Abstract
As coronavirus disease 2019 (COVID-19) vaccine booster campaigns progress worldwide, new reports of complications following COVID-19 vaccination have emerged. We herein report a case of new-onset anti-glomerular basement membrane (GBM) disease concomitant with myeloperoxidase-antineutrophil cytoplasmic antibody positivity concurrent with high levels of interleukin (IL)-26 following the second dose of the Pfizer-BioNTech COVID-19 vaccine. The temporal association with vaccination in this case suggests that an enhanced neutrophilic immune response through IL-26 may have triggered necrotizing glomerulonephritis and a T-cell-mediated immune response to GBMs, leading to the development of anti-GBM antibodies, with an enhanced B-cell response after the vaccination triggering anti-GBM IgG and the onset of anti-GBM disease.
Keywords: anti-GBM disease, ANCA-associated glomerulonephritis, IL-26, mRNA-based COVID-19 vaccine, pharmacovigilance
Introduction
As coronavirus disease 2019 (COVID-19) vaccine booster campaigns progress worldwide, new reports of adverse events following mRNA-based COVID-19 vaccination have emerged, such as cases of anti-glomerular basement membrane (GBM) disease or anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) (1,2). The pathogenesis of anti-GBM disease has been well defined at the molecular level, but the factors that initiate the autoimmune process remain unclear (3).
AAV is a multisystem autoimmune disease, with neutrophil extracellular traps (NETs) involved in its pathogenesis (4). Interleukin (IL)-26 is a member of the IL-10 family of cytokines that participates in inflammatory signaling through directly binding DNA to facilitate cellular transduction and intracellular inflammation signaling (5). Recently, it has been shown that IL-26 binds to NETs to induce the secretion of inflammatory cytokines (IL-1β and IL-6) and chemokines (IL-8) by myeloid cells in ANCA-associated glomerulonephritis (6).
We herein report a case of new-onset anti-GBM disease concomitant with myeloperoxidase (MPO)-ANCA positivity with high levels of serum IL-26 following the receipt of the second dose of the Pfizer-BioNTech COVID-19 vaccine.
Case Report
A 67-year-old man with a history of pulmonary tuberculosis at 25 years old was admitted with a fever and anuria 6 weeks after his second vaccination. He had reported protracted systemic reactions with a low-grade fever and gross hematuria two weeks after the first dose. He received a second dose three weeks after the first dose. One month after the second dose, he developed a fever, anasarca, and anuria, which lasted over the next two weeks. He had received 6 months of chemotherapy for the treatment of pulmonary tuberculosis at 25 years old. There was no history of smoking or medication use, including propylthiouracil. Results of annual medical reviews had been normal, with serum creatinine levels of 0.6 mg/dL and normal urinalysis findings.
On admission, his blood pressure was 162/98 mmHg, and a physical examination revealed generalized edema. Laboratory tests revealed a white blood cell (WBC) count of 12,900/mm3, serum creatinine of 14.6 mg/dL, and albumin of 1.6 g/dL. A serologic evaluation revealed a C-reactive protein level of 37.0 mg/dL, positive anti-GBM IgG (>3,500 U/mL, reference range 3.0> U/mL), positive MPO-ANCA IgG (268 U/mL, reference range 3.5> U/mL), and positive IFN-γ release assays for tuberculosis. The levels of complement C3 and C4 were within the reference ranges, and testing for proteinase 3-ANCA, anti-nuclear antibody, hepatitis B virus, hepatitis C virus were negative. Polymerase chain reaction and serology testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were also negative. A urinalysis revealed blood (3+) and protein (3+) at 4,377 mg/dL, and urine microscopy showed >100 red blood cells per high-power field (>10% dysmorphic) and 100 WBCs per high-power field with granular casts. Computed tomography of the chest revealed nodules in the apex segment of the right upper lobe and bilateral mild pleural effusion, without pulmonary involvement. Three sputum smear examinations with Ziehl-Neelsen staining for the diagnosis of tuberculosis over a three-day period were negative. In addition, there were no negative culture results for those sputum specimens.
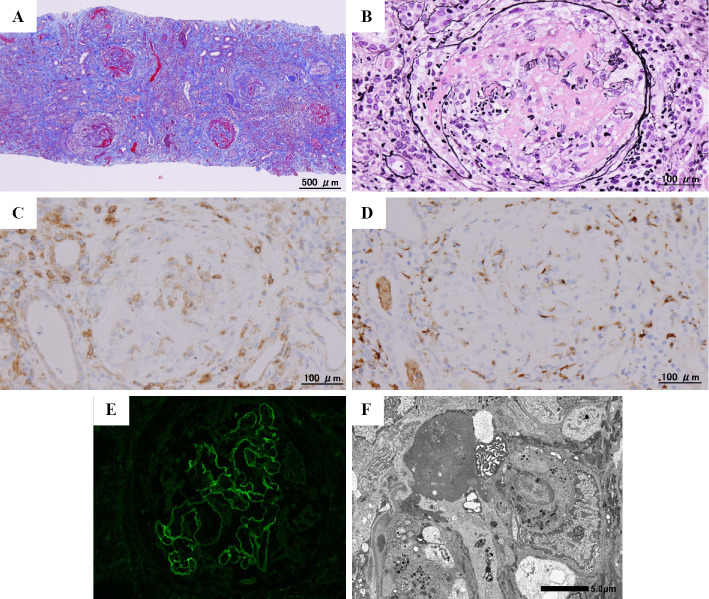
A kidney biopsy was performed 52 days after the second vaccination. Light microscopy of the kidney biopsy specimen showed cellular crescents and fibrinoid necrosis involving 43 of 45 glomeruli, with CD4 T cells and macrophages scattered throughout the glomeruli. Cortical tubules displayed diffuse acute epithelial injury with interstitial inflammation. Interstitial fibrosis and tubular atrophy were moderate. Immunofluorescence showed linear staining of GBMs for IgG1. Electron microscopy revealed disruption of GBMs and diffuse effacement of podocyte foot processes without immune complex-mediated deposits, leading to a diagnosis of anti-GBM glomerulonephritis (Fig. 1). Further investigations revealed a high serum IL-26 level of 517.1 pg/mL on an enzyme-linked immunosorbent assay (reference range: not detectable), IL-1β of 34.5 pg/mL, IL-6 of 1,577.9 pg/mL, tumor necrosis factor-α (TNF-α) of 300.6 pg/mL, granulocyte colony-stimulating factor (G-CSF) of 126.6 pg/mL, IL-8 of 615.4 pg/mL, and chemokine (C-X-C motif) ligand (CXCL) 1 of 1,480.9 pg/mL using the Bio Plex multi-plex system (Bio-Rad Laboratories, Hercules, USA). He carried human leukocyte antigen (HLA) DRB1*04:03 and DRB1*14:05 alleles.
Figure 1.
Kidney biopsy findings. Light microscopy of (A) the renal cortex showing multiple glomeruli with fibrinoid necrosis and crescentic formations associated with rupture of Bowman’s capsule (Masson trichrome, original magnification ×40) and of (B) a representative glomerulus showing fibrinoid necrosis, a circumferential cellular crescent, and destruction of the glomerular tuft and Bowman’s capsule with leukocytes, including neutrophils (Jones methenamine silver, original magnification ×200). Immunohistochemical staining for (C) CD4 and (D) CD68 showing scattered CD4 T cells and macrophages throughout the glomeruli as well as adjacent tubules and interstitium (original magnification ×200, respectively). Immunofluorescence for (E) IgG1 of glomeruli showing global linear staining along glomerular basement membranes (original magnification ×200). (F) Electron microscopy of the glomerulus showing disruption of the glomerular basement membranes, diffuse foot process effacement, and the absence of electron-dense deposits (original magnification, ×6,000).
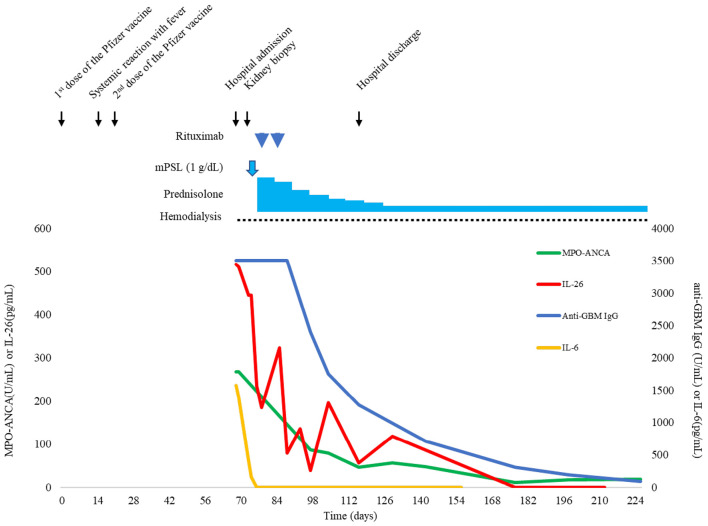
Intravenous methylprednisolone 1 g daily for 3 days was initiated the day after the kidney biopsy followed by oral prednisolone 1 mg/kg daily. Ten days after admission, he was treated with 2 doses of rituximab at 375 mg/m2 once a week. Hemodialysis was initiated on day 2 after admission. Prednisolone was tapered by 0.1 mg/kg/day weekly, and the patient was discharged after 50 days in hospital. The patient received 300 mg of isoniazid daily for latent tuberculosis infection over 9 months from the start of treatment for anti-GBM disease. Currently, he continues maintenance hemodialysis while on oral prednisolone at 10 mg/day. Nine months after discharge, neither pulmonary involvement nor relapse occurred, with serum levels of anti-GBM IgG, MPO-ANCA and inflammatory cytokines/chemokines, including IL-26, gradually decreasing or resolving (Fig. 2).
Figure 2.
Timeline of clinical events and trends in the serum IgG antibody to GBM titer, MPO-ANCA titer, serum IL-26 concentration, and serum IL-6 concentration from the time of vaccination until the four-month follow-up after discharge.
Discussion
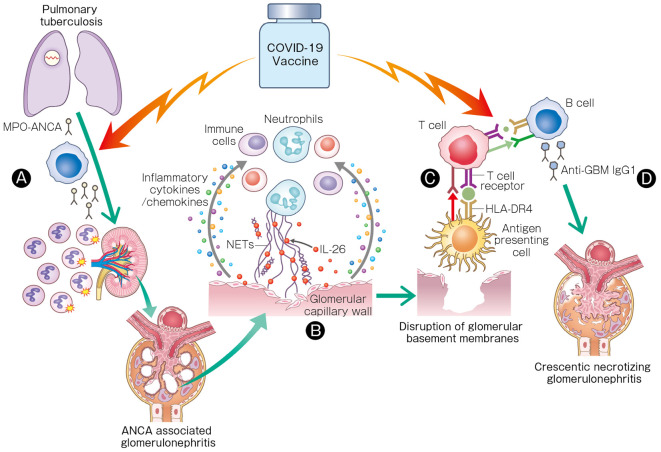
Several recent case reports have described the new onset of anti-GBM disease or AAV following COVID-19 vaccination (2,7,8). However, the mechanism concerning the contribution of the mRNA-based vaccines to these diseases remains unknown. To our knowledge, this is the first report of anti-GBM disease concomitant with ANCA positivity accompanied by a high serum IL-26 level following the second dose of the Pfizer-BioNTech COVID-19 vaccine. The temporal association with vaccination in this case suggests that an enhanced neutrophilic immune response through IL-26 may have triggered necrotizing glomerulonephritis and a T-cell-mediated immune response to GBMs, leading to the development of anti-GBM antibodies, with an enhanced B-cell response after the vaccination triggering anti-GBM IgG and the onset of anti-GBM disease (Fig. 3).
Figure 3.
Hypothetical schematic illustration of the role of IL-26 and the mRNA-based COVID-19 vaccine in anti-GBM disease concomitant with MPO-ANCA following vaccination. (A) Latent MPO-ANCA, which might have developed following pulmonary tuberculosis, may have been enhanced following receipt of the SARS-CoV-2 mRNA vaccine, triggering ANCA-associated glomerulonephritis. (B) IL-26 potentiates recruitment of immune cells to necrotizing lesions through inflammatory cytokines and chemokines, leading to ANCA-associated glomerulonephritis. (C) The T-cell-mediated immune response to peptides derived from the ruptured GBM through HLA-DR4 from HLA DRB1*04: 03 on antigen-presenting cells may have induced the development of anti-GBM antibodies. (D) The COVID-19 vaccine may have been responsible for triggering extremely high titers of anti-GBM IgG1 and the onset of anti-GBM disease.
The possible mechanisms underlying the new-onset adverse effects following mRNA-based COVID-19 vaccination reportedly include molecular mimicry, vaccine adjuvants, and polyclonal activation followed by the production of particular autoantibodies. Recently, Irure-Ventura et al. reported that 2 out of 35 patients who developed ANCA after SARS-CoV-2 vaccination developed ANCA and AAV after receiving the first dose of the Pfizer-BioNTech COVID-19 vaccine. One of the two cases developed ANCA six days after the first dose (9). A secondary effect from the polyclonal activation induced by the vaccine may be attributable to the production of ANCA by potential autoreactive clones specific for MPO. AAV has been known to occur under a variety of circumstances, including with infections (4,10). Certain infectious agents such as Mycobacterium tuberculosis may lead to development of ANCA (11,12). In our case, latent MPO-ANCA, which might have developed following pulmonary tuberculosis, may have been enhanced following receipt of the SARS-CoV-2 mRNA vaccine, potentially triggering ANCA-associated glomerulonephritis. Whether or not our case can be attributed to pulmonary tuberculosis-induced ANCAs is speculative but intriguing and warrants further investigation, given emerging cases of vaccination-induced AAV.
IL-26 directly binds neutrophil-derived extracellular DNA to facilitate intracellular inflammation signaling in a stimulator of interferon genes (STING)- and inflammasome-dependent manner (5). It is possible that an enhanced neutrophilic immune response through IL-26 after the vaccination may have been responsible for the ANCA-associated glomerulonephritis in our case. Recently, Poli et al. demonstrated that IL-26 is one of the most significant inflammatory mediators in active AAV (6). High levels of IL-26 are detected in the sera of active AAV patients with glomerulonephritis. Furthermore, IL-26 binds to NETs, and IL-26-DNA complexes induce secretion of inflammatory cytokines (TNF-α, IL-1β, IL-6, and G-CSF) and chemokines (IL-8 and CXCL1) by myeloid cells in the crescentic necrotizing lesions of ANCA-associated glomerulonephritis (6). In our case, IL-26 may have potentiated the recruitment of immune cells to the necrotizing lesions through inflammatory cytokines and chemokines, leading to ANCA-associated glomerulonephritis.
Anti-GBM disease is caused by autoimmunity to the α3 chain of type IV collagen of GBM. Susceptibility to anti-GBM disease is strongly associated with HLA-DRB1 genes, for which HLA-DRB1*15 and DRB1*04 are particularly responsible (13). In our case, the T-cell-mediated immune response to peptides derived from the ruptured GBM through HLA-DR4 from HLA DRB1*04:03 on antigen-presenting cells may have induced the development of anti-GBM antibodies. Furthermore, the vaccine is supposed to elicit CD4 cytokine responses involving type 1 helper T cells (14). It is possible that the enhanced B-cell response after the second dose of the vaccine was responsible for triggering the extremely high titers of anti-GBM IgG1 and the subsequent onset of anti-GBM disease.
Whether AAV predisposes patients to the development of anti-GBM disease or if ANCA positivity occurs in the course of anti-GBM disease is unclear at present (15). Patients with anti-GBM disease may be classified into at least two clusters based on clinical presentation: older adults presenting with kidney involvement alone with a high proportion of ANCA positivity and younger individuals with pulmonary involvement and a lower proportion of double positivity (16). The recent confirmation of spatial and temporal clustering of anti-GBM disease suggests that environmental factors, including certain infections, may trigger the disease in susceptible individuals (17,18). We suspect that genetic susceptibility to anti-GBM disease and the history of pulmonary tuberculosis, followed by vaccination, may have triggered the secondary autoimmunity in our case.
Anti-GBM disease is a monophasic non-relapsing illness. However, this may not be so in patients with dual positivity for both ANCA and anti-GBM antibodies. The relapse of clinical features while anti-GBM antibodies are still present is common, and although relapses can also occur in double-positive cases, such instances are often in the context of coexistent ANCA positivity (19). In the present case, decreases in serum IL-26 levels were correlated to a decline in the serum levels of anti-GBM IgG and MPO-ANCA, with no relapses. IL-26 might be a specific biomarker of disease activity. Furthermore, we recently developed a humanized neutralizing anti-IL-26 monoclonal antibody (mAb) for therapeutic use (20). Humanized anti-IL-26 mAb might be a useful therapeutic agent for the treatment of AAV.
This case highlights the association between an aberrant immune response to a vaccine and development of anti-GBM disease concomitant with MPO-ANCA positivity through IL-26. A deeper analysis of the immune response through IL-26 may provide better insight into the mechanism underlying the development of AAV and anti-GBM disease. However, at present, there is insufficient evidence to postulate causality, as it may have been coincidental that mRNA-based SARS-CoV-2 vaccine administration closely preceded the new-onset anti-GBM disease with ANCA positivity. Given the possibility of further usage of mRNA-based vaccines against viral infections, strict pharmacovigilance will be important to determine the true frequency and potential causality between these vaccines and small-vessel vasculitides.
The authors declare that they have obtained consent from the patient reported in this article for the publication of information about him that appears within this Case Report.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. September 1, 2022 [Internet]. Available from: https://coronavirus.jhu.edu/map.html
- 2.Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int 100: 959-965, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellmark T, Segelmark M. Diagnosis and classification of Goodpasture's disease (anti-GBM). J Autoimmun 48-49: 108-112, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Nachman PH. ANCA glomerulonephritis and vasculitis. Clin J Am Soc Nephrol 12: 1680-1691, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shabgah AG, Abdelbasset WK, Rahman HS, et al. A comprehensive review of IL-26 to pave a new way for a profound understanding of the pathobiology of cancer, inflammatory diseases and infections. Immunology 165: 44-60, 2022. [DOI] [PubMed] [Google Scholar]
- 6.Poli C, Augusto JF, Dauvé J, et al. IL-26 confers proinflammatory properties to extracellular DNA. J Immunol 198: 3650-3661, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 78: 611-613, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RK, Ellis BK. Concurrent antiglomerular basement membrane nephritis and antineutrophil cytoplasmic autoantibody-mediated glomerulonephritis after second dose of SARS-CoV-2 mRNA vaccination. Kidney Int Rep 7: 127-128, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irure-Ventura J, Belmar-Vega L, Fernández-Fresnedo G, et al. Increased induction of de novo serum ANCA and ANCA-associated vasculitis after mass vaccination against SARS-CoV-2. iScience 25: 104847, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frangou E, Vassilopoulos D, Boletis J. Boumpas DT. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): implications for the pathogenesis and treatment. Autoimmun Rev 18: 751-760, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Suárez LF, Cabiedes J, Villa AR, van der Woude FJ, Alcocer-Varela J. Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology (Oxford) 42: 223-229, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Elkayam O, Bendayan D, Segal R, et al. The effect of anti-tuberculosis treatment on levels of anti-phospholipid and anti-neutrophil cytoplasmatic antibodies in patients with active tuberculosis. Rheumatol Int 33: 949-953, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Fisher M, Pusey CD, Vaughan RW, Rees AJ. Susceptibility to anti-glomerular basement membrane disease is strongly associated with HLA-DRB1 genes. Kidney Int 51: 222-229, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 586: 594-599, 2020. [DOI] [PubMed] [Google Scholar]
- 15.Levy JB, Hammad T, Coulthart A, Dougan T, Pusey CD. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int 66: 1535-1540, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Alchi B, Griffiths M, Sivalingam M, Jayne D, Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant 30: 814-821, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Canney M, O'Hara PV, McEvoy CM, et al. Spatial and temporal clustering of anti-glomerular basement membrane disease. J Am Soc Nephrol 11: 1392-1399, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prendecki M, Candice Clarke C, Cairns T, et al. Anti-glomerular basement membrane disease during the COVID-19 pandemic. Kidney Int 98: 780-781, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy JB, Lachmann RH, Pusey CD. Recurrent Goodpasture's disease. Am J Kidney Dis 27: 573-578, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Hatano R, Itoh T, Otsuka H, et al. Humanized anti-IL-26 monoclonal antibody as a novel targeted therapy for chronic graft-versus-host disease. Am J Transplant 22: 2804-2820, 2022. [DOI] [PubMed] [Google Scholar]