval (CI) 1.36 to 2.71; P = 0.02, I2 = 63%; 6 studies, 320 participants; number needed to treat for an additional beneficial outcome (NNTB) 3; moderate‐certainty evidence). Fecal microbiota transplantation probably results in a slight reduction in serious adverse events; however, the CIs around the summary estimate were wide (RR 0.73, 95% CI 0.38 to 1.41; P = 0.24, I² = 26%; 6 studies, 320 participants; NNTB 12; moderate‐certainty evidence). Fecal microbiota transplantation may result in a reduction in all‐cause mortality; however, the number of events was small, and the CIs of the summary estimate were wide (RR 0.57, 95% CI 0.22 to 1.45; P = 0.48, I2 = 0%; 6 studies, 320 participants; NNTB 20; low‐certainty evidence). None of the included studies reported colectomy rates.
Authors' conclusions
In immunocompetent adults with rCDI, FMT likely leads to a large increase in the resolution of recurrent Clostridioides difficile infection compared to alternative treatments such as antibiotics. There was no conclusive evidence regarding the safety of FMT for the treatment of rCDI as the number of events was small for serious adverse events and all‐cause mortality. Additional data from large national registry databases might be required to assess any short‐term or long‐term risks with using FMT for the treatment of rCDI. Elimination of the single study that included some immunocompromised people did not alter these conclusions. Due to the low number of immunocompromised participants enrolled, conclusions cannot be drawn about the risks or benefits of FMT for rCDI in the immunocompromised population.
Keywords: Adult, Child, Humans, Anti-Bacterial Agents, Anti-Bacterial Agents/therapeutic use, Clostridioides, Clostridioides difficile, Clostridium Infections, Clostridium Infections/drug therapy, Clostridium Infections/microbiology, Dysbiosis, Fecal Microbiota Transplantation, Fecal Microbiota Transplantation/adverse effects, Quality of Life, Recurrence, Treatment Outcome
Plain language summary
Stool transplantation for treatment of repeated Clostridioides difficile infection
Review question
We reviewed the evidence about the effect of stool transplant compared to currently used treatments such as antibiotics for the treatment of recurrent C difficile diarrhea in adults and children.
What is Clostridioides difficile infection and how is it treated?
Clostridioides difficile (C difficile) infection is a common bacterial illness that can cause life‐threatening diarrhea (runny stools). Evidence suggests that an unhealthy mixture of gut bacteria called dysbiosis may increase the risk of repeated or multiple C difficile infections. Changing from an unhealthy to a healthier balance of gut bacteria through treatment may protect people from becoming sick with C difficile, or prevent repeated infections with this bacterium. Stool administration from healthy donors to people who have had multiple infections with C difficile, known as fecal microbiota transplantation (FMT), is an intervention that seeks to change an unhealthy mixture of gut microbes into a healthy balance of gut microbes.
What did we want to find out?
We wanted to discover whether using FMT in people with multiple C difficile infections leads to a higher percentage of resolution of the infection compared to commonly used therapies such as antibiotics and whether FMT may cause harm.
What did we do?
We searched medical databases for clinical trials looking at stool transplantation compared to currently used treatments such as antibiotics for the treatment of recurrent C difficile diarrhea in adults and children.
What did we find?
We found six clinical trials of 320 adults that met criteria for inclusion in this review that assessed the efficacy and safety of stool transplantation for the treatment of repeated C difficile infection. Two studies were conducted in Denmark, and one each in the Netherlands, Italy, Canada, and the US. The time of follow‐up after the treatment with FMT ranged from eight weeks to 17 weeks. The amount of stool, route of administration, number of administrations, type of donor, and what type of treatment the comparison group received varied among the studies. Five studies excluded people who had weak immune systems (immunocompromised people); one study included people with weak immune systems and apparently normal immune systems (immunocompetent people).
Key results
Stool transplantation probably leads to a larger increase in resolution of repeated infections of C difficile than the other treatments studied. Other treatments included antibiotics such as vancomycin, which are commonly prescribed for this infection. These same studies looked at the rate of serious side effects and risk of death from FMT. Fecal microbiota transplantation likely leads to a small decrease in serious side effects; however, these effects were few. Fecal microbiota transplantation may decrease the risk of death in people with rCDI; however, there were few deaths in either group. Elimination of one study that included some immunocompromised people did not alter these conclusions, but, based on the low number of immunocompromised people enrolled in the included studies, conclusions could not be drawn about the benefits or harms of FMT for rCDI in the immunocompromised population at this time.
What are the limitations of the evidence?
We rated the overall certainty of the evidence using a set of criteria that takes into account the type of studies, potential flaws in how the studies were run, how similar or different reporting of the results was between studies, how studies measured the effect of the intervention, and mathematical confidence in the combined results. Based on these criteria, we judged the overall certainty of the evidence supporting stool transplants as more effective than other treatments for the resolution of repeated C difficile infection as moderate. The certainty of evidence for serious side effects was moderate and the certainty of evidence for deaths was low.
Study funding sources
None of the included studies was funded by a drug manufacturer or an agency that had a commercial interest in FMT.
How up to date is this evidence?
The evidence is current to 31 March 2022.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Fecal microbiota transplantation (FMT) compared to control in adults with recurrent Clostridioides difficile infection (rCDI).
| Fecal microbiota transplantation (FMT) compared to control in adults with recurrent Clostridioides difficile infection (rCDI) | ||||||
| Patient or population: adults with recurrent Clostridioides difficile infection (rCDI) Setting: inpatient and outpatient Intervention: fecal microbiota transplantation (FMT) Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with fecal microbiota transplantation (FMT) | |||||
| Resolution of rCDI follow‐up: range 8 weeks to 17 weeks | 401 per 1000 | 770 per 1000 (545 to 1000) | RR 1.92 (1.36 to 2.71) | 320 (6 RCTs) | ⊕⊕⊕⊝ Moderatea,b,c | FMT likely results in a large increase in resolution of rCDI. |
| Serious adverse events follow‐up: range 8 weeks to 17 weeks | 225 per 1000 | 164 per 1000 (85 to 317) | RR 0.73 (0.38 to 1.41) | 320 (6 RCTs) | ⊕⊕⊕⊝ Moderated | FMT probably results in a slight reduction in serious adverse events; however, the CIs around the summary estimate were wide and included a possibility of increased risk of serious adverse events. |
| All‐cause mortality follow‐up: range 8 weeks to 17 weeks | 96 per 1000 | 55 per 1000 (21 to 140) | RR 0.57 (0.22 to 1.45) | 320 (6 RCTs) | ⊕⊕⊝⊝ Lowe | FMT may result in a reduction in all‐cause mortality; however, the CIs around the summary estimate were wide and possible risk of increased mortality could not be ruled out. |
| Colectomy | 0 per 1000 | 0 per 1000 (0 to 0) | Not estimable | (0 studies) | ‐ | None of the included studies reported this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_432555373835069207. | ||||||
a We did not downgrade for risk of bias for this outcome. Even though two included studies in this analysis did not describe their methods of randomization in clear detail, the study groups were balanced at the start of the study. A sensitivity analysis by excluding these studies from the meta‐analysis for this outcome did not change the direction or statistical significance of the summary estimate. We also acknowledge that five of the six studies were open‐label. The outcome was defined with a combination of clinical symptoms and negative test in most of the studies so it is less likely that lack of blinding biased the results. b Even though the statistical heterogeneity based on I2 values was 63% in the pooled analysis, the direction of effect was in favor of FMT in five out of six studies included in the analysis. Therefore, we did not downgrade for statistical heterogeneity. c Downgraded one level due to imprecision. The CIs around the summary estimate were wide and included a small to a very large increase of resolution of rCDI. d Downgraded one level due to imprecision. The number of events was small and the CIs around the summary estimate were wide. e Downgraded two levels due to imprecision. The number of events was small and the CIs around the summary estimate were very wide and included a possibility of lower or increased risk of mortality.
Background
Description of the condition
Clostridioides difficile (formerly known as Clostridium difficile) is a spore‐forming, gram‐positive, obligate anaerobic bacillus bacterium (Lawson 2016). It is acquired via fecal‐oral transmission of spores shed in the stools of infected or colonized people, which can be transmitted via contact with any surface. C difficile is the most frequently reported nosocomial pathogen in the US, as healthcare facilities such as hospitals, nursing homes, and childcare facilities are major sources of transmission (Leffler 2015; Red Book 2018). C difficile infection (CDI) is defined by the presence of diarrheal symptoms, and a stool test positive for C difficile toxins, detection of toxigenic C difficile, or colonoscopic or histopathologic findings revealing pseudomembranous colitis (Crobach 2018; McDonald 2018; Red Book 2018). Asymptomatic C difficile colonization is the detection of the organism without the symptoms of the disease (Crobach 2018). Asymptomatic C difficile colonization is especially common in children under two years of age and testing in this age group is discouraged unless other infectious and non‐infectious causes of diarrhea have been excluded (McDonald 2018). The known risk factors for CDI include antimicrobial therapy, proton pump inhibitor therapy, prolonged nasogastric tube placement, gastrostomy and jejunostomy tube placement, inflammatory bowel disease, gastrointestinal tract surgery, chronic kidney disease, repeated enemas, advanced age, organ transplantation, and immunocompromised states (Crobach 2018; Davidovics 2019; McDonald 2018; Red Book 2018). Treatment with antibiotics increases the risk of CDI, as antibiotics decrease the taxonomic richness, diversity, and evenness of the intestinal microbiota community, providing a niche for C difficile to flourish, as toxigenic strains of C difficile are favored by disturbances in the ecology of intestinal microbiota (Chang 2008; Dethlefsen 2008; Fekety 1993).
First‐line treatment of CDI involves antibiotics (Davidovics 2019; McDonald 2018; Leffler 2015; Red Book 2018). Once an individual has developed CDI, they are at risk for recurrent C difficile infections (rCDI), which occur in 20% to 30% of individuals treated with antibiotics for an initial episode of CDI and rates increase up to 60% after the second recurrence (Davidovics 2019; Kelly 2008). Recurrent C difficile infections may occur either from the germination of spores from prior CDI or from reinfection with a different strain of C difficile acquired from human or environmental contacts (Bakken 2011; Fekety 1993). The definition of rCDI is an episode that fulfills the criteria for CDI (both diarrheal symptoms and either positive laboratory testing, colonoscopic or histopathologic findings of pseudomembranous colitis [or both]) and occurs between two and eight weeks after treatment for a previous episode of CDI, provided that the symptoms of the earlier episode initially resolved (McDonald 2007; McDonald 2018). This definition excludes any repeat positive laboratory result for C difficile within two weeks after the last specimen that tested positive, as this likely represents a continuation of the same CDI case (McDonald 2007). Treatment failure of CDI is defined as no response after one week of treatment with appropriate antibiotics (Shannon‐Lowe 2010; Vardakas 2012). One systematic review for the treatment of CDI found a treatment failure rate of 22.4% for metronidazole and 14.2% for vancomycin (Vardakas 2012).
Description of the intervention
Most current guidelines recommend further antibiotics for the treatment of a first and second recurrence of non‐severe CDI (Al Momani 2018; McDonald 2018; Mullish 2018; Red Book 2018), before recommending FMT (Bakken 2011; McDonald 2018). However, this approach might be changing following a recommendation of FMT after the first recurrence of CDI in the 2021 American College of Gastroenterology guidelines (ACG Clinical Guidelines 2021). Fecal microbiota transplantation has been defined as the administration of fecal material containing distal gut microbiota from a healthy donor to a person with a disease or condition related to dysbiosis or an alteration in their normal gut microbiota (Kelly 2015). Fecal microbiota transplantation involves the selection and screening of a donor and the appropriate selection and preparation of the recipient. There is no universally agreed‐upon donor screening method, but most centers perform an interview to screen for chronic disease states along with blood and stool tests to rule out a variety of infectious diseases (Woodworth 2017). Stool specimens are also commercially available from stool banks. After appropriate screening, donor stool is collected, mixed with a solvent, and sometimes filtered, then either administered on the same day or frozen for later use. The patient is usually given a laxative or undergoes a bowel lavage prior to the procedure (Cammarota 2017; Davidovics 2019). An FMT can be administered via a colonoscopy, an enema, orally ingested capsules, a gastrostomy tube, a jejunostomy tube, or a temporary nasoduodenal or nasogastric tube (Cammarota 2017; Davidovics 2019; McDonald 2018; Imdad 2018; Jiang 2018a; Kao 2017; Lee 2016). The US Food and Drug Administration (FDA) considers FMT as an investigational procedure and requires an Investigational New Drug application for any use of FMT other than treatment of rCDI, where the FDA exercises enforcement discretion (FDA 2013).
How the intervention might work
Exposure to C difficile spores alone, either through new inoculation or asymptomatic carriage, is thought to be insufficient to cause CDI, necessitating coexisting dysbiosis for CDI and rCDI to occur (Kociolek 2016). Dysbiosis is broadly defined as any alteration in the composition of resident commensal bacteria communities as compared to the communities found in healthy individuals. Dysbiosis leads to loss of microbial diversity and beneficial microbes, and expansion of potentially harmful microbes (Petersen 2014). Individuals with conditions correlated with dysbiosis have higher CDI rates than the general population, including those who have recently received antibiotics, people with inflammatory bowel disease, and people receiving chemotherapy (Johnsen 2018; McDonald 2018; Petersen 2014; Razik 2016). Treatment for non‐severe, uncomplicated CDI and rCDI in low‐risk patients includes discontinuation of antibiotics that may have caused or exacerbated dysbiosis and initiation of antibiotics with activity against C difficile such as vancomycin and fidaxomicin (McDonald 2018; Red Book 2018). However, antibiotics can potentiate further dysbiosis, leading to additional episodes of rCDI (Davidovics 2019; Kelly 2008; Kociolek 2016). The ideal treatment of rCDI should attempt to restore a healthy, diverse intestinal microbiota milieu that will protect against further episodes of rCDI (Kelly 2008; Kociolek 2016). While probiotics are a potential mechanism to change the host microbiome, they are not thought to be effective as monotherapy for active CDI or to prevent rCDI, and high‐quality, robust evidence to support their use is lacking (ACG Clinical Guidelines 2021; Davidovics 2019; Kelly 2008; McDonald 2018). Fecal microbiota transplantation is likely the most effective treatment for rCDI and has become part of the standard‐of‐care treatment algorithms for rCDI in both adults and children (ACG Clinical Guidelines 2021; Bakken 2011; Davidovics 2019; Kellermayer 2019; McDonald 2018). Fecal microbiota transplantation attempts to correct dysbiosis by altering the recipient's microbiome via the 'transplantation' of a healthy donor's microbiota (Cammarota 2017), which in the case of rCDI, can eliminate the niche that C difficile is able to exploit. Fecal microbiota transplantation significantly decreases dysbiosis and increases gut microbial diversity in individuals with rCDI (Kelly 2016; Khanna 2017).
While FMT has the potential to correct dysbiosis, there is concern that pathogenic micro‐organisms could be introduced, causing undesirable outcomes (Alang 2015; Cammarota 2017). Serious adverse events, including mortality, septic shock, aspiration pneumonia, and toxic megacolon have been reported (Kelly 2014; Link 2016; Solari 2014). The FDA has issued a safety alert regarding the risk of serious adverse events including mortality from the transmission of multiple‐drug‐resistant organisms (FDA 2020a), and provided additional guidance in regard to the risk of infection from SARS‐CoV‐2 (strain of coronavirus that causes COVID‐19 [coronavirus disease 2019]) (FDA 2020b).
Why it is important to do this review
Clostridioides difficile was associated with almost 250,000 infections and approximately 12,800 deaths in the US in 2017 alone (CDC 2019). It is the most common healthcare‐associated infection and the leading cause of gastroenteritis‐associated death; the cost of managing CDI was estimated at 1 billion US dollars in the US in 2017 (CDC 2019; Lessa 2015). While there is a paucity of data on the incidence of CDI from outside North America, Europe, and the Western Pacific, one meta‐analysis estimated the worldwide incidence rate of healthcare facility‐associated CDI rate for patients of all ages to be 2.24 per 1000 admissions per year (Balsells 2019).
Data from observational studies show that FMT might cure more than 90% of cases with rCDI (Kassam 2013; Quraishi 2017). Such high efficacy of an intervention to cure a recurrent disease is very appealing; however, these findings need to be confirmed with data from randomized controlled trials (RCTs). Data from RCTs to define the efficacy of FMT against the standard of care have recently become available (Cammarota 2015; Hota 2017; Hvas 2019; Kelly 2016; Rode 2021; van Nood 2013). Thus, there is a need to assess this evidence in a systematic review and meta‐analysis. While systematic reviews have been performed on the efficacy of FMT for rCDI, most have included observational studies, and none have used Cochrane methodology while simultaneously incorporating the Cochrane RoB 2 tool and the GRADE criteria (Drekonja 2015; Hui 2019; Khan 2018; Quraishi 2017). Therefore, we conducted a comprehensive, up‐to‐date systematic review to assess the efficacy of donor‐based FMT versus other treatments for the treatment of rCDI.
Objectives
To evaluate the benefits and harms of donor‐based fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile infection in immunocompetent people.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs assessing FMT for the treatment of rCDI. We included trials with multiple arms, as long as these included an intervention and comparison group that addressed the primary question for this review. We planned to include both cross‐over and cluster‐randomized trials; however, there were none that met criteria for inclusion. We excluded observational studies, case reports, and case series.
Types of participants
We included studies of participants with rCDI. We considered the definition of CDI as any person with watery or frequent (or both) stools (more than two or three loose stools per day), who simultaneously had either a positive stool test for C difficile or colonoscopic or histopathologic findings (or both) of pseudomembranous colitis (McDonald 2007; McDonald 2018). A case met criteria for rCDI when the person met criteria for CDI, received treatment for CDI with antibiotics known to have activity against C difficile (generally metronidazole, vancomycin, fidaxomicin, or a combination of these), their diarrhea initially resolved, then the diarrhea recurred with any C difficile test simultaneously being positive. This would theoretically occur in a period of two to eight weeks from the previously documented positive C difficile stool test (McDonald 2007; McDonald 2018). We considered both children and adults. We included participants in both hospital and community settings. We did not include studies that exclusively enrolled immunocompromised people. We excluded studies that relied on clinical symptoms without laboratory confirmation when defining rCDI, as one study observed that approximately 25% (29/117) of participants with presumed rCDI referred for work‐up for FMT were found to have a non‐CDI diagnosis, with irritable bowel syndrome and inflammatory bowel disease being the most common alternative diagnoses (Jackson 2016).
Studies differed in the number of rCDI episodes prior to offering FMT to participants. We included studies in our analysis that provided FMT for rCDI regardless of the number of recurrences, but excluded studies where the participant received FMT as treatment for their first case of CDI, as this is not the standard of care at the time of this analysis. In defining rCDI, we did not insist on studies documenting a negative microbiologic test after treatment of CDI before the development of a recurrence as a 'test of cure' in asymptomatic participants, as this is not the standard of care, and a person might carry C difficile without having active symptoms (Davidovics 2019; McDonald 2018).
There are areas of ambiguity regarding CDI testing based on the limitations of available testing modalities. One area of ambiguity is the differentiation between true CDI/rCDI and carriers of C difficile who develop frequent or watery bowel movements (or both) for another reason but test positive for C difficile (Crobach 2018). Another challenge is how to compare C difficile testing strategies used in different trials, as there are a wide variety of testing modalities available, which vary in sensitivity and specificity. There is currently no gold standard laboratory test method available, and the evidence base to optimize testing is weak (Crobach 2018; McDonald 2018; Red Book 2018). Therefore, we accepted any form of positive stool testing for documentation of CDI and rCDI. A third area of ambiguity concerns differentiating between the 'recurrence' of the same C difficile infection from a second infection with a different strain of C difficile. One small study of people with rCDI found that 33% (6/18) of suspected rCDI episodes were due to infection with a different C difficile strain, while 67% (12/18) were true recurrences of the same strain of C difficile (Tang-Feldman 2003). As these two entities are practically indistinguishable without additional ribotyping, and the difference is clinically irrelevant with regard to treatment, we did not differentiate between these two entities, with the understanding that some 'recurrences' were likely new infections.
We included trials regardless of length of follow‐up; we planned that if the last recorded follow‐up date was shorter than eight weeks, it will be included in the eight‐week outcome data. The eight‐week time point is relevant as post‐FMT, this is the maximum time frame in which recurrence of symptoms may be considered as a recurrence of CDI (McDonald 2007). Theoretically, if recurrence of diarrheal symptoms and a repeat positive test for C difficile occur more than eight weeks after the previous positive test, this is consistent with a new CDI infection after a successful FMT as opposed to an episode of rCDI (McDonald 2007; McDonald 2018).
Types of interventions
We included studies that evaluated FMT for the treatment of rCDI. Fecal microbiota transplantation has been defined as the administration of fecal material containing distal gut microbiota from a healthy donor to a person with a disease or condition related to dysbiosis, or an alteration in their normal gut microbiota (Kelly 2015). We excluded studies that combined FMT with antibiotic treatment during or after the FMT but included studies that used antibiotics prior to FMT. The control group included those who received placebo, the standard of care antibiotic medications, other controls, autologous FMT, or no intervention. Furthermore, we included studies irrespective of the type of stool used (fresh versus thawed, previously frozen stool), volume of stool used, route of administration, number of FMT administrations (single versus multiple infusions), and the number of recurrences of CDI prior to FMT (as long as there was at least one recurrence).
For studies with multiple intervention groups (e.g. factorial design), we included the data such that the only difference between the two groups was donor FMT versus no‐donor FMT.
Types of outcome measures
Primary outcomes
Proportion of participants with a resolution of rCDI: we considered a participant fulfilling the definition of resolution of rCDI if studies reported either of the two criteria: diarrheal symptoms did not recur after treatment or repeat C difficile testing was negative.
Serious adverse events, as per the author's definition of a serious adverse event.
Secondary outcomes
A priori planned secondary outcomes:
Treatment failure: symptoms of CDI did not resolve after FMT treatment or that reoccurred within two weeks post‐FMT.
All‐cause mortality.
Proportion of participants who withdrew from the study.
Rate of new CDI infection after a successful FMT, with renewal of diarrheal symptoms and a repeat positive test for C difficile more than eight weeks after the previous positive test (McDonald 2007; McDonald 2018).
Any adverse event.
Quality of life score.
Colectomy.
We considered the primary and secondary outcomes at the longest follow‐up before the trial was open for analysis. We anticipated that trials would have a follow‐up period of at least six weeks. Additional details on definitions of certain primary and secondary outcomes discussed in protocol are available in Appendix 1.
Search methods for identification of studies
Electronic searches
We searched the following databases from their inception using the methods in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022):
Cochrane Central Register of Controlled Trials (CENTRAL, via Ovid; Issue 3, 2022) (Appendix 2);
MEDLINE (1946 via Ovid) (Appendix 3);
Embase (1974 via Ovid) (Appendix 4);
Conference Proceedings Citation Index (Appendix 5);
ISRTN Registry (www.isrctn.com/; Appendix 5).
The literature was conducted on 16 February 2021, and updated on 31 March 2022. We searched the Cochrane Gut Group Specialized Register in February 2021 only and not in March 2022.
Searching other resources
We searched ClinicalTrials.gov (www.clinicaltrials.gov/) for ongoing trials. We also searched the reference sections of previously published randomized trials and meta‐analyses on this topic. We contacted authors of published and ongoing studies to seek new or additional data when needed. Of note, ICTRP and ClinicalTrials.gov are both indexed in CENTRAL.
Data collection and analysis
Selection of studies
Two review authors (SHA and AI) independently screened the titles and abstracts of records retrieved from the search to identify potentially eligible studies. The same review authors reviewed the full text of all studies deemed potentially eligible and made a final decision as to inclusion or exclusion. They resolved any discrepancies by discussion and consensus or by consulting a senior review author if disagreement persisted. We used Covidence software to screen titles and abstracts.
Data extraction and management
Teams of two review authors (from SHA, MM, AI) independently extracted the following data into a pretested Microsoft Excel data extraction form (MS Excel 2018): study authors, date of publication, journal, site of the study, age of participants, definition of the study population (inclusion/exclusion criteria), details of intervention (type, volume, frequency, route of administration of fecal microbiota transplant, source), outcomes (primary and secondary outcomes), and risk of bias.
We extracted data on an intention‐to‐treat basis, which considers the initial allocation of participants to an intervention or control group irrespective of whether the participants received the intervention or completed the follow‐up (Gupta 2011).
Assessment of risk of bias in included studies
We used the Cochrane RoB 2 tool (current version 22 August 2019) to assess the risk of bias for outcomes of interest in all included studies in the analysis (Higgins 2020; Sterne 2019). The tool considers the following domains:
bias arising from the randomization process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in the measurement of the outcome;
bias in the selection of the reported result.
The RoB 2 tool also assesses overall risk of bias for an outcome. We used the RoB 2 assessment forms in an Excel tool to assess the risk of bias for each outcome (available at riskofbiasinfo.org). At least two review authors (SHA and MM) answered the signaling questions in the RoB 2 tool for each domain to assess the risk of bias separately for all included studies, for all outcomes reported in the summary of findings table, and the authors compared their assessments. The overall risk of bias was determined based on signaling question responses and any conflicts were discussed with one review author (AI) to reach a final decision. We present the risk of bias summary for each outcome in the results section and provided details regarding the justification for the risk of bias assessment in a supplemental data file. The risk of bias for each outcome was categorized as high risk of bias, some concerns, or low risk of bias. We assessed the risk of bias for outcomes included in the summary of findings table only, namely, resolution of rCDI, serious adverse events, and all‐cause mortality. We had planned to assess the risk of bias for the outcome of colectomy; however, none of the included studies reported on this outcome, so this assessment was not completed.
Measures of treatment effect
We calculated the risk ratio (RR) and associated 95% confidence interval (CI) for all dichotomous outcomes. All analyses from RCTs were conducted using an intention‐to‐treat analysis. We planned to calculate a pooled mean difference (MD) for the continuous outcomes and report them with a 95 % CI, but we did not identify any continuous outcomes.
Unit of analysis issues
If we had encountered cross‐over trials that were eligible for inclusion, we planned to include data from the first segment of the trial only, before the cross‐over occurred. If we had encountered any cluster‐randomized trials that were eligible for inclusion, we had planned to synthesize the findings from individually and cluster‐randomized trials into a single meta‐analysis. We planned to use the cluster adjusted values as reported by the study authors. If the authors did not adjust for the cluster design, we planned to adjust for this by decreasing the effective sample size per guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). However, we did not find any cross‐over trials or cluster‐randomized trials that met criteria for inclusion.
For trials with multiple arms, we aimed to include the data in a way that the intervention group received donor‐based FMT and the control group received interventions that did not include donor‐based FMT. For example, if a study had three study arms and one group received a donor‐based FMT, a second group received antibiotic therapy with vancomycin and a third group received antibiotic therapy with fidaxomicin, we included the data in the analysis as donor‐based FMT group versus vancomycin group and fidaxomicin group.
Dealing with missing data
Attrition is an important factor that can impact the validity of studies, and differential dropout rates between study groups can lead to biased estimates of effect size (Dumville 2006). We described the missing data, including dropouts and reasons for dropout, as reported by the study authors. We analyzed data from RCTs on an intention‐to‐treat basis, assuming participants with missing values for the outcomes were treatment failures. For the outcome of resolution of rCDI, this meant that participants lost to follow‐up were considered as not having experienced a resolution of rCDI and for the outcomes of serious adverse events and mortality, the participants lost to follow‐up were considered as having experienced those outcomes.
We anticipated that study authors may not have reported the standard deviation (SD) for means for continuous outcomes. If SDs had not been available for a mean value, we planned to contact the study authors to request this information. If we were unable to obtain the missing SD from the study authors, we would have calculated the SD from the available data, such as standard error or interquartile range. If no estimates of variance were available for a mean value, we would have used the SD from a similar study with similar sample size, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). However, as our analyses included no continuous outcomes, we did not have to implement these procedures.
Assessment of heterogeneity
We assessed the clinical, methodologic, and statistical heterogeneity amongst studies. We assessed methodologic heterogeneity by comparing components of the risk of bias assessment. We assessed statistical heterogeneity based on forest plots, the I2 statistic, and the P value for the Chi2 test. We considered heterogeneity to be significant if the P value for Chi2 was less than 0.10 or the I2 statistic was greater than 60%. We planned to explore potential explanations for heterogeneity using subgroup analyses to explore the distribution of important factors such as maximum number of doses of FMT, route of administration, and the source of FMT, but the number of studies was too small to complete the planned subgroup analyses.
Assessment of reporting biases
We planned to assess potential publication bias based on the symmetry of a funnel plot. We planned to construct funnel plots if the pooled analysis included at least 10 studies. However, there were no analyses with 10 or more studies, so we did not construct any funnel plots.
Data synthesis
We combined data from RCTs for meta‐analysis using Review Manager 5 (Review Manager 2014) and Review Manager Web (RevMan Web 2020). We pooled the data to obtain a summary estimate in the form of RR for dichotomous outcomes with 95% CIs. We used the random‐effects model to pool data but completed a sensitivity analysis employing a fixed‐effect model on all primary outcomes to see if this changed the conclusions. We used the intention‐to‐treat analysis from individual studies. If the intention‐to‐treat analysis was not reported in the study, we constructed the analysis using the raw values reported in the study. We considered the intention‐to‐treat analysis as the analysis for an outcome based on initial allocation to the intervention and control group after randomization, irrespective of whether a participant received the intervention or was lost to follow‐up. For the outcome of resolution of rCDI, this meant that participants lost to follow‐up were considered as not having experienced a resolution of rCDI and for the outcomes of serious adverse events and mortality, the participants lost to follow‐up were considered as having experienced those outcomes. We planned to pool continuous data to obtain a pooled MD with 95% CI if all the studies reported the continuous outcome in the same unit. If the studies used different units to report the continuous outcome, we planned to use the standardized mean difference (SMD) with a 95% CI; however, no relevant continuous outcomes were identified. We calculated the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) for all primary and selected secondary outcomes and reported the results for outcomes where the GRADE certainty was at least moderate level.
Subgroup analysis and investigation of heterogeneity
We planned to explore potential explanations for heterogeneity using subgroup analyses. We planned the following a priori subgroup analyses.
Clinical setting: outpatient versus hospitalized participants.
Storage of stool: fresh stool (of non‐stool bank origin) versus frozen then thawed stool (of stool bank origin).
Type of donor: related versus unrelated donor.
Source of stool: single donor versus pooled donor source of FMT.
Route of administration: upper (nasogastric, nasoduodenal, capsule) versus lower (enema, colonoscopy).
All subgroup analyses were at the study and not at the individual level. None of the subgroup analyses were conducted because the number of studies was small (fewer than 10).
Sensitivity analysis
We planned the following a priori sensitivity analyses.
Fixed‐effect model versus random‐effects model.
Studies with high risk of bias versus those with low risk of bias/some concerns.
None of the included studies were at high risk of bias so the second of these planned sensitivity analyses was not conducted.
Summary of findings and assessment of the certainty of the evidence
We assessed the overall certainty of the evidence supporting the primary and selected secondary outcomes using the GRADE criteria (Guyatt 2011). This method of evidence evaluation takes into consideration the impact of the type of studies and each study's risk of bias, indirectness, imprecision, inconsistency, and potential publication biases, providing a rating of the overall certainty of the evidence as high, moderate, low, or very low. We presented the GRADE evaluations as part of Table 1 for the outcomes of resolution of rCDI, serious adverse events, and all‐cause mortality. We had planned to present the GRADE evaluation for the outcome of colectomy, however, none of the included studies reported on this outcome, so this evaluation was not completed for this outcome. We considered the overall risk of bias for each outcome in our grading of the evidence. We provided explanations in the footnotes of the summary of findings table about our decision related to the allocation of certainty of the evidence for a certain outcome.
Results
Description of studies
Results of the search
An initial search was conducted on 16 February 2021 which was updated on 31 March 2022. We identified 1741 records. After removing 476 duplicates, we retained 1265 records for title and abstract screening. After excluding 1194 evidently irrelevant records we assessed 71 full‐text records. We excluded 33 studies (41 reports) for reasons outlined in the Characteristics of excluded studies table. Three studies are awaiting classification and 13 studies are ongoing studies. We included six studies (14 reports) in the review (Cammarota 2015; Hota 2017; Hvas 2019; Kelly 2016; Rode 2021; van Nood 2013). This is summarized in the PRISMA flow diagram (Figure 1).
1.
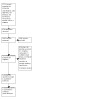
PRISMA flow diagram.
Included studies
Six RCTs assessed FMT for the treatment of rCDI (Cammarota 2015; Hota 2017; Hvas 2019; Kelly 2016; Rode 2021; van Nood 2013). See Characteristics of included studies table for full details.
Study type
All the studies were individual RCTs. Five studies were open‐label and one study was a double‐blinded (Kelly 2016). Four studies were single‐center (Cammarota 2015; Hota 2017; Hvas 2019; van Nood 2013), and two were multicenter studies (Kelly 2016; Rode 2021). Three studies had two intervention groups (Cammarota 2015; Hota 2017; Kelly 2016), and three had more than two intervention groups (Hvas 2019; Rode 2021; van Nood 2013).
We combined all the comparisons groups without donor‐based FMT as one group for a meta‐analysis of donor‐based FMT versus control and the details of this analysis are available in the notes section of each study in the Characteristics of included studies table.
Country
The included studies were conducted in five different countries, with two studies conducted in Denmark (Hvas 2019; Rode 2021), and one each in Canada (Hota 2017), the Netherlands (van Nood 2013), Italy (Cammarota 2015), and the US (Kelly 2016).
Study population
Five studies excluded people who were severely immunocompromised; one study did not explicitly describe it as an exclusion criterion (Hvas 2019). Three studies excluded people who were admitted to intensive care units (Cammarota 2015; Hota 2017; van Nood 2013). Two studies excluded people with severe fulminant colitis (Hota 2017; Hvas 2019). All the studies excluded pregnant women.
Age and gender
All studies were conducted on adults. The percentage of men in the studies ranged from 20% (Kelly 2016) to 57% (van Nood 2013). The mean age of participants ranged from 52 years (Kelly 2016) to 73 years (Cammarota 2015; Hota 2017; Rode 2021).
History of prior medication treatment
All six studies included people who had previously received some form of antibiotic treatment for CDI or rCDI (or both). Two studies included people who had previously been treated with vancomycin (Hota 2017; Kelly 2016). Three studies included people who had previously been treated with vancomycin or metronidazole (or both) (Cammarota 2015; van Nood 2013; Rode 2021). One study included people who had previously been treated with vancomycin or metronidazole or fidaxomicin (or a combination of these) (Hvas 2019).
Use of immunosuppressive medications
Five studies excluded people who were immunocompromised (Cammarota 2015; Hota 2017; Kelly 2016; Rode 2021; van Nood 2013), with only Hvas 2019 including 10 participants who were receiving immunosuppressive therapy out of the total of 64 enrolled, which were similarly distributed between the FMT group (4/24 or 17%) and comparison group (6/40 or 15%).
Intervention
Indications for fecal microbiota transplantation
All six studies used FMT for the treatment of rCDI. Four studies required a person to have had at least one recurrence of CDI (Cammarota 2015; Hvas 2019; van Nood 2013; Rode 2021), while one study enrolled only people who had two or more recurrences (Hota 2017), and one study only enrolled people who had three or more recurrences (Kelly 2016). The overall reported number of rCDI episodes prior to inclusion in the respective trials differed between studies, with a range of the mean from approximately three (Cammarota 2015) in one study to as high as six episodes in another study (Rode 2021).
Donors
All six studies used feces produced by apparently healthy donors. In three studies, the donors were not related to the study participants (Hvas 2019; Rode 2021; van Nood 2013), and in three studies some of the donors were related to the recipients and some were not (Cammarota 2015; Hota 2017; Kelly 2016). All studies used one donor for each FMT and did not use pooled stool from multiple donors to perform one FMT.
Route of administration
The route of administration was to the upper gastrointestinal tract via a nasoduodenal tube in one study (van Nood 2013). One study used either nasojejunal or colonoscopic delivery depending on a clinical determination of whether the patient could tolerate a colonoscopy (Hvas 2019). Two studies used administration by enema (Hota 2017; Rode 2021), and two used only colonoscopic delivery (Cammarota 2015; Kelly 2016).
Number of administrations of fecal microbiota transplantation
Three studies limited the FMT recipients to a single administration of FMT within the primary analysis (Hota 2017; Hvas 2019; Kelly 2016), whereas the other three studies allowed multiple FMT administrations (Cammarota 2015; Rode 2021; van Nood 2013). Within van Nood 2013, participants with pseudomembranous colitis were potentially allowed to receive an unlimited number of administrations, as the revised protocol allowed for repeat administrations until visible pseudomembranes on colonoscopy were resolved.
Weight of stool
The weight of stool used in each FMT administration ranged from 50 g (Hota 2017; Hvas 2019; Rode 2021) to a mean of 152 g (Cammarota 2015).
Volume of stool
The volume of FMT delivered in an administration ranged from 170 mL (Rode 2021) to 500 mL (Cammarota 2015; Hota 2017; Kelly 2016; van Nood 2013). Hvas 2019 did not explicitly state the volume of FMT delivered.
Colonic lavage
A colonic lavage was part of the protocol in five studies (Cammarota 2015; Hvas 2019; Kelly 2016; Rode 2021; van Nood 2013). Hota 2017 did not perform colonic lavage.
Follow‐up
The follow‐up time for measurement of the primary outcome ranged from eight weeks (Hvas 2019; Kelly 2016) to 17 weeks (Hota 2017).
Comparison
Three studies had two non‐FMT comparator arms, one of which was a vancomycin regimen (Hvas 2019; Rode 2021; van Nood 2013). The other comparator arm included vancomycin combined with bowel lavage in van Nood 2013, treatment with a 10‐day regimen of fidaxomicin in Hvas 2019, and a combination of vancomycin followed by a daily enema for three consecutive days containing a mixture of 12 well‐characterized gut bacterial strains sensitive to either metronidazole or ampicillin (a treatment termed bacteriotherapy) in Rode 2021.
Five studies had a comparison group that received vancomycin (Cammarota 2015; Hota 2017; Hvas 2019; Rode 2021; van Nood 2013). Two studies used a tapering dose after 14 days of standard therapy (Cammarota 2015; Hota 2017), while the other used the standard dose without a taper (Hvas 2019; Rode 2021; van Nood 2013).
Excluded studies
Twenty‐one excluded studies used an ineligible comparator such as high‐dose versus low‐dose FMT, comparing various FMT delivery systems, and comparing different types of FMT (fresh, frozen, lyophilized, lactobacillus‐enriched). Six studies did not fulfill the criteria based on the study design. One study provided the intervention for an ineligible indication. See the Characteristics of excluded studies table for details.
Studies awaiting classification
Four studies are awaiting classification (Dubberke 2018; Kao 2019; NCT03353506; NCT03548051).
One study was terminated and details of the results were not available even after contact with investigators (NCT03548051). Kao 2019 was a small pilot study that met the inclusion criteria but there was insufficient information for us to complete the risk of bias assessment and include the data in the analysis. NCT03353506 was a small pilot study that has been completed but there appeared to be no published data at the time of this publication.
Dubberke 2018 may qualify for inclusion in subsequent versions of this systematic review and meta‐analysis. Based on the proprietary nature and our lack of access to the exact methods of collection of donor stool, processing, and shipping of the RBX2660 microbiota suspension, it is unclear at the time of the publication of this text whether RBX2660 microbiota suspension technically qualifies as FMT. We will contact study authors for further clarification in this regard.
Ongoing studies
Thirteen studies are ongoing (Drekonja 2021; EUCTR2015‐003062‐82‐DK; NCT02255305; NCT02774382; NCT03005379; NCT03053505; NCT03806803; NCT03970200; NCT04885946; NCT04960306; NCT05077085; NCT05201079; NCT05266807).
Risk of bias in included studies
We included the risk of bias assessment in the forest plots for each of the outcomes included in Table 1 and discussed the risk of bias in the Effects of interventions section for each of these outcomes. We also included a supplemental data (Microsoft Excel) file with details of the risk of bias assessment data. A brief summary of the risk of bias assessment across the outcomes is described below.
The results of risk of bias assessments were similar across outcomes in the included studies. Even though we had concerns about the lack of description of randomization methods for two studies (Hota 2017; Hvas 2019), these studies were preregistered and they had randomized groups that looked similar at baseline, so we did not assign a higher risk of bias for them for any of the outcomes considered in the risk of bias assessment. Five studies were open‐label (Cammarota 2015; Hota 2017; Hvas 2019; Rode 2021; van Nood 2013). We decided that lack of blinding in these studies did not increase the risk of bias because the outcomes of rCDI resolution, serious adverse events, and mortality were fairly objective hence the assessment of these outcomes was unlikely to be influenced by knowledge of intervention received. Two studies performed a per‐protocol analysis rather than an intention‐to‐treat analysis (Hota 2017; van Nood 2013). All other studies performed an intention‐to‐treat analysis in addition to per‐protocol or modified intention‐to‐treat analysis. We recreated the intention‐to‐treat analysis where studies reported a per‐protocol analysis. We did not assign a high risk of bias due to deviations from allocated groups. For the outcome of resolution of rCDI, this meant that participants lost to follow‐up were considered as not having experienced a resolution of rCDI and for the outcomes of serious adverse events and mortality, the participants lost to follow‐up were considered as having experienced those outcomes. We performed sensitivity analyses comparing the intention‐to‐treat results with the as‐available values for all outcomes in the summary of findings table (Analysis 1.3; Analysis 1.7; Analysis 1.11). All included studies were registered on a trial registry and we had low concern for selective reporting of outcomes.
1.3. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 3: Resolution of rCDI: sensitivity analysis: as‐available analysis
1.7. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 7: Serious adverse events: sensitivity analysis: as‐available analysis
1.11. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 11: All‐cause mortality: sensitivity analysis: as‐available analysis
Effects of interventions
See: Table 1
Primary outcomes
Proportion of participants with resolution of recurrent C difficile infections
All six included studies reported data on the proportion of participants with the resolution of rCDI. The data included 320 participants, 133 in the FMT group and 187 in the control group. Pooled results showed that treatment with FMT likely leads to a large increase in the proportion of participants with a resolution of rCDI with FMT compared to control (RR 1.92, 95 % CI 1.36 to 2.71; P = 0.02, I² = 63%; NNTB 3; moderate‐certainty evidence; Analysis 1.1; Figure 2; Table 1). We downgraded the certainty of evidence due to imprecision.
1.1. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 1: Resolution of rCDI: intention‐to‐treat analysis
2.

Forest plot of comparison: 1 Fecal microbiota transplantation (FMT) vs control for the treatment of recurrent Clostridioides difficile infections (rCDI), outcome: 1.1 Resolution of rCDI.
Sensitivity and subgroup analyses
A fixed‐effect model had a similar result to the primary random‐effects model used in this review (RR 1.92, 95 % CI 1.58 to 2.34; P = 0.02, I2 = 63%; 6 studies, 320 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 2: Resolution of rCDI: sensitivity analysis: fixed‐effect model
A post‐hoc sensitivity analysis using as‐available data found similar results to the intention‐to‐treat analysis used in this review (RR 1.89, 95 % CI 1.31 to 2.73; P = 0.008, I² = 68%; 6 studies, 313 participants; Analysis 1.3).
We performed a sensitivity analysis excluding immunocompromised participants. Of note, Hvas 2019 was the only study that enrolled immunocompromised participants, and this study did not present the data in a way that allowed us to distinguish results between immunocompromised and immunocompetent participants. Excluding this study, the analysis found similar results to the analysis that included immunocompromised participants for resolution of rCDI (RR 1.81, 95 % CI 1.23 to 2.66; P = 0.02, I² = 65%; 5 studies, 256 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 4: Resolution of rCDI: sensitivity analysis: excluding immunocompromised participants
We had planned to conduct a sensitivity analysis comparing studies with a high risk of bias versus those with low risk of bias/some concerns; however, there were no studies with high risk of bias.
We did not conduct any of the planned subgroup analyses as there were too few studies.
Serious adverse events
All six included studies reported data on serious adverse event rate. The data included 320 participants, 133 in the FMT group and 187 in the control group. The pooled results showed that FMT probably results in a slight reduction in serious adverse events; however, the CIs around the summary estimate were wide so we downgraded the certainty of evidence one level due to imprecision (RR 0.73, 95% CI 0.38 to 1.41; P = 0.24, I² = 26%; NNTB 12; moderate‐certainty evidence; Analysis 1.5; Figure 3; Table 1).
1.5. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 5: Serious adverse events: intention‐to‐treat analysis
3.

Forest plot of comparison: 1 Fecal microbiota transplantation (FMT) vs control for the treatment of recurrent Clostridioides difficile infections (rCDI), outcome: 1.2 Serious adverse events.
Sensitivity and subgroup analyses
A fixed‐effect model had a similar result to the primary random‐effects model used in this review (RR 0.64, 95 % CI 0.38 to 1.09; P = 0.24, I2 = 26%; 6 studies, 320 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 6: Serious adverse events: sensitivity analysis: fixed‐effect model
A post‐hoc sensitivity analysis using as‐available data found similar results to the intention‐to‐treat analysis used in this review (RR 0.72, 95 % CI 0.37 to 1.38; P = 0.32, I² = 14%; 6 studies, 314 participants; Analysis 1.7).
We performed a post‐hoc sensitivity analysis excluding immunocompromised participants. Of note, Hvas 2019 was the only study that enrolled immunocompromised participants, and this study did not present the data in a way that allowed us to distinguish results between immunocompromised and immunocompetent participants. Excluding this study, the analysis showed similar results to the analysis that included immunocompromised participants for SAE (RR 0.72, 95 % 0.30 to 1.74; P = 0.16, I² = 39%; 5 studies, 256 participants; Analysis 1.8).
1.8. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 8: Serious adverse events: sensitivity analysis: excluding immunocompromised participants
We had planned to conduct a sensitivity analysis comparing studies with a high risk of bias versus those with low risk of bias/some concerns; however, there were no studies with high risk of bias.
We did not conduct any of the planned subgroup analyses as there were too few studies.
Secondary outcomes
Treatment failure
None of the included studies explicitly reported treatment failure.
All‐cause mortality
All six studies reported data on all‐cause mortality. The data included 320 participants, 133 in the FMT group, and 187 in the control group. Pooled data showed that FMT may lower all‐cause mortality; however, the CIs around the summary estimates were wide so we downgraded the certainty of the evidence because of very serious imprecision. None of the included studies were at high risk of bias for this outcome (RR 0.57, 95% CI 0.22 to 1.45; P = 0.48, I² = 0%; NNTB 20; low‐certainty evidence; Analysis 1.9; Table 1).
1.9. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 9: All‐cause mortality: intention‐to‐treat analysis
Sensitivity and subgroup analysis
A fixed‐effect model showed a similar result to the primary random‐effects model used in this review (RR 0.52, 95 % CI 0.22 to 1.23; P = 0.48, I² = 0%; 6 studies, 320 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 10: All‐cause mortality: sensitivity analysis: fixed‐effect model
A post‐hoc sensitivity analysis using as‐available data found similar results to the intention‐to‐treat analysis used in this review (RR 0.50, 95 % CI 0.17 to 1.46; P = 0.68, I² = 0%; 6 studies, 314 participants; Analysis 1.11).
We performed a post‐hoc sensitivity analysis excluding immunocompromised participants. Of note, Hvas 2019 was the only study that enrolled immunocompromised participants, and this study did not present the data in a way that allowed us to distinguish results between immunocompromised and immunocompetent participants. Excluding this study, the analysis showed similar results to the analysis that included immunocompromised participants for all‐cause mortality (RR 0.57, 95 % 0.22 to 1.45; P = 0.48, I² = 0%; 5 studies, 256 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 12: All‐cause mortality: sensitivity analysis: excluding immunocompromised participants
We had planned to conduct a sensitivity analysis comparing studies with a high risk of bias versus those with low risk of bias/some concerns; however, there were no studies with high risk of bias.
We did not conduct any of the planned subgroup analyses as there were too few studies.
Proportion of participants who withdrew from the study
Six studies reported data on the number of participants who withdrew from the study. The data included 320 participants, 133 in the FMT group and 187 in the control group. The rates of withdrawal from the study were similar in both the groups (RR 0.75, 95 % CI 0.17 to 3.28; P = 0.52, I² = 0%; 6 studies, 320 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI), Outcome 13: Withdrawals
Rate of new Clostridioides difficile infection
None of the studies reported the rate of new CDI infections.
Any adverse event
All six studies reported data on any adverse events. A total of 111 participants in the FMT group experienced 189 adverse events, whereas 163 participants in the control group experienced 164 adverse outcomes. Because one participant could experience multiple simultaneous mild adverse events that were not mutually exclusive, the planned statistical analyses would not have been valid. Therefore, Table 2 shows a breakdown of adverse events extracted from the text of the primary studies. The most commonly described mild adverse events in the FMT group were abdominal pain, bloating, and diarrhea.
1. List of adverse events.
| Adverse event | Cammarota 2015 | Hota 2017 | Hvas 2019 | Kelly 2016 | van Nood 2013 | Rode 2021 | ||||||
| FMT (n = 20) | Control (n = 19) | FMT (n = 16) | Control (n = 14) | FMT (n = 24) | Control (n = 40) | FMT (n = 22) | Control (n = 24) | FMT (n = 17) | Control (n = 26) | FMT (n = 34) | Control (n = 64) | |
| Abdominal distention | — | — | 9 (56%) | 8 (57%) | — | — | — | — | — | — | — | — |
| Abdominal pain/cramping | 12 (63%) | — | 10 (63%) | 14 (100%) | 1 (4%) | — | — | — | 7 (41%) | — | 11 (32%) | 18 (28%) |
| Anasarca/edema | — | — | 1 (6%) | — | — | — | — | — | — | — | — | 2 (3%) |
| Anemia | — | — | — | — | — | — | — | — | — | — | — | 1 (2%) |
| Anorexia | — | — | 6 (38%) | 5 (36%) | — | — | — | — | — | — | — | 1 (2%) |
| Belching | — | — | — | — | — | — | — | — | 3 (18%) | — | 1 (3%) | 1 ( 2%) |
| Bloating | 12 (63%) | — | 9 (56%) | 13 (93%) | 5 (21%) | — | — | — | — | 1 (4%) | 12 (35%) | 16 (25%) |
| Bloody stools | — | — | 3 (19%) | 2 (14%) | — | — | — | — | — | — | — | 1 (2%) |
| Bowel perforation | — | — | 1 (6%) | — | — | — | — | — | — | — | — | — |
| Chest pain | — | — | — | — | — | — | — | — | — | — | — | 1 (2%) |
| Chills | — | — | — | — | — | — | — | — | — | — | — | 2 (3%) |
| Choledocholithiasis | — | — | — | — | — | — | — | — | 1 (6%) | — | — | — |
| Constipation | — | — | — | — | 1 (4%) | — | — | — | 3 (18%) | 3 (12%) | — | — |
| Cough | — | — | — | — | — | — | — | — | — | — | 1 (3%) | — |
| Dehydration | — | — | — | — | — | — | — | — | — | — | — | 1 (2%) |
| Diarrhea | 19 (95%) | — | 10 (63%) | 8 (57%) | 3 (13%) | — | — | — | 15 (88%) | 1 (4%) | 5 (15%) | 15 (23%) |
| Dizziness | — | — | — | — | — | — | — | — | 1 (6%) | — | 1 (3%) | 2 (3%) |
| Dyspepsia | — | — | — | — | — | — | — | — | 1 (4%) | — | 1 (2%) | |
| Dyspnea | — | — | — | — | — | — | — | — | — | — | — | 2 (3%) |
| Epistaxis | — | — | — | — | — | — | — | — | — | — | 2 (6%) | — |
| Fatigue | — | — | 9 (56%) | 13 (93%) | — | — | — | — | — | — | 5 (15%) | 3 (5%) |
| Fecal incontinence | — | — | 7 (44%) | 7 (50%) | — | — | — | — | — | — | 1 (3%) | 2 (3%) |
| Fever | — | — | 3 (19%) | 1 (7%) | — | — | — | — | 1 (6%) | — | 2 (6%) | 4 (6%) |
| Flatulence | — | — | — | — | — | — | — | — | — | — | 7 (21%) | 3 (5%) |
| GI cancer diagnosed incidentally | — | — | — | — | — | — | 1 (5%) | — | — | — | — | — |
| GI cancer recurrence | — | — | — | — | — | — | 1 (5%) | — | — | — | — | — |
| Headache | — | — | — | — | — | — | — | — | — | — | 2 (6%) | 1 (2%) |
| Hematoma | — | — | — | — | — | — | — | — | — | — | — | 1 (2%) |
| Hypoglycemia | — | — | — | — | — | — | — | — | — | — | — | — |
| Joint pain | — | — | — | — | — | — | 1 (5%) | — | — | 1 (4%) | — | 4 (4%) |
| Nausea/vomiting | — | — | 4 (25%) | 6 (43%) | — | — | — | — | 1 (6%) | — | 4 (12%) | 5 (8%) |
| Neck swelling | — | — | — | — | — | — | — | — | — | — | 1 (3%) | — |
| Pneumonia | — | — | — | — | — | — | — | — | — | — | — | 1 (2%) |
| Pulmonary nodule | — | — | — | — | — | — | 1 (5%) | — | — | — | — | — |
| Rash | — | — | — | 3 (21%) | — | — | — | — | — | — | 1 (3%) | 1 (2%) |
| Rectal pain with defecation | — | — | — | — | — | — | — | — | — | — | 1 (3%) | 1 (2%) |
| Seizure | — | — | — | — | — | — | — | — | — | — | 1 (3%) | — |
| Sepsis like | — | — | — | — | 1 (4%) | — | — | — | — | — | — | — |
| Small bowel bacterial overgrowth | — | — | — | — | 1 (4%) | — | — | — | — | — | — | — |
| UTI | — | — | — | 1 (7%) | — | — | — | — | 1 (6%) | 1 (4%) | 1 (3%) | — |
| Weight gain | — | — | — | — | — | — | 1 (5%) | — | — | — | — | — |
FMT: fecal microbiota transplantation; GI: gastrointestinal; UTI: urinary tract infection.
Quality of life score
None of the studies reported quality of life scores.
Colectomy
None of the studies reported data on colectomy rates.
Post‐hoc secondary outcomes
Microbiome outcomes
Three studies reported analysis of microbiome outcomes in FMT recipients. Table 3 gives the summary of methods used to assess the microbiome‐related outcomes as well as a summary of the key findings from the included studies.
2. Microbiome outcomes.
| Study | Methods and main findings of microbiome analysis |
| Hota 2017 | Diversity indices were analyzed using Student t‐tests interrogating the V4 hypervariable region of the 16S ribosomal RNA locus of bacterial DNA in samples from 19 donors and 3 recipients with successful outcomes. Fecal microbiota composition and diversity of the 19 donors were consistently high, with no significant difference between those associated with recipient success or failure of resolution of rCDI. Increased fecal microbiota diversity was found post‐FMT in the analysis of 3 recipients who had resolution of rCDI after FMT. |
| Kelly 2016 | DNA extraction, 16S ribosomal RNA gene amplification, and sequencing were performed on donors and participants ≥ 5 days before and 2 and 8 weeks after FMT. Shannon indices and abundance‐based coverage estimate parameters were calculated to assess alpha diversity, while beta diversity and abundances of genera were analyzed using analysis of similarity and Kruskal–Wallis analysis. All participants had marked dysbiosis prior to FMT. This persisted in those who received autologous FMT while those receiving donor FMT had a restoration of alpha diversity, a pattern seen in those who had success with rescue FMT after initial failure of treatment. This study had 2 sites, and analysis showed differences in the pre‐FMT microbiomes between sites in both donors and recipients pre‐FMT. |
| van Nood 2013 | The study used paired‐samples Student t‐tests to examine statistical significance of a change in microbiota diversity. Wilcoxon signed‐rank tests were performed to determine microbial groups in fecal samples before and after FMT infusion. The Simpson's Reciprocal Index of 9 pre‐FMT patients was low (mean 57, SD 26) and increased within 2 weeks after infusion to 179, SD 42 (P < 0.001), which became indistinguishable from the diversity level of the donors (mean 172, SD 54). This persisted throughout the follow‐up period for those who completed follow‐up testing. A principal component analysis indicated a major shift in the participants' microbiota after FMT towards that of the donors. There was a statistically significant change in multiple groups of intestinal bacteria (P < 0.05). |
FMT: fecal microbiota transplantation; rCDI: recurrent Clostridioides difficile infections; RNA: ribonucleic acid.
Discussion
Summary of main results
This review synthesized findings from six RCTs, consisting of 320 participants, which assessed the benefits and harms of FMT in the treatment of immunocompetent adults with rCDI. There is moderate‐certainty evidence that in immunocompetent adults with rCDI, the use of FMT likely leads to a large increase in resolution of rCDI in FMT‐receiving participants compared to controls. Fecal microbiota transplantation likely decreases the rates of serious adverse and may reduce all‐cause mortality; however, the summary estimates for these outcomes were imprecise. Elimination of the study that included some immunocompromised participants did not alter these conclusions, but, based on the low number of immunocompromised participants enrolled in the included studies, conclusions could not be drawn about the benefits or harms of FMT for rCDI in the immunocompromised population at this time. Data were not available for all the prespecified outcomes. The number of included studies was small and, therefore, we did not complete any of the planned subgroup analyses.
Overall completeness and applicability of evidence
The use of FMT for the treatment of rCDI seems biologically plausible. Data from observational studies have shown that the risk of CDI is increased in people with dysbiosis, such as after the use of antibiotics, proton pump inhibitors, immunosuppression, and hospitalization (Crobach 2018; Fekety 1997). The use of FMT seems to reverse the dysbiosis as shown in some of the included studies in this review where the microbiome of the responders seemed to mirror the donors, as summarized in Table 3 (Hota 2017; Kelly 2016; van Nood 2013).
Many observational studies have been published on this topic, and support FMT as efficacious for the treatment of rCDI; however, these studies did not meet criteria for inclusion in this review. All included studies in this review were preregistered on a trial registry and five were stopped early due to futility. All studies contributed data to the primary outcome and five studies showed convincing evidence in favor of the intervention that was depicted in the summary estimate of the meta‐analysis for the outcome of the resolution of rCDI. The data on serious adverse events and all‐cause mortality from the included studies showed that FMT may be safe in the short term for the treatment of rCDI. However, it is important to note that the number of events was small and the CIs of the summary estimate included both a decreased and a possible increased risk for these outcomes. Randomized controlled trials may not be the ideal study design to assess the risk of serious adverse events and long‐term outcomes, and database registries with a larger sample size and longer follow‐up may be more useful for this purpose. One recent report from the FMT national registry in the US reported effectiveness and safety data for 259 participants at one‐ and six‐month follow‐ups, confirming effectiveness and showing a favorable safety profile of FMT for treatment of rCDI (Kelly 2021). The most commonly reported adverse events in the Kelly 2021 study were abdominal pain, diarrhea, and bloating, similar to those reported in the included studies in this review. Moreover, the US FDA recently issued a safety alert about the use of FMT due to reports of cases of transmission of multiple‐drug‐resistant organisms and mortality in people who received FMT (FDA 2019; FDA 2020a).
The longest follow‐up in any of the included studies was 17 weeks, so this review does not provide evidence regarding the long‐term safety of FMT. Evidence regarding the long‐term safety of FMT was reported in one recent observational cohort study that included data from 609 people who received FMT (Saha 2021). This study reported safety data at one and two years after FMT. Diarrhea and constipation were commonly reported symptoms in this cohort after FMT. The study also reported that 73 people who received an FMT developed a new diagnosis over the period of follow‐up; however, these diagnoses were all deemed as unrelated to FMT and this paper did not include a comparator group so no solid conclusion about the risk of developing new diagnoses as a result of FMT could be drawn from this study (Saha 2021).
We had planned five a priori subgroup analyses, but none of these could be conducted due to the low number of studies that met the inclusion criteria. Therefore, we cannot comment if the efficacy and safety of FMT will differ based on clinical setting (outpatient versus hospitalized people); storage of stool (fresh stool of non‐stool bank origin versus frozen then thawed stool of stool bank origin); type of donor (related versus unrelated); source of stool (single donor versus pooled donor source of FMT); route of FMT delivery (to the upper gastrointestinal tract including nasogastric, nasoduodenal, and capsule routes versus delivery to the lower gastrointestinal tract via enema and colonoscopy).
While our protocol allowed for the inclusion of both children and adults with rCDI (Imdad 2021), all six studies that met the criteria for inclusion excluded children from enrolling. Therefore, the results of this review are applicable to the adult population only. Five studies excluded people who were severely immunocompromised. Therefore, the results supported by this review should be used with caution for people who are severely immunocompromised. Similarly, all the studies excluded pregnant women, and the use of FMT during pregnancy should be used with extreme caution.
Quality of the evidence
The GRADE criteria consider the type of studies, risk of bias, indirectness, inconsistency (i.e. unexplained heterogeneity), imprecision, and potential publication bias (Guyatt 2011). Using the GRADE criteria, the overall certainty of the evidence was moderate for resolution of rCDI and serious adverse events, and low for all‐cause mortality.
Five studies were open‐label (Cammarota 2015; Hota 2017; Hvas 2019; Rode 2021; van Nood 2013). We did not assign these studies a high risk of bias because the outcomes of rCDI resolution, serious adverse events, and mortality were considered objective.
Some included studies performed a per‐protocol analysis rather than an intention‐to‐treat analysis. We recreated the intention‐to‐treat analysis where applicable and did not assign a high risk of bias due to deviations from allocated groups. We created the intention‐to‐treat analysis for studies where data for follow‐up were missing. For the outcome of resolution of rCDI, this meant that participants lost to follow‐up were considered as not having experienced a resolution of rCDI and for the outcomes of serious adverse events and mortality, the participants lost to follow‐up were considered as having experienced those outcomes. We performed a sensitivity to assess our assumption and the summary estimate were similar between recreated intention‐to‐treat analyses and as‐available analyses.
Five studies were stopped early due to futility (Cammarota 2015; Hota 2017; Kelly 2016; Rode 2021; van Nood 2013). Four of these studies determined that further recruitment of participants would not change the results and that FMT is an effective intervention compared to control (Cammarota 2015; Kelly 2016; Rode 2021; van Nood 2013). One study was stopped due to lack of effect (Hota 2017). We did not consider early termination of trials as the high risk of bias because of the apparent reproducibility of similar results across trials including the one that was completed (Hvas 2019).
In summary, even though we noted some issues in risk of bias assessment as noted above, we did not downgrade the certainty of the evidence for risk of bias for any of the outcomes. All six studies were well conducted, and the measured outcomes were fairly objective. Consequently, it is less likely that the observed effect of FMT for treatment of rCDI is because of bias in the included studies.
The outcome of resolution of rCDI had a statistical heterogeneity of 63% based on the I2 value. We did not downgrade the certainty of evidence due to inconsistency for this outcome because this statistical heterogeneity was likely due to differences in the magnitude of effect as the direction of effect was in favor of the intervention in five of the studies, and it was clinically meaningful. However, we downgraded the certainty of evidence due to imprecision as the CIs around the summary estimate were wide.
We downgraded the certainty of the evidence for serious adverse events and all‐cause mortality for imprecision because the number of events was small, and the confidence of the summary estimate included both a reduced and potentially increased risk of the outcome.
Potential biases in the review process
This review used standard methodologic procedures expected by Cochrane (Higgins 2020). We searched for both published studies and ongoing studies. As the number of included studies was fewer than 10, we could not perform analyses to assess for potential publication bias.
Three studies had more than one non‐FMT comparator group. We combined these subgroups to obtain a donor‐based FMT versus non‐donor‐based FMT comparison. We specified this approach in our protocol (Imdad 2021), and described these decisions for each study in the Characteristics of included studies table. To investigate this approach further, we planned to conduct a post hoc subgroup analysis based on comparator but as there were fewer than 10 studies, this subgroup analysis was not conducted. An alternative approach to assess the efficacy of FMT versus other treatments would be to perform a network meta‐analysis and one recent network‐analysis indicated that FMT might be the best therapy among all the available therapies to treat rCDI (Dembrovszky 2020).
The FMT group in our analysis combined studies that only allowed a single FMT infusion with studies that allowed for the potential of multiple FMT infusions. One recent RCT showed that multiple FMT infusions might help cure the relapsing colitis related to rCDI (Ianiro 2018). Therefore, we may have overestimated the efficacy of a single FMT infusion by grouping it with studies that allowed for multiple FMT infusions if the participants' rCDI symptoms did not resolve with the first infusion. We had planned to perform a post‐hoc subgroup analysis based on the number of FMTs allowed but were unable to conduct this as the number of included studies was fewer than 10 and it was very unlikely that an investigation of heterogeneity would produce useful findings unless there are at least 10 studies in a meta‐analysis.
Agreements and disagreements with other studies or reviews
Multiple systematic reviews have been published on the efficacy and safety of FMT for the treatment of rCDI (Baunwall 2020; Pomares Bascuñana 2021) including network meta‐analyses (Dembrovszky 2020; Rokkas 2019). The reviews by Baunwall 2020 and Pomares Bascuñana 2021 considered both randomized and non‐randomized studies but came to a similar conclusion as the network meta‐analyses (Dembrovszky 2020; Rokkas 2019), which also agree with the findings of our systematic review and meta‐analysis that FMT is likely to be highly efficacious for the treatment of rCDI.
The objectives of our review were mainly related to the efficacy and safety of FMT for the treatment of rCDI. Even though we planned several subgroup analyses to differentiate characteristics of FMT in terms of the optimal route of administration, frequency, type of donor, and other variables, unfortunately, there were not enough studies in these subgroup analyses to make any conclusive statements. Other reviews and studies have addressed some of these questions. For example, Ramai 2021 assessed the different routes of administration of FMT for the treatment of rCDI and included 26 studies with 1309 participants. Fecal microbiota transplantation was found to be highly efficacious irrespective of the route of administration; however, the administration via colonoscopy seems to have the highest cure rate of about 94.8% (95% CI 92.4% to 96.8%) while the nasogastric tube had lower cure rate of 78.1% (95% CI 71.6% to 84.1%). The number of studies in Ramai 2021 was small in each of the subgroups other than colonoscopy subgroup, so the observed difference in the nasogastric tube may be explained by a paucity of studies. The raw data in our analysis showed approximately a 77% cure rate of rCDI treated with FMT. This appears to be roughly similar but slightly lower than the cure rate found in other systematic reviews and network meta‐analyses, where resolution of rCDI rates ranged between 82% and 91% (Baunwall 2020; Dembrovszky 2020; Pomares Bascuñana 2021; Ramai 2021).
Authors' conclusions
Implications for practice.
In immunocompetent adults with recurrent Clostridioides difficile infection (rCDI), fecal microbiota transplantation (FMT) probably leads to a large increase in the resolution of rCDI compared to alternative treatments such as antibiotics. Fecal microbiota transplantation probably leads to a small decrease in the rates of serious adverse events and may decrease all‐cause mortality in people with rCDI; however, the number of events was small and an increased risk of these outcomes cannot be ruled out. Additional data from large national registry databases may be required to assess the potential short‐term and long‐term risks with using FMT for treatment of rCDI in clinical practise. Based on the low number of immunocompromised participants enrolled in the included studies, conclusions cannot be drawn about the benefits or harms of FMT for rCDI in the immunocompromised population at this time.
Implications for research.
Five of the included studies excluded people who were immunocompromised and additional data from clinical trials might be required in people with rCDI who have HIV, solid organ transplant, stem cell transplant, those undergoing chemotherapy (Abu‐Sbeih 2019), and those on long‐term immunosuppressive medications. Similarly, there is paucity of data on safety of FMT use in people with fulminant colitis requiring admission to the intensive care unit as most of the included studies excluded such individuals. None of the included studies enrolled children; however, given the efficacy of FMT for rCDI reported in this and other studies, it would be morally dubious to recommend studies with a comparator arm with no treatment in this population. The safety data reported in the included studies were based on short‐term follow‐up, and the same safety profile was confirmed in a recent publication from data from a national registry (Kelly 2021); however, future studies with a comparator arm are needed to establish the long‐term safety of FMT (Saha 2021). Finally, new therapies based on particular strains of bacteria that may reverse the dysbiosis require further investigations as such therapies can simplify the bacteriotherapy for rCDI by eliminating the need for donors and minimizing the risk of exposure to potentially harmful micro‐organisms (Rode 2021).
History
Protocol first published: Issue 2, 2021
Risk of bias
Risk of bias for analysis 1.1 Resolution of rCDI: intention‐to‐treat analysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we performed analysis on an intention‐to‐treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | This was an open‐label study. We did not think that the measurement of the resolution of rCDI was at high risk of bias because the definition required two negative stool tests for C. difficile toxin. | Low risk of bias | The clinical trial was registered at clinicaltrial.gov (NCT02148601). The authors followed the pre‐specified plan of analysis. The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. There were no concerns about selective reporting for this outcome. | Low risk of bias | No major concerns were noted in any of the domains of risk of bias for the outcomes of resolution of rCDI from this study. Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to objective measurement with C. difficile stool testing. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we created an intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that the participants excluded by the study authors in the analysis did not have the outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to the protocol. We included all participants in the intention‐to‐treat analysis and assumed that those excluded were failures (had an episode of rCDI). We did a sensitivity analysis to assess our assumption and the results were similar. So the missing data is less likely to create a bias for this outcome. | Low risk of bias | The study was open‐label but we did not consider it as high risk of bias for this domain as the outcome definition included an objective measure with stool C. difficle testing. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). The authors followed the pre‐specified plan of analysis. We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of the randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open‐label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Hvas 2019 | Some concerns | No information was available on allocation sequence and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | Quote from article: ''All 64 randomized patients received the allocated treatment.'' The study was not blinded however we do not believe there were significant deviations from intended intervention due to non‐blinding as all participants received allocated intervention and authors of the study performed intention to treat analysis. |
Low risk of bias | There was no attrition and data were available for all the participants for this outcome. | Low risk of bias | The study was open‐label but the outcome measured was defined objectively. We did not think that outcome of the resolution of rCDI was at high risk of bias because the definition of the outcome included laboratory‐confirmed CDI. | Low risk of bias | Comment: The trial was registered at clinicaltrial.gov (NCT02743234). The authors followed the pre‐specified plan of analysis. We did not have any concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process and that study was open‐label, we did not consider this study was at high risk of bias for this outcome. All the study groups were similar at the start of the trial and the outcome measured was objective in nature. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention‐to‐treat analysis hence low risk of bias due to deviation from intended intervention. | Low risk of bias | Two patients were lost to follow‐up in the control group and one patient in the intervention group. All the participants were considered in the intention to treat analysis in our review assuming that patients who were lost to follow up were failures. We did a sensitivity analysis by considering those participants for whom the data were available and the results were similar. So we think that missing data is less likely to bias this outcome. | Low risk of bias | The study was double‐blind, hence we do not have any concerns about the risk of bias in the measurement of this outcome. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT01703494). The authors followed the pre‐specified plan of analysis. We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted double‐blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that the participants who did not complete the study did not have the outcome. | Low risk of bias | The majority of missing data was due to mortality (2 in the FMT group and 11 in the comparison group). We created the intention‐to‐treat analysis basis by analyzing the participants in which they were randomized irrespective if they received the intervention and were loss to follow‐up. For participants with loss to follow up, we considered them to have failures meaning they did not have resolution of rCDI. In order to investigate our assumption, we did a sensitivity analysis just on available cases and the summary estimate has a slight reduction in effect (from RR 1.63 to 1.58) but the direction of effect was same and width of confidence did not change much. We, therefore, think that this outcome was not at high risk of bias due to missing data. | Low risk of bias | The study was open‐label but the outcome measured was defined objectively. We did not think that measurement of resolution of rCDI was at high risk of bias because the definition of the outcome included the absence of laboratory‐confirmed CDI in case diarrhea was present. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). The authors followed the pre‐specified plan of analysis. We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to the objective definition of the outcome and intention to treat analysis. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention‐to‐treat basis by assuming that participants who did not complete the study did not have the outcome. | Low risk of bias | Only two participants did not complete the study and one of them was mortality. We created the intention to treat analysis assuming the two participants with loss to follow up were failures, i.e. did not have resolution of rCDI. In order to investigate our assumptions, we did a sensitivity analysis and the effect size remained the same. We, therefore, do not think that this outcome was at high risk of bias due to missing data from this study. | Low risk of bias | The study was open‐label. Resolution of rCDI was defined based on the absence of diarrhea or persistent diarrhea that could be explained by other causes with three consecutive negative stool tests for C. difficile toxin. We did not have any major concerns about bias in the measurement of this outcome due to the objective definition of the outcome. | Low risk of bias | The trial was registered in the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome or analysis reporting for this outcome. The authors followed the pre‐specified plan of analysis. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to objective measurement with C. difficile stool testing and intention to treat analysis. |
Risk of bias for analysis 1.5 Serious adverse events: intention‐to‐treat analysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | The study was open‐label however, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. The clinical trial was registered at clinicaltrial.gov (NCT02148601). There were no concerns about selective reporting for this outcome. | Low risk of bias | No major concerns were noted in any of the domains of risk of bias for the outcomes of serious adverse events from this study. Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we included the intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that the participants excluded by the study authors in the analysis also achieved this outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to protocol. We included all participants in the intention‐to‐treat analysis and assumed that were failures meaning they experience the outcome. We did a sensitivity analysis based on as available cases and the results were similar. Therefore, missing data from this study is less likely to create a bias for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of the randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open‐label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Hvas 2019 | Some concerns | No information was available on allocation sequence and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there were significant deviations from the intended treatment due to non‐blinding as all participants received allocated intervention and authors of the study performed intention to treat analysis. | Low risk of bias | There was no attrition and data were available for all the participants for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT02743234). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open‐label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention to treat analysis. | Low risk of bias | Two patients were lost to follow‐up in the control group (one of these telephonic contact only and no serious adverse events were reported) and one patient in the intervention group. All the participants were considered in the intention to treat analysis assuming they achieved this outcome. So we think that this outcome was not at high risk of bias due to missing the data from this study. We did a sensitivity analysis to assess our assumption and the results were similar. So the missing data is less likely to create a bias for this outcome. | Low risk of bias | The study was double‐blind, hence we did not have any concerns about the risk of bias in measurement of this outcome. | Low risk of bias | Comment: The trial was registered at clinicaltrial.gov (NCT01703494). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted doudle blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that the participants who did not complete the study did also achieve this outcome. | Low risk of bias | The majority of missing data was due to mortality (2 in the FMT group and 11 in the comparison group). We analyzed the data on intention to treat analysis and considered all the participants who were randomized. We assumed that participants who were lost to follow‐up experienced the outcome. We did a sensitivity analysis to assess if our assumption changed the overall summary estimate and the summary effect size remained the same (RR 0.75 to 0.73). We therefore think that there is a low risk of bias in the measurement of this outcome due to missing data from this study. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgment hence the assessment is not likely to be influenced by knowledge of intervention received. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The authors analyzed data on a modified intention‐to‐treat basis with the exclusion of one patient who required high‐dose prednisolone treatment after randomization but before the study treatment was initiated. The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that the participants who did not complete the study did also achieve this outcome. | Low risk of bias | Only two participants (one in each group) did not complete the study and one of them was due to mortality. We created the intention to treat analysis by assuming that two participants who were lost to follow‐up had the event. We did a sensitivity analysis based on available cases only and the results were similar. We, therefore, think that the risk of bias is low due to missing data for this outcome from this study. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
Risk of bias for analysis 1.7 Serious adverse events: sensitivity analysis: as‐available analysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | The study was open‐label however, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. The clinical trial was registered at clinicaltrial.gov (NCT02148601). There were no concerns about selective reporting for this outcome. | Low risk of bias | No major concerns were noted in any of the domains of risk of bias for the outcomes of serious adverse events from this study. Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we included the intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that the participants excluded by the study authors in the analysis also achieved this outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to protocol. We included all participants in the intention‐to‐treat analysis and assumed that were failures meaning they experience the outcome. We did a sensitivity analysis based on as available cases and the results were similar. Therefore, missing data from this study is less likely to create a bias for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of the randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open‐label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Hvas 2019 | Some concerns | No information was available on allocation sequence and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there were significant deviations from the intended treatment due to non‐blinding as all participants received allocated intervention and authors of the study performed intention to treat analysis. | Low risk of bias | There was no attrition and data were available for all the participants for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT02743234). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open‐label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention to treat analysis. | Low risk of bias | Two patients were lost to follow‐up in the control group (one of these telephonic contact only and no serious adverse events were reported) and one patient in the intervention group. All the participants were considered in the intention to treat analysis assuming they achieved this outcome. So we think that this outcome was not at high risk of bias due to missing the data from this study. We did a sensitivity analysis to assess our assumption and the results were similar. So the missing data is less likely to create a bias for this outcome. | Low risk of bias | The study was double‐blind, hence we did not have any concerns about the risk of bias in measurement of this outcome. | Low risk of bias | Comment: The trial was registered at clinicaltrial.gov (NCT01703494). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted double blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that the participants who did not complete the study did also achieve this outcome. | Low risk of bias | The majority of missing data was due to mortality (2 in the FMT group and 11 in the comparison group). We analyzed the data on intention to treat analysis and considered all the participants who were randomized. We assumed that participants who were lost to follow‐up experienced the outcome. We did a sensitivity analysis to assess if our assumption changed the overall summary estimate and the summary effect size remained the same (RR 0.75 to 0.73). We therefore think that there is a low risk of bias in the measurement of this outcome due to missing data from this study. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The authors analyzed data on a modified intention‐to‐treat basis with the exclusion of one patient who required high‐dose prednisolone treatment after randomization but before the study treatment was initiated. The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that the participants who did not complete the study did also achieve this outcome. | Low risk of bias | Only two participants (one in each group) did not complete the study and one of them was due to mortality. We created the intention to treat analysis by assuming that two participants who were lost to follow‐up had the event. We did a sensitivity analysis based on available cases only and the results were similar. We, therefore, think that the risk of bias is low due to missing data for this outcome from this study. | Low risk of bias | The study was open‐label. However, the outcome of serious adverse events (such as serious illness requiring hospitalization or life‐threatening events) is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as serious adverse events are observer‐reported outcomes not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
Risk of bias for analysis 1.9 All‐cause mortality: intention‐to‐treat analysis.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The clinical trial was registered at clinicaltrial.gov (NCT02148601). The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. There were no concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. Moreover, intention to treat analysis was performed. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we included the intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that two participants excluded by the study authors in the analysis did experience this outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to protocol. We included all participants in the intention‐to‐treat analysis and assumed that those excluded had the outcome. We did a sensitivity analysis on as available cases and the results were similar. We therefore think that missing data is less likely to create a bias for this outcome in this study. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Hvas 2019 | Some concerns | No information was available on allocation sequence and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there were significant deviations from the intended treatment due to non‐blinding as all participants received allocated intervention and authors of the study performed intention to treat analysis. | Low risk of bias | There was no attrition and data were available for all the participants for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered on clinicaltrial.gov (NCT02743234). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process and that study was open label, we did not consider this study was at high risk for bias for this outcome. All the study groups were similar at the start of the trial and that outcome measured was objective in nature. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention to treat analysis. | Low risk of bias | Two patients were lost to follow‐up in the control and one patient in the intervention group. All participants were considered in the intention to treat analysis. A sensitivity analysis based on as available cases was similar. | Low risk of bias | The study was double‐blind, hence we did not have any concerns about the risk of bias in the measurement of this outcome. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT01703494). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted double blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention‐to‐treat basis by assuming that the participants who did not complete the study also achieved this outcome. | Low risk of bias | We analyzed data on an intention‐to‐treat basis assuming that patients with loss to follow up experienced the outcome. A sensitivity analysis based on as available cases did not change the summary estimate significantly. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to the objective nature of outcomes measured and intention to treat analysis. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The authors analyzed data on a modified intention‐to‐treat basis with the exclusion of one patient who required high‐dose prednisolone treatment after randomization but before the study treatment was initiated. The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention‐to‐treat basis by assuming that the participants who did not complete the study also achieved this outcome. | Low risk of bias | All participants were included in the intention to treat analysis while assessing all‐cause mortality. We also did an as‐available analysis to account for any missing data which was similar in effect size and direction. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
Risk of bias for analysis 1.10 All‐cause mortality: sensitivity analysis: fixed‐effect model.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The clinical trial was registered at clinicaltrial.gov (NCT02148601). The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. There were no concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. Moreover, intention to treat analysis was performed. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we included the intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that two participants excluded by the study authors in the analysis did not have the outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to protocol. We included all participants in the intention‐to‐treat analysis and assumed that those excluded did not experience the outcome. This missing data is less likely to create a bias for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Hvas 2019 | Some concerns | No information was available on allocation sequence and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there were significant deviations from the intended treatment due to non‐blinding as all participants received allocated intervention and authors of the study performed intention to treat analysis. | Low risk of bias | There was no attrition and data were available for all the participants for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT02743234). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process and that study was open label, we did not consider this study was at high risk for bias for this outcome. All the study groups were similar at the start of the trial and that outcome measured was objective in nature. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention to treat analysis. | Low risk of bias | Two patients were lost to follow‐up in the control and one patient in the intervention group. All participants were considered in the intention to treat analysis. | Low risk of bias | The study was double‐blind, hence we did not have any concerns about the risk of bias in the measurement of this outcome. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT01703494). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted double blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that two participants who did not complete the study did not have the outcome. | Low risk of bias | We analyzed data on an intention‐to‐treat basis, hence there is a low risk of bias from missing data on two participants. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to the objective nature of outcomes measured and intention to treat analysis. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The authors analyzed data on a modified intention‐to‐treat basis with the exclusion of one patient who required high dose prednisolone treatment after randomization but before the study treatment was initiated. The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that two participants who did not complete the study did not have the outcome. | Low risk of bias | All participants were included in the intention to treat analysis while assessing all‐cause mortality. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
Risk of bias for analysis 1.12 All‐cause mortality: sensitivity analysis: excluding immunocompromised participants.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Cammarota 2015 | Low risk of bias | Quote from article: "Blocked randomization of subjects was performed by an external person not involved in the study. An online random number generator software was used to provide random permuted blocks with a block size of six and an equal allocation ratio; the sequence was concealed until the interventions were assigned." Comment: We think the allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis. | Low risk of bias | There was no missing data at the end of the study period. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The clinical trial was registered at clinicaltrial.gov (NCT02148601). The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) Statement. There were no concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. Moreover, intention to treat analysis was performed. |
| Hota 2017 | Some concerns | No information was available on sequence generation and concealment of allocation sequence. However, the baseline characteristics of both groups were similar. We contacted the authors for further information on the randomization process but did not get a reply. | Low risk of bias | The study was not blinded however we do not believe there was a significant deviation from intended intervention due to non‐blinding nor did this affect the outcome measured. The authors of the study performed a per‐protocol analysis and we included the intention to treat analysis by considering all the participants who were randomized to FMT and vancomycin group and assumed that two participants excluded by the study authors in the analysis did not have the outcome. | Low risk of bias | Two participants were excluded from the vancomycin group, one withdrew and one was withdrawn by the investigator due to non‐compliance to protocol. We included all participants in the intention‐to‐treat analysis and assumed that those excluded did not experience the outcome. This missing data is less likely to create a bias for this outcome. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The study was registered at clinicaltrial.gov (NCT01226992). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though we had some concerns about the lack of description of methods of randomization process, we did not think that this study was at high risk of bias. The two groups were balanced at the start of the trial. Even though this was an open label study the outcome measured was objective hence low risk of bias from non‐blinding. |
| Kelly 2016 | Low risk of bias | Quote from article: "Patients were equally allocated to the donor and autologous FMT groups via block randomization by C difficile positivity at baseline, with stratification by study site." Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | This was a double‐blinded study and the authors performed an intention to treat analysis. | Low risk of bias | Two patients were lost to follow‐up in the control and one patient in the intervention group. All participants were considered in the intention to treat analysis. | Low risk of bias | The study was double‐blind, hence we did not have any concerns about the risk of bias in the measurement of this outcome. | Low risk of bias | The trial was registered at clinicaltrial.gov (NCT01703494). We did not have any concerns about selective reporting for this outcome. | Low risk of bias | This was a very well conducted double blinded study with no major concerns about risk of bias for any of the domains considered. |
| Rode 2021 | Low risk of bias | Quote from article: "Computer‐generated stratified randomization in blocks of six was used with allocation concealment in sealed opaque envelopes with sequential numbers for each stratum. " Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that two participants who did not complete the study did not have the outcome. | Low risk of bias | We analyzed data on an intention‐to‐treat basis, hence there is a low risk of bias from missing data on 2 participants. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at ClinicalTrials.gov (NCT02774382). We did not have any major concerns about selective reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome due to the objective nature of outcomes measured and intention to treat analysis. |
| van Nood 2013 | Low risk of bias | Quote from protocol: "Patients will be randomized by a computer according to their first, second or >2 relapses, and hospitalization status." Quote from Supplementary Appendix: ''To achieve adequate allocation concealment, each patient was randomized by applying automated biased coin minimization in ALEA with stratification for hospitalization status (clinical or outpatient) and the number of previous recurrences (1, 2, >2). The coin bias factor was set at 3, the bias coin lower threshold at 2. Study physicians at the coordinating center in charge of randomization were unaware of the model specifications used.'' Comment: The allocation sequence was random and appropriately concealed in this study and the baseline characteristics of the two groups were comparable. |
Low risk of bias | The authors analyzed data on a modified intention‐to‐treat basis with the exclusion of one patient who required high dose prednisolone treatment after randomization but before the study treatment was initiated. The study was not blinded however we do not believe there was a deviation from intended intervention due to non‐blinding nor did this affect the outcome measured as we included the analyzed data on an intention to treat basis by assuming that two participants who did not complete the study did not have the outcome. | Low risk of bias | All participants were included in the intention to treat analysis while assessing all‐cause mortality. | Low risk of bias | The study was open‐label. However, the outcome of all‐cause mortality is an observer‐reported outcome not involving judgement hence there is a low risk of bias. | Low risk of bias | The trial was registered at the Netherlands Trial Register number, NTR1177. We did not have any concerns about selective outcome reporting for this outcome. | Low risk of bias | Even though the study was open‐label, we did not have any major concerns about bias in the measurement of this outcome as all‐cause mortality is an observer‐reported outcome not involving judgement hence the assessment is not likely to be influenced by knowledge of intervention received. |
Acknowledgements
Cochrane Gut supported the review authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Paul Moayyedi; McMaster University, Canada; Co‐ordinating Editor of the Cochrane Gut Group;
Managing Editor (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale and Sam Hinsley, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service;
Copy Editor (copy‐editing and production): Anne Lawson, Central Production Service, Cochrane;
Peer‐reviewers (provided comments and recommended an editorial decision): Christian Lodberg Hvas, Aarhus University Hospital, Denmark (clinical/content review); Muhammad Abdel‐Gawad, Al‐Azhar University, Assiut, Egypt (clinical/content review); Rapat Pittayanon, Chulalongkorn University, Bangkok, Thailand (clinical/content review); George Lillington (consumer review), Rachel Richardson, Associate Editor, Cochrane Evidence Production and Methods directorate (methods review);
One additional peer reviewer provided search peer review, but chose not to be publicly acknowledged.
The search strategies were designed and run by Yuhong Yuan (Information Specialist, Cochrane Gut) on 16 February 2021.
The literature search was updated on 31 March 2022 by Abigail Smith (SUNY Upstate Medical University, Health Sciences Library, Syracuse, New York, USA).
Appendices
Appendix 1. Definitions of treatment failure, continuation of same CDI episode, rCDI, and new CDI
There is no uniformly agreed definition of treatment failure/recurrence after FMT, and studies varied with their definitions (Mullish 2018). The definition of rCDI is an episode that fulfils the criteria for CDI (both diarrheal symptoms and positive laboratory testing) and occurs between 2 and 8 weeks after treatment of a previous episode of CDI, provided that the symptoms of the earlier episode have resolved (McDonald 2007; McDonald 2018). This definition excludes any repeat positive laboratory result for Clostridioides within 2 weeks after the last specimen that tested positive, as this likely represents a continuation of the same CDI case (McDonald 2007). Treatment failure of CDI is defined as no response after 1 week of treatment with appropriate antibiotics (Shannon‐Lowe 2010; Vardakas 2012). If the diarrhea resolves, then restarts after 8 weeks, they will be considered to have a new CDI infection (McDonald 2007; McDonald 2018).
Appendix 2. CENTRAL search strategy (via Ovid)
Search run on 16 February 2021
(bacteriotherap* or colonic restoration or flora reconstitution or RBX2660).tw,kw.
FMT.ab.
((Fecal or Faecal or microbiota or microflora or feces or faeces or stool) adj3 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
((bacteria or bacterio*) adj2 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
or/1‐4
exp Clostridium Infections/
Clostridium difficile/
(Clostridium difficile or Clostridioides difficile or "C.difficile" or "CDAD" or "CDI" or Peptoclostridium difficile or pseudomembranous colitis).tw,kw.
(antibiotic* adj2 (diarrhea or diarrhoea)).tw,kw.
or/6‐9
5 and 10
Search run on 31 March 2022
#1 MeSH descriptor: [Fecal Microbiota Transplantation] explode all trees
#2 (bacteriotherap* OR "colonic restoration" OR "flora reconstitution" OR RBX2660):ti,ab,kw
#3 FMT:ab
#4 ((Fecal OR Faecal OR microbiota OR microflora OR feces OR faeces OR stool) NEAR/3 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)):ti,ab,kw
#5 ((bacteria OR bacterio*) NEAR/2 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)):ti,ab,kw
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH descriptor: [Clostridium Infections] explode all trees
#8 MeSH descriptor: [Clostridioides difficile] explode all trees
#9 ("Clostridium difficile" OR "Clostridioides difficile" OR "C.difficile" OR CDAD OR CDI OR "Peptoclostridium difficile" OR "pseudomembranous colitis"):ti,ab,kw
#10 (antibiotic* NEAR/2 (diarrhea or diarrhoea)):ti,ab,kw
#11 #7 OR #8 OR #9 OR #10
#12 #6 AND #11
Custom date range: 16 February 2021 to 31 March 2022
Appendix 3. MEDLINE (via Ovid)
Search run on 16 February 2021
Fecal Microbiota Transplantation/
(bacteriotherap* or colonic restoration or flora reconstitution or RBX2660).tw,kw.
FMT.ab.
((Fecal or Faecal or microbiota or microflora or feces or faeces or stool) adj3 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
((bacteria or bacterio*) adj2 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
or/1‐5
exp Clostridium Infections/
Clostridium difficile/
(Clostridium difficile or Clostridioides difficile or "C.difficile" or "CDAD" or "CDI" or Peptoclostridium difficile or pseudomembranous colitis).tw,kw.
(antibiotic* adj2 (diarrhea or diarrhoea)).tw,kw.
or/7‐10
6 and 11
randomized controlled trial.pt.
controlled clinical trial.pt.
random*.ab.
placebo.ab.
trial.ab.
groups.ab.
drug therapy.fs.
or/13‐19
exp animals/ not humans.sh.
20 not 21
12 and 22
Note: lines 13‐22 Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format. Minor revision was made: randomised.ab. and randomly.ab. was replaced by "random*.ab" to capture terms such as randomized, randomization.
Search run on 31 March 2022
1 exp Fecal Microbiota Transplantation/
2 (bacteriotherap* or colonic restoration or flora reconstitution or RBX2660).tw,kw.
3 FMT.ab.
4 ((Fecal or Faecal or microbiota or microflora or feces or faeces or stool) adj3 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
5 ((bacteria or bacterio*) adj2 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
6 or/1‐5
7 exp Clostridium Infections/
8 exp Clostridioides difficile/
9 (Clostridium difficile or Clostridioides difficile or "C.difficile" or "CDAD" or "CDI" or Peptoclostridium difficile or pseudomembranous colitis).tw,kw.
10 (antibiotic* adj2 (diarrhea or diarrhoea)).tw,kw.
11 or/7‐10
12 6 and 11
13 randomized controlled trial.pt.
14 controlled clinical trial.pt.
15 random*.mp.
16 placebo.ab.
17 trial.ab.
18 groups.ab.
19 drug therapy.fs.
20 or/13‐19
21 exp animals/ not humans.sh.
22 20 not 21
23 12 and 22
24 limit 23 to dt=20210216‐20220331
Appendix 4. Embase (via Ovid)
Search run on 16 February 2021
fecal microbiota transplantation/
feces microflora/ and exp therapy/
(bacteriotherap* or colonic restoration or flora reconstitution or RBX2660).tw,kw.
FMT.ab.
((Fecal or Faecal or microbiota or microflora or feces or faeces or stool) adj3 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
((bacteria or bacterio*) adj2 (transplant* or transfus* or implant* or instillation or donor* or enema or reconstitution or infusion* or therap* or transfer* or treat*)).tw,kw.
or/1‐6
Clostridium difficile infection/ or clostridioides difficile/
pseudomembranous colitis/
(Clostridium difficile or Clostridioides difficile or "C.difficile" or "CDAD" or "CDI" or Peptoclostridium difficile or pseudomembranous colitis).tw,kw.
(antibiotic* adj2 (diarrhea or diarrhoea)).tw,kw.
or/8‐11
7 and 12
random:.tw.
placebo:.mp.
double‐blind:.tw.
or/14‐16
exp animal/ not human/
17 not 18
13 and 19
Note: Line 14‐17. Hedge Best balance of sensitivity and specificity filter for identifying "therapy studies" in Embase. hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Search run on 31 March 2022
#25 #24 AND [16‐02‐2021]/sd NOT [31‐03‐2022]/sd
#24 #15 AND #23
#23 #19 NOT #22
#22 #20 NOT #21
#21 'human'/exp
#20 'animal'/exp
#19 #16 OR #17 OR #18
#18 'double blind':ti,ab
#17 placebo
#16 random*:ti,ab
#15 #9 AND #14
#14 #10 OR #11 OR #12 OR #13
#13 (antibiotic* NEAR/2 (diarrhea OR diarrhoea)):ti,ab,kw
#12 'clostridium difficile':ti,ab,kw OR 'clostridioides difficile':ti,ab,kw OR 'c.difficile':ti,ab,kw OR 'cdad':ti,ab,kw OR 'cdi':ti,ab,kw OR 'peptoclostridium difficile':ti,ab,kw OR 'pseudomembranous colitis':ti,ab,kw
#11 'clostridioides difficile'/exp
#10 'clostridium difficile infection'/exp
#9 #1 OR #4 OR #5 OR #6 OR #7 OR #8
#8 ((bacteria OR bacterio*) NEAR/2 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)):ti,ab,kw
#7 ((fecal OR faecal OR microbiota OR microflora OR feces OR faeces OR stool) NEAR/3 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)):ti,ab,kw
#6 fmt:ab
#5 bacteriotherap*:ti,ab,kw OR 'colonic restoration':ti,ab,kw OR 'flora reconstitution':ti,ab,kw OR rbx2660:ti,ab,kw
#4 #2 AND #3
#3 'therapy'/exp
#2'feces microflora'/exp
#1 'fecal microbiota transplantation'/exp
Appendix 5. Conference Proceedings Citation Index and ISRCTN
Search strategy run on 31 March 2022
Conference Proceedings Citation Index
9 #5 AND #8
8 #6 OR #7
7 TS=( (antibiotic* NEAR/2 (diarrhea OR diarrhoea)))
6 TS=("Clostridium difficile" OR "Clostridioides difficile" OR "C.difficile" OR CDAD OR CDI OR "Peptoclostridium difficile" OR "pseudomembranous colitis")
5 #1 OR #2 OR #3 OR #4
4 TS=(((bacteria OR bacterio*) NEAR/2 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)))
3 TS=(((Fecal OR Faecal OR microbiota OR microflora OR feces OR faeces OR stool) NEAR/3 (transplant* OR transfus* OR implant* OR instillation OR donor* OR enema OR reconstitution OR infusion* OR therap* OR transfer* OR treat*)))
2 AB=(FMT)
1 TS=(bacteriotherap* OR "colonic restoration" OR "flora reconstitution" OR RBX2660)
ISRCTN
("fecal transplant" OR "faecal transplant" OR "stool transplant" OR "stool therapy" OR "fecal microbial transplant" OR "fecal microbiota transplant" OR "fecal microbiota transplantation" OR "faecal microbiota transplant" OR "faecal microbiota transplantation" OR FMT OR bacteriotherapy OR "colonic restoration" OR "flora reconstitution" OR RBX2660) AND ("Clostridium difficile" OR "Clostridioides difficile" OR "C.difficile" OR CDAD OR CDI OR "Peptoclostridium difficile" OR "pseudomembranous colitis")
Data and analyses
Comparison 1. Fecal microbiota transplantation (FMT) versus control for the treatment of recurrent Clostridioides difficile infections (rCDI).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cammarota 2015.
| Study characteristics | |
| Methods | Single center, open‐label, randomized controlled clinical trial conducted in Italy |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Fresh donor feces solution infusion after pretreatment with vancomycin for 3 days and bowel lavage 1 or 2 days before FMT; n = 20 Route: colonoscopy Frequency: every 3 days if the participant had pseudomembranous colitis until resolution Weight of stool: mean 152 g Volume per treatment: 500 mL Donor: healthy relative or unrelated volunteers Comparison Standard vancomycin 125 mg orally 4 times daily for 10 days, followed by a pulse regimen (125–500 mg/day every 2–3 days) for ≥ 3 weeks; n = 19 Donor screening
|
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes | Recurrence after treatment was defined as diarrhea (≥ 3 loose or watery stools per day for ≥ 2 consecutive days, or ≥ 8 loose stools in 48 hours) unexplainable by other causes, with or without positive stool toxin within 10 weeks from end of therapy. This is different from a recurrence of new CDI per our protocol. The authors performed analysis on an intention‐to‐treat basis. The trial was stopped 1 year earlier. Funding: (quote) "The study was in part funded by the Catholic University of Rome, Line D‐1 research funding". |
Hota 2017.
| Study characteristics | |
| Methods | Single‐site, open‐label, randomized controlled trial conducted in Canada |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Fresh donor feces solution enema given 48 hours after pretreatment with vancomycin for 14 days; n = 16 Route: enema Frequency: single Weight of stool: 50 g Volume per treatment: 500 mL Donor: healthy relative or unrelated volunteers Comparison Vancomycin 14 days of standard dosing (125 mg orally every 6 hours) followed by a taper over 4 weeks; n = 14 Donor screening Healthy adult aged ≥ 18 years screened using a self‐screening questionnaire of behaviors associated with risk for blood‐borne pathogens, study physician assessment, and blood and stool testing for potentially transmissible infections and screening were developed in consultation with Health Canada. |
| Outcomes |
Primary outcome
Secondary outcomes
Safety outcomes
|
| Notes | Recurrence was described as symptomatic, laboratory‐confirmed CDI within 120 days of the intervention and this is different from recurrence of new CDI per our protocol. The study authors performed a per‐protocol analysis. We created an intention‐to‐treat analysis by considering all the participants who were randomized to FMT and vancomycin group. We also performed a sensitivity analysis on an as‐available basis. The authors reported lack of resolution of rCDI and our primary outcome was resolution of rCDI. We subtracted the ones whose rCDI did not resolve from the total randomized to obtain the participants with resolution of rCDI. Funding for the study: (quote) "This work was supported by the Physicians Services Incorporated Foundation (grant number PSI 10‐2021); Public Health Ontario; University of Toronto Department of Medicine Integrating Challenge Grant; University Health Network; and Sinai Health System (in kind)." |
Hvas 2019.
| Study characteristics | |
| Methods | Single center, randomized, active‐comparator, open‐label clinical trial conducted in Denmark |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Frozen‐thawed single‐donor solution of donor feces was applied after pretreatment with vancomycin for 4–10 days and bowel lavage 1 or 2 days before FMT; n = 24 Route: nasoduodenal or colonoscopy (depending on tolerance) Frequency: up to 2 times if needed Weight of stool: 50 g Volume per treatment: – Donor: healthy unrelated volunteer Comparison (2 groups)
Donor screening
Recurrence was described as clinical relapse and a positive C difficile test result before or at 8 weeks after the allocated treatment, this is different from recurrence of new CDI per our protocol. |
| Outcomes |
Primary outcome
Secondary outcome
Safety outcomes
|
| Notes | The study author performed an intention‐to‐treat analysis. For our analysis, we used the total of the 2 comparison groups as the single control group (fidaxomicin + vancomycin). Authors reported resolution of rCDI based on 2 definitions. We included the definition based on resolution of diarrhea + negative test for Cdifficile The data on serious adverse events were taken from the supplementary document. Funding: (quote) "This study was financed by the Danish Regions (grant 14/217). The funder had no access to the data and had no influence on the study presentation." |
Kelly 2016.
| Study characteristics | |
| Methods | Dual‐center, double‐blind, randomized controlled trial conducted in the US |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Fresh donor feces solution infusion after bowel lavage the day before FMT; n= 22 Route: colonoscopy Frequency: single Weight of stool: mean 64 g Volume per treatment: 500 mL Donor: healthy relative or unrelated volunteers screened using questionnaires, and blood and stool laboratory testing. Comparison Autologous feces solution infusion after bowel lavage the day before FMT; n = 24 Donor screening
|
| Outcomes |
Primary outcome
Secondary outcome
Safety endpoints
|
| Notes | Study authors described late CDI recurrence as after 8 weeks, which is similar to the recurrence of new CID per our protocol. Study authors performed analysis on an intention‐to‐treat basis. We also recreated the analysis on an as‐available basis taking into account any missing data. Funding: National Institute of Diabetes and Digestive and Kidney Diseases |
Rode 2021.
| Study characteristics | |
| Methods | Open‐label, multicenter randomized controlled trial conducted in Denmark |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Frozen–thawed donor feces solution given 36 hours after pretreatment with vancomycin for 1–14 days; n = 34 Route: enema Frequency: 1–3 infusions Weight of stool: 50 g Volume per treatment: 170 mL Donor: healthy relative or unrelated volunteers Comparison
Donor screening Used frozen donor stool from a donor stool bank with extensively tested universal donors recruited from the Danish Blood Donor Corps. |
| Outcomes |
Primary outcome
Secondary outcome
Safety outcomes
|
| Notes | Study authors did not comment on the recurrence of a new CDI. Study authors analyzed the primary endpoint on intention‐to‐treat, modified intention‐to‐treat, and per‐protocol basis. We added both intention‐to‐treat and as‐available analysis for primary outcomes to the review. For our analysis, we used the total of the 2 comparison groups as the control. Funding: (quote) "This study was funded by Ministeriet Sundhed Forebyggelse, The Research Council for Naestved/Ringste /Slagelse Hospital, Hvidovre Hospital, The Research fund of the Department of Infectious Disease, Hvidovre Hospital, The Christenson‐Cesons Family Foundation and the Region Sjælland. None of the funders had any influence on designing the study, analysing data or writing the manuscript." |
van Nood 2013.
| Study characteristics | |
| Methods | Open‐label, randomized controlled trial conducted in the Netherlands |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Fresh donor feces solution infusion after pretreatment with vancomycin for 4–5 days and bowel lavage the day before FMT; n = 17 Route: nasoduodenal tube Frequency: up to 2 times if needed Weight of stool: mean 141 g Volume per treatment: 500 mL Donor: healthy unrelated volunteers Comparison
Donor screening
|
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes | Study authors performed analysis on a modified intention‐to‐treat basis with the exclusion of 1 participant who required high‐dose prednisolone treatment after randomization but before the study treatment was initiated. We recreated an intention‐to‐treat analysis and also included an as‐available analysis. For our analysis, we used the total of the 2 comparison groups as the control. Funding: (quote) "Supported by grants from the Netherlands Organization for Health Research and Development (ZonMW, 170881001; VENI grant, MN: 016096044) and a Spinoza Award (to Dr. de Vos) from the Netherlands Organization for Scientific Research." |
CDI: Clostridioides difficile infection; EBV: Epstein‐Barr virus; EIA: enzyme immunoassay; FMT: fecal microbiota transplantation; GI: gastrointestinal; ICU: intensive care unit; MDR: multiple drug‐resistant; MRSA: methicillin‐resistant Staphylococcus aureus; n: number of participants; PCR: polymerase chain reaction; VRE: vancomycin‐resistant enterococci.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allegretti 2016 | Wrong comparator; low‐ and high‐dose FMT capsules. |
| Allegretti 2019 | Wrong comparator; this is a comparison of FMT delivered via gastric release and targeted colonic release capsules |
| Cicerone 2017 | Wrong comparator; full publication not available but seemed to not have a 'no FMT group' and, therefore, it would not qualify as a randomized controlled trial. |
| Dupont 2017 | Wrong comparator; encapsulated lyophilized FM given once or on 2 successive days vs frozen FM product given by single retention enema. |
| Fischer 2015 | Wrong comparator; low‐ and high‐dose FMT capsules. |
| Friedman‐Korn 2018 | Wrong study design; prospective cohort observational study. |
| Garza‐Gonzalez 2019 | Wrong comparator; comparison of FMT vs FMT enriched with Lactobacillus. |
| Ianiro 2018 | Wrong comparator; single‐infusion FMT, including a vancomycin antibiotic regimen plus a single administration of feces by colonoscopy; or multiple‐infusion FMT, including a vancomycin antibiotic regimen plus multiple fecal infusions. |
| Jiang 2017 | Wrong comparator; comparison of fresh, frozen, and lyophilized FMT. |
| Jiang 2018b | Wrong comparator; comparison of lyophilized fecal microbiota vs frozen FMT. |
| Jiang 2018c | Wrong comparator; lower GI administration (retention enema with frozen product) versus upper GI route (oral administration of lyophilized product in enteric‐coated capsules). |
| JPRN‐UMIN000016900 | Wrong study design; single arm non‐randomized trial. |
| JPRN‐UMIN000019181 | Wrong study design; single arm non‐randomized trial. |
| JPRN‐UMIN000020766 | Wrong study design; single arm non‐randomized trial. |
| Kao 2017 | Wrong comparator; FMT by oral capsule or colonoscopy at 1:1 ratio. |
| Kates 2020 | Wrong indication; FMT was given to participant with prior history of CDI while on antibiotics to prevent recurrence of rCDI, not to treat rCDI. |
| Lee 2016 | Wrong comparator; comparison of fresh vs frozen FMT. |
| Lee 2019 | Wrong study design; retrospective study. |
| Martinez 2018 | Wrong study design; observational study. |
| NCT01398969 | Wrong comparator; fresh vs frozen‐and‐thawed FMT. |
| NCT01704937 | Wrong comparator; FMT delivery by nasogastric tube or colonoscopy. |
| NCT02254811 | Wrong comparator; delivery via capsules vs colonoscopy. |
| NCT02318992 | Wrong comparator; comparison of fresh vs frozen vs lyophilized FMT. |
| NCT03298048 | Wrong comparator; comparison of low‐ vs mid‐ vs high‐dose FMT. |
| NCT03427229 | Wrong comparator; aimed to assess if multiple‐infusion FMT is more effective than single‐infusion FMT in curing severe CDI. |
| NCT03804736 | Wrong comparator; comparison of FMT vs FMT enriched with Lactobacillus |
| Satokari 2015 | Wrong comparator; this is a comparison of fresh vs frozen feces for FMT and had no control group; also it was a retrospective non‐randomized study. |
| Youngster 2014 | Wrong comparator; comparison of FMT administered via colonoscopy vs nasogastric tube. |
CDI: Clostridioides difficile infection; FM: fecal microbiota; FMT: fecal microbiota transplantation; GI: gastrointestinal; rCDI: recurrent Clostridioides difficile infection.
Characteristics of studies awaiting classification [ordered by study ID]
Dubberke 2018.
| Methods | Randomized, double‐blind, placebo‐controlled, phase 2b study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Group A: 2 enemas of RBX2660 (microbiota suspension) administered 7 days apart Group C: 1 enema of RBX2660 (microbiota suspension) and 1 enema of placebo (a suspension of saline and cryoprotectant) administered 7 days apart Comparison Group B: 2 enemas of placebo (a suspension of saline and cryoprotectant) administered 7 days apart |
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes | Based on the proprietary nature and our lack of access to procedures between the collection of donor stool and shipping of the RBX2660 microbiota suspension, it is unclear at the time of this publication whether the RBX2660 'microbiota suspension' technically qualifies as FMT per se. We will contact the study authors to request additional details and this study may qualify for inclusion in future versions of this systematic review and meta‐analysis. |
Kao 2019.
| Methods | Randomized, controlled, pilot study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Single dose of 15 capsules of lyophilized donor stool Comparison Single dose of 15 capsules of lyophilized sterile fecal filtrate |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | While this study meets criteria and has been completed there was not enough information for us to complete the risk of bias assessment and include the data in the analysis. |
NCT03353506.
| Methods | Double‐blind, randomized, controlled, pilot study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 dose of 15 lyophilized fecal microbiota transplant capsules Comparison 1 dose of 15 lyophilized sterile fecal filtrate capsules |
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes | It appears this small pilot study has been completed but there are no published data we are aware of. |
NCT03548051.
| Methods | Multicenter, randomized, placebo‐controlled, partially blinded trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 100 g of thawed processed stool diluted into 250 mL of saline and delivered by retention enema given 1–3 hours after loperamide 4 mg orally × 1 Comparison 250 mL of saline delivered by retention enema given 1–3 hours after loperamide 4 mg orally × 1 |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | Terminated (low enrollment) |
CDAD: Clostridioides difficile‐associated diarrhea; CDI: Clostridioides difficile infection; EIA: enzyme immunoassay; FMT: fecal microbiota transplantation; PCR: polymerase chain reaction; rCDI: recurrent Clostridioides difficile infection.
Characteristics of ongoing studies [ordered by study ID]
Drekonja 2021.
| Study name | Microbiota or placebo after antimicrobial therapy for recurrent Clostridioides difficile at home: a clinical trial with novel home‐based enrollment |
| Methods | Randomized controlled trial |
| Participants | Aged ≥ 18 years |
| Interventions | Intervention: oral capsule‐delivered FMT Control: oral capsule‐delivered placebo |
| Outcomes |
Primary outcomes
Secondary outcome
Other outcome
Safety outcomes
|
| Starting date | 29 December 2016 |
| Contact information | jane.zhang@va.gov tassos.kyriakides@va.gov |
| Notes |
EUCTR2015‐003062‐82‐DK.
| Study name | Rectal enema with a mix of gut bacteria, rectal enema with fecal material from a healthy donor or oral given vancomycin for the treatment of patients with recurrent diarrhea caused by infection with the bacteria Clostridium Difficile |
| Methods | Randomized controlled trial (not blinded) |
| Participants | Adults aged ≥ 18 years |
| Interventions | Intervention: FMT Participants were pretreated with oral vancomycin 125 mg 4 times a day for 7–14 days. This was discontinued 36 hours prior to FMT. Frozen donor stool from a donor stool bank was administered by rectal enema once, but with a possibility to repeat it up to twice within 14 days after the first infusion. The indication for repetition was ongoing or new‐onset diarrhea (≥ 3 loose or liquid stools per day), as judged by a trial physician, without new testing for C difficile. They used a different donor when repeating FMTs. Control 1: RBT The standardized laboratory‐based bacterial mixture used for RBT consisted of 12 bacterial strains suspended in 200 mL isotonic saline with concentrations of 5 × 1010 bacteria of each strain. Included strains: Escherichia coli MT‐1108‐1, E coli MT‐1109, Enterococcus cassiliflavus, Enterococcus gallinarum, Bacteroides thetaiotaomicron, Bacteroides ovatus, Bacteroides vulgatus, Clostridium bifermentans, C innocuum, Coprobacillus cateniformis, LactobacilIus rhamnosus, and LactobacilIus gasserii. Participants were pretreated with oral vancomycin 125 mg 4 times a day for 7–14 days. This was discontinued 12 hours prior to RBT. RBT was administered by rectal enema with 3 infusions on 3 consecutive days for all participants in this group. Control 2: oral vancomycin All participants in the vancomycin group received monotherapy with oral capsule vancomycin 125 mg 4 times daily for 14 days. Furthermore, participants with ≥ 2 recurrences of CDI were treated with an additional 5 weeks of tapering as recommended in guidelines. The tapering regimen included oral vancomycin 125 mg twice daily for 1 week, 125 mg once daily for 1 week, 125 mg every other day for 1 week, and 125 mg every third day for 2 weeks. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 1 May 2017 |
| Contact information | Clinical Trial Information, Department of Medicine, Zealand University Hospital, Køge, Denmark, +45 23345235, aala@regionsjaelland.dk |
| Notes |
NCT02255305.
| Study name | FMT versus antimicrobials for initial treatment of recurrent CDI |
| Methods | Open‐label, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 50 g of fecal material given via retention enema after pretreatment with antimicrobials targeting Cdifficile Comparison Antimicrobials targeting C difficile |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2015 |
| Contact information | Becky Smith MD, Principal Investigator, NorthShore University HealthSystem |
| Notes | No results posted |
NCT02774382.
| Study name | Rectal bacteriotherapy, fecal microbiota transplantation or oral vancomycin treatment of recurrent Clostridium difficile infections |
| Methods | Randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 1 May 2017 |
| Contact information | Andreas M Petersen MD, Principal Investigator, Hvidovre University Hospital |
| Notes | Estimated enrollment: 450 Status: unknown |
NCT03005379.
| Study name | Microbiota or placebo after antimicrobial therapy for recurrent C. difficile at home (MATCH) |
| Methods | Randomized clinical trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Oral capsule‐delivered FMT Comparison Oral capsule‐delivered placebo |
| Outcomes |
Primary outcomes Recurrent CDI (definite or probable) or death within 56 days of randomization. Definite recurrence defined as any of the following: new onset of > 3 loose or watery stools in 24 hours for 2 consecutive days; other clinical symptoms including ileus, toxic mega colon, or colectomy plus laboratory confirmation of C difficile from a stool specimen. Probable recurrence defined as the same clinical manifestations as above, but without laboratory confirmation of C difficile (stool test not sent, negative result, or uninterpretable result) Secondary outcomes
|
| Starting date | 15 November 2018 |
| Contact information | Dimitri M Drekonja MD, study chair, Minneapolis VA Health Care System, Minneapolis, Minnesota |
| Notes | Estimated enrollment: 390 |
NCT03053505.
| Study name | A novel faecal microbiota transplantation system for treatment of primary and recurrent Clostridium difficile infection (FMTREAT) |
| Methods | 2‐arm, interventional, prospective, open‐label, multicenter trial |
| Participants |
Inclusion criteria Group "R" (non‐randomized group)
Group "F" (randomized group):
In all cases, primary consideration must be given to the severity and pace of the patient's CDI when deciding whether early use of FMT is appropriate to prevent further clinical deterioration. Exclusion criteria
|
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | January 2017 |
| Contact information | Gergely G Nagy, Study Chair, University of Debrecen |
| Notes | No results available |
NCT03806803.
| Study name | Multicentre blinded comparison of lyophilized sterile fecal filtrate to lyophilized fecal microbiota transplant in recurrent Clostridioides difficile infection |
| Methods | Double‐blind, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention Lyophilized fecal microbiota transplant capsules Comparison Lyophilized cell free fecal slurry, free of any live bacteria |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | January 2019 |
| Contact information | Dina Kao MD, Principal Investigator, University of Alberta |
| Notes | No results posted |
NCT03970200.
| Study name | PMT for severe‐CDI |
| Methods | Randomized, open label, comparative, phase 2 study |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 16 January 2020 |
| Contact information | Brendan J Kelly MD, Hospital of the University of Pennsylvania |
| Notes | Estimated enrollment: 90 Still recruiting |
NCT04885946.
| Study name | Fecal microbiota transplantation for early Clostridioides difficile infection (EarlyFMT) |
| Methods | Double‐blind, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparator
Open‐label for screened, but not randomized participants with fulminant CDI (considered unethical to give placebo) |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | May 2021 |
| Contact information | Christian L Hvas PhD, Consultant, Aarhus University Hospital |
| Notes | No results posted |
NCT04960306.
| Study name | Fecal filtrate as a treatment option of multiple recurrent Clostridioides difficile infection (FILTRATE) |
| Methods | Triple‐blinded, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 5–8 encapsulated lyophilized fecal filtrate transplantations in enterosolvent, size '0' capsules Control 5–8 encapsulated lyophilized conventional FMTs in enterosolvent, size '0' capsules |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | July 2021 |
| Contact information | Hegyi Péter MD, PhD, DSc, Principal Investigator, University of Pecs, Hungary |
| Notes | No results posted |
NCT05077085.
| Study name | Bezlotoxumab versus FMT for multiple recurrent CDI (BSTEP) |
| Methods | Open‐label, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparator
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | October 2021 |
| Contact information | Joffrey van Prehn MD, PhD, Clinical Microbiologist, Leiden University Medical Center |
| Notes | No results posted |
NCT05201079.
| Study name | Recurrent Clostridioides difficile infection treatment with capsules of lyophilised faecal microbiota vs fidaxomicin |
| Methods | Open‐label, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | January 2022 |
| Contact information | Javier Cobo MD, Principal Investigator, Hospital Universitario Ramon y Cajal, Madrid, Spain |
| Notes | No results posted |
NCT05266807.
| Study name | Fecal microbiota transplantation versus vancomycin or fidaxomicin in Clostridioides difficile infection first recurrence (FENDER) |
| Methods | Open‐label, randomized controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Comparison
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | March 2022 |
| Contact information | Benoit Guery MD, Principal Investigator, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland |
| Notes | No results posted |
ANC: absolute neutrophil count; CD: Clostridioides difficile; CDI: Clostridioides difficile infection; CTCAE V5.0: Common Terminology Criteria for Adverse Events Version 5.0; EIA: enzyme immunoassay; ESCMID: European Society of Clinical Microbiology and Infectious Diseases; FMT: fecal microbiota transplantation; GI: gastrointestinal; ICU: intensive care unit; PCR: polymerase chain reaction; RBT: rectal bacteriotherapy; rCDI: recurrent Clostridioides difficile infection; WBC: white blood cell count.
Differences between protocol and review
We made the following changes from our protocol (Imdad 2021).
We had planned to compare "stool bank" versus "non‐stool bank" storage of FMT. These titles were changed to "fresh stool (of non‐stool bank origin)" versus "frozen then thawed stool (of stool bank origin)" to be more specific regarding the storage/handling of the stool. This subgroup analysis was not conducted due to the low number of included studies.
Individuals with inflammatory bowel disease (IBD) experience CDI at a higher rate than the general population, have a higher rate of rCDI, are often on immunosuppressive medication, and show a less robust increase in gut microbial diversity after FMT than people without IBD (Khanna 2017; Razik 2016). For these reasons, we had planned to exclude participants with IBD from our analysis, however, as only Hvas 2019 explicitly stated that it included people with IBD, and they were less than 25% of those in the study, we decided not to exclude this study, to maximize the total number of participants in our study rather than exclude a study based on a small number of participants with IBD. To assess whether this could have impacted our results significantly we completed sensitivity analyses on all outcomes in the summary of findings table excluding the Hvas 2019 study, none of which found the exclusion of this study to impact the outcomes significantly.
The data on 'treatment failure' and 'new CDI after successful treatment' could not be distinguished from 'recurrence of CDI', so these outcomes were not available from the included studies. We expected differences between included studies in the definitions of treatment efficacy, treatment failure, and what they defined as rCDI as opposed to a new case of CDI after a previously successful FMT. We planned to standardize the definitions of treatment failure, rCDI, and new CDI across all studies as per the definitions in Appendix 1. However, we found no papers that used these definitions nor were we able to apply these time‐bound definitions to the raw data from the included studies based on the way the studies themselves reported the results. We used the definition of efficacy as defined by the included studies, as long as it encompassed a resolution of symptoms after treatment, as we planned a priori.
We planned to assess the risk of bias for the outcomes of treatment failure, colectomy, and mortality; however, data were only available for mortality. We additionally assessed the risk of bias for serious adverse events and resolution of rCDI.
We initially planned to use a fixed‐effect model to synthesize data; however, we decided to use the random‐effects model to adjust for any heterogeneity across the studies. We completed sensitivity analyses to determine if this impacted the outcomes reported in the summary of findings table significantly; this showed minimal impact.
We had planned a sensitivity analysis comparing studies with a high risk of bias versus those with low risk of bias/some concerns; however, there were no studies at high risk of bias.
We did not conduct any of the planned subgroup analysis as the number of included studies was fewer than 10 and it is very unlikely that an investigation of heterogeneity will produce useful findings unless there are at least 10 studies in a meta‐analysis.
Contributions of authors
The contributions of authors based on tasks of the review are as follows.
Conception of the review: AI
Design of the review; NZM, AI, MN, SA, JPZ, ETS
Co‐ordination of the review: AI, NZM
Search and selection of studies for inclusion in the review: SHA, MM, NZM, AI
Collection of data for the review: SHA, MM, AI
Assessment of the risk of bias in the included studies: SHA, MM, AI
Analysis of data: SHA, MM, AI
Assessment of the certainty in the body of evidence: AI
Interpretation of data: AI, MN, SA, ETS, JPZ, NZM
Writing of the review: NZM, AI
All the authors reviewed and agreed to the manuscript before submission. AI is the guarantor of the review.
Sources of support
Internal sources
-
None, Other
None
External sources
-
National Institute of Allergy and Infectious Diseases K23 award (No.1K23AI156132‐01), USA
Dr. Maribeth Nicholson is supported by a grant from NIH
Declarations of interest
NZM: none.
AI: none.
SA: none.
MM: none.
ETS: none.
JZ: Dr Zackular's research laboratory receives research funding from BioNTech, but Dr Zackular does not receive any compensation for this work.
SA: none.
MN: National Institute of Allergy and Infectious Diseases K23 award [No. 1K23AI156132‐01] to MRN. Dr Nicholson serves on a data and safety monitoring board for Summit Therapeutics.
New
References
References to studies included in this review
Cammarota 2015 {published data only}
- Cammarota G, Masucci L, Ianiro G, Bibbo S, Dinoi G, Costamagna G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Alimentary Pharmacology and Therapeutics 2015;41(9):835-43. [DOI: 10.1111/apt.13144] [DOI] [PubMed] [Google Scholar]
Hota 2017 {published data only}
- Hota SS, Sales V, Tomlinson G, Salpeter MJ, McGeer A, Coburn B, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an open-label, randomized controlled trial. Clinical Infectious Diseases 2017;64(3):265-71. [DOI: 10.1093/cid/ciw731] [DOI] [PubMed] [Google Scholar]
Hvas 2019 {published data only}
- Hvas CL, Jørgensen SM, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology 2019;156(5):1324-32. [DOI: 10.1053/j.gastro.2018.12.019] [DOI] [PubMed] [Google Scholar]
Kelly 2016 {published data only}
- Kelly CR, Khoruts A, Staley C, Sadowsky MJ, Abd M, Alani M, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection – a randomized trial. Annals of Internal Medicine 2016;165(9):609-16. [DOI: 10.7326/M16-0271] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C, Kelly CR, Brandt LJ, Khoruts A, Sadowsky MJ. Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. mBio 2016;7(6):e01965-16. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rode 2021 {published data only}
- Rode AA, Chehri M, Krogsgaard LR, Heno KK, Svendsen AT, Ribberholt I, et al. Randomised clinical trial: a 12-strain bacterial mixture versus faecal microbiota transplantation versus vancomycin for recurrent Clostridioides difficile infections. Alimentary Pharmacology and Therapeutics 2021;53(9):999-1009. [DOI: 10.1111/apt.16309] [DOI] [PubMed] [Google Scholar]
van Nood 2013 {published data only}
- Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. New England Journal of Medicine 2013;368(5):407-15. [DOI: 10.1056/NEJMoa1205037] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allegretti 2016 {published data only}
- Allegretti JR, Fischer M, Papa E, Elliott R J, Klank J, Mendolia G, et al. Fecal microbiota transplantation delivered via oral capsules achieves microbial engraftment similar to traditional delivery modalities: safety, efficacy and engraftment results from a multi-center cluster randomized dose-finding study. American Gastroenterological Association Abstracts 2016;150(4):S540. [DOI: 10.1016/S0016-5085(16)31855-8] [DOI] [Google Scholar]
- Allegretti JR, Fischer M, Papa E, Elliott R J, Klank J, Mendolia G, et al. Fecal microbiota transplantation delivered via oral capsules achieves microbial engraftment similar to traditional delivery modalities: safety, efficacy and engraftment results from a multi-center cluster randomized dose-finding study. Digestive Disease Week 2016;1:S540. [Google Scholar]
Allegretti 2019 {published data only}
- Allegretti JR, Fischer M, Sagi SV, Bohm ME, Fadda HM, Ranmal SR, et al. Fecal microbiota transplantation capsules with targeted colonic versus gastric delivery in recurrent Clostridium difficile infection: a comparative cohort analysis of high and lose dose. Digestive Diseases and Sciences 2019;64:1672-8. [DOI: 10.1007/s10620-018-5396-6] [DOI] [PubMed] [Google Scholar]
Cicerone 2017 {published data only}
- Cicerone C, Bruno G, Lamonaca L, D'Abramo A, Oliva A, Zingaropoli MA. Fecal microbiota transplantation via enema for recurrent Clostridium difficile infection modulates the inflammatory host response and restores intestinal dysbiosis. Digestive and Liver Disease 2017;49:e103. [DOI: 10.1016/S1590-8658(17)30354-7] [DOI] [Google Scholar]
Dupont 2017 {published data only}
- Dupont H, Jiang ZD, Alexander A, Ajami N, Petrosino JF, DuPont AW, et al. Lyophilized fecal microbiota transplantation capsules for recurrent Clostridium difficile infection. Open Forum Infectious Diseases 2017;4(Suppl 1):S381. [DOI: 10.1093/ofid/ofx163.943] [DOI] [Google Scholar]
Fischer 2015 {published data only}
- Fischer M, Allegretti JR, Smith M, Klank MJ, Mendolia G, Vo E, et al. A multi-center, cluster randomized dose-finding study of fecal microbiota transplantation capsules for recurrent Clostridium difficile infection. United European Gastroenterology Journal 2015;3(6):565-66. [DOI: 10.1177/2050640615616068] [DOI] [Google Scholar]
Friedman‐Korn 2018 {published data only}
- Friedman-Korn T, Livovsky DM, Maharshak N, Aviv CN, Paz K, Bar-Gil SA, et al. Fecal transplantation for treatment of Clostridium difficile infection in elderly and debilitated patients. Digestive Diseases and Sciences 2018;63:198-203. [DOI: 10.1007/s10620-017-4833-2] [DOI] [PubMed] [Google Scholar]
Garza‐Gonzalez 2019 {published data only}
- Garza-Gonzalez E, Mendoza-Olazaran S, Morfin-Otero R, Ramirez-Fontes A, Rodriguez-Zulueta P, Flores-Trevino S, et al. Intestinal microbiome changes in fecal microbiota transplant (FMT) vs. FMT enriched with Lactobacillus in the treatment of recurrent Clostridioides difficile infection. Canadian Journal of Gastroenterology and Hepatology 2019;2019:4549298. [ARTICLE ID: 4549298] [DOI: 10.1155/2019/4549298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ianiro 2018 {published data only}
- Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection – single versus multiple infusions. Alimentary Pharmacology and Therapeutics 2018;48:152-9. [DOI] [PubMed] [Google Scholar]
- Ianiro G, Masucci L, Quaranta G, Simonelli C, Rizzatti G, Lopetuso L, et al. Randomized clinical trial: single-infusion FMT versus multiple-infusion FMT for the treatment of severe C. difficile infection. Digestive and Liver Disease 2018;50(2 Suppl):e66-7. [Google Scholar]
Jiang 2017 {published data only}
- Jiang ZD, Ajami NJ, Petrosino JF, Jun G, Hanis CL, Shah M, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridium difficile infection – fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Alimentary Pharmacology and Therapeutics 2017;45:899-908. [DOI: 10.1111/apt.13969] [DOI] [PubMed] [Google Scholar]
Jiang 2018b {published data only}
- Jiang ZD, Jenq RR, Ajami NJ, Petrosino JF, Alexander AA, Shi KS, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLOS One 2018;13(11):e0205064. [DOI: 10.1371/journal.pone.0205064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2018c {published data only}
- Jiang ZD, Jenq R, Ajami N, DuPont HL. The effect of type of inoculum and administration route of fecal microbiota transplantation in subjects with recurrent Clostridium difficile infection. Gastroenterology 2018;154(6):S892. [Google Scholar]
JPRN‐UMIN000016900 {published data only}
- JPRN-UMIN000016900. Fecal microbiota transplantation for Clostridium difficile infection. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000016900 (first received 24 March 2015).
JPRN‐UMIN000019181 {published data only}
- JPRN-UMIN000019181. Fecal microbiota transplantation for refractory Clostridium difficile infection. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000019181 (first received 1 November 2015).
JPRN‐UMIN000020766 {published data only}
- JPRN-UMIN000019181. Fecal microbiota transplantation for Clostridium difficile infection. trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000020766 (first received 27 January 2016).
Kao 2017 {published data only}
- Kao D, Roach B, Beck P, Hotte N, Madsen K, Louie T. A dual center, randomized trial comparing colonoscopy and oral capsule delivered fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection: preliminary results. American Journal of Gastroenterology 2015;110:S553. [Google Scholar]
- Kao D, Roach B, Hotte N, Silva M, Madsen K, Beck P, et al. A prospective, dual center, randomized trial comparing colonoscopy versus capsule delivered fecal microbiota transplantation in the management of recurrent Clostridium difficile infection. Canadian Journal of Gastroenterology and Hepatology 2016;318(20):71-2. [Google Scholar]
- Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection a randomized clinical trial. Journal of the American Medical Association 2017;318(20):1985-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kates 2020 {published data only}
- Kates AE, Gaulke I, De Wolfe T, Zimbric M, Haight K, Watson L, et al. Fecal microbiota transplantation for patients on antibiotic treatment with C. difficile infection history (GRAFT): study protocol for a phase II, randomized, double-blind, placebo-controlled trial to prevent recurrent C. difficile infections. Contemporary Clinical Trials Communications 2020;18:6. [DOI: 10.1016/j.conctc.2020.100576] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03621657. The GRAFT study: gut recolonization by fecal transplantation (GRAFT) [A phase II randomized, double-blind placebo-controlled trial to determine if fecal microbiota transplantation is efficacious for hospitalized patients with C. difficile infection history during antibiotic treatment]. clinicaltrials.gov/ct2/show/NCT03621657 (first received 8 august 2018). [NATIONAL CLINICAL TRIAL NUMBER: NCT03621657]
Lee 2016 {published data only}
- Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection a randomized clinical trial. Journal of the American Medical Association 2016;315(2):142-9. [DOI: 10.1001/jama.2015.18098] [DOI] [PubMed] [Google Scholar]
Lee 2019 {published data only}
- Lee CH, Chai J, Hammond K, Jeon SR, Patel Y, Goldeh C, et al. Long-term durability and safety of fecal microbiota transplantation for recurrent or refractory Clostridioides difficile infection with or without antibiotic exposure. European Journal of Clinical Microbiology & Infectious Diseases 2019;38:1731-5. [DOI: 10.1007/s10096-019-03602-2] [DOI] [PubMed] [Google Scholar]
Martinez 2018 {published data only}
- Martinez C, Edwards J, Hassoun A. Commercialized fecal microbiota transplantation provides efficacious treatment of Clostridium difficile infection. Infectious Diseases 2018;50(11-2):864-7. [DOI: 10.1080/23744235.2018.1500709] [DOI] [PubMed] [Google Scholar]
NCT01398969 {published data only}
- NCT01398969. Multi-centre trial of fresh vs. frozen-and-thawed FMT for recurrent CDI. clinicaltrials.gov/ct2/show/NCT01398969 (first received 11 July 2011). [NATIONAL CLINICAL TRIAL NUMBER: NCT01398969]
NCT01704937 {published data only}
- NCT01704937. Fecal microbiota transplant (FMT) for relapsing C. difficile infection in adults and children using a frozen inoculum. clinicaltrials.gov/ct2/show/NCT01704937 (first received 12 October 2012). [NATIONAL CLINICAL TRIAL NUMBER: NCT01704937]
NCT02254811 {published data only}
- NCT02254811. FMT delivered by capsule versus colonoscopy for recurrent C. diff [A prospective, multi-center, randomized trial of fecal microbiota transplantation (FMT) delivered by capsule vs colonoscopy in the management of recurrent Clostridium difficile infection (CDI)]. clinicaltrials.gov/ct2/show/NCT02254811 (first received 2 October 2014). [NATIONAL CLINICAL TRIAL NUMBER: NCT02254811]
NCT02318992 {published and unpublished data}
- NCT02318992. Fresh, frozen or lyophilized fecal microbiota transplantation for multiple recurrent C. difficile associated diarrhea. clinicaltrials.gov/ct2/show/NCT02318992 (first received 18 December 2014). [NATIONAL CLINICAL TRIAL NUMBER: NCT02318992]
NCT03298048 {published and unpublished data}
- NCT03298048. Dose ranging study of the safety and efficacy of orally administered lyophilized fecal microbiota product (PRIM-DJ2727) for the treatment of recurrent Clostridium difficile infection (Cdi). clinicaltrials.gov/ct2/show/NCT03298048 (first received 29 September 2017). [NATIONAL CLINICAL TRIAL NUMBER: NCT03298048]
NCT03427229 {published data only}
- NCT03427229. Fecal microbiota transplantation for severe Clostridium difficile infection [Randomized clinical trial: single versus multiple-infusion fecal microbiota transplantation for severe Clostridium difficile infection]. clinicaltrials.gov/ct2/show/NCT03427229 (first received 9 February 2018). [NATIONAL CLINICAL TRIAL NUMBER: NCT03427229]
NCT03804736 {published data only}
- NCT03804736. Fecal microbiota transplant by oral capsules enriched with Lactobacilli for recurrent Clostridium difficile infection. clinicaltrials.gov/ct2/show/NCT03804736 (first received 15 January 2019). [NATIONAL CLINICAL TRIAL NUMBER: NCT03804736]
Satokari 2015 {published data only}
- Satokari R, Mattila E, Kainulainen V, Arkkila PE. Simple faecal preparation and efficacy of frozen inoculum in faecal microbiota transplantation for recurrent Clostridium difficile infection – an observational cohort study. Alimentary Pharmacology & Therapeutics 2015;41(1):46-53. [DOI: 10.1111/apt.13009] [DOI] [PubMed] [Google Scholar]
Youngster 2014 {published data only}
- Youngster I, Sauk J, Pindar C, Wilson RG, Kaplan JL, Smith MB, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clinical Infectious Diseases 2014;58(11):1515-22. [DOI: 10.1093/cid/ciu135] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dubberke 2018 {published data only}
- Dubberke ER, Lee C, Orenstein R, Khanna S, Hecht G, Fraiz J. Efficacy and safety of RBX2660 for the prevention of recurrent Clostridium difficile infection: results of the PUNCH CD 2 trial. Open Forum Infectious Diseases 2016;3(Suppl 1):1341. [DOI: 10.1093/ofid/ofw172.1044] [DOI] [Google Scholar]
- Dubberke ER, Lee CH, Orenstein R, Khanna S, Hecht G, Gerding DN. Results from a randomized, placebo-controlled clinical trial of a RBX2660 – a microbiota-based drug for the prevention of recurrent Clostridium difficile infection [A Phase 2B Prospective, Randomized, Double-blinded, Placebo-controlled Clinical Study Demonstrating the Efficacy and Safety of Rebiotix RBX2660 (Microbiota Suspension) for the Treatment of Recurrent Clostridium Difficile Infection]. Clinical Infectious Diseases 2018;67(8):1198-204. [DOI: 10.1093/cid/ciy259] [DOI] [PubMed] [Google Scholar]
- Hecht GA, Orenstein R, Dubberke ER, Lee C, Khanna S. Lack of association with patient demographics and outcomes in PUNCH CD2, a randomized controlled trial of RBX2660, a microbiota-based drug for recurrent Clostridium difficile infection. American Gastroenterological Association Abstracts 2017;152(5):S951-2. [DOI: 10.1016/S0016-5085(17)33238-9] [DOI] [Google Scholar]
- NCT02299570. Microbiota restoration therapy for recurrent Clostridium difficile infection (Punchcd2) [A phase 2b prospective, randomized, double-blinded, placebo-controlled clinical study demonstrating the efficacy and safety of Rebiotix RBX2660 (microbiota suspension) for the treatment of recurrent Clostridium difficile infection]. clinicaltrials.gov/ct2/show/NCT02299570 (first received 24 November 2014). [NATIONAL CLINICAL TRIAL NUMBER: NCT02299570]
- Ramesh MS, Khanna S, Messer J, Adams M. Durable prevention of recurrent C. difficile infection with RBX2660: results of the PUNCH CD 2 trial. American Journal of Gastroenterology 2016;111:S93. [Google Scholar]
Kao 2019 {published data only}
- Kao DH, Roach B, Walter J, Loebenberg R, Wong K. Effect of lyophilized sterile fecal filtrate vs lyophilized donor stool on recurrent Clostridoides difficile infection: preliminary results from a randomized, double-blind pilot study. American Gastroenterological Association Abstracts 2019;156(6):S-900. [Google Scholar]
NCT03353506 {published data only}
- NCT03353506. Lyophilized fecal transplant vs lyophilized fecal filtrate in recurrent C diff infection [A prospective double blind randomized pilot study comparing the efficacy of lyophilized fecal microbiota transplantation (FMT) to lyophilized sterile fecal filtrate in the management of recurrent Clostridium difficile infection (CDI)]. clinicaltrials.gov/ct2/show/NCT03353506 (first received 27 November 2017). [NATIONAL CLINICAL TRIAL NUMBER: NCT03353506]
NCT03548051 {unpublished data only}
- NCT03548051. Safety and efficacy of FMT in individuals with one or more recurrences of Clostridium difficile associated disease (CDAD) [Phase 1/2 placebo controlled, partially-blinded clinical trial to assess the safety and efficacy of microbial restoration by enema with banked and thawed processed stool in individuals with one or more recurrences of Clostridium difficile associated disease (CDAD)]. clinicaltrials.gov/ct2/show/NCT03548051 (first received 6 June 2018).
References to ongoing studies
Drekonja 2021 {published data only}
- Drekonja DM, Shaukat A, Zhang JH, Reinink AR, Nugent S, Dominitz JA, et al. Microbiota or placebo after antimicrobial therapy for recurrent Clostridioides difficile at home: a clinical trial with novel home-based enrollment. Clinical Trials 2021;18(5):622-9. [DOI: 10.1177/17407745211021198] [DOI] [PubMed] [Google Scholar]
EUCTR2015‐003062‐82‐DK {published data only}
- EUCTR2015-003062-82-DK. Rectal bacteriotherapy, faecal microbiota transplantation or oral vancomycin for the treatment of recurrent Clostridium difficile infection: a randomised controlled trial. clinicaltrialsregister.eu/ctr-search/trial/2015-003062-82/DK (first received 11 January 2016).
NCT02255305 {unpublished data only}
- NCT02255305. FMT versus antimicrobials for initial treatment of recurrent CDI [Fecal microbiota transplantation versus standard medical therapy for initial treatment of recurrent Clostridium difficile infection]. clinicaltrials.gov/ct2/show/NCT02255305 (first received 2 October 2014).
NCT02774382 {unpublished data only}
- NCT02774382. Rectal bacteriotherapy, fecal microbiota transplantation or oral vancomycin treatment of recurrent Clostridium difficile infections. clinicaltrials.gov/ct2/show/NCT02774382 (first received 17 May 2016).
NCT03005379 {published and unpublished data}
- NCT03005379. Microbiota or placebo after antimicrobial therapy for recurrent C. difficile at home (MATCH). clinicaltrials.gov/ct2/show/NCT03005379 (first received 29 December 2016).
NCT03053505 {unpublished data only}
- NCT03053505. A novel faecal microbiota transplantation system for treatment of primary and recurrent Clostridium difficile infection (FMTREAT) [Two-arm, interventional, prospective, open-label, multi-center trial to evaluate the safety & effectiveness of FMT for treatment of adult patients with primary or recurrent CDI, using a novel, standardized microbiota transplantation system]. clinicaltrials.gov/ct2/show/NCT03053505 (first received 15 February 2017).
NCT03806803 {unpublished data only}
- NCT03806803. Multicentre blinded comparison of lyophilized sterile fecal filtrate to lyophilized fecal microbiota transplant in recurrent Clostridioides difficile infection [A multicenter double blind randomized study comparing the efficacy of lyophilized sterile fecal filtrate to lyophilized fecal microbiota transplantation (FMT) in the management of recurrent Clostridioides difficile infection (CDI)]. clinicaltrials.gov/ct2/show/NCT03806803 (first received 16 January 2019). [NATIONAL CLINICAL TRIAL NUMBER: NCT03806803]
NCT03970200 {published and unpublished data}
- NCT03970200. PMT for severe-CDI [A phase II, randomized trial to evaluate the safety and efficacy of fecal microbiota transplantation using the Penn microbiome therapy products for severe or severe-complicated/fulminant Clostridium difficile infection]. clinicaltrials.gov/ct2/show/NCT03970200 (first received 16 January 2020).
NCT04885946 {unpublished data only}
- NCT04885946. Fecal microbiota transplantation for early Clostridioides difficile infection (EarlyFMT). clinicaltrials.gov/ct2/show/NCT04885946 (first received 12 May 2021).
NCT04960306 {unpublished data only}
- NCT04960306. Fecal filtrate as a treatment option of multiple recurrent Clostridioides difficile infection (FILTRATE) [Fecal filtrate versus conventional microbiota transplantation in the treatment of multiple recurrent Clostridioides difficile infection (FILTRATE): a protocol of a randomized, controlled trial]. clinicaltrials.gov/ct2/show/NCT04960306 (first received 13 July 2021).
NCT05077085 {unpublished data only}
- NCT05077085. Bezlotoxumab versus FMT for multiple recurrent CDI (BSTEP) [New treatment strategy for patients with multiple recurrent Clostridioides difficile infection with bezlotoxumab as first option]. clinicaltrials.gov/ct2/show/NCT05077085 (first received 13 October 2021).
NCT05201079 {unpublished data only}
- NCT05201079. Recurrent Clostridioides difficile infection treatment with capsules of lyophilised faecal microbiota vs fidaxomicin [A randomised, controlled, open-label phase III clinical trial in patients with recurrent Clostridioides difficile (CD) infection, to evaluate the efficacy and safety of capsules of lyophilised faecal microbiota vs fidaxomicin]. clinicaltrials.gov/ct2/show/NCT05201079 (first received 21 January 2022).
NCT05266807 {unpublished data only}
- NCT05266807. Fecal microbiota transplantation versus vancomycin or fidaxomicin in Clostridioides difficile infection first recurrence (FENDER) [Fecal microbiota transplantation versus vancomycin or fidaxomicin in Clostridioides difficile infection first recurrence: a randomized controlled, open-label, multicenter phase III clinical trial]. clinicaltrials.gov/ct2/show/NCT05266807 (first received 4 March 2022).
Additional references
Abu‐Sbeih 2019
- Abu-Sbeih H, Ali FS, Wang Y. Clinical review on the utility of fecal microbiota transplantation in immunocompromised patients. Current Gastroenterology Reports 2019;21(4):8. [DOI] [PubMed] [Google Scholar]
ACG Clinical Guidelines 2021
- Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. American Journal of Gastroenterology 2021;116(6):1124-47. [DOI] [PubMed] [Google Scholar]
Al Momani 2018
- Al Momani LA, Abughanimeh O, Boonpheng B, Gabriel JG, Young M. Fidaxomicin vs vancomycin for the treatment of a first episode of Clostridium difficile infection: a meta-analysis and systematic review. Cureus Journal of Medical Science 2018;10(6):e2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alang 2015
- Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infectious Diseases 2015;2(1):ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakken 2011
- Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clinical Gastroenterology and Hepatology 2011;9(12):1044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balsells 2019
- Balsells E, Shi T, Leese C, Lyell I, Burrows J, Wiuff C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. Journal of Global Health 2019;9(1):010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baunwall 2020
- Baunwall SM, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, et al. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine 2020;23(29-30):100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cammarota 2017
- Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017;66(4):569-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2019
- US Department of Health and Human Services, CDC. Antibiotic resistance threats in the United States, 2019. cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed prior to 19 January 2023).
Chang 2008
- Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. Journal of Infectious Diseases 2008;197(3):435-8. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 15 December 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Crobach 2018
- Crobach MJ, Vernon JJ, Loo VG, Kong LY, Péchiné S, Wilcox MH, et al. Understanding Clostridium difficile colonization. Clinical Microbiology Reviews 2018;31(2):e00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidovics 2019
- Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, Hansen R, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: a joint position paper from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2019;68(1):130-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dembrovszky 2020
- Dembrovszky F, Gede N, Szakács Z, Hegyi P, Kiss S, Farkas N, et al. Fecal microbiota transplantation may be the best option in treating multiple Clostridioides difficile infection: a network meta-analysis. Infectious Diseases & Therapy 2020;10:201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dethlefsen 2008
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLOS Biology 2008;6(11):e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drekonja 2015
- Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, et al. Fecal microbiota transplantation for Clostridium difficile infection: a systematic review. Annals of Internal Medicine 2015;162(9):630-8. [DOI] [PubMed] [Google Scholar]
Dumville 2006
- Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ 2006;332(7547):969-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2013
- US Department of Health and Human Services, Food and Drug Administration: Center for Biologics Evaluation and Research. Enforcement policy regarding investigational new drug requirements for use of fecal microbiota for transplantation to treat Clostridium difficile infection not responsive to standard therapies. fda.gov/media/86440/download (accessed 12 March 2022).
FDA 2019
- US Food and Drug Administration. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi-drug resistant organisms. fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse (accessed 12 March 2022).
FDA 2020a
- FDA. Fecal microbiota for transplantation: safety alert – risk of serious adverse events likely due to transmission of pathogenic organisms. fda.gov/safety/medical-product-safety-information/fecal-microbiota-transplantation-safety-alert-risk-serious-adverse-events-likely-due-transmission (accessed 12 March 2022).
FDA 2020b
- FDA. Safety alert regarding use of fecal microbiota for transplantation and additional safety protections pertaining to SARS-CoV-2 and COVID-19. fda.gov/vaccines-blood-biologics/safety-availability-biologics/safety-alert-regarding-use-fecal-microbiota-transplantation-and-additional-safety-protections (accessed 12 March 2022).
Fekety 1993
- Fekety R, Shah AB. Diagnosis and treatment of Clostridium difficile colitis. JAMA 1993;269(1):71-5. [PubMed] [Google Scholar]
Fekety 1997
- Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clinical Infectious Diseases 1997;24(3):324-33. [DOI] [PubMed] [Google Scholar]
Gupta 2011
- Gupta SK. Intention-to-treat concept: a review. Perspectives in Clinical Research 2011;2(3):109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Hui 2019
- Hui W, Li T, Liu W, Zhou C, Gao F. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: an updated randomized controlled trial meta-analysis. PLOS One 2019;14(1):e0210016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Ianiro 2018
- Ianiro G, Masucci L, Quaranta G, Simonelli C, Lopetuso LR, Sanguinetti M, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy plus vancomycin for the treatment of severe refractory Clostridium difficile infection – single versus multiple infusions. Alimentary Pharmacology & Therapeutics 2018;48(2):152-9. [DOI] [PubMed] [Google Scholar]
Imdad 2018
- Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD012774. [DOI: 10.1002/14651858.CD012774.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiang 2018a
- Jiang ZD, Jenq RR, Ajami NJ, Petrosino JF, Alexander AA, Ke S, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLOS One 2018;13(11):e0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnsen 2018
- Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterology & Hepatology 2018;3(1):17-24. [DOI] [PubMed] [Google Scholar]
Kao 2017
- Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2017;318(20):1985-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassam 2013
- Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. American Journal of Gastroenterology 2013;108(4):500-8. [DOI] [PubMed] [Google Scholar]
Kellermayer 2019
- Kellermayer R. Fecal microbiota transplantation: great potential with many challenges. Translational Gastroenterology and Hepatology 2019;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2008
- Kelly CP, LaMont JT. Clostridium difficile – more difficult than ever. New England Journal of Medicine 2008;359(18):1932-40. [DOI] [PubMed] [Google Scholar]
Kelly 2014
- Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. American Journal of Gastroenterology 2014;109(7):1065-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2015
- Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 2015;129(1):223-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2021
- Kelly CR, Yen EF, Grinspan AM, Kahn SA, Atreja A, Lewis JD, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology 2021;160(1):183-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2018
- Khan MY, Dirweesh A, Khurshid T, Siddiqui WJ. Comparing fecal microbiota transplantation to standard-of-care treatment for recurrent Clostridium difficile infection: a systematic review and meta-analysis. European Journal of Gastroenterology & Hepatology 2018;30(11):1309-17. [DOI] [PubMed] [Google Scholar]
Khanna 2017
- Khanna S, Vazquez-Baeza Y, González A, Weiss S, Schmidt B, Muñiz-Pedrogo DA, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome 2017;5(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kociolek 2016
- Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nature Reviews Gastroenterology & Hepatology 2016;13(3):150-60. [DOI] [PubMed] [Google Scholar]
Lawson 2016
- Lawson PA, Citron DM, Tyrrell KL, Finegold SM. Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O'Toole 1935) Prévot 1938. Anaerobe 2016;40:95-9. [DOI] [PubMed] [Google Scholar]
Lee 2016
- Lee CH, Steiner T, Petrof EO, Smieja M, Roscoe D, Nematallah A, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 2016;315(2):142-9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Featherstone R, Littlewood A, Marshall C, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from training.cochrane.org/handbook.
Leffler 2015
- Leffler DA, Lamont JT. Clostridium difficile infection. New England Journal of Medicine 2015;372(16):1539-48. [DOI] [PubMed] [Google Scholar]
Lessa 2015
- Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, et al. Burden of Clostridium difficile infection in the United States. New England Journal of Medicine 2015;372(9):825-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Link 2016
- Link A, Lachmund T, Schulz C, Weigt J, Malfertheiner P. Endoscopic peroral jejunal fecal microbiota transplantation. Digestive & Liver Disease 2016;48(11):1136-9. [DOI] [PubMed] [Google Scholar]
McDonald 2007
- McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK, Ad Hoc Clostridium difficile Surveillance Working Group. Recommendations for surveillance of Clostridium difficile-associated disease. Infection Control & Hospital Epidemiology 2007;28(2):140-5. [DOI] [PubMed] [Google Scholar]
McDonald 2018
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases 2018;66(7):987-94. [DOI] [PubMed] [Google Scholar]
MS Excel 2018 [Computer program]
- Microsoft Excel. Microsoft Corporation, 2018. Available from office.microsoft.com/excel.
Mullish 2018
- Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut 2018;67(11):1920-41. [DOI] [PubMed] [Google Scholar]
Petersen 2014
- Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cellular Microbiology 2014;16(7):1024-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomares Bascuñana 2021
- Pomares Bascuñana RÁ, Veses V, Sheth CC. Effectiveness of fecal microbiota transplant for the treatment of Clostridioides difficile diarrhea: a systematic review and meta-analysis. Letters in Applied Microbiology 2021;73(2):149-58. [DOI] [PubMed] [Google Scholar]
Quraishi 2017
- Quraishi MN, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Alimentary Pharmacology & Therapeutics 2017;46(5):479-93. [DOI] [PubMed] [Google Scholar]
Ramai 2021
- Ramai D, Zakhia K, Fields PJ, Ofosu A, Patel G, Shahnazarian V, et al. Fecal microbiota transplantation (FMT) with colonoscopy is superior to enema and nasogastric tube while comparable to capsule for the treatment of recurrent Clostridioides difficile infection: a systematic review and meta-analysis. Digestive Diseases and Sciences 2021;66(2):369-80. [DOI] [PubMed] [Google Scholar]
Razik 2016
- Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen GC. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. American Journal of Gastroenterology 2016;111(8):1141-6. [DOI] [PubMed] [Google Scholar]
Red Book 2018
- American Academy of Pediatrics. Clostridium difficile. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors(s). Red Book: 2018 Report of the Committee on Infectious Diseases. 31st edition. Itasca (IL): American Academy of Pediatrics, 2018:288-92. [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Rokkas 2019
- Rokkas T, Gisbert JP, Gasbarrini A, Hold GL, Tilg H, Malfertheiner P, et al. A network meta-analysis of randomized controlled trials exploring the role of fecal microbiota transplantation in recurrent Clostridium difficile infection. United European Gastroenterology Journal 2019;7(8):1051-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saha 2021
- Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology 2021;160(6):1961-9. [DOI] [PubMed] [Google Scholar]
Shannon‐Lowe 2010
- Shannon-Lowe J, Matheson NJ, Cooke FJ, Aliyu SH. Prevention and medical management of Clostridium difficile infection. BMJ 2010;340:c1296. [DOI] [PubMed] [Google Scholar]
Solari 2014
- Solari PR, Fairchild PG, Noa LJ, Wallace MR. Tempered enthusiasm for fecal transplant. Clinical Infectious Diseases 2014;59(2):319. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;28(366):l4898. [DOI] [PubMed] [Google Scholar]
Vardakas 2012
- Vardakas KZ, Polyzos KA, Patouni K, Rafailidis PI, Samonis G, Falagas ME. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. International Journal of Antimicrobial Agents 2012;40(1):1-8. [DOI] [PubMed] [Google Scholar]
Woodworth 2017
- Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, et al. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent Clostridium difficile infection. Journal of Clinical Microbiology 2017;55(4):1002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Imdad 2021
- Imdad A, Minkoff NZ, Tanner-Smith EE, Zackular JP, Acra S, Nicholson MR. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile ( Clostridium difficile). Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013871. [DOI: 10.1002/14651858.CD013871] [DOI] [PMC free article] [PubMed] [Google Scholar]


