Abstract
A 73-year-old woman in complete remission from localized small-cell lung cancer associated with Lambert-Eaton myasthenic syndrome (LEMS) 22 years earlier was referred to our hospital and diagnosed with non-small-cell lung cancer. After three courses of pembrolizumab, an immune checkpoint inhibitor, the patient complained of muscle weakness, fatigue, ptosis, and dysarthria. The anti-voltage-gated calcium channel antibody level was elevated, and waxing was observed on a high-frequency repetitive stimulation test using an electromyogram. We diagnosed her with recurrence of LEMS as an immune-related adverse event (irAE) induced by pembrolizumab. After intravenous immunoglobulin therapy, the patient's symptoms improved, and she was discharged.
Keywords: Lambert-Eaton myasthenic syndrome, immune checkpoint inhibitor, immune-related adverse event, non-small-cell lung cancer
Introduction
Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune disease of the neuromuscular junction characterized by proximal muscle weakness, depressed tendon reflexes, and autonomic dysfunction. The diagnosis is confirmed based on characteristic electromyographic findings. The majority of LEMS patients have cancer, especially small-cell lung cancer (SCLC). Most patients also have antibodies circulating to the P/Q-type voltage-gated calcium channel (VGCC) (1).
Although there have been several reports of LEMS after treatment with immune checkpoint inhibitors (ICIs), such as pembrolizumab, there have been no case reports of recurrence of LEMS after complete remission as an immune-related adverse event (irAE) associated with immune reactivation by ICIs.
We herein report a case of LEMS recurrence after pembrolizumab treatment in a patient with a history of LEMS.
Case Report
A 73-year-old woman had developed limited-stage SCLC associated with LEMS and achieved complete remission in response to chemoradiation therapy 22 years ago. LEMS was diagnosed by proximal weakness, depressed tendon reflexes, cerebellar ataxia, and dry mouth with waxing in the repetitive stimulation test as well as an extremely high level of anti-P/Q-type VGCC antibody (2,472.9 pmol/L). With LEMS in remission, she was referred to our department in February 2021 with persistent bloody sputum. At our hospital, she was diagnosed with squamous cell carcinoma with lymph node metastasis (c-stage IIIB, cT2bN3M0 in the 8th TNM classification). She was started on combination therapy with pembrolizumab (200 mg) plus carboplatin (area under the curve: 4.5 mg/mL/min) and nab-paclitaxel (90 mg/m2 body surface area).
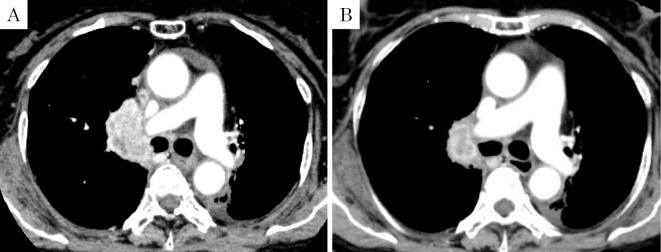
Three cycles of combination therapy every four weeks resulted in a partial response (Fig. 1) and decreases in serum carcinoembryonic antigen (CEA) and cytokeratin 19 fragment (CYFRA) levels. However, thyroid dysfunction [grade 2 Common Terminology Criteria for Adverse Events (CTCAE) v4] and uveitis (grade 2 CTCAE v4) appeared as irAEs, so we decided to discontinue pembrolizumab treatment following the fourth cycle.
Figure 1.
A: Enhanced chest CT on admission. B: Enhanced chest CT after three courses of immune-chemotherapy. CT: computed tomography
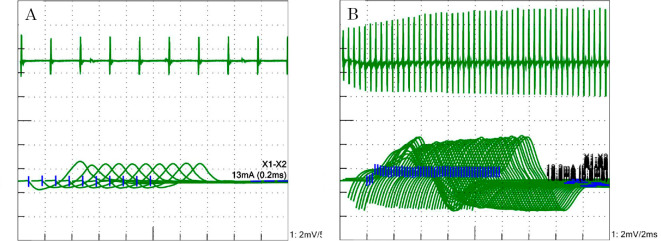
After four cycles of chemotherapy (three months after starting pembrolizumab), the patient complained of muscle weakness, ptosis, and dysarthria. Left eyelid ptosis, weakness in the left orbicularis oculi and bilateral proximal limb muscles, dysarthria, depressed Achilles tendon reflexes, and ataxia in the left upper limb and trunk were observed; apparent autonomic symptoms were not observed. In the edrophonium test, there were no appreciable changes in her ptosis. The serum anti-acetylcholine receptor antibody level was negative, while the anti-P/Q-type VGCC antibody level was as high as 124.9 pmol/L. A cerebrospinal fluid examination did not reveal any significant findings. The repetitive stimulation test showed waning with low-frequency stimulation (3 Hz) (Fig. 2A) and waxing with high-frequency stimulation (20 Hz) (Fig. 2B). Brain magnetic resonance imaging did not reveal any mass or recent infarction. Based on these results, we diagnosed her with LEMS recurrence as an irAE caused by pembrolizumab.
Figure 2.
A: The repetitive stimulation test showed waning from the trapezius muscle caused by the 3-Hz stimulation of the left accessory nerve. B: The repetitive stimulation test showed waxing from the abductor digiti minimi muscle caused by the 20-Hz stimulation of the right ulnar nerve.
Because the patient developed bulbar palsy, high-dose intravenous immunoglobulin (IVIG) therapy (20 g/day for 5 days) was started. Following pembrolizumab discontinuation and IVIG therapy, the patient's neurological symptoms improved. The chemotherapy was then continued without ICIs. Eight months after starting chemotherapy, the patient died due to worsening of the primary disease.
Discussion
ICIs are known to cause a variety of irAEs. The most common neurological irAEs, such as myositis, Guillain-Barré syndrome, myasthenic syndrome (2), and LEMS, have been reported in a few cases. Among these, most LEMS cases occur with SCLC and before treatment. The current case is rare because LEMS recurred in a patient with non-SCLC (NSCLC) and during ICI treatment.
To our knowledge, there have only been five reports of LEMS as irAEs caused by ICIs, including four cases of SCLC and one case of squamous cell carcinoma (3-7). The reported ICIs were nivolumab in two cases and nivolumab plus ipilimumab, atezolizumab, and pembrolizumab in one case each. There are also reports of the use of ICIs in patients who had already developed LEMS at the start of ICI treatment (8,9). Sakaguchi et al. reported a case of safe ICI use in SCLC complicated by LEMS (8). In contrast, Dohrn et al. reported a case of worsening neurological symptoms after avelumab treatment for Merkel cell carcinoma (9). Various ICIs may cause LEMS as irAEs; however, the safety of ICI administration to patients with LEMS is unknown. Although LEMS is often accompanied by SCLC, there have been no reports of relapsed LEMS during ICI administration in NSCLC after complete remission of SCLC associated with LEMS, as in this case. To our knowledge, this is the first case of LEMS relapse with pembrolizumab treatment after 22 years of complete remission.
The relapse of LEMS may be associated with squamous cell carcinoma; however, the final diagnosis is often LEMS associated with irAE caused by ICI. In our case, LEMS emerged when the patient was recovering from squamous cell carcinoma. There have been several previous reports of LEMS accompanied by squamous cell carcinoma (10-13), and the progression of primary lung cancer or its metastasis was correlated with the onset of LEMS, which was not seen in our case. Our patient had a history of LEMS 22 years earlier and was in remission. Therefore, we speculate that ICI administration activated immunity, consequently activating the anti-P/Q-type VGCC antibody. The anti-P/Q-type VGCC antibody level at the recurrence of LEMS was lower than that at the initial onset of LEMS, so remission was easily induced by IVIG therapy alone. Previous reports have shown that LEMS can develop as an irAE after anti-cytotoxic treatment based on an increase in the anti-P/Q-type VGCC antibody level, and indeed, similar findings were seen in the present case (3-6).
In general, pyridostigmine, corticosteroids, IVIG, and 3,4-diaminopyridine have been used for the treatment of LEMS. Although a treatment algorithm has been reported for LEMS (14), there are no treatment guidelines for LEMS induced as an irAE. In the present case, IVIG was highly effective in treating LEMS, suggesting that IVIG may be effective in the treatment of LEMS as an irAE induced by ICIs. However, if a patient's response to IVIG is poor, other drugs should be considered, such as corticosteroids, which are often used for the treatment of irAEs.
The incidence of LEMS as an irAE is expected to increase in the future. Patients with anti-P/Q-type VGCC antibodies and SCLC without the symptoms of LEMS have been previously reported (15). Therefore, it is necessary to pay close attention to the onset of LEMS in cases with a history of SCLC.
We herein report the first case of recurrence of LEMS as an irAE induced by ICIs 22 years after complete remission of SCLC associated with LEMS. Our case suggests the effectiveness of IVIG against LEMS as an irAE induced by ICIs.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We wish to thank the patient and her family. We are also grateful to Drs. Ami Hashimoto, Yuichiro Tominaga, and Momoka Nagae for their helpful discussions.
References
- 1.Sanders DB. Lambert-Eaton myasthenic syndrome: diagnosis and treatment. Ann N Y Acad Sci 998: 500-508, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Marini A, Bernardini A, Gigli GL, et al. Neurologic adverse events of immune checkpoint inhibitors: a systematic review. Neurology 96: 754-766, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani Y, Tanaka N, Enami T, Minami S, Okazaki T, Komuta K. Lambert-Eaton myasthenic syndrome caused by nivolumab in a patient with squamous cell lung cancer. Case Rep Neurol 10: 346-352, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal K, Agrawal N. Lambert-Eaton myasthenic syndrome secondary to nivolumab and ipilimumab in a patient with small-cell lung cancer. Case Rep Neurol Med 2019: 5353202, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill AJ, Gandhy S, Lancaster E. Nivolumab-associated Lambert-Eaton myasthenic syndrome and cerebellar dysfunction in a patient with a neuroendocrine tumor. Muscle Nerve 63: E18-E21, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Anderson CJ, Guidon AC, Khan FB, et al. Case report of Lambert Eaton myasthenic syndrome in a patient with small cell lung cancer on immune checkpoint inhibitor therapy. OBM Neurobiol 5: 9, 2021. [Google Scholar]
- 7.Kunii E, Owaki S, Yamada K, et al. Lambert-Eaton myasthenic syndrome caused by atezolizumab in a patient with small-cell lung cancer. Intern Med 61: 1739-1742, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi T, Kokubo Y, Furuhashi K, et al. An extensive-stage small-cell lung cancer case with preexisting Lambert-Eaton myasthenic syndrome successfully treated with an immune checkpoint inhibitor. Clin Lung Cancer 23: e273-e275, 2022. [DOI] [PubMed] [Google Scholar]
- 9.Dohrn MF, Schöne U, Küppers C, et al. Immunoglobulins to mitigate paraneoplastic Lambert Eaton myasthenic syndrome under checkpoint inhibition in Merkel cell carcinoma. Neurol Res Pract 2: 52, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tchouhadjian C, Barlesi F, Doddoli C, et al. Difficulties associated with Lambert-Eaton syndrome. Rev Mal Respir 21: 1167-1170, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Katada E, Nakamura T, Watanabe H, Matsukawa N, Ojika K, Sobue G. Lambert-Eaton myasthenic syndrome associated with pulmonary squamous cell carcinoma and circulating anti-PQ-type voltage-gated calcium channel antibody. Clin Neurol 50: 17-19, 2010(in Japanese). [DOI] [PubMed] [Google Scholar]
- 12.Dai Y, Li P, Yan S, Xia X, Li Z, Xia M. Lung squamous carcinoma with two paraneoplastic syndromes: dermatomyositis and Lambert-Eaton myasthenic syndrome. Clin Respir J 10: 495-499, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Martin-Moutot N, De Haro L, Seagar M. Distinct evolution of calcium channel antibody types in Lambert-Eaton myasthenic syndrome. J Neuroimmunol 197: 47-53, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Newsom-Davis J. A treatment algorithm for Lambert-Eaton myasthenic syndrome. Ann N Y Acad Sci 841: 817-822, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Zalewski NL, Lennon VA, Lachance DH, Klein CJ, Pittock SJ, Mckeon A. P/Q- and N-type calcium-channel antibodies: oncological, neurological, and serological accompaniments. Muscle Nerve 54: 220-227, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]