Abstract
Anti-melanoma differentiation-associated gene 5 (MDA-5) antibody-positive dermatomyositis is a disease with a poor prognosis associated with rapid progressive interstitial pneumonia. Autoimmune diseases have occasionally been reported to occur after hematopoietic stem cell transplantation (HSCT). We experienced a case of anti-MDA-5 antibody-positive dermatomyositis after HSCT. In this case, a sufficient dose of cyclophosphamide could not be administered due to an impaired bone marrow function. We discuss the complications of autoimmune diseases after HSCT.
Keywords: MDA-5 antibody-positive dermatomyositis, hematopoietic stem cell transplantation
Introduction
Dermatomyositis (DM) positive for anti-melanoma differentiation-associated gene 5 (MDA-5) antibody is known to be associated with interstitial pneumonia with a rapidly progressive diffuse alveolar damage pattern and is also characterized by a lack of muscle symptoms (1).
Recently, it has been shown that strong immunosuppression early in the course of the disease can improve the survival rate. This regimen includes methylprednisolone pulse therapy and frequent intravenous cyclophosphamide administration (IVCY), and its combination with calcineurin inhibitors in addition to high-dose prednisolone has been proposed (2).
We experienced a case of DM with positive anti-MDA-5 antibody during remission after hematopoietic stem cell transplantation (HSCT) for acute transformation of chronic myeloid leukemia. Although there have been several reports of cases of autoimmune disease after HSCT (3-7), there have been no reports of secondary cases of DM with anti-MDA-5 antibody positivity.
We herein report this case, including the course of treatment.
Case Report
A 56-year-old man who was being treated with dasatinib in the Department of Hematology for chronic myeloid leukemia onset in August 2015. Subsequently, the patient underwent acute transformation, and an interrelated peripheral blood stem cell transplant (human leukocyte antigen-full match) was performed in April 2016. His condition remained in molecular genetic remission after HSCT. In July 2016, the patient developed liver injury and was diagnosed with hepatic graft-versus-host disease (GVHD) based on liver biopsy results. He was treated with prednisolone (PSL) 1 mg/kg and mycophenolate mofetil (MMF) 2,000 mg/day. His liver GVHD improved, and these immunosuppressants were terminated in August 2017.
In September 2017, this patient had oral sores, finger arthralgia, and facial and hand skin rash, and his blood examination showed increased liver enzymes. Chest computed tomography (CT) also showed multiple ground-glass opacities in both lungs. Immunosuppression was restarted with PSL 5 mg/day, but the symptoms did not improve, and the patient was referred to our department in September. Dermatomyositis was suspected based on cutaneous findings, and the patient was later found to be positive for anti-MDA-5 antibodies and was admitted to our department due to rapidly progressing respiratory deterioration.
Medical history: hypertension, type 2 diabetes, chronic myelogenous leukemia (after acute transformation and bone marrow transplant)
Preference history: smoking/never, drinking/occasional drinking
Family history: none of note
Allergy history: none of note
Present condition on admission: height 161.0 cm, weight 54.5 kg, blood pressure 116/69 mmHg, heart rate 88 beats/min, body temperature 35.8°C, respiratory rate 18 breaths/min, SpO2 92% (room air). No abnormal heart sounds were noted. Dry rale was heard extensively in the lung fields. An abdominal examination showed no abnormal findings, and no abnormal findings were noted neurologically either. No tender or swollen joints were noted. There was a heliotrope rash on the face, Gottron papules on both fingers, and redness on the nail beds (Fig. 1). Manual muscle strength test showed no abnormalities.
Figure 1.
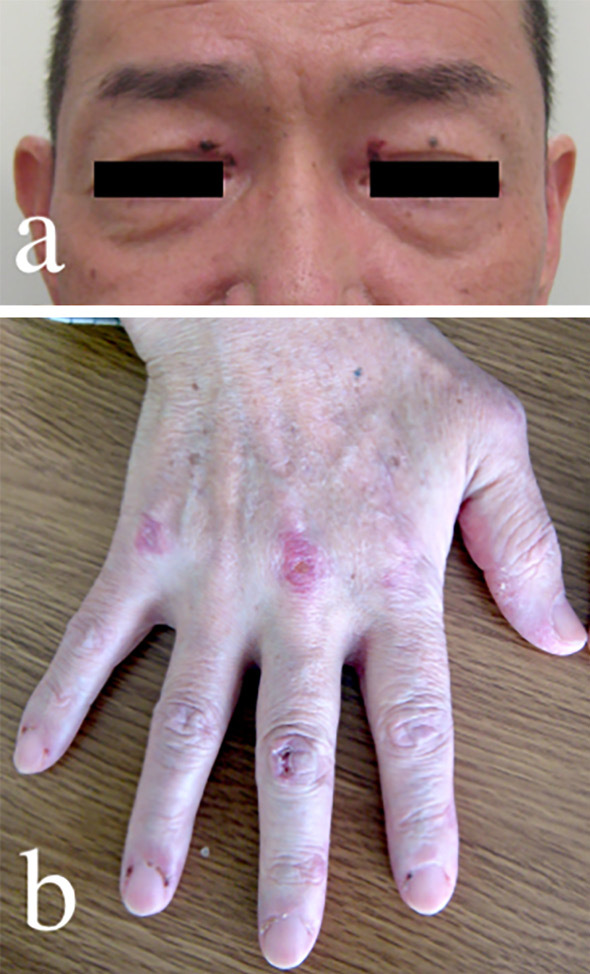
Heliotopic rash (a) and Gottron papule (b).
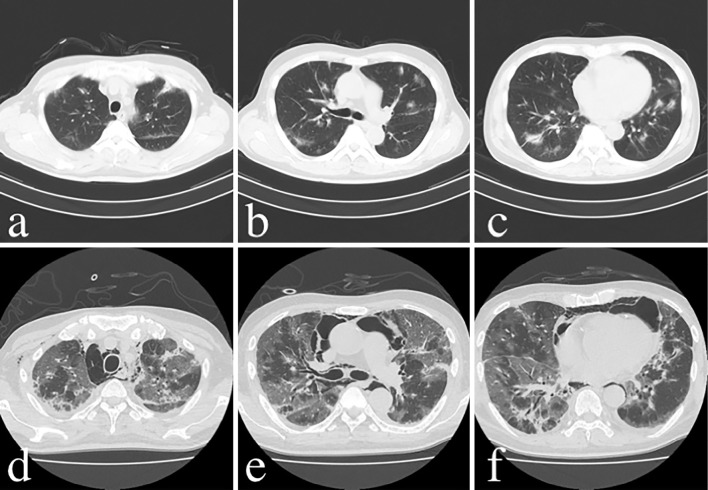
As shown in Table, laboratory data at the first visit to our department (one week before admission) showed an elevated erythrocyte sedimentation rate and C-reactive protein, and ferritin was also elevated at 1,164 ng/mL. Creatine kinase 88 U/L and myoglobin 64 ng/mL were normal, but only aldolase was mildly increased at 6.3 U/L. Krebs von den Lungen (KL)-6 and surfactant protein (SP-A) were mildly elevated at 601 U/mL and 62.7 ng/mL, respectively. Regarding autoantibodies, anti-nuclear antibodies were less than 40-fold positive according to a fluorescent antibody assay, but mildly positive by Enzyme-Linked Immunosorbent Assay (5.3 C.O.I). Anti-MDA-5 antibody was positive at more than 150 (index). Chest CT showed infiltrative and frosted shadows with multiple bronchial translucencies distributed in the subpleural space and around the bronchial vascular bundles (Fig. 2a-c).
Table.
Laboratory Data at the Time of Anti-melanoma Differentiation-associated Gene 5 Antibody-positive Amyotrophic Dermatomyositis.
| Normal range | Normal range | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 3,500-8,500 /μL | 8,100 | PT | 75-130% | 112.6 | |||||
| Neutrophils | 42-77% | 82.0 | APTT | 23-35 s | 32.1 | |||||
| Basophils | 0.1-2% | 0.0 | Fbg | 150-350 mg/dL | 409 | |||||
| Eosinocytes | 0.5-6% | 1.0 | ||||||||
| Lymphocytes | 18-49% | 10.0 | IgG | 870-1,700 mg/dL | 646 | |||||
| Monocytes | 3-9% | 6.5 | IgA | 110-410 mg/dL | 34 | |||||
| Myelocytes | - | 0.5 | IgM | 46-260 mg/dL | 68 | |||||
| RBC | 370-510 ×104/μL | 312 | ||||||||
| Hb | 11.3-15.4 g/dL | 10.4 | CH50 | 30-50 U/mL | 99 | |||||
| Ht | 34.0-46.3% | 30.9 | C3 | 69-128 mg/dL | 118 | |||||
| MCV | 82-100 fL | 99 | C4 | 14-36 mg/dL | 46 | |||||
| MCH | 27.5-34.5 pg | 33.3 | ||||||||
| MCHC | 32.0-35.5 g/dL | 33.7 | CMV (C7HRP) | Negative | ||||||
| PLT | 140-340 ×109/L | 241 | CMV IgG antibody | <2 | 92.2 | |||||
| CMV IgM antibody | <0.8 | <0.8 | ||||||||
| ESR | <10 mm/h | 85.8 | EBV-VCA IgG antibody | <×10 | ×40 | |||||
| EBV-VCA IgM antibody | <×10 | <×10 | ||||||||
| BS | 60-100 mg/dL | 49 | EBV-EBNA antibody | <×10 | <×10 | |||||
| HbA1c | 4.6-6.2% | 6.0 | IgM-HA antibody | <0.8 S/CO | 0.09 | |||||
| Na | 138-146 mEq/L | 142 | β-D-glucan | <11 pg/mL | <6.0 | |||||
| K | 3.5-5 mEq/L | 4.5 | Cryptococcus antibody | Negative | ||||||
| Cl | 100-100 mEq/L | 103 | Candida antibody | Negative | ||||||
| UN | 8-20 mg/dL | 16 | Aspergillus antibody | Negative | ||||||
| Cre | 0.4-0.8 mg/dL | 0.94 | IGRA (T-SPOT) | Negative | ||||||
| TP | 6.5-8.2 g/dL | 6.3 | ||||||||
| Alb | 3.8-5.0 g/dL | 3.5 | FANA | <×40 | <40 fold | |||||
| A/G | 1.25-2.14 | 1.25 | ANA (ELISA) | <0.9 C.O.I | 5.3 | |||||
| AST | 13-35 U/L | 52 | Anti-mitochondrial antibody | <×20 | Negative | |||||
| ALT | 5-35 U/L | 29 | Anti-dsDNA antibody | <10 IU/mL | <10 | |||||
| ALP | 107-340 U/L | 1,764 | Anti-U1-RNP antibody | <10 U/mL | <2.0 | |||||
| γGTP | 8-45 U/L | 381 | Anti-SS-A antibody | <10 U/mL | 1.1 | |||||
| LD | 112-230 U/L | 333 | Anti-Scl-70 antibody | <10 U/mL | <1.0 | |||||
| CK | 45-165 U/L | 88 | Anti-centromere antibody | <10 | <5 | |||||
| Myoglobin | <154 ng/mL | 64 | Anti-ARS antibody | <25 | <5.0 | |||||
| Aldolase | 2.1-6.1 U/L | 6.3 | Anti-MDA-5 antibody | <32 | >150 | |||||
| CRP | <0.3 mg/dL | 2.853 | Anti-Mi-2 antibody | <53 | <5 | |||||
| Ferritin | 17-321 ng/mL | 1,164 | Anti-TIF1-γ antibody | <32 | <5 | |||||
| Rheumatoid factor | <15 IU/mL | 5 | ||||||||
| SP-A | <43.8 ng/mL | 62.7 | Anti-CCP antibody | <4.5 U/mL | <0.5 | |||||
| SP-D | <110 ng/mL | 17.5 | PR3-ANCA | <3.5 U/mL | <1.0 | |||||
| KL-6 | <500 U/mL | 601 | MPO-ANCA | <3.5 U/mL | <1.0 |
Figure 2.
Time course of chest CT images. Initial presentation: infiltrative and frosted shadows with multiple bronchial translucencies distributed around the subpleural and bronchovascular bundles (a-c). 24th day: Exacerbation of interstitial pneumonia and mediastinal emphysema (d-f).
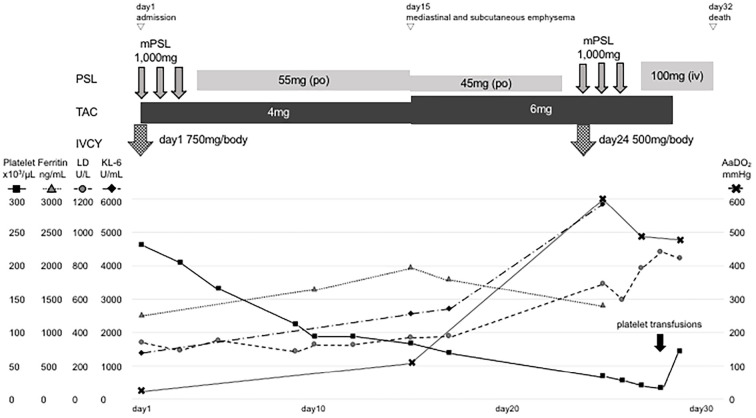
The clinical course of the patient after admission is shown in Fig. 3. Based on the typical cutaneous symptoms, appearance of interstitial pneumonia, and slight increase in muscle enzymes, the patient was diagnosed with anti-MDA-5 antibody-positive clinical amyopathic dermatomyositis (CADM) and rapidly progressive interstitial pneumonia (RP-ILD) caused by CADM. He was treated with methylprednisolone pulse therapy at 1,000 mg for 3 days and 750 mg/body of IVCY from the first admission day. The patient was treated with PSL 1 mg/kg/day (55 mg/day) and tacrolimus 4 mg/day from the fourth day of treatment. His respiratory status and skin symptoms showed an improving trend, and his respiratory status was maintained at 92% SpO2 with nasal oxygen at 2 L/min.
Figure 3.
The clinical course of the patient after admission. Intensive immunosuppressive therapy was administered immediately after admission. The patient’s oxygenating capacity (shown in AaDO2) was poorly improved, and he showed marked thrombocytopenia after treatment. LD: lactate dehydrogenase, KL-6: Krebs von den lungen-6, PSL: prednisolone, TAC: tacrolimus, IVCY: intravenous cyclophosphamide, mPSL: methylprednisolone, AaDO2: alveolar arterial difference of oxygen
On the 15th day, poor oxygenation (SpO2 88%/nasal canula O2 5 L/min) appeared, and the alveolar arterial difference of oxygen (AaDO2) worsened from 32.0 mmHg on day 1 to 108.8 mmHg. The patient was also complicated by mediastinal and subcutaneous emphysema that was noted on simple chest X-ray (Fig. 4). PSL was reduced from 55 to 45 mg/day, and tacrolimus increased from 4 to 6 mg/day because of the weakening of tissue connectivity caused by steroid administration. Subsequently, the subcutaneous emphysema expanded to the right anterior chest and left neck but did not enlarge thereafter. However, his interstitial pneumonia worsened, and his AaDO2 further increased to 604.1 mmHg. After the first IVCY, his platelet count was markedly decreased (232×109/L → 84×109/L), so the second IVCY was reduced to 500 mg/body and was administered on the same day as mPSL pulse therapy (1,000 mg for 3 days) from the 24th day. However, the interstitial pneumonia did not improve (Fig. 2d-f), and the poor oxygenation progressed, making recovery difficult.
Figure 4.
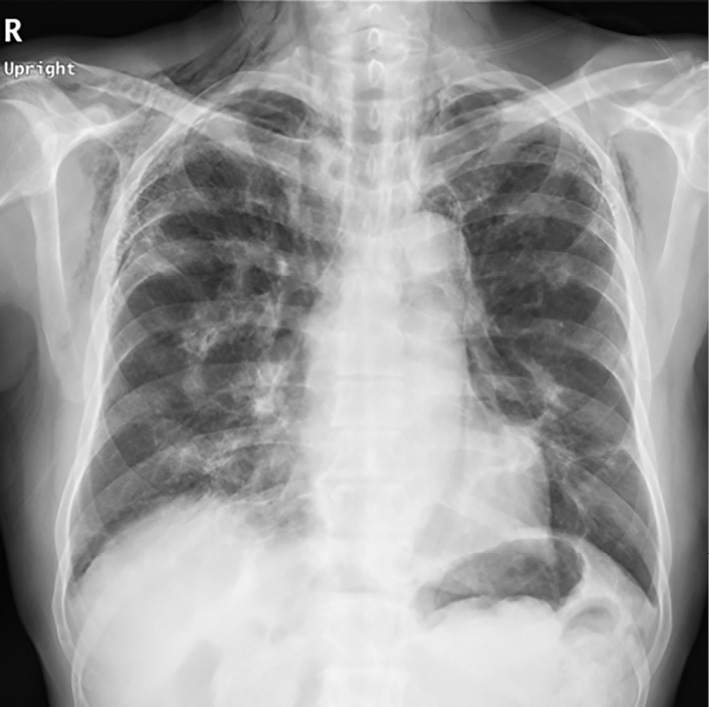
Chest simple radiograph: The findings on the 15th day showed subcutaneous and mediastinal emphysema.
As shown in Fig. 3, AaDO2 remained at 472.3 mmHg after the second IVCY and mPSL pulse therapy, showing only minor improvement. Therefore, platelet transfusions were performed, and treatment continuation was planned, but on the 31st day, the patient died from respiratory failure.
Discussion
In recent years, the pathogenesis of idiopathic inflammatory myopathy (DM and polymyositis) has been classified based on autoantibodies. Anti-MDA-5 antibody-positive DM is characterized by lack of muscle symptoms and RP-ILD (8-10). Patients with DM positive for anti-MDA-5 antibodies have significantly higher serum ferritin levels than 790 ng/mL, and ferritin levels have been shown to be related to the prognosis of interstitial pneumonia (11). It has also been suggested that anti-MDA-5 antibodies themselves may indicate disease activity (12). In our case, the pre-treatment serum ferritin level was 1,164 ng/mL, and the anti-MDA-5 antibody value was 150 (index).
CT findings of RP-ILD associated with anti-MDA-5 antibody-positive DM have been reported to begin with infiltrative shadows and ground-glass opacity (GGO) around the vascular bronchial bundles, thickening of the interlobular septa, and linear irregular shadows, progressing to GGO and consolidation with bronchial translucency extending to the entire lung field (11). Skin manifestations of anti-MDA-5 antibody-positive DM are characterized by skin ulcers (e.g., around nails and elbows), painful palm papules, alopecia, and oral ulcers (13). These characteristics also occurred in the present case, making it a typical case of RP-ILD associated with anti-MDA-5 antibody-positive DM.
Many cases of autoimmune diseases after HSCT have been reported. Autoimmune hemolytic anemia (3,4), autoimmune thrombocytopenic purpura (4), hyperthyroidism (5), membranous nephropathy (5), and myasthenia gravis (7)have been reported. To our knowledge, however, no reports of cases of dermatomyositis after HSCT have been published, but some cases of polymyositis without interstitial pneumonia were reported as cases of chronic GVHD (14-21).
Various pathogenesis of autoimmune disease after HSCT has been hypothesized. In post-transplant microvariant nephrotic syndrome, real-time polymerase chain reaction (PCR) revealed that the onset of the disease was associated with an increase in tumor necrosis factor-α and interferon-γ produced by T cells (22), and it has been speculated that cytokines produced by activated T cells due to abnormal cellular immunity may be involved. In addition, since the incidence of autoimmune thrombocytopenic purpura after transplantation is frequently observed during the period of reduction or discontinuation of immunosuppressant drugs, the involvement of recovery of the donor-derived lymphocyte function, especially humoral immunity, has been speculated (23). Similarly, post-transplant myasthenia gravis has been reported to occur after the reduction or discontinuation of immunosuppressive drugs and has been characterized as being more common in patients with chronic GVHD than in those without. (7). The discontinuation or reduction of immunosuppressive drugs also coincides with the replacement of CD8+ T cells with thymus-derived T cells and the onset of autoimmune disease after HSCT. Patients with GVHD reportedly often have positive autoantibodies (24), and it is speculated that an increase in naïve T cells with autoreactivity may trigger the development of autoimmune diseases in patients with conditions that are conducive to the development of autoimmune diseases.
Another possible mechanism other than discontinuation of immunosuppressive drugs is immune reconstitution inflammatory syndrome (IRIS). The pattern of viral reactivation is similar in GVHD and drug-induced hypersensitivity syndrome (DIHS) (25,26), and it has been suggested that they share the same mechanism of IRIS (27). IRIS was originally proposed as a concept to explain opportunistic infections that occur during the recovery process of immune responses in human immunodeficiency virus-positive patients after initiation of antiretroviral therapy. IRIS is a phenomenon in which immune responses to pathogens (bacteria and viruses) that have been growing in the host under immunosuppressed conditions do not show obvious clinical symptoms when immunity is suppressed, with symptoms of infection worsening when the immune status is restored. In the host body, various viral reactivations occur coincident with recovery from an immunosuppressive state, with a concomitant decrease in the number of regulatory T cells being noted (28). Dysfunction of regulatory T cells in IRIS restores the immune response and may lead to the development of autoimmune diseases in the presence of self-responsive naïve T cells.
Several cases of complication with myositis after HSCT have been reported. All were reported to be polymyositis, without complications of skin lesions or interstitial pneumonia, and without the appearance of antibodies specific for myositis (14-21). Reported myositis after HSCT is recognized as a manifestation of chronic GVHD and often responds well to prednisolone. To our knowledge, there have been no reports of cases of dermatomyositis with rapidly progressive interstitial pneumonia positive for anti-MDA-5 antibodies as in the present case, making it a rare case.
Autoimmune diseases that can develop after HSCT include autoimmune hemolytic anemia, autoimmune thrombocytopenic purpura, hyperthyroidism, membranous nephropathy, and myasthenia gravis, as mentioned above. What they all have in common is that they show some relationship between autoantibodies and disease development. Although typical autoimmune connective tissue diseases are also positive for autoantibodies, there are few diseases in which autoantibodies are clearly involved in the onset or course of the disease. Many autoantibodies are treated as disease markers during the diagnosis. However, autoantibodies have also been found to be associated with disease activity in systemic lupus erythematosus, such as anti-dsDNA antibody and anti-MDA-5 antibody-positive DM. Whether the reason why these diseases are not reported as autoimmune diseases that develop after HSCT is simply due to the low incidence of the diseases or whether there are other factors involved is unclear at present.
Gono et al. compared the clinical characteristics of anti-MDA-5 antibody-positive DM between surviving (n=11) and deceased (n=9) cases and reported that A-aDO2 and serum ferritin levels were higher in the deceased cases than in the survivors at the initial diagnosis (11). In the present case, the A-aDO2 value was 32.0 mmHg at the initial visit, but it did not improve to 108.8 mmHg within 2 weeks of treatment and thereafter it deteriorated rapidly. The serum ferritin level was also 1,164 ng/mL at the initial visit and never fell below 1,000 ng/mL throughout the course of the disease. Furthermore, in a Japanese report of a multicenter cohort of 497 patients with idiopathic inflammatory myopathy, 76 patients died of directly related respiratory failure (29). The independent risk factors for mortality from interstitial pneumonia in this cohort were an age at the onset ≥60 years old, positive anti-MDA-5 antibody, CRP level ≥1 mg/dL, and SpO2 ≤95%. The present patient's findings were compatible with all of these factors except for age, suggesting that the patient had a poor prognosis from the outset.
For anti-MDA-5 antibody-positive DM, a highly effective regimen has been presented (2). Unfortunately, our patient had undergone HSCT, and although he was relatively young (56 years old), his platelet count did not recover after the initial IVCY, suggesting that his bone marrow function was not sufficient to withstand strong cytotoxic immunosuppression.
The prognosis of anti-MDA-5 antibody-positive DM depends greatly on the success or failure of induction of remission in the initial treatment. The regimen used in our case, which was proposed by Tsuji et al., combines multiple immunosuppressive agents from the start. This regimen showed a significantly higher 6-month survival rate than the step-up regimen group, in which conventional immunosuppressive agents were added sequentially (89% vs. 33%; p<0.0001) (2). As mentioned above, various autoimmune diseases have been reported to be complicated after HSCT, but autoimmune hemolytic anemia (3,4), autoimmune thrombocytopenic purpura (4), hyperthyroidism (5), and membranous nephropathy (6) have been reported in many cases with good outcomes with steroids and antithyroid medication. This suggests that the prognosis of autoimmune disease complications after HSCT may be influenced by whether or not drugs affecting the bone marrow function are required for treatment. Therapies other than drugs that affect the bone marrow function, such as cyclophosphamide and plasma exchange (30), may also be effective for anti-MDA-5 antibody-positive DM, and further case studies are needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52: 1571-1576, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol 72: 488-498, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Vicent M, Sanz J, Fuster JL, et al. Autoimmune hemolytic anemia (AIHA) following allogeneic hematopoietic stem cell transplantation (HSCT): a retrospective analysis and a proposal of treatment on behalf of the Grupo Espanol De Trasplante de Medula Osea en Ninos (GETMON) and the Grupo Espanol de Trasplante Hematopoyetico (GETH). Transfus Med Rev 32: 179-185, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Faraci M, Zecca M, Pillon M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant 20: 272-278, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Sanders JE, Hoffmeister PA, Woolfrey AE, et al. Thyroid function following hematopoietic cell transplantation in children: 30 years' experience. Blood 113: 306-308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez Garcia P, Herrera Arroyo C, Torres Gomez A, et al. Renal involvement in chronic graft-versus-host disease: a report of two cases. Bone Marrow Transplant 3: 357-362, 1988. [PubMed] [Google Scholar]
- 7.Mackey J, Desai S, Nabholtz J-M, et al. Myasthenia gravis in association with allogeneic bone marrow transplantation: clinical observations, therapeutic implications and review of literature. Bone Marrow Transplant 19: 939-942, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 49: 433-440, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti- melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49: 1713-1719, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino K, Muro Y, Sugiura K, et al. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology (Oxford) 49: 1726-1733, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 51: 1563-1570, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita T, Mizumaki K, Kano M, et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br J Dermatol 176: 395-402, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65: 25-34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker P, Chao NJ, Ben-Ezra J, et al. Polymyositis as a manifestation of chronic graft-versus-host disease. Medicine (Baltimore) 75: 279-285, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Stevens AM, Sullivan KM, Nelson JL. Polymyositis as a manifestation of chronic graft-versus-host disease. Rheumatology (Oxford) 42: 34-39, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Couriel DR, Beguelin GZ, Giralt S, et al. Chronic graft-versus-host disease manifesting as polymyositis: an uncommon presentation. Bone Marrow Transplant 30: 432-546, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Leano AM, Miller K, White AC. Chronic graft-versus-host disease-related polymyositis as a cause of respiratory failure following allogeneic bone marrow transplant. Bone Marrow Transplant 26: 1117-1120, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Anderson BA, Young PV, Kean WF, et al. Polymyositis in chronic graft vs host disease. A case report. Arch Neurol 39: 188-190, 1982. [DOI] [PubMed] [Google Scholar]
- 19.Urbano-Márquez A, Estruch R, Grau JM, et al. Inflammatory myopathy associated with chronic graft-versus-host disease. Neurology 36: 1091-1093, 1986. [DOI] [PubMed] [Google Scholar]
- 20.Prussick R, Brain MC, Walker IR, et al. Polymyositis: a manifestation of chronic graft-versus-host disease. J Am Acad Dermatol 25: 560-562, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi K, Kashihara K, Shinagawa K, et al. Myositis as a manifestation of chronic graft-versus-host disease. Intern Med 39: 482-485, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Seconi J, Watt V, Ritchie DS. Nephrotic syndrome following allogeneic stem cell transplantation associated with increased production of TNF-alpha and interferon-gamma by donor T cells. Bone Marrow Transplant 32: 447-450, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kalwak K, Gorczynska E, Wojcik D, et al. Late-onset idiopathic thrombocytopenic purpura correlates with rapid B-cell recovery after allogeneic T-cell-depleted peripheral blood progenitor cell transplantation in children. Transplant Proc 34: 3374-3377, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Sherer Y, Shoenfield Y. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant 22: 873-881, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Shiohara T, Kano Y, Hirahara K, et al. Prediction and management of drug reaction with eosinophilia and systemic symptoms (DRESS). Expert Opin Drug Metab Toxicol 13: 701-704, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Kano Y, Hiraharas K, Sakuma K, et al. Several herpesviruses can reactivate in a severe drug-induced multiorgan reaction in the same sequential order as in graft-versus-host disease. Br J Dermatol 155: 301-306, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Shiohara T, Kurata M, Mizukawa Y, et al. Recognition of immune reconstitution syndrome necessary for better management of patients with severe drug eruptions and those under immunosuppressive therapy. Allergol Int 59: 333-343, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Ushigome Y, Mizukawa Y, Kimishima M, et al. Monocytes are involved in the balance between regulatory T cells and Th17 cells in severe drug eruptions. Clin Exp Allergy 48: 1453-1463, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in myositis-associated interstitial lung disease: a multicentre cohort of 497 patients. Rheumatology (Oxford) 57: 1212-1221, 2018. [DOI] [PubMed] [Google Scholar]
- 30.Saito T, Mizobuchi M, Miwa Y, et al. Anti-MDA-5 antibody-positive clinically amyopathic dermatomyositis with rapidly progressive interstitial lung disease treated with therapeutic plasma exchange: a case series. J Clin Apher 36: 196-205, 2021. [DOI] [PubMed] [Google Scholar]